Abstract
Charcoal rot, a destructive disease of soybean roots, has limited control options, and the development of resistant cultivars is the most effective, economical, and sustainable strategy. However, tropical cultivars are generally susceptible, while exotic resistant accessions are poorly adapted to tropical conditions. This study represents the initial step toward developing tropical soybean cultivars combining resistance and agronomic performance. We evaluated the agronomic performance of progenies derived from crosses between exotic lines partially resistant to Macrophomina phaseolina (PI 594302 and PI 567562A) and three commercial tropical cultivars with high yield potential (relative maturity groups (RMGs) 5.5, 6.2, and 6.9). Populations were advanced to the F6 generation and assessed for yield, plant height, first pod insertion, and agronomic value using a partial diallel design. Grain yield ranged from 18.5 to 33.2 g·plant−1 across generations. The combining ability analysis revealed predominance of additive genetic effects, highlighting PI 594302 and VMAX RR (RMG 6.2) as key parents for plant height, agronomic value, and grain yield and exhibited the greatest potential for superior progeny across generations. These results identify promising lines with high agronomic performance and adaptation, which will undergo resistance confirmation in subsequent studies, supporting the long-term goal of developing tropical soybean cultivars that combine productivity, sustainability, and tolerance to charcoal rot.
1. Introduction
Soybean (Glycine max (L.) Merr.) is among the most important and widely cultivated crops worldwide and Brazil is currently the largest soybean producer worldwide, with approximately 47 million hectares cultivated in the 2024/2025 growing season, yielding around 169 million tons and an average productivity of 3561 kg/ha [1]. In this context, soybean diseases, such as charcoal rot caused by the fungus M. phaseolina (Tassi) Goid., pose a significant challenge to the country, which has extensive areas under soybean cultivation.
Charcoal rot, caused by Macrophomina phaseolina, is a soil-borne disease of global relevance that affects more than 500 plant species, including soybean, maize, sorghum, cotton, and sunflower [2]. The pathogen survives for extended periods in the soil and crop residues as microsclerotia, which serve as the primary source of inoculum. Infection occurs mainly under high temperatures (30–35 °C) and low soil moisture, conditions that favor fungal colonization of the root and stem tissues, leading to vascular obstruction, premature senescence, and grain filling reduction. In soybean, the typical symptoms include root and lower stem discoloration, microsclerotia formation within vascular tissues, and the characteristic charcoal-like appearance of the stem cortex. These physiological damages result in decreased water uptake and accelerated leaf drop, substantially reducing grain yield under drought-prone tropical environments.
Between 2010 and 2014, soybean diseases in the United States and Canada caused annual losses ranging from 10.06 to 13.92 million tons, representing 11.7% to 14.2% of total production [3]. The main causes of these losses were cyst nematode caused by Heterodera glycines Ichinohe, seedling diseases caused by Rhizoctonia spp., Pythium spp., and Phomopsis spp., charcoal rot caused by M. phaseolina, and sudden death syndrome caused by Fusarium virguliforme O’Donnell & T. Aoki. An increase in disease pressure was observed over time, leading to greater yield losses.
Resistance to M. phaseolina has been a major focus of research due to the considerable economic losses caused by this disease. Several studies have addressed key aspects of genetic improvement. Recent studies have provided important insights into the genetic mechanisms underlying resistance, including the identification of quantitative trait loci (QTLs) and molecular markers applicable to marker-assisted selection (MAS) [4,5]. Soybean genotypes have been evaluated for resistance to charcoal rot and agronomic performance, enabling the identification of promising lines for breeding programs [6], while resistant germplasms have been characterized through seed inoculation, highlighting the importance of efficient screening techniques [7].
Furthermore, studies involving ancestral germplasm have expanded the genetic base available for breeding programs [8]. Genetic diversity analyses have demonstrated the feasibility of combining resistance to charcoal rot with high yield [9]. The integration of these approaches demonstrates significant progress in the understanding and development of genetic resistance in soybean. Each study provides unique insights that, when combined, offer a comprehensive view of the breeding strategies and management of resistance to M. phaseolina in soybean.
Breeding soybean cultivars that combine high yield potential with resistance to Macrophomina spp. remains a major challenge. Although resistance to the pathogen is essential to reduce yield losses and production risks, it is not sufficient on its own, as resistant genotypes often lack the agronomic performance and adaptation required for tropical environments. Many exotic sources of resistance provide valuable alleles against the disease but tend to produce progenies with poor yield, undesirable plant architecture, or late maturity when introgressed directly into elite backgrounds. Thus, the main difficulty lies in balancing resistance with traits such as grain yield, pod number, seed size, and adaptation to local production systems. Achieving this equilibrium requires careful selection strategies, recurrent breeding cycles, and the integration of advanced tools such as genomic selection and phenotyping platforms, ensuring that resistant lines also meet the productivity and quality standards demanded by farmers and the market.
By developing soybean varieties naturally resistant to diseases such as charcoal rot, farmers can minimize the use of chemical treatments, which not only reduces production costs but also mitigates the environmental impact of agricultural practices. This aligns with the growing demand for sustainable agricultural practices and provides a pathway toward healthier ecosystems, reducing the environmental damage of crop production while maintaining high yields and economic viability.
The integration of genetic, biochemical, and agronomic knowledge is essential for the development of cultivars resistant to charcoal rot and other soybean diseases. Thus, this study aims to evaluate the introgression of exotic soybean genotypes resistant to M. phaseolina and to characterize their genetic control in commercial tropical soybean populations. The study results may assist in the development of soybean cultivars resistant to charcoal rot, significantly contributing to sustainability by reducing reliance on chemical pesticides and herbicides.
