Estimated Impact of Achieving the Australian National Sodium Reduction Targets on Blood Pressure, Chronic Kidney Disease Burden and Healthcare Costs: A Modelling Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Specification of the Modelled Sodium Targets
2.2. Sodium Intake and Blood Pressure Data
2.3. Model Framework
2.3.1. Sodium and Blood Pressure Risk Modelling
2.3.2. Epidemiological and Multistate Lifetable Modelling
2.3.3. Healthcare Costs
2.4. Scenario and Uncertainty Analysis
3. Results
3.1. Impact of Sodium Reduction on Mean Blood Pressure
3.2. Estimated Changes in CKD Incidence by 2030 and over the Lifetime
3.3. Estimated Changes in CKD Mortality by 2030 and Lifetime
3.4. Healthy Life Years Gained between 2019–2030 and Lifetime
3.5. Healthcare Costs
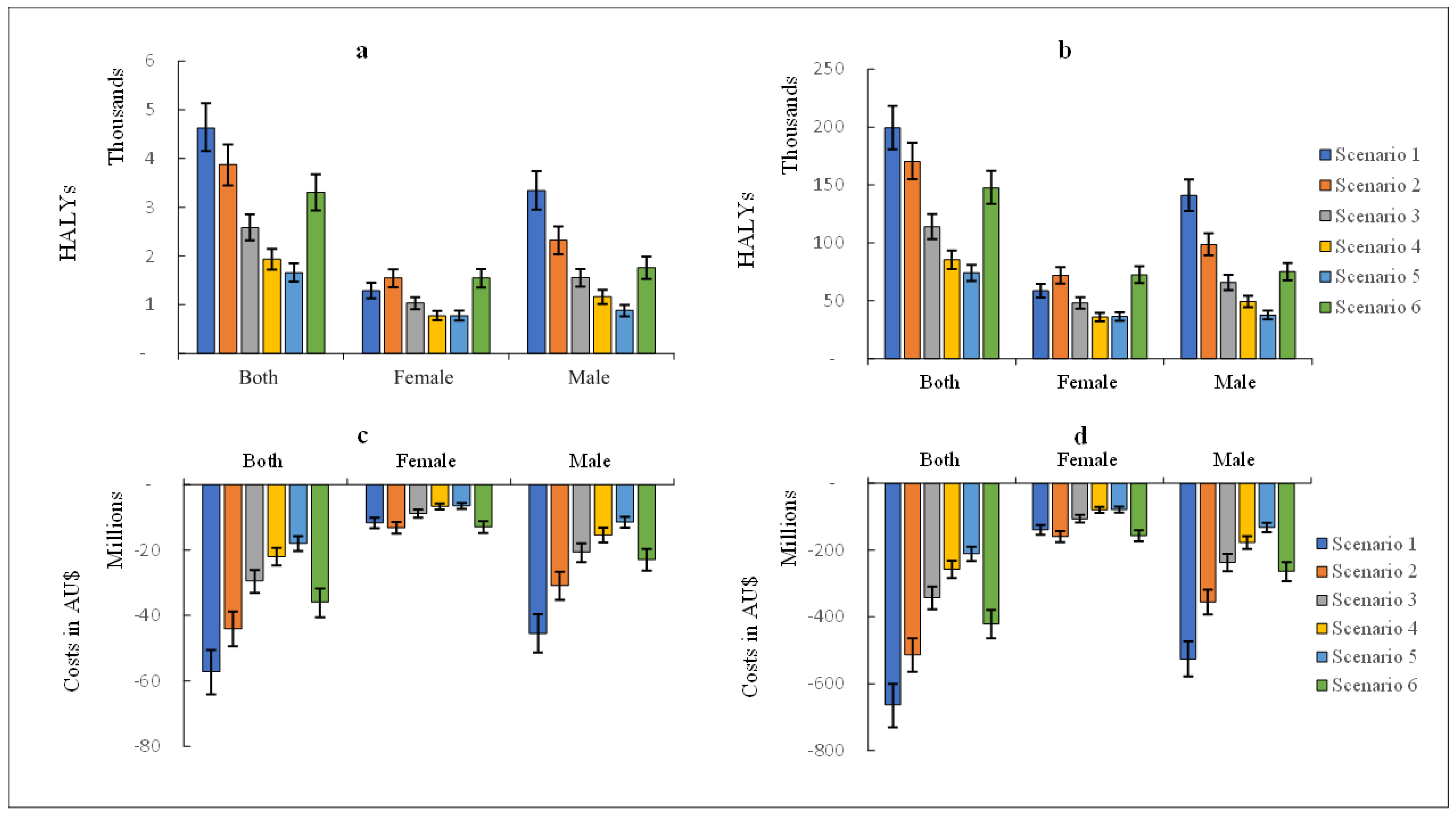
3.6. Scenario Analyses
4. Discussion
4.1. Comparison with Other Studies
4.2. Implications of the Findings
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2017 Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
- Dyer, A.R.; Elliott, P.; Shipley, M. Urinary electrolyte excretion in 24 hours and blood pressure in the INTERSALT Study. II. Estimates of electrolyte-blood pressure associations corrected for regression dilution bias. The INTERSALT Cooperative Research Group. Am. J. Epidemiol. 1994, 139, 940–951. [Google Scholar] [CrossRef]
- Filippini, T.; Malavolti, M.; Whelton, P.K.; Naska, A.; Orsini, N.; Vinceti, M. Blood Pressure Effects of Sodium Reduction: Dose-Response Meta-Analysis of Experimental Studies. Circulation 2021, 143, 1542–1567. