Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease
Abstract
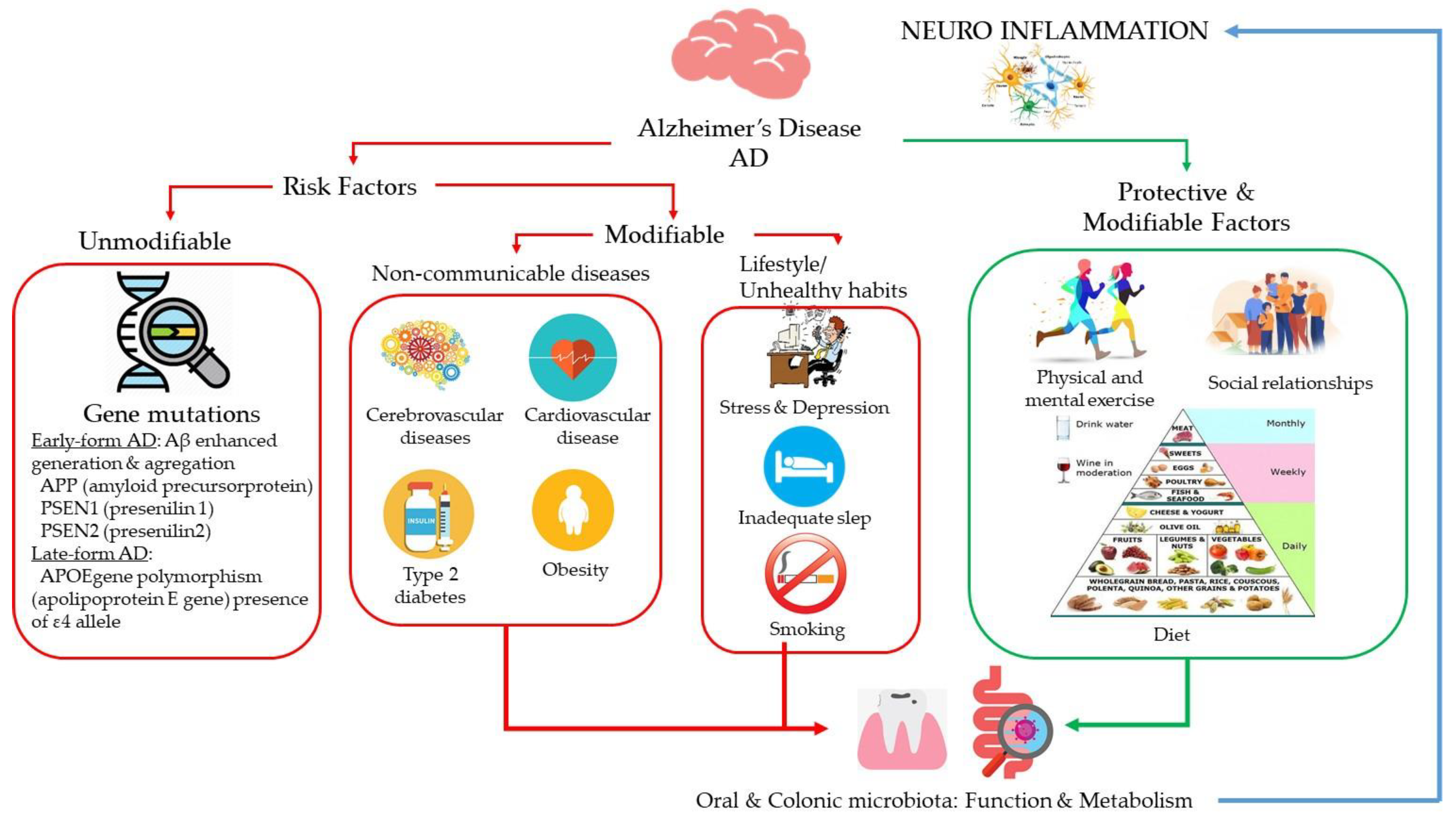
1. Introduction
2. Lifestyle and Dietary Patterns and Alzheimer’s Disease
2.1. Diet
2.2. Alcohol
2.3. Wine
3. Oral and Gut Microbiota in Alzheimer’s Disease
| Study | Design, Aims and Details | Digestive Tract Compartment | Key Findings |
|---|---|---|---|
| Exploring the association between AD, Oral Health, Microbial Endocrinology and Nutrition [104] | Scientific literature review | Oral | Healthy diet based interventions together with improved life style/behavioral changes may reduce and/or delay the incidence of AD. |
| The Microbiome and Disease: Reviewing the Links between the Oral Microbiome, Aging, and Alzheimer’s Disease [31] | Scientific literature review | Oral | Epidemiological and experimental evidence links oral bacteria found in brains and oral bacteria and tumor necrosis factor in blood in AD. Combining human genetic factors with microbiome composition greatly improves the predictive capacity for assessing disease risk. |
| The Possible Causal Link of Periodontitis to Neuropsychiatric Disorders: More Than Psychosocial Mechanisms [105] | Scientific literature review | Oral | Periodontal bacteria/bacterial molecules can directly invade the brain either through the blood stream or via cranial nerves. In periodontitis, a periodontal pocket is filled with periodontal bacteria/bacterial molecules that form biofilms. Oral bacteria are capable of invading an intact pocket epithelium, and gain access to the circulation. |
| Oral microbiota and AD: Do all roads lead to Rome? [81] | Scientific literature review | Oral | Oral microbiota produces inflammatory mediators able to migrate into the bloodstream and affect distant tissues and organs, thus representing a source of neuro-inflammation. |
| Association between chronic periodontitis and the risk of AD: a retrospective, population-based, matched-cohort study [106] | Retrospective matched-cohort study: 9291 patients diagnosed with chronic periodontitis (1997–2004) | Oral | 10-year chronic periodontitis exposure was associated with a 1.707-fold increase in the risk of developing AD. |
| Periodontitis and Cognitive Decline in Alzheimer’s Disease [107] | Six month observational cohort study (n = 60 participants with mild to moderate AD). To determine if periodontitis in AD is associated with both increased dementia severity and cognitive decline. | Oral | Periodontitis is associated with an increased systemic pro inflammatory state, and increase in cognitive decline in AD, independent to baseline cognitive state, which may be mediated through effects on systemic inflammation. |
| Chronic P. gingivalis infection accelerates the occurrence of age-related granules in APOE−/− mice brains [85] | Age-related granules in the apolipoprotein E gene knockout (APOE−/−) B6 background mice brains following chronic gingival infection with P.gingivalis for 24 weeks. | Oral | Periodontal bacterial infection results in injury of the hippocampus, thereby increasing blood-brain barrier permeability to toxic vascular components. Early appearance of age-related granules in APOE−/− mice following inflammation-mediated tissue injury, accompanied by loss of cerebral blood-brain barrier integrity |
| Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue [84] | Postmortem study, identifying the major periodontal disease bacteria components in brain tissue from 12 h postmostem delay (n = 10 AD cases for tissue from brains and 10 non-AD-related control with similar or greater postmortem interval). | Oral | LPS from periodontal bacteria can access the AD brain during life as labeling in the corresponding controls, with equivalent/longer postmortem interval. Demonstration of a known chronic oral-pathogen-related virulence factor reaching the human brains suggests and inflammatory role in the existing AD pathology |
| Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors [32] | Postmortem study, identifying P. gingivalis DNA and gingipains, toxic proteases in AD brains | Oral | Immunohistochemical analyses using tissue microarrays showed that gingipain immunoreactivity in AD brains and that gingipain immunoreactivity significantly correlates with tau and ubiquitin loads and AD diagnosis. Using quantitative Polymerase Chain Reaction, the authors identified P. gingivalis DNA in the AD brains which were lysine gingipain-positive |
