Abstract
The sustainable management of water bodies with hydropower plants (HPPs) and protected rheophilic fish species is challenging. The key question is whether impounded rivers can still provide habitat for protected rheophilic fish species, including Natura 2000 species. We investigated hydro-morphological conditions and fish communities, focusing on the bottom-dwelling Danube longbarbel gudgeon (Romanogobio uranoscopus) and the medium-distance migrating cactus roach (Rutilus virgo) in the Brežice HPP system on the Sava River in Slovenia. Fish sampling using an electric bottom trawl in the HPP impoundment, electrofishing in the nearshore, and video surveillance in the fish pass revealed a diverse and distinctive fish community. This community reflected rheophilic conditions in the upper impoundment and fish pass, and lentic conditions in the lower impoundment. These findings provide evidence that impounded rivers, when complemented by well-designed mitigation measures, can sustain rheophilic fish species, including the Danube longbarbel gudgeon and cactus roach. Maintaining rheophilic habitat within the impoundment, combined with a functioning river-like side channel, is crucial. However, at Brežice HPP, changes in the management of the fish pass water inflow are necessary to ensure adequate and consistent hydraulic conditions and water temperatures. Applying a knowledge co-creation approach, which requires productive interaction among scientists, managers and policy makers, could help to find the best solutions for sustainable water ecosystem management.
1. Introduction
Hydropower is the world’s largest source of climate-friendly energy source and will play an important role in the future energy system as the need to integrate environmental and socio-economic aspects of sustainability increases [1]. Rivers, including several large European rivers, have long been used as energy sources. In Europe, the first river engineering works began centuries ago and most major European large rivers are now dammed [2]. By damming a river, the head is available to generate power which is a source of energy production in hydropower plants (HPP) worldwide [3]. Dams contribute to energy supply, but also cause various pressures on the river ecosystem, including fish habitats. The construction of dams alters the hydro-morphological and physico-chemical characteristics of rivers, including connectivity, hydrological regime, sediment transport, morphology, water temperature regime, and dissolved gases, significantly impacting fish habitats [4]. The change from lotic to lentic conditions following dam construction leads to a change in fish habitats [5,6], though the effects are dependent on HPP size and type. For example, the effects of a run-of-river HPP with little or no storage differ from the effects of typical large storage HPP that use a dam to store water in an impoundment [7].
Freshwater habitats cover only about 0.8% of the Earth’s surface but harbour a disproportionately large diversity of species. One third of all described vertebrates, including ∼50% of fish species, are found in freshwater habitats [8,9]. In Europe, the Natura 2000 (N2K) network, based on the Birds Directive (79/409/EEC, 2009/147/EC) and the Habitats Directive (92/43/EEC) [10,11], is one of the world’s largest protected area systems targeting species and habitat types, including freshwater fish, with the aim of conserving biodiversity. In Slovenia, the N2K network covers 37.9% of the national territory [12]. Although most N2K sites in Slovenia are forests (almost 70%), they also include some large rivers with HPPs [13,14]. In the lowlands, river impoundment results in large areas of water that are often important secondary habitats that still harbour protected species [14]. The key question is whether impounded rivers can also provide a habitat for rheophilic fish species, including N2K species such as the Danube longbarbel gudgeon (Romanogobio uranoscopus) and the cactus roach (Rutilus virgo). There are 97 freshwater fish species in Slovenia, including 75 native species [15,16], of which 24 species are listed in Annex II and eight in Annex V of the Habitat Directive as N2K species, including the Danube longbarbel gudgeon and the cactus roach [11]. Both species have similar ecological requirements; they are rheophilic with a strong preference for running waters, release their eggs on or into gravel or stony substrate, feed predominantly on macroinvertebrates, and move predominantly over medium distances [17]. Both species are also found in large rivers [18]. In the Slovenian Danube River catchment, however, the cactus roach occurs in main river channels with moderate flow, but migrates to tributaries during the spawning season, laying eggs not only on or into gravel or stony substrate, but also on plants [19]. It has been reported that the cactus roach can migrate up to 150 km [20]. The Danube longbarbel gudgeon is a typical inhabitant of shallow, stony river sections in the submontane zone, found in sites with a fast current of 0.7 to 1.15 m/s, where the bottom is covered with medium-sized stones [21]. However, the cactus roach, can also be found in slow-flowing rivers, e.g., with a current of 0.1 m/s [22], or even in lakes [20].
The construction of an HPP, particularly through river impoundment, significantly alters natural river flows and fish. These impacts include the disruption of fish migration routes and the alteration of natural fish habitats, affecting fish populations and diversity [4,23], and may run counter to the objectives of the EU Habitats Directive [11]. Adverse effects are often partially remedied by a range of possible mitigation measures to conserve fish species, including sustainable operation of impoundments, habitat compensation in tributaries, artificial rearing and release of fish, and the construction of fish passage facilities [4]. Often, appropriate measures to be implemented depend on the extent of changes caused by the HPP and the fish species present [24,25]. The efficiency of measures also depends on both implementation and management. Fish passage facilities are a potentially effective technical approach to reconnect ecological corridors disrupted by river impoundments and to restore the longitudinal connectivity of rivers [4]. It is the earliest, and most widespread measure for the conservation of migratory fish in impounded rivers [26]. In some dammed rivers, river-like side channels are created to serve as fish passes for migration, and even as additional habitat for fish reproduction [27].
In this study, we analysed fish data collected in the Brežice HPP system in the Sava River in Slovenia with a particular focus on two N2K fish species: Danube longbarbel gudgeon and cactus roach. Fish assemblages were collected in the impoundment and in the river-like side channel that serves as a fish pass, using different sampling approaches and video surveillance. The aim of this study was to investigate: (1) whether the Brežice HPP impoundment still provides habitat for the N2K species Danube longbarbel gudgeon and cactus roach and, if so, (2) under which conditions, and (3) whether the fish passage channel also serves as an additional habitat supporting both N2K fish species, with the identification of required functional improvements, and (4) the method that might help to find the best solutions for sustainable water ecosystem management when the main sustainability challenges are to reconcile fish conservation and water use.
2. Materials and Methods
2.1. Study Area
The Sava River, a right tributary of the Danube River, has a catchment area of around 97,713 km2 (Figure 1). Most of the upstream catchment (10,872 km2) lies in Slovenia, where several HPPs and a nuclear power plant (NPP) have been built. The Brežice HPP is the fifth and most downstream HPP in the cascade in Slovenia, with an impoundment built in 2019 (elevation 154 m, average annual water discharge 207 m3/s, installed capacity 47.4 MW) (Table 1). It is a run-of-river HPP with three installed vertical units—Kaplan turbines with a total nominal flow of 500 m3/s, five spillways, and an average annual production of 161 GWh. The impoundment covers an area of 3.17 km2 and has a storage capacity of 19.3 million m3. The width of the impoundment ranges from 100 to over 700 m. Its depth changes gradually in the downstream direction from 2 m in the upper section to a maximum of 18 m near the dam. Part of the Brežice HPP facility is also a fish pass, which was built as a combination of a river-like side channel (approx. 700 m long and 3 to 5 m wide) located in the lower part, and a short fish ladder (approx. 100 m long and 2 m wide) with a vertical slot in the upper part of the fish pass facility. The discharge through the fish pass facility varies seasonally and ranges from 0.5–0.8 m3/s.
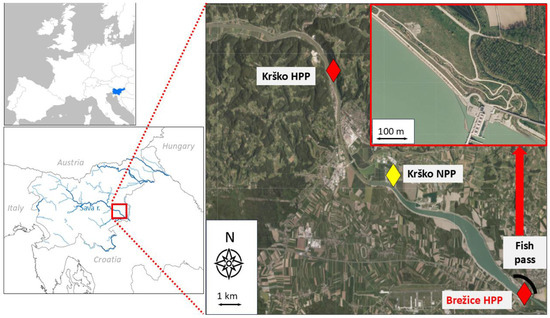
Figure 1.
