Abstract
Acrylonitrile–butadiene–styrene (ABS) has been widely used as an engineering thermoplastic, and the increasing post-consumer waste of ABS plastics calls for efficient and sustainable recycling technologies. The recent advances in ABS recycling technologies were investigated to enhance material recovery, purity, and environmental performance. Thermo-oxidative degradation compromises mechanical integrity during reprocessing, while minor reductions in molecular weight increase melt flow rates. Surface modification techniques such as boiling treatment, Fenton reaction, and microwave-assisted flotation facilitate the selective separation of ABS from mixed plastic waste by enhancing its hydrophilicity. Dissolution-based recycling using solvent and anti-solvent systems enables the recovery of high-purity ABS, though some additive losses may occur during subsequent molding. Magnetic levitation and triboelectrostatic separation provide innovative density and charge-based sorting mechanisms for multi-plastic mixtures. Thermochemical routes, including supercritical water gasification and pyrolysis, generate fuel-grade gases and oils from ABS blends. Mechanical recycling remains industrially viable when recycled ABS is blended with virgin resin, whereas plasma-assisted mechanochemistry has emerged as a promising technique to restore mechanical properties. These recycling technologies contribute to a circular plastic economy by improving efficiency, reducing environmental burden, and enabling the reuse of high-performance ABS materials.
1. Introduction
Plastics consist of a wide range of synthetic materials or organic compounds, of which resins are mixed with additives to enhance properties and performance. The global plastic market size was estimated at USD 624.8 billion in 2023 and is expected to continue to grow at a compound annual growth rate of 4.2% from 2024 to 2030 [1]. Recent data indicate that recycling rates for non-fiber engineering polymers remain modest: globally, only 18% of such plastics were recycled in 2014, with Europe reaching approximately 30% and China around 25% [2]. Within the European Union, the overall plastics recycling rate was approximately 32.5% in 2020, with a policy target to increase this to 50% by 2030 [3]. Understanding the value of recycled engineering polymers, the global recycled engineering plastics market was estimated at USD 4.72 billion in 2024 and is projected to grow to USD 6.30 billion by 2030, with an annual growth rate of approximately 5% [4]. Specifically for ABS, the recycling market reached USD 1.5 billion in 2023 and is expected to more than double to USD 3.2 billion by 2032, at a robust CAGR of 8.5% [5]. These statistics underscore both the current underperformance in recycling and the strong momentum toward more ambitious recycling targets globally [6,7].
Directive EU/2018/852 [8] was adopted to prevent waste generation and promote the reuse, recycling, and recovery of packaging waste. In the same year, the European Union launched its plastics strategy for a circular economy, setting an ambitious target. By 2030, all plastic packaging placed on the EU market must be either reusable or recyclable. The Directive further specifies binding recycling targets, requiring that at least 50% of plastic packaging waste be recycled by 2025, with the target rising to a minimum of 55% by 2030 [6,7,9].
Although plastics are increasingly substituting metals, glass, wood, natural rubber, and other traditional materials, they are also among the most heavily regulated substances. This is largely because many of their additives and wastes are subject to strict national and international controls due to environmental and human health concerns. For instance, the Basel Convention restricts the transboundary movement of plastic waste, while plasticizers such as phthalates are regulated due to their endocrine-disrupting effects. Halogenated flame retardants, including polybrominated diphenyl ethers (PBDEs) and hexabromocyclododecane (HBCD), are listed under the Stockholm Convention on Persistent Organic Pollutants (POPs). Similarly, blowing agents such as perfluorocarbons (PFCs) are controlled under the Montreal Protocol owing to their ozone-depleting potential and greenhouse gas effects [10,11,12,13,14].
In addition to these restrictions, plastics incorporate a wide range of functional additives to improve processability and durability. Fillers reinforce the polymer matrix, thermal stabilizers enable processing at elevated temperatures, and plasticizers increase flexibility and pliability. Flame retardants suppress combustion, while UV stabilizers prevent degradation under ultraviolet radiation. These additives are typically compounded with resins into formulations that are processed into final products. However, heavy-metal-based stabilizers and pigments, such as those containing lead and cadmium, have been progressively banned in many regions under regulatory frameworks, including the European Union’s Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) and the United States’ Toxic Substances Control Act (TSCA) [1,15,16,17,18].
Acrylonitrile-butadiene-styrene (ABS) is a terpolymer, shown in Figure 1, which is used in various fields like automotive, electronics, and 3D printing. Figure 2 shows the monomeric units of ABS, which have the following chemical formulas: acrylonitrile (C3H3N), butadiene (C4H6), and styrene (C8H8), each playing a critical role in defining the overall properties of the final polymer structure. As an amorphous thermoplastic, ABS can be melted and remolded multiple times with minimal quality loss, making it highly recyclable. ABS is synthesized through the polymerization of styrene and acrylonitrile in the presence of polybutadiene, which imparts enhanced toughness, rigidity, and thermal stability. However, despite its advantageous properties, ABS exhibits a relatively high coefficient of friction and wear rate, thereby limiting its applicability in tribological systems [2,19,20,21]. To address these limitations, ABS can be alloyed with other plastics, such as polyvinyl chloride (PVC) or polycarbonate (PC), to achieve enhanced flame resistance, good processability, improved chemical resistance, and ultraviolet (UV) stability, rendering it suitable for industrial applications. Its processing is typically carried out through injection molding and extrusion methods [22,23,24].
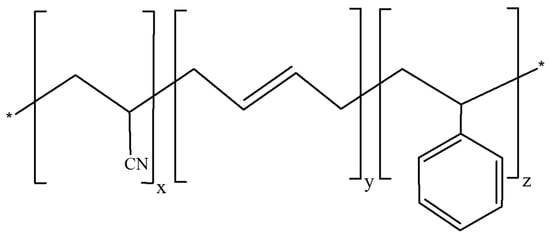
Figure 1.
Molecular structures of ABS polymer (x, y, and z denote the numbers of acrylonitrile, butadiene, and styrene repeating units, respectively).
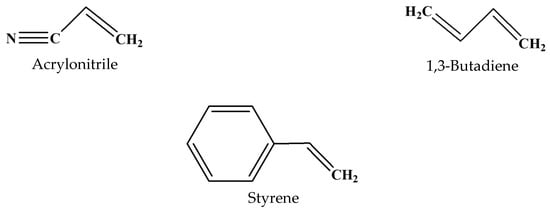
Figure 2.
Molecular structures of monomer species in ABS.
ABS was developed initially in the 1940s by IG Farben under the guidance of Dr. Hermann Staudinger. It has subsequently established itself as one of the most widely used thermoplastics worldwide. Its versatile nature gives it substantial strength, durability, and rigidity, making it suitable for a diverse range of applications [2,25]. By the 1960s, ABS had become a popular choice for engineering plastics, replacing metals and other less efficient materials in many applications due to its lightweight, durability, and cost-effectiveness [23,26,27]. ABS plastic is one of the world’s seven major plastics. Its global market size will grow from 10.04 million tons in 2023 to 12.21 million tons in 2025, with a compound annual growth rate (CAGR) of 4.00%. The major manufacturers are Chi Mei Corporation (Taiwan, China), LG Chem (Seoul, Republic of Korea), INEOS (London, UK), Formosa Plastics (Taiwan, China), and PetroChina Co., Ltd. (Beijing, China). [28,29].
ABS demonstrates excellent resistance to dilute acids and alkalis, moderate resistance to aliphatic hydrocarbons, but comparatively low resistance to aromatic hydrocarbons, halogenated hydrocarbons, and alcohols. ABS plastic is composed of two distinct phases. The first one is a rubbery phase, comprising polybutadiene (PB), and the other one is a rigid phase, composed of the styrene-acrylonitrile (SAN) copolymer. The SAN phase is a rigid and glassy matrix made from styrene and acrylonitrile copolymerized together. It is the continuous phase in which rubbery polybutadiene particles are dispersed. Styrene imparts properties such as gloss, processability, and rigidity, whereas acrylonitrile contributes to chemical resistance, thermal stability, and polarity, due to the nitrile (–CN) group. As a matrix, the SAN phase gives ABS its mechanical strength and chemical resistance, while polybutadiene improves impact strength and toughness. These two phases make a significant contribution to compatibility. In polymer blending, for instance, with PVC, the polar nitrile groups (-CN) in acrylonitrile allow dipole–dipole interactions with other polar polymers like PVC. This makes the SAN phase the primary site for interfacial adhesion in ABS blends. ABS is soluble in polar solvents, such as esters, ketones, such as acetone, chloroform, and ethylene dichloride, but it exhibits poor resistance to chlorinated solvents and aldehydes. ABS is also susceptible to stress cracking when exposed to certain greases and can degrade under prolonged exposure to sunlight [30,31].
