Stratification of Volunteers According to Flavanone Metabolite Excretion and Phase II Metabolism Profile after Single Doses of ‘Pera’ Orange and ‘Moro’ Blood Orange Juices
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling of Orange Juices
2.2. Quantification of Soluble Sugar, Organic Acids, and Dietary Fiber
2.3. Quantification of Flavanones in Orange Juice
2.4. Study Population
2.5. Study Design
2.6. Intestinal Permeability Analysis
2.7. Biochemical Parameters
2.8. Plasma LPS and Zonulin
2.9. Identification and Quantification of Urine Metabolites
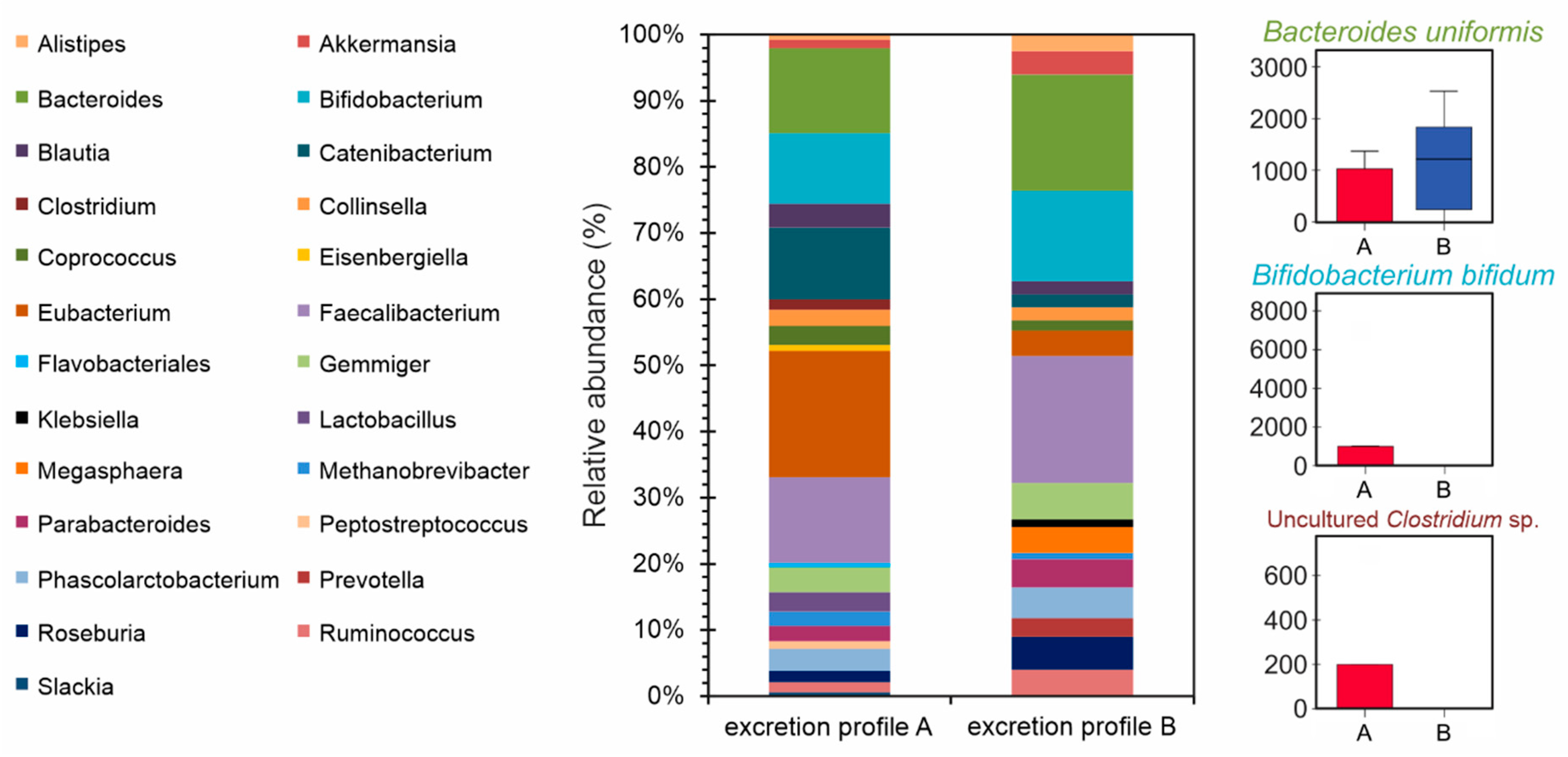
2.10. Gut Microbiota Profiling
2.11. Statistical Analysis
3. Results
3.1. Chemical Composition and Flavonoid Content of the Orange Juices and Biochemical and Anthropometric Profiles of the Volunteers
3.2. Identification of Metabolites in the Urine after the Consumption of Orange Juice
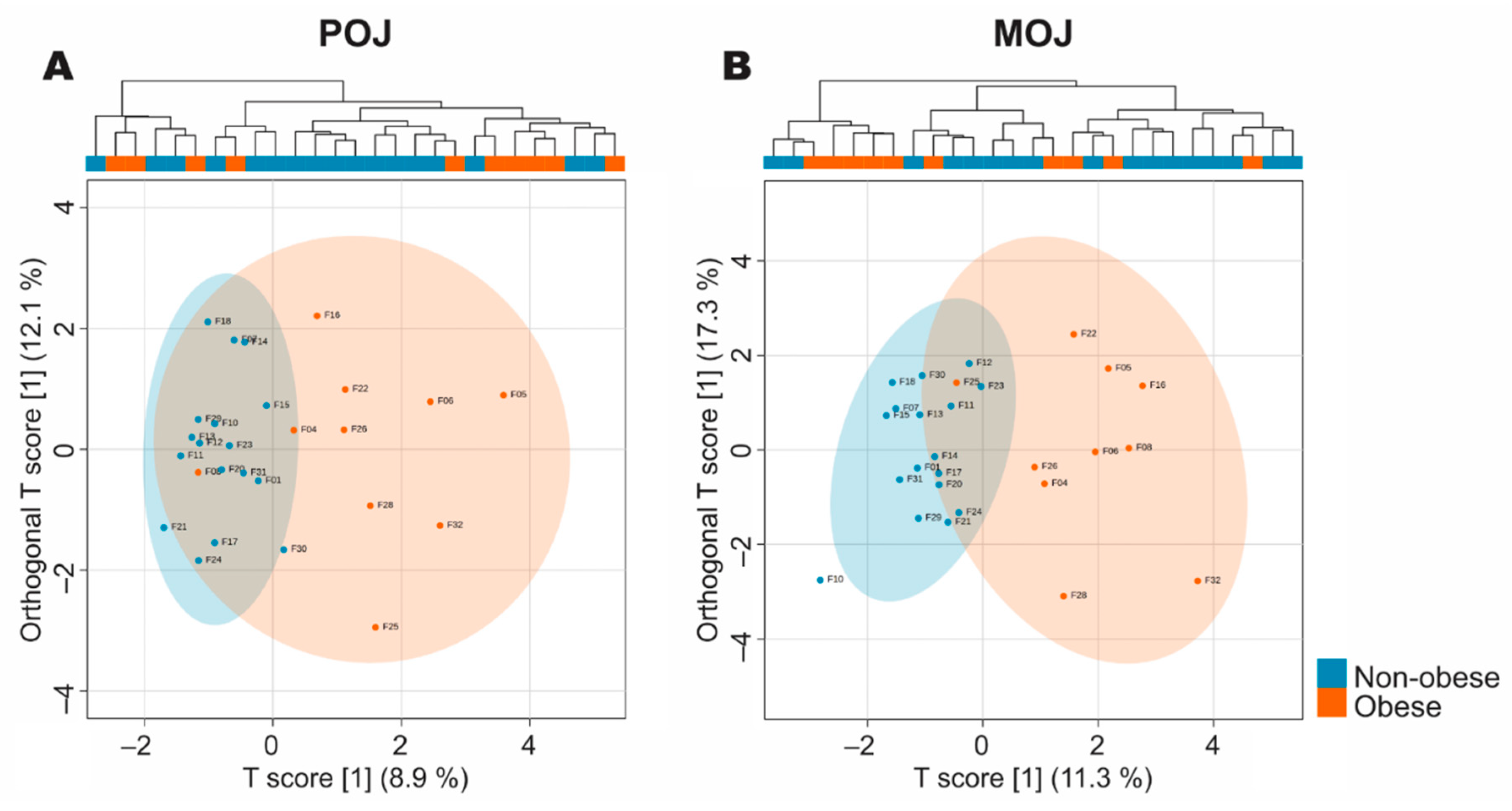
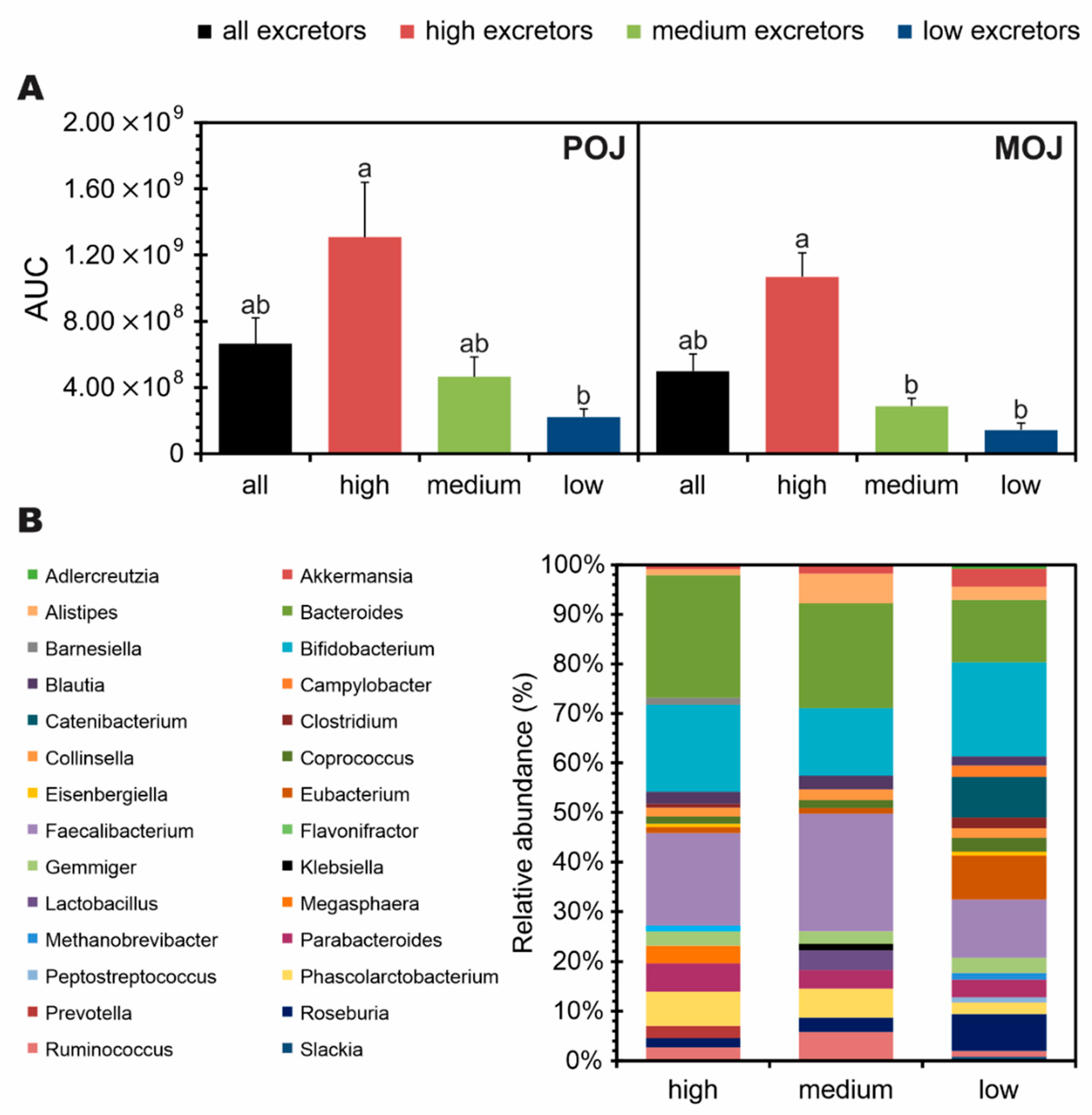
3.3. Volunteer Stratification According to Total Excretion of Flavanone Metabolites
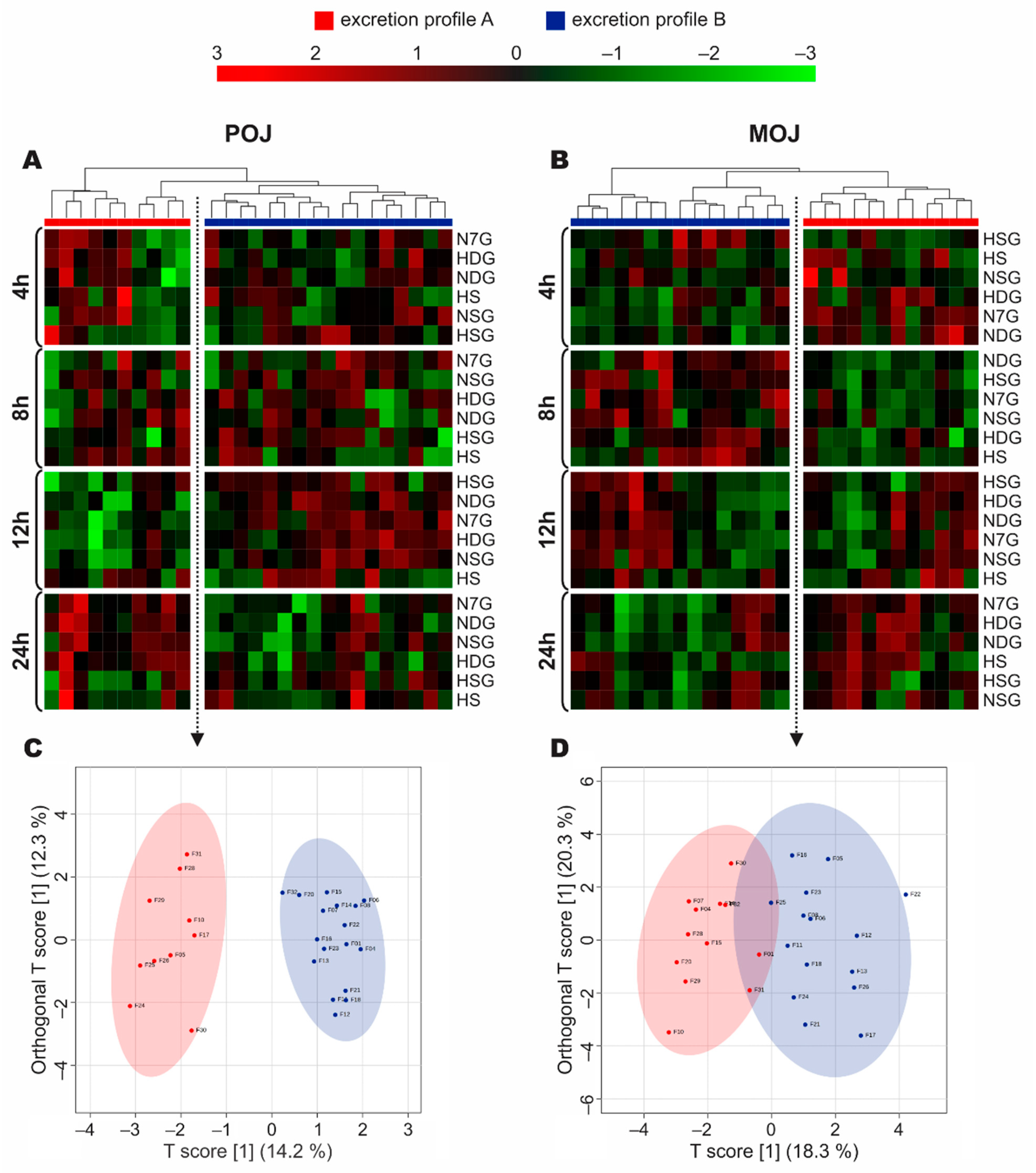
3.4. Stratification of Volunteers According to Phase II Metabolism
3.5. Time-Dependent Excretion Curve According to Excretion Profile
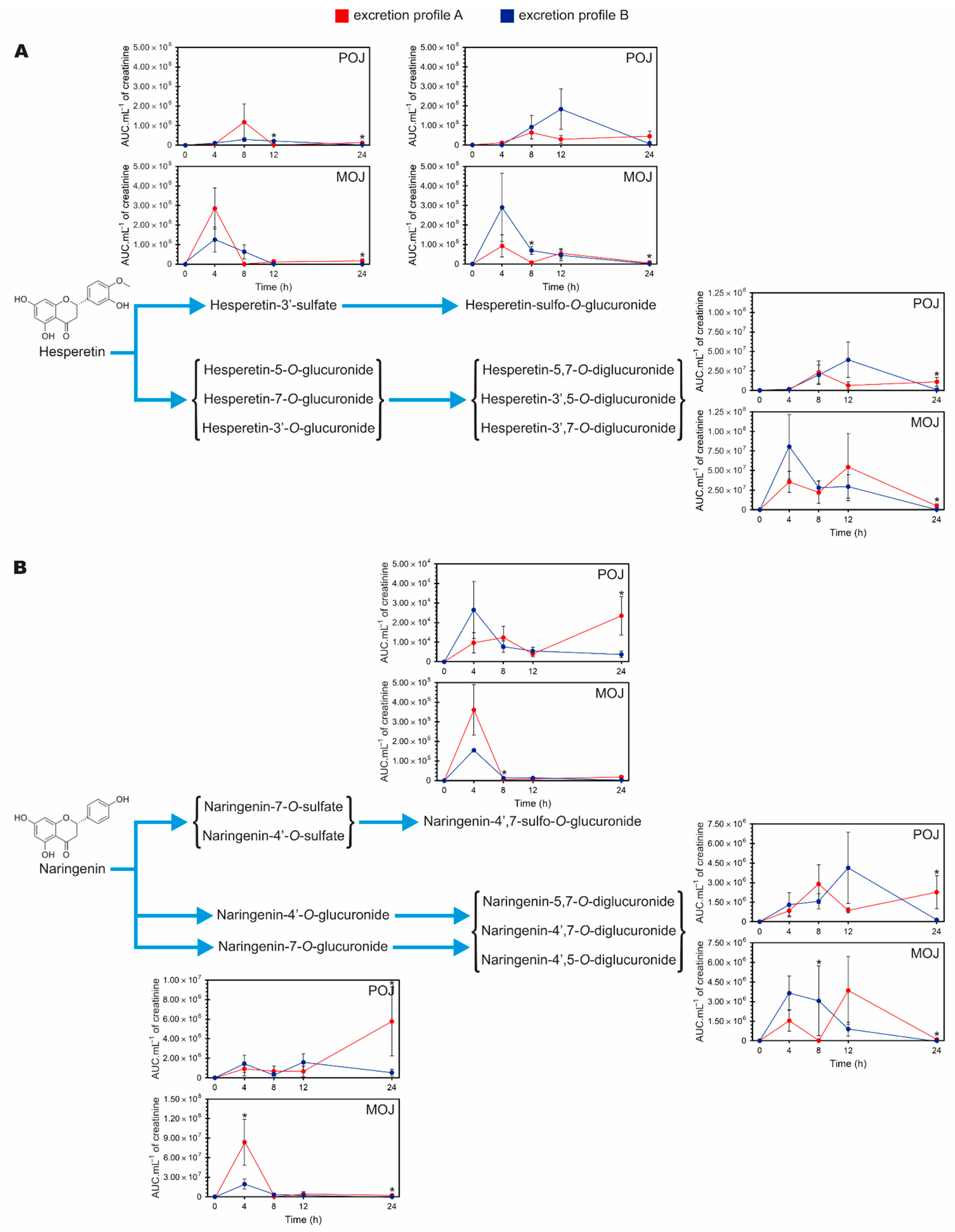
3.6. Accumulation of Excreted Metabolites Over a 24 h Period
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Martí, N.; Mena, P.; Cánovas, J.A.; Micol, V.; Saura, D. Vitamin C and the Role of Citrus Juices as Functional Food. Nat. Prod. Commun. 2009, 4, 677–700. [Google Scholar] [CrossRef]
- Milenkovic, D.; Deval, C.; DuBray, C.; Mazur, A.; Morand, C. Hesperidin Displays Relevant Role in the Nutrigenomic Effect of Orange Juice on Blood Leukocytes in Human Volunteers: A Randomized Controlled Cross-Over Study. PLoS ONE 2011, 6, e26669. [Google Scholar] [CrossRef]
- Pla-Pagà, L.; Companys, J.; Calderón-Pérez, L.; Llauradó, E.; Solà, R.; Valls, R.M.; Pedret, A. Effects of hesperidin consumption on cardiovascular risk biomarkers: A systematic review of animal studies and human randomized clinical trials. Nutr. Rev. 2019, 77, 845–864. [Google Scholar] [CrossRef]
- Valls, R.M.; Pedret, A.; Calderón-Pérez, L.; Lluradó, E.; Pla-Pagà, L.; Companys, J.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of hesperidin in orange juice on blood and pulse pressures in mildly hypertensive individuals: A randomized controlled trial (Citrus study). Eur. J. Nutr. 2020, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tripoli, E.; Guardia, M.L.; Giammanco, S.; Majo, D.D.; Giammanco, M. Citrus flavonoids: Molecular structure, biological activity and nutritional properties: A review. Food Chem. 2007, 104, 466–479. [Google Scholar] [CrossRef]
