Abstract
This study investigates the population dynamics of the freshwater bream (Abramis brama) in the Lower Danube River between 2021 and 2025, focusing on growth parameters, mortality rates, length-based recruitment estimates, and the influence of hydrological and water physico-chemical factors. A total of 685 individuals were collected, with an average total length of 31–32 cm and a balanced sex ratio. Growth parameters estimated using the von Bertalanffy Growth Function (VBGF) revealed an asymptotic length (L∞) ranging from 39.9 cm (2021) to 55.7 cm (2024) and growth coefficients (k) between 0.80 and 1.40 year−1. The total mortality (Z) varied from 2.19 to 5.24 year−1, while the exploitation rate (E) reached a maximum of 0.73 in 2025, indicating increased fishing pressure. Length-based recruitment analyses showed a unimodal seasonal pattern, with peak recruitment occurring between June and October and maximum monthly values recorded in September 2025 (29.89%). Pearson correlations indicated that recruitment was positively related to water temperature (r = 0.65) and negatively to average water level (r = –0.63). Recruitment estimates are derived from length-frequency back-calculation and reflect proxies of cohort entry into the exploited stock rather than direct juvenile abundance. These results indicate a consistent seasonal pattern of cohort entry within the exploited component of the population and highlight the role of temperature and river discharge in modulating length-based recruitment signals under variable hydrological conditions.
1. Introduction
The bream (Abramis brama, Linnaeus, 1758) is a key species in freshwater ecosystems, playing a significant role in maintaining trophic balance. Through its omnivorous diet, the bream influences the structure of phytoplankton communities and benthic invertebrate populations [1,2,3].
In addition to its role in the food chain of aquatic ecosystems, the bream contributes to nutrient cycling by redistributing organic material between sediments and the water column [1], thereby influencing water quality. This process facilitates the recycling of organic matter and sustains the dynamics of benthic and pelagic communities. Moreover, bream has proven to be a bioindicator of the ecological status of aquatic habitats [4], due to its high capacity to accumulate heavy metals [5,6,7,8] and its sensitivity to hydrological, physicochemical, and anthropogenic changes. Moreover, bream responds rapidly to chemical and physicochemical stress—through antioxidative, detoxification, and endocrine pathways—highlighting its sensitivity and adaptive capacity in disturbed river environments [9,10].
The river level and water temperature, as well as their variations, are key variables influencing fish habitat and behavior [11,12,13,14]. As a poikilothermic species, breams are particularly vulnerable to sudden temperature fluctuations [15]. Experimental evidence shows that temperature is a key driver of embryonic and larval performance in bream. Optimal development occurs only within a narrow thermal window (10–27 °C). Even small deviations from this range significantly increase mortality and reduce recruitment potential [16].
In the context of length-based stock assessment, recruitment refers to the inferred entry of cohorts into the exploitable population, estimated indirectly from adult length-frequency distributions [17,18].
In population ecology, recruitment broadly describes the contribution of new individuals or cohorts to a population and is a key driver of population dynamics, integrating the effects of growth, mortality, and environmental forcing [17,19,20]. Length-based recruitment analyses allow the identification of seasonal and interannual patterns in cohort entry signals, which can be evaluated in relation to hydroclimatic variability and population processes [21,22,23,24].
Although recruitment has been examined in various marine and freshwater species [25,26,27,28,29,30], many of these studies are relatively dated, and research specifically addressing recruitment patterns of fish in the Danube River is virtually absent. Despite the ecological importance of the Romanian sector of the Danube—often considered a key wetland corridor for fish populations [31]—comprehensive assessments of recruitment drivers in this region remain scarce.
Most available studies predate major hydrological alterations and recent climatic oscillations, and very few integrate biological information with multi-year hydrological and physicochemical datasets. To date, no recent investigation has evaluated how temperature, water level variation and water quality jointly influence length-based recruitment dynamics of A. brama in this large transboundary system. This scientific gap limits our understanding of how environmental factors modulate cohort dynamics within exploited stocks and constrains fisheries management under increasing hydrological instability
Therefore, the present study aims to evaluate the population structure, growth parameters and length-based recruitment variability of A. brama in the Romanian sector of the Lower Danube (2021–2025), and to assess how hydrological and physicochemical conditions influence seasonal and interannual cohort entry patterns inferred from length-frequency data.
2. Materials and Methods
2.1. Study Area
Data were collected through scientific fishing along the entire Romanian stretch of the Danube River, from Moldova Nouă (km 1048, coordinates N 44.9818, E 21.3191) to Galați (km 150, coordinates N 45.4361, E 28.1098) during the period 2021–2025 (Figure 1), between April and October, to maintain temporal comparability across years. Sampling was conducted at five locations (Figure 1): between Baziaș and Moldova Veche (rkm 1047–1071, Iron Gates dam sector), Calafat (rkm 795, mid-upper reach), Giurgiu (km 493, mid-lower reach), Brăila–Chiscani (rkm ~170–190, Small Brăila Island Wetland Complex), and Galați (rkm ~150, Siret/Prut River influence).
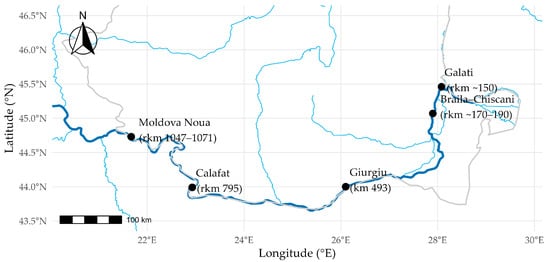
Figure 1.
Study area—Lower Danube River (original). The dark blue line represents the main course of the Danube River, while light blue lines indicate major tributaries. Grey lines show national boundaries. Colored points mark the sampling sites, with labels indicating river kilometer (rkm) positions.
Fixed and drift gillnets (one of each) were operated concurrently, with comparable gear types, mesh-size ranges, deployment duration, and seasonal timing applied across years to allow standardized interannual comparisons of length-based population parameters. Nets were set for approximately 12 h to standardize fishing effort and improve comparability among sites.
The fishing equipment complied with regulatory standards and to ensure sustainable sampling in accordance with Order No. 342/2008 [32], which establishes a minimum legal capture size of 40 cm for bream. Although scientific fishing allows the use of a wide range of gear types, the selection of fishing equipment in this study was intentionally aligned with commercial fishing practices in order to characterize population parameters and cohort signals relevant to the exploited stock. Fixed gillnets had total lengths ranging from 100 to 200 m, vertical heights between 2.5 and 3.5 m, and mesh sizes of 40–60 mm (knot to knot). Drift gillnets exhibited lengths between 150 and 200 m, heights from 2.5 to 4.0 m, and mesh sizes ranging from 40 to 80 mm (knot to knot). Data from both gear types were pooled for length-based analyses, as both nets targeted the same exploited component of the population and analyses did not rely on gear-specific catch efficiency.
2.2. Sampling and Data Handling
Specimens of bream were measured with an ichyometer (with a precision of ±0.01 cm) for total length (cm). Individual weight was recorded with the help of a scale (±0.0 g).
