Abstract
The combination of resistance exercise and creatine supplementation has been shown to decrease body fat percentage in adults ≥ 50 years of age. However, the effect on adults < 50 years of age is currently unknown. To address this limitation, we systematically reviewed the literature and performed several meta-analyses comparing studies that included resistance exercise and creatine supplementation to resistance exercise and placebo on fat mass and body fat percentage Twelve studies were included, involving 266 participants. Adults (<50 years of age) who supplemented with creatine and performed resistance exercise experienced a very small, yet significant reduction in body fat percentage (−1.19%, p = 0.006); however, no difference was found in absolute fat mass (−0.18 kg, p = 0.76). Collectively, in adults < 50 years of age, the combination of resistance exercise and creatine supplementation produces a very small reduction in body fat percentage without a corresponding decrease in absolute fat mass.
1. Introduction
There has been a significant increase in the prevalence of adiposity in young adults [1] which could lead to the development of adverse health conditions such as obesity, cardiovascular disease, and type 2 diabetes later in life [2,3]. From an overall health and longevity perspective, lifestyle interventions that help regulate fat mass are likely important for promoting a healthier metabolic phenotype over time [4,5].
A recent systematic review and meta-analysis involving over 800 healthy adults (≥19 years) showed that resistance exercise (≥4 times per week for up to 2 years) decreased fat mass by 0.55 kg (95% CI: −0.75 to −0.34; p < 0.0001) and body fat percentage by 1.46% (95% CI: −1.78 to −1.14; p < 0.0001) over time [6]. These small, yet beneficial changes, may be related to the stimulating effects of resistance exercise on the resting metabolic rate [7], excess post-oxygen consumption [8], and circulating levels of non-esterified fatty acids and by decreasing the respiratory quotient (indicating increased adipocyte lipolysis and/or intramuscular triglyceride oxidation) [9]. In addition to these small benefits of resistance exercise, there is some evidence that creatine supplementation may also lead to a reduction in body fat over time. We previously performed a meta-analysis showing that healthy older adults (n = 609; 19 studies; ≥50 years) who supplemented with creatine (≥2 g/day) and performed resistance exercise (2–3 times/week for up to 1 year) experienced a significant reduction in body fat percentage (0.55%; CI: −1.08 to −0.03; p = 0.04), but no differences were observed in regard to fat mass (−0.50 kg; 95% CI: −1.15 to 0.15; p = 0.13) compared to resistance exercise alone [10]. However, the generalizability of these findings is limited because older adults have a high degree of variability in their responsiveness and adherence to resistance exercise and creatine supplementation [11].
Interestingly, in children (n = 9) suffering from cancer (acute lymphoblastic leukemia), creatine significantly reduced body fat percentage over time (p < 0.05) [12], whereas other studies revealed no effect [13,14,15,16,17,18,19,20,21,22,23,24]. A limitation of most individual studies is that it is typically difficult to obtain adequate statistical power to detect the small differences between creatine and placebo over time due to small sample sizes. Combining studies into a meta-analysis helps overcome this limitation by assessing a large cohort of individuals. However, the meta-analytic effects of resistance exercise and creatine supplementation in adults < 50 years of age are currently unknown. This is important to determine because a common belief held by many exercising individuals is that creatine supplementation may increase fat mass over time [25], which is likely a deterrent to supplement with creatine. Therefore, the purpose of this systematic review and meta-analysis was to determine the effects of resistance exercise and creatine supplementation vs. resistance exercise alone on measures of fat mass (i.e., absolute body fat mass and body fat percentage) in adults < 50 years of age, while accounting for several confounders, including creatine dose and duration, and health status.
2. Materials and Methods
The PRISMA (preferred reporting items for systematic reviews and meta-analyses) standards were followed to conduct this systematic review and meta-analysis [26] and the protocol was registered in the PROSPERO (International Prospective Register of Systematic Reviews) database (CRD:42023416700).
