Tillage System as a Practice Affecting the Quality of Soils and Its Sustainable Management
Abstract
1. Introduction
- -
- the contents of organic carbon, nitrogen, and soluble organic matter;
- -
- sorption properties;
- -
- the activities of selected enzymes involved in the biogeochemistry of C, N, and P in soil.
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Physicochemical Properties of Soil
- −
- Soil texture was determined according to the pipette method [41]. Soil was treated to remove carbonates and organic matter. After dissolution of CaCO3 with 2 M HCl dm−3 and oxidation of the organic carbon with 30% H2O2, there followed repeated washing, and, finally, the samples were dispersed using Na(PO3)6. Silt, sand, and clay fractions were determined. The fraction content is expressed in %.
- −
- pH was determined in 1M KCl potentiometrically [42];
- −
- Hydrolytic acidity (Hh) and total exchangeable base cations (TEB) were assessed by the Kappen method [43];
- −
- Cation exchange capacity (CEC) was calculated based on TEB and Hh, and the degree of base saturation (BS) of the sorption complex was calculated from CEC and TEB.
2.2.2. Content of Organic Carbon, Total Nitrogen, and Dissolved Organic Matter
2.2.3. Activity of Enzymes
- −
- The activity of dehydrogenases (DEH) was determined by the Thalmann [44] method after incubating the sample with 2,3,5-triphenyltetrazolium chloride and measuring the absorbance of triphenylformazan (TPF) at 546 nm; results are expressed in mg TPF kg−1 24 h−1.
- −
- Catalase (CAT) was determined by the method of Johnson and Temple [45] using 0.3% hydrogen peroxide solution as the substrate. The remaining H2O2 was determined by titration with 0.02 M KMnO4 under acidic conditions.
- −
- The activity of peroxidases (PER) was determined by the method of Barth and Bordeleau [46] by measuring the amount of purpurogallin (PPG) formed by the oxidation of pyrogallol in the presence of H2O2.
- −
- The activities of alkaline phosphatase (AlP) and acid phosphatase (AcP) were determined based on the detection of p-nitrophenol (pNP) released after incubation (37 °C, 1 h) at pH ~6.5 for acid phosphatase and pH ~11.0 for alkaline [47].
- −
- β-glucosidase (BG) was measured by the method of Eivazi and Tabatabai [48], using p-nitrophenyl-β-D-glucopyranoside as a substrate. Concentrations of p-Nitrophenol were determined by direct reading of the sample at 400 nm after alkalisation with Tris/NaOH buffer (pH 10.0) and CaCl2.
- −
- The activity of proteases was determined using the method of Ladd and Butler [49], where the concentration of the amino acid tyrosine (Tyr) was determined in soil samples after incubation with sodium caseinate. Absorbance was measured with a spectrophotometer at λ = 680.
2.3. Statistical Analyses
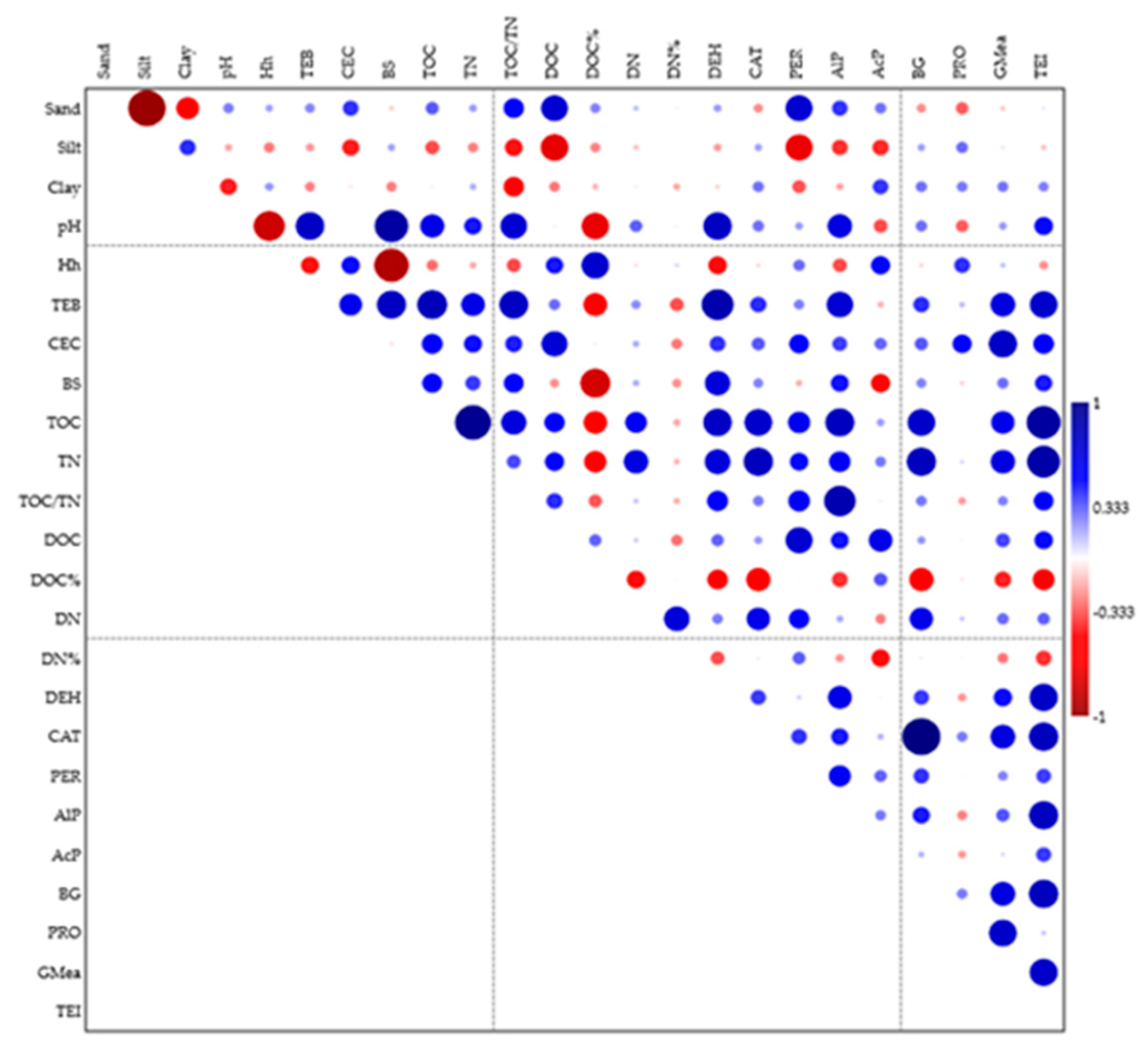
3. Results and Discussion
3.1. Selected Physico-Chemical Properties
3.2. Content of Organic Carbon, Total Nitrogen and Dissolved Organic Matter
3.3. The Activity of Enzymes in Soil
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartmann, M.; Frey, B.; Mayer, J.; Mäder, P.; Widmer, F. Distinct soil microbial diversity under long-term organic and conventional farming. ISME J. 2015, 9, 1177–1194. [Google Scholar] [CrossRef]
- Wiesmeier, M.; Urbanski, L.; Hobley, E.; Lang, B.; von Lutzow, B.; Marin-Spiotta, E.; van Wesemael, B.; Rabot, E.; Ließ, M.; Garcia-Franco, N.; et al. Soil organic carbon storage as a key function of soils—A review of drivers and indicators at various scales. Geoderma 2019, 333, 149–162. [Google Scholar] [CrossRef]
