Abstract
As a key freshwater wetland in the Beijing–Tianjin–Hebei core area, Baiyangdian Lake’s ecological health is strategically significant for regional ecological security, prompting this study to explore how sediment phosphorus forms drive its phytoplankton communities. The research adopted sequential extraction technology, morphological identification, and multivariate statistics in Baiyangdian Lake. Results showed sediment phosphorus was dominated by highly active exchangeable phosphorus (Ex-P, ~60%, with higher levels around villages of lake center and western areas), with occluded phosphorus (Oc-P, ~23%) as the second most abundant form. Ex-P was the core factor shaping phytoplankton communities, directly increasing biomass density (r = 0.38, p < 0.05) and explaining 17.92% of community variation. Bacillariophyta was the dominant group (43.3%), while calcium-bound phosphorus (Ca-P) maintained diversity and aluminum-bound phosphorus (Al-P) inhibited evenness (r = −0.35, p < 0.05). Active phosphorus directly affected, and inactive phosphorus indirectly regulated, phytoplankton patterns, clarifying the unique phosphorus structure of northern carbonate-type lakes and filling research gaps. It is suggested to include Ex-P and Ca-P in aquatic ecological monitoring and prioritize sediment passivation and riparian restoration in high-Ex-P areas to mitigate algal bloom risks.
1. Introduction
Lake eutrophication is one of the major global water environmental problems. Shallow lakes in China are generally facing the challenge of eutrophication, with most lakes experiencing varying degrees of eutrophication [1]. As a limiting nutrient for primary productivity in water bodies, the occurrence forms and release mechanisms of phosphorus in sediments directly determine the structure and function of lake ecosystems [2]. Located in the core area of Beijing–Tianjin–Hebei, Baiyangdian Lake is the only large freshwater lake wetland in North China, undertaking important ecological functions such as flood regulation, water purification, and biodiversity conservation, known as the “Kidney of North China” [3]. However, in the past 30 years, affected by multiple factors such as climate change, accelerated urbanization, and agricultural non-point source pollution, the problem of water eutrophication in Baiyangdian Lake has become increasingly prominent. Although a series of pollution control measures have been implemented since the establishment of Xiong’an New Area, the concentration of key pollutants in inflowing rivers has decreased significantly, the concentration of chlorophyll a (Chla) in the surface water of the lake remains at a high level [4], indicating that the nutrient limitation for algal growth has not been completely lifted, and the release of internal sediment pollution may become a key bottleneck in the current eutrophication prevention and control [5].
As a “source” and “sink” of nutrients in lakes, the diversity of phosphorus occurrence forms in sediments determines the bioavailability and environmental risks of phosphorus [6]. According to differences in chemical properties, sediment phosphorus can be divided into Ex-P, Al-P, iron-bound phosphorus (Fe-P), Ca-P, Oc-P, and organic phosphorus (OP). Among them, Ex-P is physically adsorbed on the sediment surface, has high migration activity, and is the main contributor to phosphorus exchange at the water–sediment interface [7]. Al-P and Fe-P are significantly affected by environmental factors such as pH and redox potential, and are easily released under acidic or reducing conditions [8]. Ca-P is usually enriched in sediments with high carbonate content, with a slow but long-lasting release rate [9]. OP can only be used by phytoplankton after being decomposed into inorganic phosphorus by microorganisms, and its ecological effect causes lag [6]. As primary producers in aquatic ecosystems, the community structure of phytoplankton is sensitive to the response of sediment phosphorus forms. By analyzing the correlation between different forms of phosphorus and phytoplankton abundance and diversity, the ecological effect of internal nutrient release can be revealed, providing a scientific basis for eutrophication prevention and control [10].
International research on the relationship between sediment phosphorus forms and phytoplankton started early. Many studies have confirmed that different forms of phosphorus in sediments have significant regulatory effects on the proliferation and community succession of phytoplankton. Among them, Ex-P and Fe-P are easy to be utilized by organisms and become core factors affecting phytoplankton biomass [8,11,12]. In recent years, with the development of high-resolution analysis technology, synchrotron radiation X-ray fluorescence spectroscopy (SRXRF) and microelectrode technology have been applied to phosphorus form research, further revealing the role of iron-phosphorus oxides in algal bloom outbreaks [13]. In addition, long-term monitoring by European scholars has found that the accumulation of Ca-P in lake sediments is positively correlated with the long-term stability of phytoplankton communities, confirming the ecological regulatory value of inactive phosphorus forms [11]. Domestic research on lake sediment phosphorus forms and their correlation with phytoplankton is mainly concentrated in typical eutrophic lakes in China, such as Taihu Lake, Chaohu Lake, and Erhai Lake. These lakes are representative of eutrophic water bodies in the middle and lower reaches of the Yangtze River and the Yunnan–Guizhou Plateau. All three lakes have an extensive research foundation, serving as important reference systems for related studies on eutrophication and phosphorus biogeochemical cycling. Studies on Taihu Lake have shown that the proportions of Ca-P and Fe/Al-P in sediments of algal-type lake areas are significantly higher than those in macrophyte-type lake areas, and the total phosphorus (TP) content in surface sediments is positively correlated with phytoplankton biomass in overlying water. Among them, easily released Fe-P is the key internal phosphorus source driving cyanobacterial blooms [14]. Long-term follow-up surveys of Erhai Lake have found that changes in sediment phosphorus concentration indirectly regulate phytoplankton community structure by affecting the phosphorus content of submerged macrophytes. The increase in the dominance of Ceratophyllum demersum under high phosphorus conditions will reduce community stability, thereby changing the algal competition pattern [15]. However, the weakly alkaline environment of northern carbonate lakes creates a unique phosphorus form structure, and existing studies mostly focus on the description of total phosphorus, with limited analysis of the functional differentiation of forms. For northern carbonate-type lakes, existing studies have pointed out that their alkaline environment will cause a large release of endogenous phosphorus from sediments, resulting in significant differences in phosphorus form structure compared with southern acidic lakes [16,17]. However, there are no quantitative research reports on the driving mechanism of the high proportion of Ex-P on phytoplankton in such lakes.
Baiyangdian Lake is the largest natural freshwater lake in the North China Plain, playing an irreplaceable role in maintaining the environmental health of the Baiyangdian Lake basin. Affected by natural and human factors, the Baiyangdian Lake wetland is facing severe crises such as water shortage, wetland area shrinkage, aggravated eutrophication, water environmental pollution, and aquatic ecological degradation [18]. Since the establishment of Xiong’an New Area, the aquaculture of aquatic products, livestock, and poultry in the main lake area of Baiyangdian Lake has been fully banned, and the sewage discharge standards of inflowing rivers and villages inside the lake area have been correspondingly improved. However, pollutants discharged in historical periods, especially nitrogen and phosphorus nutrients, accumulate in sediments and are released into water bodies, which can still lead to the deterioration of water quality in Baiyangdian Lake [19]. Previous studies have mostly focused on the total phosphorus content of sediments, lacking refined analysis of phosphorus forms, especially the quantification of the spatial distribution of different forms of phosphorus and their relationship with phytoplankton [20]. Therefore, this study, through the analysis of phosphorus forms, combined with phytoplankton identification and statistical analysis methods, clarifies the main occurrence forms and spatial distribution characteristics of phosphorus in Baiyangdian Lake sediments, and reveals the quantitative relationship and mechanism between different forms of phosphorus and phytoplankton community structure. The research results will fill the gap in quantitative research on the aquatic ecological effects of sediment phosphorus forms in northern carbonate-type shallow lakes, and provide a targeted paradigm for internal pollution prevention and control in similar lakes.
2. Materials and Methods
2.1. Sample Collection and Pretreatment
2.1.1. Collection of Sediment Samples
Baiyangdian Lake is located in the central part of the North China Plain (38°43′–39°02′ N, 115°38′–116°07′ E), with a total area of 366 km2 and an average water depth of 2.5 m, belonging to a typical northern shallow lake. The research area selects the waters around the villages in the lake, which includes typical habitats such as reed marshes (accounting for about 40%), open water (35%), and farmland ditches (25%). There are 12 natural villages around, with a permanent population of about 23,000 people. The problems of agricultural non-point source pollution and direct discharge of domestic sewage are relatively prominent [21]. The sediment type is mainly silty clay, with a pH value of 7.8–8.6 and an organic matter content of 1.2–3.5%, showing typical carbonate-type lake sediment characteristics.
The sampling was conducted in November 2023 (autumn), a stable period for phytoplankton communities in Baiyangdian Lake. After the peak growth season in summer, the phytoplankton community structure tends to be balanced in autumn, which is conducive to analyzing the long-term correlation between sediment phosphorus forms and community composition. Meanwhile, autumn is a critical transition stage for phosphorus biogeochemical cycling in sediments (from summer release to winter deposition), and one-time comprehensive sampling during this period can accurately reflect the intrinsic link between sediment phosphorus fractions and phytoplankton dynamics. The sampling was carried out as a one-time fieldwork to ensure the consistency of environmental conditions (e.g., temperature, water level) during sample collection.
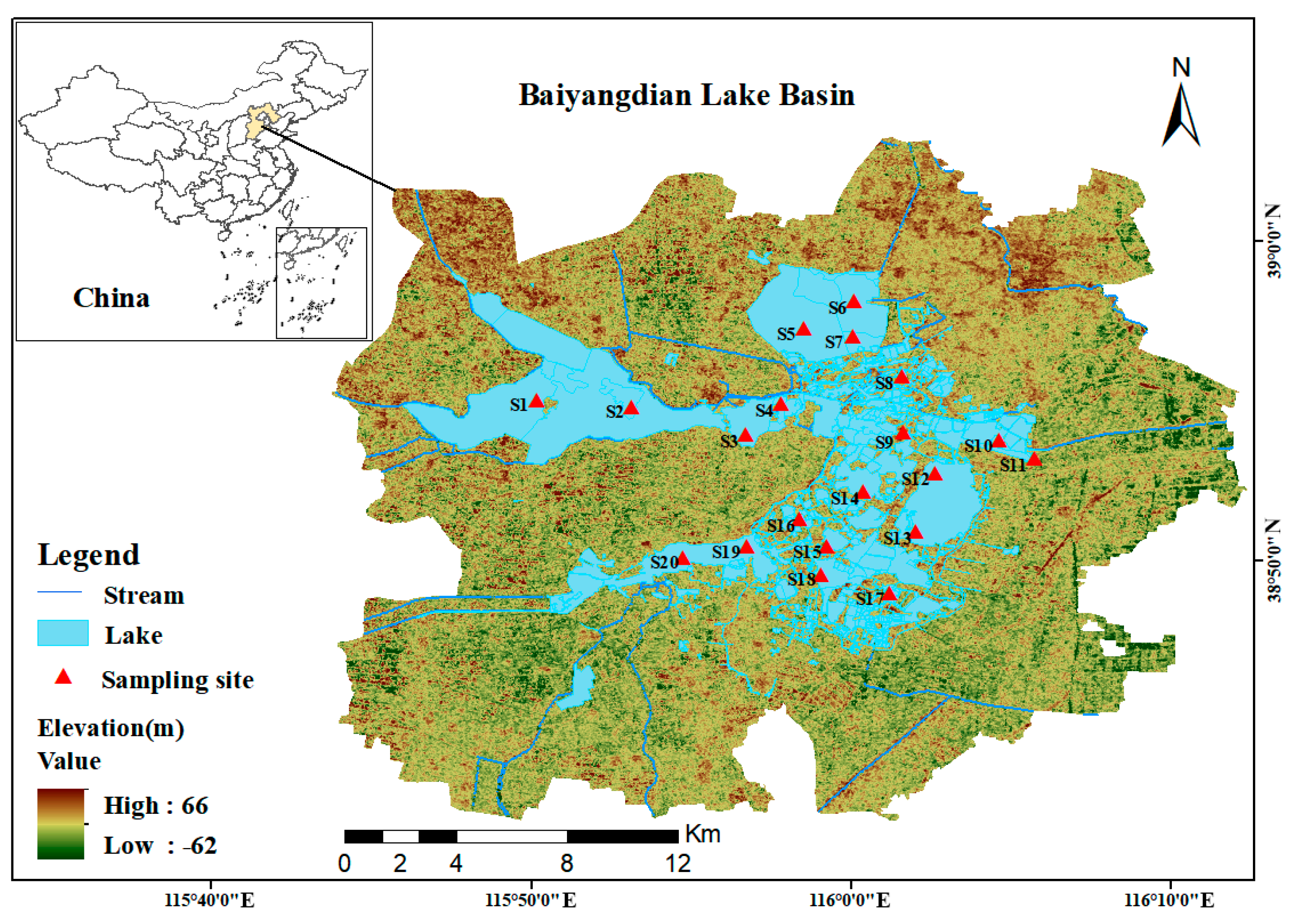
According to the requirement in technical guidelines (Technical guidelines for water ecological monitoring-aquatic organism monitoring and evaluation of lakes and reservoirs (Trial) (HJ 1296–2023), Ministry of Ecology and Environment, Beijing, China, 2023) [22] that “when the area of a lake or reservoir is between 50 km2 and 500 km2, 10–15 sampling points should be set up”, 10–15 sampling points need to be set in the 366 km2 water area of Baiyangdian Lake. Considering factors such as different habitat types, water flow directions, surrounding environments of Baiyangdian Lake, and the key monitoring areas of previous studies, to ensure that the sampling points are representative and can fully reflect the overall situation of phytoplankton and water quality in Baiyangdian Lake, a total of 20 sampling points were selected in this study (Figure 1). Surface sediment samples of 0–10 cm were collected using a Peterson grab sampler (manufactured by Petersen Technology Co., Ltd., Wuhan, China). Three duplicate samples were collected at each sampling point, mixed uniformly, and placed in polyethylene bags. The latitude and longitude coordinates were recorded on site using a handheld GPS (manufactured by Delixi Electric Co., Ltd., Wenzhou, China), and water quality parameters such as water temperature, pH, and dissolved oxygen were measured by using the multi-parameter water quality analyzer (manufactured by Hengxin Instrument Co., Ltd., Dongguan, China) synchronously. After the samples were transported back to the laboratory, they were dried in a constant temperature drying oven (manufactured by Supo Instrument Co., Ltd., Shaoxing, China) at 60 °C for 72 h to constant weight, ground through a 60-mesh sieve (aperture 0.25 mm), and stored in sealed bags for later use.