| Microbiota and Aging. A Review and Commentary [108] | Scientific literature review | Oral and Intestinal | Oral microbiota is especially important because of the opportunities for access to the brain through the olfactory nerve at the roof of the nose or through the abundant innervations of the oral cavity by the trigeminal and other cranial nerves. Communication in the gut-brain-axis is regulated by many intermediaries including diverse metabolites of the microbiota. Microbial changes have been observed in several geriatric diseases, like AD. Individuals with high frailty scores had a significant reduction on lactobacilli species when compared to non-frail individuals suggesting potential mechanisms by which the microbiota promote human health span and aging. |
| Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): detection of lipopolysaccharide (LPS) in AD hippocampus [93] | Scientific literature review | Intestinal | Presence of gastrointestinal tract microbiome-derived lipopolysaccharide (LPS) in brain lysates from the hippocampus and superior temporal lobe neocortex of AD brains. Presence of bacterial LPS hippocampal cases exhibited up to a 26-fold increase in LPS over age-matched controls. |
| Gut Microbiota and Their Neuroinflammatory Implications in Alzheimer’s Disease [109] | Scientific literature review | Intestinal | Impact of the microbiota of elderly people and the neuro-inflammatory roles they may have in AD, by different mechanisms: (1) role of the intestinal microbiota in homeostatic communication between the microbiota–gut–brain axis; (2) mechanisms of signal dysfunction; and (3) impact of signal dysfunction induced by the microbiota on AD |
| Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels [110] | Triple-transgenic mouse model of AD (3xTg-AD) mice in the early stage of AD were treated with a probiotic formulation, thereby affecting the composition of gut microbiota and its metabolites | Intestinal | Treated mice with a probiotic formulation showed partial restoration of two impaired neuronal proteolytic pathways (the ubiquitin proteasome system and autophagy). Their cognitive decline was decreased compared with controls, due to a reduction in brain damage and reduced accumulation of amyloid beta aggregates. Modulation of the microbiota induces positive effects on neuronal pathways that are able to slow down the progression of AD |
| Transferring the blues: depression-associated gut microbiota induces neuro-behavioral changes in the rat [89] | Thirty four patients with major depression and thirty three matched healthy controls were evaluated for the study of changes in gut microbiota, including fecal microbiota transplantation from depressed patients to microbiota-depleted rats | Intestinal | Fecal microbiota transplantation from depressed patients to microbiota-depleted rats can induce behavioral and physiological features characteristic of depression in the recipient animals, including anhedonia and anxiety-like behaviors, as well as alterations in tryptophan metabolism. |
| Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet [90] | To identify gut microbiota-metabolomics signatures preceding dementia in genetically prone (3xTg-AD) mice | Intestinal | 3xtg mice showed brain hypometabolism typical of pre-demented stage and lacked the physiological bacterial diversity between caecum and colon seen in controls. Cluster analyses revealed distinct profiles of microbiota, and serum and fecal metabolome across groups. Elevation in Firmicutes-to-Bacteroidetes abundance, and exclusive presence of Turicibacteraceae, Christensenellaceae, Anaeroplasmataceae and Ruminococcaceae, and lack of Bifidobacteriaceae, were also observed. Metabolome analysis revealed a deficiency in unsaturated fatty acids and choline, and an overabundance in ketone bodies, lactate, amino acids, trimethylamine and trimethylamine N-oxide in 3xTg-AD mice. These metabolic alterations were correlated with high prevalence of Enterococcaceae, Staphylococcus, Roseburia, Coprobacillus and Dorea, and low prevalence of Bifidobacterium, which, in turn, related to cognitive impairment and cerebral hypometabolism |
