Phytoestrogens and Health Effects
Abstract
1. Introduction
2. Definition and Origin
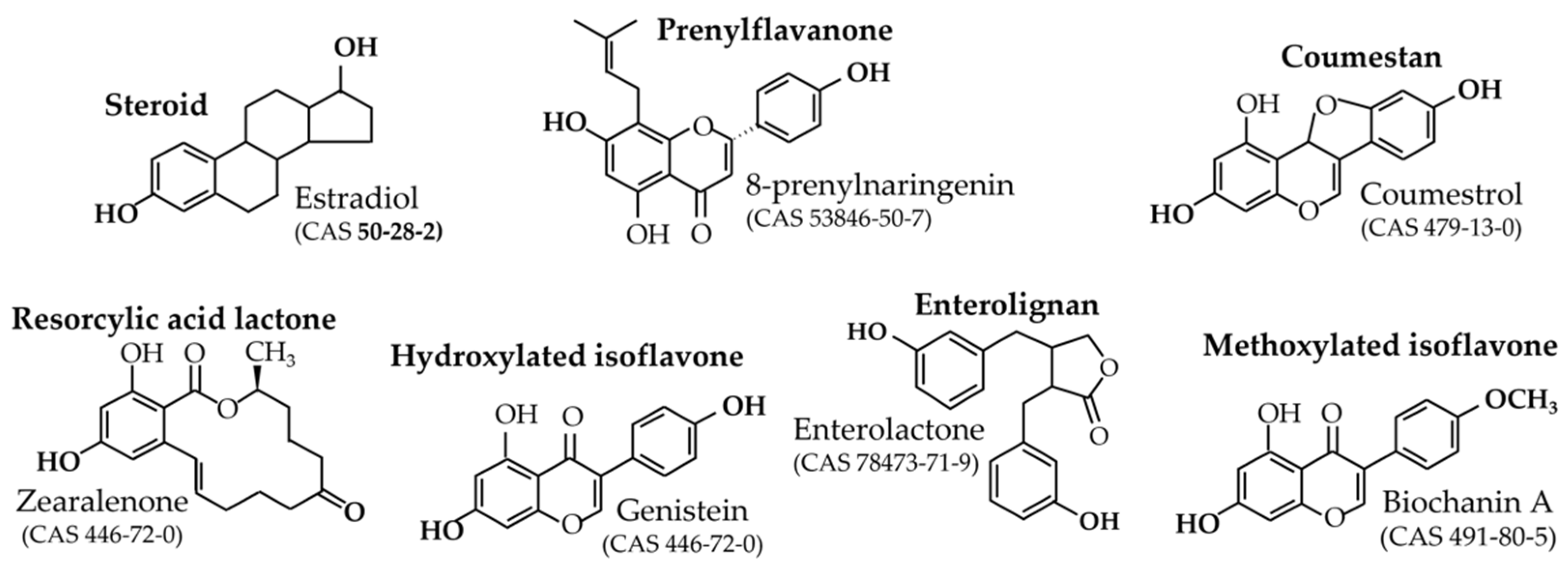
2.1. Definition and Relative Potencies
2.2. Origin and Role in Plants
- -
- Mycotoxins;
- -
- Phytoalexins;
- -
- Non-estrogenic native compounds requiring gut-flora metabolism to become active.
- Prenyl-flavanones
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Enterolignans
2.3. Aromatic and Medicinal Plants
3. Human Exposure and Bioavailability
3.1. Exposure According to Diet
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Lignans
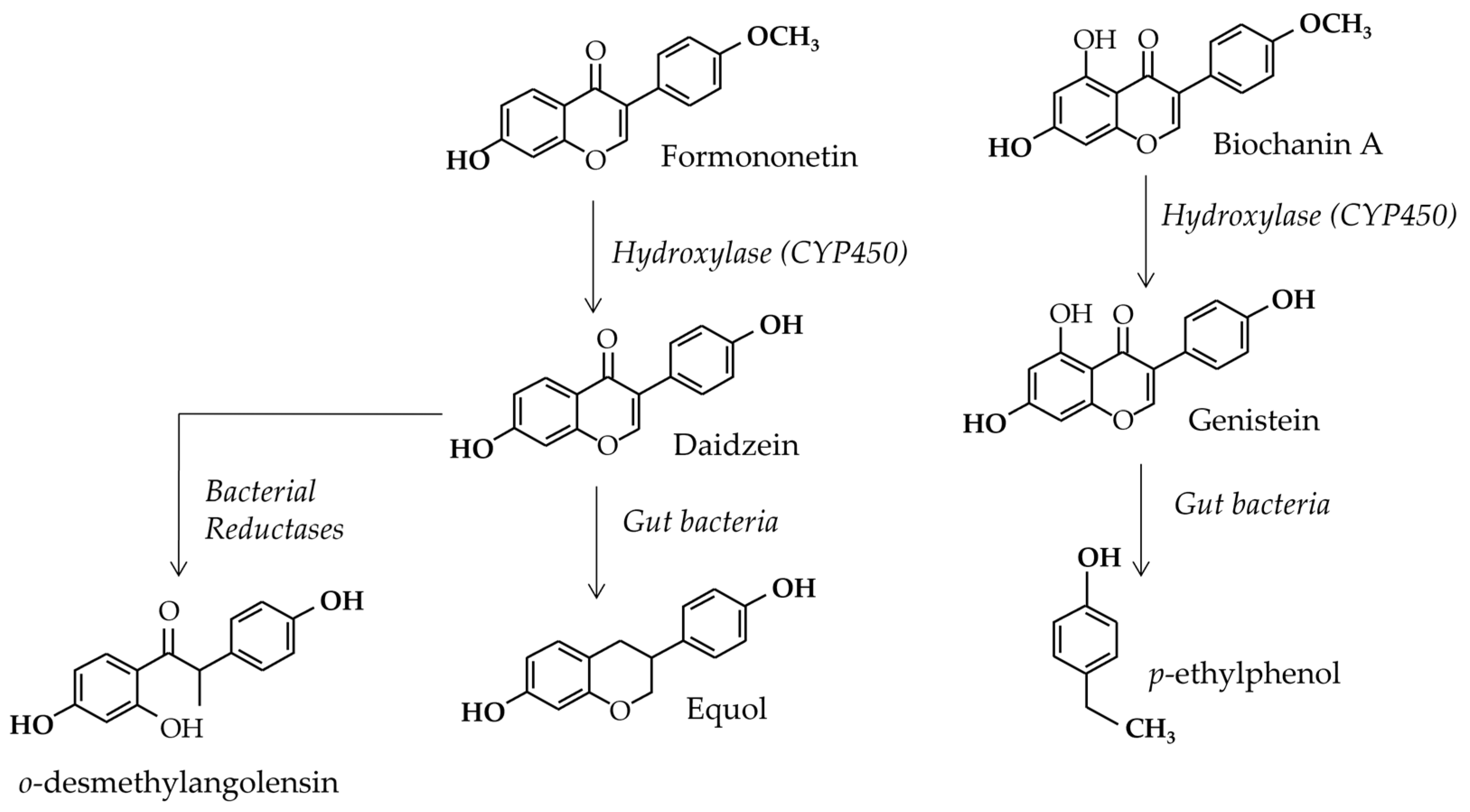
3.2. Gut Flora Involvement
3.3. Blood Concentrations
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Lignans
- Methoxylated isoflavones
4. Beneficial Effects
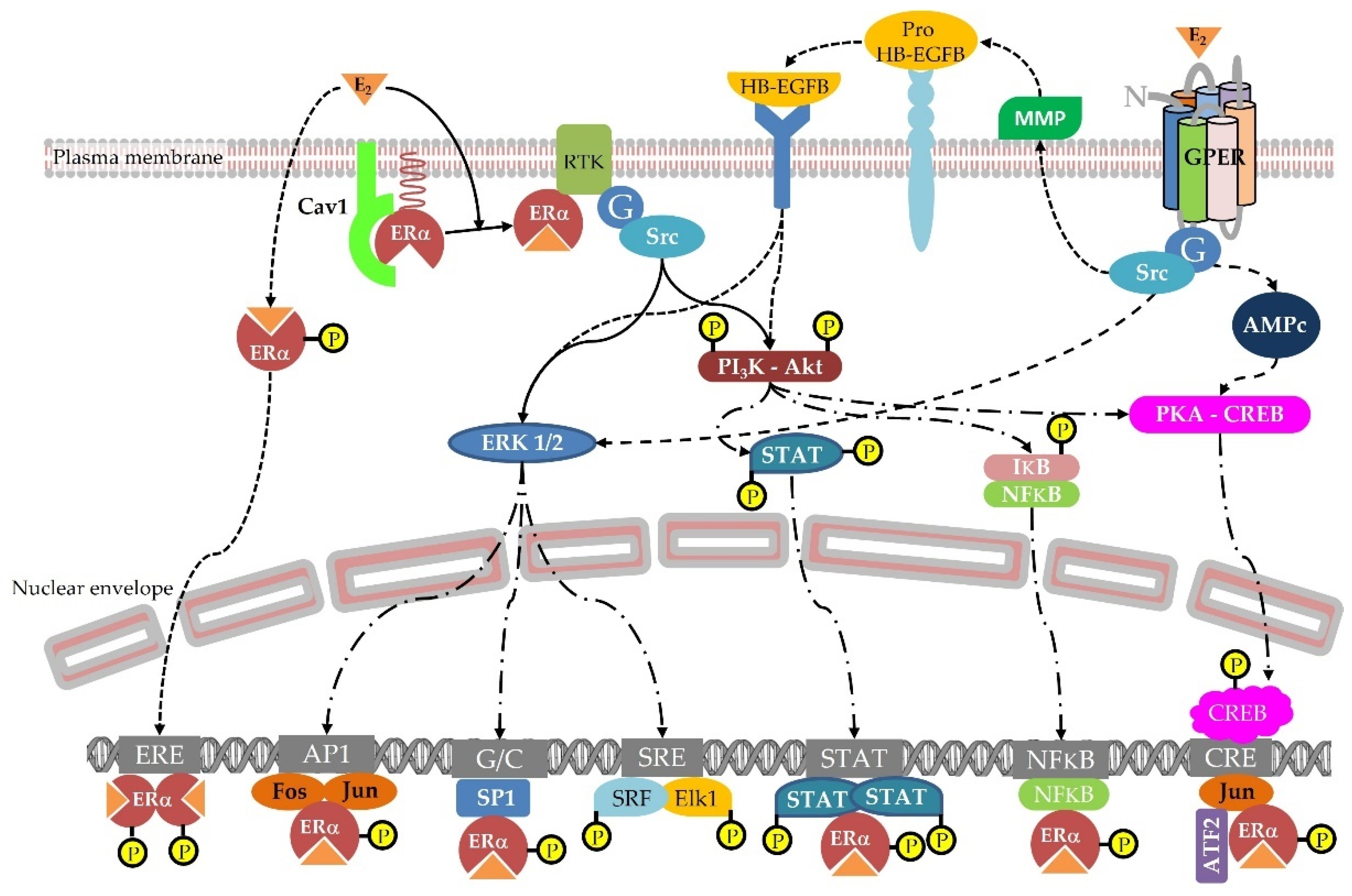
4.1. Hormonal Effects
4.1.1. Menopausal Symptoms
- 8-prenylnaringenin
- Isoflavones
- Lignans
4.1.2. Bone Health
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Lignans
4.1.3. Estrogen Responsive Tissues
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Lignans
4.2. Metabolic Beneficial Effects
4.2.1. Effect on Cholesterol
- Isoflavones
- Lignans
4.2.2. Effect on Metabolic Syndrome
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Lignans
4.2.3. Effects on Diabetes
- 8-Prenylnaringenin
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Lignans
5. Adverse Effects
5.1. Reference Doses
5.2. Hormonal Based Effects
5.2.1. Pituitary Interactions
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactones
- Isoflavones
- Lignans
5.2.2. Estrogen Based Toxic Effects
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactone
- Isoflavones
- Lignans
5.2.3. Thyroid Based Toxic Effects
- 8-prenylnaringenin
- Coumestrol
- Resorcylic acid lactone
- Isoflavones
- Lignans
5.2.4. Androgen Based Toxic Effects
6. Taste interactions: A New Endpoints for Phytoestrogens
6.1. Phytostrogens and Taste Receptor
6.2. Phytoestrogen and Taste Preference Modulation
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tibenda, J.J.; Yi, Q.; Wang, X.; Zhao, Q. Review of phytomedicine, phytochemistry, ethnopharmacology, toxicology, and pharmacological activities of Cymbopogon genus. Front. Pharmacol. 2022, 13, 997918. [Google Scholar] [CrossRef]
- Domínguez-Martín, E.M.; Tavares, J.; Rijo, P.; Díaz-Lanza, A.M. Zoopharmacology: A Way to Discover New Cancer Treatments. Biomolecules 2020, 10, 817. [Google Scholar] [CrossRef] [PubMed]
- Semwal, D.K.; Semwal, R.B.; Vermaak, I.; Viljoen, A. From arrow poison to herbal medicine--the ethnobotanical, phytochemical and pharmacological significance of Cissampelos (Menispermaceae). J. Ethnopharmacol. 2014, 155, 1011–1028. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.; Gou, J.; Liao, Q.; Li, Y.; Zhou, Q.; Bi, G.; Li, C.; Du, R.; Wang, X.; Sun, T.; et al. The Taxus genome provides insights into paclitaxel biosynthesis. Nat. Plants 2021, 7, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Perkin, A.G.; Newbury, F.G. LXXIX.—The colouring matters contained in dyer’s broom (Genista tinctoria) and heather (Calluna vulgaris). J. Chem. Soc. Trans. 1899, 75, 830–838. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Bingel, A.S.; Cordell, G.A.; Crane, F.A.; Fong, H.H.S. Potential value of plants as sources of new antifertility agents I. J. Pharm. Sci. 1975, 64, 535–598. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Bingel, A.S.; Cordell, G.A.; Crane, F.A.; Fong, H.H.S. Potential value of plants as sources of new antifertility agents II. J. Pharm. Sci. 1975, 64, 717–754. [Google Scholar]
- Chen, M.N.; Lin, C.C.; Liu, C.F. Efficacy of phytoestrogens for menopausal symptoms: A meta-analysis and systematic review. Climacteric 2015, 18, 260–269. [Google Scholar] [CrossRef]
- Ţiţ, D.M.; Pallag, A.; Iovan, C.; Furău, G.; Furău, C.; Bungău, S. Somatic-vegetative Symptoms Evolution in Postmenopausal Women Treated with Phytoestrogens and Hormone Replacement Therapy. Iran J. Public Health 2017, 46, 1528–1534. [Google Scholar]
- Tit, D.M.; Bungau, S.; Iovan, C.; Nistor Cseppento, D.C.; Endres, L.; Sava, C.; Sabau, A.M.; Furau, G.; Furau, C. Effects of the Hormone Replacement Therapy and of Soy Isoflavones on Bone Resorption in Postmenopause. J. Clin. Med. 2018, 7, 297. [Google Scholar] [CrossRef]
- Sleiman, H.K.; de Oliveira, J.M.; Langoni de Freitas, G.B. Isoflavones alter male and female fertility in different development windows. Biomed. Pharmacother. 2021, 140, 111448. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, V.S.; Popa, A.; Alexandru, A.; Manole, E.; Neagu, M.; Pop, S. Dietary Phytoestrogens and Their Metabolites as Epigenetic Modulators with Impact on Human Health. Antioxidants 2021, 10, 1893. [Google Scholar] [CrossRef] [PubMed]
- Pike, A.C.; Brzozowski, A.M.; Hubbard, R.E.; Bonn, T.; Thorsell, A.G.; Engström, O.; Ljunggren, J.; Gustafsson, J.A.; Carlquist, M. Structure of the ligand-binding domain of oestrogen receptor beta in the presence of a partial agonist and a full antagonist. EMBO J. 1999, 18, 4608–4618. [Google Scholar] [CrossRef] [PubMed]
- May, F.E.B. Novel drugs that target the estrogen-related receptor alpha: Their therapeutic potential in breast cancer. Cancer Manag. Res. 2014, 6, 225–252. [Google Scholar] [CrossRef]
- Adlanmerini, M.; Solinhac, R.; Abot, A.; Fabre, A.; Raymond-Letron, I. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc. Natl. Acad. Sci. USA 2014, 111, E283–E290. [Google Scholar] [CrossRef] [PubMed]
- Arnal, J.-F.; Lenfant, F.; Metivier, R.; Flouriot, G.; Henrion, D.; Adlanmerini, M.; Fontaine, C.; Gourdy, P.; Chambon, P.; Katzenellenbogen, B.; et al. Membrane and nuclear estrogen receptor alpha actions: From tissue specificity to medical implications. Physiol. Rev. 2017, 97, 1045–1087. [Google Scholar] [CrossRef]
- Qiu, Y.A.; Xiong, J.; Yu, T. Role of G Protein-Coupled Estrogen Receptor in Digestive System Carcinomas: A Minireview. OncoTargets Ther. 2021, 14, 2611–2622. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C.; Breton, B.; Bennetau, B.; Corraze, G. Effect of genistein-enriched diets on the endocrine process of gametogenesis and on reproduction efficiency of the rainbow trout Oncorhynchus mykiss. Gen. Comp. Endocrinol. 2001, 121, 173–187. [Google Scholar] [CrossRef]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006, 114, 567–572. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, H. Genistein increases gene expression by demethylation of WNT5a promoter in colon cancer cell line SW1116. Anticancer Res. 2010, 30, 4537–4545. [Google Scholar]
- Clavel, T.; Lippman, R.; Gavini, F.; Doré, J.; Blaut, M. Clostridium saccharogumia sp. nov. and Lactonifactor longoviformis gen. nov., sp. nov., two novel human faecal bacteria involved in the conversion of the dietary phytoestrogen secoisolariciresinol diglucoside. Syst. Appl. Microbiol. 2007, 30, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Schröder, C.; Matthies, A.; Engst, W.; Blaut, M.; Braune, A. Identification and expression of genes involved in the conversion of daidzein and genistein by the equol-forming bacterium Slackia isoflavoniconvertens. Appl. Environ. Microbiol. 2013, 79, 3494–3502. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.-F.; Wu, T.-L.; Du, S.-S.; Wu, Z.-R. The Antifungal Mechanism of Isoxanthohumol from Humulus lupulus Linn. Int. J. Mol. Sci. 2021, 22, 10853. [Google Scholar] [CrossRef]
