Abstract
Depression is a common mood disorder associated with childbirth and is hypothesized to be affected by low vitamin D. This systematic review identified two randomized controlled trials (RCT) of vitamin D supplementation for the treatment or prevention of depressive symptoms in the perinatal period, as well as 18 observational studies of vitamin D exposure and depression in the antenatal and postnatal periods. Both RCTs claimed an improvement in depressive symptoms in the vitamin D group, although the sample sizes were too small to draw firm conclusions. The case-control and cohort studies had mixed findings and were limited by study quality. There were inconsistent results within the few studies with a more robust methodology or within samples restricted to women likely to have depression. The current evidence is inconclusive due to the poor quality and heterogeneity of studies, likely contributing to the contradictory findings. Given there are already numerous RCTs of prenatal vitamin D supplementation, we recommend adding an appropriate measure of depression in the perinatal period to assist in resolving the uncertainty.
1. Introduction
Postpartum depression (PPD) is common, with 19% of mothers experiencing depression within 12 weeks of birth and another 10–20% of women experiencing PPD within the first year [1,2], and for 8% of women symptoms persist beyond a year [3]. Women with depression in the perinatal period may experience mood disturbances (including sadness, loss of pleasure, guilt, or worthlessness), sleep disturbances (unrelated to their pregnancy or infant), appetite disturbances, weight loss, and suicidal ideation. PPD has adverse implications for mother-infant attachment and child development [4,5,6].
The prevalence of depression in the perinatal period is similar across race, parity, age, education, and socioeconomic status [1] and there is no clear cause [7,8]. Consequently, it is not possible to accurately predict which mothers will develop depression or determine how best to prevent PPD in the perinatal period. However, some evidence suggests that low vitamin D may increase the risk of mood disorders such as PPD [9,10,11].
Vitamin D is obtained when the skin is exposed to sunlight, and to a lesser extent from diet. Vitamin D, from diet or skin synthesis, is then hydroxylated to 25-hydroxyvitamin D (25OHD), the major circulating form of vitamin D and the best indicator of vitamin D status [12]. To be fully activated, 25OHD must undergo a second hydroxylation to 1,25-dihydroxyvitamin D. This active form is a nuclear steroid that binds to the vitamin D receptors [13] that are present in many tissues, including the human brain [14], providing biological plausibility for a role in neurological functioning. Initial suggestions that vitamin D plays a role in mood disorders arose from seasonal affective disorder, a mood disorder with symptoms of depression that occurs in the winter months, where vitamin D synthesis by sunlight is low [15]. Meta-analyses of observational studies have reported an association between low 25OHD and mood disorders such as depression [9,10]. The meta-analysis of trials of vitamin D supplementation and depression suggests some benefit, although the results are variable, and in most instances, the study quality is poor [16]. Vitamin D supplementation is not currently recommended for the treatment of depressive symptoms due to the low quality of the evidence; however, depression in the perinatal period is absent from this literature [16].
Pregnancy and lactation may be a demanding time in terms of nutrient requirements, where maternal nutrient reserves may become depleted to ensure adequate nutrition for the developing baby [17]. Increased prevalence of poor vitamin D status has been reported in pregnant women, based on low 25OHD, in many populations globally [18,19]. Suboptimal vitamin D status during pregnancy or postpartum may contribute to symptoms of depression. If effective, ensuring women have sufficient vitamin D may be a simple, safe, and cheap method of preventing, or reducing symptoms of depression in the perinatal period [20,21]. However, reviews of vitamin D in the perinatal period have reported conflicting inconclusive results [22,23,24,25,26], and have not actively included depression as an outcome of interest [18,27], or have not included all concurrently published studies [11,26], and to date all reviews have been limited to observational studies [24,25,26]. We aim to conduct the first systematic review of trials and observational studies of vitamin D and depression during the perinatal period and postpartum. We will determine whether there is consistency between the recently published trials capable of providing causal evidence for a role of vitamin D in antenatal and postnatal depression and the body of observational evidence.
2. Materials and Methods
We conducted our systematic review according to the guidelines outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis [28]. This systematic review is registered on the PROSPERO registry (https://www.crd.york.ac.uk/prospero/; ID CRD42022328361, last edited on 10 May 2022).
Published articles were eligible for inclusion in this review if they were a trial of vitamin D supplementation during the antenatal or postpartum period, or if they were an observational study of vitamin D (25OHD) status, vitamin D intake, or vitamin D exposure), and the study included a measure of depressive symptoms (such as clinical diagnoses of a depressive disorder, use of medication for a depressive disorder in the perinatal period, and questionnaires measuring depressive symptoms). Animal studies and manuscripts not published in English were excluded.
We searched PubMed for eligible articles, with weekly search alerts to capture new potentially eligible publications for inclusion up until October 2021. Reference lists of eligible articles, as well as any similar reviews were also screened for relevant manuscripts. Titles of articles were screened, followed by abstracts and full text where needed to determine eligibility.