- Peterson, J.J.; Dwyer, J.T.; Beecher, G.R.; Bhagwat, S.A.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in oranges, tangerines (mandarins), tangors, and tangelos: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S66–S73. [Google Scholar] [CrossRef]
- Anacleto, S.L.; Milenkovic, D.; Kroon, P.A.; Needs, P.W.; Lajolo, F.M.; Hassimotto, N.M.A. Citrus flavanone metabolites protect pancreatic-β cells under oxidative stress induced by cholesterol. Food Funct. 2020, 11, 8612–8624. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, H.; Sia, C.L.; Upadhyay, M.; Korzeniewski, K.; Viswanathan, P.; Abuaysheh, S.; Mohanty, P.; Dandona, P. Orange juice neutralizes the proinflammatory effect of a high-fat, high-carbohydrate meal and prevents endotoxin increase and Toll-like receptor expression. Am. J. Clin. Nutr. 2010, 91, 940–949. [Google Scholar] [CrossRef]
- Rangel-Huerta, O.D.; Aguilera, C.M.; Martin, M.V.; Soto, M.J.; Rico, M.C.; Vallejo, F.; Tomas-Barberan, F.; Perez-De-La-Cruz, A.J.; Gil, A.; Mesa, M.D. Normal or High Polyphenol Concentration in Orange Juice Affects Antioxidant Activity, Blood Pressure, and Body Weight in Obese or Overweight Adults. J. Nutr. 2015, 145, 1808–1816. [Google Scholar] [CrossRef]
- Aptekmann, N.P.; Cesar, T.B. Long-term orange juice consumption is associated with low LDL-cholesterol and apolipoprotein B in normal and moderately hypercholesterolemic subjects. Lipids Health Dis. 2013, 12, 119. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxidative Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed]
- Kerimi, A.; Gauer, J.S.; Crabbe, S.; Cheah, J.W.; Lau, J.; Walsh, R.; Cancalon, P.F.; Williamson, G. Effect of the flavonoid hesperidin on glucose and fructose transport, sucrase activity and glycaemic response to orange juice in a crossover trial on healthy volunteers. Br. J. Nutr. 2019, 121, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Barrett, E.C.; Bresnahan, K.A.; Barraj, L.M. 100 % Fruit juice and measures of glucose control and insulin sensitivity: A systematic review and meta-analysis of randomized controlled trials. J. Nutr. Sci. 2017, 6, e59. [Google Scholar] [CrossRef] [PubMed]
- Morand, C.; DuBray, C.; Milenkovic, D.; Lioger, D.; Martin, J.F.; Scalbert, A.; Mazur, A. Hesperidin contributes to the vascular protective effects of orange juice: A randomized crossover study in healthy volunteers. Am. J. Clin. Nutr. 2011, 93, 73–80. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2097. [Google Scholar] [CrossRef]
