Abstract
Elevated serum cholesterol is a major risk factor for coronary heart diseases. Some Lactobacillus strains with cholesterol-lowering potential have been isolated from artisanal food products. The purpose of this study was to isolate probiotic Lactobacillus strains from traditional yoghurt (dahi) and yogurt milk (lassi) and investigate the impact of these strains on the blood lipid profile and anti-obesity effect in a high cholesterol high fat diet model in Wistar rats. Eight candidate probiotic strains were chosen based on in vitro probiotic features and cholesterol reduction ability. By 16S rDNA sequencing, these strains were identified as Limosilactibacillus fermentum FM6, L. fermentum FM16, L. fermentum FM12, Lacticaseibacillus rhamnosus FM9, L. fermentum Y55, L. fermentum Y57, L. rhamnosus Y59, and L. fermentum Y63. The safety of these strains was investigated by feeding 2 × 108 CFU/mL in saline water for 28 days in a Wistar rat model. No bacterial translocation or any other adverse effects were observed in animals after administration of strains in water, which indicates the safety of strains. The cholesterol-lowering profile of these probiotics was evaluated in male Wistar rats using a high-fat, high-cholesterol diet (HFCD) model. For 30 days, animals were fed probiotic strains in water with 2 × 108 CFU/mL/rat/day, in addition to a high fat, high cholesterol diet. The cholesterol-lowering effects of various probiotic strains were compared to those of statin. All strains showed improvement in total cholesterol, LDL, HDL, triglycerides, and weight gain. Serum cholesterol levels were reduced by 9% and 8% for L. rhamnosus FM9 and L. fermentum Y57, respectively, compared to 5% for the statin-treated group. HDL levels significantly improved by 46 and 44% for L. rhamnosus FM9 and L. fermentum Y57, respectively, compared to 46% for the statin-treated group. Compared to the statin-treated group, FM9 and Y57 significantly reduced LDL levels by almost twofold. These findings show that these strains can improve blood lipid profiles as effectively as statins in male Wistar rats. Furthermore, probiotic-fed groups helped weight control in animals on HFCD, indicating the possible anti-obesity potential of these strains. These strains can be used to develop food products and supplements to treat ischemic heart diseases and weight management. Clinical trials, however, are required to validate these findings.
1. Introduction
Elevated serum cholesterol levels are a major causal factor in coronary heart disease (CHD). According to the WHO, cardiovascular diseases will remain a leading cause of death and will affect around 23.6 million people worldwide by 2030 []. A patient with hypercholesterolemia has a three-fold increased risk of heart attack compared to an individual with normal cholesterol levels []. This is linked to the food consumption patterns of people in these countries, who tend to eat high-fat, low-fiber diets. High-fat diets, particularly those high in saturated fatty acids, may raise blood cholesterol levels, increase the risk of atherosclerosis, and cause coronary heart disease []. To control blood cholesterol levels, medicines like statins have been used. However, this approach has negative effects, including muscle pain, liver damage, neurological disorders, increased blood sugar, and miscarriages []. For this reason, there is a need for developing safer and cost-effective therapies for treating hypercholesterolemia. Probiotics have been proposed as an alternative approach for lowering serum cholesterol levels []. The use of probiotics in the development of functional foods is a promising field. Probiotics have been shown to lower blood cholesterol levels by up to 45% []. Fermented milk products are an essential part of diets in various parts of world. Lactobacillus, Lactococcus, Leuconostoc, Pediococcus, Bacillus, Propionibacterium, and Bifidobacterium are among those bacteria which are associated with fermented dairy products []. In pre-clinical and clinical studies, milk products have been studied, demonstrating that various lactic acid bacteria lower cholesterol serum levels [,]. Traditional fermented foods contain distinct microbial communities that are influenced by artisanal manufacturing techniques and local environmental conditions [,]. Furthermore, these findings have demonstrated that artisanal dairy products might be valuable sources for isolating bacterial strains with beneficial probiotic properties []. The probiotic Lacticaseibacillus rhamnosus CK102 (reclassified from Lactobacillus rhamnosus) isolated from fermented yoghurt lowered total cholesterol, high density lipoprotein cholesterol (HDL-c), and triglycerides (TG) by 27.9%, 28.7%, and 61.6%, respectively in a murine model [,]. The relation between cardiovascular diseases and total cholesterol levels have been proven by epidemiological studies []. Similarly, in another study, Limosilactobacillus fermentum MCC2760 (reclassified from Lactobacillus fermetnum) lowered cholesterol levels, TG, and low-density lipoprotein cholesterol (LDL-c) levels in a mice model [,].
In a rat model, L. fermentum PH5 improved blood lipid profiles by lowering cholesterol (67.21%), triglycerides (66.21%), and LDL cholesterol (63.25%). Both L. fermentum PH5 and PD2 lowered cholesterol levels in the liver, however L. fermentum PH5 performed better than the latter [].
Although the cholesterol-reducing cholesterol potential of Lactobacillus spp. is well known, the comparative data of such strains with clinically used drugs (statin) is less known. This study aimed to investigate the probiotic potential of Lactobacillus strains isolated from lassi (butter milk) and dahi (traditional yogurt), particularly their role in shaping the lipid profile in high cholesterol high-fat diet model with Wistar rats. Furthermore, we wanted to compare the impact of these probiotics on serum lipid profile with that of a clinically used drug, i.e., a statin.
2. Materials and Methods
2.1. Isolation of Lactobacillus from Yogurt and Fermented Milk
Eighty-three samples of artisanal yogurt and traditional fermented milk were collected using standard microbiology procedures from different cities, including Islamabad, Jehlum, Lahore, Khanewal, and Sukkur. After yogurt production, the samples were collected and stored at 4 °C and transported at refrigerator temperature in the Food Microbiology and Biotechnology lab, NUST. The samples were homogenized in PBS and inoculated in MRS broth for partial enrichment. The overnight enriched samples were serially diluted and spread on MRS agar plates supplemented with 1% CaCO3. The plates were incubated under anaerobic conditions at 37 °C for 48 h. Colonies were selected based on clear zone formation due to acid production on MRS plates, a property generally attributed to acid producers. Gram-positive rod-shaped and catalase-negative isolates were chosen for further characterization [].