The included studies were reviewed, and pertinent information was summarized in tables. Information of interest included descriptions of the study design (trial, case-control, and cohort), sample population (characteristics, size of sample, inclusion, and exclusion criteria), intervention details (timing, dose, and duration of any vitamin D supplements, and inclusion of other nutrients) or details of vitamin D exposure (such as timing of measurement, analytical method, definition of status, and definition of deficiency, if any), timing and measurement of depressive symptoms, as well as results. We also noted limitations and possible bias, such as small sample, suboptimal exposure, or outcome measures, or other indications of poor study quality (for example, inadequate consideration of confounders).
Trials of vitamin D supplementation were considered separately to observational studies. Observational studies were subdivided into case-control studies or cohort studies, and results of explorations in clinical samples are discussed separately from general (non-clinical) samples. Given the growing concerns around the effects of insufficient vitamin D, we considered the results of analyses of vitamin D status as a continuous variabl, and categorical variable separately, with an emphasis on explorations of deficient or insufficient vitamin D status. Where unadjusted and adjusted analyses are reported, only the fully adjusted results are considered here.
3. Results
Our search identified a total of 2810 manuscripts, of which there were 21 eligible articles (see Figure 1 for flow); two RCTs [29,30]; and 18 observational studies [29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49], including one trial that also reported associations between depression and vitamin D status at trial entry [29] and two cohorts that reported results across multiple publications [42,43,47,48]. One potentially eligible case-control study was excluded, as it was reported as a dissertation from 2013 and had not been peer reviewed [50].
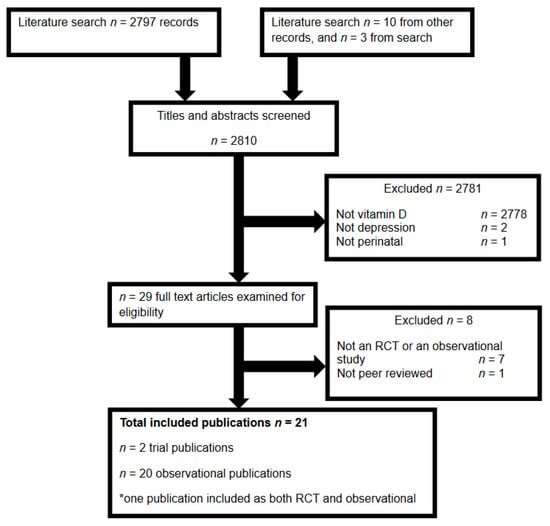
Figure 1.
Flow of publications through the literature search and screening for eligibility.
3.1. Trials of Vitamin D and Depression in the Perinatal Period
Although we identified many perinatal vitamin D RCTs, only two assessed symptoms of depression and both were conducted in Iran, (Table 1) [29,30].

Table 1.
Randomized controlled trials of vitamin D supplementation in the antenatal and postpartum period and depression.
3.1.1. Trial Design, Randomization, and Blinding
The two vitamin D trials identified were described as double-blind RCTs, one conducted during pregnancy and the other postpartum [29,30]. No primary outcome was specified in the prenatal trial [29] and the postnatal trial had joint primary outcomes of PPD and serum concentrations of 25OHD [30]. Both trials included a control group that was given a placebo [29,30]. One trial involved two randomization groups [29] and the other involved three randomization groups [30], to which women were randomized via block design, although specific details regarding randomization techniques were not reported in either trial. Blinding information was absent in the report for the prenatal trial, apart from stating it was single-blinded, and women likely knew their group allocation [29]. Women and staff in the postnatal trial were likely blinded through use of two-digit identification numbers to identify participants and supplement bottles (packed by a non-study staff) [30].
3.1.2. Trial Sample Details
The trials differed in their target sample and recruitment strategy, although both were conducted in small samples of Iranian mothers with less than 100 per group [29,30]. Pregnant women approached at a prenatal hospital clinic were excluded if they had depression or were likely to have depression [29]. The postnatal trial recruited a sample of women from a psychiatric outpatient clinic if they had depressive symptoms but were not using antidepressants [30]. Women were excluded if they had sufficient vitamin D status [30].
3.1.3. Vitamin D Interventions
Both trials randomized women to oral vitamin D supplements or a placebo [29,30]. Pregnant women were provided with 50 µg of vitamin D/3 from two tablets per day throughout the last trimester of pregnancy [29], or received 1250 µg vitamin D3 as fortnightly supplements postpartum (exact timing not reported) over an 8-week period [30]. The postpartum intervention had three randomization groups, one of which also received calcium [30], whilst women in the prenatal trial may have been taking a regular multivitamin containing vitamin D [29].