Handling of specimens complied with national legislation on animal protection for scientific purposes [33] and European regulations [34]. Given the limited data, lengths were grouped into classes with different intervals (2021–2023: 2 cm; 2024–2025: 5 cm) to ensure data consistency and statistical comparability across years, allowing analysis of the full time series without significant loss of information. These length classes were subsequently used in the FISAT II software (version 1.2.2) to estimate growth parameters, mortality rates, and annual recruitment values. Recruitment estimates derived from length-frequency data, therefore represent indirect proxies of cohort entry into the exploited stock and do not reflect direct juvenile abundance or age structure.
2.3. Growth Parameters and Mortality Rates—Recruitment Patterns
The von Bertalanffy growth function (VBGF) was applied to estimate growth parameters from length-frequency data [35]. The following parameters were calculated: asymptotic length (L∞, cm), growth coefficient (k, year−1), and the growth performance index (Φ′). The growth performance index (Φ′) is a measure of the efficiency of individual fish growth and was calculated using the formula: Φ′ = log10(K) + 2 × log10(L∞) [36]. For each year, the coefficients a and b of the length-weight relationship were determined through linear regression.
Estimating growth parameters allows for understanding how individuals or populations grow in size or weight in relation to environmental conditions and food availability, providing essential insights for ecosystem health assessment and predictions of environmental impacts on biodiversity.
Mortality rates are key parameters describing negative aspects of fish population dynamics [37]. Stock losses occur due to natural causes (natural mortality, M, year−1) and fishing (fishing mortality, F, year−1). The total annual mortality rate (Z, year−1) was estimated using length-based catch curves [38] in FISAT II. Natural mortality (M, year−1) was calculated using Pauly’s empirical formula [39]. The annual average water temperature (T) was set according to the mean Danube water data, obtained from the National Institute of Hydrology and Water Management—I.N.H.G.A. [40].
The fishing mortality (F, year−1) was obtained from the relationship: F = Z − M [41].
The exploitation rate was calculated as E = F/Z, reflecting the degree of fishing pressure. Quantifying factors leading to fish mortality is crucial for stock assessment and sustainable fisheries management [42].
Recruitment percentage was estimated using FISAT II through Modal Progression Analysis (MPA) by back-calculation. This length-based approach infers temporal patterns of cohort entry into the exploited stock by back-calculation from observed size classes. In data-limited fisheries where age structure and juvenile abundance data are unavailable, length-based methods have been widely used to assess recruitment variability and population dynamics [43,44,45]. Accordingly, recruitment estimates in the present study should be interpreted as indirect proxies of cohort entry into the exploited population rather than direct measures of juvenile abundance or reproductive success [46,47]. Length-based recruitment models describe the temporal distribution of inferred cohort entry signals into the exploited stock. A unimodal pattern indicates a single dominant period of cohort entry within a year, while bimodal patterns suggest multiple cohort entry signals. A more uniform distribution reflects continuous or weakly defined cohort entry signals inferred from length-frequency data, rather than direct reproductive activity [48].
2.4. Hydrological and Physico-Chemical Parameters Variation Assessment of Hydrological and Physico-Chemical Parameters
The analysis of environmental variability was based on daily water level and temperature data of the Danube River collected between 2021 and 2025, obtained from I.N.H.G.A. [40]. Data processing involved calculating the seasonal average values for the analyzed station. The hydrological data for the Danube River from 2021–2024 were previously used in an earlier publication [49], where they were analyzed for a different objective. In the present study, these data are reintegrated to assess the influence of hydrological factors on the recruitment pattern of the bream, thereby justifying their reuse within a distinct analytical framework.
Water samples from the analyzed area were collected to monitor the physico-chemical parameters, using specific analytical kits and standardized procedures. Samples were stored in refrigerated containers and preserved at 4 °C until analysis. The pH was measured using a Consort C532 multi-parameter analyzer (Consort, Turnhout, Belgium). Nitrogen and phosphorus compounds were quantified according to the Standard Methods for the Examination of Water and Wastewater [50], using a LANGE DR 2800 spectrophotometer (Hach Lange GmbH, Düsseldorf, Germany) as part of the water quality analysis kit.
The hydrological and physico-chemical parameters of the water were analyzed on a seasonal scale (winter, spring, summer, autumn). The mean values and the coefficient of variation for each season were correlated with the monthly recruitment percentages.
Environmental factors were examined individually using seasonal aggregation and correlation analyses, allowing the relative influence of each variable on length-based recruitment signals to be evaluated within an observational framework.
2.5. Statistical Analysis
The following software programs were used: FISAT II, XLStat Basic version 2024.4.0, and RStudio 2025.09.1 version. The graphical representations were also relized in RStudio. All data series were tested for normality using the Shapiro–Wilk test, which justified the use of nonparametric tests for comparisons between years when the data did not follow a normal distribution. In this context, differences between years in the length class distribution were analyzed using the Kruskal–Wallis test, appropriate for nonparametric data, while the Chi-squared test was applied to the F/M ratio, comparing the observed values to the theoretical 1:1 ratio.
To assess the variations in length-based recruitment estimates of the exploited bream population during the period 2021–2025, a one-way ANOVA was applied to the monthly recruitment percentages, using a significance level of α = 0.05, with df_between = 4 and df_within = 55, to test for significant differences between years.
Pearson correlations were performed between the annual recruitment rates, physico-chemical parameters, and hydrological characteristics of the Danube River to determine the influence of environmental factors on bream stocks during the analyzed period.
3. Results
Between 2021 and 2025, a total of 685 specimens of bream were collected, with a total biomass of 456.44 kg, distributed relatively evenly over time (Table 1). The mean total length remained around 31–32 cm, while the coefficient of variation (CV) increased from 8.10% in 2021 to 14.54% in 2025.

Table 1.
Morphometric characteristics of Abramis brama (2021–2025).
The Shapiro–Wilk test applied to the length-frequency data showed values significantly below the 0.05 threshold for most years, confirming the non-normal distribution of the data (Supplementary Table S1).
The Kruskal–Wallis test did not reveal significant differences between years in the length-class distribution (χ2 = 4.04, df = 4, p = 0.401), while the length-class distribution showed a clear dominance of the 28–34 cm interval in all years, particularly in 2022 and 2024 (Figure 2).
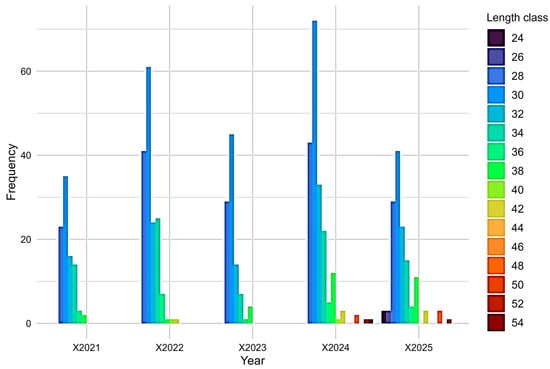
Figure 2.