2.2. Gastro Intestinal Tract (GIT) Related Stress
2.2.1. Acid and Bile Tolerance
Isolated lactic acid bacteria (LAB) strains were subjected to gastrointestinal tract (GIT) related stresses. The acid and bile tolerance of the isolates was assessed in 96 well plates []. For acid tolerance, 150 µL of MRS broth (HiMedia, Mumbai, India) was adjusted to pH2, and 50 µL of overnight bacterial culture was added to each well. Similarly, MRS broth (HiMedia, Mumbai, India) with 0.3% bile salts (Oxoid, Hampshire, UK) and 50 µL of overnight LAB culture was added to each well for a bile tolerance test. The cell suspensions were incubated anaerobically at 37 °C for 4 h, and growth was measured by absorbance at the 620 nm wavelength. All experiments were performed in triplicate.
2.2.2. Phenol Tolerance
Phenol may be produced by gut microbiota, which can inhibit probiotics, therefore, phenol tolerance by LAB is important for their survival in GIT. Overnight-grown bacterial cultures were inoculated in MRS broth supplemented with 0.4% phenol. Optical density (OD) was measured at 620 nm wavelength in a microplate reader (AMP Platos RII, Graz, Austria) [].
2.2.3. Lysozyme Tolerance
The lysozyme tolerance test was performed as described previously []. The overnight-grown bacterial cultures were harvested by centrifugation at 3500× g for 5 min, washed subsequently with phosphate buffer saline (PBS), and re-suspended in PBS. The cell suspension (10 µL) was then transferred into a PBS solution containing lysozyme (20 mg/L) (BioWorld, Visalia, CA, USA). A bacterial culture without lysozyme was used as control. LAB isolates were incubated at 37 °C. OD was measured at 600 nm wavelength after 0 and 90 min to estimate the survival.
2.3. In Vitro Safety Assessment
2.3.1. Antibiotic Susceptibility Assay
The disc diffusion method was used to evaluate antibiotic susceptibility of LAB isolates in accordance with clinical and laboratory institute standards. The antibiotics widely used in clinical practice such as Tetracycline 30 µg, Kanamycin 30 µg, Ciprofloxacin 5 µg, Chloramphenicol 30 µg, Gentamicin 10 µg, Amoxicillin/clavulanic acid 30 µg, Streptomycin 30 µg, and Vancomycin 30 µg were used in this study [].
2.3.2. DNase Activity
Isolates were streaked on DNase agar (Oxoid, Hampshire, UK) and incubated for 48–72 h at 37 °C to observe the clear zone for the DNase activity. Salmonella enterica was used as a positive control [].
2.3.3. Hemolytic Activity
Hemolytic activity of the isolates was assessed using blood base agar containing 7% v/v sheep blood. The presence of a pale decolorized halo surrounds the colony after 48 h of incubation at 37 °C was considered positive for hemolytic activity. Staphylococcus aureus was used as a positive control [].
2.4. DNA Isolation and Identification
The selected isolates were identified by partial sequencing of the 16S rRNA genes. The DNA extraction was performed as described previously []. The extracted DNA was used as a template in PCR for 16S rRNA gene using Lactobacillus specific; primers S-17 (AGAGTTTGATTCTGGCTCAG) and A-17 (CAC CGCTACACATGGAG) with the following PCR conditions: 1 cycle of 94 °C for 5 min followed by 35 cycles of 94 °C for 30 s, 52 °C for 45 s, and 72 °C for 30 s, and finally one cycle of 7 min at 72 °C. The purified PCR products of the selected LAB isolates were sequenced. The strains were identified by comparing the sequences with the GenBank database using the Basic Local Alignment Search Tool [].
2.5. In Vitro Cholesterol Assimilating Activity
The capacity of probiotic Lactobacillus strains to assimilate cholesterol in MRS broth was investigated as previously described []. Cholesterol-PEG 600 (Sigma Aldrich, St. Louis, MI, USA) was added to MRS broth at a final concentration of 100 μg/mL. The 1% (v/v) inoculum of each overnight probiotic culture was added and incubated at 37 °C for 24 h. The bacterial cultures were centrifuged at 4000 rpm for 10 min at 4 °C, and the supernatants with non- assimilated cholesterol were collected []. Cholesterol assimilation was calculated by the formula:
where A is the cholesterol that remained with the pellet (as a percentage), B is the absorbance of the sample containing the cells, and C is the absorbance of the sample without bacterial cells. The isolates with the highest cholesterol assimilating potential were selected for in vivo trials. The Lactobacillus plantarum ATCC 14917 (KWIK-STIK, UK) was used as a reference strain only for cholesterol assimilation activity.
A = (B/C) × 100
2.6. Bile Salt Hydrolase Activity
The protocol for bile salt hydrolase (BSH) activity measurement was used as described previously with some modifications []. Six mm wells were made in MRS agar plates supplemented with 0.3% w/v bile salts and 0.03% w/v CaCl2. The overnight LAB culture (10 µL) was added and incubated at 37 °C for 72 h in anaerobic conditions. The halos around the wells were observed in order to determine BSH activity. The isolates grown on MRS agar without bile salts were used as the negative control. The experiment was performed in triplicates.
2.7. Safety and Survival of Lactobacillus Strains in GIT 2.7.1. Tagging of Isolates
Eight isolates were selected on the basis of in vitro cholesterol assimilation ability and bsh activity. All selected isolates were tagged with rifampicin at 200 µg/mL [].
Experimental Groups
Forty-five male Wistar rats aged seven to eight weeks were purchased. These rats were individually housed at 25–30 °C with a 12 h light-dark cycle. The animals were randomly divided into nine groups, with five rats per group. Each group had a similar initial average body weight. The control group was fed with a standard commercial diet and water. The remaining eight groups were provided with the standard commercial diet/normal diet (ND) and 2 × 108 CFU/mL of probiotic dosage in distilled water. For survival assessment, the fecal samples from each group were collected every third day, and the samples were enumerated using the spread plate method. The animals were euthanized after 30 days, and blood samples were collected in a sterile tube. The liver, spleen, small intestine, and large intestine were collected and homogenized in PBS. The homogenized samples were serially diluted, and CFUs were determined by the spread plate method [].
2.8. Hypocholesterolemic Potential of Lactobacillus Strains in the Rat Model
2.8.1. Experimental Groups
Eight strains were selected to evaluate their effect on blood lipid profile. Fifty-five male Wistar male rats aged seven to eight weeks were divided into 11 groups with five animals each. The mean weight of each group ranged between 90 to 110 g. A high-fat high cholesterol diet and normal diet composition are shown (Table 1). The HFCD group was fed with high fat and high cholesterol diet with a feed composition of 79% commercial feed, 10% animal fat, 10% sucrose, and 1% feed grade cholesterol. The SDHF group was fed with a high-fat diet and statin drugs (10 mg/kg body weight), and the ND group was fed with a normal diet (standard commercial feed). The remaining eight groups were provided a high-fat diet with one probiotic strain from each: FM9, Y57, FM6, Y55, FM16, Y59, FM12, and Y63. The probiotic dose of 2 × 108 CFU/mL was administered every day for 30 days in drinking water for each group. The rats were euthanized on the 30th day of dose administration, and a complete anatomical analysis was done.