3.1.4. Depression Outcome Measure and Timing in Trials
Both trials measured depression using the Iranian version of the Edinburgh Postnatal Depression Scale (EPDS). The EPDS is an appropriate, widely used questionnaire that can screen for depressive symptoms specifically in the postpartum period with high sensitivity (68–95%) and high specificity (78–96%) against a clinical psychiatric diagnosis of PPD [51,52]. It is typically self-completed and a score of more than 12 is most commonly used to indicate a probable depressive disorder [53,54]. In both trials, the EPDS was administered through an interview with study staff, and women were considered at risk of depression if they scored >13 [29,30]. In the non-depressed sample, women completed the EPDS three times, once during pregnancy and twice postpartum [29], whilst women in the depressed sample completed the EPDS at the end of the intervention (8 weeks after enrolment) [30].
3.1.5. Trial Efficacy
Vitamin D supplementation in both trials resulted in a decrease in EPDS scores in the treatment group(s) compared with the control group [29,30]. In the non-depressed population, depression scores also decreased in the control group, however, not to the same extent as the vitamin D group [29].
3.2. Observational Studies of Vitamin D and Depression in the Perinatal Period
There were 21 publications with an exploration of vitamin D and depression in the perinatal period (summarized in Table 2) [29,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. Two cohorts reported outcomes across more than one manuscript [42,43,47,48]. One RCT of vitamin D also reported the association between depressive symptoms and vitamin D status and has been included as an observational study (Table 2) as well as a trial (Table 1) [29].

Table 2.
Observational studies of vitamin D and symptoms of depression during pregnancy and postpartum.
3.2.1. Observation Study Designs
None of the observational studies appeared to be designed and conducted specifically to assess the association between vitamin D and symptoms of depression in the perinatal period. Most were established as general pregnancy cohorts [31,34,35,41,42,47,49]. One was a prenatal vitamin D RCT [29], one was a convenience sample of a larger study that appeared to be a vitamin D dose-response trial [33], and two studies were originally prenatal omega-3 RCTs [37,40]. Three studies were case-control studies that appropriately compared the vitamin D status of women classified as depressed to women not classified as depressed [34,44,45], and three cohort studies analyzed their sample as though they conducted a case-control study [32,36,49].
3.2.2. Observation Study Recruitment and Sample Details
Most studies were conducted in high income countries such as the United States [31,33,39,40,43,48,49], Australia [35,37,38], Denmark [34], The Netherlands [41], and Japan [42,47]. There were a few studies in Iran [29], India [44], China [32], and Turkey [36,46]. Most studies recruited women presenting at antenatal clinics [29,37,39,40,41,42,43,46,47,48,49], whilst others recruited women in hospital after birth [32] or from postpartum or public health clinics [44,45]. Two studies did not specify how or when women were originally recruited [33,34] and another did not recruit women, but accessed medical records for all births in a specific region and time [38]. Sample sizes of the original studies ranged from 126 to 91,000 women, but most had <300 women. Inclusion and exclusion criteria varied and were not clear in some studies [36,41,42,46].
Women with PPD
All case-control studies targeted clinical samples as cases [34,44,45]. The largest was originally a national birth cohort of 91,000 women, where cases were women who filled a prescription for an anti-depressive medication within the first year of birth women who had not filled a prescription were controls [34]. Women were classified in another study according to EPDS score (≥10 = cases, <10 = controls) at the study entry, although women were excluded if they appeared to have depression [44]. In the smallest study, cases were not defined, but appeared to be based on a depression questionnaire at enrolment [45].
One cohort only included women who appeared to be at-risk of depression but excluded women with current depressive disorder or anti-depressant medication use [40]. Of the three cohort studies that analyzed their sample as case-controls, two defined cases using EPDS score (≥12 = cases) but excluded women at risk of developing PPD or who underwent prenatal psychiatric care [32,36]. The third study based the definition of cases on a screening tool of depressive symptoms [49]. In all three studies that were not true case-control studies by design, women without PPD (considered as controls) were not matched to cases [32,36,49].
Women without PPD
There were 12 studies conducted in general populations [29,31,33,35,37,38,39,41,42,43,46,47,48], half of which actively excluded women if they were under psychiatric care during their pregnancy or had psychiatric illness [29,39,43,46,48], had a history of depression [43], or if the women had risk factors for developing depression or their infant was admitted to a neonatal intensive care unit [29,45].
3.2.3. Observation Study Vitamin D Exposure Assessment
25OHD in blood is considered to be the most relevant indicator of vitamin D status. Liquid chromatography-tandem mass spectroscopy (LC-MS/MS) [55] is becoming increasingly employed to measure nutrient status in blood and this was the reported method of five studies [31,34,35,37,39]. One study claimed to have analyzed vitamin D metabolite ratio using LC-MS/MS [49] and other mechanisms included rapid direct radioimmunoassay [33,40], chemiluminescence immunoassay [29,43,48], enzyme-linked immunosorbent assay [36,44,45], enzyme immunoassay [41], high performance liquid chromatography [46], and E601 modular analyzer [32]. One of the larger studies did not specify how 25OHD status was measured [38]. Diet is a poor indicator of vitamin D as sunlight is generally the primary source, yet in a Japanese study dietary intake (of fish and eggs but not vitamin D supplements) was used to define vitamin D exposure [42,47].
