Protective Effects of Coumestrol on Metabolic Dysfunction and Its Estrogen Receptor-Mediated Action in Ovariectomized Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgery
2.2. Histological Staining
2.3. Determination of Thiobarbituric Acid Reactive Substances (TBARS)
2.4. Gene Expression Analysis
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
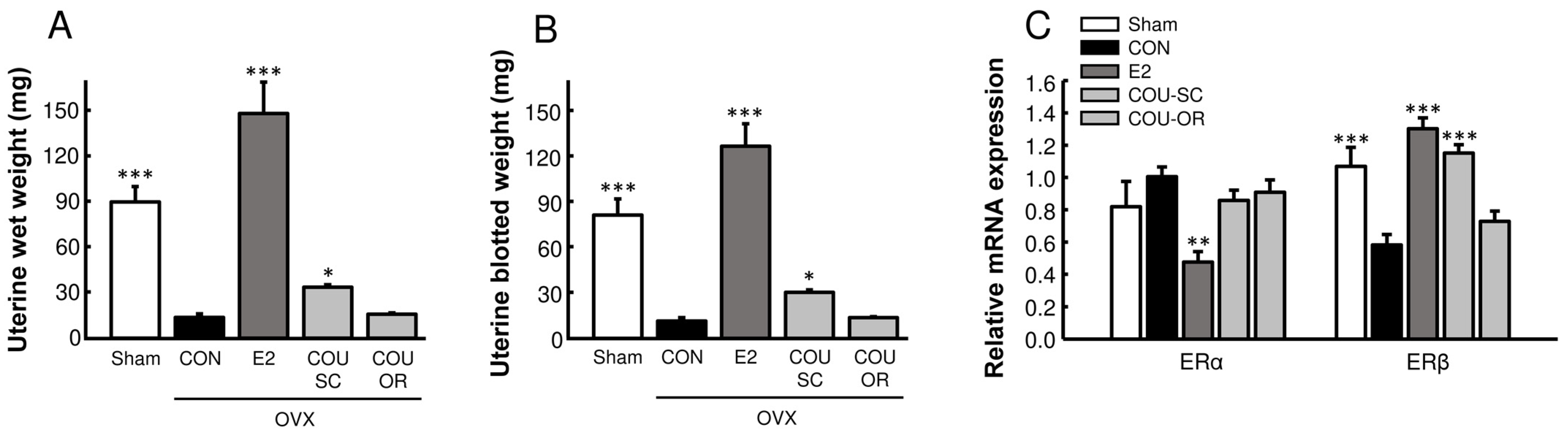
3.1. Effects of Coumestrol Treatments on Uterine Growth in OVX Mice
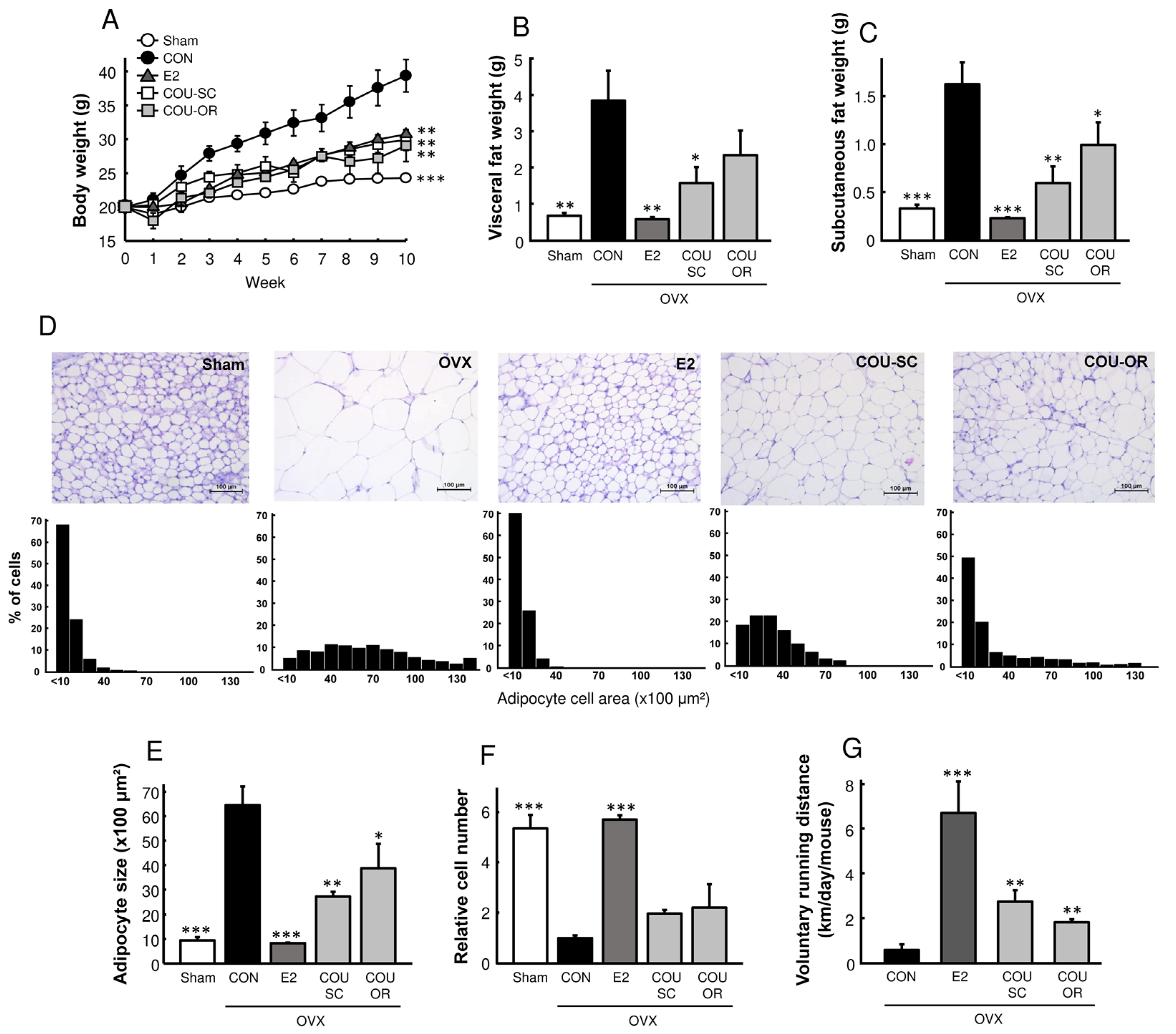
3.2. Coumestrol Prevents Adiposity in HFD-Fed OVX Mice
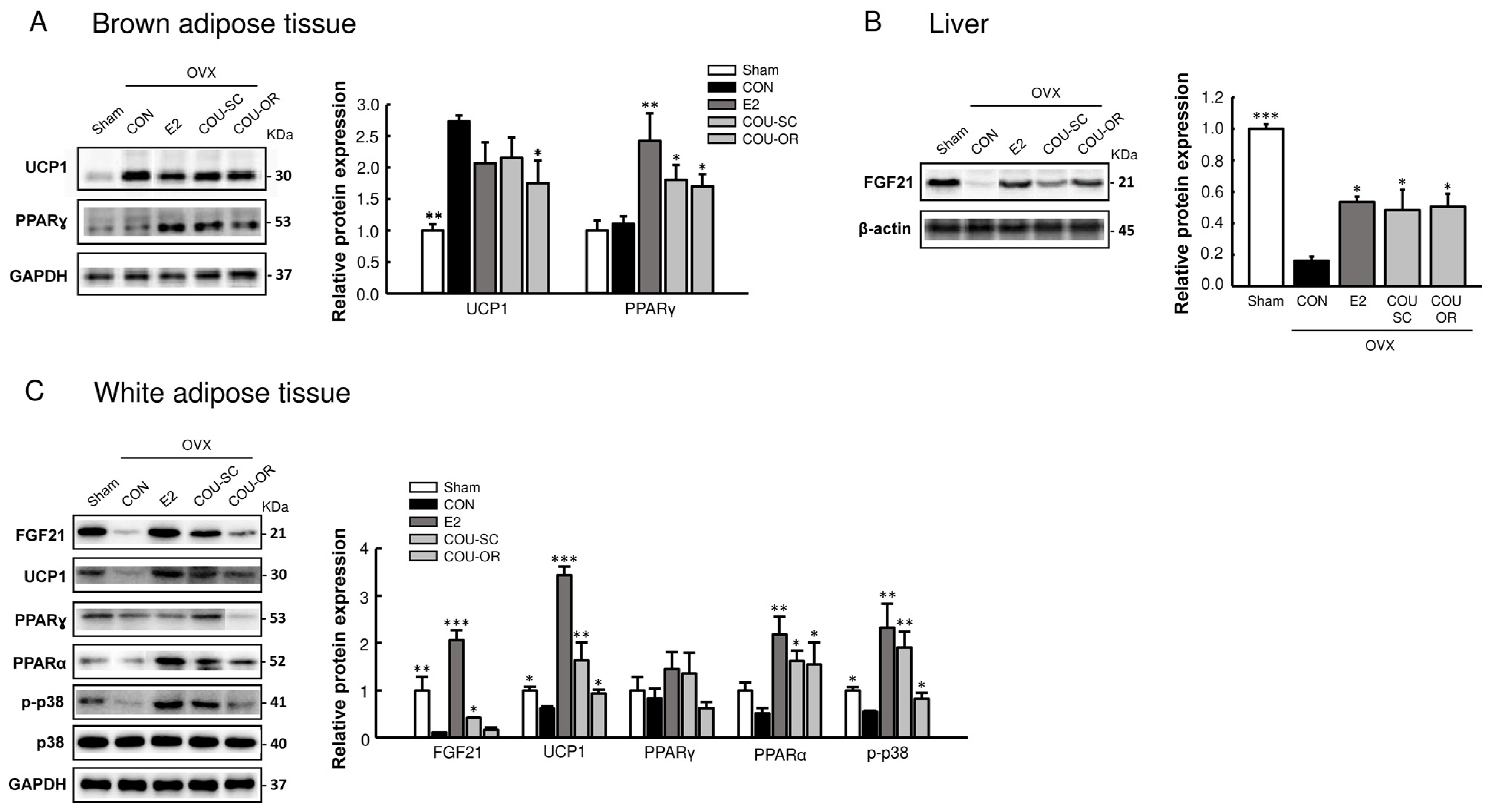
3.3. Coumestrol Modulates Protein Expressions Involved in Browning of White Fat
3.4. Coumestrol Normalizes the Phosphorylation of PI3K and Akt in the Skeletal Muscle and Liver of OVX Mice
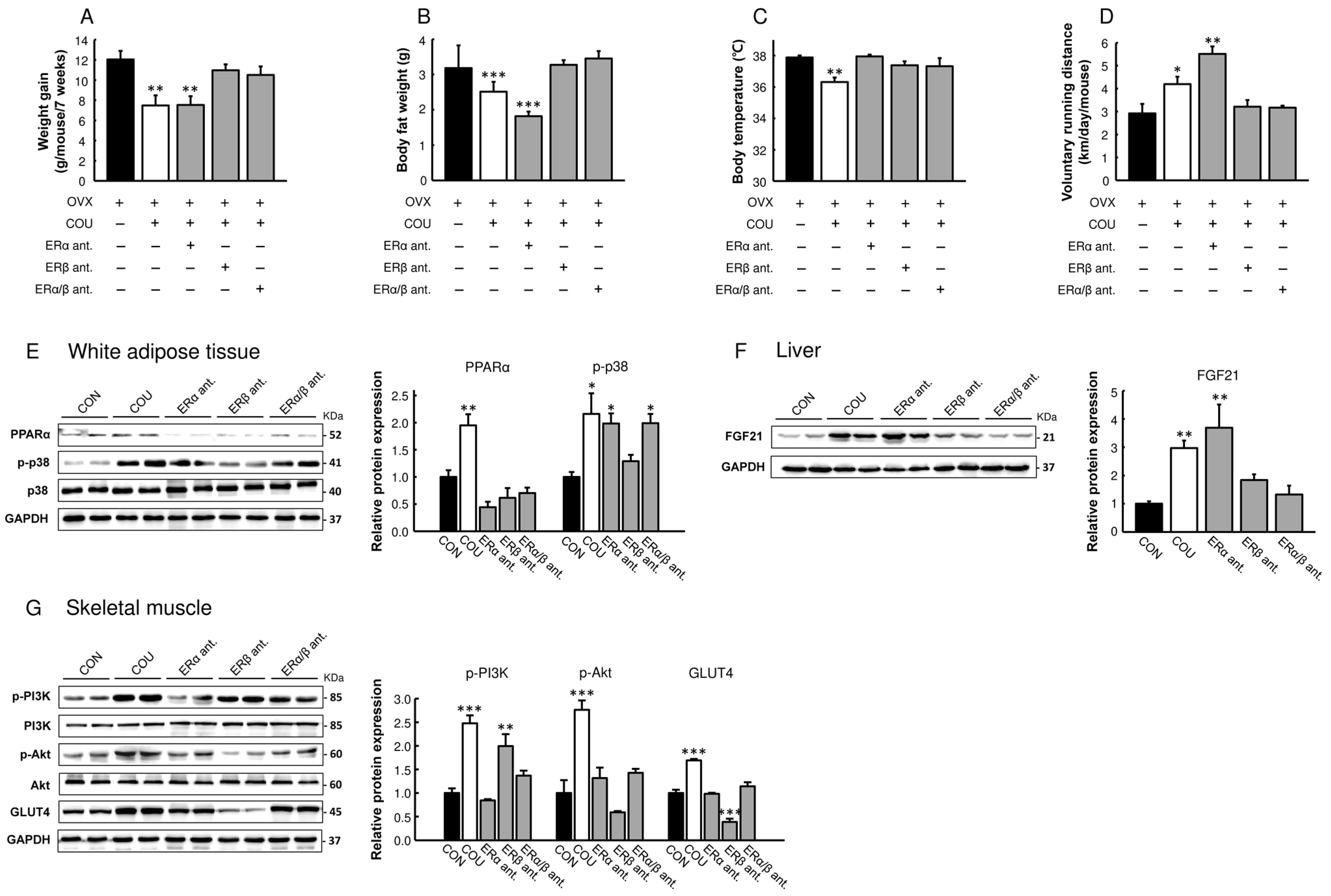
3.5. Metabolic Effects of Coumestrol in OVX Mice Are Primarily Mediated by ERβ
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mauvais-Jarvis, F. Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol. Metab. 2011, 22, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.M.; Besselink, E.; Henning, S.M.; Go, V.L.W.; Heber, D. Phytoestrogens Induce Differential Estrogen Receptor Alpha- or Beta-Mediated Responses in Transfected Breast Cancer Cells. Exp. Biol. Med. 2005, 230, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C.K.; et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef] [PubMed]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of Phytoestrogens with Estrogen Receptors ALPHA and BETA. Biol. Pharm. Bull. 2001, 24, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Poluzzi, E.; Piccinni, C.; Raschi, E.; Rampa, A.; Recanatini, M.; Ponti, F. Phytoestrogens in Postmenopause: The State of the Art from a Chemical, Pharmacological and Regulatory Perspective. Curr. Med. Chem. 2014, 21, 417–436. [Google Scholar] [CrossRef]