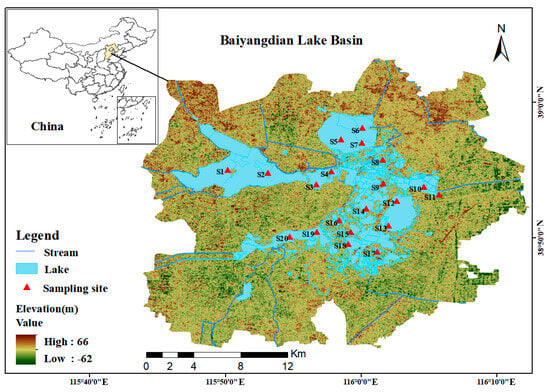
Figure 1.
Location of sampling points in Baiyangdian Lake in this study (S1, Zaozhadian 1; S2, Zaozhadian 2; S3, Nanliuzhuang; S4, Xiaotianzhuang; S5, Dianbei 1; S6, Dianbei 2; S7, Dianbei 3; S8, Zhaozhuang; S9, Guangdiancun; S10, Dianqu 1; S11, Zaolinzhuang; S12, Dianqu 2; S13, Dianqu 3; S14, Quantou; S15, Datianzhuang; S16, Beitianzhuang; S17, Dashuliuzhuang; S18, Xilizhuang; S19, Duancun; S20, Dianxi).
2.1.2. Collection of Phytoplankton Samples
The sampling technical requirements were implemented in accordance with the water quality standard (Technical Specifications for Surface Water Environmental Quality Monitoring (HJ 91.2-2022), Ministry of Ecology and Environment, Beijing, China, 2022) [23]. A 25# plankton net (mesh size 64 μm) (manufactured by Petersen Technology Co., Ltd., Wuhan, China) was used to tow at a speed of 20–30 cm/s in an “∞” shape 0.15 m underwater for 3 min. The collected algal samples were immediately transferred to a 100 mL specimen bottle, and 3 mL of formalin solution (volume fraction 40%) was added for fixation. Quantitative sample collection: 1 L of surface water was collected using an organic glass water sampler (model: 5 L, manufactured by Henan Xinchangyuan Experimental Equipment Co., Ltd., Xinxiang, China), 15 mL of Lugol’s solution was added for fixation, and after standing for 24 h, the supernatant was removed by siphon method. The remaining 30 mL was transferred to a quantitative bottle for cell counting.
2.2. Analysis Methods
2.2.1. Determination of Sediment Phosphorus Forms
The European Union Standards, Measurements and Testing (SMT) protocol was used for sequential extraction of phosphorus forms, with specific steps as follows [24]:
Ex-P: Weigh 0.5 g of dried sample into a 100 mL centrifuge tube, add 50 mL of 1 mol/L MgCl2 solution (pH = 8.0), shake in a constant temperature shaker (rotation speed 150 r/min) (model: Y-4, manufactured by Changzhou Ruihua Instrument Manufacturing Co., Ltd., Changzhou, China) for 2 h, centrifuge at 4500 r/min (model: TDL-80-2B, manufactured by Shanghai Anting Scientific Instruments Co., Ltd., Shanghai, China) for 10 min. The supernatant was filtered through a 0.45 μm cellulose acetate filter membrane, and the phosphorus content was determined by molybdenum antimony anti-spectrophotometry (wavelength 700 nm).
Al-P: Add 50 mL of 0.5 mol/L NH4F solution (pH = 8.2) to the residual sediment in the centrifuge tube, shake for 1 h, centrifuge and filter, then determine the phosphorus content in the filtrate.
Fe-P: Add 50 mL of mixed solution (0.1 mol/L NaOH + 0.05 mol/L Na2CO3), shake for 4 h, centrifuge and filter, then determine.
Ca-P: Add 35 mL of mixed solution (0.3 mol/L Na3Cit + 1 mol/L NaHCO3) and 1.125 g of Na2S2O4, magnetically stir for 15 min, then add 15 mL of 0.5 mol/L NaOH solution, shake for 8 h, centrifuge and filter, then determine.
Oc-P: Add 50 mL of 1 mol/L HCl solution, shake for 16 h, centrifuge and filter, then determine.
OP: Transfer the residual sample to a porcelain crucible, ash in a muffle furnace at 550 °C for 2 h, cool, add 50 mL of 1 mol/L HCl solution, shake for 16 h, centrifuge and filter, then determine.
TP determination: TP was estimated by summing up all forms of phosphorus.
2.2.2. Identification and Counting of Phytoplankton
Qualitative samples were identified under a microscope (magnification 400×) (model: XSP-H1600, manufactured by Shenzhen Aoswei Optical Instrument Co., Ltd., Shenzhen, China) with reference to “Freshwater Algae of China-Systematics, Taxonomy and Ecology” [25]. At least 50 fields of view were observed for each sample to ensure the completeness of species identification. Quantitative samples were counted in full using a 0.1 mL plankton counting chamber (model: 40/160, manufactured by Puxi Optical Components Co., Ltd., Wuxi, China) under a microscope. Each sample was counted twice, and the average value was taken.
2.2.3. Calculation of Community Characteristic Indices
The ecological indices in Table 1 were used to analyze the phytoplankton community structure [26,27,28].

Table 1.
Phytoplankton community characteristic indices.
2.3. Data Processing and Analysis
Excel 2021 (Version 2111) was used for data entry and preliminary statistics. Origin 2022b (Version 9.95) was used to draw spatial distribution maps and correlation matrix maps. Determine whether environmental factors conform to a normal distribution through the Kolmogorov–Smirnov (K-S) normality test. SPSS 20.0 was used for one-way analysis of variance (One-way ANOVA) and Pearson correlation analysis, with the significance level set at p < 0.05 and the extremely significant level at p < 0.01. Canoco 5.0 software was used for redundancy analysis (RDA) to determine the driving effect of phosphorus forms on phytoplankton community structure. Sensitivity tests were conducted by re-performing RDA ordination after sequentially excluding individual sampling points. If the rate of change in the explanatory power of a variable is between −15% and −30% (the negative sign indicates a decrease in explanatory power after removal), it indicates that the variable is a core driving factor and the model is sensitive to it. If the rate of change is between −5% and 5%, it indicates that the variable has a weak impact on the model and can be considered for exclusion (to simplify the model). Spatial interpolation was performed using the Kriging method in ArcGIS 10.8 to present the spatial distribution characteristics of phosphorus forms.
3. Results
3.1. Distribution Characteristics of Sediment Phosphorus Forms
3.1.1. Composition Characteristics of Phosphorus Forms
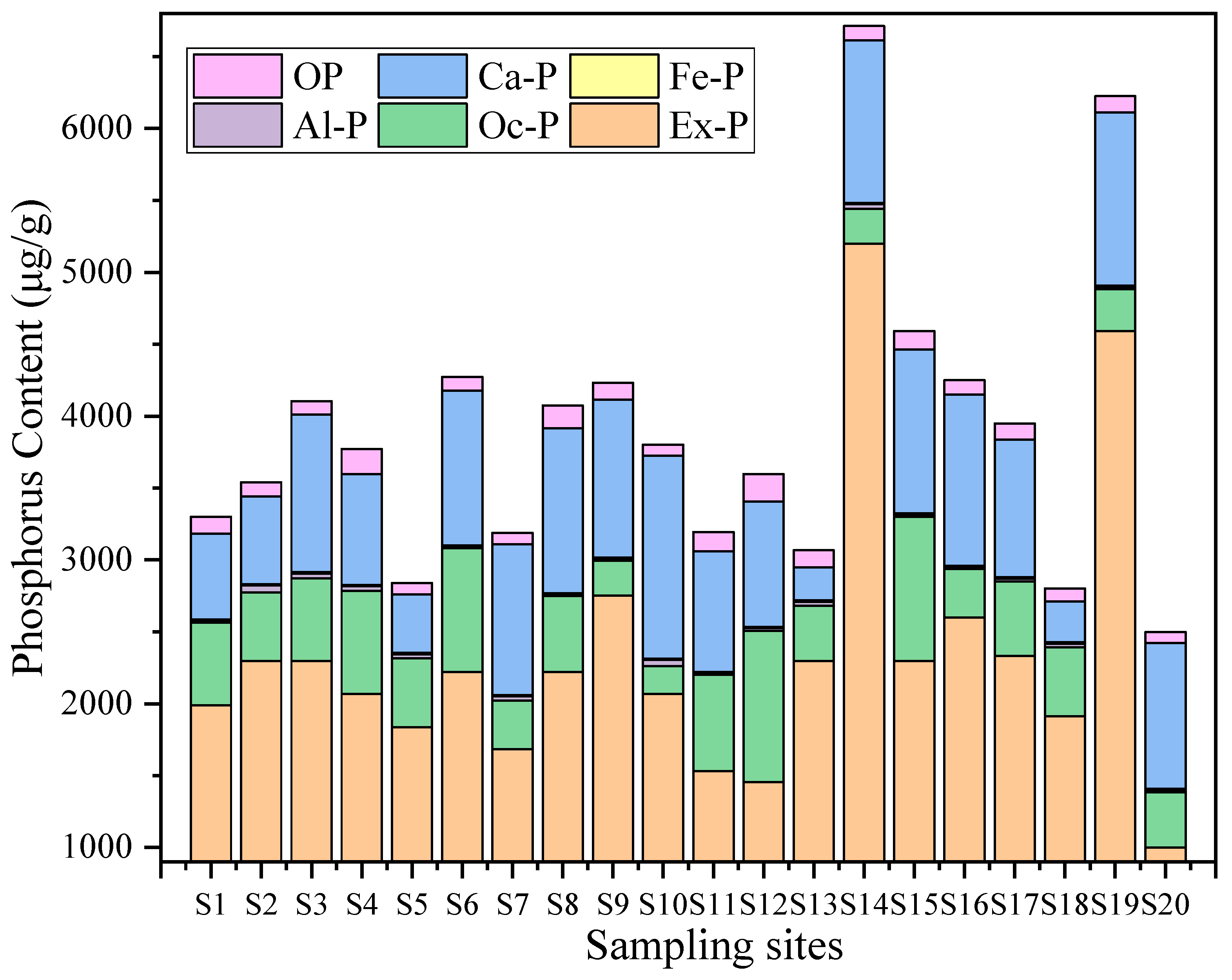
As shown in Table 2 and Figure 2, we assessed differences in the content of various phosphorus forms in Baiyangdian Lake sediments using one-way analysis of variance (ANOVA), with statistical significance determined by a criterion of p < 0.05. Note that the comparison here refers to variations among different phosphorus forms (rather than among sampling stations). The results showed significant differences in the content of various phosphorus forms (p < 0.05). Ex-P had the highest content, accounting for 45.2–77.5% of TP, with an average of 59.8%. Oc-P was the second, accounting for 15.3–35.7% of TP, with an average of 23.2%. Ca-P content accounted for 10.1–18.5% of TP, with an average of 13.3%. The contents of Al-P, Fe-P, and OP were relatively low, accounting for less than 5% of TP, which were 0.6%, 0.3%, and 2.9% respectively.

Table 2.
Statistics of phosphorus form contents in Baiyangdian Lake sediments (μg/g, n = 20).
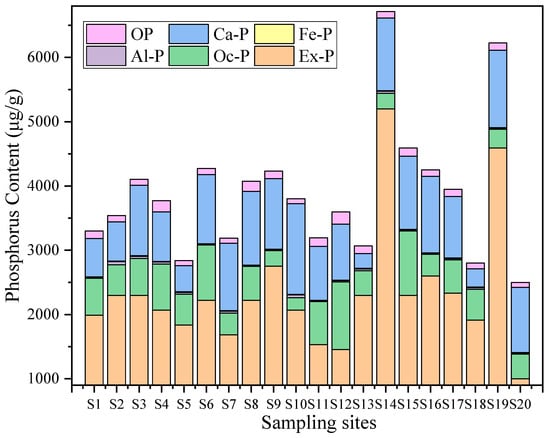
Figure 2.
Composition characteristics of different phosphorus forms at each sampling point in Baiyangdian Lake (Fe-P (yellow) is not distinguishable in the stacked bar chart due to its extremely low proportions of total phosphorus).