Length-class distribution for the period 2021–2025.
The mean individual weight remained relatively constant (≈0.68 kg) until 2024, then decreased to 0.59 kg in 2025. The sex ratio (F/M) decreased from 1.07:1 in 2021 to 0.86:1 in 2025 (Figure 3). Chi-squared analysis of the sex ratio over the period 2021–2025 yielded p-values well above the standard significance threshold (α = 0.05), indicating no statistical evidence to reject the null hypothesis that the female-to-male ratio is equal (1:1) in any of the years analyzed. Therefore, the population can be considered sex-balanced throughout the study period.
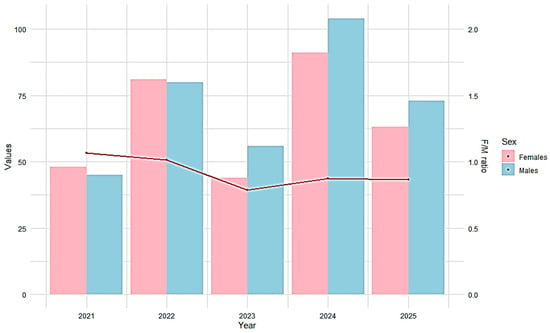
Figure 3.
Sex ratio for A. brama (2021–2025). Pink and blue bars indicate the number of females and males, respectively, and the red line shows the female-to-male (F/M) ratio (right y-axis).
3.1. Growth Parameters and Mortality Rates for Abramis brama—Recuitment Patterns Estimated Growth Parameters, Mortality Rates, and Recruitment Patterns of Abramis brama
The growth parameters estimated using the von Bertalanffy function indicated an increase in asymptotic length L∞ from 39.90 cm in 2021 to 55.65 cm in 2024, followed by a slight decrease to 52.50 cm in 2025. The growth coefficient decreased between 2021 and 2024, suggesting slower growth of larger individuals, then rose again to 1.40 y−1 in 2025. The parameter remained negative, while was relatively constant (3.27–3.59).
Natural mortality (; y−1) ranged from 0.97 in 2024 to 1.43 in 2025, with a slight increase in 2025. Total mortality (; y−1) fluctuated between 2.19 (2024) and 5.24 (2025), reflecting significant interannual variations.
Fishing mortality (; y−1) showed pronounced variability, with low values in 2022 (0.95) and a peak in 2025 (3.81), leading to an increase in the exploitation rate () up to 0.73 in the same year.
The ratio, ranging from 1.02 to 1.22, suggests a relatively stable relationship between natural mortality and growth rate (Table 2).

Table 2.
Growth and mortality parameters of A. brama (2021–2025).
The recruitment analysis revealed a clear seasonal pattern. ANOVA applied to recruitment over the period 2021–2025 indicated no significant differences between years (), suggesting that the recruitment signal did not differ significantly among years. Each year, the recruitment pattern was unimodal.
Recruitment began gradually in January–March (<3%), increasing progressively in April–May (1.2–4.8%). The peak period occurred between June and October, with maxima in August (15.77–22.79%), September (21.87–29.89%), and October (17.19–31.93%). In November–December, recruitment declined sharply, reaching zero in December for all years (Table 3, Figure 4). This pattern indicates a dominant seasonal period of inferred cohort entry into the exploited stock, concentrated between June and October.

Table 3.
Monthly recruitment percentage of A. brama (2021–2025).
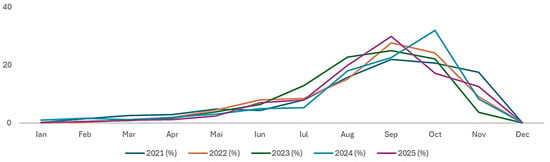
Figure 4.
Monthly recruitment percentage trend (2021–2025).
3.2. Hydrological and Physico-Chemical Parameters Variation
The hydrological parameters of the Danube River showed both seasonal and interannual variations. The mean water level was highest during January–June, peaking in January 2024 (598.52 cm), and lower during the autumn months. In 2025, the reduced levels in the early months should be considered when interpreting the overall trends (Table 4, Figure 5). The mean water temperature exhibited a clear seasonal pattern, with minima in January–February (3.20–7.54 °C) and maxima in July–August (25.86–27.33 °C), along with a slight increase in spring and summer of 2024–2025, suggesting local climatic variations.

Table 4.
Hydrological parameters of the Danube River (2021–2025).
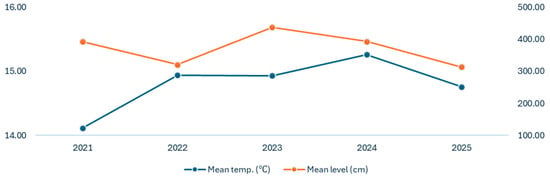
Figure 5.
Danube River’s hydrological parameters trends (2021–2025).
The physicochemical water parameters (pH, organic matter, CCO-Mn, Ca2+, Mg2+, hardness, nitrogen compounds, and chlorides) showed variations between 2021 and 2024 (Table 5).

Table 5.
Annual mean values for main physico-chemical parameters of the Danube River (2021–2024).
3.3. Influence of Hydrological and Physico-Chemical Factors on Length-Based Recruitment Signals
Seasonal correlations highlighted a positive relationship between recruitment and mean temperature (Ts, r = 0.65) and a negative relationship with mean water level (Hs, r = −0.63), while the other parameters exhibited weak correlations (|r| < 0.6) (Figure 6).
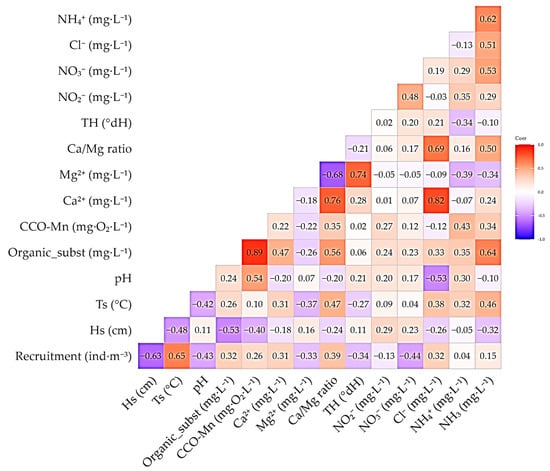
Figure 6.
Pearson Heatmap.
4. Discussion
The analyzed bream population showed relatively stable mean total length over 2021–2025, while the increasing variability observed in the later years indicates a more heterogeneous size structure within the exploited stock, consistent with the presence of multiple size classes. Individuals in the 28–34 cm interval dominated the catches across all years, indicating that the sampled component of the population was mainly composed of medium-sized fish. In 2022 and 2024, the higher frequencies within these classes suggest stronger representation of these size groups in the catches, whereas in 2025 a slight decline in the medium-size classes was accompanied by increased representation of smaller fish (24–26 cm) at the lower end of the sampled range. Very large individuals (>46 cm) were rare or absent, which may reflect natural mortality, size-selective fishing, or limitations in reaching larger sizes under local conditions [51,52,53,54].