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Trieu, K.; Yoshimura, S.; Neal, B.; Woodward, M.; Campbell, N.R.C.; Li, Q.; Lackland, D.T.; Leung, A.A.; Anderson, C.A.M.; et al. Effect of dose and duration of reduction in dietary sodium on blood pressure levels: Systematic review and meta-analysis of randomised trials. BMJ 2020, 368, m315. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.E.; O’Reilly, S.; Brinkman, M.; Hodge, A.; Giles, G.G.; English, D.R.; A Nowson, C. Relationship of urinary sodium and sodium-to-potassium ratio to blood pressure in older adults in Australia. Med. J. Aust. 2011, 195, 128–132. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. High Blood Pressure: AIHW, Australian Government. 2019. Available online: https://www.aihw.gov.au/reports/risk-factors/high-blood-pressure/contents/high-blood-pressure (accessed on 15 October 2022).
- He, F.J.; Tan, M.; Ma, Y.; MacGregor, G.A. Salt Reduction to Prevent Hypertension and Cardiovascular Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 632–647. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Blood Pressure Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Blood Pressure in Chronic Kidney Disease. Kidney Int. 2021, 99, S1–S87. [Google Scholar] [CrossRef]
- Humalda, J.K.; Navis, G. Dietary sodium restriction: A neglected therapeutic opportunity in chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2014, 23, 533–540. [Google Scholar] [CrossRef]
- McMahon, E.J.; Campbell, K.L.; Bauer, J.D.; Mudge, D.W.; Kelly, J.T. Altered dietary salt intake for people with chronic kidney disease. Cochrane Database Syst. Rev. 2021, 6, Cd010070. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME). IHME 2019. Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 10 January 2022).