2. Materials and Methods
2.1. Plant Material
The parents used in this study included two exotic genotypes, PI 594302 (relative maturity group (RMG: 0) and PI 567562A (RMG: 3), both partially resistant to charcoal rot [10]. These genotypes are not fully adapted to tropical conditions and belong to the crossing group exotic resistance (ER). Three Brazilian cultivars (BRS 282, VMAX RR, and BMX APOLO RR), which possess agronomic traits of interest but are susceptible to this disease, belong to the crossing group adapted tropical (AT). The accessions PI 594302 and PI 567562A, originating from Japan and China, respectively, were provided by the Embrapa Soybean Germplasm Bank through a Scientific Cooperation Agreement between institutions.
The conventional cultivar BRS 282 has an average height of 100 cm, a medium cycle of 130 to 142 days, a determinate growth habit, white flowers, gray pubescence, belongs to the RMG 6.9, and is resistant to stem canker and the root-knot nematodes Meloidogyne javanica and Meloidogyne incognita.
The VMAX RR, a transgenic cultivar with glyphosate tolerance, has an approximate height of 110 cm, a medium cycle of 126 days, an indeterminate growth habit, white flowers, gray pubescence, belongs to RMG 6.2, and is resistant to lodging, stem canker, frog eye leaf spot and the cyst nematodes Meloidogyne javanica and Meloidogyne incognita.
The BMX APOLO RR, a transgenic cultivar with glyphosate tolerance, has a medium height, a medium cycle of 126 days, an indeterminate growth habit, white flowers, gray pubescence, RMG 5.5, and is resistant to lodging, stem canker, and bacterial pustule.
The parental line cultivars were used as checks for the genotypes evaluated in this study. Crosses were carried out using a partial diallel design, with crossing group ER consisting of exotic genotypes with partial resistance, and crossing group AT composed of Brazilian cultivars.
2.2. Experimental Design
The populations obtained from the crosses advanced from the F1 to the F2 generation in a greenhouse at the Department of Agricultural Production Sciences, Plant Production Sector, Faculty of Agricultural and Veterinary Sciences, São Paulo State University (UNESP/FCAV), located in Jaboticabal, São Paulo, Brazil. In the F2:3, F2:4, and F2:5 generations, these populations were conducted using the modified pedigree method and evaluated in the experimental area of the Teaching, Research, and Extension Farm during the 2019/20, 2020/21, and 2021/22, respectively. The experimental area is located in Jaboticabal (21°15′22″ S, 48°18′58″ W; 605 m altitude) on a Typic Hapludox (clayey Oxisol) with pH 6.2, organic matter 28 g·dm−3, and base saturation of 58%. Mean annual rainfall is 1420 mm, and the average temperature is 23.4 °C. During the three seasons, rainfall distribution and temperature remained within normal ranges for the region. Crop management followed standard soybean production practices, including pest and weed control, recommended fertilization (2-25-25 NPK at 300 kg·ha−1), and inoculation with Bradyrhizobium japonicum.
Field experiments were conducted using Federer’s augmented block design [10]. In the 2019/20 growing season, 128 progenies were evaluated. Each experimental plot consisted of a single 3 m row per genotype, with 0.5 m spacing between rows and a planting density of 15 seeds per meter. In 2020/21, 363 progenies were evaluated, and in 2021/22, 307 progenies were assessed. For both seasons, each experimental plot consisted of a single 5 m row per genotype, with 0.5 m row spacing and a planting density of 15 seeds per meter. Fertilization, crop management, cultural practices, and phytosanitary control were carried out according to soybean production recommendations [11].
The agronomic characteristics evaluated were grain yield per plant (GY, in grams/plant), agronomic value (AV), which was assigned based on a visual rating scale ranging from 1 to 5 where 1 corresponds to plants with poor agronomic traits (low vigor, short or high height, lodging, thin stem or poor pod set) and 5 corresponds to plants with excellent agronomic traits (high vigor, upright architecture, and uniform pod distribution), height of first pod insertion (HPI, in centimeters) and plant height at maturity (PHM, in cm). In the 2019/20 season, 10 individual plants per plot were evaluated; in the 2020/21 and 2021/22 seasons, five plants per plot were analyzed.
At this stage, progeny selection was based exclusively on agronomic performance, as none of the populations had yet undergone resistance screening. The aim was to discard poorly adapted or low-yielding genotypes before proceeding to disease resistance validation. This sequential approach ensures that only progenies combining adequate agronomic standards are advanced to resistance testing, maximizing efficiency in subsequent evaluation phases.
2.3. Statistical Analyses
Each generation was conducted in distinct cropping seasons (2019/20, 2020/21, and 2021/22) under field conditions. To account for potential environmental variation among years, analyses of variance were performed separately by generation, and homogeneity of residual variances was tested using Levene’s test prior to combined interpretation. This procedure allowed environmental effects to be controlled statistically and ensured that observed differences were primarily genetic. The normality of the residuals was verified using the Shapiro–Wilk test, and the homogeneity of variances was assessed using Levene’s test. Subsequently, individual analyses of variance (ANOVA) were performed for each trait in each year/generation. The coefficient of variation was also estimated. Diallel analysis for each year/generation was conducted using the model proposed by Griffing [12]. All statistical analyses were performed using the R program [13].