3.1.2. Spatial Distribution Characteristics
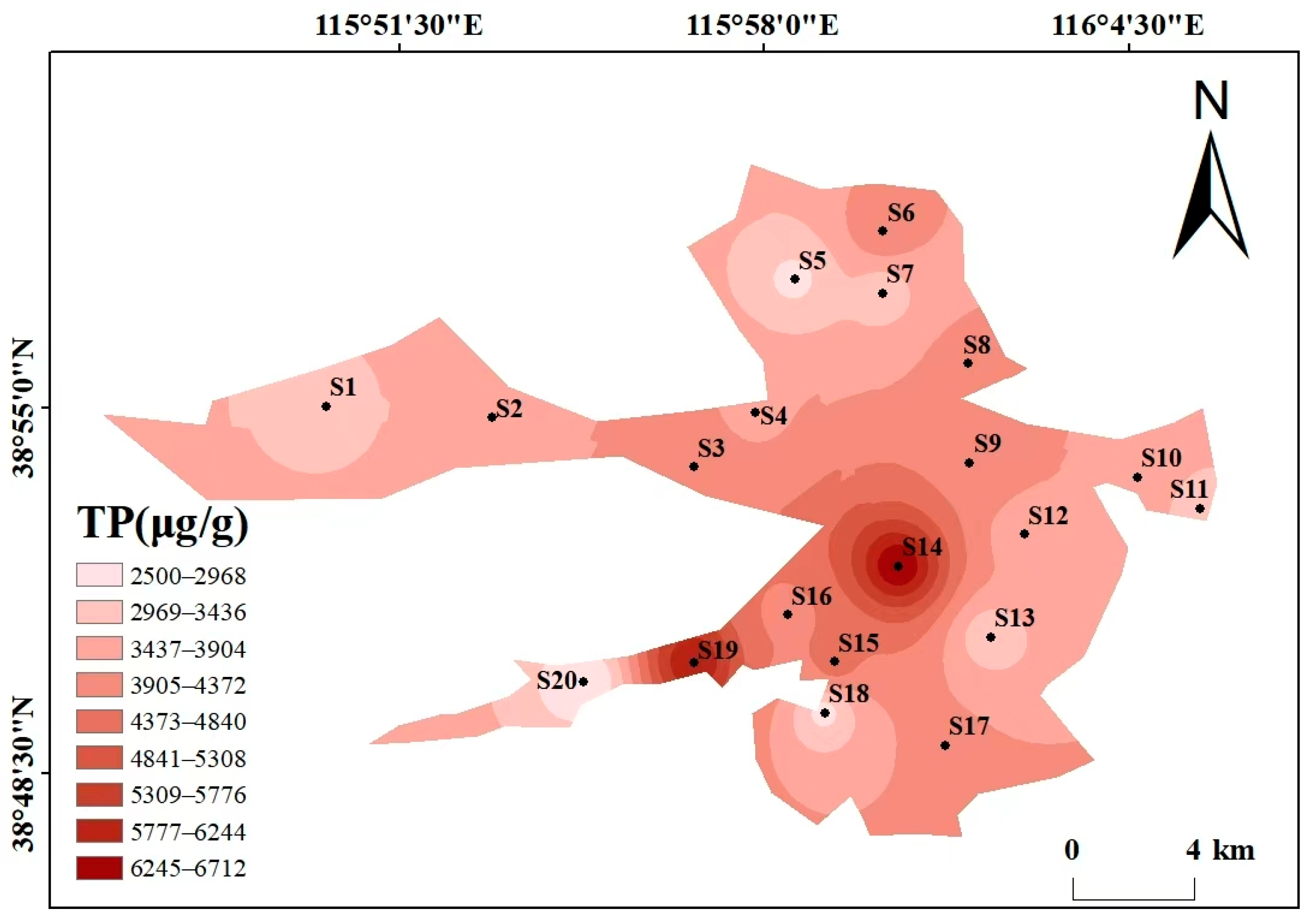
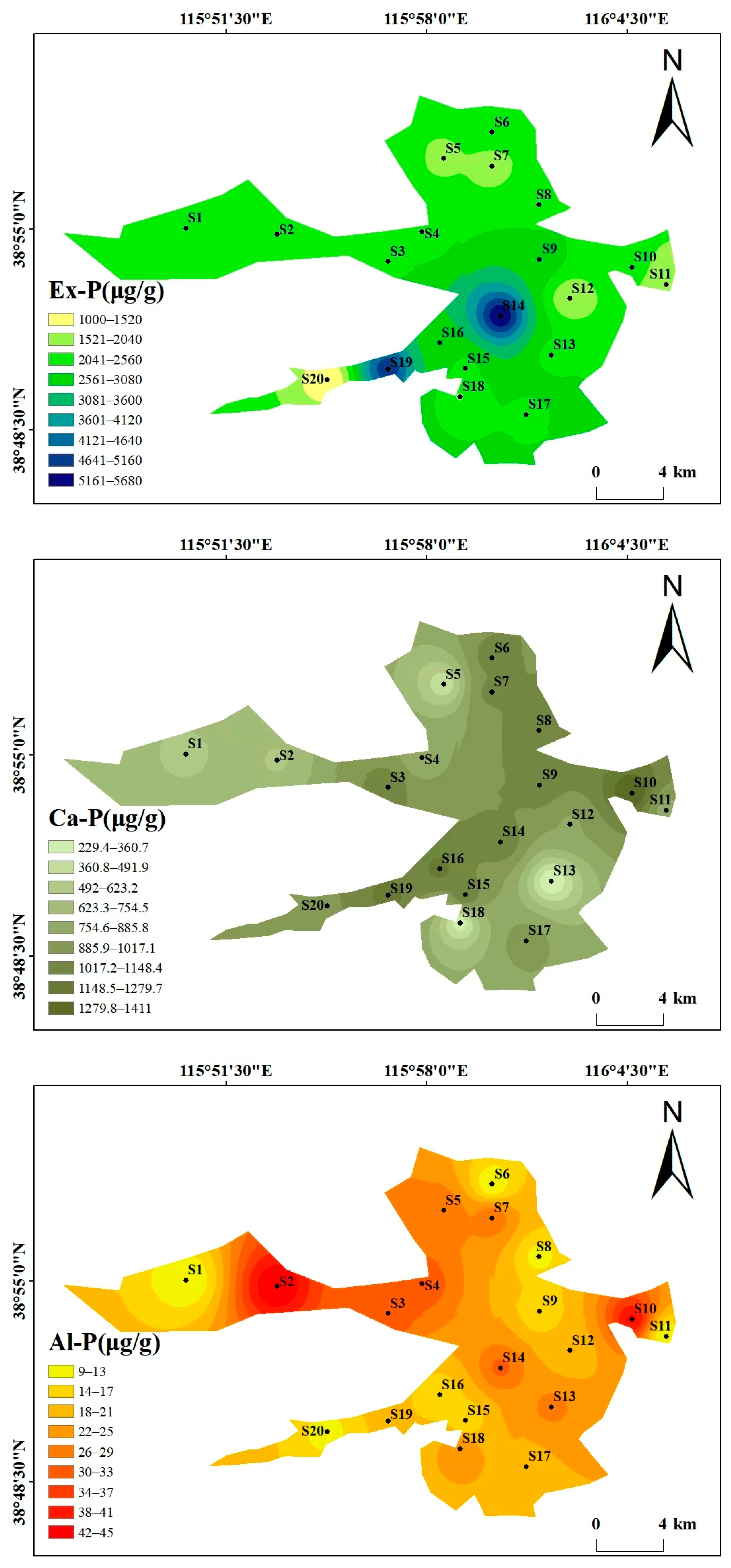
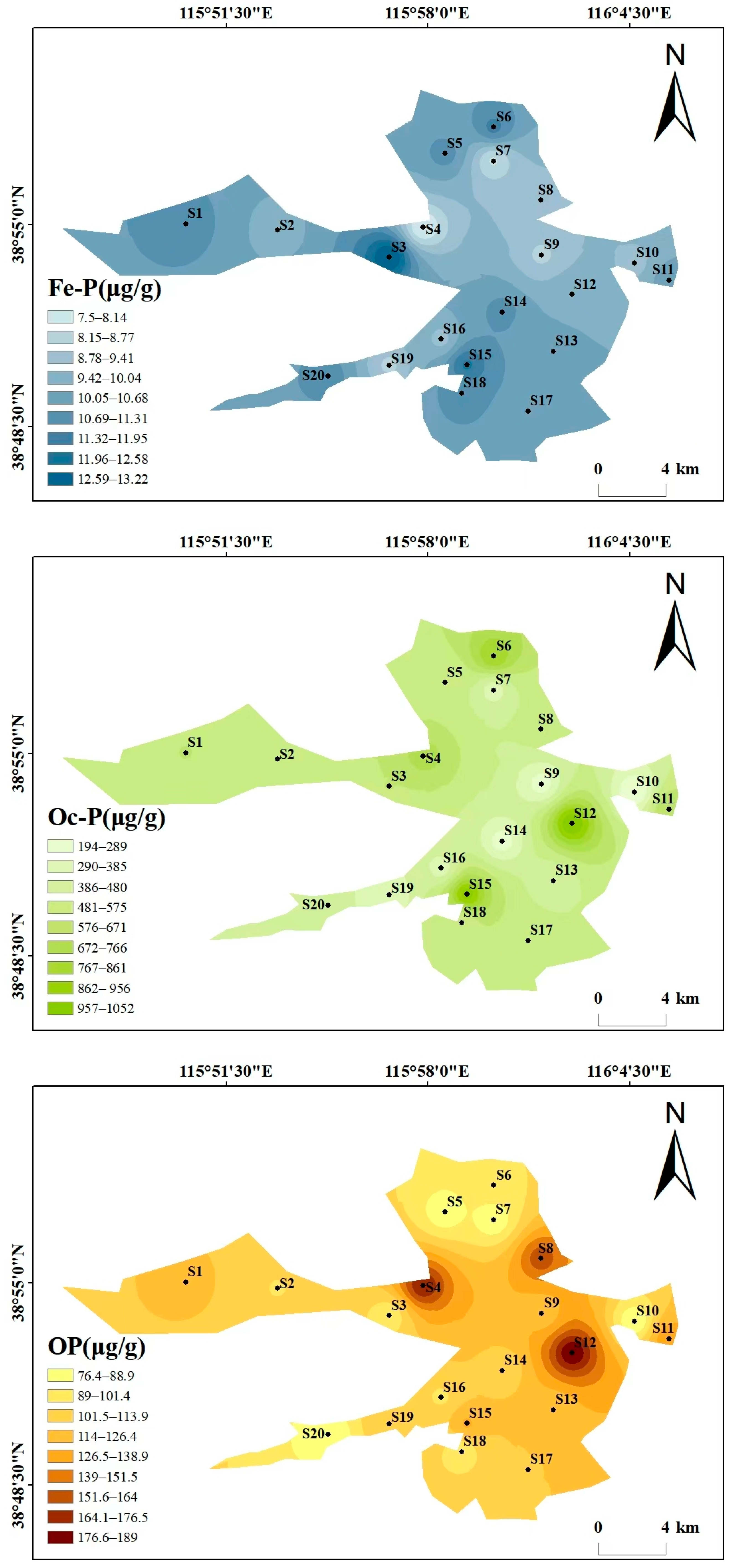
Beyond the overall phosphorus composition (Section 3.1.1), the spatial distribution also reflects human and natural impacts (Figure 3). As can be seen from the spatial distribution maps of various phosphorus forms in Figure 3, the spatial distribution of different phosphorus forms varies significantly, showing heterogeneity. The high-value areas were concentrated in some specific sampling points, and the overall distribution is characterized by “local enrichment and regional differentiation”. The total phosphorus (TP) content ranges from 2499.34 to 6713.62 μg/g, with high values concentrated at S14 (6713.62 μg/g) and S19 (6225.78 μg/g), and low values in the southwest and north (S20 and S5). The distribution patterns of the high-value areas of Ex-P and Ca-P are consistent with that of TP, both being at S14 and S19, showing the characteristic of “high around the center of the lake and villages in the southwest, and low in areas far from villages”, which reflects the impact of domestic sewage discharge and agricultural runoff. The contents of Al-P, Fe-P, and Oc-P are generally low, with small spatial fluctuations. Among them, the high value of Al-P is at S2 (45.44 μg/g), the high value of Fe-P is at S3 (13.22 μg/g), and the high value of Oc-P is at S12 (1052 μg/g), with no obvious concentrated distribution areas. The high values of OP appear in the central (S4, 175.6 μg/g) and eastern (S12, 189 μg/g) regions, and the low value is in the northern region (S7). The differences in the spatial distribution of different phosphorus forms may be related to the intensity of regional human activities and hydrological conditions. There may be inputs such as domestic sewage and agricultural non-point sources around S14 and S19, resulting in high Ex-P and TP. In contrast, S20 is far from major pollution sources, and the water exchange is active, leading to less phosphorus accumulation.