| Reduction of Alzheimer’s disease Beta-amyloid pathology in the absence of gut microbiota [111] | Preclinical study: conventionally-raised transgenic APPPS1 mice aged 8-months | Intestinal | In the intestine of conventionally-raised transgenic APPPS1 mice aged 8-months, there is a significant reduction in bacteria belonging to the phyla Firmicutes and Actinobacteria with respect to an increase of Bacteroidetes and Tenericutes, supporting evidence of the role of amyloid and related bacterial accumulation in the pathogenesis of cognitive damage. |
| Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly [94] | Cognitively impaired patients with (n = 40, Amy+) and with no brain amyloidosis (n = 33, Amy-) and also in a group of controls (n = 10, no brain amyloidosis and no cognitive impairment). Studying the association of brain amyloidosis with gut microbiota taxa with pro- and anti-inflammatory activity | Intestinal | Clinical evidence of gut microbiota bacteria alterations in patients with brain amyloidosis. Abundance of the pro-inflammatory genus Escherichia/Shigella was significantly increased in Amyþ compared with Amy patients. Significant reduction in E. rectale (butyrate producer with key protective roles against inflammation) abundance in Amyþ compared with Amy_ subjects. Cognitive impairment is associated with a reduction in certain anti-inflammatory bacteria belonging to the phyla Firmicutes and Bacteroidetes compared to an increase of other pro-inflammatory bacteria of phylum Proteobacteria. |
| Gut microbiota is altered in patients with Alzheimer’s disease [112] | Fecal samples from 43 AD patients and 43 age- and gender-matched cognitively normal controls were evaluated by sequencing techniques to ascertain if the composition of gut microbiota was different between the two groups | Intestinal | Several bacteria taxa in AD patients were different from those in controls at taxonomic levels, such as Bacteroides, Actinobacteria, Ruminococcus, Lachnospiraceae, and Selenomonadales. These findings suggest that gut microbiota is altered in AD patients and may be involved in the pathogenesis of AD. |
| Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway [96] | Prospective study (n = 108 nursing home elders, 5 months), metagenomic sequencing and in vitro T84 intestinal epithelial cell functional assays | Intestinal | Clinical parameters as well as numerous microbial taxa and functional genes act as predictors of AD dementia in comparison to elders without dementia. Less abundance of butyrate-producing species: Butyrivibrio (B. hungatei and B. proteoclasticus), Eubacterium (E. eigens, E. hallii and E. rectale) and Clostridium sp. SY8519, R.hominis and F.prausnitzzi in AD patients, as well as greater abundance of O. splanchnicus, Odoribacter sp., K. pneumoniae, B. fragilis, and E. lenta and Desulfovibrio genus (D. fairfieldensis). |
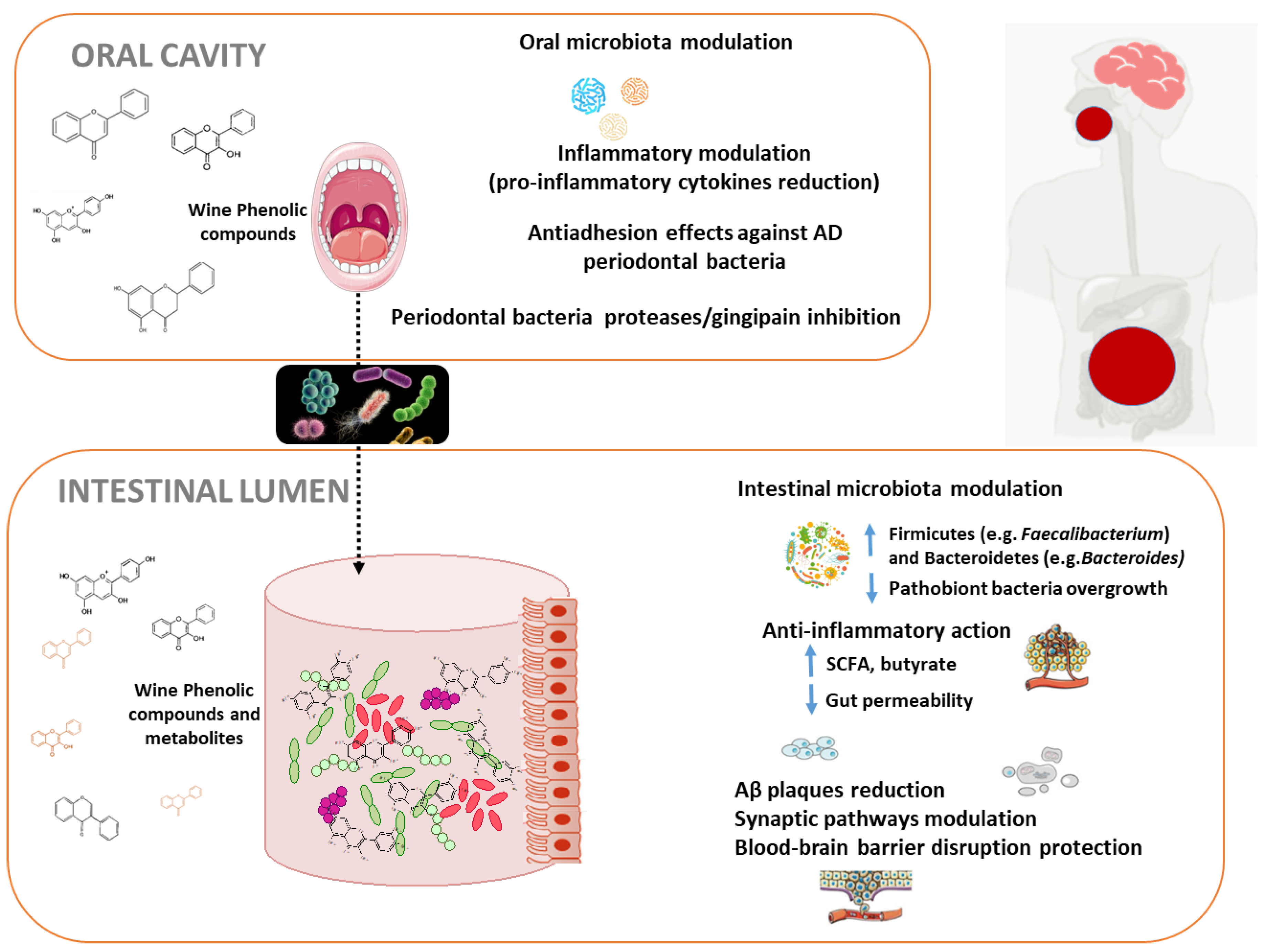
4. Microbiome Modulation by Diet/Wine Polyphenols and Alzheimer’s Disease
4.1. Oral Microbiota Modulation by Wine Polyphenols and Alzheimer’s Disease
4.2. Intestinal Microbiota Modulation by Wine Polyphenols and Alzheimer’s Disease
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid-β |
| BBB | Blood-Brain Barrier |
| CI | Confidence Interval |
| APOE | Apolipoprotein E |
| DASH | Dietary Approaches to Stop Hypertension |
| LPS | lipopolysaccharide |
| MD | Mediterranean diet |
| MIND | Mediterranean–DASH Intervention for Neurodegenerative Delay |
| RR | Relative Risk |
| SCFA | Short-Chain Fatty Acids |
| 3xTg-AD | Triple-transgenic mouse model of AD |
| WHO | World Health Organization |
References
- Available online: https://www.nia.nih.gov/health/what-happens-brain-alzheimers-disease (accessed on 29 July 2020).
- Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2019, 15, 321–387. [Google Scholar]
- Selkoe, D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001, 81, 741–766. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef]
- Winblad, B.; Amouyel, P.; Andrieu, S.; Ballard, C.; Brayne, C.; Brodaty, H.; Cedazo-Minguez, A.; Dubois, B.; Edvardsson, D.; Feldman, H.; et al. Defeating Alzheimer’s disease and other dementias: A priority for European science and society. Lancet Neurol. 2016, 15, 455–532. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Global Action Plan on the Public Health Response to Dementia 2017–2025; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Available online: https://aspe.hhs.gov/system/files/pdf/NatPlan2018.pdf (accessed on 29 July 2020).