2.1. Search Strategy
From the inception to April 2023, two separate reviewers (K.P. and F.R.) searched PubMed, Scopus, Web of Science, and the Cochrane library, using the following keywords: “creatine supplementation” OR “creatine” OR “creatine monohydrate” AND “body fat*” OR “body composition”. The following inclusion criteria were used: (1) studies had to be randomized controlled trials (RCTs); (2) the mean age of participants was <50 years irrespective of health status; (3) the intervention group was receiving creatine monohydrate and resistance exercise, and the comparator group was receiving resistance exercise with placebo; (4) the evaluation of fat mass was performed via dual X-ray absorptiometry (DXA), bioelectrical impedance (BIA), hydrodensitometry, magnetic resonance imaging (MRI), a computed tomography (CT) scan, or air displacement plethysmography (Bod Pod); (5) there was a minimum study duration of 4 weeks; and all of these were (6) irrespective of the language written. Studies were excluded if they: (1) were not RCTs; (2) provided only the abstract; (3) had subjects with any kind of dietary restrictions (i.e., vegans/vegetarians); or (4) were articles written by the same authors using identical populations that may have included data related to our outcomes of interest.
2.2. Data Extraction and Risk of Bias
Data was independently extracted by two investigators (K.P. and F.R.). The name of the first author, publication date, country of origin, study design, participant age, sex, and health status, sample size, outcomes assessed, dose and duration of creatine supplementation, fat mass assessment tool, and dietary intake assessment were among the information that was extracted. A third investigator (D.G.C.) settled disagreements between the authors. Version 2 of the Cochrane risk-of-bias 2 instrument for randomized trials (RoB2) was used to evaluate the quality of the included studies, and it was reviewed by two independent reviewers (K.P. and S.C.F.). The appraisal of the risk of bias using the RoB2 tool included the assessment of the following domains of bias in RCTs: (1) the randomization process, (2) the deviations from intended interventions, (3) the missing outcome data, (4) the measurement of the outcome, and (5) the selection of the reported result. The study quality was categorized as either having a low risk of bias, considerable concerns, or a high risk of bias using the RoB2 tool rating system.
2.3. Statistical Analysis
The mean differences between groups were calculated by comparing changes in outcomes from baseline to follow-up, treating quantitative data as continuous measurements. Standardized mean differences were employed when measurement units were inconsistent (e.g., body fat percentage changes mixed with absolute body fat kilogram changes) and could not be changed to the units needed for the analyses. The inverse-variance approach and the random-effects model were used to determine statistical significance. Standard deviations and missing data for any changes between the baseline and follow-up outcome data were determined by deriving a correlation coefficient of 0.5, considering that a value of standard deviation change from baseline derived from an included study was not provided.
Utilizing the overlap of their 95% confidence intervals (CIs) and expressing the results as a measurement of Cochran’s Q (χ2 test) and I2, the statistical heterogeneity of the outcome measurements across the included studies was evaluated. Low heterogeneity was considered when the I2 levels were <50%, moderate heterogeneity between 50% and 74.9%, and high heterogeneity ≥ 75%. Subgroup analyses based on age (<40 years vs. 41–49 years), sex (males only vs. females only vs. mixed sexes), fat mass assessment tool (DXA vs. BIA vs. Hydrodensitometry vs. Bod Pod), body mass index (BMI) (<25 kg/m2 vs. ≥25 kg/m2), creatine monohydrate duration (<8 weeks vs. ≥8 weeks) and dose (≤5 g/d vs. >5 g/d), and resistance exercise frequency (≤3x/week vs. >3x/week) were performed. Additionally, sensitivity analyses were performed to evaluate the robustness of the reported statistical results by discounting the effects of a lack of dietary intake assessment, participants with comorbidities, and studies with an increased risk of bias. The meta-analyses were synthesized using Cochrane’s Review Manager (RevMan 5.4.1) software.