Table 1.
Composition of diets used in the study.
2.8.2. Serum Lipid Analysis
The total cholesterol, triglycerides, HDL, and LDL levels were measured on the 0 and 30th days of the experiment. Blood was collected by cardiac puncture at 0 day and 30th day. A commercial kit method (Sigma-Aldrich, St. Louis, MO, USA) was used to quantify serum lipids.
2.9. Ethical Approval
The ethical approval (IRB-95) for trials was taken from ASAB Institutional Review Board on 6 December 2018.
2.10. Statistical Analysis
All the data were expressed as the standard error of mean ± SEM. Significance was measured by performing an ANOVA followed by Bonferroni’s post-hoc test. Data were analyzed using GraphPad Prism (version 5.01) (GraphPad Software, San Diego, CA, USA). Significance was accepted at p < 0.05 and p < 0.001.
3. Results
3.1. In Vitro GIT Stress Tolerance and Safety Assessment
The survival of isolates in the GIT is an essential feature of probiotics. All the selected isolates were tolerant to GIT related stress, including acid, bile, phenol, and lysozyme stress (Figure S1) (Supplementary data). The tolerance to such gastric stress indicated their potential to survive in GIT, a key attribute for probiotics. All the selected strains, FM9, Y57, FM6, Y55, FM16, Y59, FM12, and Y63, survived in broth with pH2 and 0.3% bile for 4 h. All the LAB isolates were subsequently evaluated for their phenol resistance, where these showed increased tolerance to 0.4% phenol with OD values >1.000 after 24 h of incubation. All the tested LAB isolates showed significant resistance to lysozyme (20 mg/L) after 90 min (Figure S1).
3.2. Safety Assessment
DNase, Hemolytic, and Antibiotic Susceptibility Assay
All 8 selected LAB isolates showed negative hemolytic (γ-hemolysis) results and DNase activity. However, a variable resistance was observed for different tested antibiotics. No zone was observed against Gentamycin, Vancomycin, Kanamycin, and Streptomycin, demonstrating complete resistance to these antimicrobials in these LAB isolates (Table 2). Most of the isolates were susceptible to tetracycline and amoxycillin/clavulanic acid.

Table 2.
Antibiotic susceptibility profile of LAB isolates.
3.3. Molecular Identification of LAB Strains
By comparing the 16S rRNA nucleotide sequences of strains, LAB isolates were identified as Limosilactobacillus fermentum FM6, Lacticaseibacillus rhamnosus FM9, L. fermentum FM12, L. fermentum FM16, L. fermentum Y55, L. fermentum Y57, L. rhamnosus Y59, and L. fermentum Y63 with the GenBank accession numbers, MW521133, MW556565, MW556566, MW521133, MW566788, MW563932, MW563933, MW563934, respectively.
3.4. In Vitro Cholesterol Assimilation
Selected Lactobacillus strains isolated from traditional yogurt and buttermilk were evaluated for their cholesterol-lowering capability. A variable (37–49%) cholesterol assimilation was found in the Lactobacillus strains (Figure 1). Among nine strains, positive control L. plantarum ATCC 14917 exhibited the highest cholesterol-lowering potential (49%), followed by FM6 (43.19%), FM9 (41%), and Y59 (41%) (Figure 1).
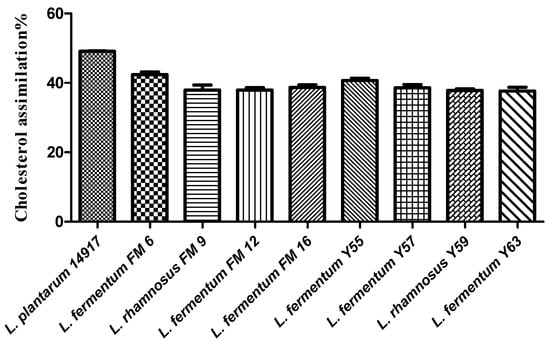
Figure 1.
Cholesterol assimilation (%) of isolates in MRS broth supplemented with bile.
3.5. In Vivo Survival and Safety Assessment
Survival and Colonization Preference in GIT
The rifampicin-tagged isolates were recovered in the fecal sample, indicating that they survived in the GIT. The bacterial count gradually increased from the first trial and remained stable on the 19th day (Figure 2). However, no bacteria were retrieved from the negative control group, indicating that no cross-contamination occurred between groups during the trial.
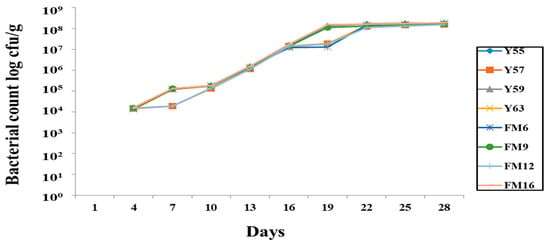
Figure 2.
Recovery of rifampicin-tagged LAB isolates from male Wistar rat feces.
All strains showed colonization in both the small and large intestines; however, strains showed better colonization in the large intestine than in the small intestine. Maximum colonization obtained by L. fermentum FM6 and L. rhamnosus Y59 were 2.55 × 107 cfu/g and 2.72 × 107 cfu/g, respectively, in the large intestine. No colonization was observed in the control group, blood, liver, or spleen samples from all groups, indicating that bacteria were not translocated from the gut to these organs, highlighting the potential safety of these strains (Figure 3).
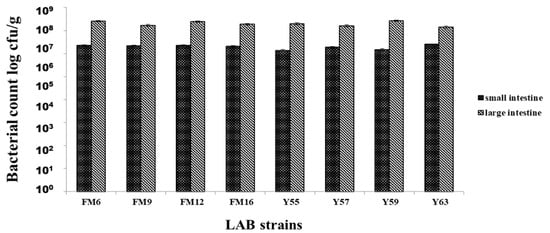
Figure 3.
Adhesion of LAB strains to small & large intestines of rats after 30 days.