Recycling ABS from electronic and electrical equipment (EEE) poses challenges, of which difficulty arises from toxic additives such as brominated flame retardants (5–25 wt%) and heavy metals (0.5–3 wt%). These substances are harmful to both the environment and human health. The recycled ABS obtained from waste electronic and electrical equipment (WEEE) often results in degraded mechanical properties and notably increased embrittlement. This degradation is primarily attributed to the cumulative effects of repeated reprocessing, wherein complex mechanisms, such as thermo-mechanical stress, oxidative reactions, and polymer chain scission, progressively compromise the material’s structural integrity. Moreover, the presence of diverse additives, including flame retardants, can significantly alter degradation pathways, further complicating the recycling process. Accordingly, the development of effective recycling strategies must prioritize both the preservation of material properties and adherence to environmental safety standards. [32,33,34,35].
The recycling technologies for waste plastics are illustrated in Figure 3. Mechanical recycling physically reprocesses plastics into new products without changing the chemical structure. Chemical depolymerization involves breaking down polymers into monomers via glycolysis, aminolysis, alcoholysis, and acidolysis. Enzymatic recycling uses biocatalysts to degrade polymers into reusable building blocks selectively. However, acrylonitrile-butadiene-styrene (ABS) is a thermoplastic terpolymer composed of three monomers with distinct functions. In particular, acrylonitrile imparts chemical resistance, and butadiene provides toughness and impact resistance. Styrene contributes rigidity and processability. This heterogeneous structure makes ABS challenging to recycle enzymatically, because most enzymes naturally target specific bonds, for instance, ester linkages in PET. ABS has carbon–carbon backbones and styrene–acrylonitrile domains, which are not easily cleaved biologically [36,37,38]. Consequently, enzymatic recycling of ABS plastics remains at an early research stage, which is not elaborated in detail in this review. Gasification and liquefaction convert plastics into fuel gases or liquid hydrocarbons under high temperatures and pressure [29,39,40]. Hydrogenolysis has recently emerged as one of the chemical catalytic depolymerization methods, particularly effective for polyolefins such as polypropylene (PP) and polyethylene (PE). Using the metal hybrid catalysts such as ruthenium nanoparticles supported on carbon (Ru/C) or Ni-based heterogeneous catalysts under mild hydrogenolysis conditions yields relatively high efficiency of C5–C32 iso-alkanes without solvent. The Ru/C catalyst is recyclable and effective for depolymerizing both high molecular weight and post-consumer PP waste [41,42]. A sunlight-driven thermocatalytic process using a Ni-based catalyst effectively upcycles mixed plastic waste into methane (98% yield) and HCl (91% yield). The method handles complex real-world plastic mixtures, including polyolefins, polyesters, and PVC [43,44,45].
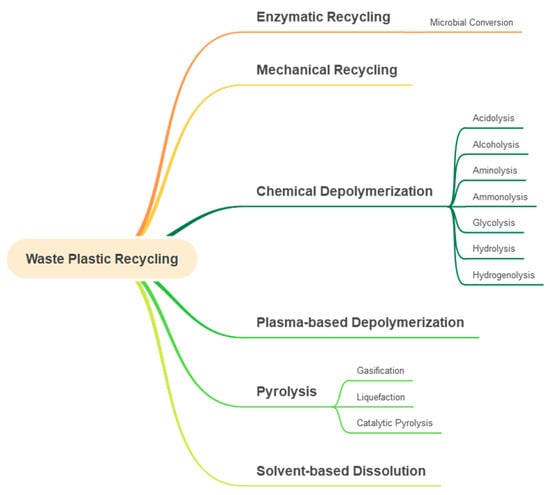
Figure 3.
Diagram of waste plastic recycling technologies.
2. Recycling of ABS Plastics
Although acrylonitrile-butadiene-styrene (ABS) is recyclable through various methods, including mechanical recycling, where it is ground and reprocessed into new products, and chemical recycling, where it is broken down into its monomers for reuse. These recycling methods help conserve resources, reduce energy consumption, and minimize environmental pollution. However, challenges such as material degradation during recycling and difficulties in separating components pose limitations [46,47,48]. As presented in Figure 4, recycling ABS plastic poses significant technical challenges due to its chemical complexity. As a copolymer composed of three distinct monomers, such as acrylonitrile, butadiene, and styrene, its heterogeneous structure complicates chemical depolymerization and pyrolysis. Conventional recycling methods, such as thermal depolymerization and chemical recovery, are energy-intensive and often reliant on high temperatures and catalytic processes. These methods incur significant operational costs and environmental impacts due to energy consumption and emissions of harmful by-products [2,49,50,51,52].
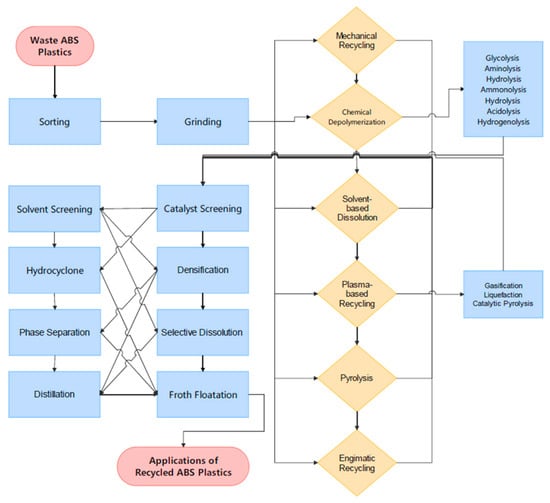
Figure 4.
Diagram and flowchart of ABS plastic recycling.
To meet growing demands for tailored material performance, ABS is frequently blended with other polymers. Blending ABS with high-impact polystyrene (HIPS) enhances impact resistance, making it ideal for products like electronics casings and toys. ABS/polyvinyl chloride (PVC) blends improve chemical resistance and durability, suitable for applications such as piping and automotive components. ABS/polypropylene (PP) combinations provide lightweight and chemically resistant properties, making them useful in packaging and household items. ABS/polycarbonate (PC) blends offer superior impact and heat resistance, often found in helmets and electronic housings. Although these blends improve mechanical properties, they introduce recycling challenges. Component separation and compatibility during reprocessing are particularly difficult. Throughout its lifecycle and recycling processes, ABS undergoes degradation, resulting in chain scission and crosslinking. During reprocessing, volatile components may be released, leading to void formation in the recycled material and reducing impact strength. Therefore, recycling of plastic waste by blending and compounding techniques has been studied for engineering plastic waste. For instance, ABS and polycarbonate (PC) come from waste electrical and electronic equipment (WEEE). ABS/PC mixtures are quite complex since the system is a physical blend of two plastic resins with different properties [53,54,55].
2.1. Chemical Depolymerization
Chemical recycling, also referred to as feedstock recycling, involves the depolymerization of plastics into their original monomers or other fundamental chemical building blocks, which can then be repolymerized into new plastic products. This approach enables the recovery of materials with properties comparable to those of virgin polymers. Common techniques include pyrolysis, solvolysis, catalytic depolymerization, gasification, and the dissolution and precipitation method, each targeting specific types of plastic waste and degradation mechanisms.
One widely studied method is solvent-based recycling, in which polymers such as ABS are selectively dissolved in solvents like acetone. This allows for the effective separation of contaminants and non-dissolved materials through filtration, followed by recovery and reuse of the purified polymer through precipitation or solvent evaporation. This approach minimizes degradation and environmental impact. Advanced techniques like sequential extraction have also been employed to recycle flame-retardant ABS blends effectively. Although ABS recycling offers environmental and economic benefits, it presents challenges. First of all, ABS contains multiple components that can complicate the recycling process. Secondly, recycled ABS may suffer from reduced mechanical properties due to heat and processing. ABS also has compatibility issues. Chemical compatibility between different polymers in blends can limit recycling potential [56,57,58,59].
Pyrolysis decomposes ABS at high temperatures into monomers or oils. Solvolysis uses solvents to break down ABS into reusable components. The dissolution and precipitation method dissolves ABS in solvents, filters impurities, and precipitates pure polymers. In regard to environmental and global climate change, recycling ABS reduces greenhouse gas emissions, conserves resources, and creates economic opportunities. However, it is energy-intensive and can release pollutants, necessitating improvements in recycling technologies to mitigate environmental impact [60,61,62,63].
2.2. Thermo-Chemical Oxidation
Severe reprocessing and thermo-oxidation cause progressive degradation of the polybutadiene (PB) phase in acrylonitrile–butadiene–styrene (ABS) copolymers. Chemically, this degradation is initiated by free radical oxidation mechanisms, where the allylic hydrogen atoms in the PB chains are abstracted, forming carbon-centered radicals. These radicals react readily with oxygen, leading to the formation of peroxy radicals (ROO·) and subsequently hydroperoxides (ROOH). Thermal decomposition of these hydroperoxides generates further radicals that propagate degradation.