3. Results
3.1. Literature Search
In the initial literature search, 3028 publications were found. Of these, 486 duplicate publications were eliminated, leaving 2542 distinct publications, from which 2134 were deemed ineligible based on titles and abstracts, and another 376 publications were not retrieved due to irrelevant study designs and outcomes of interest. In total, 32 RCTs investigating the effects of creatine monohydrate on body fat in adults aged < 50 years were found. After further examination of the remaining publications, five of these used skinfold calipers for the measurement of body fat, four used creatine monohydrate in the absence of resistance training, three had a short-term treatment duration (<4 weeks), two used the Siri equation to quantify body fat, one had inadequate data, one used endurance training, one used high-intensity interval training, and three did not have a standardized resistance training protocol. Overall, 12 RCTs were included in this systematic review and meta-analysis (Figure 1), involving 266 participants (130 in the creatine monohydrate and resistance exercise group and 133 in the placebo and resistance exercise group). A detailed description of the included studies is depicted in Table 1.
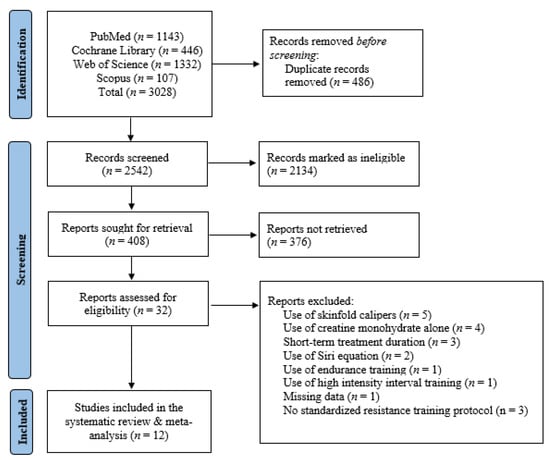
Figure 1.
Study selection flow chart.

Table 1.
Summary of study characteristics.
3.2. Creatine Supplementation and Body Fat Changes
Our main analysis showed that creatine supplementation did not significantly impact changes in absolute fat mass (kg) over time (k = 6; MD = −0.18; 95%CI, −1.32 to 0.96; I2 = 0%; p = 0.76) (Figure 2). However, creatine did produce a significant reduction in body fat percentage over time (k = 10; MD = −1.19; 95% CI, −2.03 to −0.34; I2 = 0%; p = 0.006) (Figure 3).
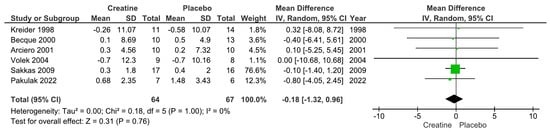
Figure 2.
Forest plots for changes in absolute fat mass (kg) [13,14,18,19,21,22].
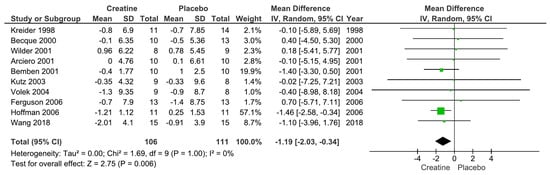
Figure 3.
Forest plot for changes in body fat percentage [13,14,16,17,18,20,22,23,24,27].