- Bennetau-Pelissero, C. Natural Estrogenic Substances, Origins, and Effects. In Bioactive Molecules in Food; Mérillon, J.M., Ramawat, K., Eds.; Reference Series in Phytochemistry; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Knuckles, B.E.; deFremery, D.; Kohler, G.O. Coumestrol content of fractions obtained during wet processing of alfalfa. J. Agric. Food Chem. 1976, 24, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.L.; Barrell, G.K.; Gash, A.; Zhao, J.; Moot, D.J. Alfalfa Coumestrol Content in Response to Development Stage, Fungi, Aphids, and Cultivar. Crop Ecol. Physiol. 2018, 110, 910–921. [Google Scholar] [CrossRef]
- Carlsen, S.C.K.; Understrup, A.; Fomsgaard, I.S.; Mortensen, A.G.; Ravnskov, S. Flavonoids in roots of white clover: Interaction of arbuscular mycorrhizal fungi and a pathogenic fungus. Plant Soil 2008, 302, 33–43. [Google Scholar] [CrossRef]
- Schoevers, E.J.; Santos, R.R.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Transgenerational toxicity of Zearalenone in pigs. Reprod Toxicol. 2012, 34, 110–119. [Google Scholar] [CrossRef]
- Modolo, L.V.; Cunha, F.Q.; Braga, M.R.; Salgado, I. Nitric oxide synthase-mediated phytoalexin accumulation in soybean cotyledons in response to the Diaporthe phaseolorum f. sp. meridionalis elicitor. Plant Physiol. 2002, 130, 1288–1297. [Google Scholar] [CrossRef]
- Zavala, J.A.; Mazza, C.A.; Dillon, F.M.; Chludil, H.D.; Ballaré, C.L. Soybean resistance to stink bugs (Nezara viridula and Piezodorus guildinii) increases with exposure to solar UV-B radiation and correlates with isoflavonoid content in pods under field conditions. Plant Cell Environ. 2015, 38, 920–928. [Google Scholar] [CrossRef]
- Setchell, K.D.; Brown, N.M.; Zimmer-Nechemias, L.; Wolfe, B.; Jha, P.; Heubi, J.E. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014, 5, 491–501. [Google Scholar] [CrossRef]
- Farnsworth, N.R.; Akerele, O.; Bingel, A.S.; Soejarto, D.D.; Guo, Z. Medicinal plants in therapy. Bull. World Health Organ. 1985, 63, 965–981. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, M.; Akram, M. Antifertility activity of medicinal plants. J. Chin. Med. Assoc. 2015, 78, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, P.; Ahmad, N.; Dhar, Y.V.; Verma, A. Estrogen receptor activation in response to Azadirachtin A stimulates osteoblast differentiation and bone formation in mice. J. Cell. Physiol. 2019, 234, 23719–23735. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Shirahatti, P.S.; Chandana Kumari, V.B.; Ramu, R.; Nagendra Prasad, M.N. Azadirachta indica A. Juss (neem) as a contraceptive: An evidence-based review on its pharmacological efficiency. Phytomedicine 2021, 88, 153596. [Google Scholar] [CrossRef]
- Kargozar, R.; Azizi, H.; Salari, R. A review of effective herbal medicines in controlling menopausal symptoms. Electron. Physician 2017, 9, 5826–5833. [Google Scholar] [CrossRef]
- Słupski, W.; Jawień, P.; Nowak, B. Botanicals in Postmenopausal Osteoporosis. Nutrients 2021, 13, 1609. [Google Scholar] [CrossRef]
- Shahmohammadi, A.; Ramezanpour, N.; Mahdavi Siuki, M.; Dizavandi, F.; Ghazanfarpour, M.; Rahmani, Y.; Tahajjodi, R.; Babakhanian, M. The efficacy of herbal medicines on anxiety and depression in peri- and postmenopausal women: A systematic review and meta-analysis. Post Reprod. Health 2019, 25, 131–141. [Google Scholar] [CrossRef]
- Zava, D.T.; Dollbaum, C.M.; Blen, M. Estrogen and progestin bioactivity of foods, herbs, and spices. Proc. Soc. Exp. Biol. Med. 1998, 217, 369–378. [Google Scholar] [CrossRef]
- Liu, J.; Burdette, J.E.; Xu, H.; Gu, C.; van Breemen, R.B.; Bhat, K.P.; Booth, N.; Constantinou, A.I.; Pezzuto, J.M.; Fong, H.H. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J. Agric. Food Chem. 2001, 49, 2472–2479. [Google Scholar] [CrossRef]
- Kiyam, R. Estrogenic terpenes and terpenoids: Pathways, functions and applications. Eur. J. Pharmacol. 2017, 815, 405–415. [Google Scholar] [CrossRef]
- Oerter Klein, K.; Janfaza, M.; Wong, J.A.; Chang, R.J. Estrogen bioactivity in fo-ti and other herbs used for their estrogen-like effects as determined by a recombinant cell bioassay. J. Clin. Endocrinol. Metab. 2003, 88, 4077–4079. [Google Scholar] [CrossRef]
- Howes, M.-J.R.; Houghton, P.J.; Barlow, D.J.; Pocock, V.J.; Milligan, S.R. Assessment of estrogenic activity in some common essential oil constituents. J. Pharm. Pharmacol. 2002, 54, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Contini, A.; di Belle, D.; Azzarà, A.; Giovanelli, S.; D’Urso, G.; Piaggi, S.; Pinto, B.; Pistelli, L.; Scarpato, R.; Testi, S. Assessing the cytotoxic/genotoxic activity and estrogenic/antiestrogenic potential of essential oils from seven aromatic plants. Food Chem. Toxicol. 2020, 138, 111205. [Google Scholar] [CrossRef] [PubMed]
- Fouyet, S.; Olivier, E.; Leproux, P.; Dutot, M.; Rat, P. Evaluation of Placental Toxicity of Five Essential Oils and Their Potential Endocrine-Disrupting Effects. Curr. Issues Mol. Biol. 2022, 44, 2794–2810. [Google Scholar] [CrossRef]
- Rahimikian, F.; Rahimi, R.; Golzareh, P.; Bekhradi, R.; Mehran, A. Effect of Foeniculum vulgare Mill. (fennel) on menopausal symptoms in postmenopausal women: A randomized, triple-blind, placebo-controlled trial. Menopause 2017, 24, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Türkyilmaz, Z.; Karabulut, R.; Sönmez, K.; Can Başaklar, A. A striking and frequent cause of premature thelarche in children: Foeniculum vulgare. J. Pediatr. Surg. 2008, 43, 2109–2111. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Ardekani, M.R. Medicinal properties of Foeniculum vulgare Mill. in traditional Iranian medicine and modern phytotherapy. Chin. J. Integr. Med. 2013, 19, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Okdemir, D.; Hatipoglu, N.; Kurtoglu, S.; Akın, L.; Kendirci, M. Premature thelarche related to fennel tea consumption? J. Pediatr. Endocrinol. Metab. 2014, 27, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.M. Herbal medicines, phytoestrogens and toxicity: Risk: Benefit considerations. Proc. Soc. Exp. Biol. Med. 1998, 217, 379–385. [Google Scholar] [CrossRef]
- Kristanc, L.; Kreft, S. European medicinal and edible plants associated with subacute and chronic toxicity part I: Plants with carcinogenic, teratogenic and endocrine-disrupting effects. Food Chem. Toxicol. 2016, 92, 150–164. [Google Scholar] [CrossRef]
- Aghamiri, V.; Mirghafourvand, M.; Charandabi, M.A.S.; Nazemiyeh, H. The effect of Hop (Humulus lupulus L.) on early menopausal symptoms and hot flashes: A randomized placebo-controlled trial. Complement. Ther. Clin. Pract. 2016, 23, 130–135. [Google Scholar] [CrossRef] [PubMed]
- ARRETE ROYAL du 29 AOUT 1997. Relatif à la Fabrication et au Commerce de Denrées Alimentaires Composées ou Contenant des Plantes ou Préparations de Plantes (M.B. 21.XI.1997). Available online: https://www.health.belgium.be/sites/default/files/uploads/fields/fpshealth_theme_file/consolidated_version_rd_29_august_1997_v10-02-2017_fr.pdf (accessed on 15 December 2022).