- Peters, M.E.; Schwartz, S.; Han, D.; Rabins, P.V.; Steinberg, M.; Tschanz, J.T.; Lyketsos, C.G. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: The Cache County Dementia Progression Study. Am. J. Psychiatry 2015, 172, 460–465. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Peng, D.; Wang, X. Genetics of Alzheimer’s disease: From pathogenesis to clinical usage. J. Clin. Neurosci. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Cacace, R.; Sleegers, K.; Van Broeckhoven, C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 2016, 12, 733–748. [Google Scholar] [CrossRef] [PubMed]
- Hokama, M.; Oka, S.; Leon, J.; Ninomiya, T.; Honda, H.; Sasaki, K.; Iwaki, T.; Ohara, T.; Sasaki, T.; LaFerla, F.M.; et al. Altered expression of diabetes-related genes in Alzheimer’s disease brains: The Hisayama study. Cereb. Cortex 2014, 24, 2476–2488. [Google Scholar] [CrossRef]
- Naughton, B.J.; Duncan, F.J.; Murrey, D.A.; Meadows, A.S.; Newsom, D.E.; Stoicea, N.; White, P.; Scharre, D.W.; Mccarty, D.M.; Fu, H. Blood genome-wide transcriptional profiles reflect broad molecular impairments and strong blood-brain links in Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 93–108. [Google Scholar] [CrossRef]
- Silva, M.V.F.; de Mello Gomide Loures, C.; Vieira Alves, L.C.; de Souza, L.C.; Braga Gomes Borges, K.; das Graças Carvalho, M. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019, 26, 33. [Google Scholar] [CrossRef]
- Pasinetti, G.M. Towards prevention and therapy of Alzheimer’s disease. Mol. Asp. Med. 2015, 43–44, 1–2. [Google Scholar] [CrossRef]
- Corona, G.; Vauzour, D.; Hercelin, J.; Williams, C.M.; Spencer, J.P.E. Phenolic acid intake, delivered via moderate champagne wine consumption, improves spatial working memory via the modulation of hippocampal and cortical protein expression/activation. Antiox. Redox Signal 2013, 19, 1676–1689. [Google Scholar] [CrossRef]
- Stockley, C.S. Role of wine components in inflammation and chronic diseases. In Wine Safety, Consumer Preference, and Human Health; Moreno-Arribas, M.V., Bartolomé Sualdea, B., Eds.; Springer International Publishing: Basel, Switzerland, 2016; pp. 240–258. [Google Scholar]
- Vauzour, D.; Camprubi-Robles, M.; Miquel-Kergoat, S.; Andres-Lacueva, C.; Bánáti, D.; Barberger-Gateau, P.; Bowman, G.L.; Caberlotto, L.; Clarke, R.; Hogervorst, E.; et al. Nutrition for the ageing brain: Towards evidence for an optimal diet. Ageing Res. Rev. 2017, 35, 222–240. [Google Scholar] [CrossRef]
- Portune, K.J.; Benítez-Páez, A.; Gomez Del Pulgar, E.M.; Cerrudo, V.; Sanz, Y. Gut microbiota, diet, and obesity-related disorders-The good, the bad, and the future challenges. Mol. Nutr. Food Res. 2017, 61, 1600252. [Google Scholar] [CrossRef]
- Requena, T.; Monagas, M.; Pozo-Bayón, M.A.; Martín-Álvarez, P.J.; Bartolomé, B.; Del Campo, R.; Moreno-Arribas, M.V. Perspectives of the potential implications of wine polyphenols on human oral and gut microbiota. Trends Food Sci. Technol. 2010, 21, 332–344. [Google Scholar] [CrossRef]
- Sanz, Y.; Romaní-Perez, M.; Benítez-Páez, A.; Portune, K.J.; Brigidi, P.; Rampelli, S.; Dinan, T.; Stanton, C.; Delzenne, N.; Blachier, F.; et al. Towards microbiome-informed dietary recommendations for promoting metabolic and mental health: Opinion papers of the MyNewGut project. Clin. Nutr. 2018, 37, 2191–2197. [Google Scholar] [CrossRef]
- Morand, C.; Tomás-Barberán, F.A. Interindividual variability in absorption, distribution, metabolism and excretion of food phytochemicals should be reported. J. Agric. Food Chem. 2019, 67, 3843–3844. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food components and dietary habits: Keys for a healthy gut microbiota composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef]
- Cueva, C.; Sánchez-Patán, F.; Monagas, M.; Watson, G.; Gibson, G.R.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microb. Ecol. 2013, 83, 792–805. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gómez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolomé, B.; Moreno-Arribas, M.A. An integrated view of the effects of wine polyphenols and their relevant metabolites on gut and host health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; de Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Polyphenols in the management of brain disorders: Modulation of the microbiota-gut-brain axis. Adv. Food Nutr. Res. 2020, 91, 1–27. [Google Scholar] [PubMed]
- Esteban-Fernández, A.; Rendeiro, C.; Spencer, J.P.E.; Gigorro del Coso, D.; Gónzalez de Llano, M.D.; Bartolomé, B.; Moreno-Arribas, M.V. Neuroprotective effects of selected microbial-derived phenolic metabolites and aroma compounds from wine in human SH-SY5Y neuroblastoma cells, and their putative mechanisms of action. Front. Nutr. 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly) phenols. Biochem. Pharm. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Simon, J.E.; Wu, Q. A critical review on grape polyphenols for neuroprotection: Strategies to enhance bioefficacy. Crit. Rev. Food Sci. Nutr. 2019, 60, 597–625. [Google Scholar] [CrossRef]
- Shoemark, D.K.; Allen, S.J. The microbiome and disease: Reviewing the links between the oral microbiome, aging, and Alzheimer’s disease. J. Alzheimers Dis. 2015, 43, 725–738. [Google Scholar] [CrossRef]
- Dominy, S.D.; Lynch, C.; Ermini, F.; Benedyk, M.; Marczyk, A.; Konradi, A.; Nguyen, M.; Haditsch, U.; Raha, D.; Griffin, C.; et al. Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 2019, 5, eaau3333. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Olsen, I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral Microbiol. 2019, 11, 1563405. [Google Scholar] [CrossRef]
- Mucke, L. Neuroscience: Alzheimer’s disease. Nature 2009, 461, 895–897. [Google Scholar] [CrossRef]
- Mangialasche, F.; Kivipelto, M.; Solomon, A.; Fratiglioni, L. Dementia prevention: Current epidemiological evidence and future perspective. Alzheimers Res. Ther. 2012, 4, 6. [Google Scholar] [CrossRef]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hyppönen, E.; Kuźma, E.; Llewellyn, D.J. Association of lifestyle and genetic risk with incidence of dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef]
- Anstey, K.J.; Mack, H.A.; Cherbuin, N. Alcohol consumption as a risk factor for dementia and cognitive decline: Meta-analysis of prospective studies. Am. J. Geriatr. Psychiatry 2009, 17, 542–555. [Google Scholar] [CrossRef]
- Blondell, S.J.; Hammersley-Mather, R.; Veerman, J.L. Does physical activity prevent cognitive decline and dementia?: A systematic review and meta-analysis of longitudinal studies. BMC Public Health 2014, 14, 510. [Google Scholar] [CrossRef]