- Hendriksen, M.A.H.; Over, E.A.B.; Navis, G.; Joles, J.A.; Hoorn, E.J.; Gansevoort, R.T.; Boshuizen, H.C. Limited salt consumption reduces the incidence of chronic kidney disease: A modeling study. J. Public Health 2018, 40, e351–e358. [Google Scholar] [CrossRef]
- Aminde, L.N.; Cobiac, L.J.; Veerman, J.L. Potential impact of a modest reduction in salt intake on blood pressure, cardiovascular disease burden and premature mortality: A modelling study. Open Heart 2019, 6, e000943. [Google Scholar] [CrossRef] [PubMed]
- Aminde, L.N.; Phung, H.N.; Phung, D.; Cobiac, L.J.; Veerman, J.L. Dietary Salt Reduction, Prevalence of Hypertension and Avoidable Burden of Stroke in Vietnam: Modelling the Health and Economic Impacts. Front. Public Health 2021, 9, 682975. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Tan, M.; Wang, C.; Kent, S.; Cobiac, L.; MacGregor, G.A.; He, F.J.; Mihaylova, B. Impact of the 2003 to 2018 Population Salt Intake Reduction Program in England: A Modeling Study. Hypertension 2021, 77, 1086–1094. [Google Scholar] [CrossRef]
- Shangguan, S.; Mozaffarian, D.; Sy, S.; Lee, Y.; Liu, J.; Wilde, P.E.; Sharkey, A.L.; Dowling, E.A.; Marklund, M.; Abrahams-Gessel, S.; et al. Health Impact and Cost-Effectiveness of Achieving the National Salt and Sugar Reduction Initiative Voluntary Sugar Reduction Targets in the United States: A Microsimulation Study. Circulation 2021, 144, 1362–1376. [Google Scholar] [CrossRef]
- Nilson, E.A.F.; Pearson-Stuttard, J.; Collins, B.; Guzman-Castillo, M.; Capewell, S.; O’Flaherty, M.; Jaime, P.C.; Kypridemos, C. Estimating the health and economic effects of the voluntary sodium reduction targets in Brazil: Microsimulation analysis. BMC Med. 2021, 19, 225. [Google Scholar] [CrossRef]
- Cobiac, L.J.; Vos, T.; Veerman, J.L. Cost-effectiveness of interventions to reduce dietary salt intake. Heart 2010, 96, 1920–1925. [Google Scholar] [CrossRef] [PubMed]
- Cobiac, L.J.; Magnus, A.; Lim, S.; Barendregt, J.J.; Carter, R.; Vos, T. Which interventions offer best value for money in primary prevention of cardiovascular disease? PLoS ONE 2012, 7, e41842. [Google Scholar] [CrossRef] [PubMed]
- Trieu, K.; Coyle, D.H.; Afshin, A.; Neal, B.; Marklund, M.; Wu, J.H.Y. The estimated health impact of sodium reduction through food reformulation in Australia: A modeling study. PLoS Med. 2021, 18, e1003806. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. Australian Government Department of Health, New Zealand Ministry of Health, Nutrient Reference Values for Australia and New Zealand; National Health and Medical Research Council: Canberra, Australia, 2006.
- National Preventive Health Strategy 2021–2030; Department of Health Australian Government: Canberra, Australia, 2021.
- Land, M.A.; Neal, B.C.; Johnson, C.; Nowson, C.A.; Margerison, C.; Petersen, K.S. Salt consumption by Australian adults: A systematic review and meta-analysis. Med. J. Aust. 2018, 208, 75–81. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Australian Health Survey 2011–13: Data from the National Nutrition and Physical Activity Survey 2011–2012. 2012. Available online: https://www.abs.gov.au/statistics/microdata-tablebuilder/available-microdata-tablebuilder/australian-health-survey-nutrition-and-physical-activity (accessed on 10 January 2022).
- Australian Bureau of Statistics. National Health Survey 2017–2018: First Results: ABS. 2018. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/national-health-survey-first-results/latest-release (accessed on 10 January 2022).
- Australian Bureau of Statistics. Blood Pressure. National Health Survey: Users’ Guide 2017–2018. 2018. Available online: https://www.abs.gov.au/ausstats/abs@.nsf/Lookup/by%20Subject/4363.0~2017-18~Main%20Features~Blood%20pressure~44 (accessed on 10 January 2022).
- Briggs, A.D.M.; Wolstenholme, J.; Blakely, T.; Scarborough, P. Choosing an epidemiological model structure for the economic evaluation of non-communicable disease public health interventions. Popul. Health Metr. 2016, 14, 17. [Google Scholar] [CrossRef]
- He, F.J.; Li, J.; Macgregor, G.A. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst. Rev. 2013, 4, Cd004937. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.J.; Veerman, J.L. Categorical versus continuous risk factors and the calculation of potential impact fractions. J. Epidemiol. Community Health 2010, 64, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Lewington, S.; Clarke, R.; Qizilbash, N.; Peto, R.; Collins, R.; Prospective Studies Collabortion. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002, 360, 1903–1913. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Barendregt, J.J.; Van Oortmarssen, G.J.; Vos, T.; Murray, C.J. A generic model for the assessment of disease epidemiology: The computational basis of DisMod II. Popul. Health Metr. 2003, 1, 4. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Health Expenditure Australia 2018–2019. Health and Welfare Expenditure Series No. 66. Cat. no. HWE 80; Australian Institute of Health and Welfare (AIHW): Canberra, Australia, 2020.