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 32, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S.; et al. The Oral Bioavailability of 8-Prenylnaringenin from Hops (Humulus lupulus L.) in Healthy Women and Men is Significantly Higher than that of its Positional Isomer 6-Prenylnaringenin in a Randomized Crossover Trial. Mol. Nutr. Food Res. 2018, 62, e1700838. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.; Chen, S.N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F.; et al. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Possemiers, S.; Heyerick, A.; Robbens, V.; de Keukeleire, D.; Verstraete, W. Activation of proestrogens from hops (Humulus lupulus L.) by intestinal microbiota; conversion of isoxanthohumol into 8-prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6288. [Google Scholar] [CrossRef]
- Aichinger, G.; Bliem, G.; Marko, D. Systemically Achievable Doses of Beer Flavonoids Induce Estrogenicity in Human Endometrial Cells and Cause Synergistic Effects with Selected Pesticides. Front. Nutr. 2021, 8, 691872. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luh, C.J.; Burns, K.A.; Arao, Y.; Jiang, Z.; Teng, C.T.; Tice, R.R.; Korach, K.S. Endocrine disrupting chemicals (EDCs): In vitro mechanism of estrogenic activation and differential effects on ER target genes. Environ. Health Perspect. 2013, 121, 459–466. [Google Scholar] [CrossRef]
- Mallis, L.M.; Sarkahian, A.B.; Harris, H.A.; Zhang, M.Y.; McConnell, O.J. Determination of rat oral bioavailability of soy-derived phytoestrogens using an automated on-column extraction procedure and electrospray tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003, 796, 71–86. [Google Scholar] [CrossRef]
- Carballo, D.; Pallarés, N.; Ferrer, E.; Barba, F.J.; Berrada, H. Assessment of Human Exposure to Deoxynivalenol, Ochratoxin A, Zearalenone and Their Metabolites Biomarker in Urine Samples Using LC-ESI-qTOF. Toxins 2021, 13, 530. [Google Scholar] [CrossRef]
- Zhu, J.; Qi Zhao, Q.; Qiu, Y.; Zhang, Y.; Cui, S.; Yu, Y.; Chen, B.; Zhu, M.; Wang, N.; Liu, X.; et al. Soy Isoflavones Intake and Obesity in Chinese Adults: A Cross-Sectional Study in Shanghai, China. Nutrients 2021, 13, 2715. [Google Scholar] [CrossRef]
- Murai, U.; Sawada, N.; Charvat, H.; Inoue, M.; Yasuda, N.; Yamagishi, K.; Tsugane, S. JPHC Study Group. Soy product intake and risk of incident disabling dementia: The JPHC Disabling Dementia Study. Eur. J. Nutr. 2022, 61, 4045–4057. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.K.; Jaceldo-Siegl, K.; Knutsen, S.F.; Fan, J.; Oda, K.; Fraser, G.E. Soy isoflavone intake and the likelihood of ever becoming a mother: The Adventist Health Study-2. Int. J. Women Health 2014, 6, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Beaubernard, L.; Lamothe, V.; Bennetau-Pelissero, C. New Evaluation of Isoflavone Exposure in the French Population. Nutrients 2019, 11, 2308. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Bensaada, S.; Lamothe, V.; Lacoste, M.; Bennetau-Pelissero, C. French Population Exposure to Endocrine Disruptors in and on Plant Foodstuffs. Mendeley Data. Version 9. 2021. Available online: https://doi.org/10.17632/dk8gsx2r4j.9 (accessed on 15 December 2022).
- Sathyapalan, T.; Manuchehri, A.M.; Thatcher, N.J.; Rigby, A.S.; Chapman, T.; Kilpatrick, E.S.; Atkin, S.L. The effect of soy phytoestrogen supplementation on thyroid status and cardiovascular risk markers in patients with subclinical hypothyroidism: A randomized, double-blind, crossover study. J. Clin. Endocrinol. Metab. 2011, 96, 1442–1449. [Google Scholar] [CrossRef]
- Zaheer, K.; Humayoun Akhtar, M. An updated review of dietary isoflavones: Nutrition, processing, bioavailability and impacts on human health. Crit. Rev. Food Sci. Nutr. 2017, 57, 1280–1293. [Google Scholar] [CrossRef]
- Shinkaruk, S.; Durand, M.; Lamothe, V.; Carpaye, A.; Carpaye, A.; Martinet, A.; Chantre, P.; Vergne, S.; Nogues, X.; Moore, N.; et al. Bioavailability of glycitein relatively to other soy isoflavones in healthy young Caucasian men. Food Chem. 2012, 135, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Mathey, J.; Lamothe, V.; Coxam, V.; Potier, M.; Sauvant, P.; Bennetau-Pelissero, C. Concentrations of isoflavones in plasma and urine of post-menopausal women chronically ingesting high quantities of soy isoflavones. J. Pharm. Biomed. Anal. 2006, 41, 957–965. [Google Scholar] [CrossRef]
- Vergne, S.; Bennetau-Pelissero, C.; Lamothe, V.; Chantre, P.; Potier, M.; Asselineau, J.; Perez, P.; Durand, M.; Moore, N.; Sauvant, P. Higher bioavailability of isoflavones after a single ingestion of a soya-based supplement than a soya-based food in young healthy males. Br. J. Nutr. 2008, 99, 333–344. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Bub, A.; Möseneder, J.; Winterhalter, P.; Stürtz, M.; Kulling, S.E. Pharmacokinetics of the soybean isoflavone daidzein in its aglycone and glucoside form: A randomized, double-blind, crossover study. Am. J. Clin. Nutr. 2008, 87, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.; Brown, N.M.; Desai, P.; Zimmer-Nechemias, L.; Wolfe, B.E.; Brashear, W.T.; Kirschner, A.S.; Cassidy, A.; Heubi, J.E. Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements. J. Nutr. 2001, 131 (Suppl. S4), 1362S–1375S. [Google Scholar] [CrossRef]
- Molina-Montes, E.; Sánchez, M.J.; Zamora-Ros, R.; Bueno-de-Mesquita, H.B.; Wark, P.A.; Obon-Santacana, M.; Kühn, T.; Katzke, V.; Travis, R.C.; Ye, W.; et al. Flavonoid and lignan intake and pancreatic cancer risk in the European prospective investigation into cancer and nutrition cohort. Int. J. Cancer 2016, 139, 1480–1492. [Google Scholar] [CrossRef]
- Reger, M.K.; Zollinger, T.W.; Liu, Z.; Jones, J.; Zhang, J. Urinary phytoestrogens and cancer, cardiovascular, and all-cause mortality in the continuous National Health and Nutrition Examination Survey. Eur. J. Nutr. 2016, 55, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Marrian, G.F.; Haslewood, G.A. Equol, a new inactive phenol isolated from the ketohydroxyoestrin fraction of mares’ urine. Biochem. J. 1932, 26, 1227–1232. [Google Scholar] [CrossRef] [PubMed]
- Shutt, D.A.; Braden, A.W.H. The significance of equol in relation to the estrogenic responses in sheep ingesting clover with a high formononetin content. Aust. J. Agric. Res. 1968, 19, 545–553. [Google Scholar] [CrossRef]
- Guadamuro, L.; Dohrmann, A.B.; Tebbe, C.C.; Mayo, B.; Delgado, S. Bacterial communities and metabolic activity of faecal cultures from equol producer and non-producer menopausal women under treatment with soy isoflavones. BMC Microbiol. 2017, 17, 93. [Google Scholar] [CrossRef] [PubMed]
- Kawada, Y.; Yokoyama, S.; Yanase, E.; Niwa, T.; Suzuki, T. The production of S-equol from daidzein is associated with a cluster of three genes in Eggerthella sp. YY7918. Biosci. Microbiota Food Health 2016, 35, 113–121. [Google Scholar] [CrossRef]
- Kilkkinen, A.; Stumpf, K.; Pietinen, P.; Valsta, L.M.; Tapanainen, H.; Adlercreutz, H. Determinants of serum enterolactone concentration. Am. J. Clin. Nutr. 2001, 73, 1094–1100. [Google Scholar] [CrossRef] [PubMed]
- Clavel, T.; Henderson, G.; Alpert, C.A.; Philippe, C.; Rigottier-Gois, L.; Doré, J.; Blaut, M. Intestinal bacterial communities that produce active estrogen-like compounds enterodiol and enterolactone in humans. Appl. Environ. Microbiol. 2005, 71, 6077–6085. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef]
- Liu, J.; Mi, S.; Du, L.; Li, X.; Li, P.; Jia, K.; Zhao, J.; Zhang, H.; Zhao, W.; Gao, Y. The associations between plasma phytoestrogens concentration and metabolic syndrome risks in Chinese population. PLoS ONE 2018, 13, e0194639. [Google Scholar] [CrossRef]
- Palma-Duran, S.A.; Caire-Juvera, G.; Robles-Burgeño, M.d.R.; Ortega-Vélez, M.I.; Gutiérrez-Coronado, M.L.; Almada Mdel, C.; Chávez-Suárez, K.; Campa-Siqueiros, M.; Grajeda-Cota, P.; Saucedo-Tamayo, M.S.; et al. Serum levels of phytoestrogens as biomarkers of intake in Mexican women. Int. J. Food Sci. Nutr. 2015, 66, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Xu, J.; Jiang, K.; Liu, X.; Meng, J.; Di Mavungu, J.D.; Guo, W.; Zhang, Z.; Jing, J.; Li, H.; et al. Determination of multiple mycotoxins in paired plasma and urine samples to assess human exposure in Nanjing, China. Environ. Pollut. 2019, 248, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Gaylor, D.W.; Kodell, R.L. Dose-response trend tests for tumorigenesis, adjusted for body weight. Toxicol. Sci. 1999, 49, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Urpi-Sarda, M.; Blondeel, P.; Roche, N.; Roche, N.; Vanhaecke, L.; Possemiers, S.; Al-Maharik, N.; Botting, N.; De Keukeleire, D.; et al. Disposition of soy isoflavones in normal human breast tissue. Am. J. Clin. Nutr. 2010, 91, 976–984. [Google Scholar] [CrossRef]
- Stuedal, A.; Gram, I.T.; Bremnes, Y.; Adlercreutz, H.; Veierød, M.B.; Ursin, G. Plasma levels of enterolactone and percentage mammographic density among postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2005, 14, 2154–2159. [Google Scholar] [CrossRef]
- Rowland, I.; Faughnan, M.; Hoey, L.; Wähälä, K.; Williamson, G.; Cassidy, A. Bioavailability of phyto-oestrogens. Br. J. Nutr. 2003, 89 (Suppl. 1), S45–S58. [Google Scholar] [CrossRef]
- Cao, H.; Jing, X.; Wu, D.; Shi, Y. Methylation of genistein and kaempferol improves their affinities for proteins. Int. J. Food Sci. Nutr. 2013, 64, 437–443. [Google Scholar] [CrossRef]
- Muchiri, R.N.; van Breemen, R.B. Single-Laboratory Validation of UHPLC-MS/MS Assays for Red Clover Isoflavones in Human Serum and Dietary Supplements. J. AOAC Int. 2020, 103, 1160–1166. [Google Scholar] [CrossRef]
- Sarri, G.; Pedder, H.; Dias, S.; Guo, Y.; Lumsden, M.A. Vasomotor symptoms resulting from natural menopause: A systematic review and network meta-analysis of treatment effects from the National Institute for Health and Care Excellence guideline on menopause. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 1514–1523. [Google Scholar] [CrossRef]