- Landberg, R.; Manach, C.; Kerckhof, F.M.; Minihane, A.M.; Saleh, R.N.M.; De Roos, B.; Tomás-Barberán, F.A.; Morand, C.; Van De Wiele, T. Future prospects for dissecting inter-individual variability in the absorption, distribution and elimination of plant bioactives of relevance for cardiometabolic endpoints. Eur. J. Nutr. 2019, 58, S21–S36. [Google Scholar] [CrossRef]
- Gibney, E.; Milenkovic, D.; Combet, E.; Ruskovska, T.; Greyling, A.; González-Sarrías, A.; De Roos, B.; Tomás-Barberán, F.A.; Morand, C.; Rodríguez-Mateos, A. Factors influencing the cardiometabolic response to (poly)phenols and phytosterols: A review of the COST Action POSITIVe activities. Eur. J. Nutr. 2019, 58, 37–47. [Google Scholar] [CrossRef]
- Morand, C.; Tomás Barberán, F.A. Contribution of plant food bioactives in promoting health effects of plant foods: Why look at interindividual variability? Eur. J. Nutr. 2019, 58, 13–19. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (Poly)phenolics in Human Health: Structures, Bioavailability, and Evidence of Protective Effects Against Chronic Diseases. Antiox. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef]
- Williamson, G.; Kay, C.D.; Crozier, A. The Bioavailability, Transport, and Bioactivity of Dietary Flavonoids: A Review from a Historical Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1054–1112. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Dupont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.; Morgan, M.R.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef]
- Hollands, W.; Brett, G.M.; Dainty, J.R.; Teucher, B.; Kroon, P.A. Urinary excretion of strawberry anthocyanins is dose dependent for physiological oral doses of fresh fruit. Mol. Nutr. Food Res. 2008, 52, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Brand, W.; Van Der Wel, P.A.; Rein, M.J.; Barron, D.; Williamson, G.; Bladeren, P.J.; Rietjens, I.M. Metabolism and Transport of the Citrus Flavonoid Hesperetin in Caco-2 Cell Monolayers. Drug Metab. Dispos. 2008, 36, 1794–1802. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.; Chowrimootoo, G.; Choudhury, R.; Debnam, E.S.; Srai, S.K.; Rice-Evans, C. The small intestine can both absorb and glucuronidate luminal flavonoids. FEBS Lett. 1999, 458, 224–230. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Cassidy, A.; Minihane, A.M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Najmanova, I.; Vopršalová, M.; Saso, L.; Mladěnka, P. The pharmacokinetics of flavanones. Crit. Rev. Food Sci. Nutr. 2020, 60, 3155–3171. [Google Scholar] [CrossRef]
