A Systematic Review of Vitamin D during Pregnancy and Postnatally and Symptoms of Depression in the Antenatal and Postpartum Period from Randomized Controlled Trials and Observational Studies
Abstract
1. Introduction
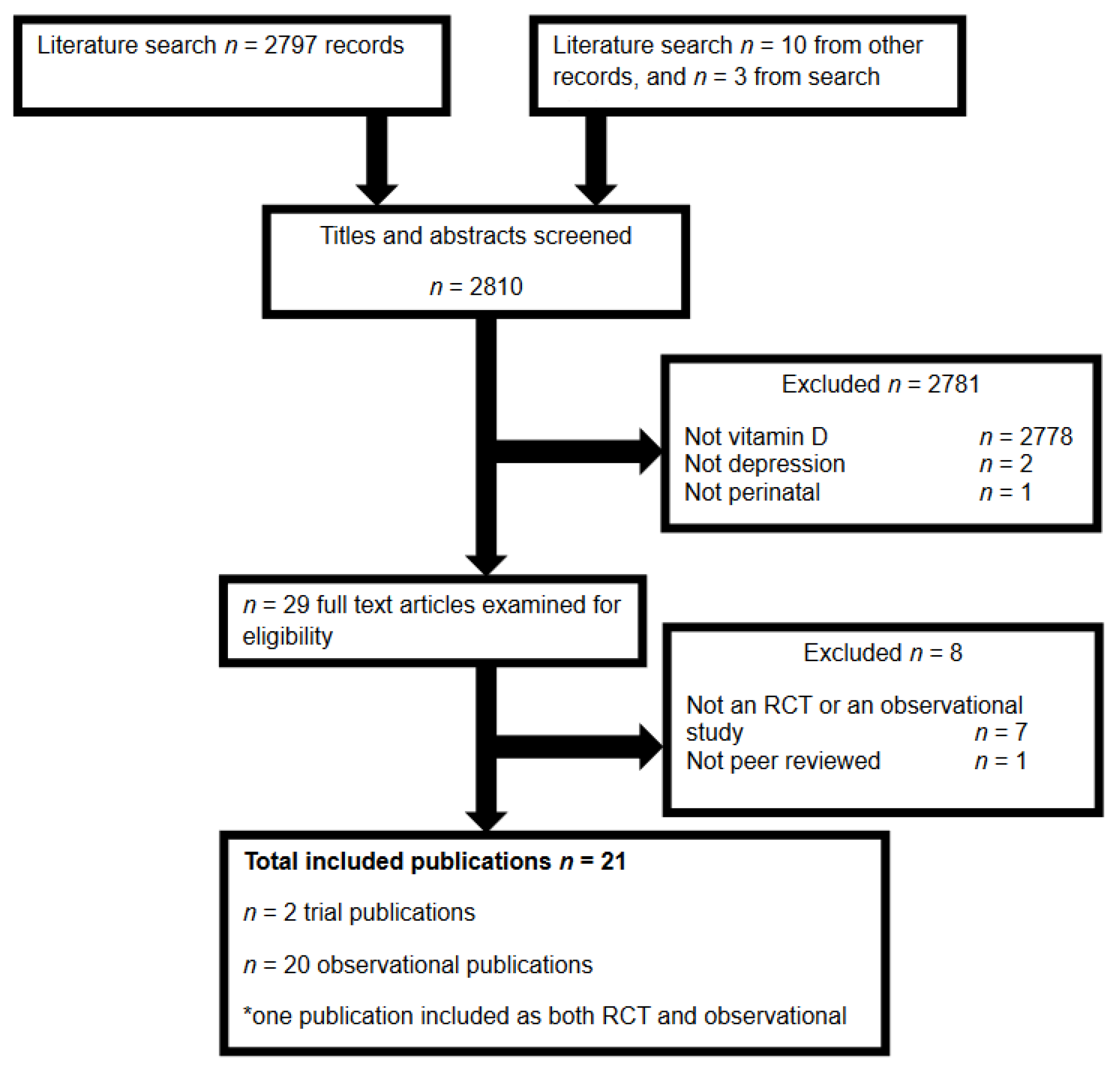
2. Materials and Methods
3. Results
3.1. Trials of Vitamin D and Depression in the Perinatal Period
3.1.1. Trial Design, Randomization, and Blinding
3.1.2. Trial Sample Details
3.1.3. Vitamin D Interventions
3.1.4. Depression Outcome Measure and Timing in Trials
3.1.5. Trial Efficacy
3.2. Observational Studies of Vitamin D and Depression in the Perinatal Period
3.2.1. Observation Study Designs
3.2.2. Observation Study Recruitment and Sample Details
Women with PPD
Women without PPD
3.2.3. Observation Study Vitamin D Exposure Assessment
3.2.4. Observation Study Depression Outcome Measure and Timing
3.2.5. Observation Study Consideration of Confounders
3.2.6. Observation Study Results
Women Considered to Have Depression
General Samples
Results according to Key Quality Indicators
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gavin, N.I.; Gaynes, B.N.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal depression: A systematic review of prevalence and incidence. Obs. Gynecol. 2005, 106, 1071–1083. [Google Scholar] [CrossRef]
- Gaynes, B.N.; Gavin, N.; Meltzer-Brody, S.; Lohr, K.N.; Swinson, T.; Gartlehner, G.; Brody, S.; Miller, W.C. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Evid. Rep./Technol. Assess. (Summ.) 2005, 119, 1–8. [Google Scholar]
- Dennis, C.L.; Heaman, M.; Vigod, S. Epidemiology of postpartum depressive symptoms among Canadian women: Regional and national results from a cross-sectional survey. Can. J. Psychiatry Rev. Can. Psychiatr. 2012, 57, 537–546. [Google Scholar] [CrossRef]
- Conroy, S.; Pariante, C.M.; Marks, M.N.; Davies, H.A.; Farrelly, S.; Schacht, R.; Moran, P. Maternal psychopathology and infant development at 18 months: The impact of maternal personality disorder and depression. J. Am. Acad. Child. Adolesc. Psychiatry 2012, 51, 51–61. [Google Scholar] [CrossRef]
- Zhu, P.; Sun, M.S.; Hao, J.H.; Chen, Y.J.; Jiang, X.M.; Tao, R.X.; Huang, K.; Tao, F.B. Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Dev. Med. Child. Neurol. 2014, 56, 283–289. [Google Scholar] [CrossRef]
- Field, T. Postpartum depression effects on early interactions, parenting, and safety practices: A review. Infant. Behav. Dev. 2010, 33, 1–6. [Google Scholar] [CrossRef]
- Beck, C.T. Predictors of postpartum depression: An update. Nurs. Res. 2001, 50, 275–285. [Google Scholar] [CrossRef]
- Bobo, W.V.; Yawn, B.P. Concise review for physicians and other clinicians: Postpartum depression. Mayo Clin. Proc. 2014, 89, 835–844. [Google Scholar] [CrossRef]
- Anglin, R.E.; Samaan, Z.; Walter, S.D.; McDonald, S.D. Vitamin D deficiency and depression in adults: Systematic review and meta-analysis. Br. J. Psychiatry 2013, 202, 100–107. [Google Scholar] [CrossRef]
- Murphy, P.K.; Wagner, C.L. Vitamin D and mood disorders among women: An integrative review. J. Midwifery Women’s Health 2008, 53, 440–446. [Google Scholar] [CrossRef]
- Fallah, M.; Askari, G.; Asemi, Z. Is Vitamin D Status Associated with Depression, Anxiety and Sleep Quality in Pregnancy: A Systematic Review. Adv. Biomed. Res. 2020, 9, 32. [Google Scholar] [CrossRef]
- Munns, C.; Zacharin, M.R.; Rodda, C.P.; Batch, J.A.; Morely, R.; Cranswick, N.E.; Craig, M.E.; Cutfield, W.S.; Hofman, P.L.; Taylor, B.J.; et al. Prevention and treatment of infant and childhood vitamin D deficiency in Australia and New Zealand: A consensus statement. Med. J. Aust. 2006, 185, 268–272. [Google Scholar] [CrossRef]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends. Endocrinol. Metab. 2002, 13, 100–105. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the Vitamin D receptor and 1a-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Bertone-Johnson, E.R. Vitamin D and the occurrence of depression: Causal association or circumstantial evidence? Nutr. Rev. 2009, 67, 481–492. [Google Scholar] [CrossRef]
- Spedding, S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. Early-Life Effects of Vitamin D: A Focus on Pregnancy and Lactation. Ann. Nutr. Metab. 2020, 76 (Suppl. 2), 16–28. [Google Scholar] [CrossRef]
- van der Pligt, P.; Willcox, J.; Szymlek-Gay, E.A.; Murray, E.; Worsley, A.; Daly, R.M. Associations of Maternal Vitamin D Deficiency with Pregnancy and Neonatal Complications in Developing Countries: A Systematic Review. Nutrients 2018, 10, 640. [Google Scholar] [CrossRef]