- European Commision. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions—The European Green Deal, Brussels. 2019. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0016.02/DOC_1&format=PDF (accessed on 11 December 2019).
- Vittori Antisari, L.; Trenti, W.; De Feudis, M.; Bianchini, G.; Falsone, G. Soil quality and organic matter pools in a temperate climate (Northern Italy) under different land uses. Agronomy 2021, 11, 1815. [Google Scholar] [CrossRef]
- Simon, L.M.; Augustine, K.O.; Holman, J.D.; Roozeboom, K.L. Long-term cover crop management effects on soil properties in dryland cropping systems. Agric. Ecosyst. Environ. 2022, 328, 107852. [Google Scholar] [CrossRef]
- Karaca, A.; Cetin, S.C.; Turgay, O.C.; Kizilkaya, R. Soil enzymes as indication of soil quality. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 22. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity—A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Jaskulska, I.; Romaneckas, K.; Jaskulski, D.; Gałęzewski, L.; Breza-Boruta, B.; Dębska, B.; Lemanowicz, J. Soil properties after eight years of the use of strip-till one-pass technology. Agronomy 2020, 10, 1596. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D. Strip-till one-pass technology in central and Eastern Europe: A MZURI Pro-Till hybrid machine case study. Agronomy 2020, 10, 925. [Google Scholar] [CrossRef]
- Jaskulska, I.; Lemanowicz, J.; Dębska, B.; Jaskulski, D.; Breza-Boruta, B. Changes in soil organic matter and biological parameters as a result of long-term strip-till cultivation. Agriculture 2023, 13, 2188. [Google Scholar] [CrossRef]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of soil organic matter dynamics to physical protection and tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D. Influence of many years’ fertilization on the dynamics of soil properties. Adv. Agric. Sci. 2003, 4, 21–35. [Google Scholar]
- Blecharczyk, A.; Małecka, I.; Sierpowski, J. Long-term effect of tillage systems on physico-chemical soil properties. Fragm. Agron. 2007, 1, 7–13. [Google Scholar]
- Aye, N.S.; Sale, P.W.G.; Tan, C. The impact of long-term liming on soil organic carbon and aggregate stability in low-input acid soils. Biol. Fertil. Soils. 2016, 52, 697–709. [Google Scholar] [CrossRef]
- Debska, B.; Kotwica, K.; Banach-Szott, M.; Spychaj-Fabisiak, E.; Tobiašová, E. Soil fertility improvement and carbon sequestration through exogenous organic matter and biostimulant application. Agriculture 2022, 12, 1478. [Google Scholar] [CrossRef]
- Menšík, L.; Hlisnikovský, L.; Pospíšilová, L.; Kunzová, E. The effect of application of organic manures and mineral fertilizers on the state of soil organic matter and nutrients in the long-term field experiment. J. Soils Sediments 2018, 18, 2813–2822. [Google Scholar] [CrossRef]
- Friedrich, T.; Derpsch, R.; Kassam, A.H. Global overview of the spread of conservation agriculture. J. Agric. Sci. Technol. 2015, 6, 1–7. [Google Scholar]
- Francaviglia, R.; Almagro, M.; Vicente-Vicente, J.L. Conservation agriculture and soil organic carbon: Principles, processes, practices and policy options. Soil Syst. 2023, 7, 17. [Google Scholar] [CrossRef]
- Stavi, I.; Bel, G.; Zaady, E. Soil functions and ecosystem services in conventional, conservation, and integrated agricultural systems. A review. Agron. Sustain. Dev. 2016, 36, 32. [Google Scholar]
- Busari, A.M.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. Int. Soil Water Conserv. Res. 2015, 3, 119–129. [Google Scholar] [CrossRef]
- Horowitz, J.; Ebel, R.; Ueda, K. Economic Information Bulletin No. 70: “No-Till” Farming Is a Growing Practice; Economic Research Service, U.S. Department of Agriculture: Washington, DC, USA, 2010.