- Vallejo, F.; Larrosa, M.; Escudero, E.; Zafrilla, M.P.; Cerdá, B.; Boza, J.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Concentration and Solubility of Flavanones in Orange Beverages Affect Their Bioavailability in Humans. J. Agric. Food Chem. 2010, 58, 6516–6524. [Google Scholar] [CrossRef]
- Tomás-Navarro, M.; Vallejo, F.; Sentandreu, E.; Navarro, J.L.; Tomás-Barberán, F.A. Volunteer Stratification Is More Relevant than Technological Treatment in Orange Juice Flavanone Bioavailability. J. Agric. Food Chem. 2014, 62, 24–37. [Google Scholar] [CrossRef]
- Brett, G.M.; Hollands, W.; Needs, P.W.; Teucher, B.; Dainty, J.R.; Davis, B.D.; Brodbelt, J.S.; Kroon, P.A. Absorption, metabolism and excretion of flavanones from single portions of orange fruit and juice and effects of anthropometric variables and contraceptive pill use on flavanone excretion. Br. J. Nutr. 2009, 101, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Roowi, S.; Mullen, W.; Edwards, C.A.; Crozier, A. Yoghurt impacts on the excretion of phenolic acids derived from colonic breakdown of orange juice flavanones in humans. Mol. Nutr. Food Res. 2009, 53, S68–S75. [Google Scholar] [CrossRef] [PubMed]
- Silveira, J.Q.; Cesar, T.B.; Manthey, J.A.; Baldwin, E.A.; Bai, J.; Raithore, S. Pharmacokinetics of Flavanone Glycosides after Ingestion of Single Doses of Fresh-Squeezed Orange Juice versus Commercially Processed Orange Juice in Healthy Humans. J. Agric. Food Chem. 2014, 62, 12576–12584. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Borges, G.; Van Der Hooft, J.; Clifford, M.N.; Del Rio, D.; Lean, M.E.; Roberts, S.A.; Kellerhals, M.B.; Crozier, A. Orange juice (poly)phenols are highly bioavailable in humans. Am. J. Clin. Nutr. 2014, 100, 1378–1384. [Google Scholar] [CrossRef]
- Aschoff, J.K.; Riedl, K.M.; Cooperstone, J.L.; Högel, J.; Bosy-Westphal, A.; Schwartz, S.J.; Carle, R.; Schweiggert, R.M. Urinary excretion of Citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study. Mol. Nutr. Food Res. 2016, 60, 2602–2610. [Google Scholar] [CrossRef]
- Nagar, S.; Walther, S.; Blanchard, R.L. Sulfotransferase (SULT) 1A1 Polymorphic Variants *1, *2, and *3 Are Associated with Altered Enzymatic Activity, Cellular Phenotype, and Protein Degradation. Mol. Pharmacol. 2006, 69, 2084–2092. [Google Scholar] [CrossRef]