This shift in size-class representation, together with the decrease in mean individual weight observed in 2025, may reflect either a redistribution toward smaller size classes within the exploited stock or a potential reduction in individual body condition. Such changes can arise from density-dependent processes, variation in food availability, or increased energetic costs associated with environmental stress or fishing pressure, and do not necessarily imply changes in recruitment intensity.
Potential sampling bias related to gear selectivity and the focus on the exploited stock may influence the relative representation of size classes and should be considered when interpreting these results.
Comparable studies report similar dimensional structures for Abramis brama in the Danube River, with total lengths ranging from 12 to 48 cm and an average of 33.86 ± 0.18 cm, and weights between 34 and 1500 g (mean 670 ± 8.83 g) [55]. Similar size ranges have been reported from other Danube sectors, including the Croatian stretch—total length 23.9–46.1 cm; body weight 310–2800 g [56] and the Serbian sector near Belgrade—total length 30–37 cm; body weight 359–687 g [57], supporting the dominance of medium-sized individuals and the low representation of very large specimens. The sex ratio (F/M) showed a steady decline after 2022, indicating a male predominance from 2023 onward. The sex ratio (F/M) showed a steady decline after 2022, indicating a numerical predominance of males from 2023 onward, which may reflect sex-specific behavior [58], spatial segregation during certain periods [59,60], and gear selectivity affecting catch composition [61,62] rather than population-level demographic processes.
However, the Chi-squared test suggests that the observed variations are not statistically significant; the population can be considered sex-balanced, with a sex ratio close to 1:1 throughout the studied period.
4.1. Growth Parameters and Mortality Rates for Abramis brama—Recuitment Patterns
The growth and mortality parameters indicate a population with moderate growth and relative stability in growth rate and mortality. The asymptotic length (L∞) increased between 2021 and 2024, with a slight decline in 2025 which may reflect interannual variability in environmental conditions or differences in cohort structure. The L∞ and K values are higher in most years than those reported for specimens from the Beni-Haroun Dam (Algeria) [63], indicating interannual variability in growth dynamics, with the highest growth coefficient (k) recorded in 2025, yet lower than values for the Croatian Danube bream [56].
In 2025, deviations in population parameters coincided with lower mean water levels and higher fishing mortality (F) and exploitation rate (E). No specific extreme anthropogenic or natural disturbance events were identified for the study area in 2025; therefore, observed changes are interpreted in relation to documented hydrological variability and exploitation intensity.
Compared to the values reported for catches from 2018–2019 in the lower Danube [55], the L∞ obtained in this study is similar, but the growth coefficient k is higher, indicating that individuals in the studied sector reach maximum size faster due to favorable local conditions, food availability, or population-specific adaptations. This pattern may be associated with favorable local conditions, food availability, or population-specific growth strategies. It is worth noting that the area investigated by Ibanescu and Dediu [55] is included within the broader range analyzed in the present study, which may explain the lower K values previously reported, given the smaller number of specimens and the more limited spatial coverage considered in that analysis.
In addition, differences in the length composition of the analyzed samples likely contributed to the observed discrepancies, as the dataset used by Ibanescu and Dediu [55] included smaller individuals (down to 12 cm), whereas the present study predominantly comprises older and larger fish, a factor known to influence length-based estimates of growth parameters.
Mortality dynamics show a direct correlation between increased fishing pressure (F, E). High fishing mortality in 2025 could suggest overpressure on adult individuals, potentially reducing the contribution of adult cohorts to subsequent length-based recruitment signals within the exploited stock [64].
In contrast, the moderate exploitation rates observed during 2022–2024 (E < 0.6), together with relatively low total mortality (Z ≈ 2.2), were associated with more stable cohort representation in the exploited population. The sharp increase in F and E in 2025, combined with high natural mortality (M = 1.43), may indicate a cumulative effect of environmental stress and exploitation [65,66]. Thus, the variations in M, F, and E over the studied period could reflect the complex interaction between anthropogenic pressure and the population resilience, providing insight into the dynamics of cohort entry into the exploitable stock.
The length-based recruitment signals exhibited a clear seasonal pattern, with minimal values during January–March (<3%) and no detectable signal in December. The dominant period of inferred cohort entry into the exploited stock occurred between June and October, with maximum values recorded in August, September, and October. This seasonal timing is broadly comparable with the extended recruitment periods reported for tropical species such as Monodactylus sebae (Cuvier, 1829) by Olopade [30], although differences in duration and intensity likely reflect contrasting climatic regimes between temperate and tropical ecosystems.
Interannual variability in the magnitude of length-based recruitment signals, including a pronounced peak in September 2025 (29.89%), suggests year-to-year differences in cohort representation within the exploited stock. Such variability is commonly observed in length-based stock assessments and may reflect the combined influence of environmental conditions [67], growth dynamics, and mortality processes acting on adult cohorts rather than direct measures of reproductive output.
The overall stability of the seasonal recruitment signal over the five-year period indicates a consistent pattern of cohort entry into the exploitable population, contrasting with the highly variable recruitment dynamics reported for many marine and coral reef fishes [25,26,27,68]. These differences are consistent with species-specific life-history strategies and the pronounced hydrological and thermal seasonality characteristic of temperate freshwater systems, such as the Danube River, unlike the relatively stable tropical environments analyzed by Olopade [30].
4.2. Analysis of the Physico-Chemical and Hydrological Variability of the Danube River
The analysis of the physico-chemical and hydrological variability of the Danube River indicates that water level and temperature directly influence habitat conditions and growth-related processes in fish populations. Higher water levels in winter and spring enhance habitat availability and lateral connectivity, whereas lower levels in autumn may limit available resources. The seasonal pattern of water temperature influences key physiological processes essential for cohort development; slight increases in mean temperature in 2024–2025 modulate growth dynamics and cohort development.
Chemical parameters were relatively stable, with slightly alkaline pH, variable organic matter, similar trends in CCO-Mn, and low nitrite and nitrate levels. Fluctuations in calcium and magnesium affect water hardness, while the increase in chlorides in 2024 may reflect anthropogenic influences or hydrological variability.
Nearly negligible concentrations of ammonium and ammonia indicate favorable conditions for aquatic fauna, suggesting that the studied system is relatively stable from a physico-chemical perspective. The results fall in the legal limits set by Order no. 161/2006 [69].
4.3. Influence of Hydrological and Physico-Chemical Factors on the Recruitment Pattern
Hydrological and physico-chemical factors appear to influence the recruitment of cohorts of bream in the Lower Danube. Positive seasonal correlations with mean water temperature are associated with a stronger representation of cohorts entering the exploited stock [39,70,71,72,73]. In contrast, the negative correlation with mean water levels indicates that higher water levels may reduce the intensity of cohort recruitment signals by altering habitats or promoting spatial redistribution within the river–floodplain system [15,74]. The patterns observed in 2025 are interpreted strictly in relation to measured hydrological variability and fishing pressure, without evidence for specific extreme anthropogenic or natural disturbance events.