- Allam, M.; Radicetti, E.; Petroselli, V.; Mancinelli, R. Meta-analysis approach to assess the effects of soil tillage and fertilization source under different cropping systems. Agriculture 2021, 11, 823. [Google Scholar] [CrossRef]
- Debska, B.; Jaskulska, I.; Jaskulski, D. Method of tillage with the factor determining the quality of organic matter. Agronomy 2020, 10, 1250. [Google Scholar] [CrossRef]
- Virto, I.; Barre, P.; Burlot, A.; Chenu, C. Carbon input differences as the main factor explaining the variability in soil organic C storage in notilled compared to inversion tilled agrosystems. Biogeochemistry 2012, 108, 17–26. [Google Scholar] [CrossRef]
- Dimassi, B.; Mary, B.; Wylleman, R.; Labreuche, J.; Couture, D.; Piraux, F.; Cohan, J.P. Long-term effect of contrasted tillage and crop management on soil carbon dynamics during 41 years. Agric. Ecosyst. Environ. 2014, 188, 134–146. [Google Scholar] [CrossRef]
- Han, P.; Zhang, W.; Wang, G.; Sun, W.; Huang, Y. Changes in soil organic carbon in croplands subjected to fertilizer management: A global metaanalysis. Nat. Sci. Rep. 2016, 6, 27199. [Google Scholar] [CrossRef]
- Yue, K.; Peng, Y.; Peng, C.; Yang, W.; Peng, X.; Wu, F. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: A metaanalysis. Sci. Rep. 2016, 6, 19895. [Google Scholar] [CrossRef]
- Armas-Herrera, C.M.; Dignac, M.-F.; Rumpel, C.; Arbelo, C.D.; Chabbi, A. Management effects on composition and dynamics of cutin and suberin in topsoil under agricultural use. Eur. J. Soil Sci. 2016, 67, 360–373. [Google Scholar] [CrossRef]
- Szostek, M.; Szpunar-Krok, E.; Pawlak, R.; Stanek-Tarkowska, J.; Ilek, A. Effect of different tillage systems on soil organic carbon and enzymatic activity. Agronomy 2022, 12, 208. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Ruis, S.J. No-tillage and soil physical environment. Geoderma 2018, 326, 164–200. [Google Scholar] [CrossRef]
- Chenu, C.; Angers, D.A.; Barré, P.; Derrien, D.; Arrouays, D.; Balesdent, J. Increasing organic stocks in agricultural soils: Knowledge gaps and potential innovations. Soil Tillage Res. 2019, 188, 41–52. [Google Scholar] [CrossRef]
- Derpsch, R.; Friedrich, T.; Kassam, A.; Li, H. Current status of adoption of no-till farming in the world and some of its main benefits. Int. J. Agric. Biol. Eng. 2010, 3, 1–25. [Google Scholar] [CrossRef]
- Cenini, V.L.; Fornara, D.A.; McMullan, G.; Ternan, N.; Carolan, R.; Crawley, M.J.; Clément, J.-C.; Lavorel, S. Linkages between extracellular enzyme activities and the carbon and nitrogen content of grassland soils. Soil Biol. Biochem. 2016, 96, 198–206. [Google Scholar] [CrossRef]
- Rahmati, M.; Eskandari, I.; Kouselou, M.; Feiziasl, V.; Mahdavinia, G.R.; Aliasgharzad, N.; McKenzie, B.M. Changes in soil organic carbon fractions and residence time five years after implementing conventional and conservation tillage practices. Soil Tillage Res. 2020, 200, 104632. [Google Scholar] [CrossRef]
- Tobiašová, E.; Lemanowicz, J.; Dębska, B.; Kunkelová, M.; Sakáč, J. The Effect of reduced and conventional tillage systems on soil aggregates and organic carbon parameters of different soil types. Agriculture 2023, 13, 818. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Haddad, S.A.; Lemanowicz, J.; Abd El-Azeim, M.M. Cellulose decomposition in clay and sandy soils contaminated with heavy metals. Int. J. Environ. Sci. Technol. 2019, 16, 3275–3290. [Google Scholar] [CrossRef]
- Tobiašová, E.; Lemanowicz, J.; Dębska, B.; Kunkelová, M.; Sakáč, J. Suitability of various parameters for the determination of the condition of soil structure with dependence to the quantity and quality of soil organic matter. Sustainability 2023, 15, 11047. [Google Scholar] [CrossRef]
- Korec, P.; Lauko, V.; Tolmáči, L.; Zubrický, G.; Mičietová, E. Region and Districts of Slovakia. A New Administrative Structure; Q111: Bratislava, Slovakia, 1997; 391p. [Google Scholar]
- Jurčová, O.; Bielek, P. Method of Soil Organic Method Balance and the Determination of Necessity of Organic Fertilization; VÚPÚ: Bratislava, Slovakia, 1997; 154p. [Google Scholar]
- van Reeuwijk, L.P. Procedures for Soil Analysis; International Soil Reference and Information Centre: Wageningen, The Netherlands, 2002; 120p. [Google Scholar]
- PN-ISO 10390; Chemical and Agricultural Analysis: Determining Soil pH. Polish Standards Committee: Warszawa, Poland, 1997.