- Australian Institute of Health and Welfare. Disease Expenditure Study: Overview of Analysis and Methodology 2018–2019. Cat. no. HWE 82; Australian Institute of Health and Welfare (AIHW): Canberra, Australia, 2021.
- Drummond, M.; Sculpher, M.; Claxton, K. Methods for the Economic Evalution of Healthcare Programmes; Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- Barendregt, J.J. EpiGear International Brisbane, Queensland. Available online: https://www.epigear.com (accessed on 13 January 2022).
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for accurate and transparent health estimates reporting: The GATHER statement. Lancet 2016, 388, E19–E23. [Google Scholar] [CrossRef]
- Nerbass, F.B.; Calice-Silva, V.; Pecoits-Filho, R. Sodium Intake and Blood Pressure in Patients with Chronic Kidney Disease: A Salty Relationship. Blood Purif. 2018, 45, 166–172. [Google Scholar] [CrossRef]
- Santos, J.A.; Tekle, D.; Rosewarne, E.; Flexner, N.; Cobb, L.; Al-Jawaldeh, A.; Kim, W.J.; Breda, J.; Whiting, S.; Campbell, N.; et al. A Systematic Review of Salt Reduction Initiatives Around the World: A Midterm Evaluation of Progress Towards the 2025 Global Non-Communicable Diseases Salt Reduction Target. Adv. Nutr. Int. Rev. J. 2021, 12, 1768–1780. [Google Scholar] [CrossRef]
- Australian Institute of Health and Welfare. Chronic Kidney Disease. Cat. no. CDK 16. Canberra: AIHW: Australian Institute of Health and Welfare. 2020. Available online: https://www.aihw.gov.au/reports/chronic-kidney-disease/chronic-kidney-disease (accessed on 20 February 2022).
- Bolton, K.A.; Webster, J.; Dunford, E.K.; Jan, S.; Woodward, M.; Bolam, B.; Neal, B.; Trieu, K.; Reimers, J.; Armstrong, S.; et al. Sources of dietary sodium and implications for a statewide salt reduction initiative in Victoria, Australia. Br. J. Nutr. 2020, 123, 1165–1175. [Google Scholar] [CrossRef]
- Australian Government Department of Health. Healthy Food Partnership Canberra: Commonwealth of Australia. 2016. Available online: https://www.health.gov.au/initiatives-and-programs/healthy-food-partnership (accessed on 20 February 2022).
- World Health Organization. WHO Global Sodium Benchmarks for Different Food Categories; World Health Organization (WHO): Geneva, Switzerland, 2021. [Google Scholar]
- Song, J.; Brown, M.K.; Cobb, L.K.; Jacobson, M.F.; Ide, N.; MacGregor, G.A.; He, F.J. Delayed Finalization of Sodium Targets in the United States May Cost Over 250,000 Lives by 2031. Hypertension 2022, 79, 798–808. [Google Scholar] [CrossRef]
- Aminde, L.N.; Cobiac, L.; Veerman, J.L. Cost-effectiveness analysis of population salt reduction interventions to prevent cardiovascular disease in Cameroon: Mathematical modelling study. BMJ Open 2020, 10, e041346. [Google Scholar] [CrossRef] [PubMed]