- Stingl, J.C.; Bartels, H.; Viviani, R.; Lehmann, M.L.; Brockmöller, J. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: A quantitative systematic review. Pharmacol. Ther. 2014, 141, 92–116. [Google Scholar] [CrossRef]
- Shiga, T.; Soares, C.A.; Nascimento, J.R.O.; Purgatto, E.; Lajolo, F.M.; Cordenunsi, B.R. Ripening-associated changes in the amounts of starch and non-starch polysaccharides and their contributions to fruit softening in three banana cultivars. J. Sci. Food Agric. 2011, 91, 1511–1516. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis, 16th ed.; AOAC International: Arlington, VA, USA, 1995. [Google Scholar]
- Brasili, E.; Chaves, D.F.; Xavier, A.A.; Mercadante, A.Z.; Hassimotto, N.M.; Lajolo, F.M. Effect of Pasteurization on Flavonoids and Carotenoids in Citrus sinensis (L.) Osbeck cv. ‘Cara Cara’ and ‘Bahia’ Juices. J. Agric. Food Chem. 2017, 65, 1371–1377. [Google Scholar] [CrossRef]
- Hillebrand, S.; Schwarz, M.; Winterhalter, P. Characterization of Anthocyanins and Pyranoanthocyanins from Blood Orange [Citrus sinensis (L.) Osbeck] Juice. J. Agric. Food Chem. 2004, 52, 7331–7338. [Google Scholar] [CrossRef]
- Lohman, T.G. Advances in Body Composition Assessment; Human Kinetics Publishers: Champaing, IL, USA, 1991. [Google Scholar]
- Christoff, A.P.; Sereia, A.F.R.; Boberg, D.R.; Moraes, R.L.V.; Oliveira, L.F.V. Bacterial identification through accurate library preparation and high-throughput sequencing. Neoprospecta Microbiome Technol. 2017, 1–5. [Google Scholar]
- Wang, Y.; Qian, P.Y. Conservative Fragments in Bacterial 16S rRNA Genes and Primer Design for 16S Ribosomal DNA Amplicons in Metagenomic Studies. PLoS ONE 2009, 4, e7401. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Christoff, A.P.; Cruz, G.N.F.; Sereia, F.R.; Yamanaka, L.E.; Silveira, P.P.; Oliveira, L.F.V. End-to-end assessment of fecal bacteriome analysis: From sample processing to DNA sequencing and bioinformatics results. BioRxiv 2019, 1–22. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Chong, J.; Wishart, D.S.; Xia, J. Using Metabo Analyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]
- Manach, C.; Milenkovic, D.; Van De Wiele, T.; Rodriguez-Mateos, A.; De Roos, B.; Garcia-Conesa, M.T.; Landberg, R.; Gibney, E.R.; Heinonen, M.; Tomás-Barberán, F.; et al. Addressing the inter-individual variation in response to consumption of plant food bioactives: Towards a better understanding of their role in healthy aging and cardiometabolic risk reduction. Mol. Nutr. Food Res. 2017, 61, 16. [Google Scholar] [CrossRef]