The subgroup analysis based on age (<40 years vs. 41–49 years) showed that creatine supplementation did not influence fat mass more than placebo (<40 years: SMD = −0.18; 95% CI, −0.44 to 0.08; I2 = 0%; p = 0.18 vs. 41–49 years: SMD = −0.05; 95% CI, −0.73 to 0.63; p = 0.88) (Figure S1). Similar results were found with regards to the fat mass assessment tool (DXA: SMD = −0.13; 95% CI, −0.46 to 0.20; I2 = 0%; p = 0.44 vs. BIA: SMD = −0.27; 95% CI, −0.99 to 0.45; p = 0.47 vs. hydrodensitometry: SMD = −0.17; 95% CI, −0.62 to 0.29; I2 = 0%; p = 0.47 vs. Bod Pod: SMD = −0.26; 95% CI, −1.35 to 0.84; p = 0.65) (Figure S2), BMI (<25 kg/m2: SMD = −0.14; 95% CI, −0.45 to 0.16; I2 = 0%; p = 0.36 vs. ≥25 kg/m2: SMD = −0.20; 95% CI, −0.61 to 0.22; I2 = 6%; p = 0.35) (Figure S3), sex (Females only: SMD = 0.08; 95% CI, −0.69 to 0.85; p = 0.84 vs. Males only: SMD = −0.19; 95% CI, −0.45 to 0.08; I2 = 0%; p = 0.17 vs. Mixed: SMD = −0.26; 95% CI, −1.35 to 0.84; p = 0.65) (Figure S4), creatine dose (<5 g: SMD = −0.12; 95% CI, −0.43 to 0.18; I2 = 0%; p = 0.43 vs. ≥5 g: SMD = −0.24; 95% CI, −0.65 to 0.17; I2 = 2%; p = 0.26) (Figure S5) and duration of supplementation (<8 weeks: SMD = −0.08; 95% CI, −0.41 to 0.25; I2 = 0%; p = 0.63 vs. ≥8 weeks: SMD = −0.28; 95% CI, −0.69 to 0.13; I2 = 20%; p = 0.18) (Figure S6), and resistance exercise frequency (≤3x/week: SMD = −0.07; 95% CI, −0.41 to 0.26; I2 = 0%; p = 0.67 vs. >3x/week: SMD = −0.27; 95% CI, −0.63 to 0.09; I2= 2%; p = 0.14) (Figure S7).
The sensitivity analysis excluding participants with health conditions (Healthy participants: SMD = −0.18; 95% CI, −0.44 to 0.08; I2 = 0%; p = 0.18 vs. Unhealthy participants: SMD = −0.05; 95% CI, −0.73 to 0.63; p = 0.88) (Figure S8), studies that did not assess for dietary intake (Assessment: SMD = −0.20; 95% CI, −0.52 to 0.11; I2 = 0%; p = 0.20 vs. No assessment: SMD = −0.10; 95% CI, −0.49 to 0.29; I2 = 0%; p = 0.61) (Figure S9), and studies with increased risk of bias (SMD = −0.19; 95% CI, −0.45 to 0.08; I2 = 0%; p = 0.17) (Figure S10) did not alter any of the findings.
3.3. Risk of Bias Assessment
Four of the studies were classified as having a low risk of bias [16,17,19,21], six studies had a moderate risk [13,18,22,23,24,27], and two studies had a high risk of bias [14,20]. These concerns primarily arose due to the absence of specific details regarding randomization procedures or treatment allocation, considering that two studies did not report whether the participants were randomized [14,20]. Lastly, in one study, the supplement was provided in a single-blind fashion [24]. A detailed description of the risk of bias assessment is depicted in Figure 4.
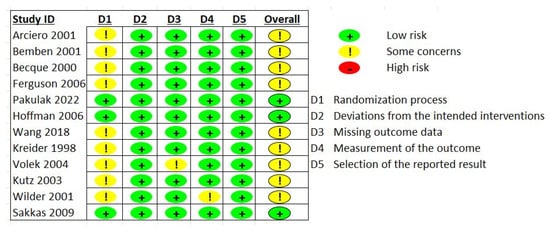
Figure 4.
Risk of bias assessment [13,14,16,17,18,19,20,21,22,23,24,27].
4. Discussion
This is the first meta-analysis to examine the efficacy of resistance exercise and creatine supplementation vs. resistance exercise alone on measures of fat mass in adults < 50 years of age. Results showed that the combination of resistance exercise and creatine supplementation (≥4 weeks) significantly reduced body fat percentage by 1.19%, without a corresponding reduction in absolute fat mass (−0.18 kg), compared to resistance exercise alone. Variables such as age (<40 years; 41–49 years), sex (females only; males only; females and males combined), fat mass assessment tool, creatine dosage (<5 g; ≥5 g) and duration of creatine supplementation (<8 weeks; ≥8 weeks), and resistance training frequency (≤3x/week; >3x/week) did not alter these findings.