- Li, L.; Lv, Y.; Xu, L.; Zheng, Q. Quantitative efficacy of soy isoflavones on menopausal hot flashes. Br. J. Clin. Pharmacol. 2014, 79, 593–604. [Google Scholar] [CrossRef]
- Hanna, K.; Day, A.; O’Neill, S.; Patterson, C.; Lyons-Wall, P. Does scientific evidence support the use of nonprescription supplements for treatment of acute menopausal symptoms such as hot flushes? Nutr. Diet 2005, 62, 138–151. [Google Scholar] [CrossRef]
- Kongkaewa, C.; Scholfielda, N.C.; Dhippayoma, T.; Dilokthornsakul, P.; Saokaew, S.; Chaiyakunapruk, N. Efficacy and safety of Pueraria candollei var. mirifica (Airy Shaw & Suvat.) Niyomdham for menopausal women: A systematic review of clinical trials and the way forward. J. Ethnopharmacol. 2018, 216, 162–174. [Google Scholar] [CrossRef]
- Lewis, J.E.; Nickell, L.A.; Thompson, L.U.; Szalai, J.P.; Kiss, A.; Hilditch, J.R. A randomized controlled trial of the effect of dietary soy and flaxseed muffins on quality of life and hot flashes during menopause. Menopause 2006, 13, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Simbalista, R.L.; Sauerbronn, A.V.; Aldrighi, J.M.; Arêas, J.A. Consumption of a flaxseed-rich food is not more effective than a placebo in alleviating the climacteric symptoms of postmenopausal women. J. Nutr. 2010, 140, 293–297. [Google Scholar] [CrossRef]
- Pruthi, S.; Thompson, S.L.; Novotny, P.J.; Barton, D.L.; Kottschade, L.A.; Tan, A.D.; Sloan, J.A.; Loprinzi, C.L. Pilot evaluation of flaxseed for the management of hot flashes. J. Soc. Integr. Oncol. 2007, 5, 106–112. [Google Scholar] [CrossRef]
- Keiler, A.M.; Helle, J.; Bader, M.I.; Ehrhardt, T.; Nestler, K.; Kretzschmar, G.; Bernhardt, R.; Vollmer, G.; Nikolić, D.; Bolton, J.L.; et al. A standardized Humulus lupulus (L.) ethanol extract partially prevents ovariectomy-induced bone loss in the rat without induction of adverse effects in the uterus. Phytomedicine 2017, 34, 50–58. [Google Scholar] [CrossRef]
- Dodge, J.A.; Glasebrook, A.L.; Magee, D.E.; Phillips, D.L.; Sato, M.; Short, L.L.; Bryant, H.U. Environmental estrogens: Effects on cholesterol lowering and bone in the ovariectomized rat. J. Steroid Biochem. Mol. Biol. 1996, 59, 155–161. [Google Scholar] [CrossRef]
- Kanno, S.; Hirano, S.; Kayama, F. Effects of the phytoestrogen coumestrol on RANK-ligand-induced differentiation of osteoclasts. Toxicology 2004, 203, 211–220. [Google Scholar] [CrossRef]
- Kanno, S.; Hirano, S.; Kayama, F. Effects of phytoestrogens and environmental estrogens on osteoblastic differentiation in MC3T3-E1 cells. Toxicology 2004, 196, 137–145. [Google Scholar] [CrossRef]
- Zong, S.; Zeng, G.; Fang, Y.; Peng, J.; Zou, B.; Gao, T.; Zhao, J. The effects of α-zearalanol on the proliferation of bone-marrow-derived mesenchymal stem cells and their differentiation into osteoblasts. J. Bone Miner. Metab. 2016, 34, 151–160. [Google Scholar] [CrossRef]
- Abdelhamid, A.M.; Kelada, I.P.; Ali, M.M.; el-Ayouty, S.A. Influence of zearalenone on some metabolic, physiological and pathological aspects of female rabbits at two different ages. Arch. Tierernahr. 1992, 42, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.; Wei, B.; Xiong, C.; Zhao, Y.; Zeng, G. The role of α-zearalanol in reversing bone loss induced by ovarian hormone deficiency in rats. J. Bone Miner. Metab. 2012, 30, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Ayed-Boussema, I.; Ouanes, Z.; Bacha, H. In vivo and in vitro induction of chromosome aberrations by alpha- and beta-zearalenols: Comparison with zearalenone. Mutat. Res. 2011, 726, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Takebayashi, J.; Mizuno, S.; Ishimi, Y.; Omori, T.; Watanabe, S. Effect of soy isoflavone extract supplements on bone mineral density in menopausal women: Meta-analysis of randomized controlled trials. Asia Pac. J. Clin. Nutr. 2010, 19, 33–42. [Google Scholar] [PubMed]
- Liu, J.; Ho, S.C.; Su, Y.X.; Chen, W.Q.; Zhang, C.X.; Chen, Y.M. Effect of long-term intervention of soy isoflavones on bone mineral density in women: A meta-analysis of randomized controlled trials. Bone 2009, 44, 948–953. [Google Scholar] [CrossRef] [PubMed]
- Dodin, S.; Lemay, A.; Jacques, H.; Légaré, F.; Forest, J.C.; Mâsse, B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: A randomized, double-blind, wheat germ placebo-controlled clinical trial. J. Clin. Endocrinol. Metab. 2005, 90, 1390–1397. [Google Scholar] [CrossRef]
- Kuhnle, G.G.C.; Ward, H.A.; Vogiatzoglou, A.; Luben, R.N.; Mulligan, A.; Wareham, N.J.; Forouhi, N.G.; Khaw, K.T. Association between dietary phyto-oestrogens and bone density in men and postmenopausal women. Br. J. Nutr. 2011, 106, 1063–1069. [Google Scholar] [CrossRef]
- Boucher, B.A.; Cotterchio, M.; Anderson, L.N.; Nancy Kreiger, N.; Kirsh, V.A.; Thompson, L.U. Use of Isoflavone supplements is associated with reduced postmenopausal breast cancer risk. Int. J. Cancer 2013, 132, 1439–1450. [Google Scholar] [CrossRef]
- Hedelin, M.; Bälter, K.A.; Chang, E.T.; Bellocco, R.; Klint, A.; Johansson, J.E.; Wiklund, F.; Thellenberg-Karlsson, C.; Adami, H.O.; Grönberg, H. Dietary intake of phytoestrogens, estrogen receptor-beta polymorphisms and the risk of prostate cancer. Prostate 2006, 66, 1512–1520. [Google Scholar] [CrossRef]
- Pazaiti, A.; Kontos, M.; Fentiman, I.S. ZEN and the art of breast health maintenance. Int. J. Clin. Pract. 2012, 66, 28–36. [Google Scholar] [CrossRef]
- Belhassen, H.; Jiménez-Díaz, I.; Arrebola, J.P.; Ghali, R.; Ghorbel, H.; Olea, N.; Hedili, A. Zearalenone and its metabolites in urine and breast cancer risk: A case-control study in Tunisia. Chemosphere 2015, 128, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gutendorf, B.; Westendorf, J. Comparison of an array of in vitro assays for the assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicolology 2001, 166, 79–89. [Google Scholar] [CrossRef] [PubMed]
- National Toxicology Program. Toxicology and Carinogenesis study of Genistein (Cas N° 446-72-0) in Sprague-Dawley rats (Feed study). Natl. Toxicol. Program Tech. Rep. Ser. 2008, 545, 1–240. [Google Scholar]
- Woo, H.D.; Park, S.; Oh, K.; Kim, H.J.; Shin, H.R.; Moon, H.K.; Kim, J. Diet and cancer risk in the Korean population: A meta- analysis. Asian Pac. J. Cancer Prev. 2014, 15, 8509–8519. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Mizoue, T.; Tanaka, K.; Tsuji, I.; Tamakoshi, A.; Matsuo, K.; Wakai, K.; Inoue, M.; Tsugane, S.; Sasazuki, S.; et al. Soy intake and breast cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2014, 44, 282–295. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61, 1600930. [Google Scholar] [CrossRef]
- Lucki, N.C.; Sewer, M.B. Genistein Stimulates MCF-7 Breast Cancer Cell Growth by Inducing Acid Ceramidase (ASAH1) Gene Expression. J. Biol. Chem. 2011, 286, 19399–19409. [Google Scholar] [CrossRef]
- Ariyani, W.; Miyazaki, W.; Amano, I.; Hanamura, K.; Shirao, T.; Koibuchi, N. Soy Isoflavones Accelerate Glial Cell Migration via GPER-Mediated Signal Transduction Pathway. Front. Endocrinol. 2020, 11, 554941. [Google Scholar] [CrossRef]
- Uifalean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy Isoflavones and Breast Cancer Cell Lines: Molecular Mechanisms and Future Perspectives. Molecules 2016, 21, 13. [Google Scholar] [CrossRef]
- Wu, Q.; Yang, Y.; Yu, J.; Jin, N. Soy isoflavone extracts stimulate the growth of nude mouse xenografts bearing estrogen-dependent human breast cancer cells (MCF-7). J. Biomed. Res. 2012, 26, 44–52. [Google Scholar] [CrossRef]
- Shike, M.; Doane, A.S.; Russo, L.; Cabal, R.; Reis-Filho, J.S.; Gerald, W.; Cody, H.; Khanin, R.; Bromberg, J.; Norton, L. The effects of soy supplementation on gene expression in breast cancer: A randomized placebo-controlled study. J. Natl. Cancer Inst. 2014, 106, dju189. [Google Scholar] [CrossRef] [PubMed]
- McMichael-Phillips, D.F.; Harding, C.; Morton, M.; Roberts, S.A.; Howell, A.; Potten, C.S.; Bundred, N.J. Effects of soy-protein supplementation on epithelial proliferation in the histologically normal human breast. Am. J. Clin. Nutr. 1998, 68 (Suppl. S6), 1431S–1435S. [Google Scholar] [CrossRef] [PubMed]
- Isidoro, B.; Lope, V.; Whelan, D.; Pedraz, C.; Sánchez-Contador, C.; Santamariña, C.; Moreo, P.; Vidal, C.; Salas-Trejo, D.; Ederra, M. Use of hormone therapy and isoflavones and mammographic density in Spain. Menopause 2016, 23, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, D.W. The role of n-3 polyunsaturated fatty acids in the prevention and treatment of breast cancer. Nutrients 2014, 6, 5184–5223. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Yin, Y.C.; Wang, J.; Jiang, Y.F. Green tea compounds in breast cancer prevention and treatment. World J. Clin. Oncol. 2014, 5, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Mylonas, I.; Jeschke, U.; Shabani, N.; Kuhn, C.; Kriegel, S.; Kupka, M.S.; Friese, K. Normal and malignant human endometrium express immunohistochemically estrogen receptor alpha (ER-alpha), estrogen receptor beta (ER-beta) and progesterone receptor (PR). Anticancer Res. 2005, 25, 1679–1686. [Google Scholar]
- Tica, A.A.; Tica, O.S.; Georgescu, C.V.; Bogdan, M.; Ciurea, T.; Mogoantă, S.Ş.; Georgescu, C.C.; Comănescu, A.C.; Bălşeanu, T.A.; Ciurea, R.N. GPER and ERα expression in abnormal endometrial proliferations. Rom. J. Morphol. Embryol. 2016, 57, 413–418. [Google Scholar]
- Cavallini, A.; Dinaro, E.; Giocolano, A.; Caringella, A.M.; Ferreri, R.; Tutino, V.; Loverro, G. Estrogen receptor (ER) and ER-related receptor expression in normal and atrophic human vagina. Maturitas 2008, 59, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yuan, F.; Gao, J.; Shan, B.; Ren, Y.; Wang, H.; Gao, Y. Oral isoflavone supplementation on endometrial thickness: A meta-analysis of randomized placebo-controlled trials. Oncotarget 2016, 7, 17369–17379. [Google Scholar] [CrossRef]