- van Schoor, N.; Lips, P. Global Overview of Vitamin D Status. Endocrinol. Metab. Clin. N. Am. 2017, 46, 845–870. [Google Scholar] [CrossRef]
- Dennis, C.L.; Dowswell, T. Psychosocial and psychological interventions for preventing postpartum depression. Cochrane Database Syst. Rev. 2013, 2, CD001134. [Google Scholar] [CrossRef]
- Dennis, C.L.; Dowswell, T. Interventions (other than pharmacological, psychosocial or psychological) for treating antenatal depression. Cochrane Database Syst. Rev. 2013, 7, CD006795. [Google Scholar] [CrossRef] [PubMed]
- Dowlati, Y.; Meyer, J.H. Promising leads and pitfalls: A review of dietary supplements and hormone treatments to prevent postpartum blues and postpartum depression. Arch. Womens Ment. Health 2021, 24, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Gould, J.F.; Best, K.; Makrides, M. Perinatal nutrition interventions and post-partum depressive symptoms. J. Affect. Disord. 2017, 224, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Aghajafari, F.; Letourneau, N.; Mahinpey, N.; Cosic, N.; Giesbrecht, G. Vitamin D Deficiency and Antenatal and Postpartum Depression: A Systematic Review. Nutrients 2018, 10, 478. [Google Scholar] [CrossRef]
- Trujillo, J.; Vieira, M.C.; Lepsch, J.; Rebelo, F.; Poston, L.; Pasupathy, D.; Kac, G. A systematic review of the associations between maternal nutritional biomarkers and depression and/or anxiety during pregnancy and postpartum. J. Affect. Disord. 2018, 232, 185–203. [Google Scholar] [CrossRef]
- Wang, J.; Liu, N.; Sun, W.; Chen, D.; Zhao, J.; Zhang, W. Association between vitamin D deficiency and antepartum and postpartum depression: A systematic review and meta-analysis of longitudinal studies. Arch. Gynecol. Obs. 2018, 298, 1045–1059. [Google Scholar] [CrossRef]
- Pérez-López, F.R.; Pilz, S.; Chedraui, P. Vitamin D supplementation during pregnancy: An overview. Curr. Opin. Obs. Gynecol. 2020, 32, 316–321. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2009, 8, 336–341. [Google Scholar] [CrossRef]
- Vaziri, F.; Nasiri, S.; Tavana, Z.; Dabbaghmanesh, M.H.; Sharif, F.; Jafari, P. A randomized controlled trial of vitamin D supplementation on perinatal depression: In Iranian pregnant mothers. BMC Pregnancy Childbirth 2016, 16, 239. [Google Scholar] [CrossRef]
- Amini, S.; Amani, R.; Jafarirad, S.; Cheraghian, B.; Sayyah, M.; Hemmati, A.A. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: A randomized double-blind clinical trial. Nutr. Neurosci. 2020, 25, 22–32. [Google Scholar] [CrossRef]
- Huang, J.Y.; Arnold, D.; Qiu, C.F.; Miller, R.S.; Williams, M.A.; Enquobahrie, D.A. Association of serum vitamin D with symptoms of depression and anxiety in early pregnancy. J. Women’s Health (2002) 2014, 23, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.W.; Liu, J.T.; Tu, W.J.; Yang, J.Q.; Cao, Y. Association between serum 25-hydroxyvitamin D levels measured 24 hours after delivery and postpartum depression. BJOG 2014, 122, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.K.; Mueller, M.; Hulsey, T.C.; Ebeling, M.D.; Wagner, C.L. An exploratory study of postpartum depression and vitamin d. J. Am. Psychiatr. Nurses Assoc. 2010, 16, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.O.; Strom, M.; Boyd, H.A.; Andersen, E.W.; Wohlfahrt, J.; Lundqvist, M.; Cohen, A.; Hougaard, D.M.; Melbye, M. Vitamin D status during pregnancy and the risk of subsequent postpartum depression: A case-control study. PLoS ONE 2013, 8, e80686. [Google Scholar] [CrossRef]
- Robinson, M.; Whitehouse, A.J.; Newnham, J.P.; Gorman, S.; Jacoby, P.; Holt, B.J.; Serralha, M.; Tearne, J.E.; Holt, P.G.; Hart, P.H.; et al. Low maternal serum vitamin D during pregnancy and the risk for postpartum depression symptoms. Arch. Womens Ment. Health 2014, 17, 213–219. [Google Scholar] [CrossRef]
- Gur, E.B.; Gokduman, A.; Turan, G.A.; Tatar, S.; Hepyilmaz, I.; Zengin, E.B.; Eskicioglu, F.; Guclu, S. Mid-pregnancy vitamin D levels and postpartum depression. Eur. J. Obs. Gynecol. Reprod. Biol. 2014, 179, 110–116. [Google Scholar] [CrossRef]
- Gould, J.F.; Anderson, A.J.; Yelland, L.N.; Smithers, L.G.; Skeaff, C.M.; Gibson, R.A.; Makrides, M. Association of cord blood vitamin D at delivery with postpartum depression in Australian women. Aust. N. Z. J. Obs. Gynaecol. 2015, 55, 446–452. [Google Scholar] [CrossRef]
- Jani, R.; Knight-Agarwal, C.R.; Bloom, M.; Takito, M.Y. The Association Between Pre-Pregnancy Body Mass Index, Perinatal Depression and Maternal Vitamin D Status: Findings from an Australian Cohort Study. Int. J. Womens Health 2020, 12, 213–219. [Google Scholar] [CrossRef]