- Cao, L.; Tan, L.; Wang, H.F.; Jiang, T.; Zhu, X.C.; Lu, H.; Tan, M.S.; Yu, J.T. Dietary patterns and risk of dementia: A systematic review and meta-analysis of cohort studies. Mol. Neurobiol. 2016, 53, 6144–6154. [Google Scholar] [CrossRef]
- Zhong, G.; Wang, Y.; Zhang, Y.; Guo, J.J.; Zhao, Y. Smoking is associated with an increased risk of dementia: A meta-analysis of prospective cohort studies with investigation of potential effect modifiers. PLoS ONE 2015, 10, e0118333. [Google Scholar] [CrossRef] [PubMed]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Zheng, G.; Xia, R.; Zhou, W.; Tao, J.; Chen, L. Aerobic exercise ameliorates cognitive function in older adults with mild cognitive impairment: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2016, 50, 1443–1450. [Google Scholar] [CrossRef]
- Gustafson, D. Adiposity indices and dementia. Lancet Neurol. 2006, 5, 713–720. [Google Scholar] [CrossRef]
- Ma, Y.; Ajnakina, O.; Steptoe, A.; Cadar, D. Higher risk of dementia in English older individuals who are overweight or obese. Int. J. Epidem. 2020. [Google Scholar] [CrossRef]
- McGrattan, A.M.; McGuinness, B.; McKinley, M.C.; Kee, F.; Passmore, P.; Woodside, J.V.; McEvoy, C.T. Diet and inflammation in cognitive ageing and Alzheimer’s disease. Curr. Nutr. Rep. 2019, 8, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimers Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Laitinen, M.H.; Ngandu, T.; Rovio, S.; Helkala, E.L.; Uusitalo, U.; Viitanen, M.; Nissinen, A.; Tuomilehto, J.; Soininen, H.; Kivipelto, M. Fat intake at midlife and risk of dementia and alzheimer’s disease: A population-based study. Dement. Geriatr. Cogn. Disord. 2006, 22, 99–107. [Google Scholar] [CrossRef]
- Morris, M.C.; Evans, D.A.; Bienias, J.L.; Tangney, C.C.; Bennett, D.A.; Aggarwal, N.; Schneider, J.; Wilson, R.S. Dietary fats and the risk of incident Azheimer´s disease. Arch. Neurol. 2003, 60, 194–200. [Google Scholar] [CrossRef]
- Liu, C.C.; Liu, C.C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer disease: Risk, mechanisms and therapy. Nat. Rev. Neurol. 2013, 9, 106–118. [Google Scholar] [CrossRef]
- Snowden, S.G.; Ebshiana, A.A.; Hye, A.; An, Y.; Pletnikova, O.; O’Brien, R.; Troncoso, J.; Legido-Quigley, C.; Thambisetty, M. Association between fatty acid metabolism in the brain and Alzheimer disease neuropathology and cognitive performance: A nontargeted metabolomic study. PLoS Med. 2017, 14, e1002266. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Mayeux, R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004, 3, 579–587. [Google Scholar] [CrossRef]
- Shield, K.D.; Parry, C.; Rehm, J. Chronic diseases and conditions related to alcohol use. Alcohol Res. 2013, 35, 155–173. [Google Scholar] [PubMed]
- Ganguli, M.; Vander Bilt, J.; Saxton, J.A.; Shen, C.; Dodge, H.H. Alcohol consumption and cognitive function in late life: A longitudinal community study. Neurology 2005, 65, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Mukamal, K.J.; Kuller, L.H.; Fitzpatrick, A.L.; Longstreth, W.T., Jr.; Mittleman, M.A.; Siscovick, D.S. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA 2003, 289, 1405–1413. [Google Scholar] [CrossRef] [PubMed]
- Ruitenberg, A.; van Swieten, J.C.; Witteman, J.C.; Mehta, K.M.; van Duijn, C.M.; Hofman, A.; Breteler, M.M. Alcohol consumption and risk of dementia: The Rotterdam Study. Lancet 2002, 359, 281–286. [Google Scholar] [CrossRef]
- Stampfer, M.J.; Kang, J.H.; Chen, J.; Cherry, R.; Grodstein, F. Effects of moderate alcohol consumption on cognitive function in women. N. Engl. J. Med. 2005, 352, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Anttila, T.; Helkala, E.L.; Viitanen, M.; Kareholt, I.; Fratiglioni, L.; Winblad, B.; Soininen, H.; Tuomilehto, J.; Nissinen, A.; Kivipelto, M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: A prospective population based study. BMJ 2004, 329, 539. [Google Scholar] [CrossRef]
- Xi, B.; Veeranki, S.P.; Zhao, M.; Ma, C.; Yan, Y.; Mi, J. Relationship of alcohol consumption to all-cause, cardiovascular, and cancer-related mortality in U.S. adults. J. Am. Coll. Cardiol. 2017, 70, 913–922. [Google Scholar] [CrossRef]
- Bucher, T.; Deroover, K.; Stockley, C. Low-alcohol wine: A narrative review on consumer perception and behavior. Beverages 2018, 4, 82. [Google Scholar] [CrossRef]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hebert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk factors for Alzheimer’s disease: A prospective analysis from the Canadian study of health and aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Truelsen, T.; Thudium, D.; Gronbaek, M. Amount and type of alcohol and risk of dementia: The Copenhagen City Heart Study. Neurology 2002, 59, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Girón, A.; Queipo-Ortuno, I.; Boto-Ordonez, M.; Muñoz-González, I.; Sánchez-Patán, F.; Monagas, M.; Martín-Álvarez, P.J.; Murri, M.; Tinahones, F.J.; Andrés-Lacueva, C.; et al. Comparative study of Microbial-Derived Phenolic Metabolites in Human Feces after Intake of Gin, Red Wine, and Dealcoholized Red Wine. J. Agric. Food Chem. 2013, 61, 3909–3915. [Google Scholar] [CrossRef]
- Nooyens, A.C.; Bueno de Mesquita, H.B.; van Gelder, B.M.; van Boxtel, M.P.; Verschuren, W.M. Consumption of alcoholic beverages and cognitive decline at middle age: The Doetinchem Cohort Study. Br. J. Nutr. 2014, 111, 715–723. [Google Scholar] [CrossRef]
- Orgogozo, J.M.; Dartigues, J.F.; Lafont, S.; Letenneur, L.; Commenges, D.; Salamon, R.; Renaud, S.; Breteler, M.B. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev. Neurol. (Paris) 1997, 153, 185–192. [Google Scholar]
- Mehlig, K.; Skoog, I.; Guo, X.; Schütze, M.; Gustafson, D.; Waern, M.; Ostling, S.; Björkelund, C.; Lissner, L. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Goteborg. Am. J. Epidemiol. 2008, 167, 684–691. [Google Scholar] [CrossRef]
- Haseeb, S.; Alexander, B.; Santi, R.L.; Liprandi, A.S.; Baran-chuk, A. What’s in wine? A clinician’s perspective. Trends Cardiov. Med. 2019, 29, 97–106. [Google Scholar] [CrossRef]
- Pasolli, E.; Asnicar, F.; Manara, S.; Zolfo, M.; Karcher, N.; Armanini, F.; Beghini, F.; Manghi, P.; Tett, A.; Ghensi, P.; et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 2019, 176, 649–662. [Google Scholar] [CrossRef]
- The Human Oral Microbiome Database (HOMD). Available online: http://www.homd.org/ (accessed on 29 July 2020).