3. Results
3.1. Diallel Analysis
At the early stages of breeding programs, the evaluation of agronomic performance is a prerequisite before resistance traits are prioritized. Populations and lines that do not meet minimum standards of yield potential, plant architecture, and adaptation are discarded, regardless of their resistance alleles. Only populations or lines with confirmed agronomic competitiveness are advanced for resistance validation, thereby ensuring that selected progenies combine productivity, stability, and tolerance. This strategy enhances the likelihood of developing cultivars capable of withstanding M. phaseolina infection while maintaining profitability and adoption in tropical production systems. Consistent with this approach, diallel analysis across generations and traits revealed significant differences at 1% F Test of both additive and non-additive gene actions for all traits. Nevertheless, General Combining Ability (GCA) effects were consistently higher than Specific Combining Ability (SCA) effects, indicating the predominance of additive genetic factors in the expression of key traits (Figure 1). This trend was evident for all traits in F2:3 and F2:4, suggesting that additive effects play a central role in guiding selection for improved performance under tropical conditions.
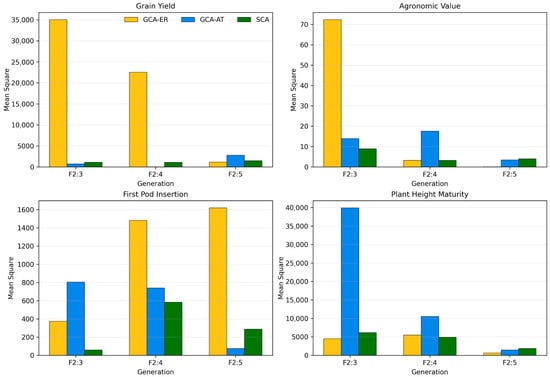
Figure 1.
Mean squares for general combining ability of exotic resistant donor parents (GCA-ER), general combining ability of adapted tropical lines (GCA-AT), and specific combining ability (SCA) across F2:3, F2:4, and F2:5 generations for grain yield (GY), agronomic value (AV, based on a visual rating scale ranging from 1 (plants with poor agronomic traits) to 5 (plants with excellent agronomic traits)), first pod insertion height (HPI), and plant height at maturity (PHM). Differences were significant at p < 0.01 according to the F-test.
The diallel analysis revealed clear differences in the relative importance of additive and non-additive genetic effects across generations and traits (Figure 1). For GY, the contribution of exotic resistant donor parents (GCA-ER) was predominant in F2:3 and F2:4, suggesting strong additive effects from exotic germplasm. However, in F2:5, the importance of GCA-ER decreased substantially, while the contributions of adapted tropical lines (GCA-AT) and SCA increased.
For AV, while GCA-ER effects were relevant in F2:3, GCA-AT and SCA dominated in F2:4 and F2:5, highlighting the increasing role of non-additive interactions in shaping this trait (Figure 1).
In HPI, GCA-AT was the main contributor in F2:3, suggesting that adapted tropical lines drive early trait expression (Figure 1). In contrast, GCA-ER showed higher influence in F2:4 and F2:5, emphasizing the potential of exotic donors to broaden genetic variability for this trait across generations.
For PHM in F2:3, GCA-AT was highly significant, reflecting an additive effect (Figure 1). Although mean squares decreased in later generations, SCA remained relevant in F2:5, suggesting that height at maturity is strongly modulated by specific parental interactions rather than purely additive effects.
Overall, the results demonstrate that additive effects play a key role in the early stages of selection, especially for grain yield and HPI, while non-additive effects become increasingly important for agronomic value and plant height in advanced generations.
3.2. Genotypes/General Combining Ability (GCA)
The GCA effects revealed significant variability among exotic resistant donor parents and adapted tropical lines across generations (F2:3, F2:4, and F2:5) for GY, AV, HPI, and PHM (Figure 2).
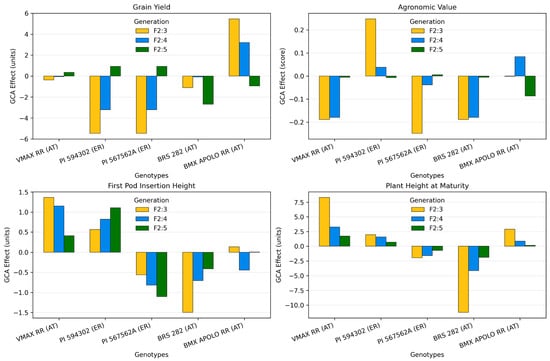
Figure 2.
GCA effects for grain yield, agronomic values, first pod insertion and plant height at maturity across generations (F2:3, F2:4, and F2:5) for soybean populations obtained by crossing among adapted tropical (AT) and exotic resistance (ER) parents. Differences were significant at p < 0.01 according to the F-test.
For GY, VMAX RR (AT) consistently exhibited positive GCA effects across generations, confirming its role as a donor of favorable alleles for yield (Figure 2). PI 567562A (ER) and BRS 282 (AT) also contributed positively in F2:5, while BMX APOLO RR (AT) and PI 594302 (ER) presented negative effects, with the latter showing strong unfavorable contributions in early generations.
Regarding AV, VMAX RR (AT) demonstrated consistently positive GCA effects across all generations (Figure 2). PI 594302 (ER) showed positive contributions in F2:3 and F2:4, while BMX APOLO RR (AT) was inconsistent among generations. Conversely, PI 567562A (ER) and BRS 282 (AT) consistently displayed negative values in F2:3 and F2:4, reinforcing their unfavorable influence on agronomic value.
For HPI, VMAX RR (AT) and PI 594302 (ER) demonstrated consistently positive GCA effects across all generations, highlighting their potential to increase pod insertion height (Figure 2). In contrast, PI 567562A (ER) and BRS 282 (AT) showed negative contributions in all generations, whereas BMX APOLO RR (AT) fluctuated between positive, neutral and negative effects.