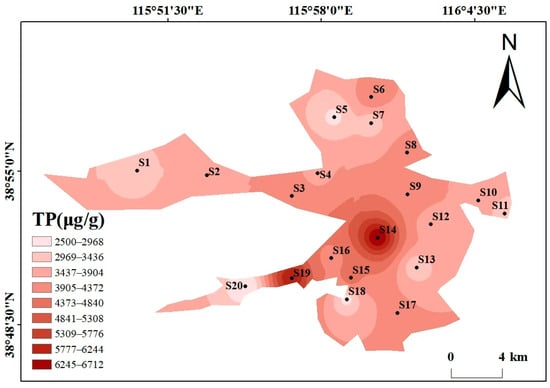
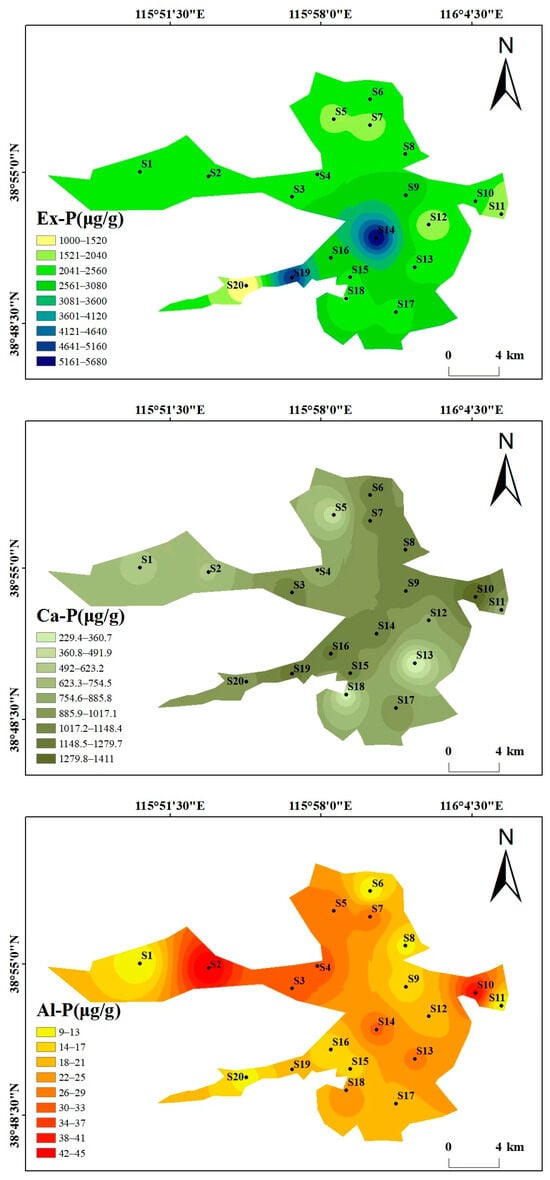
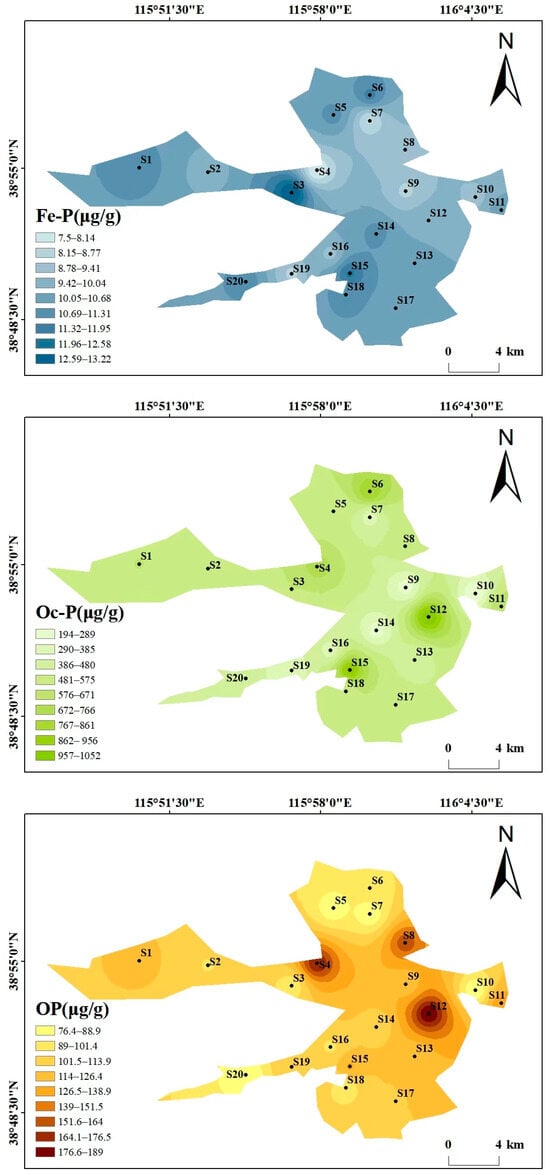
Figure 3.
Spatial distribution maps of various phosphorus forms in Baiyangdian Lake.
3.2. Characteristics of Phytoplankton Community Structure
3.2.1. Species Composition and Dominant Groups
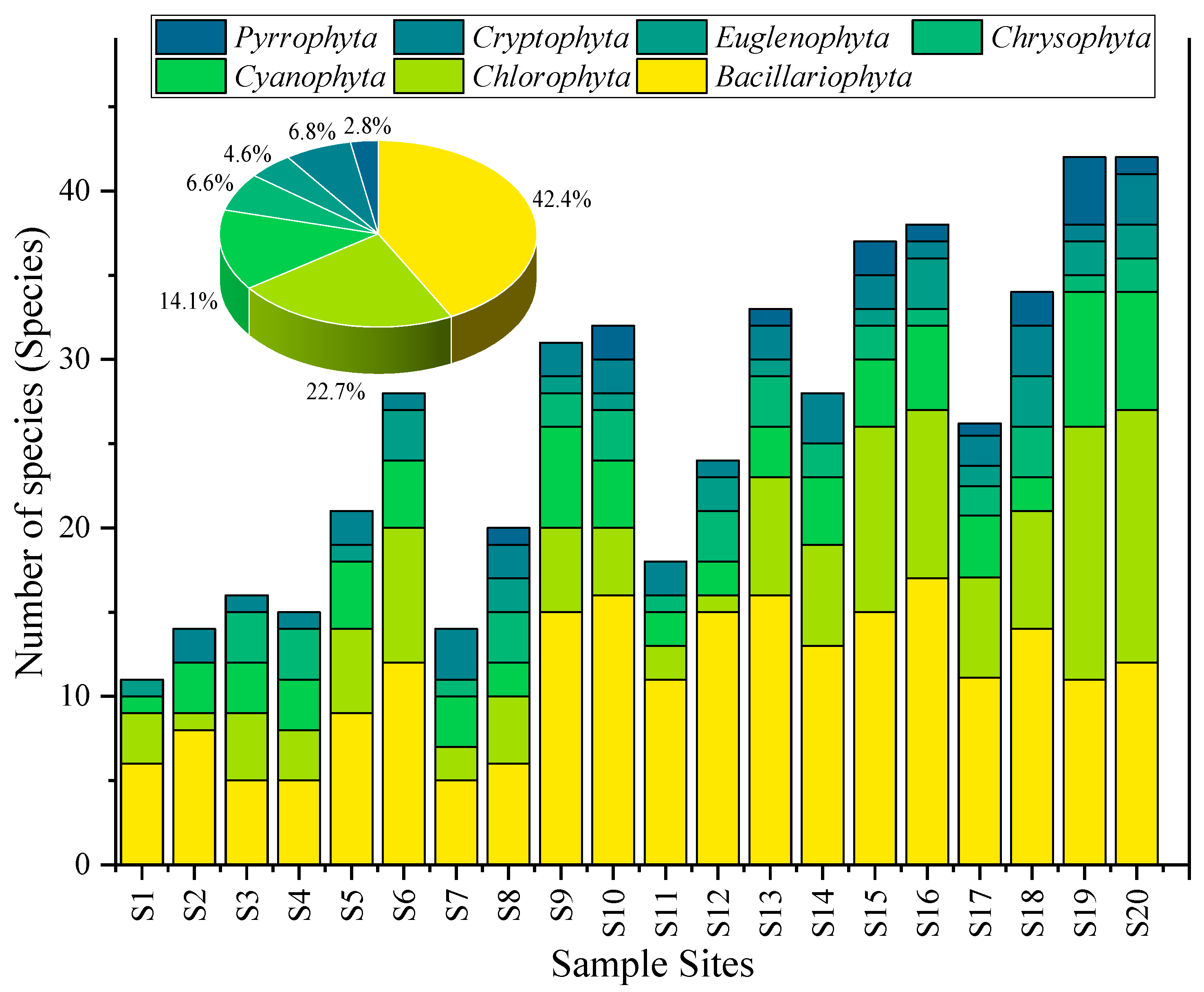
A total of 82 algal species belonging to 7 phyla were detected at 20 sampling points in Baiyangdian Lake, including 28 species of Bacillariophyta, 25 species of Chlorophyta, 13 species of Cyanophyta, 6 species of Chrysophyta, 5 species of Euglenophyta, 3 species of Cryptophyta, and 2 species of Dinophyta. It should be noted that according to the modern taxonomic system (within the framework of the phylum Ochrophyta), diatoms and golden algae belong to the class Bacillariophyceae and the class Chrysophyceae under the phylum Ochrophyta, respectively. In this study, the taxonomic expression at the “phylum level” is temporarily adopted, mainly to maintain consistency with existing studies in the Baiyangdian area (such as ref. [29,30,31]) and ensure data comparability. The results of the analysis of their community composition and dominance are not affected by the expression of taxonomic hierarchy. The distribution characteristics are shown in Table 3 and Figure 4. Bacillariophyta was the absolute dominant group, with a total count of 214 (ind./0.1 mL) at 20 sampling points, accounting for 43.3% of the total abundance of algae in each phylum (494), mainly distributed in sampling points such as S16 (17), S10 (16), and S13 (16); Chlorophyta was the second, with a total count of 113, accounting for 22.9%, and the high-value areas were S19 (15), S20 (15), and S15 (11); the total count of Cyanophyta was 69, accounting for 13.9%, and the highest values appeared in S19 (8), S20 (7), and S9 (Guangdiancun, 6); Chrysophyta (30, 6.1%), Cryptophyta (33, 6.7%), Euglenophyta (21, 4.2%), and Dinophyta (14, 2.8%) had small quantities and were only detected at some sampling points. The dominant species of Cyanophyta was Microcystis aeruginosa, accounting for 65% of the total count of Cyanophyta.

Table 3.
Statistics of the count, proportion and ecological indication significance of phytoplankton species in each class of Baiyangdian Lake (n = 20).
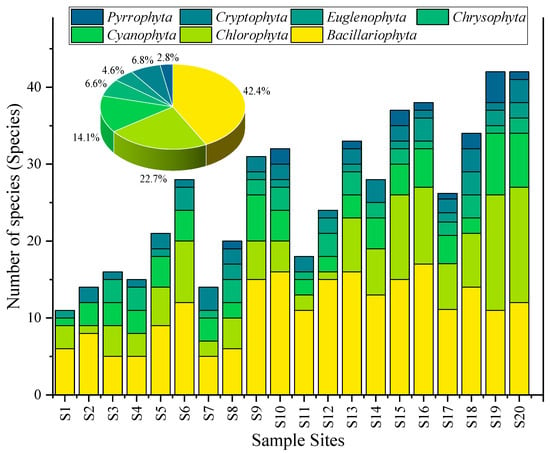
Figure 4.
Composition characteristics of different phyla of phytoplankton in Baiyangdian Lake.