Study area with the impoundment of the Brežice HPP on the Sava River in Slovenia positioned between Krško HPP and Brežice HPP with location of the Krško nuclear power plant (NPP) and the fish pass facility.

Table 1.
Main characteristics of the Brežice HPP system.
2.2. Environmental Data
Discharge and water temperature data downstream of Brežice HPP in 2022 and 2023 were determined using official data from the gauging stations Sava-Čatež and Krka-Podbočje [28]. Generally, lower discharge and higher temperature values (except minimum value) were observed in 2022. The mean annual discharge was approximately 153 m3/s in 2022 and 307 m3/s in 2023, with maximum values of 1087 m3/s and 2938 m3/s, and minimum values of 37 m3/s and 65 m3/s, respectively (Figure 2). The mean annual water temperature in 2022 and 2023 was 15.1 °C and 13.6 °C, respectively, with a maximum of 27.4 °C and 24.4 °C, and a minimum of 5.5 °C and 5.9 °C, respectively (Figure 3).
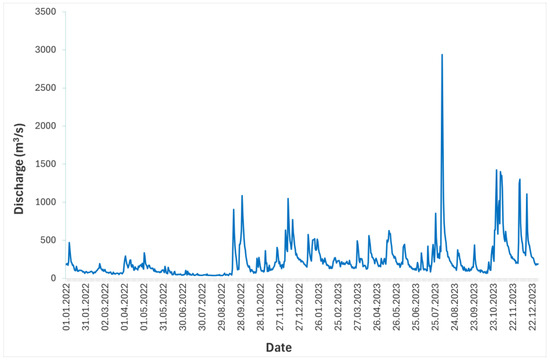
Figure 2.
Flow hydrograph of the Sava River at the Brežice HPP in 2022 and 2023.
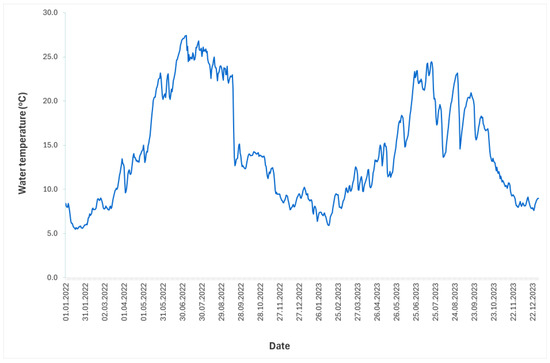
Figure 3.
Water temperature dynamics of the Sava River at the Brežice HPP in 2022 and 2023.
Water velocity and depth profiles were measured at two sections of the impoundment, in the upper section (impoundment kilometre 0.3; width 99 m and maximum depth 3 m), and in the lower section (impoundment kilometre 7; width 154 m and depth to 10 m). The weir at Krško NPP indicating the boundary between the upper and lower impoundment is located at impoundment kilometre 5.6. The water velocity and depth profiles were measured and collected using an acoustic method. Data were collected with an acoustic Doppler 3D measuring device River Surveyor (Sontec). Measurements were taken at low water level in March 2022. Depth profile data at other sections of the impoundment where fish were sampled were obtained as part of the regular bathymetric measurements of the impoundment using a NORBIT iWBMS multibeam echosounder in 2021 [29]. The size of the cross-sectional areas based on the measured depth profiles and the mean daily discharge values at Brežice HPP were used to calculate the mean flow velocity in the sampled fish sections on the day of sampling.
Water velocity and depth profiles were also measured at three sections of the river-like side channel. The data was obtained using the Son-Tec-RS5 acoustic Doppler current profiler. The measurements were carried out during normal operation in May 2023. In addition, the temperature of the surface water in the Brežice Impoundment was measured with a WTW multimeter on the day of fish sampling (29–30 June 2022). The water temperature of the fish pass was measured as the surface water temperature in the lower impoundment, as the fish pass receives surface water inflow. Nevertheless, water level and temperature were also measured in the fish pass facility 4 m downstream of the water inflow from the impoundment in the period June–July 2023. Water level and temperature were recorded every 5 min using the HOBO U-20 water level data logger (HOBO Data Loggers, Bourne, MA, USA).
2.3. Fish Sampling
Fish data were collected using different methods depending on the habitat sampled (e.g., Brežice HPP impoundment bottom, Brežice HPP impoundment nearshore, fish pass) (Table 2). At the bottom of the Brežice HPP impoundment, fish were sampled with an electric bottom trawl (E-KECE) [30]. The principle of the sampling device corresponds to that developed for large rivers [31], which combines the construction of conventional trawls and frame sledge nets. The E-KECE bottom trawl consists of a metal frame to which a 4 m long net with 0.5 cm mesh is attached. The metal frame is triangular with an opening of 0.8 m2, with a 1.6 m length of the opening along the floor and 1.0 m height of the opening. The frame was electrified with an 11.5 kW Vanguard generator (Briggs & Stratton, Milwaukee, WI, USA). During bottom trawling, direct current (approx. 350 V, 15 A) was used at intervals of 5–8 s with pauses of approx. 5 s to minimise startle and injury to the fish. Bottom trawling was conducted during the day using a 4.5 m aluminium research boat with a 30 kW Mercury engine (Mercury Marine, Fond du Lac, WI, USA). At the start of trawling, the frame was lowered to the bottom of the impoundment, with the boat moving slowly downstream. The length of the trawl haul was measured with a GARMIN GPS device (Garmin Ltd., Kansas City, MO, USA) only after the frame reached the bottom, which was easily felt while holding the centre rope. Each tow was 100 m long. Trawling was conducted during the day and away from shore due to the presence of submerged trees along the banks. Sampling sections were distributed across the entire impoundment. A total of 25 samples, each of 100 metre length, were taken along the 12.6 km long Brežice HPP impoundment (Figure 1). To achieve representative coverage of the entire impoundment, a 100 m long haul was conducted approximately every 500 m (with the exception of the area near the 5.5 km downstream Krško NPP and the area near the downstream dam). Sampling was carried out close to the shore on the bottom of the former main channel of the Sava River. Trawling was carried out over a total length of 2500 m. Sampling was performed on 29 and 30 June 2022.

Table 2.
Used fish sampling approaches according to the fish habitat.
In the nearshore zone, fish were sampled by electrofishing using the Point Abundance Sampling Method (PAST) [32,33,34,35]. However, samples were stratified by different nearshore habitats, so we used a habitat-specific PASE (HS-PASE). First, the shoreline of the impoundment was divided into 100 m long sections. Within each section, a dominant nearshore habitat was mapped. The nearshore habitats were defined on the basis of inorganic (substrate diameter) and/or organic substrate, taking into account the following categories according to [36,37]: megalithal (20–40 cm), meso- and macrolithal (2–20 cm), psammal (sand), pelal (mud), argyllal (silt, loam, clay), macro-algae, submerged macrophytes, emergent macrophytes, living parts of terrestrial plants, and xylal (tree trunks, branches and roots). HS-PASE was conducted on 50 sections representing the following four predominant nearshore habitats: submerged macrophytes, megaliths, meso- and macro-lithal, and xylal. Each selected nearshore habitat was sampled according to its coverage along the impoundment shore but in at least five sections of the impoundment. A total of 50 PASE samples were collected: 35 in the predominant submerged macrophytes, while five samples were collected in each of the other three predominant nearshore habitats. Sampling sites were randomly distributed along the left and right banks of the impoundment and were at least 100 m apart. Fishing was conducted from a boat during the day on 6 and 7 September 2022 using a portable electrofishing device, type ELT 60 GI, 300/550 V (Hans Grassl GmbH, Schönau am Königssee, Germany) with a 40 cm long anode, which provided an effective sampling area of about 1.5 m2 [38]. During electrofishing, the current was maintained for 5 s at each point. The net was swept through the area at each point, so that anaesthetised fish were recorded even when no fish were visible, reducing observer bias, and compensating for reduced water clarity. The electrofisher wore polarising goggles and a shading cap to improve vision and focus. All fish caught were identified to the species level and measured to the nearest millimetre (total length). The captured fish were stored in a large plastic container in the boat while individual fish were processed, and all fish were returned unharmed to the water at the sampled location.