In PHM, VMAX RR (AT) and PI 594302 (ER) demonstrated consistently positive GCA effects across all generations, highlighting their potential to increase plant height (Figure 2). PI 567562A (ER) exhibited strong negative GCA effects across generations, suggesting its potential for reducing plant height and mitigating lodging risk. On the other hand, PI 594302 (ER), and BMX APOLO RR (AT) contributed positively in all generations, while BRS 282 (AT) showed positive contributions in all generations.
VMAX RR emerged as a promising adapted tropical line, consistently demonstrating a positive impact on GCA for grain yield and agronomic value. Additionally, VMAX RR had a stable and positive effect on GCA for plant height at maturity, reinforcing its potential for use in breeding programs aimed at improving soybean productivity. It also contributed to higher GCA for pod insertion, which is advantageous for minimizing harvest losses, although this advantage can be reduced in taller plants.
PI 594302 shows a more pronounced impact on GCA for grain yield in the F2:3 and F2:4 generations, suggesting its potential for long-term improvement through continuous selection and breeding strategies. Its GCA for agronomic value was positive but moderate. This genotype positively influenced the GCA for height of the first pod insertion and GCA for plant height at maturity.
PI 567562A showed inconsistent variation in grain yield, although there was some evidence of a gradual increase in F2:5. The agronomic value remained consistently low. This genotype consistently showed low height for the first pod insertion, which may reduce harvest losses but might require further improvement to reach the ideal range of 10 to 15 cm. PI 567562A showed a negative impact on plant height at maturity across generations, indicating its limited usefulness for increasing height in later growth stages.
BMX APOLO RR shows generally low GCA for grain yield, particularly in F2:5, suggesting that it may not be suitable for increasing production when compared with VMAX RR. Its impact on GCA for agronomic value is minimal, with variable effects across generations. It had a variable effect on the GCA for the height of the first pod insertion and a decreasing trend in GCA for plant height at maturity.
BRS 282 shows GCA for grain yield fluctuates between negative and positive values, although it shows some potential in F2:5, demonstrating some potential for improving grain yield in later generations. The GCA for agronomic value is negative, indicating a limited contribution to the overall improvement of agronomic traits. The GCA for the height of the first pod insertion for BRS 282 is low, which could be advantageous for harvesting. GCA for plant height at maturity shows substantially negative values, suggesting that it may not be ideal for increasing plant height at this stage.
3.3. Populations/Specific Combining Ability (SCA)
The PI 594302 × VMAX RR population stands out for its consistent SCA values for grain yield, with positive and increasing estimates across generations, suggesting strong potential for yield improvement (Figure 3). SCA for AV remained stable, with a slight trend of improvement across generations, indicating a moderate enhancement of agronomic traits. The SCA for first pod insertion height is moderately positive in the later generations (F2:4, F2:5), suggesting stability in traits related to harvest efficiency. For plant height at maturity, SCA values are negative in the first two generations (F2:3, F2:4), but show improvement in F2:5, although still indicating a trend toward reduced plant stature. The PI 567562 A × VMAX RR was opposite of PI 594302 × VMAX RR with negative estimates across generations showing a worse performance.
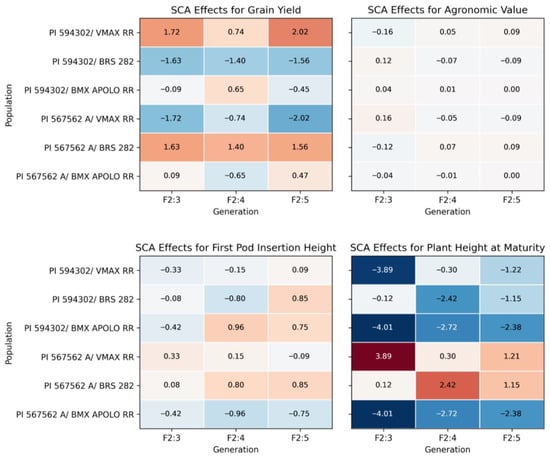
Figure 3.
Specific combining ability (SCA) effects for grain yield, agronomic values, first pod insertion and plant height at maturity across generations (F2:3, F2:4, and F2:5) for soybean populations.
The PI 567562 A × BRS 282 population demonstrates strong potential for improving productivity, with consistent and positive effects on grain yield across the three analyzed generations, indicating a favorable path for yield enhancement (Figure 3). The SCA for agronomic value (AV) is stable, with a slight trend of improvement over generations, suggesting moderate enhancement of agronomic traits. Regarding the first pod insertion height, the SCA is positive in the later generations, especially in F2:4 and F2:5, indicating good potential for reducing harvest losses. Plant height at maturity shows a moderately positive contribution, suggesting that this population may increase in stature over generations. On the other hand, the PI 594302 × BRS 282 population exhibits consistently low and negative SCA values for grain yield, agronomic value and plant height, suggesting that it is not a suitable option for improving productivity.
The PI 567562 A × BMX APOLO RR population shows variable performance across generations (Figure 3). For grain yield, the SCA was negative in F2:4 and positive in F2:5, suggesting a slight improvement in yield in subsequent generations (Figure 3). Agronomic value (AV) remained stable and negative across generations, suggesting low potential for the improvement of agronomic traits. The SCA for first pod insertion height is negatively impacted in the later generations, which may lead to harvest losses and highlights the need to improve this trait. For plant height at maturity, the SCA is consistently negative across generations, suggesting limited potential for increasing plant height. The PI 594302 × BMX APOLO RR population shows relatively stable performance for grain yield but with worse performance than PI 567562 A × BMX APOLO RR.