3.2.2. Diversity Indices
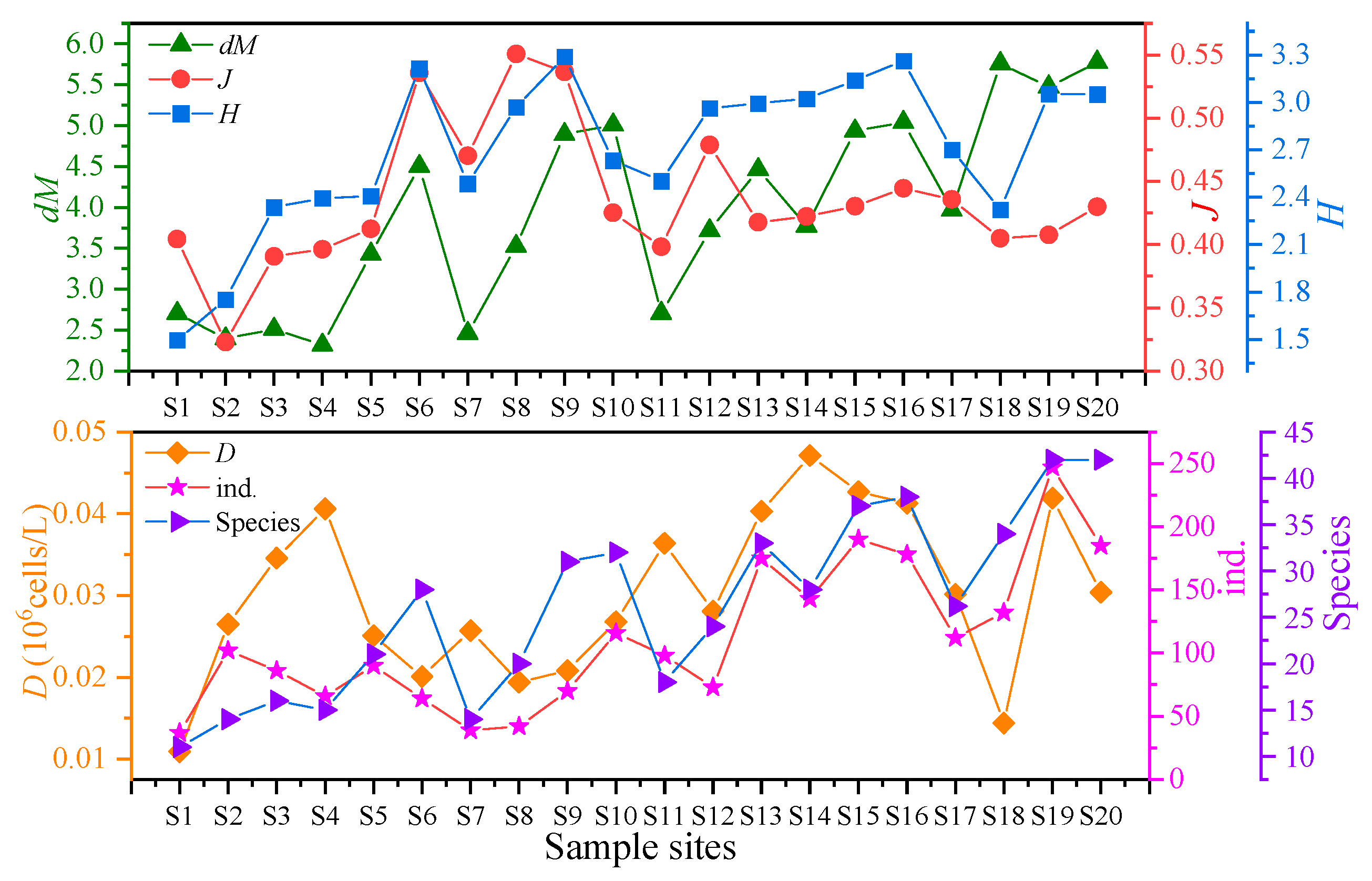
Species composition shows the basic community structure (Section 3.2.1). Diversity indices further quantify spatial differences. As shown in Table 4 and Figure 5, the phytoplankton community diversity indices in Baiyangdian Lake showed significant spatial differences. The count of species ranged from 11 to 42, with an average of 26.21 ± 10.35. Among them, S19 and S20 had the highest count of species, and S1 had the lowest. The dM index ranged from 2.316 (S4) to 5.776 (S20), with an average of 3.892 ± 1.125, which was consistent with the change trend of the count of species. S20 had the highest dM, and S4 had the lowest. The J index ranged from 0.323 (S2,) to 0.551 (S8), with an average of 0.432 ± 0.068. S8 had the highest J value, and S2 (2) had the lowest. The H index ranged from 1.496 (S1) to 3.289 (S9), with an average of 2.699 ± 0.458, indicating that the phytoplankton diversity in Baiyangdian Lake was at a medium level.

Table 4.
Statistics of phytoplankton community diversity indices in Baiyangdian Lake (n = 20).
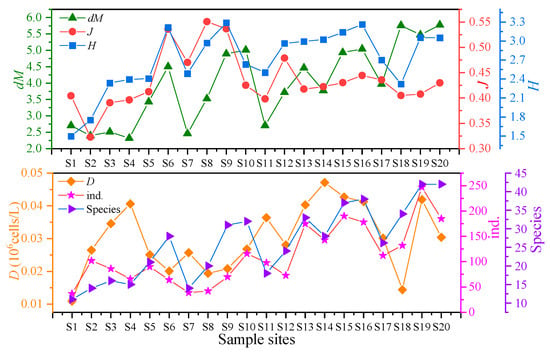
Figure 5.
Characteristics of phytoplankton diversity indices in Baiyangdian Lake. ind.: count of algal individuals; Species: count of species.
3.3. Correlation Analysis Between Phosphorus Forms and Phytoplankton
3.3.1. Pearson Correlation Analysis
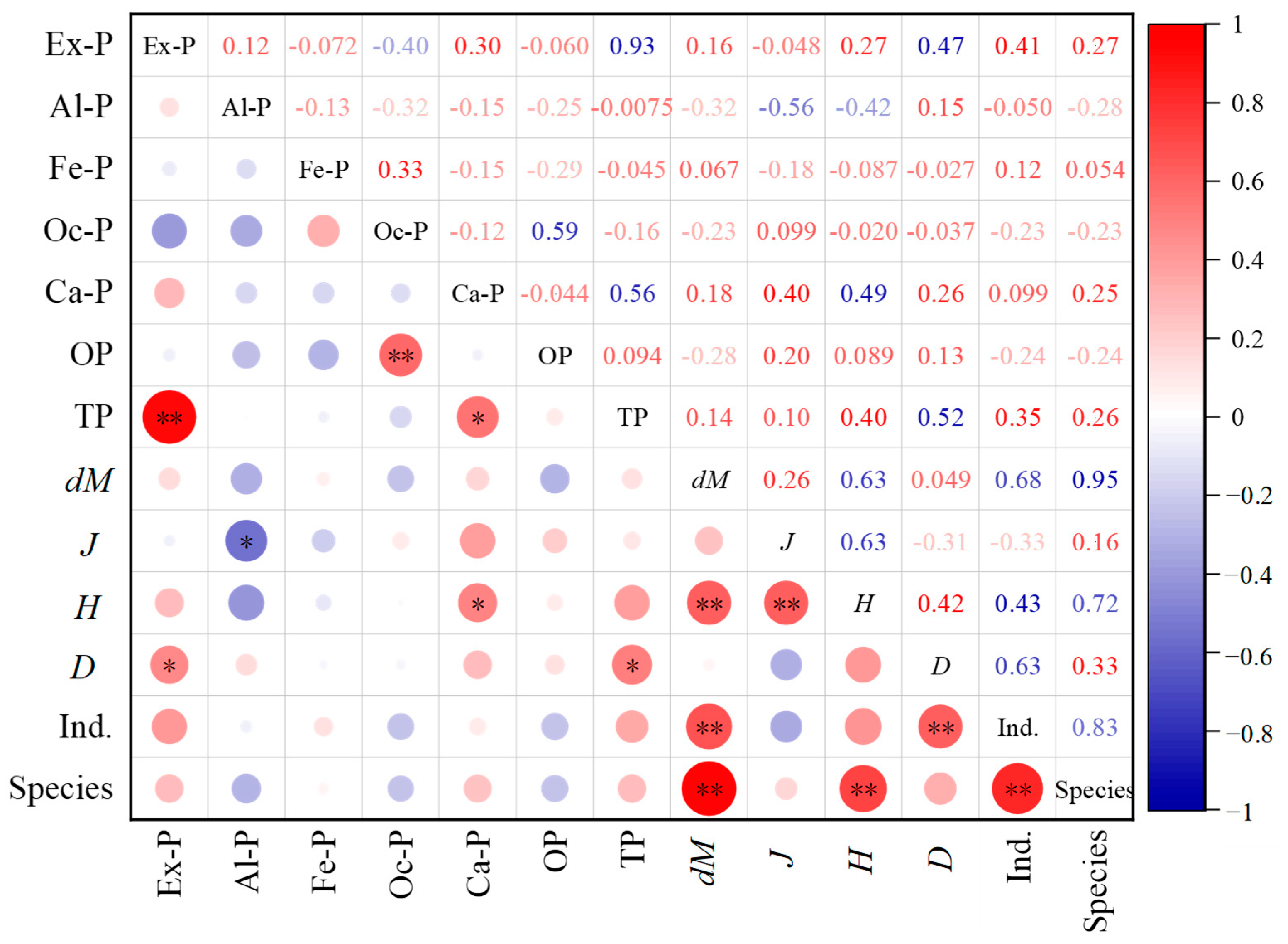
The normality of all variables has been examined using the one-sample Kolmogorov–Smirnov test. From the test results (Table S1 in the Supplementary File), it can be seen that the asymptotic significance (p-value) of all variables is greater than 0.05 (ranging from 0.055 to 0.984), indicating that all data conform to the normal distribution. Pearson correlation analysis was conducted between different phosphorus forms in Baiyangdian Lake sediments and phytoplankton community indices, and the results are shown in Figure 6. It can be seen from Figure 6 that there were significant differences in the correlation between various phosphorus forms and phytoplankton community characteristics. Among them, Ex-P was significantly positively correlated with D (r = 0.38, p < 0.05). At sampling points with high Ex-P (such as S14 and S15), D reached 0.0471 × 106 cells/L and 0.0427 × 106 cells/L, respectively, which were significantly higher than those at sampling points with low Ex-P (S1 density 0.0109 × 106 cells/L, S18 density 0.0144 × 106 cells/L). Ex-P was positively correlated with H but not significantly (r = 0.29, p > 0.05). Al-P was significantly negatively correlated with J (r = −0.35, p < 0.05). The J value of S12 (45.44 μg/g), the sampling point with the highest Al-P, was the lowest, and the count of Chlorophyta at this point (1 ind./0.1 mL) was significantly lower than the lake average (6 ind./0.1 mL); Al-P had no significant correlation with other indices (such as the count of species and biomass density) (p > 0.05). Ca-P was significantly positively correlated with H (r = 0.33, p < 0.05). The H value (3.289) of S9 (Ca-P value 1098.4 μg/g) with higher Ca-P content was the highest. Fe-P, Oc-P, and OP had no significant correlation with all phytoplankton indices (D, Species, H, J, dM) (p > 0.05). Among them, the correlation coefficient between Fe-P and biomass density was only 0.08, the correlation coefficient between Oc-P and H was 0.12, and the correlation coefficient between OP and J was −0.05, indicating that these three phosphorus forms had limited direct impact on the phytoplankton community.
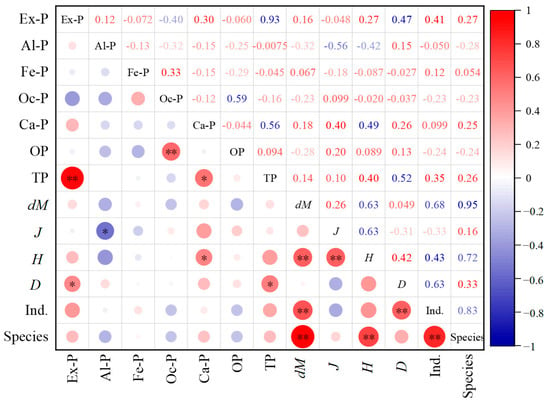
Figure 6.
Correlation heatmap between different phosphorus forms in Baiyangdian Lake sediments and phytoplankton. Lower-left circles (red = positive, blue = negative; larger/darker = stronger correlation); upper-right numbers=correlation coefficients (color/depth matches circle rules); “*” (p ≤ 0.05) and “**” (p ≤ 0.01) = significance levels.
3.3.2. Redundancy Analysis (RDA)
Pearson analysis shows pairwise correlations (Section 3.3.1). RDA further clarifies the effect of phosphorus forms. From the RDA analysis parameters in Table 5, in the ordination of sediment phosphorus forms and phytoplankton community indices, the cumulative explanation degree of the first two axes was 23.06%, and the cumulative explanation of fitted variation was 97.53%, indicating that the six phosphorus form factors could effectively explain the variation of phytoplankton community structure.

Table 5.
Parameter of redundancy analysis result in Baiyangdian Lake.
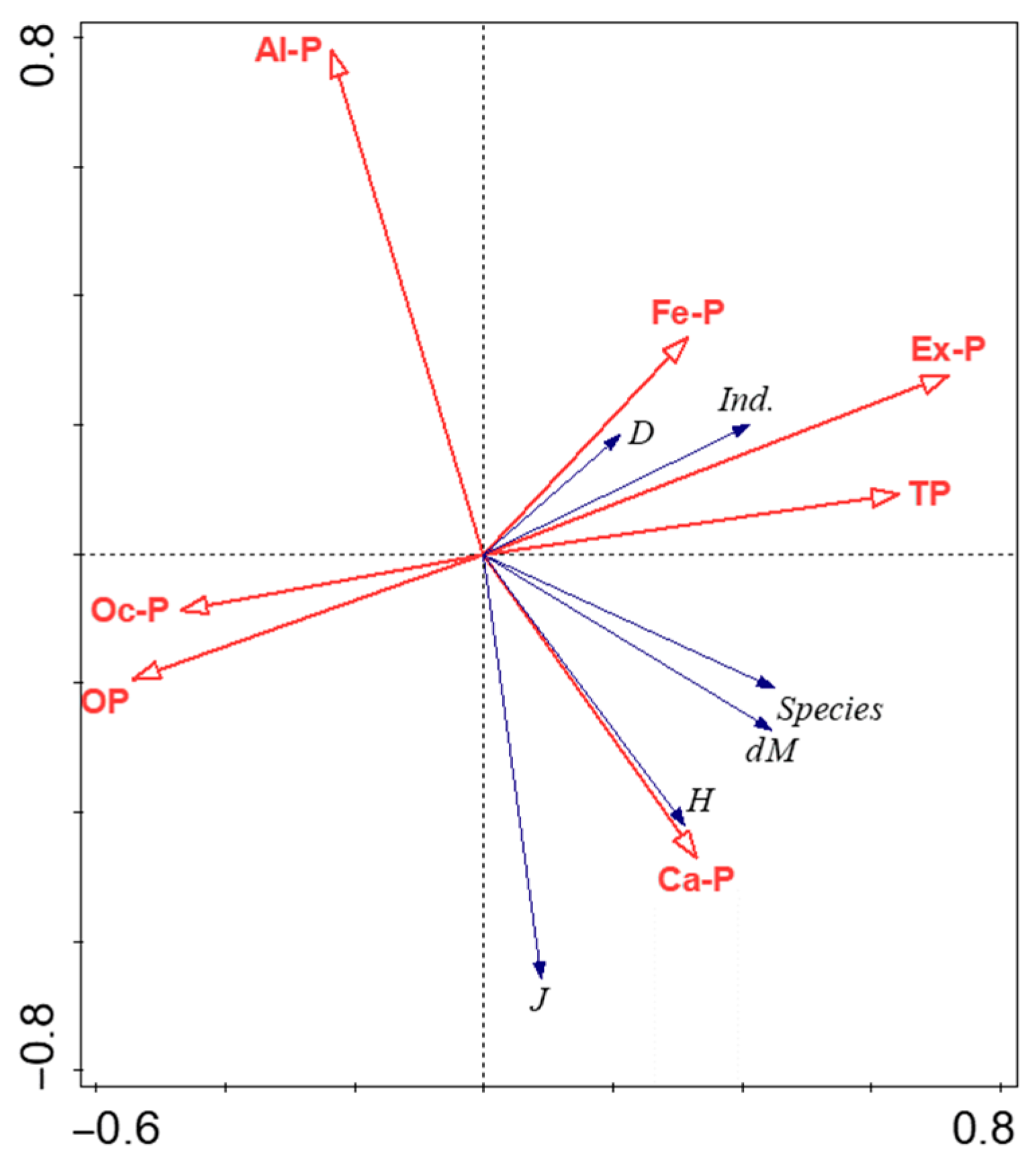
As shown in Figure 7, the RDA1 axis was the main explanatory axis, whose positive direction was significantly driven by Ex-P and the negative direction by Ca-P. Among them, the angle between the arrows of D, ind. and Ex-P was less than 45°, showing an obvious positive correlation, indicating that Ex-P played a key driving role in increasing the count of phytoplankton individuals and overall density; the angle between the arrows of H, J and Ca-P was less than 45°, showing a positive correlation, reflecting the maintaining effect of Ca-P on community diversity and evenness. The RDA2 axis was the secondary explanatory axis, whose positive direction was driven by Fe-P and the negative direction by Al-P. The angle between dM, the count of species and Fe-P was close to 45°, showing a weak positive correlation; while the angle between dM, the count of species and Al-P was greater than 90°, showing a negative correlation. In addition, the angles between the arrows of Oc-P, OP and all phytoplankton community indices were relatively large, indicating that they contributed little to the explanation of phytoplankton community structure variation and had weak correlation with community indices.