Differences between seasonal and monthly correlations highlight timing-dependent effects: in certain months (January–February), higher flows may be beneficial due to access to additional habitats, higher dissolved oxygen, and increased food distribution, while in other months (spring and summer) the same high levels may be unfavorable [67,74]. The strong positive correlation observed in October may indicate a late-season enhancement of cohort recruitment signals or conditions favoring cohort representation within the exploited stock, consistent with findings that temperature fluctuations and hydrological variability can drive asynchronous cohort recruitment patterns in freshwater species [12,68]. Thus, the relationship between water level and recruitment is non-linear and depends on the timing and local conditions cohort entry into the exploited population [75,76].
Temperature exhibits a clear biphasic effect—favorable at the beginning of the main cohort recruitment period (February–June) but detrimental during periods of high temperatures (July–August), which can induce thermal stress affect growth and mortality processes. Recruitment is not solely temperature-dependent for bream; in other species such as cod, studies [25,26] have shown a positive correlation with temperature in northern areas of the range, whereas in warmer southern regions, the relationship is reversed. Henriksen et al. [77] showed that in sandeel species (Ammodytes spp.) from the North Sea, elevated temperatures reduce cohort persistence and their representation in adult size distributions, resulting in weaker length-based recruitment signals. In contrast, Lindmark et al. [78] reported for freshwater fishes, including European perch (Perca fluviatilis, Linnaeus, 1758), that moderate warming enhances growth and cohort representation, whereas higher temperatures increase metabolic stress and mortality, ultimately weakening length-based recruitment signals. These observations indicate that the influence of temperature on recruitment can be complex and site-specific, which is relevant for interpreting recruitment patterns of the bream in the analyzed section of the Danube.
The results support the RRSM (Riverscape Recruitment Synthesis Model) hypothesis [12], which posits that recruitment dynamics are closely linked to hydrological regimes, with periods of high-water cohort dynamics through habitat availability and spatial connectivity. The biological strategy of bream, characterized by seasonal reproduction, aligns with the cohort filtering proposed by RRSM, providing a conceptual framework for interpreting cohort recruitment patterns in the lower Danube. These findings suggest that maintaining natural variability in hydrological regimes and lateral connectivity is crucial for the sustainable management of the population.
5. Conclusions
The population of Abramis brama in the analyzed section of the Danube River exhibited stable mean lengths (31–32 cm) during 2021–2025, with increased variability in recent years, indicating the presence of multiple cohorts within the exploited stock. Predominant length classes (28–34 cm) suggest a mature population dominated by medium-sized individuals within the exploited stock. Very large individuals (>46 cm) were rare, likely reflecting natural mortality, selective fishing, or growth limitations in larger fish.
The sex ratio (F/M) showed a slight decline, indicating a gradual predominance of males after 2022. Nevertheless, statistical analysis indicates that the population remains sex-balanced (approximately 1:1), without significant imbalances caused by fishing pressure or differential mortality.
Growth was moderate, with interannual variations in maximum length and growth rate. Values of growth parameters L∞, k, and M/k suggest a population well adapted to local conditions, while variations in L50 and L75 reflect differences in population structure and sexual maturation rates between years.
Recruitment modeling based on length-frequency data indicates a dominant seasonal pattern of cohort entry into the exploited population between June and October, with peak recruitment signals in August–October. Low recruitment in January–March and their absence in December confirm pronounced seasonality in cohort recruitment. The stability of recruitment patterns over the studied period suggests a resilient population structure with consistent cohort representation over time. Recruitment modeling enables the identification of critical periods for stock replenishment, providing scientific support for establishing closed seasons, protecting key habitats relevant for cohort persistence, and assessing population resilience.
Water temperature showed a positive correlation with cohort recruitment signals, indicating that warmer periods are associated with stronger recruitment estimates. Water levels showed an overall negative correlation. Physico-chemical water parameters (pH, hardness, organic matter, nitrogen, and chlorides) indicate a relatively stable environment that supports growth processes and cohort persistence within the population.
Overall, the results could suggest that the bream population in the lower Danube is stable and well adapted to local conditions, with predictable seasonal cohort recruitment. These findings provide a robust basis for fisheries management planning and long-term resource conservation.
It should be noted that recruitment estimates in this study are derived from length-frequency-based modeling and represent inferred cohort entry into the exploited component of the bream population, rather than direct observations of early life stages or recruitment processes at the population-wide level; therefore, the results should be interpreted as proxy-based indicators subject to the inherent limitations of calculated, not observed, data.
For a more comprehensive understanding of recruitment processes, future analyses should integrate less-explored biological and ecological factors, such as cohort genetic variability, physiological plasticity at the cohort level and trophic changes in key habitats.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su18031260/s1, Table S1: Shapiro–Wilk normality test results for total length frequencies (2021–2025).
Author Contributions
Conceptualization, A.D. and M.D.S.; methodology, A.D. and M.D.S.; validation, M.D.S.; formal analysis, A.D.; investigation, M.D.S.; resources, F.M.D.; data curation, M.D.S.; writing—original draft preparation, A.D.; writing—review and editing, M.D.S.; visualization, F.M.D.; supervision, F.M.D.; funding acquisition, F.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The article processing charge (APC) was paid by the Institute of Research and Development for Aquatic Ecology, Fisheries and Aquaculture (ICDEAPA), Galați, Romania.
Institutional Review Board Statement
This study involved only observational biometric measurements of wild bream (Abramis brama); no experimental manipulations, invasive procedures, or prolonged laboratory maintenance were performed. All sampling activities were conducted in accordance with the principles of animal welfare and with Directive 2010/63/EU of the European Parliament and of the Council (22 September 2010) on the protection of animals used for scientific purposes. The Bioethics Commission, constituted by Decision no. 52/16.03.2023 at the Research and Development Institute for Aquatic Ecology, Fishing and Aquaculture, Galați, supervised the measurement activities.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request.