- Skjemstad, J.O.; Gillman, G.P.; Massis, A.; Spouncer, L.R.; Leonie, R. Measurement of the exchange capacity of the cationic fraction of organic matter from soils using the modified compulsive exchange method. Soil Sci. Soc. Am. J. 2008, 39, 926–937. [Google Scholar] [CrossRef]
- Thalmann, A. Zur methodic derestimung der dehydrogenaseaktivität und boden mittels triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Johnson, J.I.; Temple, K.L. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soc. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Bartha, R.; Bordeleau, L. Cell-free peroxidases in soil. Soil Biol. Biochem. 1969, 1, 139–143. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p–nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Ladd, J.N.; Jackson, R.B. Biochemistry of ammonification. In Nitrogen in Agricultural Soil; American Society of Agronomy, Inc. Crop Science Society of America, Inc. Soil Science Society of America Inc.: Madison, WI, USA, 1982. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Garcia-Ruiz, R.; Viñegla, B.; Carreira, J.A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 2004, 36, 1637–1644. [Google Scholar] [CrossRef]
- Tan, X.; Xie, B.; Wang, J.; He, W.; Wang, X.; Wei, G. County-scale spatial distribution of soil enzyme activities and enzyme activity indices in agricultural land: Implications for soil quality assessment. Sci. World J. 2014, 2014, 535768. [Google Scholar] [CrossRef]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data anlysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Ward, J.H. Hierarchical grouping to optimize an objective function. J. Am. Stat. Ass. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Ahmad, N.; Virk, A.L.; Hafeez, M.B.; Ercisli, S.; Golokhvast, K.S.; Qi, Y.; Guo, X.; Zhang, Y.; Wang, R.; Wang, X.; et al. Effects of different tillage and residue management systems on soil organic carbon stock and grain yield of rice–wheat double cropping system. Ecol. Indic. 2024, 158, 111452. [Google Scholar] [CrossRef]
- Yuan, J.; Yan, l.; Li, G.; Sadiq, M.; Rahim, N.; Wu, J.; Ma, W.; Xu, G.; Du, M. Effects of conservation tillage strategies on soil physicochemical indicators and N2O emission under spring wheat monocropping system conditions. Sci. Rep. 2022, 12, 7066. [Google Scholar] [CrossRef]
- Mosley, L.M.; Rengasamy, P.; Fitzpatrick, R. Soil pH: Techniques, challenges and insights from a global dataset. Eur. J. Soil Sci. 2024, 75, 70021. [Google Scholar] [CrossRef]
- He, G.; Zhang, Z.; Wu, X.; Cui, M.; Zhang, J.; Huang, X. Adsorption of heavy metals on soil collected from Lixisol of typical karst areas in the presence of CaCO3 and soil clay and their competition behavior. Sustainability 2020, 12, 7315. [Google Scholar] [CrossRef]
- Stawn, D. Sorption Mechanisms of chemicals in soils. Soil Syst. 2021, 5, 13. [Google Scholar] [CrossRef]
- Bi, X.; Chu, H.; Fu, M.; Xu, D.; Zhao, W.; Zhong, Y.; Wang, M.; Li, K.; Zhang, Y. Distribution characteristics of organic carbon (nitrogen) content, cation exchange capacity, and specific surface area in different soil particle sizes. Sci. Rep. 2023, 13, 12242. [Google Scholar] [CrossRef]
- Vasudevan, D.; Bruland, G.L.; Torrance, B.S.; Upchurch, W.G.; MacKay, A.A. pH-dependent ciprofloxacin sorption to soils: Interaction mechanisms and soil factors influencing sorption. Geoderma 2009, 151, 68–76. [Google Scholar] [CrossRef]
- Lorandi, R. Evaluation of cation exchange capacity (CEC) in tropical soils using four different analytical methods. J. Agric. Sci. 2012, 4, 278–289. [Google Scholar] [CrossRef]
- Šimansky, V.; Pollakova, N. Soil organic matter and sorption capacity under different soil management practices in a productive vineyard. Arch. Agron. Soil Sci. 2014, 60, 1145–1154. [Google Scholar] [CrossRef]
- Tian, K.; Zhao, Y.; Xu, X.; Hai, N.; Huang, B.; Deng, W. Effects of long-term fertilization and residue management on soil organic carbon changes in paddy soils of China: A meta-analysis. Agric. Ecosyst. Environ. 2015, 204, 40–50. [Google Scholar] [CrossRef]
- Laufer, D.; Loibl, B.; Marlander, B.; Koch, H.J. Soil erosion and surface run-of under strip tillage for sugar beet (Beta vulgaris L.) in Centraf Europe. Soil Tillage Res. 2016, 162, 1–7. [Google Scholar] [CrossRef]