- Esteban-Fernández, A.; Zorraquín-Peña, I.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.A. The role of wine and food polyphenols in oral health. Trends Food Sci. Technol. 2017, 69, 118–130. [Google Scholar] [CrossRef]
- Lu, M.; Xuanb, S.; Wangaal, Z. Oral microbiota: A new view of body health. Food Sci. Hum. Wellness 2019, 8, 8–15. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P.D. Targeting Carbohydrates and Polyphenols for a Healthy Microbiome and Healthy Weight. Curr. Nutr. Rep. 2019, 8, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Backhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Villanueva-Millán, M.J.; Pérez-Matute, P.; Oteo, J.A. Gut microbiota: A key player in health and disease. A review focused on obesity. J. Physiol. Biochem. 2015, 71, 509–525. [Google Scholar] [CrossRef]
- Sureda, A.; Daglia, M.; Argüelles Castilla, S.; Sanadgol, N.; Nabavi, S.F.; Khan, H.; Belwalet, T.; Jeandet, P.; Marchese, A.; Pistollato, F.; et al. Oral microbiota and Alzheimer’s disease: Do all roads lead to Rome? Pharmacol. Res. 2020, 151, 104582. [Google Scholar] [CrossRef]
- Rosier, B.T.; Buetas, E.; Moya-Gonzalvez, E.M.; Artacho, A.; Mira, A. Nitrate as a potential prebiotic for the oral microbiome. Sci. Rep. 2020, 10, 12895. [Google Scholar] [CrossRef]
- Stein, P.S.; Steffen, M.J.; Smith, C.; Jicha, G.; Ebersole, J.L.; Abner, E.; Dawson, D. Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 2012, 8, 196–203. [Google Scholar] [CrossRef]
- Poole, S.; Singhrao, S.K.; Kesavalu, L.; Curtis, M.A.; Crean, S. Determining the presence of periodontopathic virulence factors in short-term postmortem Alzheimer’s disease brain tissue. J. Alzheimers Dis. 2013, 36, 665–677. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Chukkapalli, S.; Poole, S.; Velsko, I.; Crean, S.J.; Kesavalu, L. Chronic Porphyromonas gingivalis infection accelerates the occurrence of age-related granules in ApoE–/– mice brains. J. Oral. Microbiol. 2017, 9, 1270602. [Google Scholar] [CrossRef]
- Mark Welch, J.L.; Rossetti, B.J.; Rieken, C.W.; Dewhirst, F.E.; Borisy, G.G. Biogeography of a human oral microbiome at the micron scale. PNAS 2016, 113, E791–E800. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Zuang, K.; Huang, C.; Leng, L.; Zheng, H.; Gao, Y.; Chen, G.; Ji, Z.; Sun, H.; Hu, Y.; Wu, D.; et al. Neuron-specific menin deletion leads to synaptic dysfunction and cognitive impairment by modulating p35 expression. Cell Rep. 2018, 24, 701–712. [Google Scholar] [CrossRef]
- Kelly, J.R.; Borre, Y.; O’ Brien, C.; Patterson, E.; El Aidy, S.; Deane, J.; Kennedy, P.J.; Beers, S.; Scott, K.; Moloney, G.; et al. Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. J. Psychiatr. Res. 2016, 82, 109–118. [Google Scholar] [CrossRef]
- Sanguinetti, E.; Collado, M.C.; Marrachelli, V.G.; Monleon, D.; Selma-Royo, M.; Pardo-Tendero, M.M.; Burchielli, S.; Iozzo, P. Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci. Rep. 2018, 8, 4907. [Google Scholar] [CrossRef]
- Angelucci, F.; Cechova, K.; Amlerova, J.; Hort, J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019, 16, 108. [Google Scholar] [CrossRef]
- Vendrik, K.E.W.; Ooijevaar, R.E.; de Jong, P.R.C.; Laman, J.D.; van Oosten, B.W.; van Hilten, J.J.; Ducarmon, Q.R.; Keller, J.J.; Kuijper, E.J.; Contarino, M.F.; et al. Fecal microbiota transplantation in neurological disorders. Front. Cell. Infect. Microbiol. 2020, 10, 98. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory products of the human GI tract microbiome and their potential impact on Alzheimer’s disease (AD): Detection of lipopolysaccharide (LPS) in AD hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Alzheimer’s disease and gut microbiota modifications: The long way between preclinical studies and clinical evidence. Pharmacol. Res. 2018, 129, 329–336. [Google Scholar] [CrossRef]
- Harán, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory p-glycoprotein pathway. MBio 2019, 10, e00632-19. [Google Scholar]
- Bourassa, M.W.; Alim, I.; Bultman, S.J.; Ratan, R.R. Butyrate, neuroepigenetics and the gut microbiome: Can a high fiber diet improve brain health? Neurosci. Lett. 2016, 625, 56–63. [Google Scholar] [CrossRef]
- Osorio, C.; Kanukuntla, T.; Diaz, E.; Jafri, N.; Cummings, M.; Sfera, A. The post-amyloid era in alzheimer’s disease: Trust your gut feeling. Front. Aging Neurosci. 2019, 11, 143. [Google Scholar] [CrossRef]
- Koliarakis, I.; Messaritakis, I.; Nikolouzakis, T.K.; Hamilos, G.; Souglakos, J.; Tsiaoussis, J. Oral bacteria and intestinal dysbiosis in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 4146. [Google Scholar] [CrossRef]
- Agusti, A.; García-Pardo, M.P.; López-Almela, I.; Campillo, I.; Maes, M.; Romaní-Pérez, M.; Sanz, Y. Interplay between the gut-brain axis, obesity and cognitive function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef]
- Dinan, T.G.; Stanton, C.; Long-Smith, C.; Kennedy, P.; Cryan, J.F.; Cowan, C.S.M.; Cenit, M.C.; van der Kampe, J.-W.; Sanz, Y. Feeding melancholic microbes: MyNewGut recommendations on diet and mood. Clin. Nutr. 2019, 38, 1995–2001. [Google Scholar] [CrossRef]
- Plöger, S.; Stumpff, F.; Penner, G.B.; Schulzke, J.D.; Gäbel, G.; Martens, H.; Shen, Z.; Günzel, D.; Aschenbach, J.R. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann. N. Y. Acad. Sci. 2012, 1258, 52–59. [Google Scholar] [CrossRef]
- ClinicalTrials.gov Database. Available online: https://www.clinicaltrials.gov (accessed on 29 July 2020).