- Lima, S.M.; Yamada, S.S.; Reis, B.F.; Postigo, S.; Galvão da Silva, M.A.; Aoki, T. Effective treatment of vaginal atrophy with isoflavone vaginal gel. Maturitas 2013, 74, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Terashima, K.; Sato, Y.; Arai, S.; Eboshida, A. Effects of isoflavone supplement on healthy women. Biofactors 2000, 12, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T.; Chiba, Y. Evaluation of a natural S-equol supplement in treating premenstrual symptoms and the effect of the gut microbiota: An open-label pilot study. Neuropsychopharmacol. Rep. 2022, 42, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.-S.; Ge, J.; Chen, S.-W.; Xiong, Y.-Q.; Ma, S.-J.; Chen, Q. Association between Dietary Isoflavones in Soy and Legumes and Endometrial Cancer: A Systematic Review and Meta-Analysis. J. Acad. Nutr. Diet. 2018, 118, 637–651. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Chen, J.L.; Luo, Y.; Mathur, M.B.; Anagnostis, P.; Nurmatov, U.; Talibov, M.; Zhang, J.; Hawrylowicz, C.M.; Lumsden, M.A.; et al. Menopausal hormone therapy and women’s health: An umbrella review. PLoS Med. 2021, 18, e1003731. [Google Scholar] [CrossRef] [PubMed]
- Shanle, E.K.; Xu, W. Selectively targeting estrogen receptors for cancer treatment. Adv. Drug Deliv. Rev. 2010, 62, 1265–1276. [Google Scholar] [CrossRef]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; Bannon, F.; Ahn, J.V.; Johnson, C.J.; Bonaventure, A.; et al. Global surveillance of cancer survival 1995–2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Zlotta, A.R.; Kuk, C. Prevalence of prostate cancer across the globe: What can autopsy studies teach us about this peculiar disease? Arch. Esp. Urol. 2014, 67, 400–408. [Google Scholar]
- Dey, P.; Barros, R.P.; Warner, M.; Ström, A.; Gustafsson, J.Å. Insight into the mechanisms of action of estrogen receptor β in the breast, prostate, colon, and CNS. J. Mol. Endocrinol. 2013, 51, T61–T74. [Google Scholar] [CrossRef]
- Rizzardi, A.E.; Zhang, X.; Vogel, R.I.; Kolb, S.; Geybels, M.S.; Leung, Y.K.; Henriksen, J.C.; Ho, S.M.; Kwak, J.; Stanford, J.L.; et al. Quantitative comparison and reproducibility of pathologist scoring and digital image analysis of estrogen receptor β2 immunohistochemistry in prostate cancer. Diagn. Pathol. 2016, 11, 63–73. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef]
- Adlercreutz, H. Western diet and Western diseases: Some hormonal and biochemical mechanisms and associations. Scand. J. Clin. Lab. Investig. 1990, 201, 3–23. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Tuominen, J.; Pylkkänen, L.; Santti, R. Assessment of information to substantiate a health claim on the prevention of prostate cancer by lignans. Nutrients 2010, 2, 99–115. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Price, D.T.; Polascik, T.J.; Robertson, C.N.; Anderson, E.E.; Paulson, D.F.; Walther, P.J.; Gannon, M.; Vollmer, R.T. Pilot study of dietary fat restriction and flaxseed supplementation in men with prostate cancer before surgery: Exploring the effects on hormonal levels, prostate-specific antigen, and histopathologic features. Urology 2001, 58, 47–52. [Google Scholar] [CrossRef]
- Azrad, M.; Vollmer, R.T.; Madden, J.; Dewhirst, M.; Polascik, T.J.; Snyder, D.C.; Ruffin, M.T.; Moul, J.W.; Brenner, D.E.; Demark-Wahnefried, W. Flaxseed-derived enterolactone is inversely associated with tumor cell proliferation in men with localized prostate cancer. J. Med. Food 2013, 16, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Buja, A.; Pierbon, M.; Lago, L.; Grotto, G.; Baldo, V. Breast Cancer Primary Prevention and Diet: An Umbrella Review. Int. J. Environ. Res. Public Health 2020, 17, 4731–4774. [Google Scholar] [CrossRef] [PubMed]
- Carreau, C.; Flouriot, G.; Bennetau-Pelissero, C.; Potier, M. Enterodiol and enterolactone, two major diet-derived polyphenol metabolites have different impact on ERalpha transcriptional activation in human breast cancer cells. J. Steroid. Biochem. Mol. Biol. 2008, 110, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Power, K.A.; Saarinen, N.M.; Chen, J.M.; Thompson, L.U. Mammalian lignans enterolactone and enterodiol, alone and in combination with the isoflavone genistein, do not promote the growth of MCF-7 xenografts in ovariectomized athymic nude mice. Int. J. Cancer 2006, 118, 1316–1320. [Google Scholar] [CrossRef] [PubMed]
- Touillaud, M.S.; Thiébaut, A.C.; Fournier, A.; Niravong, M.; Boutron-Ruault, M.C.; Clavel-Chapelon, F. Dietary lignan intake and postmenopausal breast cancer risk by estrogen and progesterone receptor status. J. Natl. Cancer Inst. 2007, 99, 475–486. [Google Scholar] [CrossRef]
- Buck, K.; Zaineddin, A.K.; Vrieling, A.; Linseisen, J.; Chang-Claude, J. Meta-analyses of lignans and enterolignans in relation to breast cancer risk. Am. J. Clin. Nutr. 2010, 92, 141–153. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Huovinen, R.; Wärri, A.; Mäkelä, S.I.; Valentín-Blasini, L.; Sjöholm, R.; Ammälä, J.; Lehtilä, R.; Eckerman, C.; Collan, Y.U.; et al. Enterolactone inhibits the growth of 7,12-dimethylbenz(a)anthracene-induced mammary carcinomas in the rat. Mol. Cancer Ther. 2002, 1, 869–876. [Google Scholar]
- Blanco Mejia, S.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P.; et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019, 149, 968–981. [Google Scholar] [CrossRef]
- Hermsdorff, H.H.M.; Ángeles Zulet, M.; Abete, I.; Martínez, J.A. A legume-based hypocaloric diet reduces proinflammatory status and improves metabolic features in overweight/obese subjects. Eur. J. Nutr. 2011, 50, 61–69. [Google Scholar] [CrossRef]
- Crouse, J.R., 3rd; Morgan, T.; Terry, J.G.; Ellis, J.; Vitolins, M.; Burke, G.L. A randomized trial comparing the effect of casein with that of soy protein containing varying amounts of isoflavones on plasma concentrations of lipids and lipoproteins. Arch. Intern. Med. 1999, 159, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chiriboga, D.; Olendzki, B.C.; Nicolosi, R.; Merriam, P.A.; Ockene, I.S. Effect of soy protein containing isoflavones on blood lipids in moderately hypercholesterolemic adults: A randomized controlled trial. J. Am. Coll. Nutr. 2005, 24, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Eriksen, A.K.; Brunius, C.; Mazidi, M.; Hellström, P.M.; Risérus, U.; Iversen, K.N.; Fristedt, R.; Sun, L.; Huang, Y.; Nørskov, N.P.; et al. Effects of whole-grain wheat, rye, and lignan supplementation on cardiometabolic risk factors in men with metabolic syndrome: A randomized crossover trial. Am. J. Clin. Nutr. 2020, 111, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Edel, A.L.; Patenaude, A.F.; Richard, M.N.; Dibrov, E.; Austria, J.A.; Aukema, H.M.; Pierce, G.N.; Aliani, M. The effect of flaxseed dose on circulating concentrations of alpha-linolenic acid and secoisolariciresinol diglucoside derived enterolignans in young, healthy adults. Eur. J. Nutr. 2016, 55, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Peñalvo, J.L.; López-Romero, P. Urinary enterolignan concentrations are positively associated with serum HDL cholesterol and negatively associated with serum triglycerides in U.S. adults. J. Nutr. 2012, 142, 751–756. [Google Scholar] [CrossRef]
- Frankenfeld, C.L. Cardiometabolic risk factors are associated with high urinary enterolactone concentration, independent of urinary enterodiol concentration and dietary fiber intake in adults. J. Nutr. 2014, 144, 1445–1453. [Google Scholar] [CrossRef]
- Miranda, C.L.; Johnson, L.A.; de Montgolfier, O.; Elias, V.D.; Ullrich, L.S.; Hay, J.J.; Paraiso, I.L.; Choi, J.; Reed, R.L.; Revel, J.S.; et al. Non-estrogenic Xanthohumol Derivatives Mitigate Insulin Resistance and Cognitive Impairment in High-Fat Diet-induced Obese Mice. Sci. Rep. 2018, 8, 613. [Google Scholar] [CrossRef]
- Seida, A.; El-Hefnawy, H.; Abou-Hussein, D.; Mokhtar, F.A.; Abdel-Naim, A. Evaluation of Medicago sativa L. sprouts as antihyperlipidemic and antihyperglycemic agent. Pak. J. Pharm. Sci. 2015, 28, 2061–2074. [Google Scholar]
- Cano, R.; Pérez, J.L.; Dávila, L.A.; Ortega, Á.; Gómez, Y.; Valero-Cedeño, N.J.; Parra, H.; Manzano, A.; Véliz Castro, T.I.; Albornoz, M.P.D.; et al. Role of Endocrine-Disrupting Chemicals in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: A Comprehensive Review. Int. J. Mol. Sci. 2021, 22, 4807. [Google Scholar] [CrossRef] [PubMed]
- Nagl, V.; Grenier, B.; Pinton, P.; Ruczizka, U.; Dippel, M.; Bünger, M.; Oswald, I.P.; Soler, L. Exposure to Zearalenone Leads to Metabolic Disruption and Changes in Circulating Adipokines Concentrations in Pigs. Toxins 2021, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Gajęcka, M.; Tarasiuk, M.; Zielonka, Ł.; Dąbrowski, M.; Nicpoń, J.; Baranowski, M.; Gajęcki, M.T. Changes in the metabolic profile and body weight of pre-pubertal gilts during prolonged monotonic exposure to low doses of zearalenone and deoxynivalenol. Toxiconology 2017, 125, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Jungbauer, A.; Medjakovic, S. Phytoestrogens and the metabolic syndrome. J. Steroid Biochem. Mol. Biol. 2014, 139, 277–289. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Yamori, Y. Potential Effects of Soy Isoflavones on the Prevention of Metabolic Syndrome. Molecules 2021, 26, 5863. [Google Scholar] [CrossRef]
- Chen, L.R.; Chen, K.H. Utilization of Isoflavones in Soybeans for Women with Menopausal Syndrome: An Overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef]
- Woo, H.W.; Kim, M.K.; Lee, Y.H.; Shin, D.H.; Shin, M.H.; Choi, B.Y. Habitual consumption of soy protein and isoflavones and risk of metabolic syndrome in adults ≥ 40 years old: A prospective analysis of the Korean Multi-Rural Communities Cohort Study (MRCohort). Eur. J. Nutr. 2019, 58, 2835–2850. [Google Scholar] [CrossRef]