Blood 25OHD status was measured during early pregnancy [31,38,39,40,43,48], or mid-late pregnancy [29,34,35,36,46,49]. Two studies used cord blood samples [37,39] and another took a fasting maternal blood sample 24–48 h after birth [32]. In most studies, the measure of vitamin D status was prospective, prior to the assessment of depressive symptoms [32,34,35,36,37,40,46,47,48], although some measured both the exposure and outcome simultaneously [29,31,38,40,42,44] and two did not clarify the timing of exposure relative to the outcome measure [43,45]. 25OHD status was quantified differently (nmol/L, nmol/L) in the included studies and of those that explored deficient or insufficient vitamin D, definitions varied [29,32,33,34,35,36,37,39,40,43,44,45,46,48].
3.2.4. Observation Study Depression Outcome Measure and Timing
Clinical diagnosis is the most robust and accurate indication of depression; however, only one study used a tool reportedly capable of diagnosing depressive disorder: the Mini International Neuropsychiatric Interview (MINI) [40]. The MINI is an interview for major depressive disorder and anxiety symptoms, as well as generalized anxiety disorder. Another study defined cases and control based on the use of antidepressant medication [34], which is reasonably robust but would reflect serious depressive disorder and would miss women diagnosed as depressed but not willing to take medication in the perinatal period.
For research purposes, depression is often measured through brief self-completed questionnaires that screen for symptoms. The EPDS was specifically developed to measure symptoms in the postpartum period whilst accounting for common postpartum difficulties such as sleep, but is not recommended for use within 14 days of delivery [52]. The EPDS was the most commonly used tool in the included studies [29,32,33,35,36,37,38,39,44,46,47,48], although two studies inappropriately administered it within one week of birth [35,36], and one of these studies used an abbreviated, unvalidated version [35]. Cut-offs for categorization of PPD varied between studies, from >9 to ≥13.
Several other screening questionnaires were administered, although they were not necessarily designed or validated for use in the perinatal period. The Beck Depression Inventory (BDI) is a 21-item scale of depressive symptomatology that has had minimal validation for use in pregnant and postpartum women. The BDI was administered in two studies and neither specified the cut-off score use to define depression [40,45]. The Center for Epidemiological Studies Depression scale (CES-D) is another 21-item scale not adapted for use in the perinatal period, and is reported to have 60% sensitivity for detecting PPD, hence missing a large proportion of patients with PPD [56]. The CES-D was administered in four studies that all defined depression as a score ≥16 [41,42,43,49]. The Depression, Anxiety, and Stress Scale (DASS) and the Patient Health Questionnaire Depression Module (PHQ) were simultaneously administered in one study [31], although neither are well adapted to pregnancy and the DASS is considered a measure of stress and anxiety as well as depression. All depression screens used were developed in English-speaking Western samples but were translated into Chinese [32], Tamil (India) [44], Turkish [36,41,46], Iranian [29,45], Dutch [41], Arabic [41], and Japanese [42] in the included studies. Furthermore, these screening questionnaires are typically designed to be self-completed directly by the participant but were administered via interview in some studies, which may have influenced the responses [29,45].
Eight studies explored depression during pregnancy [29,31,38,39,40,41,42,43], and 14 studies targeted PPD [32,33,34,35,36,37,39,40,44,45,46,47,48,49], with several cohorts measuring symptoms in both the antenatal [39,42,43] and the postpartum periods [39,40,42,47,48]. In one study, symptoms were measured shortly after birth and likely were more reflective of depression in pregnancy [35].
3.2.5. Observation Study Consideration of Confounders
The most important confounder to consider when exploring vitamin D is that season as sun exposure is the main source of vitamin D. Several studies took season into account by including it as a potential confounder in statistical models [31,33,35,38,40,41,43], but there is only one standardized 25OHD status according to the season at the time of blood draw, which is the most robust method of accounting for the season [37]. Other key confounders that should be considered as a minimum for a study of vitamin D and depression include maternal age, education (and/or other indicators of socio-economic status), history of depression, and multivitamin or vitamin D supplement use. The minimum confounders were accounted for in the statistical models of only one study [37]. Season was absent in more than half of the studies [29,32,34,36,39,42,44,45,46,47,49]. Most studies included some but not all key confounders in their models but with no consistency; one study did not adjust for any key confounders [46], and two studies did not report adjusting for any confounding factors [29,36].
3.2.6. Observation Study Results
Women Considered to Have Depression
Among the studies conducted within samples that were classified as depressed, three case-control studies had different conclusions [34,44,45], as did three studies that analyzed their cohort as a case-control study [32,36,49]. The largest case-control study found that deficient 25OHD status did not increase the likelihood of being a case, but unexpectedly reported an increased likelihood of having PPD when 25OHD status fell into one of the higher categories (>80 nmol/L) [34]. In contrast, the other smaller case-control studies found that cases were more likely to be categorized as deficient [44,45], and have lower mean 25OHD status compared with controls [45].