- Lamb, A.R.; Lutenbacher, M.; Wallston, K.A.; Pepkowitz, S.H.; Holmquist, B.; Hobel, C.J. Vitamin D deficiency and depressive symptoms in the perinatal period. Arch. Womens Ment. Health 2018, 21, 745–755. [Google Scholar] [CrossRef]
- Williams, J.A.; Romero, V.C.; Clinton, C.M.; Vazquez, D.M.; Marcus, S.M.; Chilimigras, J.L.; Hamilton, S.E.; Allbaugh, L.J.; Vahratian, A.M.; Schrader, R.M.; et al. Vitamin D levels and perinatal depressive symptoms in women at risk: A secondary analysis of the mothers, omega-3, and mental health study. BMC Pregnancy Childbirth 2016, 16, 203. [Google Scholar] [CrossRef]
- Brandenbarg, J.; Vrijkotte, T.G.; Goedhart, G.; van Eijsden, M. Maternal early-pregnancy vitamin D status is associated with maternal depressive symptoms in the Amsterdam Born Children and Their Development cohort. Psychosom. Med. 2012, 74, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Arakawa, M. Dietary vitamin D intake and prevalence of depressive symptoms during pregnancy in Japan. Nutrition 2015, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Cassidy-Bushrow, A.E.; Peters, R.M.; Johnson, D.A.; Li, J.; Rao, D.S. Vitamin D nutritional status and antenatal depressive symptoms in African American women. J. Women’s Health (2002) 2012, 21, 1189–1195. [Google Scholar] [CrossRef]
- Pillai, R.R.; Premkumar, N.R.; Kattimani, S.; Sagili, H.; Wilson, A.B.; Sharon, L.; Rajendiran, S. Reduced Maternal Serum Total, Free and Bioavailable Vitamin D Levels and its Association with the Risk for Postpartum Depressive Symptoms. Arch. Med. Res. 2021, 52, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Abedi, P.; Bovayri, M.; Fakhri, A.; Jahanfar, S. The Relationship Between Vitamin D and Postpartum Depression in Reproductive-Aged Iranian Women. J. Med. Life 2018, 11, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, S.; Kosger, H.; Akcal, B.; Tevrizci, H.; Hizli, D.; Aldemir, S.; Namuslu, M. Is there a Relationship between Postpartum Depression and Inadequate Vitamin D in the Last Trimester? Austin Med. Sci. 2016, 1, 1–6. [Google Scholar]
- Miyake, Y.; Tanaka, K.; Okubo, H.; Sasaki, S.; Furukawa, S.; Arakawa, M. Milk intake during pregnancy is inversely associated with the risk of postpartum depressive symptoms in Japan: The Kyushu Okinawa Maternal and Child Health Study. Nutr. Res. 2016, 36, 907–913. [Google Scholar] [CrossRef]
- Accortt, E.E.; Schetter, C.D.; Peters, R.M.; Cassidy-Bushrow, A.E. Lower prenatal vitamin D status and postpartum depressive symptomatology in African American women: Preliminary evidence for moderation by inflammatory cytokines. Arch. Womens Ment. Health 2016, 19, 373–383. [Google Scholar] [CrossRef]
- Accortt, E.E.; Arora, C.; Mirocha, J.; Jackman, S.; Liang, R.; Karumanchi, S.A.; Berg, A.H.; Hobel, C.J. Low Prenatal Vitamin D Metabolite Ratio and Subsequent Postpartum Depression Risk. J. Women’s Health (2002) 2021, 30, 113–120. [Google Scholar] [CrossRef]
- Arnold, D. Early Maternal Vitamin D Concentrations in Relation to Gestational Diabetes Mellitus, Mood or Anxiety Disorders, and Preeclampsia; University of Washington: Washington, DC, USA, 2013. [Google Scholar]
- Cox, J.L.; Holden, J.M.; Sagovsky, R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. Br. J. Psychiatry 1987, 150, 782–786. [Google Scholar] [CrossRef]
- Murray, L.; Carothers, A.D. The validation of the Edinburgh Post-natal Depression Scale on a community sample. Br. J. Psychiatry 1990, 157, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Hiscock, H.; Wake, M. Infant sleep problems and postnatal depression: A community-based study. Pediatrics 2001, 107, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Murray, L. The impact of postnatal depression on infant development. J. Child Psychol. Psychiatry Allied Discip. 1992, 33, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Maunsell, Z.; Wright, D.J.; Rainbow, S.J. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin. Chem. 2005, 51, 1683–1690. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.B.; Cohn, J.F. Prevalence and correlates of postpartum depression in first-time mothers. J. Abnorm. Psychol. 1991, 100, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K. Vitamin D status during pregnancy: Maternal, fetal, and postnatal outcomes. Curr. Opin. Obs. Gynecol. 2011, 23, 422–426. [Google Scholar] [CrossRef]
- Palacios, C.; Kostiuk, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst. Rev. 2019, 7, Cd008873. [Google Scholar] [CrossRef]
