Abstract
The indiscriminate discharge of common dyes, such as malachite green (MG), poses significant risks to water quality and human health. To address this issue, a biochar (MBC) was synthesized from waste Myriophyllum aquaticum biomass (MAB) and further activated with KOH to produce micro-mesoporous biochar (KMBC) with enhanced adsorption efficiency. Characterization results demonstrated that KMBC exhibits a higher specific surface area (1632.7 m2/g) and a larger pore volume (0.759 cm3/g) compared to MBC. Batch adsorption experiments revealed that the adsorption process follows pseudo-second-order kinetics and the Langmuir isotherm model, with the theoretical maximum adsorption capacities of MBC and KMBC reaching 1772.3 mg/g and 2570.7 mg/g, respectively and the adsorption is a spontaneous, endothermic, and entropy-driven process. Key mechanisms involved in the adsorption process include hydrogen bonding, hydrophobic interactions, and surface complexation. Due to electrostatic attraction, selective adsorption experiments confirmed that MBC can effectively separate cationic dyes such as MG from mixed anionic-cationic systems. Dynamic experiments showed that the breakthrough curve data fit well with the Thomas model. In summary, MAB-derived biochar demonstrates significant potential for practical applications in the treatment of MG-contaminated wastewater.
1. Introduction
With the acceleration of industrialization, the textile printing and dyeing industry has become one of the essential pillars of global economic development. Textile printing and dyeing wastewater have such characteristics as complex composition, high color, and a significant content of organic pollutants [1]. Among these pollutants, synthetic dyes such as malachite green (MG) pose severe environmental and health risks. MG, a triphenylmethane dye, has been strictly regulated in many countries due to its toxic, carcinogenic, and mutagenic effects [2]. For instance, the European Union has set stringent limits for MG residues in food products, reducing the permissible level from 2 μg/kg to 0.5 μg/kg in 2019 [3]. The concentration of malachite green in water is usually between a few micrograms/liter and a few milligrams/liter, but may be higher in heavily polluted waters. Research has shown that the total residual levels of malachite green observed in countries including China, Iran, and the Netherlands are all 2 μg/kg higher than the authorized standard [4]. According to previous reports, MG and its metabolite leucomalachite green (LMG) have serious carcinogenic, teratogenic, mutagenic, and genotoxic effects, especially causing damage to mammalian internal organs and further promoting liver tumors. The International Agency for Research on Cancer (IARC) has classified MG and LMG as potentially harmful to humans, with LMG being categorized as Group 2B (possibly carcinogenic) [5]. The discharge of untreated MG-contaminated wastewater can severely damage aquatic ecosystems by limiting sunlight penetration and reducing oxygen levels in water bodies, ultimately threatening aquatic life [6]. The removal of MG from wastewater is critical to mitigating its environmental and health impacts. Over the past few decades, various methods, including oxidation, flocculation, electrochemical treatment, adsorption, filtration, and bioremediation, have been developed to address dye pollution [7,8,9,10]. Advanced oxidation processes (AOPs) are typically employed as primary treatment methods to degrade the bulk of organic dyes [11]. However, AOPs may not always achieve complete removal of dyes, and residual dye molecules or intermediate by-products may remain in the treated water. Adsorption is widely recognized as an effective secondary or tertiary treatment method for advanced wastewater purification due to its simple operation, effective purification, and avoidance of secondary pollution risks. Despite these advantages, the field continues to face significant challenges, most notably the development of adsorbents that simultaneously achieve high performance and economic viability.
Although numerous adsorbents, such as activated carbon, carbon nanotubes, zeolites, and metal-organic frameworks, have been explored for dye removal, many suffer from limitations such as low adsorption efficiency, high production costs, and complex preparation processes [12,13,14,15]. For example, Altun et al. used halloysite nanotube to adsorb MG and methyl violet 2B dyes and the Langmuir maximum adsorption capacities are 74.95 mg/g and 67.87 mg/g, respectively [13]. Although the adsorption of MG on some adsorbents has been studied, most studies still face challenges such as low adsorption efficiency, high raw material costs, and difficult preparation processes. Furthermore, most studies have focused on conventional adsorbents, leaving a significant gap in the exploration of sustainable and low-cost alternatives [16].
Biochar, with a large specific surface area, rich pore space, excellent adsorption performance, a vast source of raw materials, and environmental friendliness has received widespread attention in water treatment [17,18,19]. Current research on plant biochar mainly includes agricultural waste, forestry waste, and aquatic plant residues [20]. Research on aquatic plants primarily focuses on emergent plant biochar such as reeds and canna, while fewer studies are made on preparing submerged plant biochar [21,22,23,24,25,26]. Especially studies on removing organic dyes by submerged plant biochar have rarely been reported. Myriophyllum aquaticum biomass (MAB), a commonly submerged plant with strong growth capacity and adaptability, plays a pivotal role in ecological restoration, particularly in eutrophic water bodies. However, following the restoration of these water bodies, a substantial amount of waste MAB is generated, hindering the growth of other aquatic vegetation, obstructing waterways, and potentially affecting agricultural productivity [27]. Current research on the resource utilization of MAB predominantly centers around its bioremediation properties. For instance, Fakhry et al. developed a method of immobilizing MAB on polyacrylonitrile and polyvinylpyrrolidone to create mixed polymer microspheres, which exhibited a maximum adsorption capacity for rho-cyanidin O dye of 217 mg/g [28]. Barintini et al. prepared MAB into biochar material through hydrothermal carbonization, and the experimental results showed that it is feasible to prepare it into biochar material [29]. Nevertheless, the potential of MAB as a bioresource remains largely untapped. The recycling of waste resources can be realized by preparing porous biochar materials. To the best of our knowledge, despite several reports on the adsorption mechanism of MG on modified biochar, none of the available studies have focused on optimizing the adsorption factors and dynamic adsorptive performance. The dynamic adsorption performance of packing biochar fixed bed can provide a practical method for using adsorbents in chemical engineering.
Therefore, waste MAB was chosen as a precursor, and KOH as an activator to prepare biochar (MBC and KMBC) with abundant pores through a simple and environmentally friendly activated-carbonization process to achieve efficient adsorption of MG. This paper aims to (i) identify the optimal adsorption conditions and suitable adsorption models for MG removal through batch experiments.; (ii) systematically elucidate the adsorption mechanism by characterizing the surface compositions and structure of MAB-derived biochar; (iii) investigate the selective adsorption properties of MBC and KMBC for different dyes in an anionic and cationic dye coexist system; (iv) analyze the dynamic adsorption performance of MG on KMBC in a fixed-bed column. By achieving these goals, this study aims to provide sustainable and efficient solutions for dye wastewater treatment, while promoting the resource utilization of waste MAB and achieving sustainable development.
2. Materials and Methods
2.1. Biochar Preparation and Characterization
MAB collected from the pond in Conghua District, Guangzhou, Guangdong Province were used as raw materials for biochar production. All chemicals used in this study were of analytical grade unless otherwise stated. After washing the MAB several times with deionized water, it was cut into small pieces and placed in a drying oven to dry to constant weight. The dried biomass was ground and passed through a 60-mesh sieve (<0.30 mm). Place 3 g of MAB powder into 50 mL of 1 mol/L KOH solution and subject it to magnetic stirring for 12 h. After centrifuging and drying to constant weight, the obtained material was pyrolyzed in a tube furnace for 2 h (700 °C, 10 °C/min). Before the reaction started, N2 was introduced for 30 min to ensure the pyrolysis reaction without oxygen. The biochar was repeatedly washed to a neutral pH value using 1 M HCl and then dried to constant weight and passed through a 100-mesh sieve (<0.15 mm) to obtain the final sample (KMBC). The biochar (MBC) obtained without KOH as an activator was used as a control.
2.2. Batch Adsorption Test
Detailed Experimental and characterization methods instrumentation are shown in Supplementary Material Text S1 and details of the adsorption kinetics, thermodynamics, and isotherm models are provided in Supplementary Material Text S2.
2.3. Selective and Reusable Adsorption Experiment
Five dyes, including the anionic dye methyl orange (MO), naphthol green B (NgB), chrome black T (CbT), cationic dye crystal violet (CV), and MG were selected to verify the selective adsorption of biochar. UV-visible spectroscopy was used to detect the dye concentration in the solution at 463 nm, 714 nm, 540 nm, 584 nm, and 617 nm. At an initial concentration of 200 mg/L, 10 mg of adsorbent was used to adsorb for 2 h, and its single adsorption performance was compared for different dyes. A 100 mL mixed binary solution of anions and cations (300 mg/L, MG:MO = 1:1) was selected and mixed with 10 mg of biochar to evaluate the selective adsorption capacity of the adsorbent. The selective adsorption capacity of MBC and KMBC in binary dye solutions, as shown in Equation (1) [30]:
where S represents the selective adsorption capacity in the binary dye solution; c0,i and ct,i are the concentrations of the cationic dye i at the initial and t time, mg/L; c0,j and ct,j are the concentrations of the anionic dye j at the initial and t time, mg/L.