This oxidative degradation results in:
- Reduced content of carbon–carbon double bonds in the PB segments due to oxidative cleavage and addition reactions.
- Formation of oxygenated functional groups (e.g., carbonyl, hydroxyl, and epoxy groups) along the polymer backbone.
- Increased cross-linking within the rubber phase through radical recombination and addition reactions between unsaturated sites.
Such microstructural changes significantly impact the morphology of ABS. Scanning electron microscopy often reveals a coarsening or even partial disappearance of the rubber particles, while infrared spectroscopy (FTIR) detects new peaks corresponding to oxidized functional groups, notably around 1720 cm−1 for carbonyl groups. These structural modifications severely affect the mechanical properties of ABS. Impact resistance and elongation-at-break decrease significantly due to embrittlement of the rubber phase, as the rubber loses its elasticity and becomes stiffer and more brittle. Tensile strength, however, is generally less affected, since it is more dependent on the styrene-acrylonitrile (SAN) matrix, which remains comparatively stable under moderate oxidative conditions.
Optical properties are also influenced. Thermo-oxidative aging introduces yellowing due to chromophore formation from oxidative reactions in the butadiene units. Increased light scattering from microstructural changes reduces gloss and transparency, adversely affecting esthetic and functional qualities. Additionally, the melt mass-flow rate (MFR) increases slightly with repeated processing cycles. This indicates a reduction in average molecular weight, primarily from chain scission reactions in the SAN matrix and rubber phase during mechanical and thermal stress. Such chain scission occurs via β-scission of radicals or hydroperoxide decomposition, leading to shorter polymer chains and thus lower melt viscosity.
The degradation of ABS under severe processing and thermo-oxidative conditions is governed by radical-driven oxidation reactions in the polybutadiene phase, resulting in both chemical and morphological changes [32,64,65].
2.3. Triboelectrostatic Separation and Electric Charge
Recycling mixed waste plastics is challenging due to their poor material properties. A two-stage separation method based on electrostatic charge control has been developed to separate plastics with similar densities, specifically PVC, PET, and ABS. Polypropylene (PP) and high-impact polystyrene (HIPS) proved to be the most efficient materials for tribo-charging during this process.
The charge-to-mass ratio of plastics improved as air velocity increased in the tribo-charger. In the first stage, a PP-based cyclone charger yielded 99% PVC purity and 98% recovery under optimal conditions of 10 m/s air velocity, below 30% humidity, over 20 kV electrode potential, and a splitter positioned +2 cm from the center. The second stage employed a HIPS cyclone charger and achieved 98% PET purity and 95% recovery with 10 m/s airflow, more than 25 kV potential, a central splitter, and humidity below 40%. To further enhance PVC and PET purity to 99.9% and 99.3%, their recoveries had to be compromised by 20.9% and 27%, respectively, by shifting the splitter to +6 cm. Separately, triboelectrostatic separation of PP and ABS from end-of-life vehicles was studied using high-voltage separation technology. Key variables influencing sorting efficiency included voltage, friction drum speed, feed rate, electrode spacing, and angles. Optimal parameter ranges were established through single-factor and factorial experiments, supported by mathematical modeling. The optimized process achieved sorting rates above 60% and purities exceeding 95% for both PP and ABS [66,67,68,69].
Triboelectrostatic separation presents another viable method for multi-plastic mixtures, leveraging charge differences induced by friction. When two materials rub against or collide with each other, electrons are transferred from one surface to another. One material becomes positively charged, and the other becomes negatively charged, depending on its position in the triboelectric series. Charged particles are fed into a separation chamber where an external electric field, usually a high-voltage field, is applied. Based on their charge, particles are deflected toward oppositely charged electrodes and collected separately. The effectiveness of the triboelectrostatic separation device was assessed using two distinct granule mixtures, which consisted of particle sizes ranging from 2 mm to 6 mm. Each is composed in equal proportions of (1) polypropylene (PP) and high-impact polystyrene (HIPS) and (2) acrylonitrile butadiene styrene (ABS) and polyvinyl chloride (PVC). The separation efficiency was determined by evaluating the purity and recovery rates of the individual plastic fractions. The process demonstrated strong performance, with 60% to 85% of each plastic type being successfully recovered at purities exceeding 85%. While it can be efficient, it often requires multiple separation cycles to achieve high purity and involves the use of high voltage, which raises operational safety concerns [70,71,72,73].
2.4. Dissolved Air Flotation
A dissolved air flotation (DAF) system was employed to separate ABS and PS from waste plastic mixtures. Key factors such as wetting agents, frothers, conditioning time, and flotation duration were examined to optimize the process. The best separation performance was achieved using 25 mg/L tannic acid as a wetting agent and 5 mg/L terpineol as a frother, with both conditioning and flotation times set to 15 min. The process included two flotation stages. Under these conditions, PS achieved 90% purity and 97% recovery, while ABS showed 97% purity and 89% recovery. Mechanistic studies indicated that electrostatic and hydrophobic interactions were not the primary drivers. Instead, water molecules served as a mesophase, facilitating selective adsorption.
A hydrogen bonding mechanism involving hydration shells was proposed to explain the observed separation behavior. Dissolved air flotation is a simple and eco-friendly method for selectively separating PVC and ABS-containing brominated flame retardants from WEEE plastics using microwave and mild-heat treatments combined with froth flotation. Coating the plastics with powdered activated carbon (PAC) followed by microwave treatment enhanced PVC’s hydrophilicity, enabling its separation with 100% purity and recovery. A subsequent 100 s mild-heat treatment selectively separated PAC-coated ABS due to the formation of a low-density twisted structure. Using mild-heat treatment alone also allowed full recovery of both plastics, though PVC purity slightly dropped to 96.8%. The combined approach significantly improves the recycling efficiency and quality of PVC and ABS [74,75,76,77].
2.5. Dissolution-Based Recycling
A dissolution-based recycling method for acrylonitrile–butadiene–styrene (ABS) copolymer has been proposed, focusing on its impact over multiple recycling cycles by analyzing changes in chemical structure, melt viscosity, and mechanical properties such as tensile and impact strength. The optimal recycling conditions were determined to be the use of acetone as a solvent at a concentration of 0.25 g/mL, room temperature, and a dissolution time of 40 min. Analytical techniques, including FT-IR, DSC, and MFI, indicated that the dissolution process itself does not degrade ABS. However, TGA results suggest that stabilizers may be lost during dissolution, leading to degradation during subsequent injection molding. FT-IR analysis also linked the darkening of recycled ABS to butadiene degradation, although the SAN matrix retained its chemical structure despite a reduction in molecular weight. This recycling technique enables polymeric waste to be processed into usable materials, with insoluble additives and contaminants removable via fine mesh filtration, allowing recovery of pure polymer and potential reuse of additives. Solvent-based recycling is particularly effective in separating mixed plastics due to the principle of selective dissolution, accommodating polymer blends and chemically distinct polymers. The process permits polymer modification by incorporating additional components during dissolution while avoiding the shear stress-related degradation common in melt recycling. This approach is particularly relevant for additive manufacturing applications, testing different solvents, temperatures, durations, and volumes. Among the tested solvents, acetone demonstrated the highest effectiveness, outperforming chloroform and methyl ethyl ketone (MEK), primarily due to its higher polarity, which enhances reaction kinetics and strengthens molecular bonding within the ABS matrix [24,78,79,80,81].
Solvent extraction has been recognized as an effective method for recovering and purifying various plastics. As presented in Figure 5 this method is quite effective in extracting additives using a selective solvent. Its core mechanism, selective dissolution, involves dissolving either the target polymer or all others except the desired one. This technique has been widely applied to reclaim polymers from rigid plastics, packaging, electronic waste, and, more recently, multilayer films. For recycling ABS from toy waste, density separation combined with dissolution-precipitation has shown promising results. Safer solvents like acetone and water are effective in dissolving and then reprecipitating ABS. This process maintains the polymer’s structure and molecular weight, although some additive loss was detected through various characterization techniques [21,82]. Additionally, solvent-based methods reduce energy use in downstream recycling processes. Acetone and water are safer solvents to dissolve and precipitate ABS. Polymer structure and molecular weight are preserved via dissolution-precipitation. Loss of additives was identified and characterized by analytical tests. Solvent-based recycling saves energy in secondary recycling processes [24,80,83,84,85,86,87,88,89].