Three approaches were used to obtain fish data from the fish pass. First, fish species were recorded at the time of overhaul of the Krško NPP. Due to the necessary impoundment drawdown, the water discharge through the fish pass was temporally reduced to at least 20 L/s for 45 days. Prior to this, fish were removed from the fish pass on two occasions, on 1 and 8 October 2022, and transported to a nearby location. Second, quantitative electrofishing was carried out in the river-like side channel of the fish pass facility in summer (15 July) 2023. Quantitative electrofishing of 100–127 m2 (width 3–5 m, length at least 20 m) was carried out on seven sections of the near-natural fish pass watercourse. Electrofishing was carried out in accordance with the CEN standard [39]. We used a two-pass method with a backpack electrofishing device (ELT 60 GI, 300/550 V, Hans Grassl GmbH, Schönau am Königssee, Germany). The electrofisher, flanked by an assistant, collected anaesthetised fish in dip nets while zigzagging upstream. The captured fish were identified to the species level, and size (total body length, ±0.1 cm) was measured at the end of electrofishing at a specific section. The fish were then allowed to recover from the stress of handling and were released unharmed back to their capture site. Third, underwater monitoring cameras were used in the fish passage facility. We used two wide-angle cameras (100° in water) in HD resolution 3M, with IR illumination and sensor for automatic regulation (day/night IP cameras, RSTP and ONVIF protocol, PoE power supply). The camera and the whole system are waterproof and corrosion-resistant-inox 304 materials are used. For additional illumination, a waterproof reflector with RGB LED 30W was added. We used the Central Logger Server system on Linux for 24/7 continuous recording of 3 × 3 TB capacity, with establishment of appropriate security protocols, establishment of a “live” viewing mode, visualisation of the review of the recording via the web, statistical analysis, alarm-notification, remote monitoring and management of the system. Motion detector settings to start recording was used and data were privately stored. The cameras were in operation during the main migration period from March to June 2022. Moreover, they were also installed in spring and summer 2023, but data were not readable due to technical issues. The system proved to be highly efficient and reliable, allowing continuous (24/7) and non-invasive monitoring, which is a major advantage. The motion detector was used to start the recording, resulting in over 100,000 recorded events. The digital video recordings were used for species identification, but manual video processing was carried out with a focus on N2K species. A special feature of the system used is the positioning of two cameras opposite one another, allowing for better visibility and more reliable detection of organisms even in turbid conditions. This innovative solution is protected by a patent in Slovenia (SI-PATENT No 24976), the holder of which is one of the co-authors (Vidmar A.). The patent can be found in the database of the Slovenian Intellectual Property Office by entering the patent number.
2.4. Data Analyses
Habitats were defined on the basis of the sampled habitat (impoundment bottom, impoundment nearshore, fish pass) and impoundment section characterised by the abiotic conditions (surface water temperature and average current velocity) (Section 3.1). Based on the longitudinal changes in the measured environmental data, the impoundment was divided into an upper section (imp1) upstream of Krško NPP (length 5.6 km), and a lower section (imp2) downstream of the NPP (length 7.0 km). A total of five habitats were distinguished: imp1 bottom, imp2 bottom, imp1 nearshore, imp2 nearshore, and the fish pass. Prior to the analysis, the absolute abundance data (electrofishing in the fish pass) and the catch per unit effort data (bottom trawl, HS-PASE in the nearshore) were reduced to relative abundance, as different methods were used to sample fish in the different habitats. Relative abundances were expressed as the percentage of individuals of each fish species caught in the selected habitat. In addition, the distributions of fish size classes were compared among the sampled habitats. A non-parametric Kruskal-Wallis test with Dunn’s post-hoc tests were applied to detect significant (p < 0.05) differences in the length distribution of species between sampled habitats; Bonferroni correction (α = 0.05/n, where n is the number of tests) was considered for multiple testing. The fish relative abundance data were analysed using non-metric multidimensional scaling (NMS, based on the Bray-Curtis similarity measure) to examine the relatedness of fish communities between habitats. In NMS, the stress value reflects how well the ordination summarises the observed distances between samples. As the stress values were below 0.2, a solution in two-dimensional space was chosen [40]. The NMS analyses were carried out using the statistical programme PAST 2.5 [41].
3. Results
3.1. Environmental Conditions
Under normal HPP operating conditions, the average flow velocity along the impoundment decreased due to both increasing depth and width of the impoundment downstream (Figure 4). Downstream of the Krško NPP, the average current velocity was below 0.1 m/s, as the water depth was more than 8 m and increased further downstream. The combination of the measured abiotic parameters shows a clear differentiation between the upstream and downstream sections of the impoundment. The surface water temperature measured in the impoundment at end of June 2022 during the fish sampling increased downstream and fluctuated by more than 10 °C between imp1 (22.4 °C) and imp2 (33.4 °C) (Figure 4). However, the measurements of surface water temperatures were carried out on the days when the highest water temperatures were measured in the Sava River downstream of Brežice HPP in 2022 (Figure 3). In the June-July period of 2023, a similar pattern of water temperature distribution was observed with the highest water temperatures at the end of June in the fish pass, influenced by the surface water in imp2 (Figure 5). However, in the fish pass, the temperature fluctuated between 15 °C and 26 °C, which was much lower than measured in June 2022. Lower average and maximum values in 2023 were observed also downstream of Brežice HPP (Figure 3). Some higher values (up to 30 °C) were also recorded, but these were due to the sensors not being submerged as there were blockages in the tributary causing the water level to drop sharply (Figure 6). Inflow blockages caused by macrophytes and other plant debris occurred regularly, leading to significant fluctuations in the water level in the technical part of the fishway with a range of >1.5 m (Figure 6). In order to maintain water flow to the fish pass, blockages had to be cleared regularly. Measurements of the flow velocities in the depth profiles of the river-like side channel showed variability in the average (0.11 to 0.34 m/s) and maximum (0.50–0.83 m/s) flow velocities, at a discharge of 0.58 m3/s. Thus, diverse fish mesohabitats were present within the near-natural side channel, which was taken into account in the fish pass electrofishing.
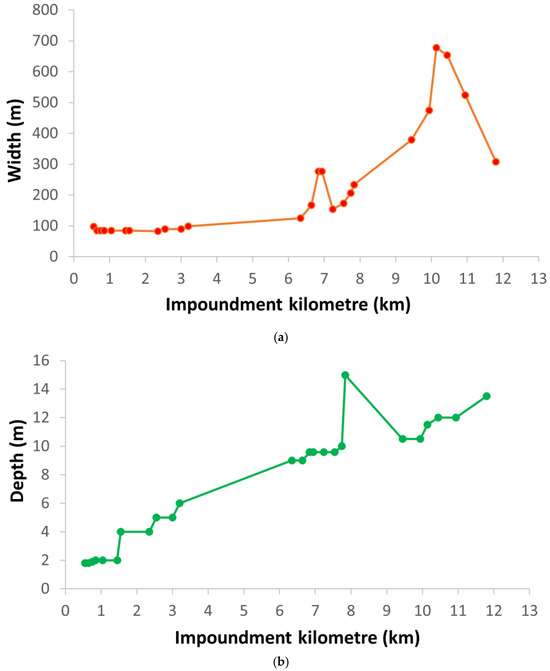
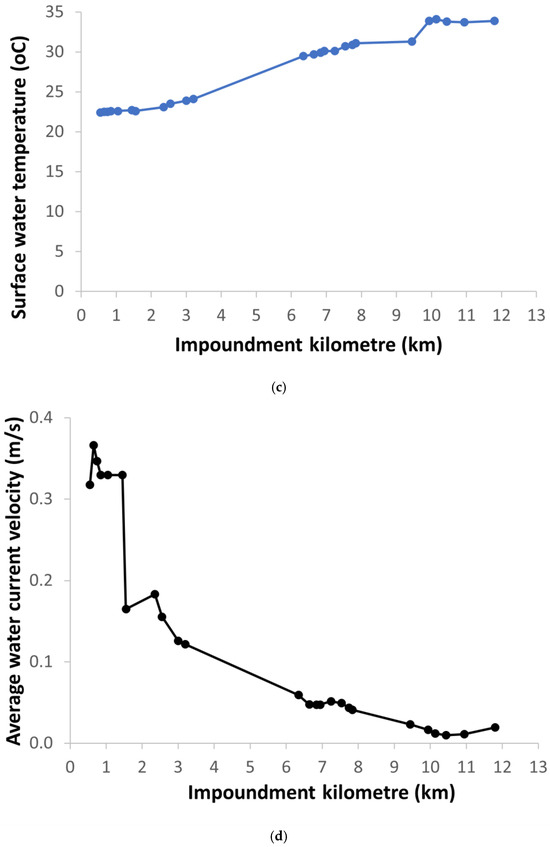
Figure 4.