3.6. Total Lipid Profile Analysis in the Rat Model
3.6.1. Cholesterol Level
On day 0, no significant difference was observed in serum cholesterol levels between the seven groups. However, the HFCD group significantly increased (31%) in cholesterol levels at day 30, whereas all probiotic groups had a moderate decrease in blood cholesterol levels. L. rhamnosus FM9 reduced serum cholesterol by 9%, while L. fermentum Y57 and L. fermentum FM6 reduced cholesterol by 8% and 7%, respectively, which was significantly better than the SDHF group, where the values were 5% (Figure 4).
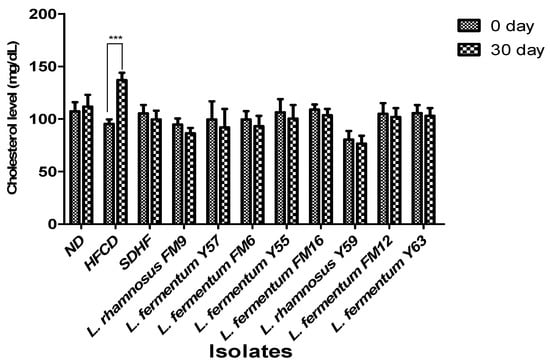
Figure 4.
Impact of probiotic feeding on total serum cholesterol levels in experimental groups (Intragroup at day 0 and 30). *** p < 0.001.
3.6.2. HDL Level
HDL levels in the HFCD group significantly decreased by 8%. The HDL level in the SDHF group, L. rhamnosus FM9, L. fermentum Y57, L. fermentum FM6, L. rhamnosus Y55, L. fermentum Y59, and L. fermentum FM16 was significantly elevated by 46%, 46%, 44%, and 44%, 37%, 22%, and 16% respectively. The data reveals that increased HDL is equally good in L. rhamnosus FM9 and L. fermentum Y57 as by statin-treated group (Figure 5).
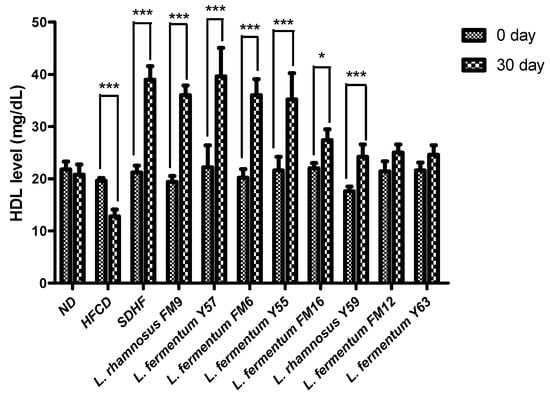
Figure 5.
Intra group comparison of HDL level at day 0 and day 30. * p < 0.05, *** p < 0.001.
3.6.3. LDL Level
The LDL significantly increased by 33% in the HFCD group, while all probiotics could decrease the LDL levels. However, a highly significant decrease was observed in the case of L. fermentum Y57, L. rhamnosus FM9, and L. fermentum FM6, with 41%, 37%, and 31% values, respectively. At the same time, statin reduced LDL levels to only 21%. This shows that the impact of L. fermentum Y57 and L. rhamnosus FM9 was much better in modulating LDL levels than atorvastatin (Figure 6).
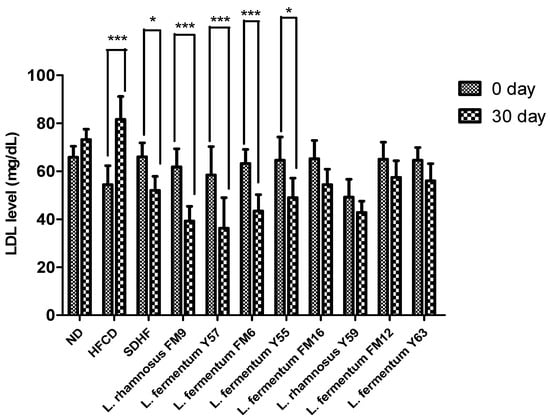
Figure 6.
Intra group comparison of LDL level at day 0 and day 30. * p < 0.05, *** p < 0.001.
3.6.4. Triglyceride Level
At day 30, there was no significant difference between the probiotic treatment groups and the statin treatment groups. However, when compared to the other groups, the HFCD group’s triglyceride level increase was extremely significant when assessed using Bonferroni’s post hoc analysis (Figure 7).
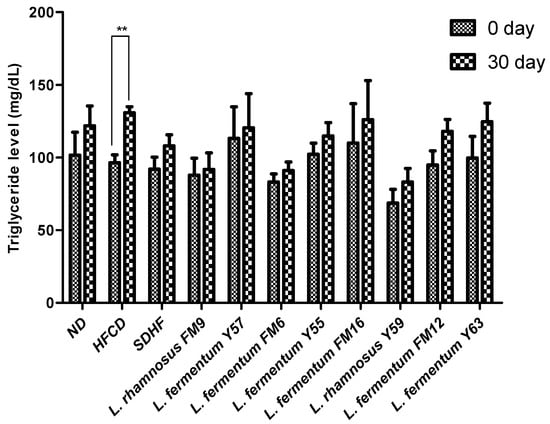
Figure 7.
Intra group comparison of Triglyceride level at day 0 and day 30. ** p < 0.01.
3.6.5. Impact of Isolates on the Average Weight of Animals in Normal Feed Regime
Weight gain is used as a standard for animal health. There was a non-significant difference in body weight between the groups at day 0; however, a healthy weight gain was observed in all test groups, including probiotic-fed groups. The percent weight gain varied in different groups, where probiotic fed groups demonstrated lower weights compared to the control group (group on normal diet), after 30 days (Figure 8A). At day 30, groups fed L. rhamnosus FM9, L. fermentum FM12, L. fermentum FM6, and L. rhamnosus Y59 strains gained considerably less weight than the control group (Figure 8B). These data indicate that these strains have a considerable impact on weight control and may have anti-obesity properties. These findings indicate that the strains may have an anti-obesity effect.
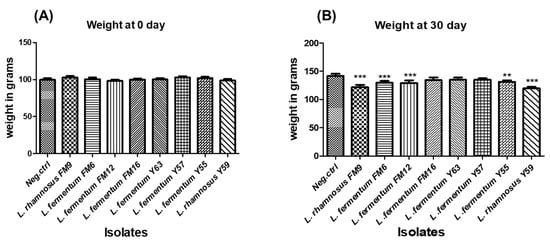
Figure 8.