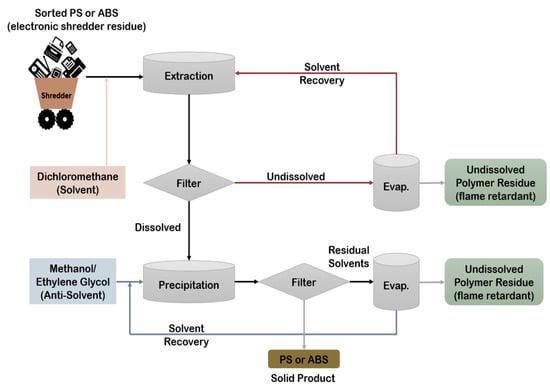
Figure 5.
Dissolution-precipitation process of ABS mixed plastics. Adapted from Ref. [21], published by MDPI, 2022.
A sustainable recycling method for waste ABS plastics involves ammoniacal leaching to selectively dissolve surface-coated metals like copper (Cu) Cu and nickel (Ni), without affecting the ABS polymer. Leaching efficiency follows the anion order CO32− > Cl− > SO42−, achieving over 99% recovery under optimized conditions. Ni can be selectively stripped with 0.5 M H2SO4, while the remaining Cu is recoverable with 1.0 M H2SO4 and further refined by pH-swing extraction. ABS-plated plastic, composed of a polymeric substrate coated with metals such as chromium (Cr), Cu, and Ni, holds considerable reuse potential due to its valuable material composition. However, efficient recycling requires a de-plating step to separate the surface metals from the ABS matrix. The effective de-plating method was developed using ammonium persulfate ((NH4)2S2O8, APS) as the primary oxidizing agent. Thermal activation of APS generates sulfate radicals (SO4•−), which facilitate the removal of metallic coatings from ABS surfaces [90,91,92].
The solvent-based tertiary recycling of ABS involves dissolving polymer waste in a solvent, filtering out undissolved impurities and additives, and subsequently recovering pure polymer material through precipitation and drying. Recent studies have utilized acetone as a solvent due to its ability to dissolve ABS at room temperature and its cost-effectiveness compared to other solvents. By optimizing dissolution conditions such as solvent type, maximum polymer concentration, dissolution temperature, and time, degradation, costs, and environmental impact were minimized during the recycling process. The dissolved polymer was recovered through a simple solvent evaporation technique [24,63,93].
An investigation into the effect of multiple recycling cycles on the mechanical properties of ABS revealed that while the solvent-based recycling process itself does not degrade ABS, the removal of some stabilizers during dissolution may lead to thermal degradation during subsequent injection molding processes. In terms of sequential technique for separating and recovering PC/ABS and bisphenol-A bis(diphenyl phosphate) (BDP) from commercially available flame-retardant PC/ABS resins, the solvent extraction method involved the use of N,N-dimethylcyclohexylamine (DMCHA) as a solvent to separate PC/ABS from BDP. The process successfully enabled the recycling of both PC/ABS and organophosphate flame retardants. Analysis of the recovered materials confirmed that this sequential approach yielded high-purity PC/ABS and BDP. Solvent-based recycling processes contribute to environmental sustainability by reducing impact and optimizing resource utilization [20,24,50,79,84].
2.6. Magnetic Levitation of ABS Plastics
Unlike traditional methods, the magnetic levitation method is unaffected by particle size and can effectively separate complex mixtures. Magnetic levitation (MagLev) separation of ABS plastics is an innovative density-based separation technique that allows for relatively precise and efficient sorting of polymers, including those with similar densities such as ABS, PS, and PVC. It utilizes the principle of magnetic buoyancy in a paramagnetic medium under a strong magnetic field. The density of ABS is 1.04–1.07 g/cm3, and other plastics such as polystyrene (PS) is 1.05 g/cm3, polycarbonate (PC) is 1.20 g/cm3, and polyvinyl chloride (PVC) is 1.38 g/cm3.
Six plastics, such as polypropylene (PP), acrylonitrile-butadiene-styrene (ABS), polyamide 6 (PA6), i.e., Nylon 6, polycarbonate (PC), polyethylene terephthalate (PET), and polytetrafluoroethylene (PTFE), i.e., Teflon, were tested in a paramagnetic liquid placed between two identical NdFeB magnets with like poles facing each other. Each type of plastic settled at a unique height based on its density and magnetic susceptibility. FT-IR spectroscopy confirmed the purity of the separated materials. The process achieved 100% purity in two-stage separation trials. Unlike flotation or hydrocyclone techniques, this method works regardless of particle size distribution. Quantitative relationships between magnetic field strength, density, and levitation height were established. Additionally, the paramagnetic medium is reusable, enhancing environmental sustainability. The sequential separation of multi-plastics and the extraction of target material from multi-plastics mixture are successfully addressed. The purities of separated multiple plastics reach approximately 100%. The process holds strong promise for scalable and eco-friendly plastic recycling [94,95,96,97].
2.7. Gasification of ABS Plastics
Gasification and pyrolysis are two prominent thermochemical conversion processes for recycling ABS (Acrylonitrile-Butadiene-Styrene) plastics into energy or value-added products. Although both involve the thermal decomposition of waste ABS plastics, they operate under different conditions, yield different products, and serve distinct objectives. Subcritical and supercritical water treatments were applied to ABS plastics for gasification purposes. ABS was used as the feedstock in supercritical water gasification experiments conducted between 450 °C and 700 °C at 23 MPa to produce fuel gas.
Gasification converts plastic waste into syngas such as H2 and CO, by partial oxidation at high temperatures, often in the presence of steam or supercritical water. In general, the temperature is 700–1200 °C for thermal gasification, whereas it is 450–700 °C for supercritical water gasification. The atmosphere is controlled by oxidants such as air, O2, and steam. Pressure is often elevated, especially in supercritical water. Higher reaction temperature, extended duration, and increased water-to-ABS ratios significantly enhanced gasification efficiency. The process showed three distinct phases: an initial rapid increase in efficiency, a plateau, and a subsequent increase. Additionally, subcritical water hydrolysis was carried out between 375 °C and 450 °C at 21 MPa to recover oil products. Over prolonged residence times, most ABS monomers transformed into more stable compounds [27,98,99].
2.8. Pyrolysis of ABS Plastics
Pyrolysis is the thermal decomposition of organic materials in the absence of oxygen. It breaks ABS down into liquid oil, gases, and solid residues such as char. The temperature is usually 400–600 °C, and the atmosphere is inert, either nitrogen or argon, while pressure is usually atmospheric or slightly elevated. It produces liquid oil, which contains styrene, acrylonitrile, phenol, BPA, etc., and gases such as CO, H2, CH4, and C2 hydrocarbons. Char also remained as the solid residue with a potential carbon value. Pyrolysis of an ABS80/PC20 blend between 400 and 550 °C at 25 °C/min for one hour yielded 40–75% pyrolysis oil, including approximately 19.9% monocyclic hydrocarbons.
ABS75/PC25 exhibited a single degradation peak at around 475 °C, while blends with higher PC content (ABS50/PC50 and ABS25/PC75) showed two distinct weight loss stages, corresponding to the breakdown of ABS and PC. A higher ABS ratio accelerated PC degradation, with radicals from ABS’s C=C bond cleavage initiating PC decomposition through hydrogen abstraction. This highlights the importance of intermolecular radical transfer in their thermal degradation synergy. Pyrolysis of PMMA/ABS blends was explored to identify major volatile products and investigate inter-polymer interactions. The presence of PMMA lowered decomposition temperature of ABS and led to the recombination of breakdown products, creating new volatile compounds. Batch pyrolysis generated significantly more gas than expected, differing from prior analytical pyrolysis studies. The resulting pyrolysis liquid was analyzed for potential as a fuel, with parameters like density, viscosity, research octane number (RON), calorific value, and gas emissions measured.
An ABS-MF (melamine formaldehyde) composite containing 87.5 wt% ABS and 12.5 wt% MF was subjected to repeated recycling cycles. While mechanical properties such as tensile strength and toughness decreased over three cycles from 42.45 MPa to 18.47 MPa, and from 1.60 to 0.1459 MPa, respectively, the MFI increased, indicating better flowability. DSC confirmed that the composite retained thermal stability, and its printability via material extrusion (MEX) remained intact. FT-IR results showed increased infrared transmittance, suggesting improved optical qualities, making the recycled ABS-MF composite a promising candidate for sensor applications [100,101,102,103,104,105].
The chemical recycling of ABS/PC blends was explored through pyrolysis as an alternative to mechanical recycling. TGA and FT-IR analysis revealed two distinct decomposition stages corresponding to ABS and PC, with flame retardants affecting the degradation pattern. CO2 emissions ranged from 6 to 8%, slightly higher (by 0.5–1%) in the flame-retarded blends. Maximum liquid yields, including phenol, styrene, and bisphenol A (BPA), were obtained at 480–500 °C. For non-flame-retarded blends, BPA was the dominant product (25–30%), whereas phenol (10–15%) was most prominent in the flame-retarded samples. Product characterization was performed using gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS), supporting pyrolysis as a promising method for recycling PC/ABS blends within a circular economy framework [53,105,106,107].