Longitudinal changes in the (a) width, (b) depth, (c) surface water temperature, and (d) average water current velocity in the Brežice HPP impoundment at the end of June 2022. Krško NPP is located at the impoundment kilometre 5.6.
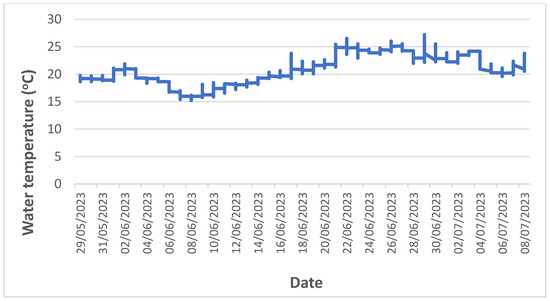
Figure 5.
Water temperature changes in the technical part of the fish pass of Brežice HPP in the period June–July 2023.
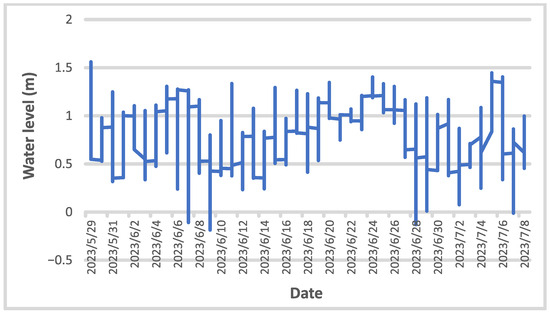
Figure 6.
Water level changes in the technical part of the fish pass of Brežice HPP in the period June-July 2023.
3.2. Fish Composition
A total of 30 fish species were recorded in five sampled habitats (Table 3). Six species were detected in only one habitat; stone loach (Barbatula barbatula), Barje sculpin (Cottus metae), Danube longbarbel gudgeon (Romanogobio uranoscopus) only in imp1 bottom, bream (Abramis brama) only in imp2 bottom, while roach (Rutilus rutilus) and Danube barbel (Barbus balcanicus) were only found in the fish pass, and the latter was recorded by video surveillance. The highest species richness was found in the fish pass with 20 species, followed by both imp1 and imp2 bottom sections with 16 and 15 species, respectively, while the lowest number of species was found using the PASE method in the imp1 and imp2 nearshore sections with 10 and 7 species, respectively. The number of the recorded species might be also related to the used sampling methodology and the fact that fish sampling was only performed during the day, with the exception of video surveillance in the fish pass.

Table 3.
Relative abundance (%) of fish species collected in the Brežice HPP system in the sampled habitats. imp1—upper part of the impoundment, imp2—lower part of the impoundment, x—species recorded in the fish pass using video surveillance only and therefore not enumerated.
Seven of the fish species recorded are N2K species, i.e., listed in the EU Habitat Directive [9]: Balkan loach (Cobitis elongata), Danube loach (Cobitis elongatoides), European bitterling (Rhodeus amarus), Danube longbarbel gudgeon (Romanogobio uranoscopus), Danube whitefin gudgeon (Romanogobio vladykovi), cactus roach (Rutilus virgo) and Balkan spined loach (Sabaneyewia balcanica). Among them, Danube whitefin gudgeon and European bitterling were the only dominant N2K species, dominating the bottom and nearshore areas of both imp1 and imp2 sections, respectively (Figure 7). Danube whitefin gudgeon dominated both imp1 and imp2 bottom sections. Danube longbarbel gudgeon was rare in the sampled habitats and restricted to the imp1 bottom, where shallower water and higher current velocities (≈0.5 m/s) were recorded. Cactus roach was also rare in the sampled habitats and was only sampled at the imp1 bottom and in the fish pass. During the upstream migration through the fish pass, specimens of cactus roach were also recorded by video surveillance, whereas gudgeons were not recorded.
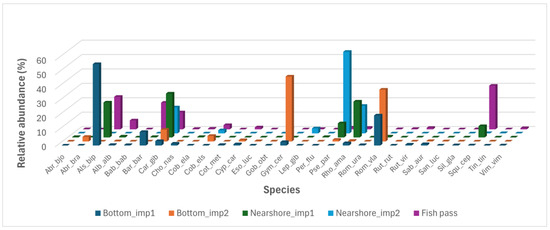
Figure 7.
Relative abundance (%) of fish species by habitat in the Brežice impoundment and fish pass. For species codes see Table 3. imp1—upper part of the impoundment, imp2—lower part of the impoundment.
Three collected species are non-native to the Sava River catchment area. Besides the Prussian carp (Carassius gibelio), there are two invasive alien species of Union concern [42]: stone moroko (Pseudorasbora parva) and pumpkinseed (Lepomis gibbosus). Stone moroko strongly dominated the imp2 nearshore, where Prussian carp was dominant and pumpkinseed played a sub-dominant role (Figure 7). Prussian carp also dominated in the fish pass and in the imp1 nearshore zone; in the latter habitat, the stone moroko was subdominant, but in the fish pass it was just as rare as the pumpkinseed.
The fish species composition varied in the sampled habitats (Figure 7 and Figure 8). Fish populations were most similar (i.e., the shortest distance between the points on the NMS graph), despite the furthest geographical separation, at the imp1 bottom and in the fish pass, where a few rheophilic fish species dominated, e.g., schneider (Alburnoides bipunctatus) and common barbel (Barbus barbus). Bottom fish populations in imp1 and imp2 most differed (highest distance between points on the NMS graph), despite the dominance of the Danube whitefin gudgeon; the main differences were in the absence of typical rheophilic species in imp2, where ruffe species characteristic of eutrophic lakes and lowland rivers dominated. Nearshore fish populations in imp1 and imp2 showed less prominent differences.
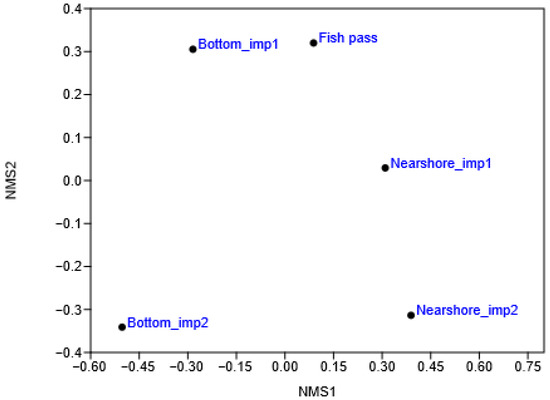
Figure 8.
Nonmetric Multidimensional Scaling ordination diagram of the sampled habitats. imp1—upper part of the impoundment, imp2—lower part of the impoundment.
The size of collected fish varied in the sampled habitats (Kruskal-Wallis test, H = 862.4, p < 0.001). Larger specimens were collected in the fish pass, whereas mostly small specimens were collected at the bottom of impoundment (Figure 9). However, in imp1 (both bottom and nearshore areas), fish were significantly larger (Dunn’s post-hoc test, p < 0.001) than in imp2. No statistically significant differences (p > 0.05) in fish size were found between bottom and nearshore habitats in either imp1 or imp2 sections.