The average weight of rats administered with different LAB at (A) day 0 and (B) day 30. ** p < 0.01, *** p < 0.001.
3.6.6. Impact of Strains on Weight in High Fat and Cholesterol Feed Regime
Weight gain was relatively modest in the test groups compared with the HFCD group. However, there was a significant difference in all probiotic groups compared to the control groups (ND: normal diet, SDHF: high-fat diet, while treating with statins, and HFCD: high-fat diet with no treatment) at day 30 (Figure 8B). These findings reveal that these strains, L. rhamnosus FM9 and L. rhamnosus Y59, also showed potential to act as a weight watcher, as it moderated the weight gain compared to the control groups (ND, HFCD, and SDHF).
When feed intake was estimated for each group, it was observed that there was no significant difference in feed intake between the groups during both trials. This indicates that weight moderation is not due to lesser feed intake.
4. Discussion
Probiotics have demonstrated the ability to lower serum triglycerides, cholesterol, and low-density lipoprotein. As a result of these findings, there is growing interest in employing probiotics to prevent coronary heart disease []. The strains FM9, Y57, FM6, Y55, FM16, Y59, FM12, and Y63 were recovered from traditional fermented milk (lassi) and artisanal yogurt in this study. These isolates demonstrated in vitro tolerance to gastrointestinal tract-related stresses (acid, bile, lysozyme, phenol) and bile salt hydrolase potential. Probiotics need to withstand gastrointestinal stress conditions, such as lysozyme, phenol, acidity, and bile, to exert a beneficial role in GIT. Our findings were consistent with a prior work in which the L. fermentum AD1 strain isolated from fermented milk demonstrated good survival at low pH, 0.3% bile salt, and 0.4% phenol isolated from fermented milk [].
Due to their long history of safe use in foods, most probiotic strains are GRAS (Generally Recognized as Safe) []. Nevertheless, researchers identified some LAB strains as opportunistic pathogens capable of infections in humans. As a result, establishing the safety of these strains is essential before using them as probiotics []. In our study, all of the initially screened isolates exhibited negative results for hemolytic, DNase activity, and susceptibility to the many antibiotics tested. These findings are consistent with recent studies in which L. fermentum NCU3087 and L. fermentum NCU3088 exhibited no hemolysis or DNase activity []. The isolated LAB should be antibiotic sensitive to avoid the transfer of undesirable antibiotic resistance to the intestinal microbiota []. According to previous findings, L. rhamnosus and L. fermentum were susceptible to methicillin, penicillin, tetracycline, levofloxacin, azithromycin, chloramphenicol, and other antibiotics []. Based on 16S rRNA gene sequence analysis, the isolates FM6, FM12, FM16, Y55, Y57, and Y63 were 99–100 percent similar to L. fermentum, while FM9 and Y59 were 100 percent similar to L. rhamnosus. Previous research has also found L. fermentum and L. rhamnosus in fermented milk [,].
Many in vitro tests are used to select cholesterol-lowering probiotics, including assessing bile salt hydrolase (BSH) activity. Prior studies [] showed that Lactobacillus strains with Bile salt hydrolase activity reduced cholesterol more effectively when administered in a rat model. The in vitro cholesterol-lowering activity was variable in our isolates, ranging from 19% to 49%. The highest cholesterol-lowering potential was observed in the FM6 strain (38%), followed by Y59 (36%). In a prior study, L. rhamnosus BFE5264 demonstrated in vitro cholesterol-lowering potential, suggesting that it could help decrease plasma total cholesterol concentrations []. However, a weak cholesterol reduction potential (5%) was observed in L. plantarum [].
The selected strains met the criteria for being identified as a potential probiotic strain and subsequently were used to investigate their probiotic effects in rats fed a high-cholesterol diet. Probiotics exert physiological functions only when they effectively survive in the host GIT and propagate through colonization []. The Lactobacillus strains in this study showed the ability to resist GIT stress when tested in the rat model. The bacterial count in the feces was low during the first week of the study. Nonetheless, the bacterial count in the feces continues to rise, indicating that these strains could survive and colonize in the GIT. Similar findings have been reported in previous studies [] where different probiotic strains effectively colonize in the GIT of the rat. In our study, colonization was seen in both small and large intestines; however, greater colonization of strains was observed in the large intestine, indicating that these strains preferred the anaerobic environment of the large intestine. Consistent with our findings, a previous study [] discovered that probiotic Lactobacillus strains prefer the large intestine. According to an earlier study, the colonization and distribution of L. plantarum MA2 were observed in the small intestine of mice []. The colonization of LAB in the gut play an important role in their beneficial effects on the host.
Another study found that L. rhamnosus SKG34 prefers colonization in the large intestine []. There was no evidence of bacterial translocation in the liver, blood, or spleen, indicating that our strains are nonpathogenic. These findings are consistent with prior research, which found that L. fermentum SM-7 did not cause translocation in the blood, liver, or kidneys and was safe to use []. In another study, L. fermentum PL9005 did not translocate from the gut to other organs in mice [].