- Powlson, D.S.; Bhogal, A.; Chambers, B.J.; Coleman, K.; Macdonald, A.J.; Goulding, K.W.T.; Whitmore, A.P. The potential to increase soil carbon stocks through reduced tillage or organic material additions in England and Wales: A case study. Agric. Ecosyst. Environ. 2012, 146, 23–33. [Google Scholar] [CrossRef]
- Kumar, S.; Kadono, A.; Lal, R.; Dick, W. Long-term no-till impacts on organic carbon and properties of two contrasting soils and corn yields in Ohio. Soil Sci. Soc. Am. J. 2012, 76, 1798–1809. [Google Scholar] [CrossRef]
- Bolan, N.S.; Adriano, D.C.; Kunhikrishnan, A.; James, T.; McDowell, R.; Senesi, N. Dissolved organic matter: Biogeochemistry, dynamics, and environmental significance in soils. Adv. Agron. 2011, 110, 1–75. [Google Scholar] [CrossRef]
- Wright, A.; Dou, F.; Hons, M.F. Soil organic C and N distribution for wheat cropping systems after 20 years of conservation tillage in central Texas. Agric. Ecosyst. Environ. 2007, 121, 376–382. [Google Scholar] [CrossRef]
- Liu, E.; Teclemariam, S.G.; Yan, C.; Yu, J.; Gu, R.; Liu, S.; He, W.; Liu, Q. Long-term efects no-tillage management practice on soil organic carbon and its fractions in the northern China. Geodema 2014, 213, 379–384. [Google Scholar] [CrossRef]
- Leinweber, P.; Schulten, H.R.; Körschens, M. Hot water extracted organic matter: Chemical composition and temporal variations in a long-term field experiment. Biol. Fertil. Soils 1995, 20, 17–23. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Rinklebe, J. Geochemical fractions of chromium, copper, and zinc and their vertical distribution in floodplain soil profiles along the Central Elbe River, Germany. Geoderma 2014, 228, 142–159. [Google Scholar] [CrossRef]
- Furtak, K.; Grządziel, J.; Gałązka, A.; Niedźwiecki, J. Analysis of soil properties, bacterial community composition, and metabolic diversity in fluvisols of a floodplain area. Sustainability 2019, 11, 3929. [Google Scholar] [CrossRef]
- Spiegel, H.; Sandén, T.; Sandén, H.; Götzinger, S.; Miloczki, J.; Kandeler, E. Changes in biological and chemical soil properties in an Austrian long-term tillage experiment. Eur. J. Soil Sci. 2025, 76, e70037. [Google Scholar] [CrossRef]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Bünemann, E., Oberson, A., Frossard, E., Eds.; Soil Biology Series; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26. [Google Scholar] [CrossRef]
- Panettieri, M.; de Sosa, L.L.; Domínguez, M.T.; Madejón, E. Long-term impacts of conservation tillage on Mediterranean agricultural soils: Shifts in microbial communities despite limited effects on chemical properties. Agric. Ecosyst. Environ. 2020, 304, 107144. [Google Scholar] [CrossRef]
- Fernández-Ortega, J.; Álvaro-Fuentes, J.; Delgado, A.; García-López, A.M.; Cantero-Martínez, C. Assessing management strategies for carbon storage in Mediterranean soils: Double-cropping, no-tillage, and nitrogen fertilization reduction. Soil Tillage Res. 2025, 249, 106496. [Google Scholar] [CrossRef]
- Mirzavand, J.; Asadi-Rahmani, H.; Moradi-Talebbeigi, R. Biological indicators of soil quality under conventional, reduced, and no-tillage systems. Arch. Agron. Soil Sci. 2022, 68, 311–324. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Piazza, G.; Pellegrino, E.; Moscatelli, M.C.; Ercoli, L. Long-term conservation tillage and nitrogen fertilization effects on soil aggregate distribution, nutrient stocks and enzymatic activities in bulk soil and occluded microaggregates. Soil Tillage Res. 2020, 196, 104482. [Google Scholar] [CrossRef]
- Wen, L.; Peng, Y.; Zhou, Y.; Cai, G.; Lin, Y.; Li, B. Effects of conservation tillage on soil enzyme activities of global cultivated land: A meta-analysis. J. Environ. Manag. 2023, 345, 118904. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, H.; Wang, Q.; Zhu, W.; Kang, Y. Soil extracellular enzyme activity linkage with soil organic carbon under conservation tillage: A global meta-analysis. Eur. J. Agron. 2024, 155, 127135. [Google Scholar] [CrossRef]
- Li, T.; Li, G.; Lu, Z.; Zhao, D.; Li, Y.; Wang, Z.; Wen, X.; Liao, Y. Crop diversification increases soil extracellular enzyme activities under no tillage: A global meta-analysis. Soil Tillage Res. 2024, 235, 105870. [Google Scholar] [CrossRef]
- Woźniak, A. Chemical properties and enzyme activity of soil as affected by tillage system and previous crop. Agriculture 2019, 9, 262. [Google Scholar] [CrossRef]