- Okereke, O.I.; Singh, A. The role of vitamin D in the prevention of late-life depression. J. Affect. Disord. 2016, 198, 1–14. [Google Scholar] [CrossRef]
- Allan, G.M.; Cranston, L.; Lindblad, A.; McCormack, J.; Kolber, M.R.; Garrison, S.; Korownyk, C. Vitamin D: A Narrative Review Examining the Evidence for Ten Beliefs. J. Gen. Intern. Med. 2016, 31, 780–791. [Google Scholar] [CrossRef][Green Version]
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef]
- Gowda, U.; Mutowo, M.P.; Smith, B.J.; Wluka, A.E.; Renzaho, A.M. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition 2015, 31, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Föcker, M.; Antel, J.; Ring, S.; Hahn, D.; Kanal, Ö.; Öztürk, D.; Hebebrand, J.; Libuda, L. Vitamin D and mental health in children and adolescents. Eur. Child. Adolesc. Psychiatry 2017, 26, 1043–1066. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.A.; Edmondson, D.; Wasson, L.T.; Falzon, L.; Homma, K.; Ezeokoli, N.; Li, P.; Davidson, K.W. Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom. Med. 2014, 76, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Mbuagbaw, L.; Samaan, Z.; Falavigna, M.; Zhang, S.; Adachi, J.D.; Cheng, J.; Papaioannou, A.; Thabane, L. Efficacy of vitamin D supplementation in depression in adults: A systematic review. J. Clin. Endocrinol. Metab. 2014, 99, 757–767. [Google Scholar] [CrossRef]
- Saggese, G.; Vierucci, F.; Prodam, F.; Cardinale, F.; Cetin, I.; Chiappini, E.; De’ Angelis, G.L.; Massari, M.; Miraglia Del Giudice, E.; Miraglia Del Giudice, M.; et al. Vitamin D in pediatric age: Consensus of the Italian Pediatric Society and the Italian Society of Preventive and Social Pediatrics, jointly with the Italian Federation of Pediatricians. Ital. J. Pediatr. 2018, 44, 51. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e01760. [Google Scholar] [CrossRef]
- Szpunar, M.J. Association of antepartum vitamin D deficiency with postpartum depression: A clinical perspective. Public Health Nutr. 2020, 23, 1173–1178. [Google Scholar] [CrossRef]
- Tan, Q.; Liu, S.; Chen, D. Poor vitamin D status and the risk of maternal depression: A dose-response meta-analysis of observational studies. Public Health Nutr. 2021, 24, 2161–2170. [Google Scholar] [CrossRef]
- Ribamar, A.; Almeida, B.; Soares, A.; Peniche, B.; Jesus, P.C.; Cruz, S.; Ramalho, A. Relationship between vitamin D deficiency and both gestational and postpartum depression. Nutr. Hosp. 2020, 37, 1238–1245. [Google Scholar] [CrossRef]
| Author, Year Sample, Design, Setting | Vitamin D Intervention | PPD Measure and Definition | Results and Limitations |
|---|---|---|---|
| Vaziri, 2016 [29] Iran n = 169, healthy women >18, singleton pregnancy 26–28 wks gestation Excluded women with a history of mental illness, EPDS > 13, substance abuse, pregnancy complications Design: single-blinded RCT Primary outcome: NR Recruited at prenatal hospital | Duration: 26–28 wks gestation until birth Trt n = 78; 50 µg vitamin D3/day Ctrl n = 75; placebo | EPDS (Iranian version, via interview) at 38–40 wks gestation EPDS (Iranian version, via interview) at 4 and 8 wks PP Depression = EPDS > 13 | N = 136 (80%) 38–40 wks: Trt depression < Ctrl 4 wks PP: Trt depression < Ctrl 8 wks PP: Trt depression < Ctrl Limitations: small sample, analyses per protocol not intention to treat (actively excluded women who took supplements irregularly or ceased supplements), EPDS conducted via interview, blinding of staff and participants unclear, randomization methods unclear, single-blinded study only, many women taking daily prenatal multivitamin with 200–400 IU vitamin D, participants apparently unlikely to develop depression at enrollment, no primary outcome specified |
| Amini, 2020 [30] Iran, n = 81 women aged 18–45 yrs, EPDS > 12 Excluded BMI ≥ 35, 25OHD status > 75 nmol/L, previous history of depression or other mental disorder, antidepressant use Design: double-blinded RCT Primary outcome: PPD and serum 25OHD Recruited at psychiatric outpatient clinic | Duration: 8 wks PP (exact timing of intervention commencement NR) Trt1 n = 27; oral 1250 µg vitamin D3/fortnight + 500 mg calcium carbonate/day Trt2 n = 27; oral 1250 µg vitamin D3/fortnight + placebo/day Ctrl n = 27; placebo | EPDS (Iranian version, via interview) at end of intervention |PPD = EPDS ≥ 12 | N = 76 (94%) Trt1 and Trt2 PPD < Ctrl Limitations: small sample, EPDS conducted via interview, calcium supplement group (Trt1) combined with vitamin D only group (Trt2) to compare to controls, randomization methods unclear |
| Author, Year Setting, Sample | Vitamin D Measure and Classification | PPD Measure and Definition Confounders | Results and Limitations |