After each MG equilibrium absorption, the biochar was separated by vacuum filtration. The regeneration process was carried out in an Erlenmeyer flask where MG in MBC or KMBC was leached using a mixture of C2H5OH and CH3COOH in a ratio of 1:1. This mixture was then sonicated for 30 min to facilitate the removal of MG. After elution, the biochar was dried at 80 °C until a constant weight was achieved. The regeneration test was repeated for five cycles, and the equilibrium adsorption capacity of MG was calculated following each cycle to assess the reusability of the biochar.
2.4. Dynamic Adsorption Experiment of Fixed Bed Column
Figure 1 shows the different bed heights obtained by filling 0.05 g, 0.1 g, and 0.2 g MBC into the fixed bed column with a 9.0 mm inner diameter and a 45.0 mm height. The corresponding actual filling heights inside the column were measured to be 2.3 mm, 4.5 mm, and 8.4 mm, respectively. The specific cross-section is 63.6 mm2. The peristaltic pump was used to control the solution with different initial concentrations (100 mg/L,150 mg/L, and 200 mg/L) to penetrate the column at various flow rates (6.4 mL/min, 8 mL/min, 9.6 mL/min) with the corresponding linear speeds (10.1, 12.6, and 15.1 cm/min). The breakthrough time (tb) is defined as the effluent concentration reaching 10% of the initial concentration. The experiment continued until the effluent concentration reached 90% of the initial concentration, which is defined as exhaust time (te).
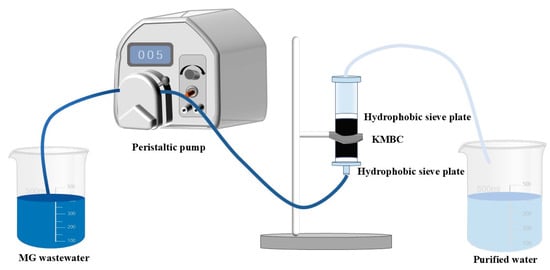
Figure 1.
Flow chart of fixed bed column experiment.
3. Results
3.1. Characterization of MBC and KMBC
Table 1 presents the elemental compositions of MAB, MBC, and KMBC. The proportions of C in MBC and KMBC have increased to 61.59% and 69.29% compared to those before pyrolysis, confirming that MAB serves as a premium carbon source for the fabrication of biochar materials [31]. Compared with MBC, the C, H, and O contents of KMBC increased and N content decreased slightly. The OH− of KOH is attached to the carbon surface, which may be the reason for the increased H and O content in KMBC. The H/C, O/C, and (O+N)/C values of MBC and KMBC are smaller than those of MAB. H/C, O/C, and (O+N)/C are usually used to characterize aromaticity, hydrophilicity, and molecular polarity, respectively. The smaller the H/C value, the stronger the aromaticity. The higher the O/C value, the better the surface hydrophilicity. The smaller the (O+N)/C value, the weaker the polarity. The decrease in polarity and the increase in aromaticity and hydrophobicity are beneficial to the adsorption of non-polar organic aromatic pollutants MG [32]. Relevant adsorption mechanisms include surface complexation and hydrophobic interactions. After carbonization, the aromaticity and hydrophobicity of biochar are greatly enhanced, and the polarity is weakened, which proves that carbonization is beneficial to the adsorption of MG by MBC and KMBC. The aromaticity, hydrophilicity, and polarity of MBC are all higher than those of KMBC. Considering that the adsorption capacity of KMBC is much higher than that of MBC in subsequent experiments, this indicates that high hydrophobicity and weaker polarity may play an essential role in the adsorption process of MG on KMBC.

Table 1.
Elemental contents of MAB, MBC, and KMBC.
The SEM, BET, XRD, and Raman characterization results are shown in Figure 2. The surface structure of MBC is relatively smooth, with granular accumulation and rod-like cellulose structure visible on the surface, and the pores are underdeveloped [33]. The KMBC material sample modified by KOH has a rough surface, rich pore structure, and many micropores and mesoporous structures. It is reported that the activation of KOH is the main factor in producing various disordered pores in carbon materials at high temperatures (6KOH + 2C = 2K + 3H2 + 2K2CO3) [34]. These ideal porous structures are conducive to increasing the adsorption capacity of the adsorbent and providing more adsorption sites for the adsorption of MG molecules [35].
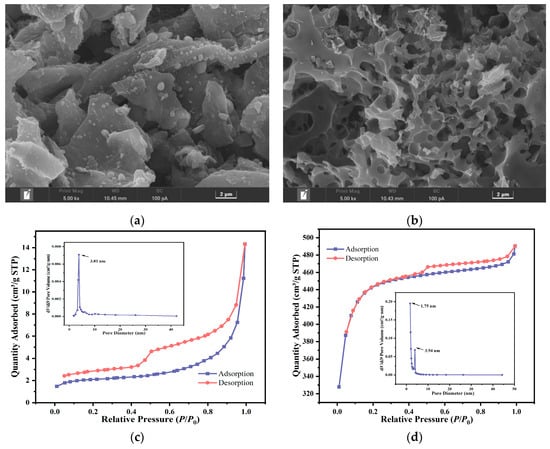
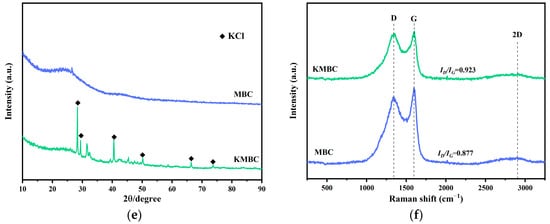
Figure 2.
(a) SEM images of MBC, (b) KMBC, N2 adsorption-desorption isotherms and the pore size distribution of (c) MBC and (d) KMBC, (e) XRD pattern, (f) Raman spectra before adsorption of MG.
The specific surface area and pore structure of the adsorbent play a pivotal role in the adsorption of pollutants. As shown in Figure 2c, the MBC adsorption and desorption curve is not closed, implying that the pore structure of MBC may be flexible. After nitrogen adsorption, the pore diameter shrinks, making desorption difficult. Thus, the desorption curve appears above the adsorption curve. A small surface area is also one of the causes of curve closure. Its isotherm is type IV, indicating the primary existence of a mesoporous structure. The adsorption hysteresis loop is H3 type, and there is no apparent saturated adsorption platform, indicating that the pore structure is very irregular [36]. It can be considered that the slit pores formed by the accumulation of flaky particles do not show adsorption saturation in the higher relative pressure region [37]. The pore size distribution is uneven, most are distributed in the mesoporous range. The specific surface area of KMBC (1632.73 m2/g) is significantly higher than that before modification, reaching more than 213 times that of MBC. According to previous reports, enhancing the biochar surface area after activation can promote interfacial reactions and provide abundant adsorption sites for dye removal [38]. The total pore volume is 33 times that before modification, and the average pore size decreased from 10.57 nm to 2.94 nm. Its isotherm rises sharply at P/P0 = 0–0.1, close to the type I isotherm, reflecting the micropore filling phenomenon on the microporous adsorbent. However, the adsorption and desorption curves of KMBC do not entirely overlap, and a hysteresis loop appears, indicating that in addition to micropores, mesopores also exist. Judging from the pore size distribution map, the pore distribution of KMBC (mean pore diameter 2.94 nm) is in the micro-mesoporous range, and most of the pores are distributed in the micropore range, further confirming its microporous structure. Considering the size of MG molecules (1.4 nm × 1.1 nm × 0.5 nm), the reduction in pore size benefits the removal of MG [39]. An H4-type hysteresis loop appears under medium pressure, corresponding to a system in which capillary condensation occurs in porous adsorbents [40]. This phenomenon is commonly observed in micro-mesoporous carbon materials and serves as a representative signature for activated carbon-type solids that harbor narrow pores.
A broad diffraction peak from 20° to 30°, as shown in Figure 2e, corresponds to the amorphous carbon in the biochar carrier. Compared to MBC, the diffraction peak of KMBC points to KCl, indicating that K is successfully loaded onto the surface of MBC [41]. KOH acts as a pore-forming agent to increase the specific surface area of biochar and reacts with MBC to generate abundant metallic potassium. With its strong permeability, metallic potassium can act on the carbon atom array and promote graphitization [42].
The degree of graphitization of the prepared biochar was measured by Raman spectroscopy (see Figure 2f) with the outcomes depicting that the two prepared biochar have two significant characteristic peaks at 1350 cm−1 (D peak) and 1580 cm−1 (G peak). The ratio of D peak to G peak (ID/IG) reflects the degree of graphitization of carbon materials, which is generally negatively correlated with the degree of graphitization. Both MBC and KMBC have a high degree of graphitization. Compared to MBC (ID/IG = 0.877), the ID/IG value of KMBC shows a slight increase, suggesting that the degree of graphitization of MBC is superior to that of KMBC [43]. However, previous studies have shown that for aromatic pollutants, the higher degree of graphitization of biochar is conducive to inducing π-π and n-π interactions with pollutants, thereby enhancing its adsorption capacity for pollutants [44].
3.2. Batch Adsorption Experiment
The MG adsorption capacity and removal rate on MAB, MBC, activated carbon (AC), and KMBC are shown in Figure 3. Among these, MAB demonstrates a relatively lower adsorption capacity (only 396.6 mg/g), accompanied by a removal rate of 19.9%. In contrast, pyrolyzed MBC exhibits a remarkable MG adsorption capacity of 1310.8 mg/g, though it still lags behind AC (1715.6 mg/g). Strikingly, KMBC displays a significantly enhanced adsorption capacity of 1997.3 mg/g after modification, surpassing even the widely employed AC. This observation highlights the efficacy of biochar derived from MAB, particularly after KOH modification, in adsorbing MG.