The three cohorts that compared the 25OHD status of women characterized as depressed and not depressed all found that vitamin D levels were lower in depressed women [32,36,49] and both studies that explored 25(OHD deficiency found that depressed women were more likely to be vitamin D deficient than non-depressed women [32,36].
A small cohort restricted to women at risk of depression detected no statistically significant increase in depressive symptoms with continuous 25OHD status, or deficient vitamin D [40].
General Samples
Of the studies in general non-clinical samples, there were eight explorations of continuous 25OHD levels [29,31,37,39,40,41,43,46,48], and nine explorations for deficient or insufficient 25OHD status [33,37,38,39,40,41,42,43,46,47], and a further two that split their sample into quartiles based on 25OHD [31,35]. Two studies with continuous 25OHD identified an increased risk of depressive symptoms as 25OHD decreased [39,41], four found no association [29,31,37,46], and one cohort reported both a negative association and no association depending on the timing of the outcome measure [43,48]. Five studies found 25OHD deficiency or insufficiency increased the risk of having depression [33,38,39,41,43], one found no increased risk (although all women were taking vitamin D supplements during pregnancy) [46], and one found conflicting results depending on whether women were randomized to omega-3 supplements or a placebo and the timing of the outcome measure [37]. PPD at 6 months was not associated with deficient 25OHD and at 6 weeks postpartum there was an increased risk of PPD in deficient women if they received the placebo [37]. Two studies that split the sample according to quartiles of 25OHD levels conversely found no association between vitamin D status and depression [31] and that the lowest quartile (25OHD < 47 nmol/L) had an increased risk of PPD [35].
Higher dietary intake of vitamin D in pregnancy was associated with lower risk of concurrent depressive symptoms [42], but not subsequent PPD [47].
Results according to Key Quality Indicators
When considering the results of the studies that assessed 25OHD using LC-MS/MS, two found no association with depression [31,34], two found a negative association between 25OHD and depression [35,39], and one conducted multiple explorations that were mixed but largely null [37]. The only two studies that used a clinical depression outcome found no increased risk of depression for low 25OHD [34,40]. The study that accounted for all key confounders found no association between PPD and continuous or deficient 25OHD in for the most part, although women with deficient 25OHD who did not receive omega-3 supplements did have an increased risk of PPD at one of the two timepoints assessed [37].
4. Discussion
This is the first systematic review of the evidence on vitamin D and PPD from both RCTs and observational studies. We found the totality of the evidence poor and inconclusive. There were only two RCTs of vitamin D supplementation, and although both claimed a benefit of vitamin D for depressive symptoms, sample sizes were insufficient to provide adequate power and intervention periods were short [29,30]. The observational studies were equally inconclusive, with some reporting a link between vitamin D and depressive symptoms in the antenatal [39,41,42,43] or postpartum period [32,33,35,36,37,39,49], and many detecting no association [29,31,40,46,47,48]. Numerous methodological limitations included insufficient samples, inappropriate exposure or outcome measures, or lack of adjustment for confounders, particularly season, which is known to be a major determinant of 25OHD status [57]. The case of vitamin D and postpartum depression highlights a missed opportunity, in which the limited contradictory observational studies were insufficient to justify including a measure of depressive symptoms in over 30 published RCTs of prenatal vitamin D supplementation [58].
Although there is biological plausibility for a role of 25OHD in the development of depression, the current literature base has been unable to demonstrate this. Our mixed, inconclusive results align with other reviews of vitamin D and depression during or outside of the perinatal period [9,23,24,25,26,59,60,61,62,63,64,65,66,67,68,69,70]. All called for further, high quality research to provide conclusive evidence, and several recommended that vitamin D supplementation only be considered for individuals with deficient 25OHD status [11,18,23,24,25,26,27,68,69,70].
Given that measures of depression in the perinatal period, such as the short self-completed EPDS questionnaire, are simple to administer, we recommend that depression is included as an outcome in current incomplete RCT’s of prenatal vitamin D as well as future trials. Future observational studies exploring associations between 25OHD status should use LC-MS/MS and standardize 25OHD for the season, as well as assessing perinatal depression with a measure suitable for use in the perinatal period, whilst adjusting for the minimum key confounders of age, education (and/or other indicators of socio-economic status), history of depression, and multivitamin or vitamin D supplement use, and ideally, smoking, body mass index, and ethnicity. Further, it would be prudent for any vitamin D RCTs to specifically target women with low or deficient 25OHD rather than a sufficient sample who is unlikely to benefit from additional vitamin D exposure. We advise that future studies of perinatal vitamin D and depressive symptoms strongly consider the PRISMA (Supplementary Materials) and CONSORT statements when designing and conducting studies.
5. Conclusions
The currently available evidence from RCTs, cohort studies, and case-control studies are insufficient to establish a role of vitamin D in the pathophysiology, prevention, or treatment of depression in the perinatal period.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu14112300/s1, PRISMA 2020 Checklist.