- Squadrito, F.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; D’Anna, R.; Arcoraci, V.; Burnett, B.P.; Minutoli, L.; et al. Genistein in the metabolic syndrome: Results of a randomized clinical trial. J. Clin. Endocrinol. Metab. 2013, 98, 3366–3374. [Google Scholar] [CrossRef]
- Irace, C.; Marini, H.; Bitto, A.; Altavilla, D.; Polito, F.; Adamo, E.B.; Arcoraci, V.; Minutoli, L.; Di Benedetto, A.; Di Vieste, G.; et al. Genistein and endothelial function in postmenopausal women with metabolic syndrome. Eur. J. Clin. Investig. 2013, 43, 1025–1031. [Google Scholar] [CrossRef]
- Usui, T.; Tochiya, M.; Sasaki, Y.; Muranaka, K.; Yamakage, H.; Himeno, A.; Shimatsu, A.; Inaguma, A.; Ueno, T.; Uchiyama, S.; et al. Effects of natural S-equol supplements on overweight or obesity and metabolic syndrome in the Japanese, based on sex and equol status. Clin. Endocrinol. 2013, 78, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Cornish, S.M.; Chilibeck, P.D.; Paus-Jennsen, L.; Biem, H.J.; Khozani, T.; Senanayake, V.; Vatanparast, H.; Little, J.P.; Whiting, S.J.; Pahwa, P. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl. Physiol. Nutr. Metab. 2009, 34, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pan, A.; Yu, Z.; Qi, Q.; Lu, L.; Zhang, G.; Yu, D.; Zong, G.; Zhou, Y.; Chen, X.; et al. Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J. Nutr. 2010, 140, 1937–1942. [Google Scholar] [CrossRef]
- Fukumitsu, S.; Aida, K.; Shimizu, H.; Toyoda, K. Flaxseed lignan lowers blood cholesterol and decreases liver disease risk factors in moderately hypercholesterolemic men. Nutr. Res. 2010, 30, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.M.; de Paula, H.; Cardoso, L.D.; Costa, N.M. Effects of brown and golden flaxseed on the lipid profile, glycemia, inflammatory biomarkers, blood pressure and body composition in overweight adolescents. Nutrition 2015, 31, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Luís, C.; Costa, R.; Rodrigues, I.; Castela, Â.; Coelho, P.; Guerreiro, S.; Gomes, J.; Reis, C.; Soares, R. Xanthohumol and 8-prenylnaringenin reduce type 2 diabetes-associated oxidative stress by downregulating galectin-3. Porto Biomed. J. 2018, 4, e23. [Google Scholar] [CrossRef]
- Park, S.; Sim, K.S.; Hwangbo, Y.; Park, S.J.; Kim, Y.J.; Kim, J.H. Naringenin and Phytoestrogen 8-Prenylnaringenin Protect against Islet Dysfunction and Inhibit Apoptotic Signaling in Insulin-Deficient Diabetic Mice. Molecules 2022, 27, 4227. [Google Scholar] [CrossRef]
- Zywno, H.; Bzdega, W.; Kolakowski, A.; Kurzyna, P.; Harasim-Symbor, E.; Sztolsztener, K.; Chabowski, A.; Konstantynowicz-Nowicka, K. The Influence of Coumestrol on Sphingolipid Signaling Pathway and Insulin Resistance Development in Primary Rat Hepatocytes. Biomolecules 2021, 11, 268. [Google Scholar] [CrossRef]
- Tang, J.; Wan, Y.; Zhao, M.; Zhong, H.; Zheng, J.S.; Feng, F. Legume and soy intake and risk of type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2020, 111, 677–688. [Google Scholar] [CrossRef]
- Barańska, A.; Błaszczuk, A.; Polz-Dacewicz, M.; Kanadys, W.; Malm, M.; Janiszewska, M.; Jędrych, M. Effects of Soy Isoflavones on Glycemic Control and Lipid Profile in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 1886. [Google Scholar] [CrossRef]
- Glisic, M.; Kastrati, N.; Gonzalez-Jaramillo, V.; Bramer, W.M.; Ahmadizar, F.; Chowdhury, R.; Danser, A.J.; Roks, A.J.; Voortman, T.; Franco, O.H.; et al. Associations between Phytoestrogens, Glucose Homeostasis, and Risk of Diabetes in Women: A Systematic Review and Meta-Analysis. Adv. Nutr. 2018, 9, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ruan, W.; Peng, Y.; Wang, D. Soy and the risk of type 2 diabetes mellitus: A systematic review and meta-analysis of observational studies. Diabetes Res. Clin. Pract. 2018, 137, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Cruz, M.; Godinez-Salas, E.T.; Sanchez-Tapia, M.; Torres-Villalobos, G.; Pichardo-Ontiveros, E.; Guizar-Heredia, R.; Arteaga-Sanchez, L.; Gamba, G.; Mojica-Espinosa, R.; Schcolnik-Cabrera, A.; et al. Genistein stimulates insulin sensitivity through gut microbiota reshaping and skeletal muscle AMPK activation in obese subjects. BMJ Open Diabetes Res. Care 2020, 8, e000948. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Sun, J.; Chen, Y.; Ye, X.; Li, H.; Yu, Z.; Wang, Y.; Gu, W.; Zhang, X.; Chen, X.; et al. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: A randomized, double-blind, cross-over trial. PLoS ONE 2007, 2, e1148. [Google Scholar] [CrossRef]
- Talaei, M.; Lee, B.L.; Ong, C.N.; van Dam, R.M.; Yuan, J.M.; Koh, W.P.; Pan, A. Urine phyto-oestrogen metabolites are not significantly associated with risk of type 2 diabetes: The Singapore Chinese health study. Br. J. Nutr. 2016, 115, 1607–1615. [Google Scholar] [CrossRef]
- Sun, Q.; Wedick, N.M.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; van Dam, R.M. Gut microbiota metabolites of dietary lignans and risk of type 2 diabetes: A prospective investigation in two cohorts of U.S. women. Diabetes Care 2014, 37, 1287–1295. [Google Scholar] [CrossRef]
- Eriksen, A.K.; Kyrø, C.; Nørskov, N.P.; Frederiksen, K.; Bach Knudsen, K.E.; Overvad, K.; Landberg, R.; Tjønneland, A.; Olsen, A. Pre-diagnostic plasma enterolactone concentrations are associated with lower mortality among individuals with type 2 diabetes: A case-cohort study in the Danish Diet, Cancer and Health cohort. Diabetologia 2019, 62, 959–969. [Google Scholar] [CrossRef]
- Dourson, M.L.; Stara, J.F. Regulatory history and experimental support of uncertainty (safety) factors. Regul. Toxicol. Pharmacol. 1983, 3, 224–238. [Google Scholar] [CrossRef]
- Pieters, M.N.; Kramer, H.J.; Slob, W. Evaluation of the uncertainty factor for subchronic-to-chronic extrapolation: Statistical analysis of toxicity data. Regul. Toxicol. Pharmacol. 1998, 27, 108–111. [Google Scholar] [CrossRef]
- ANSES. Étude de L’alimentation Totale Française 2 (EAT 2) Tome 1. Contaminants Inorganiques, Minéraux, Polluants Organiques Persistants, Mycotoxines, Phyto-Estrogènes. Avis de l’Anses; Rapport D’expertise, Edition Scientifique; ANSES: Maisons-Alfort, France, 2011. [Google Scholar]
- NIH/PubChem. Diethylstilbestrol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Diethylstilbestrol (accessed on 15 December 2022).
- Pocock, V.J.; Sales, G.D.; Milligan, S.R. Comparison of the oestrogenic effects of infant milk formulae, oestradiol and the phytoestrogen coumestrol delivered continuously in the drinking water to ovariectomised mice. Food Chem. Toxicol. 2002, 40, 643–651. [Google Scholar] [CrossRef]
- WHO-JECFA 2000. Zearalenone. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). TRS 896-JECFA 53/93. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/Home/Chemical/2730 (accessed on 15 December 2022).
- NTP. Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl. Toxicol. Program Tech. Rep. Ser. 2008, 539, 1–266. [Google Scholar]
- Lee, A.; Bensaada, S.; Lamothe, V.; Lacoste, M.; Bennetau-Pelissero, C. Endocrine disruptors on and in fruits and vegetables: Estimation of the potential exposure of the French population. Food Chem. 2022, 373, 131513. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.R.; Gua, H.; Chang, L.L.; Wang, Z.Y.; Tong, H.B.; Zou, J.M. Safety evaluation of daidzein in laying hens: Part I. Effects on laying performance, clinical blood parameters, and organs development. Food Chem. Toxicol. 2013, 55, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Wolterbeek, A.P.M.; Roberts, A.; Korte, H.; Unkila, M.; Waalkens-Berendsen, D.H. Prenatal developmental toxicity study with 7-hydroxymatairesinol potassium acetate (HMRlignan) in rats. Regul. Toxicol. Pharmacol. 2004, 40, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Soujanya, M.G.S.; Prathap Reddy, K.; Sridevi, V.; Ramachandra Reddy, P.; Sreenivasula Reddy, P. In Utero Exposure of Biochanin-A Alters Female Reproduction in Rat. J. Clin. Mol. Endocrinol. 2016, 1, 8. [Google Scholar]
- Parandin, R.; Mohammadi, L. The effects of Formononetin Derived from Red Clover During Pregnancy on Puberty, Some Reproductive Parameters and Lordosis Behavior of Female Mice. Armaghan Danesh. 2019, 24, 199–213. [Google Scholar]
- Nedresky, D.; Singh, G. Physiology, Luteinizing Hormone. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Milligan, S.R. Chapter 72—Reproductive and Estrogenic Effects of 8-Prenylnaringenin in Hops. In Beer in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2009; pp. 711–723. [Google Scholar] [CrossRef]
- Christoffel, J.; Rimoldi, G.; Wuttke, W. Effects of 8-prenylnaringenin on the hypothalamo-pituitary-uterine axis in rats after 3-month treatment. J. Endocrinol. 2006, 188, 397–405. [Google Scholar] [CrossRef]
- Rad, M.; Hümpel, M.; Schaefer, O.; Schoemaker, R.C.; Schleuning, W.D.; Cohen, A.F.; Burggraaf, J. Pharmacokinetics and systemic endocrine effects of the phyto-oestrogen 8-prenylnaringenin after single oral doses to postmenopausal women. Br. J. Clin. Pharmacol. 2006, 62, 288–296. [Google Scholar] [CrossRef]
- Odell, W.D.; Ross, G.T.; Rayford, P.L. Radioimmunoassay for Luteinizing Hormone in Human plasma or Serum: Physiological Studies. J. Clin. Investig. 1967, 46, 248–255. [Google Scholar] [CrossRef]
- Hughes, C.L., Jr. Effects of phytoestrogens on GnRH-induced luteinizing hormone secretion in ovariectomized rats. Reprod. Toxicol. 1987, 1, 179–181. [Google Scholar] [CrossRef]
- He, J.; Wei, C.; Li, Y.; Liu, Y.; Wang, Y.; Pan, J.; Liu, J.; Wu, Y.; Cui, S. Zearalenone and alpha-zearalenol inhibit the synthesis and secretion of pig follicle stimulating hormone via the non-classical estrogen membrane receptor GPR30. Mol. Cell. Endocrinol. 2018, 461, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Kriszt, R.; Winkler, Z.; Polyák, A.; Kuti, D.; Molnár, C.; Hrabovszky, E.; Kalló, I.; Szőke, Z.; Ferenczi, S.; Kovács, K.J. Xenoestrogens Ethinyl Estradiol and Zearalenone Cause Precocious Puberty in Female Rats via Central Kisspeptin Signaling. Endocrinology 2015, 156, 3996–4007. [Google Scholar] [CrossRef] [PubMed]