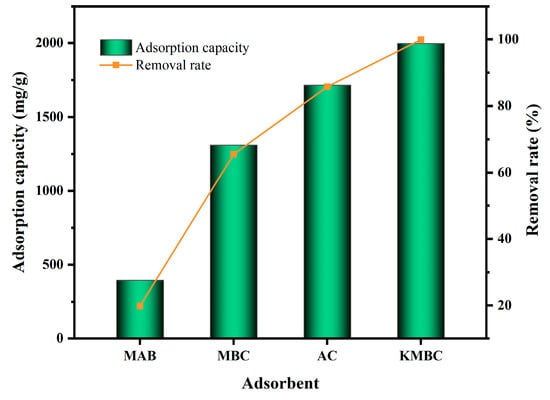
Figure 3.
Adsorption effect of MG on different adsorbents.
The effects of MBC and KMBC dosage on MG’s adsorption capacity and removal rate were examined in Figure 4a. It can be seen that the removal rate of MG by MBC and KMBC initially climbs and then plateaus as the biochar dosage increases in the range from 2 to 30 mg. While the adsorption capacity of MBC and KMBC experiences a sharp drop in the same region. This is because of the aggregation of biochar particles and a decline in the ratio of MG molecules to active sites on the biochar. At lower dosages, both MBC and KMBC exhibit remarkable adsorption properties. For instance, at a biochar dosage of 2 mg, the adsorption capacities of MBC and KMBC attain 1512.6 mg/g and 2646.6 mg/g, respectively. However, beyond 10 mg of biochar, the adsorption capacity of MBC tends to be stable. This phenomenon occurs primarily because the adsorption sites on its surface also increase accordingly as the amount of biochar added increases, and the number of MG molecules is finite causing waste of adsorbent [45].
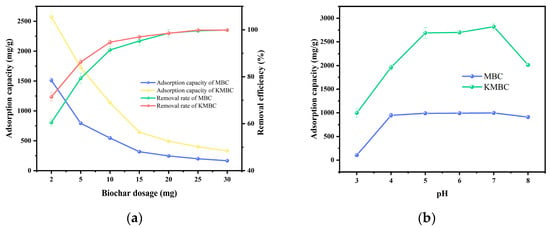
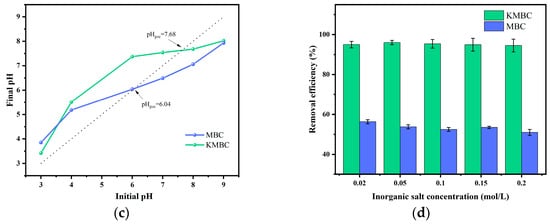
Figure 4.
(a) Effect of biochar dosage; (b) Solution pH on MG adsorption; (c) pHpzc of MBC and KMBC; (d) Effect of ion interference on the adsorption of MG by MBC and KMBC. All experiments were conducted in triplicate to ensure the accuracy and reproducibility of the results.
The initial pH value of the dye solution influences both the distribution of surface charges on the adsorbent and the solubility and ionization degree of MG in water. When the pH exceeds 8.0, the color of MG fades due to the decomposition of MG molecules under alkaline conditions [46]. Therefore, the pH range for adsorption experiments was controlled from 3 to 8. As depicted in Figure 4b, the adsorption capacity of MG on MBC and KMBC demonstrates a trend of initially increasing sharply (pH < 5), followed by stabilization (pH = 5–7), and eventually decreasing (pH > 7). MG molecules exist as positively charged species in an aqueous medium. In environments with a pH lower than 5, the elevated concentration of H+; in the solution can hinder the adsorption of MG cations due to electrostatic repulsion. With the increase in pH value, the deprotonation of functional groups on MBC and KMBC surfaces is facilitated, thereby enhancing the negative charge on the adsorbent surface. This reduction in electrostatic repulsion promotes the adsorption and removal of MG [47]. However, when the pH surpasses 7, the formation of hydrogen bonds between the OH− in the solution and the MG cations occurs, thereby limiting the adsorption capacity [48]. Therefore, the pH of the solution was selected to be 7 in subsequent experiments.
To analyze the effect of solution pH on MG adsorption, the pH drift method was first used to test the point of zero charge (pHpzc) of MBC and KMBC, and the results are shown in Figure 4c. The measured pHpzc of MBC and KMBC are 6.04 and 7.68, respectively. When the pH exceeds the pHpzc, the adsorbent surface carries a negative charge; conversely, when the pH is below the pHpzc, the adsorbent surface carries a positive charge, resulting in electrostatic repulsion with the cationic dye MG [49]. At a pH of 3, the adsorption capacity of MG on KMBC attains an impressive 1093.1 mg/g, with a corresponding MG removal efficiency of 54.7%. The remarkable MG adsorption efficacy of KMBC can be attributed to its extensive specific surface area and pore volume [50]. However, the adsorption capacity of MG on both MBC and KMBC still increases significantly with rising solution pH values, suggesting that besides pore-filling and electrostatic interactions, other removal mechanisms such as hydrogen bonding and surface complexation may also be involved. In general, the biochar prepared in this study demonstrates outstanding MG adsorption capabilities across a broad pH spectrum. This can be ascribed to the synergistic impact of various adsorption mechanisms operating in unison.
Given the high salinity characteristic of dyeing wastewater and the limited research on the influence of salinity on biochar-pollutant interactions, this study examined the effects of common inorganic salts (NaCl) found in dye wastewater on the adsorption of MG by MBC and KMBC. As shown in Figure 4d, when the salt concentration was 0.02, 0.05, 0.10, 0.15, and 0.2 mol/L, the variation in the removal rate of MG by KMBC was less than 3.2% and that by MBC was less than 15%. This indicates that the removal efficiency of MG by KMBC is not significantly affected in saline environments, confirming their salt tolerance.
3.3. Adsorption Kinetics, Isotherms, and Thermodynamics
Figure 5a illustrates the influence of adsorption time on the kinetics of MG adsorption at 25 °C, with an initial concentration of 300 mg/L. During the initial 60 min, the adsorption rates of MBC and KMBC are relatively rapid, attributable to the ample adsorption sites available on the biochar surface. Subsequently, the rates gradually decline until equilibrium is reached. Over time, the adsorption rate of MG onto MBC and KMBC decelerates, ultimately reaching adsorption equilibrium around 240 min [51,52]. As depicted in Supplementary Material Figure S1, the equilibrium adsorption capacities increase with an increase in initial MG concentration. When the initial concentration of MG changes in the range from 100 mg/L to 400 mg/L, the equilibrium adsorption capacity of MG on MBC increases from 567.4 mg/g to 1442.7 mg/g, and the equilibrium adsorption capacity of MG on KMBC increases from 1823.0 mg/g to 2645.5 mg/g. The primary reason for this is that increasing the initial concentration of MG enhances the driving force behind the adsorption of MG onto biochar.
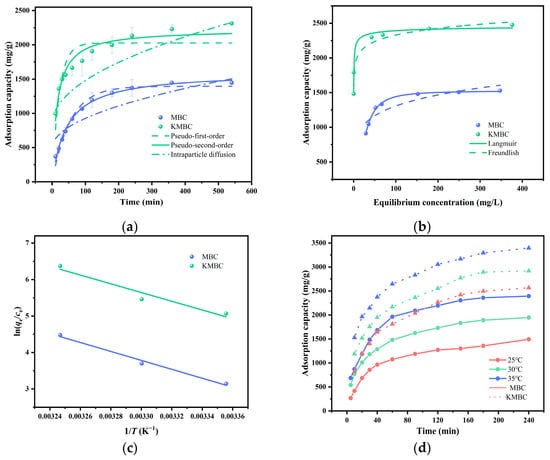
Figure 5.
(a) Adsorption kinetics, (b) adsorption isotherm, and (c) adsorption thermodynamic model of MBC and KMBC; (d) adsorption capacity of MG at different temperatures.
Table 2 presents the modeling parameters for MG adsorption kinetics, isotherm, and thermodynamics on MBC and KMBC. The adsorption of MG on both MBC and KMBC aligns more closely with the pseudo-second-order (PSO) kinetic model than with the pseudo-first-order (PFO) model and intraparticle diffusion models. Notably, the PSO kinetic model demonstrates excellent regression coefficients (R2 = 0.9999, R2 = 0.9968) and adsorption capacities (qe = 1576.7 mg/g, qe = 2671.7 mg/g) that are in close agreement with the experimental values (q = 1547.7 mg/g, q = 2645.5 mg/g). This suggests that the adsorption process likely involves chemical adsorption mechanisms, such as functional group complexation and hydrogen bonding [53]. Furthermore, as evident from the internal diffusion model fitting diagrams, a comparison between the two-step diffusion model of MBC and the internal diffusion model of KMBC reveals that the latter is delineated into the three-step model. These steps correspond to surface diffusion, mesopore diffusion, and micropore diffusion [54]. This finding substantiates the presence of numerous microporous structures within KMBC, a characteristic that aligns with the outcomes of the BET analysis.