Author Contributions
Conceptualization, J.F.G.; methodology, J.F.G., R.A.G., T.J.G. and M.M.; data curation, J.F.G.; writing—original draft preparation, J.F.G.; writing—review and editing, J.F.G., R.A.G., T.J.G. and M.M.; supervision, R.A.G., T.J.G. and M.M.; project administration, J.F.G. All authors have read and agreed to the published version of the manuscript.
Funding
M.M. was supported by the Australian National Health and Medical Research Council (NHMRC) fellowship: M.M. (Principal Research Fellow APP1061704). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Gibson and Makrides report serving on board for Trajan Nutrition until 2020.
References
- Gavin, N.I.; Gaynes, B.N.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal depression: A systematic review of prevalence and incidence. Obs. Gynecol. 2005, 106, 1071–1083. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Gavin, N.; Meltzer-Brody, S.; Lohr, K.N.; Swinson, T.; Gartlehner, G.; Brody, S.; Miller, W.C. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid. Rep./Technol. Assess. (Summ.) 2005, 119, 1–8. [Google Scholar]
- Dennis, C.L.; Heaman, M.; Vigod, S. Epidemiology of postpartum depressive symptoms among Canadian women: Regional and national results from a cross-sectional survey. Can. J. Psychiatry Rev. Can. Psychiatr. 2012, 57, 537–546. [Google Scholar] [CrossRef]
- Conroy, S.; Pariante, C.M.; Marks, M.N.; Davies, H.A.; Farrelly, S.; Schacht, R.; Moran, P. Maternal psychopathology and infant development at 18 months: The impact of maternal personality disorder and depression. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 51–61. [Google Scholar] [CrossRef]
- Zhu, P.; Sun, M.S.; Hao, J.H.; Chen, Y.J.; Jiang, X.M.; Tao, R.X.; Huang, K.; Tao, F.B. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Dev. Med. Child. Neurol. 2014, 56, 283–289. [Google Scholar] [CrossRef]
- Field, T. Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant. Behav. Dev. 2010, 33, 1–6. [Google Scholar] [CrossRef]
- Beck, C.T. Predictors of postpartum depression: An update. Nurs. Res. 2001, 50, 275–285. [Google Scholar] [CrossRef]
- Bobo, W.V.; Yawn, B.P. Concise review for physicians and other clinicians: Postpartum depression. Mayo Clin. Proc. 2014, 89, 835–844. [Google Scholar] [CrossRef]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Murphy, P.K.; Wagner, C.L. Vitamin D and mood disorders among women: An integrative review. J. Midwifery Women’s Health 2008, 53, 440–446. [Google Scholar] [CrossRef]
- Fallah, M.; Askari, G.; Asemi, Z. Is Vitamin D Status Associated with Depression, Anxiety and Sleep Quality in Pregnancy: A Systematic Review. Adv. Biomed. Res. 2020, 9, 32. [Google Scholar] [CrossRef]
- Munns, C.; Zacharin, M.R.; Rodda, C.P.; Batch, J.A.; Morely, R.; Cranswick, N.E.; Craig, M.E.; Cutfield, W.S.; Hofman, P.L.; Taylor, B.J.; et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: A consensus statement. Med. J. Aust. 2006, 185, 268–272. [Google Scholar] [CrossRef]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends. Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1a-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutr. Rev. 2009, 67, 481–492. [Google Scholar] [CrossRef]
- Spedding, S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. Early-Life Effects of Vitamin D: A Focus on Pregnancy and Lactation. Ann. Nutr. Metab. 2020, 76 (Suppl. 2), 16–28. [Google Scholar] [CrossRef]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of Maternal Vitamin D Deficiency with Pregnancy and Neonatal Complications in Developing Countries: A Systematic Review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. N. Am. 2017, 46, 845–870. [Google Scholar] [CrossRef]
- Dennis, C.L.; Dowswell, T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst. Rev. 2013, 2, CD001134. [Google Scholar] [CrossRef]
- Dennis, C.L.; Dowswell, T. Interventions (other than pharmacological, psychosocial or psychological) for treating antenatal depression. Cochrane Database Syst. Rev. 2013, 7, CD006795. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Meyer, J.H. Promising leads and pitfalls: A review of dietary supplements and hormone treatments to prevent postpartum blues and postpartum depression. Arch. Womens Ment. Health 2021, 24, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Best, K.; Makrides, M. Perinatal nutrition interventions and post-partum depressive symptoms. J. Affect. Disord. 2017, 224, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Letourneau, N.; Mahinpey, N.; Cosic, N.; Giesbrecht, G. Vitamin D Deficiency and Antenatal and Postpartum Depression: A Systematic Review. Nutrients 2018, 10, 478. [Google Scholar] [CrossRef]