2.9. Plasma Recycling of ABS
To enhance compatibility between the two polymers, plasma-assisted mechanochemistry (PMC) was employed. PMC uses a dry process combining high-energy mechanical force with plasma gases, creating chemical bonds between degraded ABS and SBS radicals. This resulted in uniform SBS dispersion within the ABS matrix and smaller particle sizes. As a result, the upcycled blends showed increased impact strength and elongation at break. Traditional methods using compatibilizers and solvents face environmental and efficiency limitations, which PMC overcomes. The process achieved better recycling efficiency compared to commercial recycled ABS blends. It also avoids solvent use, making it environmentally sustainable. Overall, the PMC method provides a promising upcycling solution for post-consumer ABS waste. This technology uses CO2 plasma gasification of ABS-based computer keyboard plastic waste to produce clean syngas. The effects of feed rate, gas flow rate, and plasma power on syngas yield and composition were investigated. Optimal conditions yielded over 90% syngas with a high LHV of 16.46 MJ/m3 and up to 46.06% energy efficiency. Economic and environmental assessments showed a competitive LCOS and low global warming potential. The byproducts, including high-calorific oil and TiO2-rich residue, offer the potential for fuel and medical applications [108,109,110,111].
2.10. Boiling Treatment, Microwave Treatment, Fenton Reaction, and Froth Flotation
Boiling treatment effectively creates a hydrophilic surface on ABS plastic. FT-IR analysis was used to explore the mechanism behind this surface modification. Surface rearrangement in the polymer is likely responsible for these changes. Variations in polymer chain mobility may explain different responses to boiling treatment. Boiling treatment influences the selective floatability of waste plastics and flotation behavior such as flotation time, frother concentration, and particle size. Insights from simple plastic flotation behavior guided separation experiments, which enabled an efficient flotation separation of boiling-treated ABS and PS with varying particle sizes. The separation method demonstrated high effectiveness and selectivity. The resulting purities were 99.78% for ABS and 95.80% for PS, indicating strong process efficiency [112,113,114,115].
A combined method using microwave and mild-heat treatment with froth flotation was also effective in separating PVC and ABS from WEEE plastics. Microwave treatment, following coating with powdered activated carbon (PAC), selectively increased PVC’s hydrophilicity, enabling its separation with 100% recovery and purity under optimal flotation conditions. Subsequently, a 100 s mild-heat treatment enabled the effective separation of PAC-coated ABS containing brominated flame retardants (BFRs), achieving 100% recovery and purity due to the formation of a twisted, low-density structure. Even without microwave use, mild-heat treatment alone of PAC-coated WEEE mixtures successfully recovered both ABS and PVC. This flotation-based technique demonstrates high efficiency in separating plastics from mixed post-consumer waste. Although flotation is relatively new in plastic recycling, its origins trace back to mineral ore separation in the early 20th century [77,116,117,118,119].
A surface treatment method based on the Fenton reaction was quite effective in enhancing the flotation separation of waste plastics. Utilizing this advanced oxidation process (AOP), hydroxyl radicals (•OH) are generated from the reaction between hydrogen peroxide (H2O2) and ferrous ions (Fe2+). This treatment selectively modified the surface properties and floatability of ABS. FT-IR and XPS analyses were conducted to examine the underlying reaction mechanism. The method proved effective for separating mixed plastic particles of various sizes. It demonstrated high recovery rates and separation efficiencies. The process is suitable for handling diverse mass fractions. Overall, Fenton treatment improves precision in plastic recycling [120,121,122,123].
Froth flotation is widely recognized as one of the most applicable techniques for separating mixed waste plastics, and has been the subject of extensive research. This method relies on buoyancy principles to separate plastics with slight density differences and is particularly effective for binary mixtures, such as PVC/PS, PVC/PET, PVC/PC, PS/PET, and PS/ABS. The introduction of frothers like methyl isobutyl carbinol (MIBC) can enhance flotation efficiency by stabilizing bubbles and improving selectivity, but also introduces added costs and environmental concerns. Surface treatment techniques such as boiling, calcium hypochlorite (CHC) treatment, sodium persulfate treatment, and surface micro-alcoholysis treatment (SMAT) have also shown promise in enhancing surface hydrophobicity or hydrophilicity to improve separation performance. However, like frothers, these treatments may not be economically viable for large-scale applications. Hydrocyclone separation, another density-based method, involves the use of a medium with a density between the plastics to be separated and can be improved through optimized flow channel design and cone angle adjustment. Nevertheless, hydrocyclones are typically limited to binary plastic mixtures. In contrast, sensor-based separation techniques including visual, hyperspectral, and impact acoustic sensors, enable the detection of various plastic types and allow for multi-material separation when integrated with mechanical systems. Despite their effectiveness, the high cost of sensors and challenges in handling irregular, crushed plastic particles with varied sizes remain limitations [95,96,114,124].
2.11. Mechanical Recycling
Mechanical recycling is the most widely adopted route for ABS recovery because of its simplicity, scalability, and cost efficiency. It involves physical reprocessing steps such as collecting, shredding, washing, drying, and remelting into new products. Mechanical recycling of ABS begins with the collection and sorting of ABS waste, which is then shredded and thoroughly cleaned to remove impurities. The cleaned material undergoes extrusion, where it is melted and formed into pellets, which are later remolded into new ABS products. This method is energy-efficient and helps retain most of the original polymer structure. Recycling of ABS plastics presents challenges during multiple recycling cycles due to thermo-oxidative degradation, chain scission, and additive loss, which can reduce toughness, impact strength, and elongation. In blends or waste streams mixed with other polymers, immiscibility leads to phase separation and compromised mechanical performance, necessitating the use of compatibilizers or advanced separation technologies [49,125].
ABS degradation during reprocessing is primarily governed by thermal oxidation of butadiene units and chain fission within the SAN domains. The rubber phase is especially vulnerable due to its low glass transition temperature and high oxygen permeability, which leads to oxidation and the formation of carbonyl or hydroxyl groups. Over successive cycles, this results in embrittlement, discoloration, and loss of toughness. Strategies to mitigate degradation include the incorporation of stabilizers, compatibilizers, and chain extenders that repair or protect polymer chains. Surface modification, froth flotation, and density-based separation methods, such as magnetic levitation and dissolved air flotation, have also been applied to improve recovery efficiency and purity, particularly for mixed plastic waste streams containing ABS and other plastics [24,82,126].
Mechanical recycling of ABS remains the dominant pathway due to its lower energy footprint of 46.5 MJ/kg, compared to the virgin production of 94.5 MJ/kg [24], and its environmental benefits are substantial. Nonetheless, property deterioration after several cycles and contamination with other plastics remain limitations. To address these, reinforcement strategies such as fiber addition, compatibilizers, or coupling with solvent-based purification are being explored. The future outlook points toward integrating mechanical recycling with selective separation and additive replenishment, enabling higher-purity recovery and extending the service life of recycled ABS in high-value applications [49,125].
Using a mixture of 70% virgin ABS and 30% recycled ABS (rABS), wiring device components were manufactured, and this recycling cycle was repeated three times to simulate long-term reuse. Mechanical tests were conducted to assess the feasibility of using rABS in mass production applications. Although there was a slight reduction in tensile and impact strength when compared to pure ABS, the overall mechanical performance remained within acceptable ranges for industrial use. The results support the viability of incorporating recycled ABS into new products, especially in non-structural or moderate-strength applications [84,127]. An industrial physical sorting process for the purification and regeneration of ABS and PS in waste refrigerators was investigated. Through the analysis of bromine and heavy metal elements, the recycled PS and ABS flakes were proven to meet the commercial standard requirements of RoHS 2.0. Recycled flakes showed very low residual glass and metal content, confirming high material purity. The findings offer practical guidance for the large-scale industrial recycling of waste ABS plastics [56,128,129].
3. Compatibility of Recycled ABS Plastics
3.1. ABS and Polypropylene (PP)
Compatibilization between polypropylene (PP) and ABS, focusing on blending waste materials to enhance plastic reuse. Blending immiscible thermoplastics like PP and ABS offers a sustainable approach for engineering applications, including potential use in 3D printing. Effective compatibilization methods include interfacial agents, morphology control, and interpenetrating networks via cross-linking. Dual compatibilizers, such as organic ones and nanofillers, perform better than single ones in enhancing compatibility and blending performance. Compatibilizers promote interchain bonding, refining morphology, and improving physicochemical properties. PP and ABS are chemically inert and difficult to blend due to high interfacial tension, requiring compatibilizers to improve adhesion. Homogeneous blending is better achieved using twin-screw extruders, compared with single-screw extruders, though they may cause chain scission under stress [130,131,132,133,134].