The minimal reduction in body fat percentage from resistance exercise and creatine supplementation in adults < 50 years is comparable to our previous meta-analysis findings in adults ≥ 50 years (body fat percentage: −0.55) [10]. Collectively, these findings help refute the common belief held by many exercising individuals that creatine supplementation increases fat mass over time [25]. However, these results are likely clinically and practically insignificant, considering that the very small reduction in body fat percentage did not correspond to a significant reduction in absolute fat mass. Although speculative, the very small reduction in body fat percentage from creatine could be attributable to changes in lean mass and/or muscle accretion over time. For example, several meta-analyses have been performed collectively showing that the combination of creatine supplementation and resistance exercise increases measures of whole-body lean tissue mass by ~1.37 kg (as measured by dual-energy X-ray absorptiometry, air-displacement plethysmography, hydrodensitometry, and bioelectrical impedance analysis) compared to placebo and resistance exercise [28,29,30,31,32]. Furthermore, Burke et al. [33] performed a systematic review and meta-analysis involving 10 studies and found significant improvements in direct measures of limb muscle hypertrophy (0.10–0.16 cm; as measured using ultrasound and peripheral quantitative computed tomography {pQCT}) in the upper- and lower-body from creatine supplementation and resistance exercise compared to resistance exercise and placebo. Interestingly, the lone study that used pQCT showed that creatine supplementation (52 weeks) increased lower-limb muscle density (Δ + 0.83 ± 1.15 mg·cm−3; p = 0.016) compared to placebo (Δ −0.16 ± 1.56 mg·cm−3). Mechanistically, these lean tissue and regional muscle improvements may be related to creatine increasing cellular hydration status, high-energy phosphate metabolism (phosphocreatine content and recovery), glycogen synthesis, satellite cell proliferation and activity, growth factor production and expression (i.e., insulin-like growth factor-1), myogenic transcription factor expression (Myf5, Mrf4, MyoD, and myogenin), protein kinases downstream in the mammalian target of the rapamycin (mTOR) signaling pathway which are involved in translation and decreasing measures of inflammation, oxidative stress (reactive oxygen species), and protein catabolism (whole-body leucine oxidation and urinary excretion of 3-methylhistidine) [31]. The significant increases in whole-body lean tissue mass, limb muscle hypertrophy, and muscle density from creatine supplementation may increase energy expenditure which could reduce body fat percentage over time [34]. However, even with the inclusion of increased weekly resistance exercise sessions, we did not observe an additional response in body fat levels. Our results indirectly support this notion as there was a statistically significant reduction in body fat percentage with only a small, non-significant change in fat mass over time. Unfortunately, the mechanistic effects of creatine, with and without resistance exercise, in healthy adults (≥18 years) are unknown.
Limitations
Results from this meta-analysis are likely affected by the measurement errors of the body composition assessment tool used and subsequent changes in lean tissue and/or total body water. Further, multiple studies did not control the dietary intake of creatine which may have influenced our findings. Moreover, considering the fundamental physiological differences underpinning men and women, we could not establish a subgroup analysis or a meta-regression to account for this confounder. Another limitation can also be attributed to the varied training status of the participants and the variability pertaining to the intensity of exercise protocols. Finally, no mechanisms of lipolysis or beta-oxidation were determined in this meta-analysis.