- Trujillo, J.; Vieira, M.C.; Lepsch, J.; Rebelo, F.; Poston, L.; Pasupathy, D.; Kac, G. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J. Affect. Disord. 2018, 232, 185–203. [Google Scholar] [CrossRef]
- Wang, J.; Liu, N.; Sun, W.; Chen, D.; Zhao, J.; Zhang, W. Association between vitamin D deficiency and antepartum and postpartum depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gynecol. Obs. 2018, 298, 1045–1059. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obs. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2009, 8, 336–341. [Google Scholar] [CrossRef]
- Vaziri, F.; Nasiri, S.; Tavana, Z.; Dabbaghmanesh, M.H.; Sharif, F.; Jafari, P. A randomized controlled trial of vitamin D supplementation on perinatal depression: In Iranian pregnant mothers. BMC Pregnancy Childbirth 2016, 16, 239. [Google Scholar] [CrossRef]
- Amini, S.; Amani, R.; Jafarirad, S.; Cheraghian, B.; Sayyah, M.; Hemmati, A.A. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: A randomized double-blind clinical trial. Nutr. Neurosci. 2020, 25, 22–32. [Google Scholar] [CrossRef]
- Huang, J.Y.; Arnold, D.; Qiu, C.F.; Miller, R.S.; Williams, M.A.; Enquobahrie, D.A. Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J. Women’s Health (2002) 2014, 23, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.W.; Liu, J.T.; Tu, W.J.; Yang, J.Q.; Cao, Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG 2014, 122, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.K.; Mueller, M.; Hulsey, T.C.; Ebeling, M.D.; Wagner, C.L. An exploratory study of postpartum depression and vitamin d. J. Am. Psychiatr. Nurses Assoc. 2010, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.O.; Strom, M.; Boyd, H.A.; Andersen, E.W.; Wohlfahrt, J.; Lundqvist, M.; Cohen, A.; Hougaard, D.M.; Melbye, M. Vitamin D status during pregnancy and the risk of subsequent postpartum depression: A case-control study. PLoS ONE 2013, 8, e80686. [Google Scholar] [CrossRef]
- Robinson, M.; Whitehouse, A.J.; Newnham, J.P.; Gorman, S.; Jacoby, P.; Holt, B.J.; Serralha, M.; Tearne, J.E.; Holt, P.G.; Hart, P.H.; et al. Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch. Womens Ment. Health 2014, 17, 213–219. [Google Scholar] [CrossRef]
- Gur, E.B.; Gokduman, A.; Turan, G.A.; Tatar, S.; Hepyilmaz, I.; Zengin, E.B.; Eskicioglu, F.; Guclu, S. Mid-pregnancy vitamin D levels and postpartum depression. Eur. J. Obs. Gynecol. Reprod. Biol. 2014, 179, 110–116. [Google Scholar] [CrossRef]
- Gould, J.F.; Anderson, A.J.; Yelland, L.N.; Smithers, L.G.; Skeaff, C.M.; Gibson, R.A.; Makrides, M. Association of cord blood vitamin D at delivery with postpartum depression in Australian women. Aust. N. Z. J. Obs. Gynaecol. 2015, 55, 446–452. [Google Scholar] [CrossRef]
- Jani, R.; Knight-Agarwal, C.R.; Bloom, M.; Takito, M.Y. The Association Between Pre-Pregnancy Body Mass Index, Perinatal Depression and Maternal Vitamin D Status: Findings from an Australian Cohort Study. Int. J. Womens Health 2020, 12, 213–219. [Google Scholar] [CrossRef]
- Lamb, A.R.; Lutenbacher, M.; Wallston, K.A.; Pepkowitz, S.H.; Holmquist, B.; Hobel, C.J. Vitamin D deficiency and depressive symptoms in the perinatal period. Arch. Womens Ment. Health 2018, 21, 745–755. [Google Scholar] [CrossRef]
- Williams, J.A.; Romero, V.C.; Clinton, C.M.; Vazquez, D.M.; Marcus, S.M.; Chilimigras, J.L.; Hamilton, S.E.; Allbaugh, L.J.; Vahratian, A.M.; Schrader, R.M.; et al. Vitamin D levels and perinatal depressive symptoms in women at risk: A secondary analysis of the mothers, omega-3, and mental health study. BMC Pregnancy Childbirth 2016, 16, 203. [Google Scholar] [CrossRef]
- Brandenbarg, J.; Vrijkotte, T.G.; Goedhart, G.; van Eijsden, M. Maternal early-pregnancy vitamin D status is associated with maternal depressive symptoms in the Amsterdam Born Children and Their Development cohort. Psychosom. Med. 2012, 74, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Dietary vitamin D intake and prevalence of depressive symptoms during pregnancy in Japan. Nutrition 2015, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Bushrow, A.E.; Peters, R.M.; Johnson, D.A.; Li, J.; Rao, D.S. Vitamin D nutritional status and antenatal depressive symptoms in African American women. J. Women’s Health (2002) 2012, 21, 1189–1195. [Google Scholar] [CrossRef]