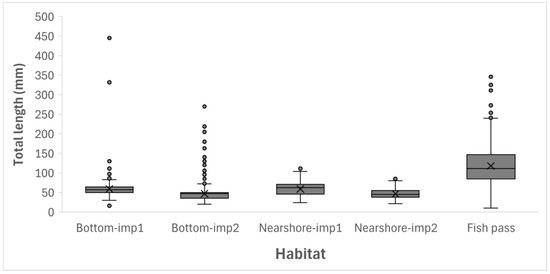
Figure 9.
Fish total length distribution and the results of Kruskal-Wallis nonparametric test for five sampled habitats in the Brežice HPP impoundment and fish pass. The box plots represent median (solid line), interquartile range (box), range of the data (whiskers), and outliers (circles). For a given habitat, box plots with different letters are significantly different (Dunn’s post hoc test, p < 0.05, using the Bonferroni correction). imp1—upper section of the impoundment, imp2—lower section of the impoundment.
4. Discussion
We found that the Brežice HPP system, with a mitigation measure of the river-like side channel, not only affects the physical habitat conditions and fish community, but also supports rheophilic N2K fish species, including several typical rheophilic bottom dwellers such as the Danube longbarbel gudgeon and medium-distance migratory fish species such as the cactus roach. However, significant differences between the upper and lower parts of the impoundment were found not just in the fish assemblage composition, but also in the size of collected fish. Chen et al. [4] reviewed river damming impacts on fish habitat and fish but did not report a significant impact on fish size distribution. We collected larger fish in the cooler imp1 section, with the characteristics of slow flowing medium-deep large river, whereas imp2 with smaller specimens showed more lentic conditions. We did not account, however, for age structure within species, as both Danube longbarbel gudgeon and cactus roach were rarely captured. Many rheophilic species including cactus roach and Danube longbarbel gudgeon were only found in imp1. To our knowledge, no records of the Danube longbarbel gudgeon in the impoundment have previously been reported in the scientific literature. On the other hand, the cactus roach has been reported to reside in very slow-flowing rivers and even lakes [20,21]. The shift from a lotic to a lentic environment following the damming of rivers leads to a decisive change in the aquatic habitats of fish species [5,6]. The situation is even more serious in rivers with cascade dams, where an upstream dam is often located in the backwater zone of the nearest downstream dam [4]. The studied Brežice HPP is the fifth in the cascade of HPPs on the Sava River. We have shown that the extent of changes in the fish community is related to changes in the lotic-lentic conditions. Rheophilic species dominate not only in the fish pass (e.g., chub, schneider), but also in the imp1 section (e.g., schneider, Danube whitefin gudgeon, common barbel) where the lotic character of the river is still present, which was confirmed on the basis of the measured habitat conditions. Our results concur with the observed differences in ecological conditions along the impoundments on the Drava River and other large rivers in Slovenia, and with the conclusions therein that the effects of barriers on aquatic communities and the ecological status of surface water bodies depend on the type of dam, its location, and the type of HPP [43,44]. Thus, the presence of lotic conditions in the upper part of the impoundment (imp1) is a key factor for the two rheophilic species, Danube longbarbel gudgeon and cactus roach, to exist in the Brežice impoundment and/or in the fish pass.
Another important finding is that the dammed river ecosystem of Brežice HPP harbours a diverse fish community, related to the gradient of abiotic conditions, e.g., water temperature, hydrological conditions (water depth, flow velocity), and substrate, all of which are key ecological factors of the fish habitat [17]. Their combination is decisive for the different fish species observed in the habitats studied and the biological diversity of the fish. The 30 recorded fish species represent nearly a third of all freshwater species recorded in Slovenia [15]. They all play an important ecological role, and some are also important from a fisheries management perspective (e.g., barbel, nase), while others are important from a biodiversity conservation perspective. Seven of the fish species recorded are N2K species as defined by the Habitats Directive [11] and are part of the N2K network, the main pillar of EU biodiversity conservation policy [45]. However, not all recorded N2K species have the same ecological preferences [18], which was reflected in their distribution and relative abundance within the Brežice HPP system. European bitterling and whitefin gudgeon were among the dominant fish species, favouring vegetation-rich nearshore areas and fine sediment bottom areas along the impoundments, respectively. In contrast, most other N2K species were rare, including the cactus roach and Danube longbarbel gudgeon. During an ichthyological survey conducted in the free-flowing section of the Sava River between Vrhovo HPP and Krško NPP (≈32 km) prior to the construction of Brežice HPP and other HPPs in the area, relatively few individuals of the Danube longbarbel gudgeon were collected, representing less than 0.5% of the total fish community sample [46]. Those authors recorded a very low proportion of Danube longbarbel gudgeon (<0.5%) in what is now the imp1 area, with only five individuals caught. The conditions at that time were also affected by the discharge of water pollution in this area, which greatly altered the fish community [45]. However, the first but further downstream dam in this area was already built in the 1980s, when Krško NPP was constructed on the Sava River, and could also affect the fish community, including the Danube longbarbel gudgeon. Prior to the construction of Krško NPP in the period 1978–1980, several fish surveys were conducted and only two individuals of Danube longbarbel gudgeon were collected in the Sava River in the area of today’s Brežice HPP, which corresponds to an equally small proportion (<0.5%) of the fish caught [47]. This could indicate that the Danube longbarbel gudgeon was a rare species in the considered section of the Sava River even prior to the construction of the barriers, or that fish sampling in large rivers is difficult. Zajicek & Wolter [48] found that rare and other species in large rivers are underestimated by electrofishing and multi-mesh gillnets. They suggested that complementary sampling of the mid-channel of large rivers and additional gear such as trawling is needed for the assessment of the Habitats Directive, migratory and rare species, as well as for the establishment of a complete species inventory, e.g., for biodiversity assessment. In the present study, we also used bottom trawls and collected several rare species (e.g., Danube longbarbel gudgeon) or species that could not be recorded in the past with other fishing methods e.g., particularly bottom-dwelling species (e.g., Barbatula barbatula, Cottus metae, Sabaneyewia balcanica) such as gudgeons and the migratory cactus roach. During the spawning season from April to May, the latter species migrates to tributaries and river arms between dense aquatic vegetation and/or to gravel beds to spawn [18]. In this study, the migration of the cactus roach in the fish pass was documented using video surveillance. Our results concur with the different habitat conditions in medium to large rivers where the cactus roach has been observed [18,20,22,31], as it was recorded in the upper section of the impoundment with moderate flow conditions and medium-deep water, but also in the river-lake side channel, where water current velocities were high (>0.8 m/s), and depth low (<1 m). Nevertheless, in both habitats, densities were relatively low, and the cactus roach was rare. Szaloky et al. [31] compared sampling with electrofishing and bottom trawling in the Danube River in Hungary, and found that cactus roach was rare with a similar relative abundances in both habitats (<0.5%). In the present study, cactus roach was not collected in the nearshore habitat of the impoundment, but we used PASE sampling method that mainly targeted juveniles during the day. Talabishka et al. [22] reported that in summer, sampling of cactus roach in the evening or at night was more efficient when juveniles (aged 0+, 1+, 2+) were observed to be actively feeding in shallow waters. Therefore, daytime sampling might underestimate fish density. Nevertheless, in the future, new methods, e.g., quantitative environmental DNA (eDNA), may allow for adequate stock assessment of fish species in large rivers and impoundments, where fish sampling remains a major challenge [49]. For the deep Brežice HPP impoundment with high fish species diversity including bottom-dwelling, rare, migratory and N2K fish species, quantitative eDNA analysis is a good step forward in the development of the appropriate fish monitoring in future research to provide evidence on the fish species composition. The eDNA approach might support the currently inadequate monitoring programmes, but need to be tailored for Brežice HPP and other impoundments in the cascade of the Sava River, and possibly for other impoundments with similar conditions. The eDNA approach was recently used to study the effect of cascade dams on freshwater ecosystems [50].