- Harding, A.; Gonder, U.; Robinson, S.J.; Crean, S.; Singhrao, S.K. Exploring the association between alzheimer’s disease, oral health, microbial endocrinology and nutrition. Front. Aging Neurosci. 2017, 9, 398. [Google Scholar] [CrossRef]
- Hashioka, S.; Inoue, K.; Miyaoka, T.; Hayashida, M.; Wake, R.; Oh-Nishi, A.; Inagaki, M. The possible causal link of periodontitis to neuropsychiatric disorders: More than psychosocial mechanisms. Int. J. Mol. Sci. 2019, 20, 3723. [Google Scholar] [CrossRef]
- Chen, C.K.; Wu, Y.T.; Chang, Y.C. Association between chronic periodontitis and the risk of Alzheimer’s disease: A retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 2017, 9, 56. [Google Scholar] [CrossRef]
- Ide, M.; Harris, M.; Stevens, A.; Sussams, R.; Hopkins, V.; Culliford, D.; Fuller, J.; Ibbett, P.; Raybould, R.; Thomas, R.; et al. Periodontitis and cognitive decline in Alzheimer’s disease. PLoS ONE 2016, 11, e0151081. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Peña, C.; Álvarez-Cisneros, T.; Quiroz-Baez, R.; Friedland, R.P. Microbiota and aging. A review and commentary. Arch. Med. Res. 2017, 48, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Giau, V.V.; Wu, S.Y.; Jamerlan, A.; An, S.S.A.; Kim, S.Y.; Hulme, J. Gut microbiota and their neuroinflammatory implications in Alzheimer’s disease. Nutrients 2018, 10, 1765. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Jan, S.; Suchodolski, J.S.; Nasuti, C.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; McCoy, K.D.; Neher, J.J.; Jucker, M.; Fak, F.; Lasser, T.; Bolmont, T. Reduction of Beta-amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Cai, M.; Zhu, C.; et al. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimers Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Márquez Campos, E.; Stehle, P.; Simon, M.C. Microbial metabolites of flavan-3-ols and their biological activity. Nutrients 2019, 11, 2260. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedye, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Tomas-Barberan, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Zorraquín-Peña, I.; Esteban-Fernández, A.; González de Llano, D.; Bartolomé, B.; Moreno-Arribas, M.V. Wine-derived phenolic metabolites in the digestive and brain function. Beverages 2019, 5, 7. [Google Scholar] [CrossRef]
- González-Sánchez, M.; Bartolome, F.; Antequera, D.; Puertas-Martín, V.; González, P.; Gómez-Grande, A.; Llamas-Velasco, S.; Herrero-San Martín, A.; Pérez-Martínez, D.; Villarejo-Galende, A.; et al. Decreased salivary lactoferrin levels are specific to Alzheimer’s disease. EBioMedicine 2020, 57, 102834. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Thurnheer, T.; Bartolomé, B.; Moreno-Arribas, M.V. Red wine and oenological extracts display antimicrobial effects in an oral bacteria biofilm model. J. Agric. Food Chem. 2014, 62, 4731–4737. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Ribeiro-Vidal, H.; Esteban-Fernández, A.; Bartolomé, B.; Figuero, E.; Moreno-Arribas, M.V.; Herrera, D. Antimicrobial activity of red wine and oenological extracts against periodontal pathogens in a validated oral biofilm model. BMC Complement. Altern. Med. 2019, 19, 145. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Zorraquín-Peña, I.; Ferrer, M.D.; Mira, A.; Bartolomé, B.; González de Llano, D.; Moreno-Arribas, M.V. Inhibition of oral pathogens adhesion to human gingival fibroblasts by wine polyphenols alone and in combination with an oral probiotic. J. Agric. Food Chem. 2018, 66, 2071–2082. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Ferrer, M.D.; Zorraquín-Peña, I.; López-López, A.; Moreno-Arribas, M.V.; Mira, A. In vitro beneficial effects of Streptococcus dentisani as potential oral probiotic for periodontal diseases. J. Periodontol. 2019, 90, 1346–1355. [Google Scholar] [CrossRef]
- Lagha, A.B.; Groeger, S.; Meyle, J.; Grenier, D. Green tea polyphenols enhance gingival keratinocyte integrity and protect against invasion by Porphyromonas gingivalis. Pathog. Dis. 2018, 76, fty030. [Google Scholar] [CrossRef]
- Kugaji, M.S.; Kumbar, V.M.; Peram, M.R.; Patil, S.; Bhat, K.G.; Diwan, P.V. Effect of Resveratrol on biofilm formation and virulence factor gene expression of Porphyromonas gingivalis in periodontal disease. APMIS 2019, 127, 187–195. [Google Scholar] [CrossRef]
- Nash, V.; Ranadheera, C.S.; Georgousopoulou, E.N.; Mellora, D.D.; Panagiotakosa, D.B.; McKunea, A.J.; Kelletta, J.; Naumovskia, N. The effects of grape and red wine polyphenols on gut microbiota—A systematic review. Food Res. Int. 2018, 113, 277–287. [Google Scholar] [CrossRef]
- Clemente-Postigo, M.; Queipo-Ortuño, M.I.; Boto-Ordoñez, M.; Coin-Aragüez, L.; Roca-Rodriguez, M.M.; Delgado-Lista, J.; Cardona, F.; Andres-Lacueva, C.; Tinahones, F.J. Effect of acute and chronic red wine consumption on lipopolysaccharide concentrations. Am. J. Clin. Nutr. 2013, 97, 1053–1061. [Google Scholar] [CrossRef]
- Barroso, E.; Martín, V.; Martínez-Cuesta, M.C.; Peláez, C.; Requena, T. Stability of saliva microbiota during moderate consumption of red wine. Arch. Oral Biol. 2015, 60, 1763–1768. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.T.; Spector, T.D. Red wine consumption associated with increased gut microbiota α-diversity in 3 independent cohorts. Gastroenterology 2020, 158, 270–272. [Google Scholar] [CrossRef]
- Nagpala, R.; Mainalia, R.; Ahmadia, S.; Wanga, S.; Singha, R.; Kavanaghc, K.; Kitzmand, D.W.; Kushugulovae, A.; Marottaf, F.; Yadava, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Espinosa-Martos, I.; Rodríguez, J.M.; Jiménez-Girón, A.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Moderate consumption of red wine modulate human intestinal inflammatory response. J. Agric. Food Chem. 2014, 62, 10567–10575. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2018, 18, 83–90. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vich Vila, A.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Belda, I.; Cueva, C.; Zorraquín-Peña, I.; Tamargo, A.; Ortiz-Álvarez, R.; Acedo, A.; Bartolomé, B.; Moreno-Arribas, M.V. A multi-omics approach for understanding the effects of moderate wine consumption on intestinal human health. 2020; submitted for publication. [Google Scholar]
- Muñoz-González, I.; Jiménez-Girón, A.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Profiling of microbial-derived phenolic metabolites in human feces after moderate red wine intake. J. Agric. Food Chem. 2013, 61, 9470–9479. [Google Scholar] [CrossRef]
- Jiménez-Girón, A.; Ibañez, C.; Cifuentes, A.; Simo, C.; Muñoz-González, I.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. Faecal metabolomic fingerprint after moderate consumption of red wine by healthy subjects. J. Proteome Res. 2015, 14, 897–905. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Ibañez, C.; Simo, C.; Bartolomé, B.; Moreno-Arribas, M.V. An ultrahigh-performance liquid chromatography-time-of-flight mass spectrometry metabolomic approach to studying the impact of moderate red-wine consumption on urinary metabolome. J. Proteome Res. 2018, 17, 1624–1635. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Ibañez, C.; Simo, C.; Bartolomé, B.; Moreno-Arribas, M.V. Metabolome-based clustering after moderate wine consumption. OENO One 2020, 3, 455–467. [Google Scholar] [CrossRef]