- Sim, K.S.; Park, S.; Seo, H.; Lee, S.-H.; Lee, H.-S.; Park, Y.; Kim, J.H. Comparative study of estrogenic activities of phytoestrogens using OECD in vitro and in vivo testing methods. Toxicol. Appl. Pharmacol. 2022, 434, 115815. [Google Scholar] [CrossRef]
- Javid, S.H.; Moran, A.E.; Carothers, A.M.; Redston, M.; Bertagnolli, M.M. Modulation of tumor formation and intestinal cell migration by estrogens in the ApcMin/+ mouse model of colorectal cancer. Carcinogenesis 2005, 26, 587–595. [Google Scholar] [CrossRef]
- Castro, C.C.; Pagnussat, A.S.; Orlandi, L.; Worm, P.; Moura, N.; Etgen, A.M.; Netto, C.A. Coumestrol has neuroprotective effects before and after global cerebral ischemia in female rats. Brain Res. 2012, 1474, 82–90. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, Q.; Li, Y.; Cui, J.; Feng, K.; Kong, X.; Xian, C.J. The higher osteoprotective activity of psoralidin in vivo than coumestrol is attributed by its presence of an isopentenyl group and through activated PI3K/Akt axis. Biomed. Pharmacother. 2018, 102, 1015–1024. [Google Scholar] [CrossRef]
- Nogowski, L. Effects of phytoestrogen-coumestrol on lipid and carbohydrate metabolism in young ovariectomized rats may be independent of its estrogenicity. J. Nutr. Biochem. 1999, 10, 664–669. [Google Scholar] [CrossRef]
- Nogowski, L.; Nowak, K.W.; Kaczmarek, P.; Maćkowiak, P. The influence of coumestrol, zearalenone, and genistein administration on insulin receptors and insulin secretion in ovariectomized rats. J. Recept. Signal Transduct. 2002, 22, 449–457. [Google Scholar] [CrossRef]