5. Conclusions
In adults < 50 years of age, the combination of resistance exercise and creatine supplementation results in a minimal reduction in body fat percentage (1.19%) with no effect on absolute fat mass compared to resistance exercise alone. Creatine supplementation combined with resistance exercise does not increase fat mass in adults < 50 years of age.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15204343/s1, Figure S1: Subgroup analysis based on age (<40 years vs. 41–49 years); Figure S2: Subgroup analysis according to fat mass assessment tool; Figure S3: Subgroup analysis based on BMI (<25 kg/m2 vs. ≥25 kg/m2); Figure S4: Subgroup analysis based on sex (females vs. males vs. mixed); Figure S5: Subgroup analysis based on creatine dose (<5 g vs. ≥5 g); Figure S6: Subgroup analysis based on duration of supplementation (<8 weeks vs. ≥8 weeks); Figure S7: Subgroup analysis based on resistance exercise frequency (≤3x/week vs. >3x/week); Figure S8: Sensitivity analysis excluding participants with health conditions; Figure S9: Sensitivity analysis excluding studies that did not assess dietary intake; Figure S10: Sensitivity analysis excluding studies with increased risk of bias.
Author Contributions
Conceptualization, D.G.C., S.C.F. and K.P; methodology, D.G.C., S.C.F., K.P. and F.R.; validation, S.C.F. and K.P.; formal analysis, S.C.F. and K.P. writing—original draft preparation, all authors; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
D.G.C. has conducted industry-sponsored research involving creatine supplementation and received creatine donations for scientific studies and travel support for presentations involving creatine supplementation at scientific conferences. In addition, D.G.C. serves on the Scientific Advisory Board for Alzchem (a company that manufactures creatine) and as an expert witness/consultant in legal cases involving creatine supplementation. S.C.F. previously served as a scientific advisor for a company that sold creatine; has received creatine donations for scientific studies; sells creatine education resources; and is a sports nutrition advisor for the International Society of Sports Nutrition. S.C.F. is a scientific advisor for BearBalanced. B.I.C. has no conflicts in terms of financial or business interests related to this manuscript. B.I.C. has received grants and contracts to conduct research on dietary supplements; has served as a paid consultant for industry; has received honoraria for speaking at conferences and writing lay articles about sports nutrition ingredients and topics; is a member of the International Protein Board which disseminates knowledge on protein and protein products; has served as an expert witness on behalf of the plaintiff and defense in cases involving dietary supplements; and receives compensation for writing and providing educational services related to exercise and nutrition-related topics. S.M.O. serves on the Scientific Advisory Board for Alzchem (a company that manufactures creatine). S.M.O. owns the patent “Sports Supplements Based on Liquid Creatine” at the European Patent Office (WO2019150323 A1) and an active patent application for “Synergistic Creatine” at the UK Intellectual Property Office (GB2012773.4). S.M.O. has served as a speaker at Abbott Nutrition, as a consultant of Allied Beverages Adriatic and IMLEK, and has received research funding related to creatine from the Serbian Ministry of Education, Science, and Technological Development, Provincial Secretariat for Higher Education and Scientific Research, AlzChem GmbH, KW Pfannenschmidt GmbH, ThermoLife International LLC, and Hueston Hennigan LLP. K.P. and F.R. declare no conflicts.