- Shier, F.L.; Rossiter, R.C. Clover disease. Practical findings and recommendations for control. J. Agri. West Aust. 1949, 26, 111–116. [Google Scholar]
- Findlay, J.K.; Buckmaster, J.M.; Chamley, W.A.; Cumming, I.A.; Hearnshaw, H.; Goding, J.R. Release of luteinizing hormone by oestradiol-17 and a gonadotrophin-releasing hormone in ewes affected with clover disease. Neuroendocrinology 1973, 11, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Bingham, S.; Setchell, K.D. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am. J. Clin. Nutr. 1994, 60, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Gardner-Thorpe, D.; O’Hagen, C.; Young, I.; Lewis, S.J. Dietary supplements of soya flour lower serum testosterone concentrations and improve markers of oxidative stress in men. Eur. J. Clin. Nutr. 2003, 57, 100–106. [Google Scholar] [CrossRef]
- Hooper, L.; Ryder, J.J.; Kurzer, M.S.; Lampe, J.W.; Messina, M.J.; Phipps, W.R.; Cassidy, A. Effects of soy protein and isoflavones on circulating hormone concentrations in pre- and post-menopausal women: A systematic review and meta-analysis. Hum. Reprod. Update 2009, 15, 423–440. [Google Scholar] [CrossRef]
- Sawagado, L.; Houdebine, L.M. Identification of the lactogenic compound present in beer. Ann. Biol. Clin. 1988, 46, 129–134. [Google Scholar]
- Xia, Y.; Chen, M.; Zhu, P.; Lu, C.; Fu, G.; Zhou, X.; Chen, D.; Wang, H.; Hang, B.; Wang, S.; et al. Urinary phytoestrogen levels related to idiopathic male infertility in Chinese men. Environ. Int. 2013, 59, 161–167. [Google Scholar] [CrossRef]
- Jefferson, W.N.; Patisaul, H.B.; Williams, C.J. Reproductive consequences of developmental phytoestrogen exposure. Reproduction 2012, 143, 247–260. [Google Scholar] [CrossRef]
- Whitten, P.L.; Lewis, C.; Russell, E.; Naftolin, F. Potential adverse effects of phytoestrogens. J. Nutr. 1995, 125 (Suppl. S3), 771S–776S. [Google Scholar] [PubMed]
- Rivera-Núñez, Z.; Barrett, E.S.; Szamreta, E.A.; Shapses, S.A.; Qin, B.; Lin, Y.; Zarbl, H.; Buckley, B.; Bandera, E.V. Urinary mycoestrogens and age and height at menarche in New Jersey girls. Environ. Health 2019, 18, 24. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Tao, F.B.; Liu, D.Y.; Xu, Y.Y.; Hao, J.H.; Sun, Y.; Su, P.Y. Effects of growth environments and two environmental endocrine disruptors on children with idiopathic precocious puberty. Eur. J. Endocrinol. 2012, 166, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Kinkade, C.W.; Rivera-Núñez, Z.; Gorcyzca, L.; Aleksunes, L.M.; Barrett, E.S. Impact of Fusarium-Derived Mycoestrogens on Female Reproduction: A Systematic Review. Toxins 2021, 13, 373. [Google Scholar] [CrossRef]
- Li, L.; Zhang, T.; Ren, X.; Li, B.; Wang, S. Male reproductive toxicity of zearalenone-meta-analysis with mechanism review. Ecotoxicol. Environ. Saf. 2021, 221, 112457. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Wu, H.; Wang, Q.; Zhou, X.; Zhang, Y.; Wu, W.; Wang, Y.; Ren, Z.; Li, H.; Ling, Y.; et al. Simultaneous determination of formononetin, biochanin A and their active metabolites in human breast milk, saliva and urine using salting-out assisted liquid-liquid extraction and ultra-high performance liquid chromatographyelectrospray ionization tandem mass spectrum. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1145, 122108. [Google Scholar] [CrossRef]
- Setchell, K.D.; Zimmer-Nechemias, L.; Cai, J.; Heubi, J.E. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am. J. Clin. Nutr. 1998, 68 (Suppl. S6), 1453S–1461S. [Google Scholar] [CrossRef]
- McCarver, G.; Bhatia, J.; Chambers, C.; Clarke, R.; Etzel, R.; Foster, W.; Hoyer, P.; Leeder, J.S.; Peters, J.M.; Rissman, E.; et al. NTP-CERHR expert panel report on the developmental toxicity of soy infant formula. Birth Defects Res. B Dev. Reprod. Toxicol. 2011, 92, 421–468. [Google Scholar] [CrossRef]
- Chin, H.B.; Kelly, A.; Adgent, M.A.; Patchel, S.A.; James, K.; Vesper, H.W.; Botelho, J.C.; Chandler, D.W.; Zemel, B.S.; Schall, J.I.; et al. Reproductive Hormone Concentrations and Associated Anatomical Responses: Does Soy Formula Affect Minipuberty in Boys? J. Clin. Endocrinol. Metab. 2021, 106, 2635–2645. [Google Scholar] [CrossRef]
- Gilchrist, J.M.; Moore, M.B.; Andres, A.; Estroff, J.A.; Badger, T.M. Ultrasonographic patterns of reproductive organs in infants fed soy formula: Comparisons to infants fed breast milk and milk formula. J. Pediatr. 2010, 156, 215–220. [Google Scholar] [CrossRef]
- Adgent, M.A.; Daniels, J.L.; Edwards, L.J.; Siega-Riz, A.M.; Rogan, W.J. Early-life soy exposure and gender-role play behavior in children. Environ. Health Perspect. 2011, 119, 1811–1816. [Google Scholar] [CrossRef]
- Strom, B.L.; Schinnar, R.; Ziegler, E.E.; Barnhart, K.T.; Barnhart, K.T.; Sammel, M.D.; Macones, G.A.; Stallings, V.A.; Drulis, J.M.; Nelson, S.E.; et al. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. JAMA 2001, 286, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Upson, K.; Harmon, Q.E.; Laughlin-Tommaso, S.K.; Umbach, D.M.; Baird, D.D. Soy-based Infant Formula Feeding and Heavy Menstrual Bleeding Among Young African American Women. Epidemiology 2016, 27, 716–725. [Google Scholar] [CrossRef]
- Upson, K.; Adgent, M.A.; Wegienka, G.; Baird, D.D. Soy-based infant formula feeding and menstrual pain in a cohort of women aged 23–35 years. Hum. Reprod. 2019, 34, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Lin, Z.; Vásquez, E.; Luan, X.; Guo, F.; Xu, L. High soy isoflavone or soy-based food intake during infancy and in adulthood is associated with an increased risk of uterine fibroids in premenopausal women: A meta-analysis. Nutr. Res. 2019, 71, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Harlid, S.; Adgent, M.; Jefferson, W.N.; Panduri, V.; Umbach, D.M.; Xu, Z.; Stallings, V.A.; Williams, C.J.; Rogan, W.J.; Taylor, J.A. Soy Formula and Epigenetic Modifications: Analysis of Vaginal Epithelial Cells from Infant Girls in the IFED Study. Environ. Health Perspect. 2017, 125, 447–452. [Google Scholar] [CrossRef]
- Felício, J.S.; Leite de Alcântara, A.; Janaú, L.C.; de Moraes, L.V.; de Oliveira, M.C.N.I.; de Lemos, M.N.; de Souza Neto, N.J.K.; Neto, J.F.A.; da Silva, W.M.; de Souza, Í.J.A.; et al. Association of Soy and Exclusive Breastfeeding with Central Precocious Puberty: A Case-Control Study. Front. Endocrinol. 2021, 12, 667029. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.K.; Gustavo, A.F.S.E.; Gonçalves, R.B.; Bolfi, F.; Mendes, A.L.; dos Santos Nunes-Nogueira, V. Association between a soy-based infant diet and the onset of puberty: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251241. [Google Scholar] [CrossRef]
- Segovia-Siapco, G.; Pribis, P.; Oda, K.; Sabaté, J. Soy isoflavone consumption and age at pubarche in adolescent males. Eur. J. Nutr. 2018, 57, 2287–2294. [Google Scholar] [CrossRef]
- Cheng, G.; Remer, T.; Prinz-Langenohl, R.; Blaszkewicz, M.; Degen, G.H.; Buyken, A.E. Relation of isoflavones and fiber intake in childhood to the timing of puberty. Am. J. Clin. Nutr. 2010, 92, 556–564. [Google Scholar] [CrossRef]
- Goldberg, M.; D’Aloisio, A.A.; O’Brien, K.M.; Zhao, S.; Sandler, D.P. Early-life exposures and age at thelarche in the Sister Study cohort. Breast Cancer Res. 2021, 23, 111. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Xu, Y.; Liu, X.; Wang, X.; Shan, S.; Crabbe, M.J.C.; Zhao, L.; Fang, H.; Cheng, G. Prospective association of dietary soy and fibre intake with puberty timing: A cohort study among Chinese children. BMC Med. 2022, 20, 145. [Google Scholar] [CrossRef] [PubMed]
- Imai, H.; Nishikawa, H.; Suzuki, A.; Kodama, E. Secondary Hypogonadism due to Excessive Ingestion of Isoflavone in a Man. Intern. Med. 2022, 61, 2899–2903. [Google Scholar] [CrossRef]
- Chandrareddy, A.; Muneyyirci-Delale, O.; McFarlane, S.I.; Murad, O.M. Adverse effects of phytoestrogens on reproductive health: A report of three cases. Complement. Ther. Clin. Pract. 2008, 14, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Chavarro, J.E.; Toth, T.L.; Sadio, S.M.; Hauser, R. Soy food and isoflavone intake in relation to semen quality parameters among men from an infertility clinic. Hum. Reprod. 2008, 23, 2584–2590. [Google Scholar] [CrossRef] [PubMed]
- Toshima, H.; Suzuki, Y.; Imai, K.; Yoshinaga, J. Endocrine disrupting chemicals in urine of Japanese male partners of subfertile couples: A pilot study on exposure and semen quality. Int. J. Hyg. Environ. Health 2012, 215, 502–506. [Google Scholar] [CrossRef]
- Mumford, S.L.; Kim, S.; Chen, Z.; Barr, D.B.; Buck-Louis, G.M. Urinary Phytoestrogens Are Associated with Subtle Indicators of Semen Quality among Male Partners of Couples Desiring Pregnancy. J. Nutr. 2015, 145, 2535–2541. [Google Scholar] [CrossRef]
- Yuan, G.; Liu, Y.; Liu, G.; Wei, L.; Wen, Y.; Huang, S.; Guo, Y.; Zou, F.; Cheng, J. Associations between semen phytoestrogens concentrations and semen quality in Chinese men. Environ. Int. 2019, 129, 136–144. [Google Scholar] [CrossRef]
- Beaton, L.K.; McVeigh, B.L.; Dillingham, B.L.; Lampe, J.W.; Duncan, A.M. Soy protein isolates of varying isoflavone content do not adversely affect semen quality in healthy young men. Fertil. Steril. 2010, 94, 1717–1722. [Google Scholar] [CrossRef]
- Povey, A.C.; Clyma, J.-A.; McNamee, R.; Moore, H.D. Phytoestrogen intake and other dietary risk factors for low motile sperm count and poor sperm morphology. Andrology 2020, 8, 1805–1814. [Google Scholar] [CrossRef]
- Andrews, M.A.; Schliep, K.C.; Wactawski-Wende, J.; Stanford, J.B.; Zarek, S.M.; Radin, R.G.; Sjaarda, L.A.; Perkins, N.J.; Kalwerisky, R.A.; Hammoud, A.O.; et al. Dietary factors and luteal phase deficiency in healthy eumenorrheic women. Hum. Reprod. 2015, 30, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- Index Mundi. Total Fertility Rate, Country Comparison. Available online: https://www.indexmundi.com/g/r.aspx?v=31 (accessed on 15 December 2022).