The cholesterol-lowering ability of selected strains was validated in a rat model. This study revealed that L. rhamnosus FM9, L. fermentum Y57, and L. fermentum FM6 increased serum HDL levels by 46%, 46%, and 44%, respectively. These findings were comparable to those reported previously, where administration of L. rhamnosus and L. fermentum elevated HDL-c levels by 15.23% and 20.12%, respectively []. In another study, no significant effect was observed by the L. fermentum strains isolated from human feces on HDL in a mice model []. This impact may be strain-dependent and a source of isolation []. In our study, the supplementation of L. fermentum Y57, L. rhamnosus FM9, and L. fermentum FM6 showed a significant decrease in serum LDL by 41%, 37%, and 31%, respectively. According to a previous study, L. fermentum strains showed a significant decline in LDL levels, at 51.07%, 49.73%, 57.96%, and 63.25%, respectively. []. According to [], decreased levels of cholesterol and increased levels of HDL content may reduce the conversion of intermediate-density lipoprotein to LDL-C particles, resulting in a decrease in serum LDL-C concentration. This could be the reason for the probiotics’ LDL-C lowering effect. However, in previous reports, probiotic strains did not affect LDL levels in a rat model []. There was no significant difference in triglyceride levels observed on day 30 in probiotic treatment groups and statins, while the level of triglycerides was significantly high in the HFCD group. These results were in agreement with the previous study where Lactobacillus strains have no impact on triglyceride levels in rats after a four week trial []. However, according to a previous study, the administration of L. fermentum strains decreases triglycerides by 66.3%, 65.3%, 50.5%, 70.7%, and 66.2%, respectively []. According to Harisa et al., [] the hypotriglyceridemic effect of probiotics may be attributed to the activation of lipases, a decrease in intestinal lipid absorption, or an increase in lipid catabolism and/or antioxidant activity. Lipoprotein lipase is responsible for TG metabolism, which results in normalization of its plasma level, while the increase in triglyceride levels could potentially be attributed to high fat stimulation of hepatic very low density lipoprotein (VLDL) synthesis []. A previous study also reported that L. rhamnosus had no impact on triglycerides []. In an earlier study, L. fermentum SM-7 was isolated from a fermented milk drink (koumiss), and it significantly reduced total serum cholesterol (TC) and LDL. However, it did not increase HDL-C significantly in the mice model []. The mechanism by which Lactobacillus strains lower cholesterol levels and improve lipid profiles remains unknown. However, this is presumed due to the probiotics’ ability to modulate rats’ gut microbiota’s growth and fermentative products []. There are four well-documented mechanisms by which probiotics lower cholesterol levels in the blood. These include (1) the probiotic bacteria’s ability to assimilate cholesterol molecules in the small intestine; (2) the ability of probiotic microbes to enzymatically deconjugate bile acid using bile salt hydrolase (BSH). Because bile acid is mostly dissolved in the conjugated form, only a small portion is absorbed in the intestine, and the rest is excreted in the feces. The absorbed cholesterol is then used to synthesize new bile acids (a homeostatic response), resulting in a decrease in serum cholesterol level; (3) the conversion of cholesterol to coprostanol by cholesterol reductase of lactobacillus strains; and (4) products of the Lactobacilli fermentation process in the form of short chain fatty acids [].
The administration of Lactobacillus strains FM6, FM9, FM12, Y55, and Y59 in the HFCD mice significantly decreased the weight gain compared to the control group in a 30-day trial, which indicates that it reduces fat deposition and obesity. Similar findings were observed in a previous study where Lactobacillus strains significantly reduced body weight in a rat model []. In another study, some of the Lactobacillus strains do not affect the bodyweight of the mice []. According to a previous study, the administration of L. fermentum CRL1446 resulted in a significant reduction in body weight in mouse models, reaching values similar to the control []. The selected probiotic strains in this study revealed the potential to improve serum lipid profile and obesity control. However, two strains, L. rhmanosus FM6 and L. fermentum Y59, prove to be the best candidates in reducing total cholesterol, triglycerides, LDL, and improving HDL since their performance is as good as the statins treated group. Probiotic consumption also improved weight control in animals on a high-fat, high cholesterol diet. Although the potential of Lactobacillus in improving lipid profiles is well known, however, its comparison with clinically used drugs is not well known. This study suggests that L. rhamnosus FM9 and L. fermentum Y57 strains exhibit great potential in improving the lipid profile, which is as good as statin.
5. Conclusions
This study demonstrates that L. rhmanosus FM9 and L. fermentum Y57 best reduce hypercholesterolemia among all tested probiotic strains. These strains’ impact on cholesterol reduction and overall lipid profile was equally as good as statins in Wistar rats fed high fat, high cholesterol diets after 30 days. These strains also improved weight control and may have anti-obesity potential. Our study also has some limitations: we used rats, and the observed effects in an animal model may not be the same in humans. Therefore, clinical trials need to be conducted to validate the efficacy and safety of the strain for its use in managing high cholesterol in humans. Furthermore, these strains can be a source of an adjunct or a starter culture in different food products such as fermented milk, yogurt, and other dairy foods.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu14081654/s1, Figure S1: GIT related stress of LAB isolates.
Author Contributions
H.Z. and N.u.A.; investigation & original draft preparation, A.A. (Abdulrahman Alshammari) and S.A. (Saeed Alghamdi) formal analysis, H.R.; manuscript writing and validation, A.A. (Amjad Ali); conceptualization and data curation, A.S.; investigation and validation, S.D.T.; writing original draft, S.A. (Samina Akbar) and M.A. (Maryum Arif); visualization, data curation, M.A. (Metab Alharbi); review manuscript, resources, data curation, A.R.; Conceptualization, funding acquisition, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by HEC, NRPU grant no: 10102, HEC, Pakistan, and Researchers Supporting Project number: RSP2022R462 King Saud University, Riyadh, Saudi Arabia’.
Institutional Review Board Statement
The study was conducted according to the guidelines as approved by the Institutional Review Board of Atta ur Rahman School of Applied Biosciences (ASAB), NUST, 44000, Islamabad (6 December 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- WHO Expert Committee on the Selection, Use of Essential Medicines, World Health Organization. The Selection and Use of Essential Medicines: Report of the WHO Expert Committee, 2013 (Including the 18th WHO Model List of Essential Medicines and the 4th WHO Model List of Essential Medicines for Children); World Health Organization: Geneva, Switzerland, 2014; Volume 985.