Acknowledgments
This work was supported by the technical assistance of the ADER 14.1.1 project—Studies on monitoring and evaluation of habitats specific to fishery resources to determine the total allowable catch, fishing effort, sustainability, and stock conservation in relation to current climate changes.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Breukelaar, A.W.; Lammens, E.H.; Breteler, J.G.K.; Tatrai, I. Effects of benthivorous bream (Abramis brama) and carp (Cyprinus carpio) on sediment resuspension and concentrations of nutrients and chlorophyll a. Freshw. Biol. 1994, 32, 113–121. [Google Scholar] [CrossRef]
- Kakareko, T. The diet, growth and condition of common bream, Abramis brama (L.) in Włocławek Reservoir. Acta Ichthyol. Piscat. 2001, 31, 37–53. [Google Scholar] [CrossRef]
- Khristenko, D.S.; Kotovska, G.O. Length–weight relationship and condition factors of freshwater bream Abramis brama (Linnaeus, 1758) from the Kremenchug Reservoir, Middle Dnieper. Turk. J. Fish. Aquat. Sci. 2017, 17, 71–77. [Google Scholar] [CrossRef]
- Teubner, D.; Paulus, M.; Veith, M.; Klein, R. Biometric parameters of the bream (Abramis brama) as indicators for long-term changes in fish health and environmental quality—Data from the German ESB. Environ. Sci. Pollut. Res. Int. 2015, 22, 1620–1627. [Google Scholar] [CrossRef] [PubMed]
- Farkas, A.; Salánki, J.; Specziár, A. Age- and size-specific patterns of heavy metals in the organs of freshwater fish Abramis brama L. populating a low-contaminated site. Water Res. 2003, 37, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Salánki, J.; V.-Balogh, K.; Berta, E. Heavy metals in animals of Lake Balaton. Water Res. 1982, 16, 1147–1152. [Google Scholar] [CrossRef]
- Farkas, A.; Salánki, J.; Varanka, I. Heavy metal concentrations in fish of Lake Balaton. Lakes Reserv. Res. Manag. 2000, 5, 271–279. [Google Scholar] [CrossRef]
- Kostić, J.; Kolarević, S.; Kračun-Kolarević, M.; Aborgiba, M.; Gačić, Z.; Lenhardt, M.; Vuković-Gačić, B. Genotoxicity Assessment of the Danube River Using Tissues of Freshwater Bream (Abramis brama). Environ. Sci. Pollut. Res. 2016, 23, 20783–20795. [Google Scholar] [CrossRef]
- Tenji, D.; Micic, B.; Sipos, S.; Miljanovic, B.; Teodorovic, I.; Kaisarevic, S. Fish Biomarkers from a Different Perspective: Evidence of Adaptive Strategy of Abramis brama (L.) to Chemical Stress. Environ. Sci. Eur. 2020, 32, 47. [Google Scholar] [CrossRef]
- Reinen, J.; Suter, M.J.-F.; Vögeli, A.C.; Fernandez, M.F.; Kiviranta, H.; Eggen, R.I.L.; Vermeulen, N.P.E. Endocrine Disrupting Chemicals—Linking Internal Exposure to Vitellogenin Levels and Ovotestis in Abramis brama from Dutch Surface Waters. Environ. Toxicol. Pharmacol. 2010, 30, 209–223. [Google Scholar] [CrossRef]
- Aadland, L.P. Stream habitat types: Their fish assemblages and relationship to flow. N. Am. J. Fish. Manag. 1993, 13, 790–806. [Google Scholar] [CrossRef]
- Humphries, P.; King, A.; McCasker, N.; Kopf, R.K.; Stoffels, R.; Zampatti, B.; Price, A. Riverscape recruitment: A conceptual synthesis of drivers of fish recruitment in rivers. Can. J. Fish. Aquat. Sci. 2020, 77, 213–225. [Google Scholar] [CrossRef]
- Seneviratne, S.I.; Zhang, X.; Adnan, M.; Badi, W.; Dereczynski, C.; Di Luca, A.; Ghosh, S.; Iskandar, I.; Kossin, J.; Lewis, S.; et al. Weather and climate extreme events in a changing climate. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021. [Google Scholar] [CrossRef]
- Mariu, A.; Chatha, A.M.M.; Naz, S.; Khan, M.F.; Safdar, W.; Ashraf, I. Effect of Temperature, pH, Salinity and Dissolved Oxygen on Fishes. J. Zool. Syst. 2023, 1, 1–12. [Google Scholar] [CrossRef]
- Houde, E.D. Recruitment Variability. In Fish Reproductive Biology; Jakobsen, T., Fogarty, M.J., Megrey, B.A., Moksness, E., Eds.; Wiley: Hoboken, NJ, USA, 2016; Chapter 3. [Google Scholar] [CrossRef]
- Kucharczyk, D.; Luczynski, M.; Kujawa, R.; Czerkies, P. Effect of Temperature on Embryonic and Larval Development of Bream (Abramis brama L.). Aquat. Sci. 1997, 59, 214–224. [Google Scholar] [CrossRef]
- Pepin, P. Reconsidering the impossible—Linking environmental drivers to growth, mortality, and recruitment of fish. Can. J. Fish. Aquat. Sci. 2016, 73, 205–215. [Google Scholar] [CrossRef]
- Subbey, S.; Devine, J.A.; Schaarschmidt, U.; Nash, R.D.M. Modelling and forecasting stock–recruitment: Current and future perspectives. ICES J. Mar. Sci. 2014, 71, 2307–2322. [Google Scholar] [CrossRef]
- Gaillard, J.M.; Coulson, T.; Festa-Bianchet, M. Recruitment. In Encyclopedia of Ecology; Jørgensen, S.E., Fath, B.D., Eds.; Elsevier: Oxford, UK, 2008; Volume 4, pp. 2982–2986. [Google Scholar] [CrossRef]
- Lorenzen, K. Population dynamics and potential of fisheries stock enhancement: Practical theory for assessment and policy analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 171–189. [Google Scholar] [CrossRef]
- Allen, M.S.; Hightower, J.E. Fish Population Dynamics: Mortality, Growth, and Recruitment. In Inland Fisheries Management in North America, 3rd ed.; American Fisheries Society: Bethesda, MD, USA, 2010; pp. 43–79. [Google Scholar]
- Danilov, M.B.; Kriksunov, E.A.; Bobyrev, A.E.; Sheremet’ev, A.D.; Mel’nik, M.M.; Severin, S.O.; Vasilev, P.V.; Chistov, S.V. Population Dynamics of the Bream Abramis brama in Lake Peipus. J. Ichthyol. 2020, 60, 593–607. [Google Scholar] [CrossRef]
- Ding, H.; Zhang, Z.; Xie, C.; Liu, C.; Chen, F.; Huang, D.; Li, Z.; Guo, Y.; Chen, Y. An Assessment of European Bream Abramis brama (Linnaeus, 1758) Fishery in the Downstream of the Irtysh River in China. J. Freshw. Ecol. 2019, 34, 107–122. [Google Scholar] [CrossRef]
- Caserta, V.; Punt, A.E.; Arneri, A.; Berginc, T.; Kec, V.C.; Costantini, I.; de Felice, A.; Donato, F.; Leonori, I.; Santojanni, A.; et al. Incorporating key environmental drivers in European anchovy (Engraulis encrasicolus) stock assessment model in the Adriatic Sea. ICES J. Mar. Sci. 2025, 82, fsaf171. [Google Scholar] [CrossRef]
- Neill, W.H.; Miller, J.M.; Van Der Veer, H.W.; Winemiller, K.O. Ecophysiology of marine fish recruitment: A conceptual framework for understanding interannual variability. Neth. J. Sea Res. 1994, 32, 135–152. [Google Scholar] [CrossRef]
- Planque, B.; Fox, C.J. Interannual variability in temperature and the recruitment of Irish Sea cod. Mar. Ecol. Prog. Ser. 1998, 172, 101–105. [Google Scholar] [CrossRef]
- Sundby, S. Recruitment of Atlantic cod stocks in relation to temperature and advection of copepod populations. Sarsia 2000, 85, 277–298. [Google Scholar] [CrossRef]
- Hufnagl, M.; Huebert, K.B.; Temming, A. How does seasonal variability in growth, recruitment, and mortality affect the performance of length-based mortality and asymptotic length estimates in aquatic resources? ICES J. Mar. Sci. 2013, 70, 329–341. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, C.; Xu, B.; Xue, Y.; Ren, Y.; Chen, Y. Accounting for seasonal growth in per-recruit analyses: A case study of four commercial fish in coastal China seas. Front. Mar. Sci. 2021, 8, 567240. [Google Scholar] [CrossRef]
- Olopade, O.A. Stock assessment of the African moony fish (Monodactylus sebae) (Cuvier, 1829) in the New Calabar, Nigeria. Int. J. Appl. Biol. 2022, 6, 161–171. [Google Scholar] [CrossRef]
- Ibănescu, D.; Popescu, A.; Nica, A. Estimation of growth and mortality parameters of the Pontic shad (Alosa immaculata Bennett, 1835) in Romanian section of the Danube River. Sci. Pap.–Anim. Husb. Ser. 2016, 67, 165–169. [Google Scholar]
- Ministry of Agriculture and Rural Development. Order No. 342/2008 Regarding the Minimum Individual Dimensions of Living Aquatic Resources in the Public Domain of the State, by Species, That Can Be Captured from the Aquatic Environment. Official Gazette, No. 410/2.06.2008. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/268545 (accessed on 3 March 2025).