Table 2.
The adsorption model parameters of MG on MBC and KMBC.
The adsorption isotherms of MBC and KMBC on different initial concentrations of MG at 298 K are shown in Figure 5b. The adsorption isotherm fitting results at other temperatures are shown in Supplementary Material Figure S2. The Langmuir isotherm model (R2 = 0.9992, 0.9997) demonstrates a higher correlation coefficient than Freundlich, indicating that the adsorption process is single-layer and uniformly distributed on the biochar surface. According to the Langmuir fitting results, the theoretical maximum adsorption capacities (qm) of MBC and KMBC are 1772.3 mg/g and 2570.7 mg/g, respectively, which align with our experimental adsorption results of 1823.0 mg/g and 2645.5 mg/g. The dimensionless constant separation factor RL value of KMBC is generally smaller than MBC, suggesting that KMBC has a higher thermodynamic advantage [55]. Notably, the RL values of both biochar decreased with the increase in the initial concentration of MG, indicating that the adsorption process of MG on the adsorbent is more preferential at high concentrations, which is consistent with our experimental results of adsorption kinetics.
Enthalpy change (ΔH0) represents the heat absorbed or released during a chemical reaction, where ΔH0 > 0 is an endothermic process. The entropy change (ΔS0) measures a system’s energy and degree of randomness or disorder, indicating its proximity to thermodynamic equilibrium. The change in Gibbs free energy (ΔG0) reveals the total energy change of the system during the reaction process and product formation, determining whether the reaction is spontaneous [56]. Table 3 reveals that ΔG0 is less than zero, and a rise in adsorption temperature leads to a marked decline in ΔG0, suggesting that elevated temperatures facilitate the adsorption process of MG onto biochar. Furthermore, the ΔG0 of KMBC is typically lower than that of MBC, indicating that MG is more readily adsorbed onto KMBC. ΔH0 is more than zero, indicating that the adsorption of MG on the biochar is an endothermic reaction, and the adsorption is positively correlated with temperature. As depicted in Figure 5d, the adsorption capacity of MG on MBC and KMBC increased correspondingly with rising adsorption temperature, suggesting that the adsorption reaction is endothermic. The entropy change (ΔS0) is positive, signifying an increase in disorder or randomness of the adsorption process [57]. The significant increase in ΔS⁰ drives the spontaneity of the reaction despite the endothermic nature. The adsorption thermodynamic results show that the adsorption of MG by MBC and KMBC is a spontaneous process characterized by endotherm and entropy increase. It has great potential as a valuable raw material for bioenergy production.

Table 3.
The Thermodynamic adsorption parameters of MG on MBC and KMBC.
3.4. Selective and Reusable Adsorption
To investigate the selective adsorption performance of MBC and KMBC for dyes in wastewater, anionic and cationic dyes were chosen as model pollutants for the adsorption experiments. As depicted in Figure 6a, distinct differences in the adsorption efficacy emerge between MBC and KMBC under identical conditions. Both adsorbents exhibit the highest adsorption capacity for the dye MG, surpassing that of the other four dyes by a significant margin. It can be seen that KMBC has good adsorption performance for both the anionic dye MO (1575.69 mg/g) and the cationic dye MG (1731.92 mg/g), indicating that it has no apparent selectivity and has relatively good adsorption properties for most dyes. The adsorption performance of MBC for cationic dyes is significantly higher than that of anionic dyes. The equilibrium adsorption capacity of CV and MG (318.71 and 721.74 mg/g) is significantly greater than that of MO (88.07 mg/g), CbT (100.18 mg/g), and NgB (63.26 mg/g), indicating that the prepared MBC exhibits excellent adsorption capacity for cationic dyes. However, its adsorption capacity for anionic dyes may not be ideal. This may be related to its smaller specific surface area and fewer adsorption sites, which further illustrates that electrostatic adsorption plays an essential role in the adsorption of MBC to adsorbates [58].
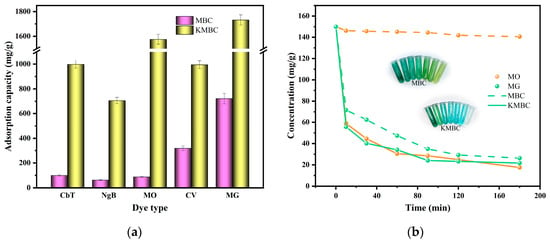
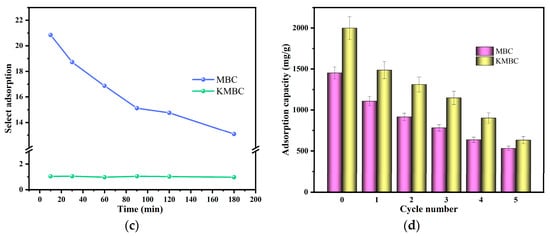
Figure 6.
(a) The adsorption capacity of different dyes on MBC and KMBC; (b) the selective adsorption of MG/MO mixture dyes; (c) the ideal adsorption selectivity of MG against MO at different times; (d) the effect of recycling on MG adsorption capacity.
In addition, the selective adsorption of binary solutions containing cationic and anionic dyes (MG/MO) in this experiment is shown in Figure 6b. The results show that for MBC, the concentration of MG dye in the mixture decreases significantly with the increases in adsorption time. Conversely, the MO concentration remains relatively stable. After 180 min of absorption, the color of the mixture gradually changes from dark cyan to light yellow-green. It shows that MBC can separate cationic dyes from mixed dye solutions. In the case of KMBC, both the MO and MG concentrations decline swiftly with the increase in adsorption time, resulting in a transparent mixed solution without any apparent selective adsorption. The selective adsorption ability of cationic dye MG against anionic dye MO on MBC and KMBC was calculated and displayed in Figure 6c. For MBC, the selective adsorption capacity of MG/MO binary dye solutions decreases significantly as the adsorption time increases. The possible reason is that as the adsorption time increases, the adsorption sites on the surface of MBC are occupied by MG molecules. Meanwhile, the content of easily adsorbed MG molecules in the solution decreased remarkably, while the number of anionic dyes MO molecules decreased more slowly, which may also lead to a decrease in selective adsorption capacity. KMBC’s selective adsorption capacity has no noticeable change trend (stable around 1), which also proved that KMBC has excellent adsorption capacity for both anionic and cationic dyes, and the electrostatic attraction mechanism can be ignored.
The reusability of adsorbents is an important indicator that must be considered in wastewater management, which is beneficial to reducing costs and secondary pollution in practical applications. The adsorption capacity of MG during each cycle is shown in Figure 6d. The results show that after five cycles of adsorption, the adsorption capacity of MG on MBC and KMBC can still reach more than 500 mg/g, proving that both have good reusability and the biochar retained significant adsorption capacity even after multiple uses. The mechanism of carbon regeneration involves the disruption of the interactions between the adsorbent and the adsorbed dye molecules. In the case of MG, which is primarily adsorbed through hydrophobic interactions, hydrogen bonding, and π-π interactions, the use of ethanol as a polar solvent effectively weakens these interactions, facilitating the release of MG from the biochar surface. For spent biochar that cannot be regenerated, safe disposal methods, such as landfill disposal or incineration, can be employed in accordance with local environmental regulations.
3.5. Column Adsorption Experiment
Mathematical models are frequently employed to depict the intricacies of laboratory-scale dynamic column adsorption. The calculated model parameters offer valuable insights that can significantly inform the large-scale implementation of adsorption engineering, as well as the practical design of fixed beds in industrial applications. This part used a bed height of 4.5 mm, a flow rate of 8.0 mL/min, and an initial MG concentration of 100 mg/L as the benchmark to study the impact of changing a single condition on the breakthrough curve. The Thomas, Yoon Nelson, and Adams Bohart models were employed to fit the KMBC adsorption column, with the fitting parameters shown in Table 4 (see Supplementary Material Figure S3 and Text S3 for the fitting curve and detailed formulas) [59]. In cases where the bed height is lower and the inlet water concentration and rate are higher, the tb and te are shortened, and the period to achieve equilibrium is also reduced. This results in breakthrough curves becoming more acute and shifting to the left (Figure 7a–c). Simultaneously, the rate constants (KT, KY, and KA) exhibit a positive correlation with the initial concentration and inlet flow rate, whereas they demonstrate an inverse correlation with the bed height. This suggests that increasing the initial concentration and flow rate while reducing the bed height can be beneficial in minimizing mass transfer resistance.

Table 4.
Parameters of column adsorption models.
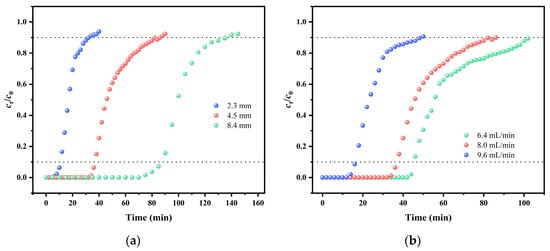
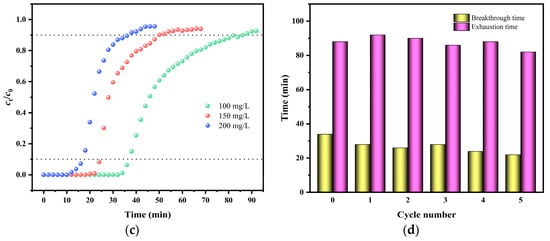
Figure 7.