- Mas-Capdevila, A.; Teichenné, J.; Domenech-Coca, C.; Caimari, A.; Del Bas, J.M.; Escoté, X.; Crescenti, A. Effect of Hesperidin on Cardiovascular Disease Risk Factors: The Role of Intestinal Microbiota on Hesperidin Bioavailability. Nutrients 2020, 12, 1488. [Google Scholar] [CrossRef]
- Bredsdorff, L.; Nielsen, I.L.; Rasmussen, S.E.; Cornett, C.; Barron, D.; Bouisset, F.; Offord, E.; Williamson, G. Absorption, conjugation and excretion of the flavanones, naringenin and hesperetin from α-rhamnosidase-treated orange juice in human subjects. Br. J. Nutr. 2010, 11, 1602–1609. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Ludwig, I.A.; Polyviou, T.; Malkova, D.; García, A.; Moreno-Rojas, J.M.; Crozier, A. Identification of Plasma and Urinary Metabolites and Catabolites Derived from Orange Juice (Poly)phenols: Analysis by High-Performance Liquid Chromatography–High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2016, 64, 5724–5735. [Google Scholar] [CrossRef]
- Kay, C.D.; Mazza, G.J.; Holub, B.J. Anthocyanins Exist in the Circulation Primarily as Metabolites in Adult Men. J. Nutr. 2005, 135, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Stalmach, A.; Edwards, C.A.; Wightman, J.D.; Crozier, A. Gastrointestinal stability and bioavailability of (poly)phenolic compounds following ingestion of Concord grape juice by humans. Mol. Nutr. Food Res. 2012, 56, 497–509. [Google Scholar] [CrossRef] [PubMed]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Su, W.; Bai, Y.; Chen, T.; Yan, Z.; Wang, J.; Su, M.; Zheng, Y.; Peng, W.; Yao, H. Urinary metabolite profiling of flavonoids in Chinese volunteers after consumption of orange juice by UFLC-Q-TOF-MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Oliver, C.M.; Weerakkody, R.; Singh, T.; Conlon, M.; Borges, G.; Sanguansri, L.; Lockett, T.; Roberts, S.A.; Crozier, A.; et al. Chronic administration of a microencapsulated probiotic enhances the bioavailability of orange juice flavanones in humans. Free Radic. Biol. Med. 2015, 84, 206–214. [Google Scholar] [CrossRef]
- Braune, A.; Blaut, M. Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 2016, 7, 216–234. [Google Scholar] [CrossRef]
- Cortés-Martín, A.; Selma, M.V.; Tomás-Barberán, F.A.; González-Sarrías, A.; Espín, J.C. Where to Look into the Puzzle of Polyphenols and Health? The Postbiotics and Gut Microbiota Associated with Human Metabotypes. Mol. Nutr. Food Res. 2020, 64, e1900952. [Google Scholar] [CrossRef]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-Pass Metabolism via UDP-Glucuronosyltransferase: A Barrier to Oral Bioavailability of Phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef]