- Lasota, J.; Błońska, E.; Piaszczyk, W. State of soil enzymatic activity in relationship to some chemical properties of Brunic Arenosols. Soil Sci. Ann. 2021, 72, 140641. [Google Scholar] [CrossRef]
- Shao, X.; Yang, W.; Wu, M. Seasonal dynamics of soil labile organic carbon and enzyme activities in relation to vegetation types in Hangzhou Bay Tidal Flat Wetland. PLoS ONE 2015, 11, e0142677. [Google Scholar] [CrossRef]
- Daunoras, J.; Kačergius, A.; Gudiukaitė, R. Role of soil microbiota enzymes in soil health and activity changes depending on climate change and the type of soil ecosystem. Biology 2024, 13, 85. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Relationship between carbon and nitrogen availability and extracellular enzyme activities in soil. Pedobiologia 2009, 53, 87–98. [Google Scholar] [CrossRef]
- Chaves, V.B.S.; Guimarães, T.M.; Bezerra, A.C.T.P.; da Costa, C.H.M.; Cruz, S.C.S. Enzymatic activity in different crop succession systems in the Cerrado Region. Agronomy 2024, 14, 810. [Google Scholar] [CrossRef]
- de Almeida, R.F.; Naves, E.R.; da Mota, R.P. Soil quality: Enzymatic activity of soil β-glucosidase. Glob. J. Agric. Res. Rev. 2015, 3, 146–450. [Google Scholar]
- Fernández-Ortega, J.; Álvaro-Fuentes, J.; Talukder, R.; Lampurlanés, J.; Cantero-Martínez, C. The use of double-cropping in combination with no-tillage and optimized nitrogen fertilization improve crop yield and water use efficiency under irrigated conditions. Field Crops Res. 2023, 301, 109017. [Google Scholar] [CrossRef]
- Tian, L.; Dell, E.; Shi, W. Chemical composition of dissolved organic matter in agroecosystems: Correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Song, Y.; Song, C.; Yang, G.; Miao, Y.; Wang, J.; Guo, Y. Changes in labile organic carbon fractions and soil enzyme activities after marshland reclamation and restoration in the Sanjiang Plain in Northeast China. Environ. Manag. 2012, 50, 418–426. [Google Scholar] [CrossRef]
- Uwituze, Y.; Nyiraneza, J.; Fraser, T.D.; Dessureaut-Rompré, J.; Ziadi, N.; Lafond, J. Carbon, nitrogen, phosphorus, and extracellular soil enzyme responses to different land use. Front. Soil Sci. 2022, 2, 814554. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Zhao, J.; Xiao, K.; Wang, K. Effects of nitrogen addition on activities of soil nitrogen acquisition enzymes:A meta-analysis. Agric. Ecosyst. Environ. 2018, 252, 126–131. [Google Scholar] [CrossRef]
- Chen, T.; Cheng, R.; Xiao, W.; Shen, Y.; Wang, L.; Sun, P.; Zhang, M.; Li, J. Nitrogen addition enhances soil nitrogen mineralization through an increase in mineralizable organic nitrogen and the abundance of functional genes. J. Soil Sci. Plant Nutr. 2024, 24, 975–987. [Google Scholar] [CrossRef]
- Landi, L.; Renella, G.; Giagnoni, L.; Nannipieri, P. Activities of proteolytic enzymes. Methods Soil Enzymol. 2011, 9, 247–260. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Chmiel, M.J.; Dziedzic, K.; Taras, H. Assessment of soil quality after biochar application based on enzymatic activity and microbial composition. Int. Agrophys. 2019, 33, 331–336. [Google Scholar] [CrossRef]
- Banach-Szott, M.; Debska, B.; Tobiasova, E. Properties of humic acids depending on the land use in different parts of Slovakia. Environ. Sci. Pollut. Res. 2021, 28, 58068–58080. [Google Scholar] [CrossRef]


| EF * | Shallow humus horizon; huge heterogeneity of soil substrate; natural vegetation in flooded forests; higher underground water before water flow regulation; the composition of the microbial community is strongly influenced by anthropogenic cultivation; the initial process of organic carbon accumulation is dominant |
| MF | Deep humus horizon; specific hydromorphic regime; rich in a clay content and organic substances of high quality with dominance of humic acids; originally humus accumulation under hydrophilic vegetation with humification as a dominant process; high abundance and diversity of soil organisms |
| HC | Deep humus horizon with intensive humification; originally rich grass vegetation supported the creation and accumulation of humus substances of high quality; the presence of areas with a longer period of dryness results in the dominance of organisms that adapted to these conditions; ammonium can briefly release and nitrates can accumulate |