These populations exhibit varied characteristics, with some showing a trend toward improved grain yield, while others face challenges in enhancing productivity. The PI 594302 × VMAX RR, PI 594302 × BRS 282 and PI 567562 A × BMX APOLO RR populations demonstrate greater consistency in agronomic traits, whereas the other ones show more unstable performance and face challenges in terms of grain yield improvement.
Across the F2:3, F2:4, and F2:5 generations, grain yield exhibited significant variability among the crosses (Figure 4). The highest values were consistently observed in the PI 594302 × VMAX RR and PI 567562A × VMAX RR populations, surpassing 30 g plant−1 in F2:3 and F2:4. However, a reduction was detected in F2:5, suggesting the influence of segregating loci and potential non-additive genetic effects. In contrast, the cross PI 567562A × BRS 282 showed the lowest yield values in F2:3, reinforcing the existence of contrasting combining abilities among parental lines but with good performance in F2:5.
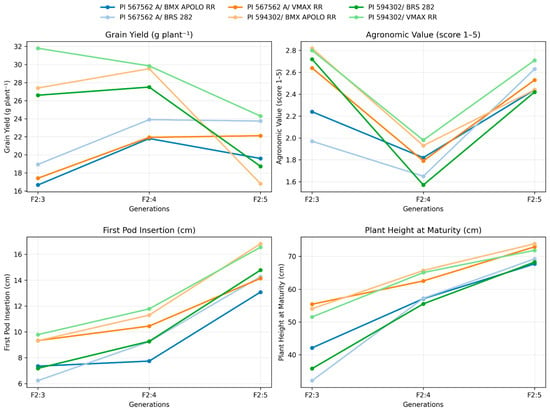
Figure 4.
Grain yield means, agronomic values, first pod insertion and plant height at maturity across generations (F2:3, F2:4, and F2:5) for soybean populations.
Agronomic value scores declined markedly in F2:4 across all populations, followed by a recovery in F2:5 (Figure 4). This trend indicates environmental influence and selection pressure during intermediate generations. PI 594302 × VMAX RR and PI 567562A × BRS 282 again displayed superior performance, highlighting their potential as donors of desirable agronomic traits in advanced breeding cycles.
The first pod insertion height increased progressively from F2:3 to F2:5 (Figure 4). Crosses involving PI 594302, particularly with VMAX RR and BMX APOLO RR, reached the highest values in F2:5 (>15 cm). This result is favorable for mechanized harvesting, since higher pod insertion reduces grain losses. Conversely, PI 567562A × BMX APOLO RR presented the lowest insertion heights, suggesting limited suitability for environments requiring mechanical harvest.
Plant height at maturity demonstrated a consistent upward trajectory across generations (Figure 4). PI 594302 × BMX APOLO RR, PI 567562A × VMAX RR and PI 594302 × VMAX RR showed the greatest increases, reaching nearly 70 cm in F2:5, while crosses with BRS 282 had intermediate heights. The linear trend suggests additive genetic control, reinforced by the stability across environments and generations.
4. Discussion
The introgression of exotic germplasm into elite tropical cultivars represents a central challenge in soybean breeding, achieving equilibrium between introducing resistance alleles from non-adapted sources and maintaining high agronomic performance in tropical environments [14]. This study addressed this challenge at its initial stage, assessing the agronomic performance and combining ability of segregating generations derived from crosses between exotic partially resistant parents and elite tropical cultivars. The results provide a framework for understanding how additive and non-additive gene actions influence the success of early selection when exotic germplasm is incorporated into adapted backgrounds.
The diallel analysis revealed a predominance of additive genetic effects across generations, demonstrating that traits such as grain yield, agronomic value, and plant height are largely predictable from parental performance [15]. This finding reinforces that additive variance, which responds efficiently to selection, is a key component for achieving simultaneous progress in productivity and adaptation [16]. The exotic group (ER), particularly PI 594302, contributed favorable alleles for yield and architecture in early generations, while the adapted tropical group (AT) provided environmental stability and adaptation [17]. The consistent superiority of crosses involving PI 594302 × VMAX RR exemplifies this complementarity, confirming that favorable additive effects from resistant exotic donors can be expressed without compromising agronomic potential.
For plant architecture traits such as first pod insertion and plant height, heritability was higher than for yield components, with greater contribution from the adapted parents (GCA-AT) [14]. However, the positive shift in GCA-ER observed in some generations suggests that exotic donors can enhance specific traits when recombination is effective [15]. This generational shift reflects the dynamic process of balancing genetic variability and selection pressure: as selection advances, genetic variance decreases, and stable recombinants emerge that integrate both productivity and adaptation. Positive specific combining ability (SCA) effects, particularly in PI 594302 × VMAX RR, indicate complementary gene interactions that can generate transgressive segregants—individuals surpassing their parents in agronomic performance due to novel allele combinations. These results emphasize the relevance of recombination and continued selection to capture and fix favorable allele combinations before genetic variance narrows in advanced generations.
Although resistance to M. phaseolina was not directly evaluated in this stage, both PI 594302 and PI 567562A are well-documented sources of partial resistance based on previous greenhouse and QTL studies [15,18,19]. The superior agronomic performance of their progenies therefore likely reflects successful introgression of alleles potentially linked to resistance mechanisms, such as lignin deposition and antioxidative defense pathways. This finding is crucial because, in many breeding programs, introducing exotic resistance sources often leads to a loss of agronomic adaptation. Here, however, the data indicate that resistance-associated alleles can be introgressed without yield penalties, a significant advance for tropical soybean improvement.