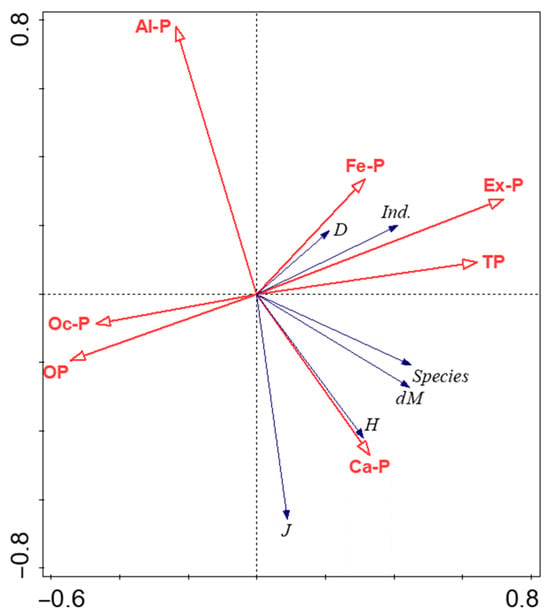
Figure 7.
RDA ordination diagram of sediment phosphorus forms and phytoplankton community parameters (characteristics) in Baiyangdian Lake. Note: Ex-P: exchangeable phosphorus; Al-P: aluminum-bound phosphorus; Fe-P: iron-bound phosphorus; Oc-P: occluded phosphorus; Ca-P: calcium-bound phosphorus; OP: organic phosphorus; dM: Margalef species richness index; J: Pielou evenness index; H: Shannon–Wiener diversity index; D: algal cell density; ind.: count of algal individuals; Species: count of species. Red arrows represent sediment phosphorus forms, and blue arrows represent phytoplankton-related indicators. The length of the arrow indicates the strength of the explanatory power of the variable for the community structure, and the angle between the arrows indicates the correlation between variables (the smaller the angle, the stronger the correlation). The cross-shaped vertical/horizontal dashed lines in the figure are the coordinate axes of the RDA ordination axes, which are used to distinguish the distribution relationships of variables in different directions.
From the results of RDA sensitivity analysis (Table S2 in Supplementary File), after removing Ex-P, the cumulative explained variance of “Axis1 + Axis2” decreased by 5.91 percentage points (from 23.06% to 17.15%) At the same time, the pseudo-canonical correlation coefficient of Axis2 decreased from 0.6223 to 0.5022, indicating that Ex-P is a core sensitive factor regulating phytoplankton communities. After removing factors such as Ca-P and Fe-P, the decrease in cumulative explained variance was less than 1 percentage point, and the change in the pseudo-canonical correlation coefficient of Axis2 was also relatively slight. In addition, except for Ex-P, the fluctuation range of the core indicators of the model after removing other factors was small, indicating that the model is not sensitive to disturbances of non-core factors and has good overall stability. In conclusion, the sensitivity analysis further clarified that Ex-P is a key factor in the phytoplankton community of Baiyangdian Lake, which verified the reliability of the RDA model.
4. Discussion
4.1. Spatial Heterogeneity and Factors Influencing of Sediment Phosphorus Forms
The phosphorus forms in Baiyangdian Lake sediments showed significant differences, with Ex-P accounting for the highest proportion, followed by Oc-P, Ca-P in the middle, and Al-P, Fe-P, and OP accounting for less than 5% each. This characteristic may be dominated by high-intensity human activities. The direct discharge of domestic sewage (containing sodium tripolyphosphate in detergents) from 12 natural villages in the region and agricultural non-point source pollution caused a large amount of active phosphorus to accumulate in the form of Ex-P [29,30,31]. In addition, the sediment is silty clay with a large specific surface area and high adsorption capacity, which further promotes its accumulation [33], which is consistent with the research conclusion that the phosphorus forms along the Fuhe River to Baiyangdian Lake are significantly affected by non-point source pollution [20]. The high proportion of Oc-P is the result of the superposition of soil formation process and human activities. The 4.2–6.8% Fe2O3 and 2.1–3.5% Al2O3 in the sediment can fix phosphorus through lattice encapsulation [15], and the iron and aluminum minerals carried by agricultural sediment will increase their reserves, similar to the characteristics that the phosphorus forms in the waters around the villages in Baiyangdian Lake are significantly regulated by human activities [32]. The proportion of Ca-P is closely related to the environmental characteristics of weak alkaline water (pH 7.8~8.6) and high carbonate. The chemical precipitation of calcium carbonate minerals promotes its formation [34], which is consistent with the research finding that Ca-P is the main component of inorganic phosphorus in Baiyangdian Lake sediments [30]. The low content of Al-P and Fe-P is not only restricted by the low background values of aluminum and iron in the parent material of alluvial deposits from the Yongding River and Daqing River, but also inhibited by the weak alkaline water body [12,35]. The low content of OP may be due to the relatively low water temperature in autumn, which reduces microbial activity and slows down the decomposition of organic matter, but the accumulated OP is more easily mineralized into inorganic phosphorus in the long-term weak alkaline water environment [36].
The spatial distribution of different phosphorus forms in Baiyangdian Lake shows a heterogeneous characteristic of “local enrichment and regional differentiation”. Ex-P presents a higher level around the villages of lake center and western areas. It can be seen from the figure that sampling points such as S14 and S19 are located in densely populated village areas. These areas have a high population density, the domestic sewage collection rate is less than 30% and some are directly discharged. At the same time, chemical fertilizers are intensively applied in farmland, and runoff carries phosphorus fertilizers into the sediment in the rainy season. In contrast, lake center areas such as S20 are far from major pollution sources and have active water exchange, which results in less phosphorus accumulation. The high-value areas of Ca-P, S10 and S19, are mostly in open water areas. The calcium carbonate content in sediments in such areas is high (15~20%, higher than 8~12% in reed marshes), which is conducive to phosphorus precipitation [34]. In contrast, in reed distribution areas, such as some coastal areas, reed roots absorb phosphorus and secrete organic acids, reducing the pH of surrounding sediments and inhibiting the formation of Ca-P [37]. The distribution of Al-P and Fe-P is uniform because the parent material of the sediment is silty clay formed by alluviation of the Yongding River-Daqing River, and the distribution of aluminum and iron is uniform, with little interference from external pollution [6]. The distribution of Oc-P is related to sediment particles. For example, S20 has a high clay content, which is conducive to iron and aluminum oxides encapsulating phosphorus to form Oc-P [38]. OP is controlled by the balance between organic matter input and mineralization, with small input differences in various regions so the distribution is uniform [39].
4.2. Factors Shaping Phytoplankton Community Structure
The phytoplankton community composition in Baiyangdian Lake shows significant phylum-level differentiation and spatial heterogeneity. The differences in community structure at each sampling point in this study are closely related to the intensity of human activities and the physicochemical properties of sediments. A total of 82 phytoplankton species belonging to 7 phyla were monitored, among which Chlorophyta (25 species), Cyanophyta (13 species), and Bacillariophyta (28 species) were the dominant groups, accounting for 80.5% of the total count of species, which is consistent with the general characteristic that the phytoplankton community in Baiyangdian Lake, as a eutrophic lake, is dominated by cyanobacteria and green algae [40]. From the perspective of spatial distribution, sampling points around villages such as S14 and S19 are dominated by Cyanophyta, with Microcystis aeruginosa and Aphanizomenon flos-aquae as the dominant species, which is directly related to the high Ex-P content of 4592~5200 μg/g in this region. High active phosphorus provides sufficient nutrients for cyanobacterial reproduction, and the high adsorption of silty clay sediment promotes phosphorus enrichment, laying a foundation for the formation of cyanobacterial dominance [41]. In contrast, at sampling points far from pollution sources such as S20 and S5, the proportion of Bacillariophyta increases, and Cyclotella meneghiniana becomes the dominant species. Because the TP content in these regions is only 2499.34~2840.30 μg/g, and the water exchange is active, which is more suitable for the growth of Bacillariophyta [42]. In addition, the weak alkaline water environment (pH 7.8~8.6) further inhibits the growth of Euglenophyta and Cryptophyta which prefer acidic environments, making them only sparsely distributed in local points such as S10, which is consistent with the research conclusion on the regulatory effect of pH on phytoplankton community structure in eutrophic lakes [43]. At the same time, the distribution differences of Fe2O3 (4.2~6.8%) and Al2O3 (2.1~3.5%) in sediments indirectly regulate the spatial differentiation of phytoplankton community composition by affecting the release rate of phosphorus [44].
The spatial pattern of phytoplankton diversity in Baiyangdian Lake shows a “high value in local areas, low value in edge and inlet areas” distribution, which is basically consistent with the spatial differences in pollution gradients and hydrological conditions at the sampling points. The H index ranges from 1.496 to 3.289, the dM value from 1.24 to 3.86, and the J index from 0.32 to 0.78, showing an overall characteristic of medium–low-level diversity, reflecting the ecological pressure of water eutrophication [45]. The high diversity values are mainly concentrated in the central part of the lake area and waters near villages, such as S9 (H = 3.289), S16 (H = 3.263), and S15 (H = 3.139). The possible reasons are that these areas have good hydrological connectivity and relatively active water exchange, which alleviates the pressure of eutrophication to a certain extent. At the same time, exogenous inputs around the villages may bring more abundant nutrient substrates, supporting the coexistence of more phytoplankton groups [11]. However, the diversity at edge inlet points of the lake area such as S1 (H = 1.496) and S2 (H = 1.752) is relatively low. This may be because these areas are mostly the initial areas of inflow water into the lake, with relatively concentrated pollution loads, weak water mobility, and high eutrophication levels. A single dominant phytoplankton group occupies a dominant position, inhibiting species diversity [21].
4.3. Effects of Different Phosphorus Forms on the Differentiation of Phytoplankton Community Structure
4.3.1. Direct Effects of Active Phosphorus
The average content of Ex-P in Baiyangdian Lake sediments reaches (2332.3 ± 970.4) μg/g, accounting for as high as 59.8% of TP, which is much higher than that of similar shallow lakes in China such as Taihu Lake and Chaohu Lake, forming a huge active phosphorus pool. Its ecological effect is mainly realized through the direct pathway of rapid release-algal response. Ex-P is physically adsorbed on the sediment surface and is significantly affected by water disturbance. The disturbance intensity of wind-driven current in Baiyangdian Lake in summer is relatively high, and the release rate of Ex-P is 4~6 times that of Al-P and Fe-P [21]. This release characteristic is consistent with the “active phosphorus preferential migration” theory proposed by Ruttenberg [6], that is, physically adsorbed phosphorus is easy to exchange into the overlying water through the water–sediment interface under disturbance conditions.
After a large amount of active phosphorus enters the overlying water, the concentration of dissolved reactive phosphorus (SRP) in the water can rise to 0.15~0.25 mg/L, which is much higher than the half-saturation constant for algal growth (0.5~3.0 μmol/L) [10], which can directly meet the nutritional needs of algal photosynthetic growth. This is highly consistent with the result of Pearson correlation analysis that Ex-P is significantly positively correlated with phytoplankton biomass density (r = 0.38, p < 0.05). For example, the phytoplankton densities at high Ex-P sampling points S14 (5200 μg/g) and S19 (4592 μg/g) reached 0.0471 × 106 cells/L and 0.0392 × 106 cells/L, respectively, which were significantly higher than 0.0125 × 106 cells/L at low Ex-P sampling point S20 (1000 μg/g).