|---|---|---|---|
| Murphy, 2010 [33] U.S.A.: prospective cohort (original study design NR) N = NR, women taking 20, 2400 or 6400 IU vitamin D/day Excluded births <35 wks gestation, pre-existing diabetes or a multiple birth Recruitment setting NR | Sample: serum 25OHD monthly from 4–6 wks PP to 7 mo PP Sample analysis: Radioimmunoassay Sufficient: ≥80 nmol/L Insufficient: 50–≤80 nmol/L Deficient: ≤50 nmol/L | EPDS (English and Spanish versions) measured monthly from 4–6 wks PP to 7 mo PP PPD = EPDS > 9 Confounders: season, age, education, infant sex, marital status, insurance status, infant feeding method, vitamin D dose, planned pregnancy | N = 97 (%NR) Continuous: NA Categorical: Low PP serum 25OHD increased risk of PPD during the first 7 mo PP Limitations: small likely underpowered sample, women provided with vitamin D supplements at 3 doses and sample appeared to be drawn from a dose-response trial, did not include confounders’ history of depression |
| Cassidy–Bushrow, 2012 [43] U.S.A.: cohort (original study design NR) N = 203, African-American women who spoke and read English and were in their second trimester Excluded illicit drug use, psychiatric illness Hospital obstetric clinic recruitment | Sample: serum 25OHD at first trimester (mean 9.5 wks gestation) Sample analysis: chemiluminescence immunoassay Sufficient: >50 nmol/L Insufficient: 30-50 nmol/L Deficient: <30 nmol/L | CES-D during pregnancy (timing NR) Depression = CES-D ≥ 16 Confounders: season, education, marital status, days between exposure and outcome | N = 178 (88%NR) Continuous: low 25OHD status increased risk of depression Categorical: deficient 25OHD increased risk of depression Limitations: small likely underpowered sample, women provided with vitamin D supplements with higher doses prescribed after exposure measure, did not include confounders’ history of depression, age, or supplement use |
| Brandenbarg, 2012 [41] Netherland: prospective cohort (pregnancy and child cohort) N = 8266, inclusive of race and language spoken First antenatal clinic visit recruitment | Sample: serum 25OHD at early-pregnancy (median 13 wks gestation) Sample analysis: enzyme immunoassay Sufficient: ≥50 nmol/L Insufficient: 30–49.9 nmol/L Deficient: ≤29.9 nmol/L | CES-D (Dutch, English, Arabic and Turkish version) during pregnancy at 16 wks gestation Depression = CES-D ≥ 16 Confounders: season, age, parity, ethnicity, BMI, smoking, drinking, planned pregnancy, education, cohabitation status, employment status | N = 4101 (50%) Continuous: low 25OHD status increased risk of depression Categorical: deficient 25OHD increased risk of depression Limitations: did not include confounder history of depression |
| Nielsen, 2013 [34] Denmark: case-control (nestled in a birth cohort of 91,000 women) N = 605 with PPD (filled prescription for antidepressant) N = 875 without PPD (no prescription, matched for age and year of recruitment) Singleton pregnancy with live-born infant, excluded women with previous registered mental illness or anti-depressant use in the year prior to giving birthRecruitment setting NR | Sample: serum 25OHD at mid-pregnancy (25 wks gestation) Sample analysis: LC-MS/MS Exposure categorized as <15 nmol/L, 15–24 nmol/L, 25–49 nmol/L, 50–70 nmol/L, 80–99 nmol/L, ≥100 nmol/L | Danish Register of Medicinal Product Statistics at 12 mo PP PPD = prescription for any anti-depressant medication Confounders: season, week of gestation at exposure measure, age, parity, smoking, socioeconomic status, BMI, physical activity, social support, multivitamin supplement use | N = 1480 (%NA) Continuous: NA Categorical: No increased risk of PPD at lower levels, increased risk if 25OHD < 80 nmol/L Limitations: crude measure of depression (severe depression only), did not include confounder history of depression |
| Robinson, 2014 [35] Australia: prospective cohort (pregnancy cohort) N = 2900, Caucasian women Maternity hospital recruitment | Sample: serum 25OHD at early-pregnancy (18 wks gestation) Sample analysis: LC-MS/MS Quartile 1: <47 nmol/L Quartile 2: 47–58 nmol/L Quartile 3: 59–70 nmol/L Quartile 4: >70 nmol/L | EPDS (English, revised to 6 items only) at 3 days PP PPD = EPDS > 6 Confounders: season, age, education, family income, BMI, smoking, drinking, hypertensive disease, infant sex, child admission to special care nursery, birthweight | N = 706 (24%) Continuous: NA Categorical: Low serum 25OHD increased risk of PPD at 3 days Limitations: EPDS used within 1 wk of birth (instead of recommended >14 days), used an unvalidated abbreviated version of EPDS, did not include confounders’ history of depression or supplement use |