- Zywno, H.; Bzdega, W.; Kolakowski, A.; Kurzyna, P.; Harasim-Symbor, E.; Sztolsztener, K.; Chabowski, A.; Konstantynowicz-Nowicka, K. The Influence of Coumestrol on Sphingolipid Signaling Pathway and Insulin Resistance Development in Primary Rat Hepatocytes. Biomolecules 2021, 11, 268. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kang, J.-H.; Han, J.-M.; Cho, M.-H.; Chung, Y.-J.; Park, K.H.; Shin, D.-H.; Park, H.-Y.; Choi, M.-S.; Jeong, T.-S. Anti-Obesity Effects of Soy Leaf via Regulation of Adipogenic Transcription Factors and Fat Oxidation in Diet-Induced Obese Mice and 3T3-L1 Adipocytes. J. Med. Food 2015, 18, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-N.; Ahn, S.-Y.; Song, H.-D.; Kwon, H.-J.; Saha, A.; Son, Y.; Cho, Y.K.; Jung, Y.-S.; Jeong, H.W.; Lee, Y.-H. Antiobesity effects of coumestrol through expansion and activation of brown adipose tissue metabolism. J. Nutr. Biochem. 2020, 76, 108300. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Waraich, R.S.; Liu, S.; Ferron, M.; Waget, A.; Meyers, M.S.; Karsenty, G.; Burcelin, R.; Mauvais-Jarvis, F. Developmental androgen excess programs sympathetic tone and adipose tissue dysfunction and predisposes to a cardiometabolic syndrome in female mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1321–E1330. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Ziaee, T.; Danaee, A. Protective effect of aqueous saffron extract (Crocus sativus L.) and crocin, its active constituent, on renal ischemia-reperfusion-induced oxidative damage in rats. J. Pharm. Pharm. Sci. 2005, 8, 387–393. [Google Scholar]