References
- Souza, N.A.B.; Rimes-Dias, K.A.; Costa, J.C.; Canella, D.S. Weight Gain and Change in Body Mass Index after Age 20 in the Brazilian Population and Associated Sociodemographic Factors: Data from the National Health Survey. Int. J. Environ. Res. Public Health 2022, 19, 2851. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.P.; De Souza Santos, R.; Palmer, B.F.; Clegg, D.J. Determinants of body fat distribution in humans may provide insight about obesity-related health risks. J. Lipid Res. 2019, 60, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.; Llewellyn, A.; Owen, C.G.; Woolacott, N. Predicting adult obesity from childhood obesity: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Jakicic, J.M.; Powell, K.E.; Campbell, W.W.; Dipietro, L.; Pate, R.R.; Pescatello, L.S.; Collins, K.A.; Bloodgood, B.; Piercy, K.L. Physical Activity and the Prevention of Weight Gain in Adults: A Systematic Review. Med. Sci. Sports Exerc. 2019, 51, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Fruh, S.; Williams, S.; Hayes, K.; Hauff, C.; Hudson, G.M.; Sittig, S.; Graves, R.J.; Hall, H.; Barinas, J. A practical approach to obesity prevention: Healthy home habits. J. Am. Assoc. Nurse Pract. 2021, 33, 1055–1065. [Google Scholar] [CrossRef]
- Wewege, M.A.; Desai, I.; Honey, C.; Coorie, B.; Jones, M.D.; Clifford, B.K.; Leake, H.B.; Hagstrom, A.D. The Effect of Resistance Training in Healthy Adults on Body Fat Percentage, Fat Mass and Visceral Fat: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 287–300. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie-Shalders, K.; Kelly, J.T.; So, D.; Coffey, V.G.; Byrne, N.M. The effect of exercise interventions on resting metabolic rate: A systematic review and meta-analysis. J. Sports Sci. 2020, 38, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Schuenke, M.D.; Mikat, R.P.; McBride, J.M. Effect of an acute period of resistance exercise on excess post-exercise oxygen consumption: Implications for body mass management. Eur. J. Appl. Physiol. 2002, 86, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Vechetti, I.J.; Peck, B.D.; Wen, Y.; Walton, R.G.; Valentino, T.R.; Alimov, A.P.; Dungan, C.M.; Van Pelt, D.W.; von Walden, F.; Alkner, B.; et al. Mechanical overload-induced muscle-derived extracellular vesicles promote adipose tissue lipolysis. FASEB J. 2021, 35, e21644. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Candow, D.; Krentz, J.; Roberts, M.; Young, K. Changes in Fat Mass Following Creatine Supplementation and Resistance Training in Adults ≥ 50 Years of Age: A Meta-Analysis. J. Funct. Morphol. Kinesiol. 2019, 4, 62. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.; Forbes, S.; Chilibeck, P.; Cornish, S.; Antonio, J.; Kreider, R. Variables influencing the effectiveness of creatine supplementation as a therapeutic intervention for sarcopenia. Front. Nutr. 2019, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, J.M.; Nagel, K.; Pearce, E.; Wright, M.; Barr, R.D.; Tarnopolsky, M.A. Creatine monohydrate attenuates body fat accumulation in children with acute lymphoblastic leukemia during maintenance chemotherapy. Pediatr. Blood Cancer 2008, 51, 183–187. [Google Scholar] [CrossRef]
- Arciero, P.J.; Hannibal, N.S.; Nindl, B.C.; Gentile, C.L.; Hamed, J.; Vukovich, M.D. Comparison of creatine ingestion and resistance training on energy expenditure and limb blood flow. Metabolism 2001, 50, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Becque, M.D.; Lochmann, J.D.; Melrose, D.R. Effects of oral creatine supplementation on muscular strength and body composition. Med. Sci. Sports Exerc. 2000, 32, 654–658. [Google Scholar] [CrossRef]
- Bemben, M.; Witten, M.; Carter, J.; Eliot, K.; Knehans, A.; Bemben, D. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J. Nutr. Health Aging 2010, 14, 155–159. [Google Scholar] [CrossRef]
- Ferguson, T.B.; Syrotuik, D.G. Effects of creatine monohydrate supplementation on body composition and strength indices in experienced resistance trained women. J. Strength Cond. Res. 2006, 20, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.; Ratamess, N.; Kang, J.; Mangine, G.; Faigenbaum, A.; Stout, J. Effect of creatine and beta-alanine supplementation on performance and endocrine responses in strength/power athletes. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 430–446. [Google Scholar] [CrossRef] [PubMed]