Experimental breakthrough curves for adsorption of MG on KMBC at (a) bed heights (c0 = 100 mg/g, v = 8.0 mL/min), (b) flow rates (c0 = 100 mg/g, Z = 4.5 mm), and (c) different inlet concentrations (Z = 4.5 mm, v = 8.0 mL/min); (d) the reusability tests of KMBC adsorption column (c0 = 100 mg/g, v = 8.0 mL/min, Z = 4.5 mm).
Thomas fitting shows that as the bed height increases, the corresponding qm of MG increases from 105.22 mg/g to 850.03 mg/g. The above results can be attributed to the fact that as the filling height increases, the residence time of the MG solution in the fixed bed becomes longer, allowing for more complete contact with KMBC. Consequently, the removal efficiency increases accordingly. The correlation coefficient R2 increases with the increase of adsorption bed height, indicating that the influence of axial diffusion decreases during the adsorption process. As the flow rate increases, the maximum adsorption capacity of MG decreases from 386.03 mg/g to 219.72 mg/g in Figure 7b. It can be concluded that when the flow rate is relatively low, the adsorption time of MG by KMBC becomes longer, enhancing the utilization efficiency of KMBC adsorption sites [60]. The breakthrough curves for different MG influent concentrations are illustrated in Figure 7c. As the initial concentration increases, both the tb and te decrease. This occurs because, as the initial concentration of MG rises, the concentration gradient emerges as the primary force accelerating adsorption mass transfer. The elevated concentration gradient enhances the diffusion of MG into the KMBC pores. However, as the mass transfer rate increases, the available adsorption area rapidly diminishes. The significant drop in adsorption site efficiency results in earlier tb and te, enabling the entire adsorption process to reach equilibrium more quickly.
The results of the Thomas model indicate that under the conditions of a bed height of 8.4 mm, an initial MG concentration of 100 mg/L, and a flow rate of 8.0 mL/min, the qm reaches 850.03 mg/g. The error between qm and qT can be calculated from Equation (2) and Table 4, and the error range is 2.47% ~ 23.3%. The Yoon Nelson model can predict the time required for ct/c0 = 0.5. The error between the theoretical and experimental values can be calculated, ranging from 0.02% to 17.8%. Notably, the error was under 0.2% in five of the seven experiments, emphasizing the strong alignment between the experimental results and the theoretical model.
where qT is the actual total adsorption capacity, mg/g; A is the area of the breakthrough curve; T is the total time of adsorption, min.
To verify the reusability of the fixed bed, 95% ethanol was used to desorb enriched MG in the adsorption column (3.2 mL/min), followed by rinsing with ultrapure water. This experiment selected a 4.5 mm high adsorption column (v = 8.0 mL/min) to study its reusability. As shown in Figure 7d, after five cycles, the tb and te of the adsorption column were approximately 65% and 93% of the new column, respectively, indicating that the column has good durability and practical application value.
3.6. Adsorption Mechanisms
MBC exhibits a lower specific surface area (7.65 m2/g) and total pore volume (0.022 cm3/g), indicating the lack of active sites with strong affinity for organic molecules. Therefore, the contribution of the pore-filling mechanism to MG adsorption is negligible. KMBC has a developed pore structure with many micropores/mesopores and a high specific surface area (1632.73 m2/g), providing abundant adsorption sites for the adsorption of dye molecules. In the selective adsorption experiment, KMBC demonstrates an excellent adsorption capacity for each dye, which is also attributable to its pore-filling mechanism.
Electrostatic adsorption mainly occurs between positive and negative charges or between anions and cations. The initial solution pH is 7, and the pHpzc-MBC is 6.04. When the solution pH exceeds 6.04, the MBC surface becomes negatively charged, enabling the positively charged groups of MG molecules to be effectively adsorbed through electrostatic interaction. This mechanism was validated by selective adsorption experiments, which demonstrated the preferential adsorption of cationic dyes by MBC. The relevant chemical reaction was given in Equation (3). The pHpzc-KMBC (7.68) is higher than the initial pH value of the solution, and KMBC does not exhibit obvious selective adsorption capacity for cationic dyes. Therefore, the electrostatic adsorption effect of MG on KMBC can be ignored.
where “R−” represents the negatively charged functional group (O−, COO−) on the surface of MBC.
MBC−R− + C23H25N2+ → MBC−(R)C23H25N2
The surface functional groups in the adsorbent material are one of the main factors affecting its adsorption effect. As depicted in Figure 8a, MBC and KMBC have two characteristic peaks near 3412 cm−1 and 1120 cm−1. The former peak is typically ascribed to the stretching vibration of −OH involved in hydrogen bonding, while the latter peak generally signifies the stretching vibration of the C−O bond [61]. The absorption peak of MBC at 1400 cm−1 can be attributed to the C−H bending vibration or the C−O bending vibration, indicating that biochar may contain aromatic compounds [62]. After modification of MAB by KOH, the stretching vibration peak (C–O/C=C) at 1620 cm−1 decreased, which was attributed to the decomposition of cellulose and hemicellulose [63]. Comparing the FT-IR spectra before and after the adsorption of MG on MBC and KMBC, a new peak emerges at 2920 cm−1, which corresponds to the symmetric stretching vibrations of the C−H and CH2 groups [64]. The peak at 1120 cm−1 disappeared, indicating that the C−O bonds of MBC and KMBC were involved in the reaction. Owing to the high degree of aromatization of biochar, following the adsorption of MG dye, the benzene ring exhibited a more pronounced stretching vibration peak at 573 cm−1 [65]. The peak related to −OH or N−H stretching vibration slightly shifted from 3412 cm−1 to 3430 cm−1. This indicates that MBC and KMBC have hydrogen bonding interactions during the adsorption of MG [66].
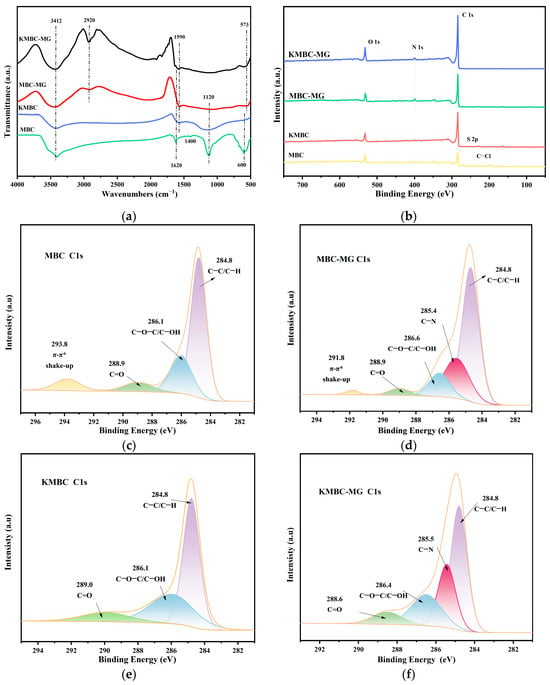
Figure 8.
(a) FT-IR spectroscopy, (b) XPS full spectrum before and after adsorption of MG; (c) C1s spectrum of MBC, (d) C1s spectrum of KMBC, (e) C1s spectrum of MBC after absorbing MG, (f) after absorbing MG C1s spectrum of KMBC.
Surface complexation is essential for removing pollutants containing aromatic structures [67]. π-π interaction mainly occurs between aromatic structures, and n-π interaction occurs between the oxygen-containing functional groups on the surface of biochar and the aromatic ring of MG molecules. After MBC and KMBC adsorb MG, the intensity of the peaks near 3400 and 1120 cm−1 changes and shifts slightly, indicating that the oxygen-containing groups of MBC and KMBC were complexed with MG. These hydroxyl groups, along with alkoxide anions, are likely the primary functional groups involved in the retention of MG through ionic or chelating interactions. The FTIR peak at 1620 cm−1 slightly shifts to 1590 cm−1, representing the vibration of the aromatic ring carbon double bond on biochar and π-π interaction. The shift of the C=C and −OH characteristic absorption peaks of MBC and KMBC after adsorption indicates the π-π interaction and n-π interaction between the benzene ring of the MG molecule and the aromatic structure and −OH functional group of biochar. The surface complexation of MBC is stronger than that of KMBC, as can be seen from elemental, XPS, and Raman analysis. Moreover, the MG molecules contain the hydrophobic functional group methyl. MBC and KMBC contain rich aromatic structures with organic hydrophobic effects. They also have rich pore structures with inner walls that attract hydrophobic MG molecules. Therefore, MG molecules can be easily attached due to their hydrophobic effect on biochar.