| Fu, 2014 [32] China: prospective cohort (PPD cohort) N = 323, women who gave birth to a full-term, singleton Excluded if psychiatric care during pregnancy City hospital recruitment at birth | Sample: serum 25OHD at 24–48 h after delivery Sample analysis: E601 modular analyzer Sufficient: >75 nmol/L Insufficient: 50–75 nmol/L Deficient: <50 nmol/L | EPDS (Chinese version) at 3 mo PP PPD = EPDS ≥ 12 Confounders: age, breastfeeding, stressful life events, education, family income, partner support, planned pregnancy, mode of delivery, previous psychiatric contact | N = 213 (66%) Continuous: 25OHD status higher in women without PPD Categorical: Low 25OHD more likely to have PPD Limitations: small likely underpowered sample, cohort analyzed as case-control, did not include confounders season, or supplement use |
| Huang, 2014 [31] U.S.A: cohort (for pregnancy migraine study) N = 500, women who sought prenatal care prior to 20 wks gestation, spoke English, >18 years Recruitment setting NR | Sample: serum 25OHD at early-pregnancy (mean 15.4 wks gestation) Sample analysis: LC-MS/MS Sufficient: ≥83 nmol/L Insufficient: 51–≤83 nmol/L Deficient: ≤50 nmol/L | DASS-21 and PHQ-9 in early pregnancy (mean 15.4 wks gestation) Depression = DASS ≥ 14 =PHQ-9 ≥ 19 Confounders: season, gestation of exposure, age, BMI, smoking, race, education, marital status | N = 498 (99.6%) Continuous: No association Categorical: No association Limitations: moderate sample size, suboptimal outcome measure, did not include confounders history of depression or supplement use |
| Gur, 2014 [36] Turkey: prospective cohort (community cohort study) N = 687, Normal pregnancy and delivery, Excluded if risk of PPD, or complications with birth or neonate University hospital recruitment | Sample: serum 25OHD at mid-pregnancy (24–28 wks gestation) Sample analysis: enzyme-linked immunosorbent assay Sufficient: >50 nmol/L Mildly deficient: 26–≤50 nmol/L Severely deficient: ≤25 nmol/L | EPDS (Turkish version) at 1 wk, 6 wks and 6 mo PP PPD = EPDS ≥ 12 Confounders: none reported | N = 179 (26%) Continuous: women with PPD had lower 25OHD Categorical: Low serum 25OHD increased risk of PPD at 1 and 6 wks and 6 mo Limitations: small likely underpowered sample, cohort analyzed as case-control, women provided with vitamin D supplements, EPDS used within 1 wk of birth (instead of recommended >14 days), EPDS completed via interview instead of self-completed, did not appear to account for any confounders |
| Gould, 2015 [37] Australia: Prospective, enrolled at ~20 wk gestation (for pregnancy omega-3 trial) N = 2399, singleton pregnancy, healthy women, <20 wks gestation Excluded illicit drug use Hospital antenatal recruitment | Sample: cord blood 25OHD at birthSample analysis: LC-MS/MS Sufficient: >50 nmol/L Insufficient: 25–50 nmol/L Deficient: <25 nmol/L | EPDS (English version) at 6 wks and 6 mo PP PPD = EPDS > 12 Confounders: season, social support, age, race, parity, BMI, education, history of depression, prenatal supplement use, prenatal smoking | N = 1040 (43%) Continuous: No association (6 wks or 6 mo) Categorical: Deficiency at 6 wks increased risk of PPD (in placebo group). No increased risk in pmega-3 group at 6 wks, and no risk at 6 mo (omega-3 or placebo group) Limitations: possible interference of omega-3 intervention |
| Miyake, 2015 [42] Japan: cross-sectional cohort (maternal-child health cohort) N = 1757 women 5–39 wks gestation Obstetric hospital recruitment | Sample: vitamin D intake at 5–39 wks gestation Sample analysis: diet history questionnaire | CES-D (Japanese version) during pregnancy at 5–39 wks gestation Depression = CES-D ≥ 16 Confounders: age, gestation, region, parity, family structure, history of depression, smoking, occupation, family income, education, BMI, intake of saturated fatty acids and omega-3 fatty acids | N = 1745 (99%) Continuous: NA Categorical: Higher dietary vitamin D intake associated with lower risk of depression Limitations: original cohort used to show increased seafood associated with less depression but did not consider this in analyses, did not asses 25OHD status, analyzed dietary intake (mainly as fish and eggs, vitamin D supplements not captured) rather than measuring sun exposure which is main source of vitamin D, dietary patterns likely varied within the 34 week period of diet assessment due to morning sickness in early pregnancy and increased intake in late pregnancy, did not include confounders’ season or supplement use |