- Jeremic, N.; Chaturvedi, P.; Tyagi, S.C. Browning of White Fat: Novel Insight Into Factors, Mechanisms, and Therapeutics. J. Cell. Physiol. 2017, 232, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Ramos, D.; Mehta, R.; Aguilar-Salinas, C.A. Fibroblast Growth Factor 21 and Browning of White Adipose Tissue. Front. Physiol. 2019, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Kroon, T.; Harms, M.; Maurer, S.; Bonnet, L.; Alexandersson, I.; Lindblom, A.; Ahnmark, A.; Nilsson, D.; Gennemark, P.; O’Mahony, G.; et al. PPARγ and PPARα synergize to induce robust browning of white fat in vivo. Mol. Metab. 2020, 36, 100964. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.-H.; Kim, K.-O.; Lee, J.-H.; Lee, H.-S. Effects of Phytoestrogens on Glucose Metabolism in C57BL/KsOlaHsd-db/db Mice. Korean J. Nutr. 2011, 44, 275–283. [Google Scholar] [CrossRef]
- Szczepańska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Badakhshi, Y.; Shao, W.; Liu, D.; Tian, L.; Pang, J.; Gu, J.; Hu, J.; Jin, T. Estrogen-Wnt signaling cascade regulates expression of hepatic fibroblast growth factor 21. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E292–E304. [Google Scholar] [CrossRef] [PubMed]
- Allard, C.; Bonnet, F.; Xu, B.; Coons, L.; Albarado, D.; Hill, C.; Fagherazzi, G.; Korach, K.S.; Levin, E.R.; Lefante, J.; et al. Activation of hepatic estrogen receptor-α increases energy expenditure by stimulating the production of fibroblast growth factor 21 in female mice. Mol. Metab. 2019, 22, 62–70. [Google Scholar] [CrossRef]
- Keinicke, H.; Sun, G.; Mentzel, C.; Fredholm, M.; John, L.M.; Andersen, B.; Raun, K.; Kjaergaard, M. FGF21 regulates hepatic metabolic pathways to improve steatosis and inflammation. Endocr. Connect. 2020, 9, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.-B.; Jeong, H.W.; Lee, S.-J.; Lee, S.-J. Coumestrol Induces Mitochondrial Biogenesis by Activating Sirt1 in Cultured Skeletal Muscle Cells. J. Agric. Food Chem. 2014, 62, 4298–4305. [Google Scholar] [CrossRef]