Based on the comprehensive XPS characterization of biochar shown in Figure 8b, two distinct absorption peaks are observed at 284 eV and 530 eV, corresponding to C1s and O1s, respectively. A new peak N1s after adsorption of MG appears in the full spectrum of MBC and KMBC with a binding energy of approximately 400 eV [68]. This observation signifies the successful adsorption of MG on MBC and KMBC. By comparing the C1s spectrum of MBC in Figure 8c with that of KMBC in Figure 8d, it can be observed that the C element predominantly exists in the form of C−C/C−H (65.3%, 55.1%) and C−O−C/C−OH (20.1%, 33.7%). The XPS results of MBC show that a π-π* shake-up peak appears at 293.8 eV, which indicates that there is an electronic transition and an unsaturated benzene ring in the conjugated π electron system of the benzene ring, which further proves that MBC has higher aromaticity than KMBC [69]. The C1s spectrum of MBC and KMBC after adsorbing MG are shown in Figure 8e,f. A distinct new peak at 285.8 eV emerges (C−N), primarily attributable to the MG dye molecules. The peak intensity of C−OH/C−O−C is notably weakened, which also indicates that these groups have hydrogen bonds with MG molecules. Moreover, the reduction of the peak intensities of C−H/C−C suggests that these groups may engage in n-π interactions with the MG molecules [70].
MG adsorption on MBC results from the joint action of electrostatic adsorption, hydrophobic interaction, hydrogen bonding, and surface interactions. Meanwhile, the MG adsorption on KMBC mainly involves pore filling, hydrophobic interaction, hydrogen bonding interaction, and surface interaction. Since the adsorption capacity of MG on KMBC is far greater than that on MBC, and KMBC has no specific selective adsorption of dyes, pore filling plays a decisive role in the adsorption process.
3.7. Comparison of Adsorption Performance
The adsorption performance of MBC and KMBC were compared with other adsorbents reported in the literature. As shown in Table 5, KMBC exhibited a maximum adsorption capacity of 2570.7 mg/g for MG, which is significantly higher than that of many previously reported adsorbents. Myriophyllum aquaticum-derived biochar, particularly the KOH-modified KMBC, is a highly promising adsorbent material capable of effectively removing malachite green from wastewater.

Table 5.
Comparison of adsorption capacities of various adsorbents for MG.
4. Conclusions
In conclusion, this paper presents biochar derived from waste Myriophyllum aquaticum, which have an efficient adsorption performance for MG in dyeing wastewater. The biochar materials derived from Myriophyllum aquaticum, particularly KMBC, exhibited remarkable adsorption capabilities for MG dye. Furthermore, KMBC’s adsorption performance demonstrates excellent stability and adaptability under various experimental conditions (e.g., different pH levels, ionic strengths, and temperatures), indicating its broad potential for treating complex dye wastewater. Compared to traditional adsorbents, KMBC not only offers higher adsorption efficiency but also boasts advantages such as low cost, environmental friendliness, and sustainability, providing an efficient and economical solution for dye wastewater treatment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17072868/s1. Refs. [78,79,80] are cited in Supplementary Materials.
Author Contributions
X.Z. (Xin Zhang): Conceptualization, Data curation, Formal analysis, Writing original draft, Investigation, Methodology, Validation. X.Z. (Xiaoping Zhang): Supervision, Writing—review & editing, Funding acquisition. W.X.: Data curation, Software. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (Grant No. 2016YFC0400702-2). The Guangdong Science and Technology Program (2020B121201003).
Institutional Review Board Statement
The study did not require ethical approval.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to [privacy].
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of the data; in the writing of the manuscript or in the decision to publish the results.
References
- Xu, Q.; Du, J.; Su, X.; Li, X.; Si, Y.; Fu, Y. Highly porous carbon with rich inherent groups for ultrahigh adsorption of organic dyes from wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2024, 703, 135288. [Google Scholar] [CrossRef]
- Wu, J.; Yang, J.; Feng, P.; Wen, L.; Huang, G.; Xu, C.; Lin, B. Highly efficient and ultra-rapid adsorption of malachite green by recyclable crab shell biochar. J. Ind. Eng. Chem. 2022, 113, 206–214. [Google Scholar] [CrossRef]
- JECFA. Joint FAO/WHO Expert Committee on Food Additives Seventy-Third Meeting. 2010. Available online: https://scholar.google.com/scholar?q=JECFA.%20Joint%20FAO%2FWHO%20Expert%20Committee%20on%20Food%20Additives%2C%20Seventy-third%20meeting%3B%208-17%20June%2020%3B%20Geneva.%202010.%20p.%2012.%20Summary%20and%20Conclusion.%20JECFA%2F73%2FSC%20 (accessed on 19 March 2025).
- Gharavi-Nakhjavani, M.S.; Niazi, A.; Hosseini, H.; Aminzare, M.; Dizaji, R.; Tajdar-Oranj, B.; Mirza Alizadeh, A. Correction to: Malachite green and leucomalachite green in fish: A global systematic review and meta-analysis. Environ. Sci. Pollut. Res. Int. 2023, 30, 48928. [Google Scholar] [CrossRef] [PubMed]
- Le Curieux, F.; Gohlke, J.M.; Pronk, A.; Andersen, W.C.; Chen, G.; Fang, J.-L.; Mitrowska, K.; Sanders, P.J.; Sun, M.; Umbuzeiro, G.A.; et al. Carcinogenicity of gentian violet, leucogentian violet, malachite green, leucomalachite green, and CI Direct Blue 218. Lancet Oncol. 2021, 22, 585–586. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Fast and efficient adsorption of methylene green 5 on activated carbon prepared from new chemical activation method. J. Environ. Manag. 2017, 188, 322–336. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Yadav, V.K.; Ali, D.; Ramar, K.; Kumar, G.; Narayanan, V. New designing (NH4)2SiP4O13 nanowires and effective photocatalytic degradation of Malachite green and antimicrobial properties. Chem. Phys. Lett. 2022, 803, 139817. [Google Scholar] [CrossRef]
- Haladu, S.A. Highly efficient adsorption of malachite green dye onto a cross-linked pH-responsive cycloterpolymer resin: Kinetic, equilibrium and thermodynamic studies. J. Mol. Liq. 2022, 357, 119115. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Yu, A.; Yu, Z. Removal of malachite green by electrochemical oxidation polymerization and electrochemical reduction precipitation: Its kinetics and intermediates. J. Solid State Electrochem. 2022, 26, 2231–2246. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Deyab, M.A.; Hassan, N.I.; Abu Ahmed, S.E. Bioremediation of malachite green dye using sodium alginate, Sargassum latifolium extract, and their silver nanoparticles. BMC Chem. 2023, 17, 108. [Google Scholar] [CrossRef]
- Korpe, S.; Bethi, B.; Sonawane, S.H.; Jayakumar, K. Tannery wastewater treatment by cavitation combined with advanced oxidation process (AOP). Ultrason. Sonochem. 2019, 59, 104723. [Google Scholar] [CrossRef]
- Ewais, H.A. Adsorption of Malachite Green Cationic Dye from Aqueous Media by Activated Carbon Modified by Nanosilver. Russ. J. Phys. Chem. A 2022, 96, S113–S121. [Google Scholar] [CrossRef]
- Altun, T.; Ecevit, H. Adsorption of malachite green and methyl violet 2B by halloysite nanotube: Batch adsorption experiments and Box-Behnken experimental design. Mater. Chem. Phys. 2022, 291, 126612. [Google Scholar] [CrossRef]
- Imessaoudene, A.; Mechraoui, O.; Aberkane, B.; Benabbas, A.; Manseri, A.; Moussaoui, Y.; Bollinger, J.-C.; Amrane, A.; Zoukel, A.; Mouni, L. Synthesis of a TiO2/zeolite composite: Evaluation of adsorption-photodegradation synergy for the removal of Malachite Green. Nano-Struct. Nano-Objects 2024, 38, 101191. [Google Scholar] [CrossRef]
- Prakash, A.; Sharma, A.; Yadav, A.; Sharma, R.K. Zirconium-based mixed ligand metal–organic framework for efficient adsorption of organic dyes. J. Nanoparticle Res. 2024, 26, 220. [Google Scholar] [CrossRef]