- Cueva, C.; Jiménez-Girón, A.; Muñoz-González, I.; Esteban-Fernández, A.; Gil-Sánchez, I.; Dueñas, M.; Martín-Álvarez, P.J.; Pozo-Bayón, M.A.; Bartolomé, B.; Moreno-Arribas, M.V. Application of a new Dynamic Gastrointestinal Simulator (SIMGI) to study the impact of red wine in colonic metabolism. Food Res. Int. 2015, 72, 149–159. [Google Scholar] [CrossRef]
- González-Sarrías, A.; Núñez-Sánchez, M.Á.; Tomás-Barberán, F.A.; Espín, J.C. Neuroprotective effects of bioavailable polyphenol-derived metabolites against oxidative stress-induced cytotoxicity in human neuroblastoma SH-SY5Y cells. J. Agric. Food Chem. 2017, 65, 752–758. [Google Scholar] [CrossRef]
- Vázquez-Fresno, R.; Llorach, R.; Perera, A.; Mandal, R.; Feliz, M.; Tinahones, F.J.; Wishart, D.S.; Andres-Lacueva, C. Clinical phenotype clustering in cardiovascular risk patients for the identification of responsive metabotypes after red wine polyphenol intake. J. Nutr. Biochem. 2016, 28, 114–120. [Google Scholar] [CrossRef]
- Bub, A.; Watzl, B.; Heeb, D.; Rechkemmer, G.; Briviba, K. Malvidin-3-glucoside bioavailability in humans after ingestion of red wine, dealcoholized red wine and red grape juice. Eur. J. Nutr. 2001, 40, 113–120. [Google Scholar] [CrossRef]
- Dueñas, M.; Muñoz-González, I.; Cueva, C.; Jiménez-Girón, A.; Sánchez-Patán, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolomé, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed Res. Int. 2015, 850902. [Google Scholar] [CrossRef]
- Carregosa, D.; Carecho, R.; Figueira, I.; Santos, C. Low-molecular weight metabolites from polyphenols as effectors for attenuating neuroinflammation. J. Agric. Food Chem. 2020, 68, 1790–1807. [Google Scholar] [CrossRef]
- Caracci, F.; Harary, J.; Simkovic, S.; Pasinetti, G.M. Grape-Derived Polyphenols Ameliorate Stress-Induced Depression by Regulating Synaptic Plasticity. J. Agric. Food Chem. 2020, 68, 1808–1815. [Google Scholar] [CrossRef]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef]
- Dal-Pan, A.; Dudonné, S.; Bourassa, P.; Bourdoulous, M.; Tremblay, C.; Desjardins, Y.; Calon, F.; Neurophenols consortium. Cognitive-Enhancing Effects of a Polyphenols-Rich Extract from Fruits without Changes in Neuropathology in an Animal Model of Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 115–135. [Google Scholar] [CrossRef]
- Vazour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Kuhnle, G.G.C.; Srai, K.S. Epicatechin and its in vivo metabolite, 3’-O-methyl epicatechin, protect human fibroblasts from oxidative-stress-induced cell death involving caspase-3 activation. Biochem. J. 2001, 354, 493–500. [Google Scholar] [CrossRef]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s Disease and in the Gut–Brain Axis. Microorganism 2020, 8, 199. [Google Scholar] [CrossRef]
- Figueira, I.; Tavares, L.; Jardim, C.; Costa, I.; Terrasso, A.P.; Almeida, A.F.; Govers, C.; Mes, J.J.; Gardner, R.; Becker, J.D.; et al. Blood–brain barrier transport and neuroprotective potential of blackberry-digested polyphenols: An in vitro study. Eur. J. Nutr. 2019, 58, 113–130. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Vingtdeux, V.; Dreses-Werringloer, U.; Zhao, H.; Davies, P.; Marambaud, P. Therapeutic potential of resveratrol in Alzheimer’s disease. BMC Neurosci. 2008, 9, S6. [Google Scholar] [CrossRef] [PubMed]
- Pasinetti, G.M. Novel role of red wine-derived polyphenols in the prevention of Alzheimer’s disease dementia and brain pathology: Experimental approaches and clinical implications. Planta Med. 2012, 78, 1614–1619. [Google Scholar] [PubMed]
- Abdel-Moneim, A.; Yousef, A.I.; Abd El-Twab, S.M.; Abdel Reheim, E.S.; Ashour, M.B. Gallic acid and p-coumaric acid attenuate type 2 diabetes-induced neurodegeneration in rats. Metab. Brain Dis. 2017, 32, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hodes, G.E.; Zhang, H.; Zhang, S.; Zhao, W.; Golden, S.A.; Bi, W.; Menard, C.; Kana, V.; Leboeuf, M.; et al. Epigenetic modulation of inflammation and synaptic plasticity promotes resilience against stress in mice. Nat. Commun. 2018, 9, 477. [Google Scholar] [CrossRef]
- Kho, A.R.; Choi, B.Y.; Lee, S.H.; Hong, D.K.; Lee, S.H.; Jeong, J.H.; Park, K.-H.; Song, H.K.; Choi, H.C.; Suh, S.W. Effects of protocatechuic acid (pca) on global cerebral ischemia-induced hippocampal neuronal death. Int. J. Mol. Sci. 2018, 19, 1420. [Google Scholar] [CrossRef]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative role of red wine polyphenols against brain pathology in Alzheimer’s and Parkinson’s disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L.; Reay, J.L.; Lietz, G.; Okello, E.J.; Wilde, A.; Haskell, C.F. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: A double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91, 1590–1597. [Google Scholar] [CrossRef]
- Moran, C.; di Palumbo, A.S.; Bramham, J.; Moran, A.; Rooney, B.; De Vito, G.; Egan, B. Effects of a six-month multi-ingredient nutrition supplement intervention of omega-3 polyunsaturated fatty acids, vitamin D, resveratrol, and whey protein on cognitive function in older adults: A randomised, double-blind, controlled trial. J. Prev. Alzheimers Dis. 2018, 5, 175–183. [Google Scholar] [PubMed]
- Rotches-Ribalta, M.; Urpi-Sarda, M.; Llorach, R.; Boto-Ordonez, M.; Jauregui, O.; Chiva-Blanch, G.; Perez-Garcia, L.; Jaeger, W.; Guillen, M.; Corella, D.; et al. Gut and microbial resveratrol metabolite profiling after moderate long-term consumption of red wine versus dealcoholized red wine in humans by an optimized ultra-high-pressure liquid chromatography tandem mass spectrometry method. J. Chromatogr. A 2012, 1265, 105–113. [Google Scholar] [CrossRef]
- Rotches-Ribalta, M.; Andres-Lacueva, C.; Estruch, R.; Escribano, E.; Urpi-Sarda, M. Pharmacokinetics of resveratrol metabolic profile in healthy humans after moderate consumption of red wine and grape extract tablets. Pharm. Res. 2012, 66, 375–382. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno-Arribas, M.V.; Bartolomé, B.; Peñalvo, J.L.; Pérez-Matute, P.; Motilva, M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients 2020, 12, 3082. https://doi.org/10.3390/nu12103082
Moreno-Arribas MV, Bartolomé B, Peñalvo JL, Pérez-Matute P, Motilva MJ. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients. 2020; 12(10):3082. https://doi.org/10.3390/nu12103082
Chicago/Turabian StyleMoreno-Arribas, M. Victoria, Begoña Bartolomé, José L. Peñalvo, Patricia Pérez-Matute, and Maria José Motilva. 2020. "Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease" Nutrients 12, no. 10: 3082. https://doi.org/10.3390/nu12103082
APA StyleMoreno-Arribas, M. V., Bartolomé, B., Peñalvo, J. L., Pérez-Matute, P., & Motilva, M. J. (2020). Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients, 12(10), 3082. https://doi.org/10.3390/nu12103082