| Miyake, 2016 [47] Japan: cross-sectional cohort (maternal-child health cohort, from [42]) N = 1757 women 5-39 wks gestation Obstetric hospital recruitment | Sample: vitamin D dietary intake at 5–39 wks gestation Sample analysis: diet history questionnaire | EPDS at 3–4 mo PP PPD = EPDS ≥ 9 Confounders: age, gestation, region, parity, family structure, history of depression, occupation, education, BMI, smoking, cesarean delivery, infant sex, birth weight, total energy intake | N = 1319 (75%) Continuous: NA Categorical: No association of low dietary vitamin D with PPD Limitations: as above, inconsistent confounders to above |
| Accortt, 2016 [48] U.S.A.: prospective cohort (cohort from [43]) N = 203, African-American women who spoke and read and were in their second trimester Excluded illicit drug use, psychiatric illness Hospital obstetric clinic recruitment | Sample: serum 25OHD at first trimester (mean 9.5 wks gestation) Sample analysis: chemiluminescence immunoassay Sufficient: NR Insufficient: NR Deficient: ≤25 nmol/L, ≤37.5 nmol/L, and ≤75 nmol/L | EPDS at 4-6 wks PP PPD = not defined, used continuous EPDS score Confounders: season, age, education, marital status, history of depression, BMI | N = 91 (45%) Continuous: No association Categorical: No increased risk from deficiency Limitations: designed for exploring combined effect of vitamin D and inflammatory biomarkers, small likely underpowered sample, women provided with vitamin D supplements, did not include confounder supplement use |
| Gunduz, 2016 [46] Turkey: prospective cohort N = 91, women with full-term singleton, took 500 IU vitamin D throughout pregnancy Excluded mental health problems University maternity clinic recruitment | Sample: serum 25OHD at 36 wks gestation Sample analysis: high performance liquid chromatography Sufficient: NA Insufficient: <32 nmol/L Deficient: <20 nmol/L | EPDS at 6 wks PP PPD = EPDS ≥ 10 Confounders: infant crying, relationship with the partner, infant weight gain, feeding type | N = 87 (94%) Continuous: No association Categorical: No increased risk from deficiency Limitations: small likely underpowered sample, women provided with vitamin D supplements, did not include any key confounders |
| Vaziri, 2016 [29] Iran: cross sectional (vitamin D RCT) N = 169, healthy women >18, singleton pregnancy 26–28 wks gestation, living with husband Excluded history of mental illness, EPDS > 13, substance abuse, pregnancy complications Prenatal hospital recruitment | Sample: serum 25OHD 26–28 at wks gestation Sample analysis: chemiluminescence immunoassay | EPDS (Iranian version, via interview) at 26–28 ks gestation Confounders: none reported | N = 136 (80%) Continuous: No association Categorical: NA Limitations: small likely underpowered sample, EPDS completed via interview instead of self-completed, did not appear to account for any confounders |
| Williams, 2016 [40] U.S.A.: prospective cohort (for pregnancy omega-3 trial to prevent depression) N = 126, pregnant women at risk of depression, with singleton pregnancy 12–20 wks gestation Excluded current depression or antidepressant medication use, substance abuse Prenatal clinic recruitment | Sample: serum 25OHD at 12–20 wks and 34–36 wks Sample analysis: radioimmunoassay Sufficient: ≥50 nmol/L Deficient: <50 nmol/L | BDI and MINI at 10–20 wks, 26–28 wks and 34–36 wks gestation, and 6–8 PP PPD = NR Confounders: season, age, smoking, BMI, initiation of antidepressants, omega-3 fatty acid status | N = 105 (83%) Continuous: No association Categorical: No increased risk from deficiency Limitations: small likely underpowered sample, women provided with vitamin D supplements, did not include confounders’ education, supplement use or history of depression |
| Abedi, 2018 [45] Iran: case-control study N = 60 with PPD (definition NR) N = 60 without PPD (definition NR, matched to age and whether taking vitamin D supplements) Women 6–8 wks PP Public health clinic recruitment | Sample: serum 25OHD at PP (timing NR) Sample analysis: enzyme-linked immunosorbent assay Sufficient: >75 nmol/L Deficient: <50 nmol/L | BDI (Iranian version, via interview) at PP (timing NR) Confounders: age, education, husbands’ education, income, BMI | N = 120 Continuous: 25OHD lower among cases Categorical: deficiency more likely in cases Limitations: small likely underpowered sample, cases and controls not defined, did not include confounders’ season, supplement use or history of depression |