- Abdo, A.I.; Li, Y.; Shi, Z.; El-Saadony, M.T.; Alkahtani, A.M.; Chen, Y.; Wang, X.; Zhang, J.; Wei, H. Biochar of invasive plants alleviated impact of acid rain on soil microbial community structure and functionality better than liming. Ecotoxicol. Environ. Saf. 2024, 282, 116726. [Google Scholar] [CrossRef]
- Hanafi, M.; Bordoloi, S.; Rinta-Hiiro, V.; Oey, T.; Korkiala-Tanttu, L. Feasibility of biochar for low-emission soft clay stabilization using CO2 curing. Transp. Geotech. 2024, 49, 101370. [Google Scholar] [CrossRef]
- Hou, L.; Liang, S.; Wang, L.; Luo, D.; Guo, J. Effect of biochar-based nano-nickel catalyst on heavy crude oil upgrading and oil shale pyrolysis. Appl. Catal. O Open 2024, 195, 207011. [Google Scholar] [CrossRef]
- You, J.; Farghali, M.; Osman, A.I.; Yoshida, G.; Ihara, I. Mechanisms of biochar-mediated reduction of antibiotic-resistant bacteria and biogas production enhancement in anaerobic digesters. Biochem. Eng. J. 2024, 211, 109465. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Ding, H.; Fu, H.; Liu, J.; Chen, Y.; Dai, T.; Lou, Q.; Zhong, X.; Fan, H.; et al. Low-dose biochar added to sediment improves water quality and promotes the growth of submerged macrophytes. Sci. Total Environ. 2020, 742, 140602. [Google Scholar] [CrossRef]
- Abdu, M.; Babaee, S.; Worku, A.; Msagati, T.A.M.; Nure, J.F. The development of Giant reed biochar for adsorption of Basic Blue 41 and Eriochrome Black T. azo dyes from wastewater. Sci. Rep. 2024, 14, 18320. [Google Scholar] [CrossRef]
- Wang, F.-P.; Kang, L.-L.; Wang, Y.-J.; Wang, Y.-R.; Wang, Y.-T.; Li, J.-G.; Jiang, L.-Q.; Ji, R.; Chao, S.; Zhang, J.-B.; et al. Magnetic biochar catalyst from reed straw and electric furnace dust for biodiesel production and life cycle assessment. Renew. Energy 2024, 227, 120570. [Google Scholar] [CrossRef]
- Moradi, N.; Karimi, A. Fe-Modified Common Reed Biochar Reduced Cadmium (Cd) Mobility and Enhanced Microbial Activity in a Contaminated Calcareous Soil. J. Soil Sci. Plant Nutr. 2021, 21, 329–340. [Google Scholar] [CrossRef]
- Chen, G.; Wang, Y.; Wang, J.; Wang, J.; Yu, F.; Ma, Q.; Cheng, Z.; Yan, B.; Song, Y.; Cui, X. Production of potassium-enriched biochar from Canna indica: Transformation and release of potassium. Waste Manag. 2023, 164, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Muduli, M.; Swain, B.; Choudhary, M.; Verma, P.; Ray, S. Environmental Contaminants Remediation from Real Domestic Wastewater through a Canna-Based Bioretention Engineered System. Water Conserv. Sci. Eng. 2024, 9, 43. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Chen, S.; Ma, L.; Li, Y.; Lu, Y. Phosphate adsorption characteristics of La(OH)3-modified, canna-derived biochar. Chemosphere 2022, 286, 131773. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, N.; Zhou, Y.; Wang, F.; Zhang, Y.; Wu, Z. Growth and Physiological Responses in Myriophyllum spicatum L. Exposed to Linear Alkylbenzene Sulfonate. Environ. Toxicol. Chem. 2019, 38, 2073–2081. [Google Scholar] [CrossRef]
- Fakhry, H.; Hassan, H.M.A.; El-Aassar, M.R.; Alsohaimi, I.H.; Hussein, M.F.; Alqahtani, M.M.; El-Amier, Y.A. A Treatment of Wastewater Containing Safranin O Using Immobilized Myriophyllum spicatum L. onto Polyacrylonitrile/Polyvinylpyrrodlidone Biosorbent. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3181–3195. [Google Scholar] [CrossRef]
- Barontini, F.; Landi, M.; Silvestri, N.; Puccini, M. Hydrothermal Carbonization of Aquatic Biomass: A Promising Solution for the Invasive Species Myriophyllum Aquaticum. Chem. Eng. Trans. 2024, 109, 553–558. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Ma, L.; Zhai, L.; Xuan, J.; Liu, C.; Bai, Z. Ultra-high efficient pH induced selective removal of cationic and anionic dyes from complex coexisted solution by novel amphoteric biocomposite microspheres. Sep. Purif. Technol. 2020, 231, 115922. [Google Scholar] [CrossRef]
- Guo, J.; Zheng, L.; Li, Z.; Zhou, X.; Cheng, S.; Zhang, L.; Zhang, Q. Effects of various pyrolysis conditions and feedstock compositions on the physicochemical characteristics of cow manure-derived biochar. J. Clean. Prod. 2021, 311, 127458. [Google Scholar] [CrossRef]
- Holmes, L., Jr.; LaHurd, A.; Wasson, E.; McClarin, L.; Dabney, K. Racial and Ethnic Heterogeneity in the Association Between Total Cholesterol and Pediatric Obesity. Int. J. Environ. Res. Public Health 2016, 13, 19. [Google Scholar]
- Abilio, T.E.; Soares, B.C.; José, J.C.; Milani, P.A.; Labuto, G.; Carrilho, E.N.V.M. Hexavalent chromium removal from water: Adsorption properties of in natura and magnetic nanomodified sugarcane bagasse. Environ. Sci. Pollut. Res. Int. 2021, 28, 24816–24829. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xiang, M.; Zhu, W.; Hui, J.; Qin, H. Biomass Heteroatom Carbon/Cerium Dioxide Composite Nanomaterials Electrode for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2020, 8, 6675–6681. [Google Scholar] [CrossRef]
- Li, S.-S.; Zhou, W.-Y.; Jiang, M.; Li, L.-N.; Sun, Y.-F.; Guo, Z.; Liu, J.-H.; Huang, X.-J. Insights into diverse performance for the electroanalysis of Pb(II) on Fe2O3 nanorods and hollow nanocubes: Toward analysis of adsorption sites. Electrochim. Acta 2018, 288, 42–51. [Google Scholar] [CrossRef]
- Gado, M.; Rashad, M.; Kassab, W.; Badran, M. Highly Developed Surface Area Thiosemicarbazide Biochar Derived from Aloe Vera for Efficient Adsorption of Uranium. Radiochemistry 2021, 63, 353–363. [Google Scholar] [CrossRef]
- Li, D.; Ding, X.; Liu, X.; Cheng, J.; Jiang, Z.; Guo, Y. CO2 hydrogenation to methane over Ni/ZrO2 and Ni/CeO2 catalysts: Experimental and DFT studies. J. Mater. Sci. 2023, 58, 12584–12595. [Google Scholar] [CrossRef]
- Su, P.; Zhang, C.; Liu, Y.; Zhang, J.; Djellabi, R.; Wang, R.; Guo, J.; Zhang, R.; Guo, H.; Ding, X.; et al. Boosting PFOA photocatalytic removal from water using highly adsorptive and sunlight-responsive ZIF67/MIL-100(Fe) modified C3N4. J. Environ. Chem. Eng. 2023, 11, 110765. [Google Scholar] [CrossRef]
- Tripathi, P.K.; Gan, L.; Liu, M. Synthesis of Large Pore Carbon Nanoparticles for Removal of Malachite Green. J. Nanosci. Nanotechnol. 2016, 16, 892–897. [Google Scholar] [CrossRef]
- Skyt, P.S.; Wahlstedt, I.; Yates, E.S.; Muren, L.P.; Petersen, J.B.B.; Balling, P. Exploring the dose response of radiochromic dosimeters. J. Phys. Conf. Ser. 2013, 444, 012036. [Google Scholar] [CrossRef]
- Fan, S.S.; Wang, Y.; Wang, Z.; Tang, J.; Tang, J.; Li, X.D. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Song, C.-L.; Zhang, H.-M.; Zhong, Y.; Hu, X.-P.; Ji, S.-H.; Wang, L.; He, K.; Ma, X.-C.; Xue, Q.-K. Observation of Double-Dome Superconductivity in Potassium-Doped FeSe Thin Films. Phys. Rev. Lett. 2016, 116, 157001. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, H.; Yang, X.; Tan, X.; Wan, C.; Liu, X. Characterization of electrodes modified with sludge-derived biochar and its performance of electrocatalytic oxidation of azo dyes. J. Environ. Manag. 2022, 324, 116445. [Google Scholar] [CrossRef] [PubMed]
- Muretta, J.E.; Prieto-Centurion, D.; LaDouceur, R.; Kirtley, J.D. Unique Chemistry and Structure of Pyrolyzed Bovine Bone for Enhanced Aqueous Metals Adsorption. Waste Biomass Valorization 2023, 14, 703–722. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Chen, Y.; Cai, L.; Wang, Y.; Song, W. An abundant porous biochar material derived from wakame (Undaria pinnatifida) with high adsorption performance for three organic dyes. Bioresour. Technol. 2020, 318, 124082. [Google Scholar] [CrossRef]
- Choudhary, M.; Kumar, R.; Neogi, S. Activated biochar derived from Opuntia ficus-indica for the efficient adsorption of malachite green dye, Cu+2 and Ni+2 from water. J. Hazard. Mater. 2020, 392, 122441. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, X.; Gao, Y.; Zhang, W.; Zhou, L.; Zeng, X.; Liu, W.; Jiang, Q.; Jiang, C.; Wang, S. Iron oxide loaded biochar/attapulgite composites derived camellia oleifera shells as a novel bio-adsorbent for highly efficient removal of Cr(VI). J. Clean. Prod. 2021, 317, 128412. [Google Scholar] [CrossRef]
- Roy, S.; Mishra, S.R.; Ahmaruzzaman, M. Ultrasmall copper-metal organic framework (Cu-MOF) quantum dots decorated on waste derived biochar for enhanced removal of emerging contaminants: Synergistic effect and mechanistic insight. J. Environ. Manag. 2024, 366, 121802. [Google Scholar] [CrossRef]