- Pillai, R.R.; Premkumar, N.R.; Kattimani, S.; Sagili, H.; Wilson, A.B.; Sharon, L.; Rajendiran, S. Reduced Maternal Serum Total, Free and Bioavailable Vitamin D Levels and its Association with the Risk for Postpartum Depressive Symptoms. Arch. Med. Res. 2021, 52, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Abedi, P.; Bovayri, M.; Fakhri, A.; Jahanfar, S. The Relationship Between Vitamin D and Postpartum Depression in Reproductive-Aged Iranian Women. J. Med. Life 2018, 11, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, S.; Kosger, H.; Akcal, B.; Tevrizci, H.; Hizli, D.; Aldemir, S.; Namuslu, M. Is there a Relationship between Postpartum Depression and Inadequate Vitamin D in the Last Trimester? Austin Med. Sci. 2016, 1, 1–6. [Google Scholar]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Furukawa, S.; Arakawa, M. Milk intake during pregnancy is inversely associated with the risk of postpartum depressive symptoms in Japan: The Kyushu Okinawa Maternal and Child Health Study. Nutr. Res. 2016, 36, 907–913. [Google Scholar] [CrossRef]
- Accortt, E.E.; Schetter, C.D.; Peters, R.M.; Cassidy-Bushrow, A.E. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Arch. Womens Ment. Health 2016, 19, 373–383. [Google Scholar] [CrossRef]
- Accortt, E.E.; Arora, C.; Mirocha, J.; Jackman, S.; Liang, R.; Karumanchi, S.A.; Berg, A.H.; Hobel, C.J. Low Prenatal Vitamin D Metabolite Ratio and Subsequent Postpartum Depression Risk. J. Women’s Health (2002) 2021, 30, 113–120. [Google Scholar] [CrossRef]
- Arnold, D. Early Maternal Vitamin D Concentrations in Relation to Gestational Diabetes Mellitus, Mood or Anxiety Disorders, and Preeclampsia; University of Washington: Washington, DC, USA, 2013. [Google Scholar]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Murray, L.; Carothers, A.D. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br. J. Psychiatry 1990, 157, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, H.; Wake, M. Infant sleep problems and postnatal depression: A community-based study. Pediatrics 2001, 107, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Murray, L. The impact of postnatal depression on infant development. J. Child Psychol. Psychiatry Allied Discip. 1992, 33, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, Z.; Wright, D.J.; Rainbow, S.J. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin. Chem. 2005, 51, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.B.; Cohn, J.F. Prevalence and correlates of postpartum depression in first-time mothers. J. Abnorm. Psychol. 1991, 100, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K. Vitamin D status during pregnancy: Maternal, fetal, and postnatal outcomes. Curr. Opin. Obs. Gynecol. 2011, 23, 422–426. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, Cd008873. [Google Scholar] [CrossRef]
- Okereke, O.I.; Singh, A. The role of vitamin D in the prevention of late-life depression. J. Affect. Disord. 2016, 198, 1–14. [Google Scholar] [CrossRef]
- Allan, G.M.; Cranston, L.; Lindblad, A.; McCormack, J.; Kolber, M.R.; Garrison, S.; Korownyk, C. Vitamin D: A Narrative Review Examining the Evidence for Ten Beliefs. J. Gen. Intern. Med. 2016, 31, 780–791. [Google Scholar] [CrossRef][Green Version]
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef]
- Gowda, U.; Mutowo, M.P.; Smith, B.J.; Wluka, A.E.; Renzaho, A.M. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition 2015, 31, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Föcker, M.; Antel, J.; Ring, S.; Hahn, D.; Kanal, Ö.; Öztürk, D.; Hebebrand, J.; Libuda, L. Vitamin D and mental health in children and adolescents. Eur. Child. Adolesc. Psychiatry 2017, 26, 1043–1066. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.A.; Edmondson, D.; Wasson, L.T.; Falzon, L.; Homma, K.; Ezeokoli, N.; Li, P.; Davidson, K.W. Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom. Med. 2014, 76, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mbuagbaw, L.; Samaan, Z.; Falavigna, M.; Zhang, S.; Adachi, J.D.; Cheng, J.; Papaioannou, A.; Thabane, L. Efficacy of vitamin D supplementation in depression in adults: A systematic review. J. Clin. Endocrinol. Metab. 2014, 99, 757–767. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e01760. [Google Scholar] [CrossRef]
- Szpunar, M.J. Association of antepartum vitamin D deficiency with postpartum depression: A clinical perspective. Public Health Nutr. 2020, 23, 1173–1178. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, S.; Chen, D. Poor vitamin D status and the risk of maternal depression: A dose-response meta-analysis of observational studies. Public Health Nutr. 2021, 24, 2161–2170. [Google Scholar] [CrossRef]
- Ribamar, A.; Almeida, B.; Soares, A.; Peniche, B.; Jesus, P.C.; Cruz, S.; Ramalho, A. Relationship between vitamin D deficiency and both gestational and postpartum depression. Nutr. Hosp. 2020, 37, 1238–1245. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).