Moreover, to ensure sustainable agriculture and prolong the effectiveness of resistance to charcoal rot in production systems in tropical regions, integrated management practices are essential. Crop rotation under no-till systems has been shown to reduce the incidence of root rots, including M. phaseolina, while also increasing soybean grain yield [20]. Studies with biocontrol agents, such as Trichoderma koningiopsis, have demonstrated lower severity of charcoal rot in soybean under both greenhouse and field conditions, promoting superior plant growth compared to the infected control [21]. The integration of these cultural and biological practices with resistant cultivars enables more resilient production systems, less dependent on fungicides, with reduced environmental impact and lower production costs.
Overall, the results highlight that integrating exotic and adapted germplasm can effectively broaden the genetic base of tropical soybean while maintaining competitive agronomic performance. The predominance of additive effects supports the use of recurrent selection and careful parental choice to achieve cumulative genetic gain. The identification of promising combinations such as PI 594302 × VMAX RR provides a strong foundation for developing tropical cultivars that integrate alleles from resistant exotic donors with the high yield and adaptation of elite local backgrounds. Thus, this study establishes the agronomic groundwork for subsequent resistance screening and long-term selection aimed at generating cultivars that combine genetic resilience with superior agronomic efficiency under tropical conditions.
From a sustainability perspective, the complementary roles of exotic and adapted germplasm become evident. Exotic donors, particularly PI 594302, expand the genetic base, introduce novel alleles potentially linked to M. phaseolina resistance, and increase genetic variability for key agronomic traits. In contrast, adapted tropical cultivars such as VMAX RR provide the stability and phenotypic consistency required for local environments. The results of this study demonstrate that these parental groups can be successfully combined, confirming the agronomic feasibility of integrating resistance sources into elite tropical backgrounds without yield penalties. The confirmation of resistance will be conducted in subsequent stages, as part of a long-term strategy to develop high-performing tropical cultivars with improved resilience.
The diallel analysis revealed that additive effects predominated for most traits, particularly grain yield, agronomic value, and plant height, indicating that these traits are largely predictable from parental performance and amenable to recurrent selection. This genetic structure suggests that continuous selection and recombination can efficiently accumulate favorable alleles in advanced lines. The exotic group (ER) contributed to increasing genetic variability and enhancing yield potential, while the tropical group (AT) conferred adaptability and stability. The consistent superiority of the PI 594302 × VMAX RR cross highlights this complementarity and indicates that favorable additive loci from exotic donors can be expressed in tropical backgrounds without disrupting overall adaptation as suggested by the predominance of general combining ability observed in other studies of soybean breeding under stress-prone environments [22,23].
For plant architecture traits, such as first pod insertion and plant height, the combination of additive and specific combining ability effects suggests the presence of complementary gene interactions between exotic and adapted parents. This interaction pattern is particularly relevant in the context of transgressive segregation, in which novel allele combinations produce progenies that surpass parental performance for yield and architecture. Such effects are essential for maintaining selection progress in later generations, where non-additive variance can still be exploited to identify superior recombinants. The observed improvement in first pod insertion, coupled with moderate plant height in populations such as PI 594302 × VMAX RR and PI 594302 × BMX APOLO RR, enhances harvest efficiency and mechanical adaptability, key sustainability traits for large-scale soybean production. This finding aligns with reports that exotic × adapted crosses can introduce genetic variability for plant architecture, which can be exploited in breeding programs targeting sustainability through reduced harvest losses [24,25].
Although resistance to M. phaseolina was not directly assessed in this stage, the exotic parents used (PI 594302 and PI 567562A) are established sources of partial resistance according to previous genetic and QTL studies. Therefore, the superior agronomic performance of progenies derived from these crosses provides indirect evidence of successful introgression of resistance-associated alleles. This outcome is notable because exotic germplasm often introduces linkage drag or late maturity, yet the populations in this study maintained acceptable yield and plant architecture, demonstrating that resistance introgression can occur without compromising agronomic quality. Agronomic value constitutes a strategic tool for the development of sustainable agriculture, as it enables the identification of genotypes exhibiting integrated traits such as vigor, appropriate plant architecture, consistent development, health, and resistance to biotic and abiotic stresses. Consequently, these genotypes use available resources more efficiently, show reduced vulnerability to diseases and adverse environmental conditions, and maintain stable productivity even under conditions typical of tropical regions [26].
The evaluation of multiple generations (F2:3, F2:4, and F2:5) also provided valuable insight into the dynamics of selection and genetic stability. The decrease in variability across generations, accompanied by consistent performance in selected populations, confirms the effectiveness of early-generation selection for additive traits. However, since these generations were evaluated in different years, environmental variation likely contributed to part of the observed differences. As commonly recognized in soybean breeding programs, advancing inbred generations across successive seasons is unavoidable, but interpretations of generational trends should remain cautious until multi-year replicated data are available. Similar results have been reported in soybean breeding programs that use exotic germplasm, where intermediate generations often express instability before stabilization occurs in advanced lines [27].
Taken together, these findings reinforce that the strategic combination of exotic and adapted germplasm can broaden the genetic base and enhance sustainability-related traits without sacrificing productivity. The predominance of additive effects supports the use of recurrent selection and well-planned parental choice to promote cumulative genetic gain. The identification of stable and high-yielding combinations such as PI 594302 × VMAX RR provides a solid foundation for future selection and subsequent validation of charcoal rot resistance.