This study has shown that river-like side channels not only serve as a fish pass for migration, but also as an additional habitat that also supports rheophilic fish species. Using video surveillance in the upstream technical part of the fish pass, we documented the migration of several fish species, including N2K species such as cactus roach, which were caught in the side channel. However, the high summer water temperatures due to the inflow of the epilimnetic impounded water and the temporarily unstable hydrodynamic conditions due to the controlled water inflow in the fish pass, may not provide sufficient support for all non-migratory rheophilic fish species typical of the river section that use it as a secondary habitat. The Danube longbarbel gudgeon was not recorded in the side channel, even though the hydrodynamic conditions appear suitable. This channel may represent an important rheophilic habitat within the Brežice HPP system, which has been reduced due to the damming of the Sava River. However, regular water drawdowns due to overhaul of the Krško NPP approximately every 18 months, but usually outside of the main migratory season, result in minimal water flow (≈30 L/s) through the fish pass and unstable hydrodynamic conditions. Such conditions could be a challenge for non-migratory fish species that might use a fish pass facility as additional habitat. Danube longbarbel gudgeon is such a candidate but was not recorded in the fish pass. It is a typical inhabitant of shallow, stony rivers in the submontane or grayling and barbel zone, preferring riverbed sections with a flow velocity of between 0.70 and 1.15 m/s [20,21]. However, summer water conditions in the fish pass with high water temperatures and regular water lowering are unfavourable for the Danube longbarbel gudgeon and other fish species with similar habitat preferences. It is crucial for these species that suitable hydraulic conditions prevail in the fish pass during operation. The inlet to the fish pass is designed in such a way that a uniform water level must be maintained along the entire inlet section up to the control gates at the outlet to the technical part of the fish pass. This needs to correspond to the current water level in the impoundment, which is the only way to ensure the same energy level at the inflow to the fish pass, when the control gates are closed/opened accordingly, and the same flow conditions, regardless of the water level in the impoundment of Brežice HPP. Any manipulation of the regulating gates outside the prescribed operating conditions causes disturbances in the flow and pulsations, affecting turbulence of the water flow and causing a higher flow velocity of the water, which can exceed the swimming ability of individual species of aquatic organisms. As such, these local unpredictable conditions affect the functionality of the entire fish pass.
Coexistence or coordinated management of protected areas, e.g., N2K sites based on the Habitats Directive [11] that harbour rheophilic fish species, and HPPs have so far seemed almost impossible, as most HPPs with their impoundments alter the hydro-morphological and physico-chemical characteristics of rivers, significantly impacting fish habitats [4]. This study shows that even within HPP systems, lotic habitats can be maintained and rheophilic species can be abundant. On the other hand, we also showed that mitigation measures in the form of a river-like side channel, which serves not only as a fish pass for migration but also as additional habitat for rheophilic fish species, are not sufficient and that special attention must be paid to the functionality and operation of the fish pass by ensuring adequate hydraulic and water temperature conditions at all times. This is important for migratory fish species in the time of migration, as well as for non-migratory fish species that might dwell there year round.
Although fish sampling was conducted only during the summer and during daytime, the use of various sampling methods, including 24/7 video surveillance in the fish pass during the main migration period, contributed to a more comprehensive understanding of the fish community composition in the Brežice HPP system. Sampling in different seasons and employing additional methods (particularly eDNA) would enhance knowledge of seasonal variability in fish community composition and the abundance of both key N2K species, the Danube longbarbel gudgeon, and particularly the migratory cactus roach. The recorded spring migration of the latter in the fish pass provides evidence of migration activity within the Brežice HPP system. Thus, our findings demonstrate that impounded rivers, when complemented by well-designed mitigation measures, can sustain rheophilic fish species, including the Danube longbarbel gudgeon and the cactus roach. Maintaining rheophilic habitat within the impoundment, combined with a functioning river-like side channel, is crucial. However, at Brežice HPP, changes in the management of fish pass water inflow are necessary to ensure adequate hydraulic conditions, including during the overhaul of Krško NPP when impoundment drawdown is required. A new technical solution for the fish pass inlet should be developed to prevent any temporary reduction in water flow through the fish pass. In summer, when high surface water temperatures occur in the lower part of the Brežice HPP impoundment, the new inlet solution should also allow inflow from deeper layers where the water temperature does not exceed 25 °C. Brežice HPP is just one of several HPPs in the cascade on the Sava River, with special conditions due to the presence of Krško NPP. Further research on other HPPs should be conducted to confirm our findings, to provide evidence that impounded rivers, when complemented by well-designed mitigation measures, can sustain rheophilic fish species. The presence of different target rheophilic fish species may require species-specific measures, as is evident in our study, where several rheophilic species are present within the Brežice HPP system.
The sustainable development of human society and biodiversity are interlinked. The balance between renewable energy and the protection of biodiversity is a necessity for the benefit of humanity and for achieving the goals of the European Green Deal, which outlines the objectives for sustainable development and zero pollution by 2050 [51]. In its strategic documents, Slovenia has stated that by 2050 it will be a climate-neutral and climate-resilient society based on sustainable development [52]. In order to utilise the considerable potential of hydropower for increasing climate-friendly energy security, while reducing dependence on electricity from fossil fuels, hydropower must overcome a number of obstacles, including those related to environmental challenges for fish [53]. The main sustainability challenges are to reconcile fish conservation and water use, including hydropower generation, taking into account nature conservation and stakeholder requirements [24,50]. Hydropower is often in conflict with the nature conservation requirements listed under the N2K programme. Therefore, a holistic approach must be taken when planning HPPs to find the best practises for fish [53].
We recognise that there will always be conflicting views on hydropower and the conservation of aquatic ecosystems. Nevertheless, in order to identify best practices for fish conservation, hydropower and nature conservation need to overcome certain obstacles related to the management and conservation of aquatic ecosystems. Some authors have identified the lack of coordination between authorities as one of the main obstacles to sustainable management of natural resources [54]. We agree that greater interaction between policy sectors is needed, but also support the idea of applying a knowledge co-creation model to natural resource management that requires productive interaction among different scientists, managers and policy makers [55]. This could facilitate in finding the best solutions for those who realise that biodiversity is crucial for human well-being and that we are facing a biodiversity crisis, and for those who argue that hydropower is a climate-friendly energy and we need it to secure our future with increasing demands.
5. Conclusions
Hydro-morphological conditions and fish communities focusing on the bottom-dwelling Danube longbarbel gudgeon (Romanogobio uranoscopus) and the medium-distance migrating cactus roach (Rutilus virgo) were investigated in the Brežice HPP system on the Sava River in Slovenia. The main findings are as follows:
- The use of habitat-specific sampling methods (bottom trawling, electrofishing by wading and from a boat), along with 24/7 video surveillance in the fish pass during the main migration period, contributed to a more holistic understanding of the fish community composition in the Brežice HPP system, including both target species: Danube longbarbel gudgeon and cactus roach although fish sampling was conducted only during the summer and daytime.
- Our findings provide evidence that impounded rivers, when complemented by well-designed mitigation measures, can sustain rheophilic fish species, including the Danube longbarbel gudgeon and the cactus roach. Maintaining rheophilic habitat within the impoundment, combined with a well-functioning river-like side channel, is crucial for the bottom-dwelling Danube longbarbel gudgeon and migratory cactus roach.
- At Brežice HPP, adjustments to the management of water inflow to the fish pass are required to ensure optimal hydraulic conditions during operation. The inlet should be designed to maintain a uniform water level along its entire length up to the control gates at the outlet of the technical section of the fish pass. This water level must align with the current impoundment level to ensure consistent energy and flow conditions at the fish pass inlet, regardless of whether the control gates are open or closed.