- Romanian Parliament. Law No. 43/2014 on the Protection of Animals Used for Scientific Purposes. Official Gazette, No. 326, 6 May 2014. Available online: https://legislatie.just.ro/Public/DetaliiDocument/157944 (accessed on 28 August 2025).
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 33–79. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj/eng (accessed on 28 August 2025).
- Von Bertalanffy, L. A quantitative theory of organic growth (Inquiries on Growth Laws. II). Hum. Biol. 1938, 10, 181–213. [Google Scholar]
- Munro, J.L.; Pauly, D. A simple method for comparing the growth of fishes and invertebrates. Fishbyte 1983, 1, 5–6. [Google Scholar]
- Navodaru, I. The Estimation of the Fish and Fisheries Stocks; Dobrogea: Constanța, Romania, 2008. [Google Scholar]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations; Bull. Fish. Res. Board Can. 191: Ottawa, ON, Canada, 1975; pp. 1–382. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- National Institute of Hydrology and Water Management (INHGA). Hydrological Diagnosis and Forecast for the Danube at the Entry into Romania and Along the Romanian Sector. 2025. Available online: https://www.hidro.ro/bulletin_type/diagnoza-si-prognoza-pentru-dunare/ (accessed on 10 October 2025).
- Gulland, J.A. The Fish Resources of the Ocean; Fishing News Books: London, UK, 1971. [Google Scholar]
- Santos, R.; Peixoto, U.I.; Medeiros-Leal, W.; Novoa-Pabon, A.; Pinho, M. Growth Parameters and Mortality Rates Estimated for Seven Data-Deficient Fishes from the Azores Based on Length-Frequency Data. Life 2022, 12, 778. [Google Scholar] [CrossRef]
- Rudd, M.B.; Thorson, J.T. Accounting for variable recruitment and fishing mortality in length-based stock assessments for data-limited fisheries. Can. J. Fish. Aquat. Sci. 2018, 75, 1019–1035. [Google Scholar] [CrossRef]
- Medeiros-Leal, W.; Santos, R.; Peixoto, U.I.; Casal-Ribeiro, M.; Novoa-Pabon, A.; Sigler, M.F.; Pinho, M. Performance of length-based assessment in predicting small-scale multispecies fishery sustainability. Rev. Fish Biol. Fish. 2023, 33, 819–852. [Google Scholar] [CrossRef]
- Gulland, J.A.; Rosenberg, A.A. A Review of Length-Based Approaches to Assessing Fish Stocks; FAO Fisheries Technical Paper. No. 323; FAO: Rome, Italy, 1992; 100p, Available online: https://www.fao.org/4/t0535e/t0535e00.htm (accessed on 6 January 2026).
- Pauly, D. Some Simple Methods for the Assessment of Tropical Fish Stocks; FAO Fisheries Technical Papers No. 234; FAO: Rome, Italy, 1983; 52p. [Google Scholar]
- Moreau, J.; Cuende, F.X. On Improving the Resolution of the Recruitment Patterns of Fishes. ICLARM Fishbyte 1991, 9, 45–46. [Google Scholar]
- Gayanilo, F.C., Jr.; Sparre, P.; Pauly, D. FAO-ICLARM Stock Assessment Tools II (FiSAT II). Revised Version. User’s Guide; FAO Computerized Information Series (Fisheries). No. 8, Revised Version; FAO: Rome, Italy, 2005; 168p, Available online: https://openknowledge.fao.org/handle/20.500.14283/y5997e (accessed on 8 January 2026).
- Stroe, M.; Dobre, A.; Dima, F.M.; Dediu, L.; Macoveiu (Dobre), A.N.; Rochard, E. Effects of Climate Change on Cyprinus Carpio (Linnaeus, 1758) in the Danube River (2021–2024): Analysis of Sex Ratios, Condition and Length-Class Distribution. Sci. Pap. Ser. D Anim. Sci. 2025, 68, 789–800. [Google Scholar]
- American Public Health Association (APHA); American Water Works Association (AWWA); Water Environment Federation (WEF). Standard Methods for the Examination of Water and Wastewater, 24th ed.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Arlinghaus, R.; Lorenzen, K.; Johnson, B.; Cooke, S.; Cowx, I. Management of freshwater fisheries: Addressing habitat, people and fishes. In Freshwater Fisheries Ecology; Craig, J.F., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 557–579. [Google Scholar] [CrossRef]
- Shephard, S.; Davidson, I.C.; Walker, A.M.; Gargan, P.G. Length-based indicators and reference points for assessing data-poor stocks of diadromous trout Salmo trutta. Fish. Res. 2018, 199, 36–43. [Google Scholar] [CrossRef]
- Ahrens, R.N.M.; Allen, M.S.; Walters, C.; Arlinghaus, R. Saving large fish through harvest slots outperforms the classical minimum-length limit when the aim is to achieve multiple harvest and catch-related fisheries objectives. Fish Fish. 2020, 21, 483–510. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Hou, G.; Huang, Z.; Shi, D.; Chen, Z.; Qiu, Y. Length-Based Assessment of Fish Stocks in a Data-Poor, Jointly Exploited (China and Vietnam) Fishing Ground, Northern South China Sea. Front. Mar. Sci. 2021, 8, 718052. [Google Scholar] [CrossRef]
- Ibănescu, D.C.; Dediu, L. Growth of bream, Abramis brama (Linnaeus, 1758), in the Romanian section of the Danube River. Sci. Pap. Ser. D Anim. Sci. 2022, 65, 437–441. [Google Scholar]
- Treer, T.; Opacak, A.; Anicic, I.; Safner, R.; Piria, M.; Odak, T. Growth of bream, Abramis brama, in the Croatian section of the Danube. Czech J. Anim. Sci. 2003, 48, 251–256. [Google Scholar]
- Kostić-Vuković, J.; Kolarević, S.; Kračun-Kolarević, M.; Višnjić-Jeftić, Ž.; Rašković, B.; Poleksić, V.; Gačić, Z.; Lenhardt, M.; Vuković-Gačić, B. Temporal variation of biomarkers in common bream Abramis brama (L., 1758) exposed to untreated municipal wastewater in the Danube River in Belgrade, Serbia. Environ. Monit. Assess. 2021, 193, 465. [Google Scholar] [CrossRef] [PubMed]
- Passos, C.; Tassino, B.; Rosenthal, G.G.; Reichard, M. Reproductive Behavior and Sexual Selection in Annual Fishes. In Annual Fishes: Life History Strategy, Diversity, and Evolution; Berois, N., García, G., de Sá, R.O., Eds.; CRC Press/Taylor & Francis Group: Boca Raton, FL, USA, 2016; Chapter 12. [Google Scholar] [CrossRef]
- Millar, R.B.; Mainguy, J. A general methodology for the estimation of gillnet size-selectivity and population length frequencies. ICES J. Mar. Sci. 2025, 82, fsaf226. [Google Scholar] [CrossRef]
- Moraes, K.; Souza, A.T.; Vašek, M.; Říha, M.; Kubečka, J. Detailed Insight into Gillnet Catches: Fish Directivity and Micro Distribution. Water 2024, 16, 2683. [Google Scholar] [CrossRef]
- FAO. Sex, Maturity and Fecundity. In Manual of Fisheries Science Part 2—Methods of Resource Investigation and Their Application; Holden, M.J., Raitt, D.F.S., Eds.; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1974; Chapter 5; Available online: https://www.fao.org/4/F0752E/F0752E05.HTM (accessed on 8 January 2026).