| HL | Shallow humus horizon mixed with genetic horizon in the arable land; strong risk of soil erosion; accumulation of clay in genetic illuvial luvic horizon with dominant process of illimerisation, which results in soil compaction and worse soil structure; biological activity is relatively high with dominance of bacteria |
| ER | Shallow humus horizon influenced by soil erosion; unsuitable textural composition, usually coarse soils; high aeration and dominance of non-capillary pores; small amount of organic sources for heterotrophic microbial community; high intensity of oxidation processes; risk of nutrient leaching |
| EG | Unsuitable textural composition with clay dominance; high level of underground water and poor aeration; reduction processes are dominant; accumulation of organic sources is high but with dominant production of low molecular substances and creation of fulvic acids; usually soils with a lower pH and unsuitable soil structure |
| SP | Soil of the areas with the accumulation of water with unsuitable hydromorphic regimes; frequent fluctuation of water level, changes in oxidation, and reduction conditions in the genetic horizon, mobilisation of Fe, Al, Mn; low diversity of microbial community; special hydrogenetic development of humus-forming process with FA dominance |
| Soil Types | Sand% | Silt% | Clay% | pH KCl | ||||
|---|---|---|---|---|---|---|---|---|
| Tillage Systems | ||||||||
| RT | CT | RT | CT | RT | CT | RT | CT | |
| EF | 2.33 ± 0.07 | 7.33 ± 0.07 | 64.27 ± 1.41 | 62.04 ± 0.47 | 33.4 ± 0.41 | 30.66 ± 0.06 | 4.6 ± 0.14 | 6.29 ± 0.12 |
| MF | 19.70 ± 0.37 | 14.90 ± 0.33 | 52.44 ± 0.04 | 45.74 ± 0.86 | 27.86 ± 0.76 | 39.36 ± 0.10 | 7.16 ± 0.21 | 6.99 ± 0.12 |
| HC | 14.54 ± 0.16 | 13.77 ± 0.15 | 61.26 ± 0.01 | 59.29 ± 0.19 | 24.20 ± 0.70 | 26.94 ± 0.05 | 5.57 ± 0.18 | 6.24 ± 0.05 |
| HL | 27.07 ± 0.34 | 9.75 ± 0.30 | 51.47 ± 0.05 | 66.07 ± 0.46 | 21.46 ± 0.41 | 24.18 ± 0.10 | 6.91 ± 0.10 | 5.27 ± 0.10 |
| ER | 10.48 ± 0.76 | 9.43 ± 0.67 | 65.40 ± 0.1 | 61.92 ± 0.69 | 24.12 ± 0.55 | 28.65 ± 0.12 | 7.02 ± 0.19 | 5.57 ± 0.14 |
| EG | 7.17 ± 0.08 | 16.58 ± 0.08 | 58.05 ± 0.02 | 45.30 ± 0.29 | 4.78 ± 0.23 | 38.12 ± 0.07 | 5.55 ± 0.06 | 5.70 ± 0.10 |
| SP | 19.04 ± 0.21 | 10.45 ± 1.09 | 64.96 ± 0.11 | 63.20 ± 0.25 | 16.00 ± 0.69 | 26.35 ± 0.46 | 4.67 ± 0.08 | 6.05 ± 0.09 |
| Soil * Types | Hh (cmol(+) kg−1) | TEB (cmol(+) kg−1) | CEC (cmol(+) kg−1) | BS (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Tillage Systems | ||||||||
| RT | CT | RT | CT | RT | CT | RT | CT | |
| EF | 3.90 a ± 0.8 | 0.75 b ± 0.3 | 3.30 a ± 0.6 | 2.50 b ± 1.4 | 7.20 a ± 2.2 | 3.25 b ± 0.8 | 45.83 b ± 2.3 | 76.92 a ± 3.2 |
| MF | 1.30 a ± 0.2 | 0.37 b ± 0.1 | 5.40 a ± 0.5 | 4.80 b ± 2.2 | 6.70 a ± 1.1 | 5.17 b ± 1.1 | 80.60 b ± 3.1 | 92.84 a ± 3.0 |
| HC | 2.92 a ± 0.8 | 0.90 b ± 0.2 | 2.40 b ± 1.3 | 3.40 a ± 1.3 | 5.32 a ± 2.1 | 4.30 b ± 1.0 | 45.11 b ± 1.8 | 79.07 a ± 2.4 |
| HL | 1.45 b ± 0.1 | 2.25 a ± 0.8 | 4.30 a ± 4.1 | 2.90 b ± 1.1 | 5.75 a ± 2.2 | 5.15 a ± 1.2 | 74.78 a ± 2.6 | 56.31 b ± 2.0 |
| ER | 1.37 b ± 0.0 | 1.87 a ± 0.4 | 3.60 a ± 2.5 | 3.30 a ± 0.8 | 4.97 b ± 1.4 | 5.17 a ± 1.1 | 72.43 a ± 3.4 | 63.83 b ± 1.5 |
| EG | 1.87 a ± 0.5 | 1.87 a ± 0.4 | 1.90 b ± 1.5 | 2.90 a ± 15.1 | 3.77 b ± 1.1 | 4.67 a ± 1.3 | 50.40 b ± 2.8 | 59.96 a ± 4.1 |
| SP | 2.85 a ± 0.5 | 0.98 b ± 0.1 | 1.60 b ± 0.15 | 3.20 a ± 0.3 | 4.45 a ± 1.1 | 4.18 a ± 1.1 | 35.96 b ± 1.5 | 76.56 a ± 1.4 |
| Soil * Types | TOC (g·kg−1) | TN (g·kg−1); | TOC/TN | |||
|---|---|---|---|---|---|---|
| Tillage Systems | ||||||
| RT * | CT | RT | CT | RT | CT | |
| EF * | 25.06 a ± 0.35 | 18.73 b ± 0.22 | 2.49 a ± 0.05 | 1.86 b ± 0.04 | 10.13 a | 10.10 a |
| MF | 38.90 b ± 0.50 | 42.07 a ± 0.55 | 2.86 b ± 0.06 | 3.73 a ± 0.08 | 13.64 a | 11.30 b |
| HC | 27.63 a± 0.22 | 18.13 b ± 0.30 | 2.87 a ± 0.03 | 1.81 b ± 0.02 | 9.63 a | 10.02 a |
| HL | 31.67 a ± 0.32 | 16.53 b ± 0.20 | 2.51 a ± 0.03 | 1.57 b ± 0.04 | 12.71 a | 10.53 b |
| ER | 24.20 a ± 0.25 | 17.75 b ± 0.35 | 2.12 a ± 0.04 | 1.91 a ± 0.03 | 11.41 a | 9.32 b |
| EG | 18.63 b ± 0.35 | 29.36 a ± 0.35 | 1.84 b ± 0.03 | 2.89 a ± 0.04 | 10.13 a | 10.17 a |
| SP | 15.51 b ± 0.20 | 20.97 a ± 0.30 | 1.49 b ± 0.03 | 1.92 a ± 0.03 | 10.41 a | 10.95 a |
| Soil * Types | DOC (mg kg−1) | DOC (%) | DTN (mg kg−1) | DTN(%) | ||||