| Lamb, 2018 [39] U.S.A.: prospective cohort N = 126 women <25 wks gestation Excluded pre-existing mental conditionObstetric clinic recruitment | Sample: serum 25OHD at early pregnancy (mean 14 wks gestation), and at delivery, and at 6 wks PP Sample: cord blood at birth Sample analysis: LC-MS/MS Sufficient: >75 nmol/L Insufficient: 50-75 nmol/L Deficient: ≤50 nmol/L | EPDS at 14 wks gestation, 32 wks gestation, and at 10 wks PP PPD = EPDS ≥ 10 Confounders: history of depression, supplement use | N = 125 (99%) Continuous: 25OHD status in maternal and cord blood associated with depression Categorical: maternal deficiency associated with increased risk of depression Cord blood: NR Limitations: small likely underpowered sample did not include confounders’ season, age, or education |
| Jani, 2020 [38] Australia: retrospective cross-sectional cohort N = 17,132, all women who gave birth in the target region during the study period Excluded multiple pregnancies and missing key data Recruited via accessing medical records of births in study period | Sample: serum 25OHD at ~14 wks gestation Sample analysis: NR Sufficient: >50 nmo/L Deficient: ≤50 nmol/L | EPDS at 12-14 wks gestation Depression = EPDS ≥ 13 Confounders: season, age, parity, marital status, smoking, birthweight, maternal country of birth, employment status, domestic violence, hypertension during pregnancy | N = 13,805 (81%) Continuous: NA Categorical: maternal deficiency associated with increased risk of depression Limitations: unclear measure of 25OHD, did not include confounders’ supplement use, or history of depression |
| Accortt, 2021 [49] U.S.A.: prospective cohort (analyzed as case vs. control) N = NR, singleton pregnancy, <20 wks gestation Prenatal clinic recruitment | Sample: plasma 25OHD at 18–21 wks gestation Sample analysis: LC-MS/MS in multiple reaction monitoring mode—“vitamin D metabolites” Vitamin D ration ratio of 24,25OHD and 25OHD | CES-D at 6–10 wks PP PPD = CES-D ≥ 16 Confounders: BMI, age, smoking, race, prenatal depression | N = 89 (56% of the 160 with vitamin D status) Continuous: women with PPD had lower vitamin D ratio Categorical: NA Limitations: small likely underpowered sample, cohort analyzed as case-control, analyzed vitamin D metabolite ratio (rather than 25OHD status), did not include confounders’ season or supplement use |
| Pillai, 2021 [44] India: cross-sectional case-control N = 330 cases (EPDS ≥ 10) N = 330 controls (EPDS < 10), matched for age and BMI Excluded women with transient mood changes, postpartum blues, pre-existing depressive symptoms that commenced prior to birth Postpartum clinic recruitment | Sample: serum 25OHD at 6 wks PPSample analysis: enzyme-linked immunosorbent assay Sufficient: >75 nmol/L Deficient: ≤75 nmol/L | EPDS (English or Tamil translation) at 6 wks PP Confounders: age, BMI, socioeconomic status, marriage satisfaction, adverse events during pregnancy, fear of labor, prenatal medical conditions, kangaroo care, child care stress | N = 660 (%NA) Continuous: lower 25OHD status in controls than casesCategorical: cases more likely to be deficient than controls Limitations: women provided with vitamin D supplements, did not include confounders’ season, education, history of depression, or supplement use |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gould, J.F.; Gibson, R.A.; Green, T.J.; Makrides, M. A Systematic Review of Vitamin D during Pregnancy and Postnatally and Symptoms of Depression in the Antenatal and Postpartum Period from Randomized Controlled Trials and Observational Studies. Nutrients 2022, 14, 2300. https://doi.org/10.3390/nu14112300
Gould JF, Gibson RA, Green TJ, Makrides M. A Systematic Review of Vitamin D during Pregnancy and Postnatally and Symptoms of Depression in the Antenatal and Postpartum Period from Randomized Controlled Trials and Observational Studies. Nutrients. 2022; 14(11):2300. https://doi.org/10.3390/nu14112300
Chicago/Turabian StyleGould, Jacqueline F., Robert A. Gibson, Tim J. Green, and Maria Makrides. 2022. "A Systematic Review of Vitamin D during Pregnancy and Postnatally and Symptoms of Depression in the Antenatal and Postpartum Period from Randomized Controlled Trials and Observational Studies" Nutrients 14, no. 11: 2300. https://doi.org/10.3390/nu14112300
APA StyleGould, J. F., Gibson, R. A., Green, T. J., & Makrides, M. (2022). A Systematic Review of Vitamin D during Pregnancy and Postnatally and Symptoms of Depression in the Antenatal and Postpartum Period from Randomized Controlled Trials and Observational Studies. Nutrients, 14(11), 2300. https://doi.org/10.3390/nu14112300