- Sakurai, K.; Nakayama, A.; Watanabe, T.; Kyuma, K. Influences of aluminum ions on the determination of zpc (zero point of charge) of variable charge soils. Soil Sci. Plant Nutr. 1989, 35, 623–633. [Google Scholar] [CrossRef]
- Shahwan, T. Sorption kinetics: Obtaining a pseudo-second order rate equation based on a mass balance approach. J. Environ. Chem. Eng. 2014, 2, 1001–1006. [Google Scholar] [CrossRef]
- Xu, S.; Yu, Y.; Zhang, X.; Xue, D.; Wei, Y.; Xia, H.; Zhang, F.; Zhang, J. Enhanced Electron Delocalization Induced by Ferromagnetic Sulfur doped C(3)N(4) Triggers Selective H(2)O(2) Production. Angew. Chem. Int. Ed. Engl. 2024, 63, e202407578. [Google Scholar] [CrossRef]
- Li, X.; Jia, H.; Jiang, L.; Mou, Z.; Zhang, B.; Zhang, Z.; Chen, Y. Biochar Prepared from Steam-Exploded Bitter Melon Vine for the Adsorption of Methylene Blue from Aqueous Solution: Kinetics, Isotherm, Thermodynamics and Mechanism. Sustainability 2024, 16, 7278. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- Maceiras, R.; Feijoo, J.; Perez-Rial, L.; Alfonsin, V.; Falcon, P. Study of natural zeolites for hydrogen purification: CO2 adsorption capacity and kinetic mechanism. Fuel 2024, 376, 132732. [Google Scholar] [CrossRef]
- Henini, G.; Khurshid, H.; Laidani, Y.; Henini, S.; Umoren, S.A.; Suleiman, R.K.; Meliani, M.H. An investigation into equilibrium, Kinetics, and thermodynamics of yellow bemacid dye removal from Aqueous Solutions using pomegranate skin (PG) and Date Pedicels (DPd) as Green Adsorbents. Adsorption 2024, 30, 2099–2112. [Google Scholar] [CrossRef]
- Hao, J.; Cui, Z.; Liang, J.; Ma, J.; Ren, N.; Zhou, H.; Xing, D. Sustainable efficient utilization of magnetic porous biochar for adsorption of orange G and tetracycline: Inherent roles of adsorption and mechanisms. Environ. Res. 2024, 252 Pt 1, 118834. [Google Scholar] [CrossRef]
- Fan, S.; Tang, J.; Wang, Y.; Li, H.; Zhang, H.; Tang, J.; Wang, Z.; Li, X. Biochar prepared from co-pyrolysis of municipal sewage sludge and tea waste for the adsorption of methylene blue from aqueous solutions: Kinetics, isotherm, thermodynamic and mechanism. J. Mol. Liq. 2016, 220, 432–441. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, X.; Yao, J.; Zhan, S.; Li, H.; Zhang, J.; Qiu, Z. Synthesis of polyethyleneimine modified CoFe2O4-loaded porous biochar for selective adsorption properties towards dyes and exploration of interaction mechanisms. Sep. Purif. Technol. 2021, 277, 119474. [Google Scholar] [CrossRef]
- Hudson, A.; Murnane, J.; O’Dwyer, T.; Courtney, R. Influence of organic matter in wetland substrate on vanadium removal: A batch and column study. J. Water Process Eng. 2024, 68, 106359. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, S.; Chen, L.; Li, Z.; Wu, K.; Zhang, Y.; Su, Z.; Yin, X.; Hamza, M.F.; Wei, Y.; et al. Efficient separation of palladium from nitric acid solution by a novel silica-based ion exchanger with ultrahigh adsorption selectivity. Sep. Purif. Technol. 2023, 322, 124326. [Google Scholar] [CrossRef]
- Chen, L.; Liu, N.; Zhang, M.; Li, C.; Wu, K.; Qin, J.; Zhao, Q.; Song, J.; Liu, J.; Ye, Z. Preparation of chitosan resin by two-step crosslinking method and its adsorption for palladium in wastewater. Int. J. Biol. Macromol. 2024, 278 Pt 2, 134766. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Z.; Li, Z.; Chen, G.; Zhou, J.; Chen, Y.; Zhu, J.; Chen, Z. Rapid Separation and Efficient Removal of Cd Based on Enhancing Surface Precipitation by Carbonate-Modified Biochar. ACS Omega 2021, 6, 18253–18259. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wei, S.; Li, J.; Wang, X.; Wang, X.; Xue, B. Magnetically recyclable hydrothermal biochar functionalized with β-CD for simultaneous capturing of cationic methylene blue and auramine O in wastewater. Colloids Surf. C Environ. Asp. 2024, 2, 100033. [Google Scholar] [CrossRef]
- Mukhamatdinov, I.I.; Salih, I.S.S.; Khelkhal, M.A.; Vakhin, A.V. Application of Aromatic and Industrial Solvents for Enhancing Heavy Oil Recovery from the Ashalcha Field. Energy Fuels 2021, 35, 374–385. [Google Scholar] [CrossRef]
- Hewett, D.M.; Bocklitz, S.; Tabor, D.P.; Iii, E.L.S.; Suhm, M.A.; Zwier, T.S. Identifying the first folded alkylbenzene via ultraviolet, infrared, and Raman spectroscopy of pentylbenzene through decylbenzene. Chem. Sci. 2017, 8, 5305–5318. [Google Scholar] [CrossRef]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A comprehensive review of biochar in removal of organic pollutants from wastewater: Characterization, toxicity, activation/functionalization and influencing treatment factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Stark, M.; Ditze, S.; Drost, M.; Buchner, F.; Steinrück, H.-P.; Marbach, H. Coverage dependent disorder-order transition of 2H-tetraphenylporphyrin on Cu(111). Langmuir 2013, 29, 4104–4110. [Google Scholar] [CrossRef]
- Darby, I.; Xu, C.-Y.; Wallace, H.M.; Joseph, S.; Pace, B.; Bai, S.H. Short-term dynamics of carbon and nitrogen using compost, compost-biochar mixture and organo-mineral biochar. Environ. Sci. Pollut. Res. 2016, 23, 11267–11278. [Google Scholar] [CrossRef]
- Bardestani, R.; Biriaei, R.; Kaliaguine, S. Hydrogenation of Furfural to Furfuryl Alcohol over Ru Particles Supported on Mildly Oxidized Biochar. Catalysts 2020, 10, 934. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, B.; Zhu, B.; Li, X.; Rauhut, G.; Zeng, X. Hydrogen-Bonded π Complexes between Phosphaethyne and Hydrogen Chloride. J. Phys. Chem. Lett. 2023, 14, 4327–4333. [Google Scholar] [CrossRef]
- Piriya, R.S.; Jayabalakrishnan, R.M.; Maheswari, M.; Boomiraj, K.; Oumabady, S. Comparative adsorption study of malachite green dye on acid-activated carbon. Int. J. Environ. Anal. Chem. 2023, 103, 16–30. [Google Scholar] [CrossRef]
- Shao, J.; Wu, D. Study on the Performance of Coal Gangue-Loaded Hydroxyapatite (CG@HAP) for the Adsorption of Malachite Green. Molecules 2024, 29, 5649. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Guo, F.; Shu, R.; Dong, K.; Qiao, Q.; Liu, S.; Xu, L.; Bai, Y. Evaluation of the High Metals-Containing Coal Gasification Fine Slag as a High-Performance Adsorbent for Malachite Green Adsorption. Waste Biomass Valorization 2022, 13, 4897–4909. [Google Scholar] [CrossRef]
- Söğüt, E.G.; Karataş, Y.; Gülcan, M.; Kılıç, N.Ç. Enhancement of adsorption capacity of reduced graphene oxide by sulfonic acid functionalization: Malachite green and Zn (II) uptake. Mater. Chem. Phys. 2020, 256, 123662. [Google Scholar] [CrossRef]
- Chouli, F.; Ezzat, A.O.; Sabantina, L.; Benyoucef, A.; Zehhaf, A. Optimization Conditions of Malachite Green Adsorption onto Almond Shell Carbon Waste Using Process Design. Molecules 2024, 29, 54. [Google Scholar]
- Zhang, Y.; Qi, X.; Ma, Q.; Li, J.; Guo, X.; Qiao, J.; Wu, Y. Fluorescent β-cyclodextrin-based hydrogel for enhanced adsorption and fluorescence detection of malachite green. Sep. Purif. Technol. 2025, 363, 132065. [Google Scholar] [CrossRef]
- Ranote, S.; Chauhan, S.; Kumar, K.; Kowalczuk, M.; Chauhan, G.S. Pine needles, a forest waste biomass, driven biosorbent for malachite green dye. Biomass Convers. Biorefinery 2024, 14, 25885–25899. [Google Scholar] [CrossRef]
- Bullen, J.C.; Saleesongsom, S.; Gallagher, K.; Weiss, D.J. A Revised Pseudo-Second-Order Kinetic Model for Adsorption, Sensitive to Changes in Adsorbate and Adsorbent Concentrations. Langmuir 2021, 37, 3189–3201. [Google Scholar] [CrossRef]
- Chen, G.; Yin, Y.; Zhang, X.; Qian, A.; Pan, X.; Liu, F.; Li, R. Enhanced Adsorption of Methyl Orange from Aqueous Phase Using Chitosan-Palmer Amaranth Biochar Composite Microspheres. Molecules 2024, 29, 1836. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Ryu, S.; Loganathan, P.; Kandasamy, J.; Nguyen, T.V.; Vigneswaran, S. Arsenic adsorption by low-cost laterite column: Long-term experiments and dynamic column modeling. Process Saf. Environ. Prot. 2022, 160, 868–875. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).