3.2. ABS and Polystyrene (PS)
To restore the mechanical properties of recycled ABS (r-ABS), particularly impact resistance, various approaches have been explored. Adding styrene-ethylene/butylenes–styrene (SEBS) improves r-ABS ductility, while ABS graft powder enhances the toughness. Combining r-ABS with other polymers like polycarbonate (PC) or polyamide (PA) also recovers mechanical properties, demonstrating the potential of polymer blending for r-ABS recycling. ABS and high-impact polystyrene (HIPS) are commonly used as outer casings for computer equipment. Recycling and blending ABS and HIPS on their mechanical properties were conducted. Recycling caused negligible changes in glass-transition temperatures, tensile strengths, and tensile moduli but significantly reduced strains to failure and impact strengths. Blending small proportions of ABS and HIPS restored ductility, improving strains to failure. Simulated recycling showed that multiple passes had minimal impact on tensile properties but more noticeable effects on elongation and impact properties. Blending with small amounts of other polymers generally preserved mechanical properties, though impact properties required suitable modifiers for improvement [64,135,136,137,138,139,140].
3.3. ABS and Polymethylmethacrylate (PMMA)
Recycling of engineering plastics from dismantled automobiles was conducted by resin blending and compounding. Blending ABS, ABS/polycarbonate (70/30), and 10% polymethylmethacrylate (PMMA) with impact fillers of core–shell structure improves polymeric properties. However, polyamide remains incompatible with other polymeric resins and should be excluded from formulation. This incompatibility can lead to poor interfacial adhesion, phase separation, and inferior mechanical properties of the blend. Acrylonitrile-butadiene-styrene (ABS) copolymers make up a large portion of waste electrical and electronic equipment (WEEE) plastics due to their favorable mechanical properties and ease of processing. However, ABS is prone to oxidative aging under heat or UV exposure, resulting in mechanical degradation [48,141,142].
3.4. ABS and Polyvinyl Chloride (PVC)
The compatibility between recycled PVC from credit cards and both virgin and recycled ABS from WEEE was investigated. DSC analysis confirms partial compatibility between recycled PVC and ABS, mainly due to interactions between the polar groups of PVC and the SAN phase in ABS. Two glass transition temperatures shifting with composition indicate blend interaction. Virgin ABS provides better compatibility than recycled ABS due to stronger adhesion between its polybutadiene and SAN phases. Recycled ABS introduces interference from degraded polybutadiene, reducing compatibility. PVC can tolerate higher proportions of both virgin and recycled ABS than ABS can tolerate PVC. Tan delta curves show two peaks, reflecting varying phase behaviors depending on blend composition. Tensile strength analysis using the Equivalent Box Model shows stronger interfacial adhesion with virgin ABS blends [143,144,145].
3.5. ABS and Polycarbonate (PC)
The compatibility enhancement in ABS/polycarbonate (PC) blends was analyzed in terms of stress-whitened phases and dispersion. PC/ABS blends are widely used in automotive parts due to their excellent physical and processing properties, despite being fundamentally immiscible. Uncompatibilized blends can suffer from dispersed phase coalescence and loss of ductility during molding. Reactive compatibilizers like maleic anhydride (MAH)-functionalized polymers have been studied to improve morphology and impact strength. Blending PC/ABS with MAH-grafted ABS or epoxy-based compatibilizers enhances mechanical performance and compatibility. A sustainable filler system combining biocarbon (BC) with basalt (BF) or carbon (CF) fibers was used to reinforce PC/ABS and PBT/ABS blends. Hybrid composites with 20 wt% filler showed significant increases in stiffness up to 100%. Thermal resistance improved by 20 °C for BF/BC and 10–12 °C for CF/BC composites compared to unmodified matrices. While BC did not cause thermal degradation, mechanical performance was slightly reduced, especially after heat aging in PC/ABS blends.
The mechanical performance of recycled poly(acrylonitrile-butadiene-styrene) (RABS) and recycled polycarbonate (RPC)-rich blends without a compatibilizer is notably inferior. Minor degradation observed in RABS may contribute to the diminished mechanical properties of the blend. Incorporating 5% maleic anhydride-grafted polypropylene (MAP) as a compatibilizer enhances the interfacial adhesion between phases. This MAP addition induces a positive deviation in the mechanical performance of both RABS and RPC-rich blends. Further addition of solid epoxy results in a synergistic effect, notably improving modulus and tensile strength. The enhanced mechanical properties surpass those of virgin polycarbonate (VPC) and virgin poly(acrylonitrile-butadiene-styrene) (VABS). In RPC-rich blends, the inclusion of two nanoclays leads to additional improvements in modulus values. The thermal stability of the MAP- and epoxy-compatibilized blends is significantly enhanced compared to VABS. Nanoclay addition in the RPC-rich phase further contributes to superior thermal stability [146,147,148,149,150,151].
3.6. ABS and Nylon 6
Blending acrylonitrile–butadiene–styrene (ABS) with nylon (polyamide) combines the favorable properties of both polymers. ABS contributes ease of processing, dimensional stability, and moderate strength, while Nylon provides flexibility, chemical resistance, and exceptional toughness. However, Nylon alone absorbs moisture and has lower stiffness compared with PLA or ABS, whereas ABS lacks the high impact resistance and durability of Nylon. The hybridization of these two polymers offers a pathway to balance their complementary strengths and reduce inherent weaknesses, making ABS–Nylon blends attractive for engineering applications and additive manufacturing [152,153,154].
When reinforced with short carbon fibers (CFs), both ABS and Nylon exhibit notable improvements in mechanical properties. For instance, studies report that Nylon–CF composites achieve Young’s modulus values above 7 GPa and tensile strengths exceeding 58 MPa at optimal print orientations such as [0°/15°/−15°]. Similarly, ABS–CF composites display higher stiffness compared with neat ABS, although the magnitude of improvement is generally lower than that observed in Nylon–CF systems. Process parameters, particularly nozzle temperature, infill density, and raster orientation, play a critical role in determining the final properties, with optimized conditions yielding tensile strength improvements of 10–20% over unreinforced matrices [154,155,156].
ABS and nylon blends reinforced with carbon fibers emerge as promising candidates for high-performance 3D printing and injection molding applications. Their balanced combination of toughness, chemical resistance, and stiffness makes them suitable for automotive, aerospace, and consumer goods, where both durability and processability are required. Nevertheless, challenges such as anisotropy in fused filament fabrication (FFF and FDM), porosity, and increased nozzle wear due to abrasive fibers remain significant hurdles. Future research is expected to focus on hybrid reinforcement strategies, optimized blend ratios, and processing improvements to unlock the full industrial potential of ABS–Nylon composites [157,158,159].
4. Conclusions and Perspectives
Recent advancements in ABS recycling have demonstrated the potential of multiple approaches, including thermo-oxidative reprocessing, surface modification, flotation-assisted separation, plasma-assisted mechanochemistry, and gasification. Each of these methods offers unique advantages: flotation and triboelectrostatic techniques provide effective separation in mixed plastic streams, plasma and mechanochemical processes enable the production of compatibilizer-free blends, and gasification offers energy recovery pathways. However, these routes also face limitations in scalability, cost, or material property retention.
Among the emerging methods, dissolution-based separation appears to hold the greatest potential to become the dominant future technology. Unlike chemical depolymerization, this method preserves the polymer backbone, allowing for the recovery of high-purity ABS with minimal degradation. The ongoing development of eco-friendly solvent systems, such as ionic liquids and deep eutectic solvents, enhances both the environmental compatibility and scalability of this approach. Key challenges remain in solvent recovery, additive loss during repeated cycles, and economic feasibility, but research progress continues to address these issues.
Looking forward, the most likely perspective is a hybrid recycling landscape, where dissolution-based methods form the technological backbone for high-purity recovery, complemented by mechanical recycling for bulk applications, and advanced physical or thermal processes for niche cases. This integrated approach will be crucial in establishing industrially viable and sustainable pathways for ABS recycling and in advancing the transition toward a circular economy for plastics.
Funding
This project was supported by the Korea Institute for Advancement of Technology (KIAT) (P0026016) and the Ministry of Trade, Industry, and Energy (MOTIE) of the Republic of Korea. The APC was funded by the same research grant (P0026016). This project was also supported by a research grant from Hankyong National University in the year of 2025.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
This manuscript is a “review article”. No new data were created or analyzed in this study. Data sharing is not applicable.
Acknowledgments
The author gratefully acknowledges the support provided by the Korea Institute for Advancement of Technology (KIAT) and by Hankyong National University (HKNU).
Conflicts of Interest
The author declares no conflict of interest.
References
- Grand View Research. Plastic Market Size, Share & Trends Analysis (2024–2030). 2023. Available online: https://www.grandviewresearch.com/industry-analysis/global-plastics-market (accessed on 5 August 2025).