- Brand, W.; Boersma, M.G.; Bik, H.; Hoek-van Den Hil, E.F.; Vervoort, J.; Barron, D.; Meinl, W.; Glatt, H.; Williamson, G.; Van Bladeren, P.J.; et al. Phase II Metabolism of Hesperetin by Individual UDP-Glucuronosyltransferases and Sulfotransferases and Rat and Human Tissue Samples. Drug Metab. Dispos. 2010, 38, 617–625. [Google Scholar] [CrossRef]
- Mehboob, H.; Tahir, I.M.; Iqbal, T.; Akhter, N.; Munir, N.; Riaz, M. Genetic Polymorphism of UDP-Glucuronosyltransferase. In Genetic Polymorphisms; IntechOpen, Narasimha Reddy Parine, King Saud University: Riyadh, Saudi Arabia, 2017; pp. 159–183. [Google Scholar]
- Kasteel, E.E.J.; Darney, K.; Kramer, N.I.; Dorne, J.L.C.M.; Lautz, L.S. Human variability in isoform-specific UDP-glucuronosyltransferases: Markers of acute and chronic exposure, polymorphisms and uncertainty factors. Arch. Toxicol. 2020, 94, 2637–2661. [Google Scholar] [CrossRef]
- Peters, U.; Sinha, R.; Bell, D.A.; Rothman, N.; Grant, D.J.; Watson, M.A.; Kulldorff, M.; Brooks, L.R.; Warren, S.H.; DeMarini, D.M. Urinary mutagenesis and fried red meat intake: Influence of cooking temperature, phenotype, and genotype of metabolizing enzymes in a controlled feeding study. Environ. Mol. Mutagen. 2004, 43, 53–74. [Google Scholar] [CrossRef] [PubMed]
- Wakeling, L.A.; Ford, D. Polymorphisms in genes involved in the metabolism and transport of soy isoflavones affect the urinary metabolite profile in premenopausal women following consumption of a commercial soy supplement as a single bolus dose. Mol. Nutr. Food Res. 2012, 56, 1794–1802. [Google Scholar] [CrossRef] [PubMed]

| Variable | Non-Obese (n = 17) | Obese (n = 10) | p-Value |
|---|---|---|---|
| Age (years) | 26.76 ± 1.53 | 30.80 ± 3.02 | 0.197 |
| Body weight (kg) | 56.45 ± 1.85 b | 83.29 ± 3.22 a | 0.001 |
| BMI (kg.m−2) | 21.19 ± 0.49 b | 31.56 ± 0.88 a | 0.001 |
| Body fat (%) | 28.23 ± 1.36 b | 47.03 ± 1.74 a | 0.001 |
| Creatinine (mg.dL−1) | 0.71 ± 0.03 | 0.74 ± 0.02 | 0.376 |
| AST (U.L−1) | 18.59 ± 3.45 | 21.80 ± 3.44 | 0.545 |
| ALT (U.L−1) | 18.59 ± 6.75 | 30.00 ± 11.34 | 0.364 |
| Cholesterol (mg.dL−1) | |||
| LDL | 89.71 ± 4.29 | 123.60 ± 17.17 | 0.084 |
| HDL | 69.12 ± 2.57 | 67.50 ± 9.77 | 0.876 |
| Total | 176.94 ± 5.73 b | 217.00 ± 17.85 a | 0.016 |
| Triglycerides (mg.dL−1) | 93.88 ± 9.39 | 175.40 ± 44.57 | 0.104 |
| Glucose (mg/dL) | 85.76 ± 1.82 | 102.00 ± 14.25 | 0.287 |