Different algae have obvious differences in their ability to utilize Ex-P, which further aggravates the differentiation of community structure. Cyanophyta (such as Microcystis aeruginosa) has pseudovacuole structure, which can preferentially obtain Ex-P through vertical migration (floating to the surface for photosynthesis during the day and sinking to the sediment surface to absorb phosphorus at night) [46], so the count of cyanobacteria at S19 reached 8 (ind./0.1 mL); although Bacillariophyta has low demand for Ex-P (Ks = 0.5~1.0 μmol/L), the high Ex-P environment can alleviate its nutritional competition with cyanobacteria, and the count of Bacillariophyta at S14 still reached 11 (ind./0.1 mL). Chlorophyta has weak competitiveness due to its high Ks (2.0~3.0 μmol/L), and the count of Chlorophyta at S14 was only 3 (ind./0.1 mL), lower than the lake average (5.95 ind./0.1 mL).
RDA analysis showed that the RDA1 axis explained 17.92% of the community variation. The angle between the arrows of algal density, the count of algal individuals and Ex-P is less than 45°, which further confirmed the dominant role of Ex-P. Considering the high mobility of Ex-P, it is speculated to be a potential core factor driving changes in phytoplankton communities, and this causal relationship needs further verification through subsequent bioassay experiments (such as Ex-P gradient addition culture).
4.3.2. Potential Regulation of Inactive Phosphorus
Although the release rate of inactive phosphorus such as Ca-P, Al-P, and Fe-P is much lower than that of Ex-P, they have an important impact on the stability of phytoplankton communities through long-term effects or indirect effects. Ca-P is extremely stable in the alkaline environment (pH 7.8~8.6) of Baiyangdian Lake, with a release cycle of several years to decades [34] and a rate of about 2~3 mg/(m2·d). Its slow release can avoid the explosive growth of algae. At the same time, the released calcium ions can promote the synthesis of siliceous cell walls of Bacillariophyta [47,48], maintain the dominance of Bacillariophyta, and avoid the monopoly of cyanobacteria. Pearson analysis in this study showed that Ca-P was significantly positively correlated with H (r = 0.33, p < 0.05). For example, the H value of S9 (Ca-P value 1098.4 μg/g) reached 3.289 (the highest in the lake), with 15 Bacillariophyta, 5 Chlorophyta, and 6 Cyanophyta, and the community was balanced. In RDA analysis, the angle between H and Ca-P arrows was less than 45°, further confirming its maintaining effect. At the same time, the calcium carbonate content in sediments of open water areas in Baiyangdian Lake is high, promoting the formation of Ca-P [38], while in reed marsh areas, the pH is reduced (7.2~7.6) due to the secretion of organic acids by reed roots, inhibiting the formation of Ca-P. This habitat difference further strengthens the regulation of Ca-P on community distribution.
The ecological effect of Al-P is reflected in the inhibition of community evenness, and the core mechanism is the release and toxicity of Al3+ under acidic conditions. Although the overall pH of the water body is 7.8~8.6, the local pH at the sediment microinterface (0~2 cm) may drop below 7.0 due to the release of CO2 by microbial respiration [49], leading to the dissolution of Al3+ (0.1~0.3 mg/L). Al3+ can combine with the hydroxyl groups of polysaccharides in the cell wall of Chlorophyta, destroy the structure and inhibit the activity of photosynthetic enzymes [50]. For example, the count of Chlorophyta at S2 (45.4 μg/g), the high-value point of Al-P, was only 1, and the evenness index (J = 0.325) was lower than the average (0.436). In RDA analysis, the angle between Al-P and J arrows was greater than 90°, further verifying the inhibitory effect.
The ecological effect of Fe-P is potential and seasonal, and its release depends on anaerobic environment. There is aerobic condition in the surface sediment (0~10 cm) of Baiyangdian Lake, so the release of Fe-P is limited. However, the decomposition of dead algae in summer (mortality rate 10~15%/d) [10] leads to the deposition of organic matter in the deep layer to form an anaerobic environment, promoting the conversion of Fe3+ → Fe2+ and phosphorus release (rate 3~5 mg/(m2·d)) [30], accompanied by the release of NH4+ and S2−, forming “phosphorus-nitrogen-sulfur” coupled pollution. Although this study did not capture significant release, referring to the study on Taihu Lake [51], a similar process may exist in Baiyangdian Lake, which needs long-term monitoring and verification.
4.3.3. Group Adaptation Strategies of Phytoplankton to Phosphorus Regulation
Facing the spatial heterogeneity of sediment phosphorus forms, different groups of phytoplankton in Baiyangdian Lake have formed unique distribution patterns through morphological and physiological adaptations, strengthening the shaping effect of phosphorus forms on the community. The adaptation strategy of Bacillariophyta (accounting for 43.3%) is “calcium dependence and surface phosphorus uptake”. On the one hand, the synthesis of siliceous cell walls requires calcium ions, and the high Ca-P environment provides sufficient calcium sources, so the count of Bacillariophyta is relatively high in high Ca-P areas (S16, S19) (S16: 15 ind./0.1 mL; S19: 11 ind./0.1 mL) [47]. On the other hand, Bacillariophyta can migrate vertically day and night, sinking to absorb Ex-P at night and floating up for photosynthesis during the day [10], making it dominant in different Ex-P areas.
The adaptation of Chlorophyta focuses on the efficient utilization of resources in low-phosphorus environments, showing advantages in the western lake area (S20, 1000 μg/g) with low Ex-P. Chlorophyta possesses two major adaptive advantages. One advantage is its high Chla content, which enables efficient utilization of light energy. Notably, the western lake area features high transparency (1.5~2.0 m) and favorable photosynthetic conditions that complement this trait well. The second advantage lies in the 2~3-fold increase in phosphorus transporter expression under low-phosphorus environments [25]. This increase directly enhances phosphorus absorption for Chlorophyta. Such combined adaptive traits explain why the abundance of Chlorophyta at sampling point S20 is the highest in the lake (15 ind. /0.1 mL), ultimately forming a low-phosphorus–high-photosynthesis model.
The adaptation strategy of Cyanophyta is competitive and tolerant, making it dominant in high Ex-P areas (S14, S19). Cyanophyta has low Ks (0.8~1.2 μmol/L), and vertical migration supported by pseudovacuoles can preferentially obtain Ex-P [46]. Secrete extracellular polysaccharides (EPS) to adsorb Fe3+, promote the release of Fe-P, and EPS forms a protective layer to resist Al3+ toxicity, such as the count of Cyanophyta at S10, a high-value point of Al-P, still reach 4 per unit. It can also produce algal toxins to inhibit other algae [50], such as the proportion of Cyanophyta at S19 is 19.04%, higher than the lake average (14.12%). In addition, cyanobacteria can reduce phosphorus consumption through membrane lipid remodeling when phosphorus is limited [36], further improving their adaptability.
In summary, the specific adaptations of phytoplankton groups, together with the direct effect and indirect regulation mechanisms of phosphorus forms, jointly shape a community distribution pattern: Bacillariophyta serves as the dominant group, Chlorophyta is enriched in the western lake area, and Cyanophyta is concentrated in village-adjacent regions. This pattern provides clear targets for the prevention and control of internal pollution.
4.4. Limitation Analysis
This study has certain limitations as the sampling period only covers the autumn of 2023 (November) without involving spring and summer, which are periods with a high incidence of algal blooms, meaning it cannot fully reflect the seasonal dynamics of the relationship between phosphorus forms and phytoplankton. Correlation analyses including Pearson and RDA only reveal associations, and causal relationships have not been verified through bioassay experiments such as Ex-P addition culture. Additionally, the contribution ratios of different pollution sources like domestic sewage and agricultural non-point sources to Ex-P have not been quantified. Future research can focus on conducting continuous sampling across spring, summer, autumn, and winter to analyze the seasonal coupling laws between phosphorus forms and phytoplankton. It can also verify the driving effect of phosphorus forms on phytoplankton through indoor culture and release simulations experiments such as setting different Ex-P concentration gradients to clarify the causal relationships. Another direction is quantifying the contribution ratios of domestic sewage and agricultural non-point sources to sediment Ex-P by combining isotope tracing technology such as 32P, which can provide a basis for precise control of pollution sources.
5. Conclusions
Focusing on northern carbonate-type lakes, this study targeted 20 typical sampling points in the waters around villages in Baiyangdian Lake. It revealed the occurrence characteristics, the spatial patterns of sediment phosphorus forms, and their driving mechanisms in phytoplankton communities. Results showed sediment TP ranged from 2499.3 to 6713.6 μg/g, with an average of (3901.5 ± 1047.4) μg/g. Phosphorus forms were dominated by Ex-P (59.8%) and Oc-P (23.2%). Spatially, Ex-P was affected by human activities, being high around villages of lake center and western areas. High-value areas (S14, S19) reached 4592~5200 μg/g, while low-value areas (S20, S11) were only 1000~1532 μg/g. Ca-P was high in open water and low in reed marshes, and Al-P, Fe-P, Oc-P and OP were relatively uniformly distributed.
A total of 82 phytoplankton species (from 7 phyla) were identified, with Bacillariophyta acting as the absolute dominant group (43.3%). H significantly affected by combined phosphorus forms. The study clarified the dual regulation law: active phosphorus affects directly, and inactive phosphorus regulates indirectly. Ex-P is easily released by disturbance and significantly positively correlated with phytoplankton biomass density (r = 0.38, p < 0.05). It explains 17.92% of community variation via RDA1 axis, dominating community changes. Ca-P is significantly positively correlated with H (r = 0.33, p < 0.05) to maintain community balance. Al-P is significantly negatively correlated with evenness index (r = −0.35, p < 0.05), releasing Al3+ at the microinterface to inhibit diversity.
This study first clarified the unique phosphorus structure of nearly 60% Ex-P in northern carbonate-type lakes, filling gaps in quantitative research on sediment phosphorus’ ecological effects on phytoplankton. It provides a form-targeted paradigm for internal pollution control in similar lakes. It is suggested that Ex-P and Ca-P should be included in routine monitoring. In areas with high Ex-P content, sediment passivation and riparian zone restoration should be implemented to cut off the cycle of internal phosphorus enrichment and algal bloom outbreak. This measure will provide support for the ecological security of the Beijing–Tianjin–Hebei wetland and the functional restoration of the “Kidney of North China”.
This study takes Baiyangdian Lake in China as the research object, clarifies the dominant role of Ex-P in the phytoplankton community and the distribution of high-risk areas, directly responds to sustainability challenges such as water pollution control and ecosystem protection, and provides a practical basis for water bloom prevention and control as well as biodiversity conservation. Addressing the insufficient research on northern carbonate-type lakes, this study proposes to include Ex-P and Ca-P in routine monitoring, providing sustainability tools that meet the needs of sustainability science assessment and management. The study suggests prioritizing sediment passivation and other restoration measures in high-risk areas, which not only contributes to the control of agricultural non-point source pollution but also ensures the ecological security of Baiyangdian Lake to support the socially and environmentally sustainable development of the Beijing–Tianjin–Hebei region. Meanwhile, the study emphasizes the key role of phosphorus form balance in ecosystem stability, highlights the management idea of balancing short-term governance and long-term resilience, and aligns with the core requirements of sustainable development such as ecosystem maintenance and biodiversity conservation.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su18010310/s1. Table S1. Results of Kolmogorov–Smirnov (K–S) normality test for all variables. This table presents the asymptotic significance (p-value) of each variable (sediment phosphorus forms and phytoplankton community indices) from the K–S normality test. All p-values range from 0.055 to 0.984 (p > 0.05), indicating that the data conform to a normal distribution and support subsequent Pearson correlation analysis. Table S2. Results of RDA sensitivity analysis. This table shows the changes in cumulative explained variance and pseudo-canonical correlation coefficients of RDA ordination axes after sequentially excluding individual phosphorus form variables. The analysis verifies that Ex-P is a core sensitive factor regulating phytoplankton communities, while other factors have little impact on model stability.
Author Contributions
Q.C. contributed to the ideas, conceptual framework, draw some of the figures and tables and wrote the manuscript. X.Z., L.S., L.C. and S.W. conducted sampling, experiments and obtained data. B.L. and Q.L. performed the data statistical analyses. Y.Y. and R.X. draw some of the figures and provided revision suggestions for the text. All authors have read and agreed to the published version of the manuscript.
Funding
This research was jointly supported by the Natural Science Foundation of Hebei Province (D2023508003) and the Fundamental Research Funds for the Central Universities (3142024023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author (Q.C., cqy@ncist.edu.cn) on reasonable request.
Acknowledgments
The authors thank all the personnel who either provided technical support or helped with data collection. We also acknowledge all the reviewers for their useful comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ministry of Ecology and Environment of the People’s Republic of China (MEEPRC). National Surface Water Environmental Quality Status for the Fourth Quarter of 2024 and January–December 2024. 2025. Available online: https://www.mee.gov.cn/ywdt/xwfb/202501/t20250124_1101321.shtml (accessed on 15 September 2025).