- Aminde, L.N.; Cobiac, L.J.; Phung, D.; Phung, H.N.; Veerman, J.L. Avoidable burden of stomach cancer and potential gains in healthy life years from gradual reductions in salt consumption in Vietnam, 2019–2030: A modelling study. Public Health Nutr. 2022, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kypridemos, C.; Guzman-Castillo, M.; Hyseni, L.; Hickey, G.L.; Bandosz, P.; Buchan, I.; Capewell, S.; O’Flaherty, M. Estimated reductions in cardiovascular and gastric cancer disease burden through salt policies in England: An IMPACTNCD microsimulation study. BMJ Open 2017, 7, e013791. [Google Scholar] [CrossRef]
- Sugiura, T.; Takase, H.; Ohte, N.; Dohi, Y. Dietary Salt Intake is a Significant Determinant of Impaired Kidney Function in the General Population. Kidney Blood Press. Res. 2018, 43, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Aburto, N.J.; Ziolkovska, A.; Hooper, L.; Elliott, P.; Cappuccio, F.P.; Meerpohl, J.J. Effect of lower sodium intake on health: Systematic review and meta-analysis. BMJ 2013, 346, f1326. [Google Scholar] [CrossRef]
- Graudal, N.A.; Hubeck-Graudal, T.; Jurgens, G. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Sys. Rev. 2020, 12, CD004022. [Google Scholar] [CrossRef]
- Kovesdy, C.P.; Trivedi, B.K.; Anderson, J.E. Association of kidney function with mortality in patients with kidney disease not yet on dialysis: A historical prospective cohort study. Adv. Chronic Kidney Dis. 2006, 13, 183–188. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Van Oortmarssen, G.J.; Van Hout, B.A.; Van Den Bosch, J.M.; Bonneux, L. Coping with multiple morbidity in a life table. Math. Popul. Stud. 1998, 7, 29–49. [Google Scholar] [CrossRef]
- Gold, M.R.; Stevenson, D.; Fryback, D.G. HALYs and QALYs and DALYs, Oh My: Similarities and differences in summary measures of population health. Annu. Rev. Public Health 2002, 23, 115–134. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Population: National, State and Territory Population. 2019. Available online: https://www.abs.gov.au/statistics/people/population (accessed on 10 January 2022).
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Age (Years) | Baseline | SDT Achieved | NPHS Achieved | Baseline | SDT Achieved | NPHS Achieved |
| 25–34 | 120.3 (13.5) | 116.6 (12.7) | 118.4 (13.1) | 108.5 (15.8) | 106.7 (15.3) | 107.1 (15.4) |
| 35–44 | 121.3 (11.6) | 118.1 (10.9) | 119.5 (11.2) | 112.5 (15.7) | 111.0 (15.4) | 111.2 (15.4) |
| 45–54 | 126.5 (17.9) | 123.3 (17.1) | 124.7 (17.5) | 119.6 (17.0) | 118.1 (16.6) | 118.3 (16.7) |
| 55–64 | 132.4 (14.7) | 129.9 (14.1) | 130.8 (14.3) | 126.8 (20.6) | 125.6 (20.4) | 125.6 (20.3) |
| 65–74 | 134.9 (21.3) | 132.5 (20.7) | 133.4 (20.9) | 133.6 (17.7) | 132.5 (17.5) | 132.4 (17.4) |
| 75–84 | 136.5 (20.9) | 134.7 (20.5) | 135.1 (20.6) | 137.9 (17.0) | 137.2 (16.7) | 136.8 (16.8) |
| ≥85 | 140.2 (33.3) | 136.2 (32.8) | 137.2 (32.9) | 140.8 (28.0) | 139.2 (27.8) | 138.5 (27.7) |
| Male | Female | Total | ||||
| 2019–2030 | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) |
| SDT, 5 g/day salt target achieved | 40,518 (35,890–45,264) | 7.4 (6.5–8.2) | 18,704 (16,399–21,216) | 3.2 (2.8–3.6) | 59,223 (53,140–65,503) | 5.3 (4.7–5.8) |
| NPHS 30% relative salt reduction | 27,748 (24,194–31,258) | 5.0 (4.4–5.6) | 22,142 (19,494–24,975) | 3.8 (3.4–4.3) | 49,890 (44,377–55,569) | 4.4 (3.9–4.9) |