This study therefore represents an essential first step toward developing tropical soybean cultivars that combine disease tolerance, superior agronomic performance, and sustainability within modern breeding frameworks. The present study focused solely on agronomic performance, with resistance to M. phaseolina yet to be phenotypically validated in subsequent experiments. Additionally, as the experiments were conducted over three seasons without replicated multi-environment trials, residual environmental variability may have influenced certain trait expressions. Future studies will integrate resistance screening under controlled inoculation and multi-site evaluation to confirm genetic stability and resilience.
5. Conclusions
The incorporation of exotic germplasm effectively broadened the genetic base of tropical soybean and introduced allelic diversity potentially linked to resistance against M. phaseolina, while maintaining agronomic adaptation. The exotic donors PI 594302 and PI 567562A, previously identified as partially resistant to charcoal rot, significantly improved key agronomic traits when crossed with Brazilian cultivars, confirming their suitability for genetic introgression under tropical conditions. The consistent superiority of PI 594302 × VMAX RR across generations underscores the feasibility of combining exotic resistance sources with elite tropical backgrounds without compromising yield potential. Additive genetic effects predominated, indicating that recurrent selection and precise parental choice are effective strategies for advancing these populations. The improvement in first pod insertion contributes to mechanization efficiency and aligns with sustainability goals by reducing harvest losses and production costs. Overall, this study represents an initial stage focused on agronomic evaluation, establishing a solid foundation for subsequent validation of charcoal rot resistance and the development of high-yielding, resilient tropical soybean cultivars.
Author Contributions
Conceptualization, H.K.d.S. and G.V.M.; methodology, H.K.d.S., G.V.M. and S.H.U.-T.; validation, G.V.M. and S.H.U.-T.; formal analysis, G.V.M. and S.H.U.-T.; investigation, H.K.d.S., A.C.R.M., D.S.C., J.d.S.S., A.P.S. and T.P.G.; resources, G.V.M. and S.H.U.-T.; data curation, H.K.d.S., G.V.M. and S.H.U.-T.; writing—original draft preparation, H.K.d.S., G.V.M. and S.H.U.-T.; writing—review and editing, H.K.d.S., G.V.M. and S.H.U.-T.; supervision, S.H.U.-T.; project administration, S.H.U.-T.; funding acquisition, G.V.M. and S.H.U.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES)—Financial Code 001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data is included in the article.
Acknowledgments
The authors acknowledge CAPES and the Department of Agricultural Production Sciences, Plant Production Sector, Faculty of Agricultural and Veterinary Sciences, São Paulo State University (UNESP/FCAV), Jaboticabal, for their support in carrying out this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Companhia Nacional de Abastecimento—CONAB. Safra Brasileira de Grãos. Brasília: CONAB. Available online: https://www.conab.gov.br/info-agro/safras/graos (accessed on 9 August 2025).
- Marquez, N.; Giachero, M.L.; Declerck, S.; Ducasse, D.A. Macrophomina phaseolina: General Characteristics of Pathogenicity and Methods of Control. Front. Plant Sci. 2021, 12, 634397. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Allen, T.W.; Bradley, C.A.; Sisson, A.J.; Byamukama, E.; Chilvers, M.I.; Coker, C.M.; Collins, A.A.; Damicone, J.P.; Dorrance, A.E.; Dufault, N.S.; et al. Soybean Yield Loss Estimates Due to Diseases in the United States and Ontario, Canada, from 2010 to 2014. Plant Health Prog. 2017, 18, 19–27. [Google Scholar] [CrossRef]
- da Silva, M.P.; Klepadlo, M.; Gbur, E.E.; Pereira, A.; Mason, R.E.; Rupe, J.C.; Bluhm, B.H.; Wood, L.; Mozzoni, L.A.; Chen, P. QTL Mapping of Charcoal Rot Resistance in PI 567562A Soybean Accession. Crop. Sci. 2019, 59, 474–479. [Google Scholar] [CrossRef]
- Coser, S.M.; Reddy, R.V.C.; Zhang, J.; Mueller, D.S.; Mengistu, A.; Wise, K.A.; Allen, T.W.; Singh, A.; Singh, A.K. Genetic Architecture of Charcoal Rot (Macrophomina phaseolina) Resistance in Soybean Revealed Using a Diverse Panel. Front. Plant Sci. 2017, 8, 1626. [Google Scholar] [CrossRef] [PubMed]
- Magar, S.G.; Jadhav, P.V.; Vaidya, E.R.; Moharil, M.P.; Ghawade, R.S.; Shinde, U.D.; Sakhare, S.B.; Ghorade, R.B.; Mane, S.S. Assessment of soybean genotypes for charcoal rot disease resistance and agronomic performance. Int. J. Adv. Biochem. Res. 2024, 8, 309–313. [Google Scholar] [CrossRef]
- Ishikawa, M.S.; Ribeiro, N.R.; de Almeida, A.A.; Balbi-Peña, M.I. Identification of Soybean Genotypes Resistant to Charcoal Rot by Seed Inoculation with Macrophomina phaseolina. J. Agric. Sci. 2019, 11, 213–219. [Google Scholar] [CrossRef]
- Pawlowski, M.L.; Hill, C.B.; Hartman, G.L. Resistance to Charcoal Rot Identified in Ancestral Soybean Germplasm. Crop. Sci. 2015, 55, 1230–1235. [Google Scholar] [CrossRef]
- Amrate, P.K.; Shrivastava, M.K.; Bhale, M.S.; Agrawal, N.; Kumawat, G.; Shivakumar, M.; Nataraj, V. Identification and genetic diversity analysis of high-yielding charcoal rot resistant soybean genotypes. Sci. Rep. 2023, 13, 8905. [Google Scholar] [CrossRef] [PubMed]
- Federer, W.T. Augmented (or hoonuiaku) designs. Hawaii Plant Rec. 1956, 55, 191–208. Available online: https://ecommons.cornell.edu/server/api/core/bitstreams/7e626471-05ad-4f2b-858e-be100a513b5e/content (accessed on 20 January 2024).