|---|---|---|---|---|---|---|---|---|
| Tillage Systems | ||||||||
| RT * | CT | RT | CT | RT | CT | RT | CT | |
| EF * | 271.3 a ± 10.5 | 141.9 b ± 5.5 | 1.08 a ± 0.05 | 0.76 b ± 0.03 | 54.4 a ± 3.0 | 47.2 b ± 2.5 | 2.19 a ± 0.05 | 2.54 a ± 0.07 |
| MF | 278.4 a ± 9.3 | 177.9 b ± 8.5 | 0.72 a ± 0.03 | 0.42 b ± 0.01 | 43.7 b ± 2.1 | 85.5 a ± 8.8 | 1.53 b ± 0.03 | 2.30 a ± 0.09 |
| HC | 294.9 a ± 8.5 | 150.6 b ± 5.8 | 1.07 a ± 0.06 | 0.83 b ± 0.05 | 66.7 a ± 3.3 | 43.7 b ± 2.5 | 2.32 a ± 0.03 | 2.41 a ± 0.11 |
| HL | 280.1 a ± 10.2 | 172.5 b ± 8.5 | 0.88 b ± 0.05 | 1.04 a ± 0.07 | 45.2 a ± 1.6 | 22.7 b ± 1.8 | 1.80 a ± 0.05 | 1.45 a ± 0.08 |
| ER | 232.6 a ± 5.5 | 169.7 b ± 8.5 | 0.96 a ± 0.05 | 0.96 a ± 0.08 | 59.5 a ± 3.8 | 24.2 b ± 2.0 | 2.81 a ± 0.08 | 1.27 b ± 0.05 |
| EG | 141.2 b ± 7.5 | 275.1 a ± 11.0 | 0.76 b ± 0.04 | 0.94 a ± 0.08 | 54.0 a ± 3.0 | 32.7 b ± 2.2 | 2.93 a ± 0.10 | 1.13 b ± 0.04 |
| SP | 218.0 a ± 10.8 | 215.1 a ± 8.9 | 1.41 a ± 0.09 | 1.03 b ± 0.07 | 41.3 a ± 2.5 | 19.9 b ± 1.9 | 2.77 a ± 0.09 | 1.04 b ± 0.05 |
| Soil * Types | DEH (mg TPF kg−1 24 h−1) | CAT (mg H2O2 kg−1 h−1) | PER (mM PPG kg−1 h−1) | |||
|---|---|---|---|---|---|---|
| Tillage Systems | ||||||
| RT * | CT | RT | CT | RT | CT | |
| EF * | 3.42 a ± 0.009 | 2.36 b ± 0.008 | 1.76 a ± 0.002 | 1.52 b ± 0.009 | 2.08 a ± 0.002 | 1.86 b ± 0.004 |
| MF | 10.85 a ± 0.021 | 11.28 a ± 0.01 | 1.93 a ± 0.009 | 1.89 a ± 0.011 | 2.20 a ± 0.002 | 1.92 b ± 0.002 |
| HC | 6.54 a ± 0.042 | 6.12 a ± 0.053 | 1.48 a ± 0.005 | 0.96 b ± 0.005 | 2.11 a ± 0.005 | 1.81 b ± 0.002 |
| HL | 7.23 a ± 0.056 | 4.87 b ± 0.006 | 0.89 a ± 0.003 | 0.81 a ± 0.004 | 2.16 a ± 0.003 | 1.72 b ± 0.003 |
| ER | 9.73 a ± 0.012 | 8.09 b ± 0.072 | 1.06 a ± 0.009 | 0.86 a ± 0.002 | 1.95 a ± 0.001 | 1.62 b ± 0.002 |
| EG | 2.63 b ± 0.005 | 7.23 a ± 0.068 | 1.72 a ± 0.009 | 1.69 a ± 0.0011 | 1.98 a ± 0.002 | 1.94 a ± 0.004 |
| SP | 2.05 b ± 0.004 | 6.32 a ± 0.009 | 0.72 a ± 0.004 | 0.69 a ± 0.008 | 2.01 a ± 0.004 | 1.75 b ± 0.001 |
| Soil * Types | AlP (mM pNP kg−1h−1) | AcP (mM pNP kg−1h−1) | BG (mM pNP kg−1h−1) | PRO (mg TYR kg−1h−1) | ||||
|---|---|---|---|---|---|---|---|---|
| Tillage Systems | ||||||||
| RT * | CT | RT | CT | RT | CT | RT | CT | |
| EF * | 1.05 a ± 0.002 | 1.31 a ± 0.001 | 3.40 a ± 0.014 | 1.93 b ± 0.085 | 2.80 a ± 0.014 | 2.30 b ± 0.021 | 38.26 a ± 0.081 | 22.79 b ± 0.065 |
| MF | 3.90 a ± 0.008 | 2.14 b ± 0.005 | 4.65 a ± 0.055 | 2.21 b ± 0.091 | 2.99 b ± 0.021 | 3.37 a ± 0.008 | 18.06 a ± 0.058 | 19.87 a ± 0.041 |
| HC | 1.10 a ± 0.001 | 1.12 a ± 0.031 | 5.18 a ± 0.061 | 2.26 b ± 0.047 | 2.55 a ± 0.009 | 1.57 b ± 0.009 | 15.04 b ± 0.035 | 19.96 a ± 0.022 |
| HL | 2.14 a ± 0.002 | 0.58 b ± 0.008 | 3.07 a ± 0.034 | 3.50 a ± 0.009 | 1.94 a ± 0.008 | 1.87 a ± 0.006 | 17.42 b ± 0.009 | 20.88 a ± 0.012 |
| ER | 1.70 a ± 0.001 | 1.17 b ± 0.012 | 1.85 b ± 0.019 | 3.58 a ± 0.014 | 2.24 a ± 0.012 | 1.98 b ± 0.011 | 18.64 b ± 0.017 | 20.76 a ± 0.061 |
| EG | 1.23 b ± 0.001 | 2.15 a ± 0.038 | 3.36 b ± 0.022 | 5.91 a ± 0.052 | 2.84 a ± 0.008 | 2.50 b ± 0.025 | 17.51 b ± 0.012 | 18.35 a ± 0.016 |
| SP | 1.55 a ± 0.002 | 1.42 a ± 0.018 | 3.81 a ± 0.020 | 3.17 a ± 0.017 | 1.58 b ± 0.006 | 2.23 a ± 0.019 | 13.57 b ± 0.008 | 15.79 a ± 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemanowicz, J.; Balontayová, E.; Dębska, B.; Bartkowiak, A.; Wasilewski, P. Tillage System as a Practice Affecting the Quality of Soils and Its Sustainable Management. Sustainability 2025, 17, 2867. https://doi.org/10.3390/su17072867
Lemanowicz J, Balontayová E, Dębska B, Bartkowiak A, Wasilewski P. Tillage System as a Practice Affecting the Quality of Soils and Its Sustainable Management. Sustainability. 2025; 17(7):2867. https://doi.org/10.3390/su17072867
Chicago/Turabian StyleLemanowicz, Joanna, Erika Balontayová, Bożena Dębska, Agata Bartkowiak, and Piotr Wasilewski. 2025. "Tillage System as a Practice Affecting the Quality of Soils and Its Sustainable Management" Sustainability 17, no. 7: 2867. https://doi.org/10.3390/su17072867
APA StyleLemanowicz, J., Balontayová, E., Dębska, B., Bartkowiak, A., & Wasilewski, P. (2025). Tillage System as a Practice Affecting the Quality of Soils and Its Sustainable Management. Sustainability, 17(7), 2867. https://doi.org/10.3390/su17072867