- Vanegas, J.C.; Afeiche, M.C.; Gaskins, A.J.; Mínguez-Alarcón, L.; Williams, P.L.; Wright, D.L.; Toth, T.L.; Hauser, R.; Chavarro, J.E. Soy food intake and treatment outcomes of women undergoing assisted reproductive technology. Fertil. Steril. 2015, 103, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.F.; Sprando, R.L.; Black, T.N.; Olejnik, N.; Wiesenfeld, P.W.; Babu, U.S.; Bryant, M.; Flynn, T.J.; Ruggles, D.I. Effects of flaxseed and defatted flaxseed meal on reproduction and development in rats. Food Chem. Toxicol. 2003, 41, 819–834. [Google Scholar] [CrossRef] [PubMed]
- Phipps, W.R.; Martini, M.C.; Lampe, J.W.; Slavin, J.L.; Kurzer, M.S. Effect of flax seed ingestion on the menstrual cycle. J. Clin. Endocrinol. Metab. 1993, 77, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Huang, H.; Zhao, N.; Ni, X.; Udelsman, R.; Zhang, Y. Phytoestrogens and Thyroid Cancer Risk: A Population-Based Case-Control Study in Connecticut. Cancer Epidemiol. Biomark. Prev. 2020, 29, 500–508. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, R.; Kitts, W.D. Plasma thyroid hormone concentrations in growing beef steers implanted with estrogenic anabolic growth promotants. Growth 1984, 48, 515–526. [Google Scholar] [PubMed]
- Williams, J.E.; Ireland, S.J.; Mollett, T.A.; Hancock, D.L.; Beaver, E.E.; Hannah, S. Influence of zeranol and breed on growth, composition of gain, and plasma hormone concentrations. J. Anim. Sci. 1991, 69, 1688–1696. [Google Scholar] [CrossRef]
- Becci, P.J.; Johnson, W.D.; Hess, F.G.; Gallo, M.A.; Parent, R.A.; Taylor, J.M. Combined two-generation reproduction-teratogenesis study of zearalenone in the rat. J. Appl. Toxicol. 1982, 2, 201–206. [Google Scholar] [CrossRef]
- Ripp, J.A. Soybean-induced goiter. Am. J. Dis. Child. 1961, 102, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 2002, 110 (Suppl. S3), 349–353. [Google Scholar] [CrossRef]
- Conrad, S.C.; Chiu, H.; Silverman, B.L. Soy formula complicates management of congenital hypothyroidism. Arch. Dis. Child. 2004, 89, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Fruzza, A.G.; Demeterco-Berggren, C.; Lee Jones, K. Unawareness of the effects of soy intake on the management of congenital hypothyroidism. Pediatrics 2012, 130, e699–e702. [Google Scholar] [CrossRef] [PubMed]
- Divi, R.L.; Chang, H.C.; Doerge, D.R. Anti-thyroid isoflavones from soybean: Isolation, characterization, and mechanisms of action. Biochem. Pharmacol. 1997, 54, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Sosvorová, L.; Mikšátková, P.; Bičíková, M.; Kaňová, N.; Lapčík, O. The presence of monoiodinated derivates of daidzein and genistein in human urine and its effect on thyroid gland function. Food Chem. Toxicol. 2012, 50, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Radović, B.; Mentrup, B.; Köhrle, J. Genistein and other soya isoflavones are potent ligands for transthyretin in serum and cerebrospinal fluid. Br. J. Nutr. 2006, 95, 1171–1176. [Google Scholar] [CrossRef]
- Ariyani, W.; Iwasaki, T.; Miyazaki, W.; Yu, L.; Takeda, S.; Koibuchi, N. A Possible Novel Mechanism of Action of Genistein and Daidzein for Activating Thyroid Hormone Receptor-Mediated Transcription. Toxicol. Sci. 2018, 164, 417–427. [Google Scholar] [CrossRef]
- Fan, Y.; Qian, H.; Wu, Z.; Li, Z.; Li, X.; Zhang, Y.; Xu, Q.; Lu, C.; Wang, X. Exploratory analysis of the associations between urinary phytoestrogens and thyroid hormones among adolescents and adults in the United States: National Health and Nutrition Examination Survey 2007–2010. Environ. Sci. Pollut. Res. Int. 2022, 29, 2974–2984. [Google Scholar] [CrossRef]
- Thankamony, A.; Pasterski, V.; Ong, K.K.; Acerini, C.L.; Hughes, I.A. Anogenital distance as a marker of androgen exposure in humans. Andrology 2016, 4, 616–625. [Google Scholar] [CrossRef]
- Becker, L.A.; Kunkel, A.J.; Brown, M.R.; Ball, E.E.; Williams, M.T. Effects of dietary phytoestrogen exposure during perinatal period. Neurotoxicol. Teratol. 2005, 27, 825–834. [Google Scholar] [CrossRef]
- Nagata, C.; Iwasa, S.; Shiraki, M.; Ueno, T.; Uchiyama, S.; Urata, K.; Sahashi, Y.; Shimizu, H. Associations among maternal soy intake, isoflavone levels in urine and blood samples, and maternal and umbilical hormone concentrations (Japan). Cancer Causes Control. 2006, 17, 1107–1113. [Google Scholar] [CrossRef]
- Adgent, M.A.; Umbach, D.M.; Zemel, B.S.; Kelly, A.; Schall, J.I.; Ford, E.G.; James, K.; Darge, K.; Botelho, J.C.; Vesper, H.W.; et al. A Longitudinal Study of Estrogen-Responsive Tissues and Hormone Concentrations in Infants Fed Soy Formula. J. Clin. Endocrinol. Metab. 2018, 103, 1899–1909. [Google Scholar] [CrossRef] [PubMed]
- Pfitscher, A.; Reiter, E.; Jungbauer, A. Receptor binding and transactivation activities of red clover isoflavones and their metabolites. J. Steroid Biochem. Mol. Biol. 2008, 112, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Berensztein, E.B.; Baquedano, M.S.; Gonzalez, C.R.; Saraco, N.I. Expression of aromatase, estrogen receptor alpha and beta, androgen receptor, and cytochrome P-450scc in the human early prepubertal testis. Pediatr. Res. 2006, 60, 740–744. [Google Scholar] [CrossRef]
- Canivenc-Lavier, M.C.; Neiers, F.; Briand, L. Plant polyphenols, chemoreception, taste receptors and taste management. Curr. Opin. Clin. Nutr. Metab. Care. 2019, 22, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Intelmann, D.; Batram, C.; Kuhn, C.; Haseleu, G.; Meyerhof, W.; Hofmann, T. Three TAS2R Bitter Taste Receptors Mediate the Psychophysical Responses to Bitter Compounds of Hops (Humulus lupulus L.) and Beer. Chem. Percept. 2009, 2, 118–132. [Google Scholar] [CrossRef]
- Soares, S.; Silva, M.S.; Garcia-Estevez, I.; Groβmann, P.; Brás, N.; Brandão, E.; Mateus, N.; de Freitas, V.; Behrens, M.; Meyerhof, W. Human bitter taste receptors are activated by different classes of polyphenols. J. Agric. Food Chem. 2018, 66, 8814–8823. [Google Scholar] [CrossRef]
- Roland, W.S.U.; Vincken, J.P.; Gouka, R.J.; van Buren, L.; Gruppen, H.; Smit, G. Soy isoflavones and other isoflavonoids activate the human bitter taste receptors hTAS2R14 and hTAS2R39. J. Agric. Food Chem. 2011, 59, 11764–11771. [Google Scholar] [CrossRef]
- Ley, J.P.; Dessoy, M.; Paetz, S.; Blings, M.; Hoffmann-Lücke, P.; Reichelt, K.V.; Krammer, G.E.; Pienkny, S.; Brandt, W.; Wessjohann, L. Identification of enterodiol as a masker for caffeine bitterness by using a pharmacophore model based on structural analogues of homo-eriodictyol. J. Agric. Food Chem. 2012, 60, 6303–6311. [Google Scholar] [CrossRef]
- Grau-Bové, C.; Miguéns-Gómez, A.; González-Quilen, C.; Fernández-López, J.A.; Remesar, X.; Torres-Fuentes, C.; Ávila-Román, J.; Rodríguez-Gallego, E.; Beltrán-Debón, R.; Blay, M.T.; et al. Modulation of Food Intake by Differential TAS2R Stimulation in Rat. Nutrients 2020, 12, 3784. [Google Scholar] [CrossRef]
- Jalševac, F.; Terra, X.; Rodríguez-Gallego, E.; Beltran-Debón, R.; Blay, M.T.; Pinent, M.; Ardévol, A. The Hidden One: What We Know About Bitter Taste Receptor 39. Front. Endocrinol. 2022, 13, 854718. [Google Scholar] [CrossRef]
- Semplici, B.; Luongo, F.P.; Passaponti, S.; Landi, C.; Landi, C.; Governini, L.; Morgante, G.; De Leo, V.; Piomboni, P.; Luddi, A. Bitter Taste Receptors Expression in Human Granulosa and Cumulus Cells: New Perspectives in Female Fertility. Cells 2021, 10, 3127. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.J.; Sollars, S.I. Contributory role of sex differences in the variations of gustatory function. J. Neurosci. Res. 2017, 95, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Saluja, P.; Shetty, V.; Dave, A.; Arora, M.; Hans, V.; Madan, A. Comparative Evaluation of the Effect of Menstruation, Pregnancy and Menopause on Salivary Flow Rate, pH and Gustatory Function. J. Clin. Diagn. Res. 2014, 8, ZC81-5. [Google Scholar] [CrossRef]
- Dahir, N.S.; Calder, A.N.; McKinley, B.J.; Liu, Y.; Gilbertson, T.A. Sex differences in fat taste responsiveness are modulated by estradiol. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E566–E580. [Google Scholar] [CrossRef]
- Kouidhi, W.; Bergès, R.; Tiffon, C.; Desmetz, C.; El May, M.; Auger, J.; Canivenc-Lavier, M. Perinatal xenohormone exposure impacts sweet preference and submandibular development in male rats. Oral Dis. 2013, 19, 812–823. [Google Scholar] [CrossRef]
- Kouidhi, W.; Bergès, R.; Drouin, G.; Desmetz, C.; Auger, J.; El May, M.; Canivenc-Lavier, M.-C. Post-weaning xenohormone intake affects adult rat submandibular gland in a sex-dependent manner. Oral Dis. 2018, 24, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Flynn, K.M.; Ferguson, S.A.; Delclos, K.B.; Newbold, R.R. Effects of Genistein Exposure on Sexually Dimorphic Behaviors in Rats. Toxicol. Sci. 2020, 55, 311–319. [Google Scholar] [CrossRef] [PubMed]


| Compounds | Chemical Family | NOAEL or LOAEL * in Animal | Model Species | Theoretical Reference Dose for Human ** (RfD) | Potential Intake in France | References |
|---|---|---|---|---|---|---|
| Diethylstilbestrol | E2 analogue | NOAEL 5 mg/kg/d | Rat | 0.05 mg/kg bw/d | Drug forbidden | [194] |
| 8-prenylnaringenin | Phytoestrogen | - | Human | 400 µg/d * | No data available | [53] |
| Coumestrol | Phytoestrogen | LOAEL 100 mg/kg/d | Mouse | 0.33 mg/kg bw/d | 0.016 µg/kg bw/d | [193,195] |
| Zearalenone | Mycotoxin | NOAEL 40 µg/kg/d | Pig | 0.4 µg/kg bw/d | 0.042 µg/kg bw/d | [193,196] |
| Genistein | Phytoestrogen | LOAEL 35 mg/kg/d | Rat | 0.12 mg/kg bw/d | 0–1.5 mg/kg bw/d | [197,198] |
| Daidzein | Phytoestrogen | NOAEL 50 mg/kg/d | Hen | 0.5 mg/kg bw/d | 0–0.8 mg/kg bw/d | [198,199] |
| Enterolactone | Phytoestrogen | NOAEL 7-hydroxymatairesinol 460–740 mg/kg/d | Rat | 4.6–7.4 mg/kg bw/d | 1.64–18.2 µg/kg bw/d matairesinol | [193,200] |
| Biochanin A | Phytoestrogen | LOAEL 25 mg/kg/d | Rat | 0.083 mg/kg bw/d | 0.0003 mg/kg bw/d | [193,201] |
| Formononetin | Phytoestrogen | NOAEL 5 mg/kg/d | Mouse | 0.05 mg/kg bw/d | 0.0013 mg/kg bw/d | [193,202] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canivenc-Lavier, M.-C.; Bennetau-Pelissero, C. Phytoestrogens and Health Effects. Nutrients 2023, 15, 317. https://doi.org/10.3390/nu15020317
Canivenc-Lavier M-C, Bennetau-Pelissero C. Phytoestrogens and Health Effects. Nutrients. 2023; 15(2):317. https://doi.org/10.3390/nu15020317
Chicago/Turabian StyleCanivenc-Lavier, Marie-Chantal, and Catherine Bennetau-Pelissero. 2023. "Phytoestrogens and Health Effects" Nutrients 15, no. 2: 317. https://doi.org/10.3390/nu15020317
APA StyleCanivenc-Lavier, M.-C., & Bennetau-Pelissero, C. (2023). Phytoestrogens and Health Effects. Nutrients, 15(2), 317. https://doi.org/10.3390/nu15020317