- Itoh, T. Can sex differences in spatiotemporal distribution and age composition explain the female-biased sex ratio observed in the catch of butterfly kingfish Gasterochisma melampus? Fish. Sci. 2024, 90, 723–732. [Google Scholar] [CrossRef]
- Mounia, T.; Ramzi, H.; Bouziani, M.C.; Kaouachi, N. Length-Weight Relationships of the Bream Abramis brama (Linnaeus, 1758) in Beni-Haroun Dam of Mila City (North-East of Algeria). J. Biores. Manag. 2022, 9, 24–34. [Google Scholar]
- Rosciszewski-Dodgson, M.J.; Cirella, G.T. Environmental drivers affecting the status of top commercial fish stocks in the Baltic Sea: Review. Front. Mar. Sci. 2024, 11, 1399707. [Google Scholar] [CrossRef]
- Fréon, P.; Cury, P.; Shannon, L.; Roy, C. Sustainable exploitation of small pelagic fish stocks challenged by environmental and ecosystem changes: A review. Bull. Mar. Sci. 2005, 76, 385–462. [Google Scholar]
- Cheilari, A.; Raetz, H. The effect of natural mortality on the estimation of stock state parameters and derived references for sustainable fisheries management. In Proceedings of the ICES Annual Science Conference 2009, Copenhagen, Denmark, 21–25 September 2009; International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2009; pp. 1–12. [Google Scholar]
- Whiterod, N.S.; Hammer, M.; Vilizzi, L. Linking the recruitment and survivorship of a freshwater stream-specialist fish species to flow metrics in Mediterranean climate temporary streams. Hydrol. Sci. J. 2017, 62, 2614–2630. [Google Scholar] [CrossRef]
- Doherty, P.J.; Williams, D.M. The replenishment of coral reef fish populations. Oceanogr. Mar. Biol. 1988, 26, 487–515. [Google Scholar]
- Ministry of Environment and Water Management. Order No. 161/2006 for the Approval of the Normative Regarding the Classification of the Quality of Surface Waters for the Purpose of Determining the Ecological Status of Water Bodies. Official Gazette of Romania, No. 511, 13 June 2006. Available online: https://legislatie.just.ro/Public/DetaliiDocumentAfis/72574 (accessed on 10 October 2025).
- Haddon, M. Modelling and Quantitative Methods in Fisheries, 2nd ed.; Chapman and Hall/CRC: New York, NY, USA, 2011; 465p. [Google Scholar] [CrossRef]
- Targońska, K.; żarski, D.; Kupren, K.; Palińska-żarska, K.; Mamcarz, A.; Kujawa, R.; Skrzypczak, A.; Furgała-Selezniow, G.; Czarkowski, T.K.; Hakuć-Błażowska, A.; et al. Influence of temperature during four following spawning seasons on the spawning effectiveness of common bream, Abramis brama (L.) under natural and controlled conditions. J. Therm. Biol. 2014, 39, 17–23. [Google Scholar] [CrossRef]
- Lourenço, S.; Bueno-Pardo, J.; Vaz, A.; Primo, A.L.; Costa, F.; Pardal, M.A.; Martinho, F. Short and long-term temperature variations drive recruitment variability in marine and estuarine juvenile fishes. Mar. Pollut. Bull. 2023, 192, 115093. [Google Scholar] [CrossRef]
- Régnier, T.; Gibb, F.M.; Wright, P.J. Understanding temperature effects on recruitment in the context of trophic mismatch. Sci. Rep. 2019, 9, 15179. [Google Scholar] [CrossRef] [PubMed]
- Górski, K.; De Leeuw, J.J.; Winter, H.V.; Vekhov, D.A.; Minin, A.E.; Buijse, A.D.; Nagelkerke, L.A.J. Fish recruitment in a large, temperate floodplain: The importance of annual flooding, temperature and habitat complexity. Freshw. Biol. 2011, 56, 2210–2225. [Google Scholar] [CrossRef]
- Mikhailov, A.I.; Bobyrev, A.E.; Bulgakova, T.I.; Sheremetyev, A.D. Returning to the Question of Population Regulation: A Generalized Model of Recruitment Formation in Exploited Fish Populations. Biol. Bull. Rev. 2021, 11, 67–75. [Google Scholar] [CrossRef]
- Brosset, P.; Smith, A.D.; Plourde, S.; Castonguay, M.; Lehoux, C.; van Beveren, E. A fine-scale multi-step approach to understand fish recruitment variability. Sci. Rep. 2020, 10, 16064. [Google Scholar] [CrossRef]
- Henriksen, O.; Rindorf, A.; Brooks, M.E.; Lindegren, M.; van Deurs, M. Temperature and body size affect recruitment and survival of sandeel across the North Sea. ICES J. Mar. Sci. 2021, 78, 1409–1420. [Google Scholar] [CrossRef]
- Lindmark, M.; Audzijonyte, A.; Blanchard, J.L.; Gårdmark, A. Temperature impacts on fish physiology and resource abundance lead to faster growth but smaller fish sizes and yields under warming. Glob. Change Biol. 2022, 28, 6239–6253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.