- Martin, T.N.; Rugeri, A.P.; Beutler, A.N.; Conceição, G.M.; Fipke, G.M.; Pires, J.L.F.; Galon, L.; Cunha, V.S. Indicações Técnicas para a Cultura da Soja no Rio Grande do Sul e Em Santa Catarina, Safras 2022/2023 e 2023/2024, 1st ed.; GR: Santa Maria, Brazil, 2022; p. 136. [Google Scholar]
- Griffing, B. Concept of General and Specific Combining Ability in Relation to Diallel Crossing Systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- R Version 4.0.2. “Taking Off Again”. The R Foundation for Statistical Computing. Platform: x86_64-w64-mingw32/x64 (64-bit). 2020. Available online: https://www.r-project.org/ (accessed on 15 May 2024).
- Dallastra, A. Predição Fenotípica no Melhoramento da Soja Para Diferentes Regiões do Brasil. Thesis, Universidade Federal do Tocantins, Gurupi, Brazil, 2022. Available online: http://hdl.handle.net/11612/5586 (accessed on 1 August 2025).
- Mengistu, A.; Read, Q.D.; Little, C.R.; Kelly, H.M.; Henry, P.M.; Bellaloui, N. Severity of charcoal rot disease in soybean genotypes inoculated with Macrophomina phaseolina isolates differs among growth environments. Plant Dis. 2025, 109. [Google Scholar] [CrossRef]
- Noor, A.; Little, C.R. RNA-seq analysis reveals genes associated with defense responses to Macrophomina phaseolina in soybean. BMC Genom. 2024, 25, 1129. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.F. Métodos de Inoculação e Seleção de Cultivares de Soja para Tolerância a Macrophomina phaseolina (Tassi) Goid. Universidade Federal de Viçosa. 2019. Available online: https://locus.ufv.br/items/448b1193-532e-4c4a-ae31-14437d651d63 (accessed on 1 August 2025).
- Vennampally, N.; Kumar, S.; Kumawat, G.; Shivakumar, M.; Rajput, L.S.; Ratnaparkhe, M.B.; Ramteke, R.; Gupta, S.; Satpute, G.K.; Rajesh, V.; et al. Charcoal Rot Resistance in Soybean: Current Understanding and Future Perspectives. In Disease Resistance in Crop Plants; Wani, S.H., Ed.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Ortiz, V.; Chang, H.-X.; Sang, H.; Jacobs, J.; Malvick, D.K.; Baird, R.; Mathew, F.M.; de Jensen, C.E.; Wise, K.A.; Mosquera, G.M.; et al. Population genomic analysis reveals geographic structure and climatic diversification for Macrophomina phaseolina isolated from soybean and dry bean across the United States, Puerto Rico, and Colombia. Front. Genet. 2023, 14, 1103969. [Google Scholar] [CrossRef] [PubMed]
- Reis, E.M.; Segalin, M.; Moraes, N.L.; Ghissi, V.C. Efeitos da rotação de culturas na incidência de podridões radiciais e na produtividade da soja. Summa Phytopathol. 2014, 40, 9–15. [Google Scholar] [CrossRef]
- Bleckwedel, J.M.; Martinez, M.J.; Claps, M.P.; Lisi, V.; González, V.; Ploper, L.P.; Reznikov, S. Biological control of soybean charcoal rot by native Trichoderma koningiopsis in Tucumán, Argentina. Biol. Control 2024, 196, 105581. [Google Scholar] [CrossRef]
- Santos, M.S.; Hamawaki, O.T.; Gomes, B.H.; Filho, J.G.; de Oliveira, M.B.; da Silva, A.C.; de Faria, M.V.; Nogueira, A.P.O. Combining ability of soybean in F1 and F2 generations for earliness and grain yield. Rev. Caatinga 2025, 38, e12487. [Google Scholar] [CrossRef]
- Tkachuk, C.T. Soybean Pod Height: Influence of Genetics, Environment and Management. Manitoba Pulse & Soybean Growers. (Heritability of Pod Height, Genetics vs. Environment). 2019. Available online: https://manitobapulse.ca/ (accessed on 10 August 2025).
- Jiang, H.; Li, Y.; Qin, H.; Li, Y.; Qi, H.; Li, C.; Wang, N.; Li, R.; Zhao, Y.; Huang, S.; et al. Identification of Major QTLs Associated With First Pod Height and Candidate Gene Mining in Soybean. Front. Plant Sci. 2018, 9, 1280. [Google Scholar] [CrossRef] [PubMed]
- Soares, S.L.; Simon, G.A.; Alvares, R.C.; Silva, F.H.d.L.e. Combining performance and estimated genetic diversity among soybean parents and F1 populations. Rev. Ceres 2023, 70, 81–90. [Google Scholar] [CrossRef]
- Ebone, L.A.; Caverzan, A.; Tagliari, A.; Chiomento, J.L.T.; Silveira, D.C.; Chavarria, G. Soybean Seed Vigor: Uniformity and Growth as Key Factors to Improve Yield. Agronomy 2020, 10, 545. [Google Scholar] [CrossRef]
- Tabdeen, I.; Lamptey, S.; Karikari, B. Soybean (Glycine max (L.) Merrill) germplasm characterization on plant architecture and yield traits for potential mechanical harvest. Discov. Plants 2025, 2, 211. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).