- Deshmukh, D.; Kulkarni, H.; Srivats, D.S.; Bhanushali, S.; More, A.P. Recycling of acrylonitrile butadiene styrene (ABS): A review. Polym. Bull. 2024, 81, 1–38. [Google Scholar] [CrossRef]
- Verified Market Reports. ABS Plastic Recycling Market Insights (2023–2033). 2025. Available online: https://www.verifiedmarketreports.com/product/abs-plastic-recycling-market/?utm_source=chatgpt.com/ (accessed on 5 August 2025).
- Grand View Research. Recycled Engineering Plastics Market Size, Share & Trends Analysis (2025–2030). 2025. Available online: https://www.grandviewresearch.com/industry-analysis/recycled-engineering-plastics-market-report (accessed on 5 August 2025).
- Dataintelo Consulting. ABS Plastic Recycling Market (2025–2033). 2025. Available online: https://dataintelo.com/report/abs-plastic-recycling-market?utm_source=chatgpt.com (accessed on 5 August 2025).
- PW Consulting Chemical & Energy Research Center. Acrylonitrile Butadiene Styrene (ABS) Recyclates Market. 2025. Available online: https://pmarketresearch.com/chemi/acrylonitrile-butadiene-styrene-abs-recyclates-market/?utm_source=chatgpt.com (accessed on 5 August 2025).
- Universite Cote d’Azur (France). Paving the Way for an ABS Recycling Revolution in the EU. 2025. Available online: https://cordis.europa.eu/project/id/101058636/reporting (accessed on 25 July 2025).
- European Commission. Directive (EU) 2018/852 of the European Parliament and of the Council of 30 May 2018 Amending Directive 94/62/EC on Packaging and Packaging Waste. 2018. Available online: https://eur-lex.europa.eu/eli/dir/2018/852/oj (accessed on 5 August 2025).
- Torkelis, A.; Dvarionienė, J.; Denafas, G. The Factors Influencing the Recycling of Plastic and Composite Packaging Waste. Sustainability 2024, 16, 9515. [Google Scholar] [CrossRef]
- van der Marel, E.R. Trading Plastic Waste in a Global Economy: Soundly Regulated by the Basel Convention? J. Environ. Law 2022, 34, 477–497. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, S.; Xu, W.; Chen, C.; Chen, A.; Lu, R.; Jing, Q.; Liu, J. Exploring long-term global environmental impacts of chlorinated paraffins (CPs) in waste: Implications for the Stockholm and Basel Conventions and the global plastic treaty. Environ. Int. 2024, 185, 108527. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Ward, H.; Lin, H.X.; Tukker, A. Shift to intra-EU-OECD trade enhanced environmental benefits after Basel Convention Plastic Waste Amendments. Resour. Conserv. Recycl. 2025, 223, 108527. [Google Scholar] [CrossRef]
- Hui, Z.; Haider, A.; Khan, A. International trade and plastic waste in oceans: Legal and policy challenges. Front. Mar. Sci. 2025, 12, 1627829. [Google Scholar] [CrossRef]
- Eckert, S.; Karassin, O.; Steinebach, Y. A policy portfolio approach to plastics throughout their life cycle: Supranational and national regulation in the European Union. Environ. Policy Gov. 2024, 34, 427–441. [Google Scholar] [CrossRef]
- Andrady, A.L.; Neal, M.A. Applications and societal benefits of plastics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Choi, W.H.; Pae, K.P.; Kim, N.S. Feasibility study of closed-loop recycling for plastic generated from waste electrical and electronic equipment (WEEE) in South Korea. Energies 2023, 16, 6358. [Google Scholar] [CrossRef]
- Musa, A.; Jaseer, E.A.; Barman, S.; Garcia, N. Review on Catalytic Depolymerization of Polyolefin Waste by Hydrogenolysis: State-of-the-Art and Outlook. Energy Fuels 2024, 38, 1676–1691. [Google Scholar] [CrossRef]
- Hamad, K.; Kaseem, M.; Deri, F. Recycling of waste from polymer materials: An overview of the recent works. Polym. Degrad. Stab. 2013, 98, 2801–2812. [Google Scholar] [CrossRef]
- Walker, R.; Korey, M.; Hubbard, A.M.; Clarkson, C.M.; Corum, T.; Smith, T.; Hershey, C.J.; Lindahl, J.; Ozcan, S.; Duty, C. Recycling of CF-ABS machining waste for large format additive manufacturing. Compos. Part B Eng. 2024, 275, 111291. [Google Scholar] [CrossRef]
- Arends, D.; Schlummer, M.; Mäurer, A. Removal of inorganic colour pigments from acrylonitrile butadiene styrene by dissolution-based recycling. J. Mater. Cycles Waste Manag. 2012, 14, 85–93. [Google Scholar] [CrossRef]
- Anderson, L.; Yu, E.; Chen, W.-T. Chemical Recycling of Mixed Plastics in Electronic Waste Using Solvent-Based Processing. Processes 2022, 10, 66. [Google Scholar] [CrossRef]
- Manish; Gurjar, D.; Sharma, S.; Akash; Sarkar, M. A Review on testing methods of recycled Acrylonitrile Butadiene-Styrene. Mater. Today Proc. 2018, 5, 28296–28304. [Google Scholar] [CrossRef]
- García, M.d.G.T.; Schlatter, M.; Cabrera, F.M.; Manzanares, J.T.; Hanafi, I. Recycling of Acrylonitrile–Butadiene–Styrene Using Injection Moulding Machine. Procedia Technol. 2016, 22, 399–406. [Google Scholar] [CrossRef]
- Lu, T.; Chen, W.-T. Material recycling of Acrylonitrile Butadiene Styrene (ABS) from toy waste using density separation and safer solvents. Resour. Conserv. Recycl. 2023, 197, 107090. [Google Scholar] [CrossRef]
- Tiwari, R.; Azad, N.; Dutta, D.; Yadav, B.R.; Kumar, S. A critical review and future perspective of plastic waste recycling. Sci. Total Environ. 2023, 881, 163433. [Google Scholar] [CrossRef] [PubMed]
- Bertin, M.-P.; Marin, G.; Montfort, J.-P. Viscoelastic properties of acrylonitrile-butadiene-styrene (ABS) polymers in the molten state. Polym. Eng. Sci. 1995, 35, 1394–1406. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, C.; Zhang, H.; Zou, J.; Zhou, F.; Jin, H. The resource utilization of ABS plastic waste with subcritical and supercritical water treatment. Int. J. Hydrogen Energy 2019, 44, 15758–15765. [Google Scholar] [CrossRef]
- Mordor Intelligence. Acrylonitrile Butadiene Styrene Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). 2025. Available online: https://www.mordorintelligence.com/industry-reports/acrylonitrile-butadiene-styrene-abs-resin-market (accessed on 18 July 2025).