- Savva, C.; Korach-André, M. Estrogen Receptor beta (ERβ) Regulation of Lipid Homeostasis—Does Sex Matter? Metabolites 2020, 10, 116. [Google Scholar] [CrossRef]
- Barros, R.P.A.; Gustafsson, J.Å. Estrogen Receptors and the Metabolic Network. Cell Metab. 2011, 14, 289–299. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Y.; Zhuang, X.; Luan, F.; Zhao, C.; Cordeiro, M.N.D.S. Interaction of Coumarin Phytoestrogens with ERα and ERβ: A Molecular Dynamics Simulation Study. Molecules 2020, 25, 1165. [Google Scholar] [CrossRef]
- Han, D.-H.; Denison, M.S.; Tachibana, H.; Yamada, K. Relationship between Estrogen Receptor-Binding and Estrogenic Activities of Environmental Estrogens and Suppression by Flavonoids. Biosci. Biotechnol. Biochem. 2002, 66, 1479–1487. [Google Scholar] [CrossRef]
- Kuiper, G.G.J.M.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; Van Der Saag, P.T.; Van Der Burg, B.; Gustafsson, J.Å. Interaction of Estrogenic Chemicals and Phytoestrogens with Estrogen Receptor β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Fekri, K.; Mahmoudi, J.; Sadigh-Eteghad, S.; Farajdokht, F.; Nayebi, A.M. Coumestrol alleviates oxidative stress, apoptosis and cognitive impairments through hippocampal estrogen receptor-beta in male mouse model of chronic restraint stress. Pharm. Sci. 2021, 28, 260–274. [Google Scholar] [CrossRef]
- Diel, P.; Schulz, T.; Smolnikar, K.; Strunck, E.; Vollmer, G.; Michna, H. Ability of xeno- and phytoestrogens to modulate expression of estrogen-sensitive genes in rat uterus: Estrogenicity profiles and uterotropic activity. J. Steroid Biochem. Mol. Biol. 2000, 73, 1–10. [Google Scholar] [CrossRef]