| Insulin (mU.L−1) | 7.65 ± 1.00 b | 12.30 ± 1.74 a | 0.019 |
| HOMA-IR | 1.64 ± 0.23 b | 3.02 ± 0.46 a | 0.018 |
| Zonulin (ng.mL−1) | 32.63 ± 1.92 | 29.28 ± 2.25 | 0.280 |
| LPS (EU.mL−1) | 0.09 ± 0.04 | 0.05 ± 0.01 | 0.448 |
| Intestinal permeability | 0.02 ± 0.00 | 0.01 ± 0.01 | 0.699 |
| Compound | Peak | RT (min) | [M-H]− (m/z) | MS/MS (m/z) |
|---|---|---|---|---|
| Naringenin-diglucuronide—isomer 1 | 1 | 6.5 | 623.1285 | 447.0976/271.0690 |
| Hesperetin-diglucuronide—isomer 1 | 2 | 8.3 | 653.1352 | 477.1054/301.0735 |
| Naringenin-diglucuronide—isomer 2 | 3 | 8.8 | 623.1274 | 447.1274/271.0624 |
| Naringenin-sulfo-O-glucuronide | 4 | 8.8 | 527.0433 | 271.0585 |
| Hesperetin-diglucuronide—isomer 2 | 5 | 10.6 | 653.1362 | 477.1041/301/0721 |
| Hesperetin-sulfo-O-glucuronide | 6 | 10.6 | 557.0699 | 477.1118/301.0759 |
| Naringenin-7-O-glucuronide * | 7 | 13.5 | 447.0929 | 271.0621 |
| Naringenin-sulfate | 8 | 14.5 | 351.0180 | 271.0622 |
| Hesperetin-glucuronide | 9 | 15.0 | 477.1045 | 301.0728 |
| Hesperetin-sulfate | 10 | 15.9 | 381.0343 | 301.0790 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishioka, A.; Tobaruela, E.d.C.; Fraga, L.N.; Tomás-Barberán, F.A.; Lajolo, F.M.; Hassimotto, N.M.A. Stratification of Volunteers According to Flavanone Metabolite Excretion and Phase II Metabolism Profile after Single Doses of ‘Pera’ Orange and ‘Moro’ Blood Orange Juices. Nutrients 2021, 13, 473. https://doi.org/10.3390/nu13020473
Nishioka A, Tobaruela EdC, Fraga LN, Tomás-Barberán FA, Lajolo FM, Hassimotto NMA. Stratification of Volunteers According to Flavanone Metabolite Excretion and Phase II Metabolism Profile after Single Doses of ‘Pera’ Orange and ‘Moro’ Blood Orange Juices. Nutrients. 2021; 13(2):473. https://doi.org/10.3390/nu13020473
Chicago/Turabian StyleNishioka, Alessandra, Eric de Castro Tobaruela, Layanne Nascimento Fraga, Francisco A. Tomás-Barberán, Franco Maria Lajolo, and Neuza Mariko Aymoto Hassimotto. 2021. "Stratification of Volunteers According to Flavanone Metabolite Excretion and Phase II Metabolism Profile after Single Doses of ‘Pera’ Orange and ‘Moro’ Blood Orange Juices" Nutrients 13, no. 2: 473. https://doi.org/10.3390/nu13020473
APA StyleNishioka, A., Tobaruela, E. d. C., Fraga, L. N., Tomás-Barberán, F. A., Lajolo, F. M., & Hassimotto, N. M. A. (2021). Stratification of Volunteers According to Flavanone Metabolite Excretion and Phase II Metabolism Profile after Single Doses of ‘Pera’ Orange and ‘Moro’ Blood Orange Juices. Nutrients, 13(2), 473. https://doi.org/10.3390/nu13020473