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Beaty, K.G.; Lyng, M.; Kasian, S.E.M. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, B.S.; Yang, Z.F. Study of the lowest ecological water level of Baiyangdian Lake. Acta Ecol. Sin. 2005, 25, 1033–1040. [Google Scholar]
- Liu, X.; Shi, B.; Meng, J.; Zhou, Y.Q.; Ke, X.; Wang, T.Y. Spatio-temporal Variations in the Characteristics of Water Eutrophication and Sediment Pollution in Baiyangdian Lake. Environ. Sci. 2020, 41, 2127–2136. [Google Scholar]
- Ma, W.; Feng, J.; Zhang, J.; Wang, H.; Guo, Y.; Lyu, Y.; Chen, L.; Li, X.; Zhao, M.; Liu, S. Different responses of phytoplankton taxa to water n and p inputs in a freshwater wetland: A mesocosm study. Mar. Pollut. Bull. 2025, 216, 117895. [Google Scholar] [CrossRef]
- Ruttenberg, K.C. Development of a sequential extraction method for different forms of phosphorus in marine sediments. Limnol. Oceanogr. 1992, 37, 1460–1482. [Google Scholar] [CrossRef]
- Lü, Y.B.; Zhang, M.; Yin, H.B. Phosphorus release from the sediment of a drinking water reservoir under the influence of seasonal hypoxia. Sci. Total Environ. 2024, 917, 170490. [Google Scholar]
- Sondergaard, M.; Kristensen, P.; Jeppesen, E. Phosphorus release from resuspended sediment in the shallow and wind-exposed lake Arreso, Denmark. Hydrobiologia 1992, 228, 91–99. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Choi, Y.H.; Kwak, D.H. Effects of dissolved oxygen changes in the benthic environment on phosphorus flux at the sediment-water interface in a coastal brackish lake. Mar. Environ. Res. 2024, 196, 106439. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Phytoplankton; Cambridge University Press: Cambridge, UK, 2006; pp. 213–245. [Google Scholar]
- Sondergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506–509, 135–145. [Google Scholar] [CrossRef]
- Li, J.D.; Wang, Z.F.; Yang, H.; Liu, F.J.; Chen, X.H.; Chen, X.G.; Huang, X.G. Phosphorus forms and zinc concentrations affect the physiological ecology and sinking rate of Thalassiosira weissflogii. Mar. Pollut. Bull. 2024, 200, 116124. [Google Scholar] [CrossRef]
- Zheng, Y.; Sun, Y.; Zhang, Z.H.; Han, C.; Wang, Z.D.; Liu, C.; Li, J.; Wang, L.; Zhang, X.; Yang, Q. Evaluation of the distribution and mobility of labile phosphorus in sediment profiles of Lake Nansi, the largest eutrophic freshwater lake in northern China. Chemosphere 2023, 315, 137756. [Google Scholar] [CrossRef]
- Chen, J.; Li, D.P.; Zhu, P.Y.; Huang, Y.; Wang, R. Sedimentary Phosphorus Forms Under Disturbances and Algae in Taihu Lake. Environ. Sci. 2015, 36, 4509–4515. [Google Scholar]
- Jin, L.; Wu, Q.H.; Xie, S.J.; Chen, W.W.; Duan, C.Q.; Sun, C.Q.; Pan, Y.; Lauridsen, T.L. Phosphorus stoichiometric homeostasis of submerged macrophytes and associations with interspecific interactions and community stability in Erhai Lake, China. Water Res. 2024, 256, 121575. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sun, Y.; Lü, C.W.; Liu, E.D.; Shen, L.L. Research on phosphorus release from the surface sediments in the Daihai Lake. Acta Ecol. Sin. 2010, 30, 389–398. [Google Scholar]
- Li, J.; Ma, Y.A.; Sun, Z.Y.; Yang, J.W.; Hou, X.K.; He, L.S.; Che, F.F.; Shi, D.; Shen, D.B.; Guo, Y.Y. Characteristics and influencing factors of phosphorus release from sediments of Lake Bosten based on occurrence forms and interfacial diffusion. J. Environ. Eng. Tech. 2025, 15, 1906–1917. [Google Scholar]
- Yi, Y.J.; Lin, C.Q.; Tang, C.H. Hydrology, environment and ecological evolution of Lake Baiyangdian since 1960s. J. Lake Sci. 2020, 32, 1333–1347. [Google Scholar] [CrossRef]
- Zhu, T.Y. Investigation and Evaluation of Substrate Pollution in Zaozhaodian and Study on the Release Law of Nutrients in Sediments; Beijing Forestry University: Beijing, China, 2019. [Google Scholar]
- Li, W.Y.; Wang, S.Q.; Gu, C.K.; Tan, K.D.; Lü, J.L. Distribution characteristics and influencing factors of phosphorus forms in water-sediment from Fu River to Baiyangdian Lake. Acta Sci. Circumstantiae 2023, 43, 179–188. [Google Scholar]
- Cao, Y.; Wen, S.F.; Wang, X.; Shan, B.Q. Sediment oxygen consumption rate and oxygen deficit effect in Baiyangdian Lake. Acta Sci. Circumstantiae 2022, 42, 240–248. [Google Scholar]
- Ministry of Ecology and Environment of the People’s Republic of China (MEEPRC). HJ 1296-2023 Technical Guidelines for Water Ecological Monitoring—Aquatic Organism Monitoring and Evaluation of Lakes and Reservoirs (Trial). 2023. Available online: https://www.mee.gov.cn/ywgz/fgbz/bz/bzwb/shjbh/xgbzh/202305/t20230511_1029717.shtml (accessed on 15 September 2025).
- China National Environmental Monitoring Center (CNEMC). Technical Requirements for Aquatic Ecological Monitoring—Freshwater Phytoplankton (Trial); China National Environmental Monitoring Center: Beijing, China, 2022; No. 41. [Google Scholar]
- Ruban, V.; Pardo, P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments—A synthesis of recent works. Fresenius J. Anal. Chem. 2001, 370, 224–228. [Google Scholar] [CrossRef]
- Hu, H.J.; Wei, Y.X. Freshwater Algae of China—Systematics, Taxonomy and Ecology; Science Press: Beijing, China, 2006; pp. 213–245. [Google Scholar]
- Wang, X.; Sun, M.; Wang, J.; Zhang, Y.; Li, Y.; Yu, H.; Yang, Z. Microcystis genotype succession and related environmental factors in Lake Taihu during cyanobacterial blooms. Microb. Ecol. 2012, 64, 986–999. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, D.Y.; Wang, J.; Hu, C.; Zhao, Q.; Chen, J.; Zhang, H. Phytoplankton community structure in Lake Taiping of Anhui Province. J. Lake Sci. 2016, 28, 1066–1077. [Google Scholar] [CrossRef]
- Long, Z.Y. Study on Relationship Between Macrobenthos and Water Quality Response of Small and Medium-Sized Shallow Lakes in Western Jilin Province; Northeast Normal University: Changchun, China, 2018. [Google Scholar]
- Li, Y.Z.; Chen, H.Y.; Sun, W.C. Load estimation and source apportionment of nitrogen, phosphorus and COD in the basin of Lake Baiyang. China Environ. Sci. 2021, 1, 366–376. [Google Scholar]
- Tang, Y.S.; Wang, S.Q.; Lü, J.L.; Gu, C.K. Spatiotemporal characteristics of nitrogen and phosphorus in the water from Fu River to Lake Baiyangdian and their response to extreme rainfall. J. Lake Sci. 2025, 37, 1593–1603. [Google Scholar]
- Dong, L.M.; Liu, G.N. Phosphorus forms and its distribution characteristics in sediment cores of Baiyangdian Lake. J. Agro-Environ. Sci. 2011, 30, 711–719. [Google Scholar]
- Jeppesen, E.; Meerhoff, M.; Davidson, T.A.; Trolle, D.; Søndergaard, M.; Lauridsen, T.L. Climate change impacts on lakes: An integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. J. Limnol. 2014, 73, 88–111. [Google Scholar] [CrossRef]
- Shen, L.L.; He, J.; Lü, C.W.; Sun, Y. Phosphorus Release from Surface Sediment of the Hasuhai Lake. J. Agro-Environ. Sci. 2009, 28, 1219–1224. [Google Scholar]
- Hamilton, S.; Bruesewitz, D.; Horst, G.; Weed, D.; Sarnelle, O. Biogenic calcite-phosphorus precipitation as a negative feedback to lake eutrophication. Can. J. Fish. Aquat. Sci. 2009, 66, 343–350. [Google Scholar] [CrossRef]
- Kraal, P.; Slomp, C.P.; Van der Gaast, S.J.; Van Cappellen, P.; Seitaj, S.; Smolders, A.J.P. Iron and phosphorus speciation in sediments of a shallow eutrophic lake: Implications for internal phosphorus loading. Geochim. Cosmochim. Acta 2015, 150, 211–225. [Google Scholar]
- Hu, J.; Zhao, Q.; Zeng, P.; Tang, Q.; Sun, Q.; Yin, H. Warming accelerated phosphorus release from the sediment of Lake Chaohu during the decomposition of algal residues: A simulative study. PLoS ONE 2025, 20, e0314534. [Google Scholar] [CrossRef]
- Batty, L.C.; Younger, P.L.; Lindsay, S. Aluminium and Phosphate Uptake by Phragmites australis: The Role of Fe, Mn and Al Root Plaques. Ann. Bot. 2002, 89, 443–451. [Google Scholar] [CrossRef]
- Jiao, Y.; Yang, C.; He, W.; Liu, W.X.; Xu, F.L. The spatial distribution of phosphorus and their correlations in surface sediments and pore water in lake Chaohu, China. Environ. Sci. Pollut. Res. 2018, 25, 25906–25915. [Google Scholar] [CrossRef] [PubMed]
- Prasath, B.B.; Lin, Z.R.; Su, Y.P.; She, C.X.; Lin, H.; Zhang, C.W.; Yang, H. Adsorption-Release characteristics of phosphorus and the community of phosphorus accumulating organisms of Sediments in a shallow Lake. Sustainability 2021, 13, 11501. [Google Scholar] [CrossRef]
- Chen, T.; Du, X.; Chen, Y.Y.; Guo, X.Y.; Xiong, W. Metabarcoding profiling of phytoplankton communities associated with algal blooms and determining related drivers in Baiyangdian Lake. Environ. Sci. 2023, 44, 6116–6124. [Google Scholar]
- Zhou, X.S.; Li, N.; Sun, B.W.; Wang, X.H.; Zhao, Y.; Chen, Y.L.; Liu, M. Seasonal variation of plankton community structure in Lake Baiyangdian and the relationship with environmental factors. Water Resour. Hydropower Eng. 2021, 52, 110–119. [Google Scholar]
- Sun, W.Z. Seasonal Community Characteristics and Driving Mechanisms of Algae in Baiyangdian Lake; Hebei University: Baoding, China, 2022. [Google Scholar]
- Li, N.; Zhou, X.S.; Sun, B.W.; Gao, X.P.; Cui, W.Y. Spatiotemporal variation of phytoplankton community and its relationship with environmental factors in the Lake Baiyangdian. J. Lake Sci. 2020, 32, 772–783. [Google Scholar] [CrossRef]
- Strzepek, R.F.; Price, N.M.; Morel, F.M.M. Iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean. Nature 1994, 372, 145–149. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, H. Temporal and spatial variations of phytoplankton community in Baiyangdian Lake and their relationship with environmental factors. J. Lake Sci. 2020, 32, 845–854. [Google Scholar]
- Reynolds, C.S.; Jaworski, G.H.M.; Cmiech, H.A.; Leedale, G.F. On the annual cycle of the blue-green alga Microcystis aeruginosa Kütz. Emend. Elenkin. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1981, 293, 419–477. [Google Scholar] [CrossRef]
- Kröger, N.; Bergsdorf, C.; Sumper, M. A new calcium binding glycoprotein family constitutes a major diatom cell wall component. EMBO J. 1994, 13, 2811–2818. [Google Scholar] [CrossRef]
- Leone, G.; Vona, D.; Lo Presti, M.; Urbano, L.; Cicco, S.; Gristina, R.; Palumbo, F.; Ragni, R.; Farinola, G.M. Ca2+-in vivo doped biosilica from living Thalassiosira weissflogii diatoms: Investigation on Saos-2 biocompatibility. MRS Adv. 2017, 2, 1047–1058. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, L.J.; Xiang, J.J.; Liao, Q.G.; Zhang, D.W.; Liu, J.T. Response of the microbial community structure to the environmental factors during the extreme flood season in Poyang Lake, the largest freshwater lake in China. Front. Microbiol. 2024, 15, 1362968. [Google Scholar] [CrossRef]
- Jiang, D.X.; Hou, J.J.; Gao, W.W.; Tong, X.; Li, M.; Chu, X.; Chen, G.X. Exogenous spermidine alleviates the adverse effects of aluminum toxicity on photosystem II through improved antioxidant system and endogenous polyamine contents. Ecotoxicol. Environ. Saf. 2021, 207, 111265. [Google Scholar] [CrossRef]
- Zhu, G.W.; Qin, B.Q.; Gao, G.; Zhang, L.M.; Liu, J.R.; Hu, W.P. Fractionation of phosphorus in sediments and its relation with soluble phosphorus contents in shallow lakes located in the middle and lower reaches of Changjiang River, China. Acta Sci. Circumstantiae 2004, 24, 381–388. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.