- Kreider, R.B.; Ferreira, M.; Wilson, M.; Grindstaff, P.; Plisk, S.; Reinardy, J.; Cantler, E.; Almada, A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sports Exerc. 1998, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Pakulak, A.; Candow, D.G.; de Zepetnek, J.T.; Forbes, S.C.; Basta, D. Effects of creatine and caffeine supplementation during resistance training on body composition, strength, endurance, rating of perceived exertion and fatigue in trained young adults. J. Diet. Suppl. 2022, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Kutz, M.; Gunter, M. Creatine monohydrate supplementation on body weight and percent body fat. J. Strength Cond. Res. 2003, 17, 817–821. [Google Scholar]
- Sakkas, G.K.; Mulligan, K.; DaSilva, M.; Doyle, J.W.; Khatami, H.; Schleich, T.; Kent-Braun, J.A.; Schambelan, M. Creatine fails to augment the benefits from resistance training in patients with HIV infection: A randomized, double-blind, placebo-controlled study. PLoS ONE 2009, 4, e4605. [Google Scholar] [CrossRef] [PubMed]
- Volek, J.S.; Ratamess, N.A.; Rubin, M.R.; Gómez, A.L.; French, D.N.; McGuigan, M.M.; Scheett, T.P.; Sharman, M.J.; Häkkinen, K.; Kraemer, W.J. The effects of creatine supplementation on muscular performance and body composition responses to short-term resistance training overreaching. Eur. J. Appl. Physiol. 2004, 91, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-C.; Fang, C.-C.; Lee, Y.-H.; Yang, M.-T.; Chan, K.-H. Effects of 4-Week Creatine Supplementation Combined with Complex Training on Muscle Damage and Sport Performance. Nutrients 2018, 10, 1640. [Google Scholar] [CrossRef]
- Wilder, N.; Deivert, R.G.; Hagerman, F.; Gilders, R. The Effects of Low-Dose Creatine Supplementation Versus Creatine Loading in Collegiate Football Players. J. Athl. Train. 2001, 36, 124. [Google Scholar]
- Antonio, J.; Candow, D.G.; Forbes, S.C.; Gualano, B.; Jagim, A.R.; Kreider, R.B.; Rawson, E.S.; Smith-Ryan, A.E.; VanDusseldorp, T.A.; Willoughby, D.S.; et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021, 18, 13. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Bemben, M.G.; Bemben, D.A.; Loftiss, D.D.; Knehans, A.W. Creatine supplementation during resistance training in college football athletes. Med. Sci. Sports Exerc. 2001, 33, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.C.; Candow, D.G.; Ostojic, S.M.; Roberts, M.D.; Chilibeck, P.D. Meta-analysis examining the importance of creatine ingestion strategies on lean tissue mass and strength in older adults. Nutrients 2021, 13, 1912. [Google Scholar] [CrossRef] [PubMed]
- Chilibeck, P.D.; Kaviani, M.; Candow, D.G.; Zello, G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sport. Med. 2017, 8, 226. [Google Scholar] [CrossRef]
- Delpino, F.M.; Figueiredo, L.M.; Forbes, S.C.; Candow, D.G.; Santos, H.O. Influence of age, sex, and type of exercise on the efficacy of creatine supplementation on lean body mass: A systematic review and meta-analysis of randomized clinical trials. Nutrition 2022, 103–104, 111791. [Google Scholar] [CrossRef]
- Candow, D.G.; Chilibeck, P.D.; Forbes, S.C. Creatine supplementation and aging musculoskeletal health. Endocrine 2014, 45, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Devries, M.C.; Phillips, S.M. Creatine supplementation during resistance training in older adults—A meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 1194–1203. [Google Scholar] [CrossRef]
- Burke, R.; Piñero, A.; Coleman, M.; Mohan, A.; Sapuppo, M.; Augustin, F.; Aragon, A.A.; Candow, D.G.; Forbes, S.C.; Swinton, P.; et al. The Effects of Creatine Supplementation Combined with Resistance Training on Regional Measures of Muscle Hypertrophy: A Systematic Review with Meta-Analysis. Nutrients 2023, 15, 2116. [Google Scholar] [CrossRef] [PubMed]
- Westerterp, K.R. Control of energy expenditure in humans. Eur. J. Clin. Nutr. 2016, 71, 340–344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).