- In summer, when high surface water temperatures occur in the lower part of the Brežice HPP impoundment, the new technical solution for the water inlet should also allow inflow from deeper layers where the water temperature does not exceed 25 °C.
- Our findings indicate that the presence of various target rheophilic fish species may require species-specific measures, as was evident in our study, where several rheophilic species are present within the Brežice HPP system.
- Applying a knowledge co-creation approach, which requires productive interaction among scientists, managers and policy makers, could help to find the best solutions for sustainable water ecosystem management.
Author Contributions
Conceptualization, G.U., A.V. and A.K.; methodology, G.U., A.V., A.K.; formal analysis G.U., A.V. and A.K.; investigation, G.U., A.V., D.Z., M.Ć., R.K., M.P.U. and A.K.; writing—original draft preparation, G.U., A.V., D.Z. and A.K.; writing—review and editing, G.U., A.V., D.Z., M.Ć., R.K., M.P.U. and A.K.; visualization, G.U.; project administration, G.U.; funding acquisition, G.U., A.V. and A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research is a part of the research project “Research of Danube longbarbel gudgeon and cactus roach on the Sava River and its tributaries and research of the effectiveness of fish passes for aquatic organisms on the lower Sava River (V1-2142)” granted to Gorazd Urbanič and funded by the Slovenian Research Agency and Ministry of the Environment and Spatial Planning of the Republic of Slovenia.
Institutional Review Board Statement
Fish sampling was performed in accordance with permits issued by the Ministry of Agriculture, Forestry and Food and Ministry of the Environment and Spatial Planning of the Republic of Slovenia.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on special request.
Acknowledgments
The authors are grateful to Samo Podgornik for his assistance in the nearshore fish sampling and Linda Zanella for proofreading the manuscript. The authors would like to thank the anonymous reviewers for their useful comments to a previous version of the manuscript.
Conflicts of Interest
Author Maja Pavlin Urbanič was employed by the company URBANZERO Institute for Holistic Environmental Management Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HPP | Hydropower plant |
| N2K | Natura 2000 |
| NPP | Nuclear power Plant |
| HD | Habitats Directive |
| EU | European Union |
References
- Schleker, T.; Fjeldstad, H.-P. Hydropower and Fish—Report and Messages from Workshop on Research and Innovation in the Context of the European Policy Framework. Sci. Total Environ. 2019, 647, 1368–1372. [Google Scholar] [CrossRef] [PubMed]
- Tockner, K.; Uehlinger, U.; Robinson, C.T.; Tonolla, D.; Siber, R.; Peter, F.D. Introduction to European rivers. In Rivers of Europe, 1st ed.; Tockner, K., Robinson, C.T., Uehlinger, U., Eds.; Academic Press Publisher: London, UK, 2008; pp. 1–21. [Google Scholar]
- Best, J. Anthropogenic stresses on the world’s big rivers. Nat. Geosci. 2019, 12, 7–21. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Lin, Y.; Zhang, J.; Xia, J.; Ni, J.; Cooke, S.J.; Best, J.; He, S.; Feng, T.; et al. River damming impacts on fish habitat and associated conservation measures. Rev. Geophys. 2023, 61, e2023RG000819. [Google Scholar] [CrossRef]
- Antonio, R.R.; Agostinho, A.A.; Pelicice, F.M.; Bailly, D.; Okada, E.K.; Dias, J.H.P. Blockage of migration routes by dam construction: Can migratory fish find alternative routes? Neotrop. Ichthyol. 2007, 5, 177–184. [Google Scholar] [CrossRef]
- Liermann, C.R.; Nilsson, C.; Robertson, J.; Ng, Y. Implications of dam obstruction for global freshwater fish diversity. BioScience 2012, 62, 539–548. [Google Scholar] [CrossRef]
- Gleick, P.H. Environmental consequences of hydroelectric development: The role of facility size and type. Energy 1992, 17, 735–747. [Google Scholar] [CrossRef]
- Vardakas, L.; Perdikaris, C.; Freyhof, J.; Zimmerman, B.; Ford, M.; Vlachopoulos, K.; Koutsikos, N.; Karaouzas, I.; Chamoglou, M.; Kalogianni, E. Global Patterns and Drivers of Freshwater Fish Extinctions: Can We Learn from Our Losses? Glob. Change Biol. 2025, 31, e70244. [Google Scholar] [CrossRef]
- WWF. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; Almond, R.E.A., Grooten, M., Petersen, T., Eds.; WWF: Gland, Switzerland, 2020; 159p, Available online: https://wwfin.awsassets.panda.org/downloads/lpr_2020_full_report.pdf (accessed on 25 October 2025).
- Directive, E.B. European Council Directive 79/409/EEC of 2 April 1979 on the conservation of wild birds. Off. J. Eur. Communities 1979, 103, 1–18. [Google Scholar]
- Directive, H. European Council Directive 92/43/EEC of 21 May 1992 on the conservation of natural habitats and of wild fauna and flora. Off. J. Eur. Communities 1992, 206, 7–50. [Google Scholar]
- Commission Staff Working Document. Environmental Implementation Review. Country Report—SLOVENIA. 2025. Available online: https://ec.europa.eu/transparency/documents-register/detail?ref=SWD(2025)324&lang=en (accessed on 10 July 2025).
- Natura 2000 Forests in Slovenia. 2024. Available online: https://natura2000.gov.si/fileadmin/user_upload/Slike/Natura2000_v_Sloveniji/Infografika_Gozdovi_Nature_2000.pdf (accessed on 26 October 2025). (In Slovene)
- Natura 2000 Areas. 2024. Available online: https://natura2000.gov.si/natura-2000/natura-2000-v-sloveniji/ (accessed on 26 October 2025). (In Slovene)
- Povž, M.; Gregori, A.; Gregori, M. Freshwater Fish and Lampreys in Slovenia; Zavod Umbra: Ljubljana, Slovenia, 2015; p. 293. (In Slovene) [Google Scholar]
- Povž, M.; Jakšič, G.; Piria, M. The Updated List of the Non-Native Freshwater Fishes in Slovenia with Note of their Potential Impact in Inland Waters. Pak. J. Zool. Suppl. Ser. 2018, 13, 1–7. [Google Scholar]
- Dußling, U.; Berg, R.; Klinger, H.; Wolter, C. Assessing the ecological status of river systems using fish assemblages. In Handbuch Angewandte Limnologie; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 1–84. [Google Scholar]
- Kottelat, M.; Freyhof, J. Handbook of European Freshwater Fishes; Publications Kottelat: Cornol, Switzerland, 2007; p. 646. [Google Scholar]
- Povž, M.; Sket, B. Our Freshwater Fish; Mladinska knjiga: Ljubljana, Slovenia, 1990; p. 376. (In Slovene) [Google Scholar]
- Ćaleta, M.; Buj, I.; Mrakovčić, M.; Mustafić, P.; Zanella, D.; Marčić, Z.; Duplić, A.; Mihinjač, T.; Katavić, I. Endemic Fishes of Croatia; Croatian Environment Agency: Zagreb, Croatia, 2015; p. 116. [Google Scholar]
- Bless, R. Threatened fishes of the world: Gobio uranoscopus (Agassiz, 1828) (Cyprinidae). Environ. Biol. Fishes 1997, 49, 20. [Google Scholar] [CrossRef]
- Talabishka, E.; Didenko, A.; Velykopolskiy, I. Some biological data on cactus roach, Rutilus virgo (Heckel), in rivers of the Transcarpathian region of Ukraine. Arch. Pol. Fish. 2015, 23, 67–77. [Google Scholar] [CrossRef][Green Version]
- Barbarossa, V.; Schmitt, R.J.P.; Huijbregts, M.A.J.; Zarfl, C.; King, H.; Schipper, A.M. Impacts of current and future large dams on the geographic range connectivity of freshwater fish worldwide. Proc. Natl. Acad. Sci. USA 2020, 117, 3648–3655. [Google Scholar] [CrossRef]
- van Treeck, R.; Radinger, J.; Smialek, N.; Pander, J.; Geist, J.; Mueller, M.; Wolter, C. Comparative assessment of hydropower risks for fishes using the novel European fish hazard Index. Sustain. Energy Technol. Assess. 2022, 51, 101906. [Google Scholar] [CrossRef]
- Akstinas, V.; Virbickas, T.; Meilutyte-Lukauskiene, D.; Sarauskiene, D.; Vezza, P.; Kriauciuniene, J.; Rakauskas, V.; Steponenas, A.; Jurgelenaite, A.; Jakimavičius, D.; et al. Multicomponent assessment of the impact of hydropower cascade on fish metrics. Sci. Total Environ. 2024, 906, 167541. [Google Scholar] [CrossRef] [PubMed]
- Schilt, C.R. Developing fish passage and protection at hydropower dams. Appl. Anim. Behav. Sci. 2007, 104, 295–325. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, H.; Gessner, J.; Congiu, L.; Haxton, T.J.; Jeppesen, E.; Svenningj, J.-C.; Xie, P. To save sturgeons, we need river channels around hydropower dams. Proc. Natl. Acad. Sci. USA 2023, 120, e221786120. [Google Scholar] [CrossRef]
- Hydrological Data Archive—Daily Data; Slovenian Environment Agency: Ljubljana, Slovenia. Available online: https://vode.arso.gov.si/hidarhiv/pov_arhiv_tab.php (accessed on 10 May 2025).