- Kedev, S. European Society of Cardiology: Cardiovascular disease statistics 2017. Eur. Heart J. 2018, 39, 508–577. [Google Scholar]
- Gao, M.; Jebb, S.A.; Aveyard, P.; Ambrosini, G.L.; Perez-Cornago, A.; Carter, J.; Sun, X.; Piernas, C.J.B.M. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK Biobank: A prospective cohort study. BMC Med. 2021, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Koslik, H.J.; Meskimen, A.H.; Golomb, B.A. Physicians’ experiences as patients with statin side effects: A case series. Drug Saf.-Case Rep. 2017, 4, 1–7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, E.A.; Chang, H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. LWT-Food Sci. Technol. 2015, 62, 210–217. [Google Scholar] [CrossRef]
- Ooi, L.-G.; Liong, M.-T. Cholesterol-lowering effects of probiotics and prebiotics: A review of in vivo and in vitro findings. Int. J. Mol. Sci. 2010, 11, 2499–2522. [Google Scholar] [CrossRef]
- Ghosh, T.; Beniwal, A.; Semwal, A.; Navani, N.K. Mechanistic insights into probiotic properties of lactic acid bacteria associated with ethnic fermented dairy products. Front. Microbiol. 2019, 10, 502. [Google Scholar] [CrossRef]
- Bao, Y.; Zhang, Y.; Zhang, Y.; Liu, Y.; Wang, S.; Dong, X.; Wang, Y.; Zhang, H. Screening of potential probiotic properties of Lactobacillus fermentum isolated from traditional dairy products. Food Control 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Yadav, R.; Puniya, A.K.; Shukla, P. Probiotic properties of Lactobacillus plantarum RYPR1 from an indigenous fermented beverage Raabadi. Front. Microbiol. 2016, 7, 1683. [Google Scholar] [CrossRef]
- Bao, Q.; Yu, J.; Liu, W.; Qing, M.; Wang, W.; Chen, X.; Wang, F.; Li, M.; Wang, H.; Lv, Q. Predominant lactic acid bacteria in traditional fermented yak milk products in the Sichuan Province of China. Dairy Sci. Technol. 2012, 92, 309–319. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Technol. 2004, 15, 67–78. [Google Scholar] [CrossRef]
- Koirala, R.; Ricci, G.; Taverniti, V.; Ferrario, C.; Malla, R.; Shrestha, S.; Fortina, M.G.; Guglielmetti, S. Isolation and molecular characterization of lactobacilli from traditional fermented Dahi produced at different altitudes in Nepal. Dairy Sci. Technol. 2014, 94, 397–408. [Google Scholar] [CrossRef][Green Version]
- Sedláčková, P.; Horáčková, Š.; Shi, T.; Kosová, M.; Plocková, M. Two different methods for screening of bile salt hydrolase activity in Lactobacillus strains. Czech J. Food Sci. 2015, 33, 13–18. [Google Scholar] [CrossRef]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.; Harris, H.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Bonczar, G.; Walczycka, M.B.; Domagała, J.; Maciejowski, K.; Najgebauer-Lejko, D.; Sady, M.; Wszołek, M. Effect of dairy animal species and of the type of starter cultures on the cholesterol content of manufactured fermented milks. Small Rumin. Res. 2016, 136, 22–26. [Google Scholar] [CrossRef]
- Kumar, M.; Nagpal, R.; Kumar, R.; Hemalatha, R.; Verma, V.; Kumar, A.; Chakraborty, C.; Singh, B.; Marotta, F.; Jain, S. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012, 2012, 902917. [Google Scholar] [CrossRef]
- Thakkar, P.N.; Patel, A.; Modi, H.A.; Prajapati, J.B. Hypocholesterolemic effect of potential probiotic Lactobacillus fermentum strains isolated from traditional fermented foods in Wistar rats. Probiotics Antimicrob. Proteins 2020, 12, 1002–1011. [Google Scholar] [CrossRef]
- Tomaro-Duchesneau, C.; Jones, M.L.; Shah, D.; Jain, P.; Saha, S.; Prakash, S. Cholesterol assimilation by Lactobacillus probiotic bacteria: An in vitro investigation. BioMed Res. Int. 2014, 2014, 380316. [Google Scholar] [CrossRef]
- Turchi, B.; Mancini, S.; Fratini, F.; Pedonese, F.; Nuvoloni, R.; Bertelloni, F.; Ebani, V.V.; Cerri, D. Preliminary evaluation of probiotic potential of Lactobacillus plantarum strains isolated from Italian food products. World J. Microbiol. Biotechnol. 2013, 29, 1913–1922. [Google Scholar] [CrossRef]
- Khalil, E.S.; Manap, A.; Yazid, M.; Mustafa, S.; Alhelli, A.M.; Shokryazdan, P. Probiotic properties of exopolysaccharide-producing Lactobacillus strains isolated from tempoyak. Molecules 2018, 23, 398. [Google Scholar] [CrossRef]
- Lara-Villoslada, F.; Sierra, S.; Martín, R.; Delgado, S.; Rodríguez, J.; Olivares, M.; Xaus, J. Safety assessment of two probiotic strains, Lactobacillus coryniformis CECT5711 and Lactobacillus gasseri CECT5714. J. Appl. Microbiol. 2007, 103, 175–184. [Google Scholar] [CrossRef]
- Lindbäck, T.; Granum, P.E. Detection and purification of Bacillus cereus enterotoxins. In Food-Borne Pathogens; Springer: Berlin/Heidelberg, Germany, 2006; pp. 15–26. [Google Scholar]
- Harun-ur-Rashid, M.; Togo, K.; Ueda, M.; Miyamoto, T. Identification and characterization of dominant lactic acid bacteria isolated from traditional fermented milk Dahi in Bangladesh. World J. Microbiol. Biotechnol. 2007, 23, 125–133. [Google Scholar] [CrossRef]
- Rudel, L.L.; Morris, M. Determination of cholesterol using o-phthalaldehyde. J. Lipid Res. 1973, 14, 364–366. [Google Scholar] [CrossRef]
- Tulumoğlu, Ş.; Kaya, H.İ.; Şimşek, Ö. Probiotic characteristics of Lactobacillus fermentum strains isolated from tulum cheese. Anaerobe 2014, 30, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Plavec, T.V.; Berlec, A. Safety aspects of genetically modified lactic acid bacteria. Microorganisms 2020, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Suresh, K.; Krishnappa, S.; Bhardwaj, P. Safety concerns of Probiotic use: A review. IOSR J. Dent. Med. Sci. 2013, 12, 56–60. [Google Scholar]
- Park, J.-H.; Lee, Y.; Moon, E.; Seok, S.-H.; Baek, M.-W.; Lee, H.-Y.; Kim, D.-J.; Kim, C.-H.; Park, J.-H. Safety assessment of Lactobacillus fermentum PL9005, a potential probiotic lactic acid bacterium, in mice. J. Microbiol. Biotechnol. 2005, 15, 603–608. [Google Scholar]
- Sharma, P.; Tomar, S.K.; Sangwan, V.; Goswami, P.; Singh, R. Antibiotic resistance of Lactobacillus sp. isolated from commercial probiotic preparations. J. Food Saf. 2016, 36, 38–51. [Google Scholar] [CrossRef]