- Tinwell, H.; Soames, A.R.; Foster, J.R.; Ashby, J. Estradiol-type activity of coumestrol in mature and immature ovariectomized rat uterotrophic assays. Environ. Health Perspect. 2000, 108, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Markaverich, B.M.; Webb, B.; Densmore, C.L.; Gregory, R.R. Effects of coumestrol on estrogen receptor function and uterine growth in ovariectomized rats. Environ. Health Perspect. 1995, 103, 574–581. [Google Scholar] [CrossRef]
- Ohta, T.; Uto, T.; Tanaka, H. Effective methods for increasing coumestrol in soybean sprouts. PLoS ONE 2021, 16, e0260147. [Google Scholar] [CrossRef]
- Fields, R.L.; Barrell, G.K.; Gash, A.; Zhao, J.; Moot, D.J. Alfalfa Coumestrol Content in Response to Development Stage, Fungi, Aphids, and Cultivar. Agron. J. 2018, 110, 910–921. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Sim, K.-S.; Heo, W.; Kim, J.-H. Protective Effects of Coumestrol on Metabolic Dysfunction and Its Estrogen Receptor-Mediated Action in Ovariectomized Mice. Nutrients 2023, 15, 954. https://doi.org/10.3390/nu15040954
Park S, Sim K-S, Heo W, Kim J-H. Protective Effects of Coumestrol on Metabolic Dysfunction and Its Estrogen Receptor-Mediated Action in Ovariectomized Mice. Nutrients. 2023; 15(4):954. https://doi.org/10.3390/nu15040954
Chicago/Turabian StylePark, Song, Kyu-Sang Sim, Wan Heo, and Jun-Ho Kim. 2023. "Protective Effects of Coumestrol on Metabolic Dysfunction and Its Estrogen Receptor-Mediated Action in Ovariectomized Mice" Nutrients 15, no. 4: 954. https://doi.org/10.3390/nu15040954
APA StylePark, S., Sim, K.-S., Heo, W., & Kim, J.-H. (2023). Protective Effects of Coumestrol on Metabolic Dysfunction and Its Estrogen Receptor-Mediated Action in Ovariectomized Mice. Nutrients, 15(4), 954. https://doi.org/10.3390/nu15040954