- Kenig, M. Bathymetric measurements of the impoundment of the Hydroelectric powerplant Brežice. In Report 2021; Partner: Brežice, Slovenia, 2021; p. 16. (In Slovene) [Google Scholar]
- Olajos, P.; Kiss, B.; Sallai, Z.; E-KECE. Elektromos Fenékháló. Patent Pending. 2021. p. 8. Available online: https://www.globeecology.hu/wp-content/uploads/e-kece.pdf (accessed on 10 May 2025).
- Szalóky, Z.; György, A.I.; Tóth, B.; Sevcsik, A.; Specziár, A.; Csányi, B.; Szekeres, J.; Eros, T. Application of an electrified benthic frame trawl for sampling fish in a very large European river (the Danube River)—Is offshore monitoring necessary? Fish. Res. 2014, 151, 12–19. [Google Scholar] [CrossRef]
- Nelva, A.; Persat, H.; Chessel, D. Une nouvelle méthode d’étude des peuplements ichtyologiques dans les grands cours d’eau par échantillonnage ponctuel d’abondance. C R Hebd. Seances Acad. Sci. Sér. D Sci. Nat. 1979, 289, 1295–1298. [Google Scholar]
- Persat, H.; Copp, G.H. Electric fishing and point abundance sampling for ichthyology of large rivers. In Development in Electric Fishing; Cowx, I.G., Ed.; Blackwell: Oxford, UK, 1990; pp. 197–209. [Google Scholar]
- Grenouillet, G.; Pont, D.; Olivier, J.M. Habitat occupancy pattern of juvenile fishes in a large lowland river: Interactions with macrophytes. Arch. Für Hydrobiol. 2000, 149, 307–326. [Google Scholar] [CrossRef]
- Coop, G.H. The David Noakes article that debunked the misguided belief that absolute numbers of fish can be captured in fresh waters: A lesson for early-career scientists. Environ. Biol. Fish 2023, 106, 811–816. [Google Scholar] [CrossRef]
- Peterlin, M.; Urbanič, G. A lakeshore modification index and its association with benthic invertebrates in alpine lakes. Ecohydrology 2013, 6, 297–311. [Google Scholar] [CrossRef]
- Urbanič, G. A Littoral Fauna Index for assessing the impact of lakeshore alterations in Alpine lakes. Ecohydrology 2014, 7, 703–716. [Google Scholar] [CrossRef]
- Perrow, M.D.; Winfield, I.J.; Tomlinson, M.L.; Hardwood, A.J.P. Designing a Methodology for Surveying Fish Populations in Freshwater Lakes (NECR230); Natural England: York, UK, 2017; p. 61. [Google Scholar]
- SIST EN 14011; Water Quality—Sampling of Fish with Electricity. British Standards Institution: London, UK, 2003; p. 16.
- Clarke, K.R. Non-parametric multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar] [CrossRef]
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- EU. Regulation (EU) No 1143/2014 of the European Parliament and of the Council of 22 October 2014 on the Prevention and Management of the Introduction and Spread of Invasive Alien Species. Off. J. Eur. Union 2014, 317, 35–55. [Google Scholar]
- Urbanič, G. Ecological status assessment of rivers in Slovenia—An overview. Nat. Slov. 2011, 13, 5–16. [Google Scholar] [CrossRef]
- Urbanič, G. Hydromorphological degradation impact on benthic invertebrates in large rivers in Slovenia. Hydrobiologia 2014, 729, 191–207. [Google Scholar] [CrossRef]
- Keulartz, J. European nature conservation and restoration policy—Problems and perspectives. Restor. Ecol. 2009, 17, 446–450. [Google Scholar] [CrossRef]
- Šumer, S.; Povž, M.; Podgornik, S.; Kosi, G. Ichthyological Research on the Sava River from HPP Vrhovo to NPP Krško; Fisheries Research Institute of Slovenia: Ljubljana, Slovenia, 2004; p. 68. (In Slovene) [Google Scholar]
- Habeković, D.; Homen, Z.; Fašaić, K. Ichthyofauna of a part of the Sava River. Ribar. Jugosl. 1990, 45, 8–14. (In Croatian) [Google Scholar]
- Zajicek, P.; Wolter, C. The gain of additional sampling methods for the fish-based assessment of large rivers. Fish. Res. 2018, 197, 15–24. [Google Scholar] [CrossRef]
- Pont, D.; Meulenbroek, P.; Bammer, V.; Dejean, T.; Erős, T.; Jean, P.; Lenhardt, M.; Nagel, C.; Pekarik, L.; Schabus, M.; et al. Quantitative monitoring of diverse fish communities on a large scale combining eDNA metabarcoding and qPCR. Mol. Ecol. Resour. 2023, 23, 396–409. [Google Scholar] [CrossRef]
- Deng, C.; Huang, S.; Chen, B.; Huang, R.; Zhang, J.; Xiao, Z.; Ma, C.; Wang, Z.; Liu, X. eDNA Metabarcoding Reveals Homogenization of Fish in Fujiang Segments Isolated by Cascading Hydroelectric Stations. Animals 2025, 15, 2031. [Google Scholar] [CrossRef]
- The European Green Deal; European Commission. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; European Green Deal; European Commission: Brussel, Belgium, 2019; pp. 1–24. [Google Scholar]
- Government of the Republic of Slovenia. Integrated National Energy and Climate Plan of the Republic of Slovenia. 2020. Available online: https://energy.ec.europa.eu/system/files/2020-06/si_final_necp_main_en_0.pdf (accessed on 11 July 2025).
- Nielsen, N.; Szabo-Meszaros, M. (Eds.) A Roadmap for Best Practice Management on Hydropower and Fish—IEA Hydro Report on Annex XIII Hydropower and Fish; Zenodo: Genève, Switzerland, 2022; p. 170. [Google Scholar] [CrossRef]
- Cortina-Segarra, J.; García-Sánchez, I.; Grace, M.; Andrés, P.; Baker, S.; Bullock, C.; Decleer, K.; Dicks, L.V.; Fisher, J.L.; Frouz, J.; et al. Barriers to Ecological Restoration in Europe: Expert Perspectives. Restor. Ecol. 2021, 29, e13346. [Google Scholar] [CrossRef]
- Urbanič, G.; Politti, E.; Rodríguez-Gonzálz, P.M.; Payne, R.; Schook, D.; Alves, M.H.; Anđelković, A.; Bruno, D.; Chilikova-Lubomirova, M.; Di Lonardo, S.; et al. Riparian Zones—From Policy Neglected to Policy Integrated. Front. Environ. Sci. 2022, 10, 868527. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).