- Kotzamanidis, C.; Kourelis, A.; Litopoulou-Tzanetaki, E.; Tzanetakis, N.; Yiangou, M. Evaluation of adhesion capacity, cell surface traits and immunomodulatory activity of presumptive probiotic Lactobacillus strains. Int. J. Food Microbiol. 2010, 140, 154–163. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.-H. Identification of a novel potential probiotic Lactobacillus plantarum FB003 isolated from salted-fermented shrimp and its effect on cholesterol absorption by regulation of NPC1L1 and PPARα. Probiotics Antimicrob. Proteins 2019, 11, 785–793. [Google Scholar] [CrossRef]
- Mathara, J.M.; Schillinger, U.; Guigas, C.; Franz, C.; Kutima, P.M.; Mbugua, S.K.; Shin, H.-K.; Holzapfel, W.H. Functional characteristics of Lactobacillus spp. from traditional Maasai fermented milk products in Kenya. Int. J. Food Microbiol. 2008, 126, 57–64. [Google Scholar] [CrossRef]
- Kumar, R.; Grover, S.; Batish, V.K. Bile salt hydrolase (Bsh) activity screening of Lactobacilli: In vitro selection of indigenous Lactobacillus strains with potential bile salt hydrolysing and cholesterol-lowering ability. Probiotics Antimicrob. Proteins 2012, 4, 162–172. [Google Scholar] [CrossRef] [PubMed]
- De Champs, C.; Maroncle, N.; Balestrino, D.; Rich, C.; Forestier, C. Persistence of colonization of intestinal mucosa by a probiotic strain, Lactobacillus casei subsp. rhamnosus Lcr35, after oral consumption. J. Clin. Microbiol. 2003, 41, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, L.; Liu, F.; Li, B.; Tang, Y.; Yu, S.; Zhang, D.; Huo, G. Effect of bile salt hydrolase-active Lactobacillus plantarum KLDS 1.0344 on cholesterol metabolism in rats fed a high-cholesterol diet. J. Funct. Foods 2019, 61, 103497. [Google Scholar] [CrossRef]
- Peng, Z.; Wei, B.; Huang, T.; Liu, Z.; Guan, Q.; Xie, M.; Li, H.; Xiong, T. Screening, safety evaluation, and mechanism of two Lactobacillus fermentum strains in reducing the translocation of staphylococcus aureus in the Caco-2 monolayer model. Front. Microbiol. 2020, 11, 566473. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y. Benefits of fermented milk in rats fed by hypercholesterolemic diet (II). Korean J. Food Hyg. 1992, 7, 123–135. [Google Scholar]
- Reis, S.; Conceição, L.; Rosa, D.; Siqueira, N.; Peluzio, M. Mechanisms responsible for the hypocholesterolaemic effect of regular consumption of probiotics. Nutr. Res. Rev. 2017, 30, 36–49. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, N.; Xi, A.; Ahmed, Z.; Zhang, B.; Bai, X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2009, 84, 341–347. [Google Scholar] [CrossRef]
- Nocianitri, K.; Antara, N.; Sugitha, I.; Sukrama, I.; Ramona, Y.; Sujaya, I. The effect of two Lactobacillus rhamnosus strains on the blood lipid profile of rats fed with high fat containing diet. Int. Food Res. J. 2017, 24, 795. [Google Scholar]
- Pan, D.D.; Zeng, X.Q.; Yan, Y.T. Characterisation of Lactobacillus fermentum SM-7 isolated from koumiss, a potential probiotic bacterium with cholesterol-lowering effects. J. Sci. Food Agric. 2011, 91, 512–518. [Google Scholar] [CrossRef]
- Yoo, S.R.; Kim, Y.J.; Park, D.Y.; Jung, U.J.; Jeon, S.M.; Ahn, Y.T.; Huh, C.S.; McGregor, R.; Choi, M.S. Probiotics L. pelantarum and L. curvatus in combination alter hepatic lipid metabolism and suppress diet-Induced obesity. Obesity 2013, 12, 2571–2578. [Google Scholar] [CrossRef]
- Xie, N.; Cui, Y.; Yin, Y.-N.; Zhao, X.; Yang, J.-W.; Wang, Z.-G.; Fu, N.; Tang, Y.; Wang, X.-H.; Liu, X.-W. Effects of two Lactobacillus strains on lipid metabolism and intestinal microflora in rats fed a high-cholesterol diet. BMC Complement. Altern. Med. 2011, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Liong, M.-T. Probiotics: A critical review of their potential role as antihypertensives, immune modulators, hypocholesterolemics, and perimenopausal treatments. Nutr. Rev. 2007, 65, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Zarrati, M.; Salehi, E.; Nourijelyani, K.; Mofid, V.; Zadeh, M.J.; Najafi, F.; Ghaflati, Z.; Bidad, K.; Chamari, M.; Karimi, M.; et al. Effects of probiotic yogurt on fat distribution and gene expression of proinflammatory factors in peripheral blood mononuclear cells in overweight and obese people with or without weight-loss diet. J. Am. Coll. Nutr. 2014, 33, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Harisa, G.; Taha, E.; Khalil, A.; Salem, M. Oral administration of Lactobacillus acidophilus restores nitric oxide level in diabetic rats. Aust. J. Basic Appl. Sci. 2009, 3, 2963–2969. [Google Scholar]
- Grefhorst, A.; Elzinga, B.M.; Voshol, P.J.; Plo, T.; Kok, T.; Bloks, V.W.; van der Sluijs, F.H.; Havekes, L.M.; Romijn, J.A.; Verkade, H.J. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002, 277, 34182–34190. [Google Scholar] [CrossRef]
- Cavallini, D.C.; Suzuki, J.Y.; Abdalla, D.S.; Vendramini, R.C.; Pauly-Silveira, N.D.; Roselino, M.N.; Pinto, R.A.; Rossi, E.A. Influence of a probiotic soy product on fecal microbiota and its association with cardiovascular risk factors in an animal model. Lipids Health Dis. 2011, 10, 1–9. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, B.; Hu, J.; Nie, S.; Xiong, T.; Xie, M.J.x. Intervention of five strains of Lactobacillus on obesity in mice induced by high-fat diet. J. Funct. Foods 2020, 72, 104078. [Google Scholar] [CrossRef]
- Liang, C.; Zhou, X.H.; Jiao, Y.H.; Guo, M.J.; Meng, L.; Gong, P.M.; Lyu, L.Z.; Niu, H.Y.; Wu, Y.F.; Chen, S.W.; et al. Ligilactobacillus Salivarius LCK11 Prevents Obesity by Promoting PYY Secretion to Inhibit Appetite and Regulating Gut Microbiota in C57BL/6J Mice. Mol. Nutr. Food Res. 2021, 17, 2100136. [Google Scholar] [CrossRef]
- Russo, M.; Marquez, A.; Herrera, H.; Abeijon-Mukdsi, C.; Saavedra, L.; Hebert, E.; Gauffin-Cano, P.; Medina, R. Oral administration of Lactobacillus fermentum CRL1446 improves biomarkers of metabolic syndrome in mice fed a high-fat diet supplemented with wheat bran. Food Funct. 2020, 11, 3879–3894. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).