| A 20% relative salt reduction | 18,496 (16,182–20,817) | 3.4 (2.9–3.8) | 14,774 (12,962–16,703) | 2.6 (2.3–2.9) | 33,270 (29,672–36,947) | 3.0 (2.6–3.3) |
| A 15% relative salt reduction | 13,846 (11,988–15,597) | 2.5 (2.2–2.8) | 11,072 (9611–12,491) | 1.9 (1.6–2.2) | 24,919 (22,141–27,724) | 2.2 (1.9–2.5) |
| A 1 g/day absolute salt reduction | 10,401 (8992–11,817) | 1.9 (1.6–2.1) | 11,018 (9625–12,578) | 1.9 (1.7–2.2) | 21,419 (18,967–24,069) | 1.9 (1.6–2.1) |
| A 2 g/day absolute salt reduction | 20,838 (18,037–23,603) | 3.8 (3.3–4.2) | 22,050 (19,126–25,018) | 3.8 (3.4–4.3) | 42,888 (37,943–47,892) | 3.8 (3.4–4.2) |
| Male | Female | Total | ||||
| Lifetime | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) |
| SDT, 5 g/day salt target achieved | 266,676 (237,912–295,250) | 8.3 (7.5–9.1) | 120,133 (106,868–133,853) | 3.4 (3.0–3.7) | 386,809 (347,452–427,680) | 5.7 (5.2–6.2) |
| NPHS 30% relative salt reduction | 192,140 (171,383–214,418) | 6.0 (5.5–6.6) | 156,186 (138,862–174,500) | 4.4 (4.0–4.8) | 348,326 (312,986–386,619) | 5.2 (4.7–5.6) |
| A 20% relative salt reduction | 128,163 (113,735–142,551) | 4.0 (3.6–4.4) | 103,853 (92,544–115,616) | 2.9 (2.6–3.2) | 232,016 (207,515–257,151) | 3.4 (3.1–3.7) |
| A 15% relative salt reduction | 96,067 (85,977–106,793) | 3.0 (2.7–3.3) | 77,820 (69,266–86,774) | 2.2 (2.0–2.4) | 173,887 (156,162–192,231) | 2.6 (2.3–2.8) |
| A 1 g/day absolute salt reduction | 74,919 (66,529–83,386) | 2.3 (2.1–2.6) | 79,926 (70,776–89,192) | 2.2 (2.0–2.5) | 154,845 (138,789–171,916) | 2.3 (2.1–2.5) |
| A 2 g/day absolute salt reduction | 149,649 (133,203–166,751) | 4.7 (4.2–5.1) | 160,061 (142,105–178,186) | 4.5 (4.0–5.0) | 309,711 (276,892–342,894) | 4.6 (4.2–5.0) |
| Male | Female | Total | ||||
| 2019–2030 | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) |
| SDT, 5 g/day salt target achieved | 423 (341–496) | 0.7 (0.6–0.8) | 145 (118–171) | 0.3 (0.2–0.3) | 568 (479–652) | 0.5 (0.4–0.6) |
| NPHS 30% relative salt reduction | 313 (249–368) | 0.5 (0.4–0.6) | 198 (159–231) | 0.4 (0.3–0.4) | 511 (426–590) | 0.5 (0.4–0.5) |
| A 20% relative salt reduction | 209 (169–244) | 0.3 (0.3–0.4) | 132 (107–153) | 0.3 (0.2–0.3) | 341 (288–390) | 0.3 (0.2–0.3) |
| A 15% relative salt reduction | 157 (124–185) | 0.3 (0.2–0.3) | 99 (79–116) | 0.2 (0.1–0.2) | 255 (214–293) | 0.2 (0.2–0.3) |
| A 1 g/day absolute salt reduction | 124 (98–146) | 0.2 (0.1–0.2) | 103 (84–120) | 0.2 (0.1–0.2) | 226 (188–260) | 0.2 (0.1–0.2) |
| A 2 g/day absolute salt reduction | 248 (199–292) | 0.4 (0.3–0.5) | 205 (163–240) | 0.4 (0.3–0.5) | 453 (381–520) | 0.4 (0.3–0.5) |
| Male | Female | Total | ||||
| Lifetime | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) | Absolute Est. (95% UI) | Relative Est., % (95% UI) |
| SDT, 5 g/day salt target achieved | 15,798 (14,248–17,362) | 4.0 (3.7–4.4) | 6785 (6067–7515) | 1.7 (1.6–1.9) | 22,583 (20,414–24,752) | 2.9 (2.6–3.1) |
| NPHS 30% relative salt reduction | 11,425 (10,286–12,627) | 3.0 (2.7–3.2) | 8866 (7975–9768) | 2.2 (2.1–2.4) | 20,291 (18,441–22,276) | 2.6 (2.4–2.8) |
| A 20% relative salt reduction | 7638 (6836–8455) | 2.0 (1.8–2.1) | 5913 (5314–6523) | 1.5 (1.4–1.6) | 13,551 (12,262–14,891) | 1.7 (1.6–1.9) |
| A 15% relative salt reduction | 5730 (5125–6347) | 1.5 (1.3–1.6) | 4437 (3965–4902) | 1.1 (1.0–1.2) | 10,168 (9189–11,168) | 1.3 (1.2–1.4) |
| A 1 g/day absolute salt reduction | 9047 (8176–9913) | 1.1 (1.0–1.3) | 4565 (4103–5035) | 1.2 (1.1–1.3) | 4481 (3995–4963) | 1.2 (1.1–1.3) |
| A 2 g/day absolute salt reduction | 8924 (7995–9900) | 2.3 (2.0–2.5) | 9108 (8162–10,077) | 2.3 (2.1–2.5) | 18,032 (16,295–19,838) | 2.3 (2.1–2.5) |
| Scenario/Time Horizon | CKD HTN | CKD DM | CKD GMN | CKD Other |
| 2019–2030 | ||||
| SDT 5 g/day achieved | 1671 (36.1%) | 1003 (21.7%) | 906 (19.6%) | 1031 (22.3%) |
| NPHS 30% reduction | 1411 (36.5%) | 833 (21.5%) | 744 (19.2%) | 877 (22.7%) |
| 20% reduction | 942 (36.5%) | 556 (21.5%) | 496 (19.2%) | 582 (22.5%) |
| 15% reduction | 707 (36.6%) | 418 (21.6%) | 373 (19.3%) | 437 (22.6%) |
| 1 g/day reduction | 607 (36.7%) | 356 (21.5%) | 315 (19.1%) | 377 (22.8%) |
| 2 g/day reduction | 1208 (36.5%) | 710 (21.5%) | 628 (19.0%) | 755 (22.8%) |
| Lifetime | ||||
| SDT 5 g/day achieved | 67,808 (33.9%) | 38,074 (19.1%) | 41,044 (20.6%) | 51,923 (26.1%) |
| NPHS 30% reduction | 59,850 (35.1%) | 31,795 (18.6%) | 33,629 (19.8%) | 45,149 (26.5%) |
| 20% reduction | 39,998 (35.2%) | 21,212 (18.6%) | 22,510 (19.8%) | 29,964 (26.3%) |
| 15% reduction | 30,063 (35.2%) | 15,926 (18.7%) | 16,896 (19.8%) | 22,450 (26.3%) |
| 1 g/day reduction | 26,477 (35.7%) | 13,624 (18.4%) | 14,302 (19.3%) | 19,645 (26.6%) |
| 2 g/day reduction | 52,584 (35.6%) | 27,222 (18.4%) | 28,530 (19.3%) | 39,433 (26.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aminde, L.N.; Wanjau, M.N.; Cobiac, L.J.; Veerman, J.L. Estimated Impact of Achieving the Australian National Sodium Reduction Targets on Blood Pressure, Chronic Kidney Disease Burden and Healthcare Costs: A Modelling Study. Nutrients 2023, 15, 318. https://doi.org/10.3390/nu15020318
Aminde LN, Wanjau MN, Cobiac LJ, Veerman JL. Estimated Impact of Achieving the Australian National Sodium Reduction Targets on Blood Pressure, Chronic Kidney Disease Burden and Healthcare Costs: A Modelling Study. Nutrients. 2023; 15(2):318. https://doi.org/10.3390/nu15020318
Chicago/Turabian StyleAminde, Leopold Ndemnge, Mary Njeri Wanjau, Linda J. Cobiac, and J. Lennert Veerman. 2023. "Estimated Impact of Achieving the Australian National Sodium Reduction Targets on Blood Pressure, Chronic Kidney Disease Burden and Healthcare Costs: A Modelling Study" Nutrients 15, no. 2: 318. https://doi.org/10.3390/nu15020318
APA StyleAminde, L. N., Wanjau, M. N., Cobiac, L. J., & Veerman, J. L. (2023). Estimated Impact of Achieving the Australian National Sodium Reduction Targets on Blood Pressure, Chronic Kidney Disease Burden and Healthcare Costs: A Modelling Study. Nutrients, 15(2), 318. https://doi.org/10.3390/nu15020318