- Clark, R.A.; Shaver, M.P. Depolymerization within a Circular Plastics System. Chem. Rev. 2024, 124, 2617–2650. [Google Scholar] [CrossRef]
- Miao, Y.; von Jouanne, A.; Yokochi, A. Current technologies in depolymerization process and the road ahead. Polymers 2021, 13, 449. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, D.; Czyżewski, P.; Sykutera, D.; Bieliński, M. Recycling of ABS operating elements obtained from industry 3D printing machines. Polimery 2021, 64, 803–810. [Google Scholar] [CrossRef]
- Freymond, C.; Mackré-Delannoy, X.; Guinault, A.; Charbuillet, C.; Fayolle, B. Thermal oxidation of acrylonitrile-butadiene-styrene: Origin of the ductile/brittle transition. Polym. Degrad. Stab. 2022, 206, 110186. [Google Scholar] [CrossRef]
- Alassali, A.; Barouta, D.; Tirion, H.; Moldt, Y.; Kuchta, K. Towards a high quality recycling of plastics from waste electrical and electronic equipment through separation of contaminated fractions. J. Hazard. Mater. 2020, 387, 121741. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef]
- Oguchi, M.; Sakanakura, H.; Terazono, A. Toxic metals in WEEE: Characterization and substance flow analysis in waste treatment processes. Sci. Total Environ. 2013, 463–464, 1124–1132. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, D.; Wei, N. Enzyme discovery and engineering for sustainable plastic recycling. Trends Biotechnol. 2022, 40, 22–37. [Google Scholar] [CrossRef]
- Aguiar, M.I.S.; Sousa, A.F.; Teixeira, G.; Tavares, A.P.M.; Ferreira, A.M.; Coutinho, J.A.P. Enhancing plastic waste recycling: Evaluating the impact of additives on the enzymatic polymer degradation. Catal. Today 2024, 429, 114492. [Google Scholar] [CrossRef]
- Orlando, M.; Molla, G.; Castellani, P.; Pirillo, V.; Torretta, V.; Ferronato, N. Microbial Enzyme Biotechnology to Reach Plastic Waste Circularity: Current Status, Problems and Perspectives. Int. J. Mol. Sci. 2023, 24, 3877. [Google Scholar] [CrossRef]
- Shanker, R.; Khan, D.; Hossain, R.; Islam, M.T.; Locock, K.; Ghose, A.; Sahajwalla, V.; Schandl, H.; Dhodapkar, R. Plastic waste recycling: Existing Indian scenario and future opportunities. Int. J. Environ. Sci. Technol. 2023, 20, 5895–5912. [Google Scholar] [CrossRef]
- Li, A.; Wu, L.; Cui, H.; Song, Y.; Zhang, X.; Li, X. Unlocking a Sustainable Future for Plastics: A Chemical-Enzymatic Pathway for Efficient Conversion of Mixed Waste to MHET and Energy-Saving PET Recycling. ChemSusChem 2024, 17, e202301612. [Google Scholar] [CrossRef]
- Rorrer, J.E.; Troyano-Valls, C.; Beckham, G.T.; Román-Leshkov, Y. Hydrogenolysis of Polypropylene and Mixed Polyolefin Plastic Waste over Ru/C to Produce Liquid Alkanes. ACS Sustain. Chem. Eng. 2021, 9, 11661–11666. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Z.; Zhang, X.; Li, T.; Li, Y.; Chen, X.; Wang, K. Catalytic hydrogenolysis of plastic to liquid hydrocarbons over a nickel-based catalyst. Environ. Pollut. 2022, 313, 120154. [Google Scholar] [CrossRef]
- Wang, M.; Gao, Y.; Yuan, S.; Deng, J.; Yang, J.; Yan, J.; Yu, S.; Xu, B.; Ma, D. Complete hydrogenolysis of mixed plastic wastes. Nat. Chem. Eng. 2024, 1, 376–384. [Google Scholar] [CrossRef]
- Kumar, S.; Sajwan, D.; Sharma, D.; Krishnan, V. Reductive Upcycling of Polyolefins, Polyesters and Mixed Plastic Wastes to Valuable Chemicals: Bridging Chemical Catalysis With Plastic Waste Management. Adv. Sustain. Syst. 2025, 9, 2500003. [Google Scholar] [CrossRef]
- Li, H.; Aguirre-Villegas, H.A.; Allen, R.D.; Bai, X.; Benson, C.H.; Beckham, G.T.; Bradshaw, S.L.; Brown, J.L.; Brown, R.C.; Cecon, V.S.; et al. Expanding plastics recycling technologies: Chemical aspects, technology status and challenges. Green Chem. 2022, 24, 8899–9002. [Google Scholar] [CrossRef]
- Karahaliou, E.K.; Tarantili, P.A. Stability of ABS compounds subjected to repeated cycles of extrusion processing. Polym. Eng. Sci. 2009, 49, 2269–2275. [Google Scholar] [CrossRef]
- de Sousa Filho, V.A.; de Azevedo, A.C.S.; de Oliveira, R.; Bonfim, R.L.P.; da Silva Amaral, A.K.; da Cunha, R.B.; Agrawal, P.; Cunha, C.T.C.; de Figueiredo Brito, G.; de Mélo, T.J.A. Properties of Recycled ABS and HIPS Polymers From WEEE and Their Blends With Virgin ABS Prepared by 3D Printing and Compression Molding. J. Appl. Polym. Sci. 2025, 142, e56797. [Google Scholar] [CrossRef]
- Liu, X.; Boldizar, A.; Rigdahl, M.; Bertilsson, H. Recycling of blends of acrylonitrile–butadiene–styrene (ABS) and polyamide. J. Appl. Polym. Sci. 2002, 86, 2535–2543. [Google Scholar] [CrossRef]
- Korey, M.; Rencheck, M.L.; Tekinalp, H.; Wasti, S.; Wang, P.; Bhagia, S.; Walker, R.; Smith, T.; Zhao, X.; Lamm, M.E.; et al. Recycling polymer composite granulate/regrind using big area additive manufacturing. Compos. Part B Eng. 2023, 256, 110652. [Google Scholar] [CrossRef]
- Pelto, J.; Barreto, C.; Anwar, H.; Strobl, L.; Schlummer, M. Compatibilized PC/ABS blends from solvent recycled PC and ABS polymers from electronic equipment waste. Polym. Test. 2023, 120, 107969. [Google Scholar] [CrossRef]
- Mishra, V.; Negi, S.; Kar, S. FDM-based additive manufacturing of recycled thermoplastics and associated composites. J. Mater. Cycles Waste Manag. 2023, 25, 758–784. [Google Scholar] [CrossRef]
- Hirschberg, V.; Rodrigue, D. Recycling of polyamides: Processes and conditions. J. Polym. Sci. 2023, 61, 1937–1958. [Google Scholar] [CrossRef]
- Balart, R.; López, J.; García, D.; Salvador, M.D. Recycling of ABS and PC from electrical and electronic waste. Effect of miscibility and previous degradation on final performance of industrial blends. Eur. Polym. J. 2005, 41, 2150–2160. [Google Scholar] [CrossRef]
- Barthes, M.L.; Mantaux, O.; Pedros, M. Recycling of aged ABS from real WEEE through ABS/PC blends in the ABS-rich compositions. Adv. Polym. Technol. 2012, 31, 343–353. [Google Scholar] [CrossRef]
- Bärwinkel, S.; Seidel, A.; Hobeika, S. Morphology formation in PC/ABS blends during thermal processing and the effect of the viscosity ratio of blend partners. Materials 2016, 9, 659. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.F.; Roelands, M.C.P.; White, R.J.; van Harmelen, T.; de Wild, P.; van der Laan, G.P.; Meirer, F.; Keurentjes, J.T.F.; Weckhuysen, B.M. Beyond Mechanical Recycling: Giving New Life to Plastic Waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef]
- Merrington, A. 9—Recycling of Plastics. In Applied Plastics Engineering Handbook, 3rd ed.; Kutz, M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2024; pp. 191–217. [Google Scholar]
- Kumar, R.; Sharma, H.; Saran, C.; Tripathy, T.S.; Sangwan, K.S.; Herrmann, C. A Comparative Study on the Life Cycle Assessment of a 3D Printed Product with PLA, ABS & PETG Materials. Procedia CIRP 2022, 107, 15–20. [Google Scholar] [CrossRef]
- Spicer, A.J.; Brandolese, A.; Dove, A.P. Selective and Sequential Catalytic Chemical Depolymerization and Upcycling of Mixed Plastics. ACS Macro Lett. 2024, 13, 189–194. [Google Scholar] [CrossRef]
- Al Rashid, A.; Koç, M. Additive manufacturing for sustainability and circular economy: Needs, challenges, and opportunities for 3D printing of recycled polymeric waste. Mater. Today Sustain. 2023, 24, 100529. [Google Scholar] [CrossRef]
- Mishra, V.; Ror, C.K.; Negi, S.; Kar, S.; Borah, L.N. Development of sustainable 3D printing filaments using recycled/virgin ABS blends: Processing and characterization. Polym. Eng. Sci. 2023, 63, 1890–1899. [Google Scholar] [CrossRef]
- Kuram, E.; Ozcelik, B.; Kocoglu, H.; Ayas, H.; Dogan, M. UV and outdoor weathering of glass fiber reinforced polycarbonate/acrylonitrile-butadiene-styrene composites and recycling of aged composites. J. Thermoplast. Compos. Mater. 2025, 38, 1427–1464. [Google Scholar] [CrossRef]
- Marquez, C.; Aerts, A.; Parida, D.; Glassee, I.; Mitta, H.; Li, L.; Van Geem, K.M.; Vanbroekhoven, K.; Feghali, E.; Elst, K. Monomer recycling of virgin polycarbonate (PC), end-of-life PC and PC-ABS blends by Ni-catalyzed reductive depolymerization. Green Chem. 2025, 27, 5709–5714. [Google Scholar] [CrossRef]
- Salari, D.; Ranjbar, H. Study on the recycling of ABS resins: Simulation of reprocessing and thermo-oxidation. Iran. Polym. J. 2008, 17, 599–610. [Google Scholar]
- Vilaplana, F.; Ribes-Greus, A.; Karlsson, S. Degradation of recycled high-impact polystyrene. Simulation by reprocessing and thermo-oxidation. Polym. Degrad. Stab. 2006, 91, 2163–2170. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, M. Triboelectrostatic separation for PP and ABS plastics in end of life passenger vehicles. J. Mater. Cycles Waste Manag. 2017, 19, 884–897. [Google Scholar] [CrossRef]
- Park, C.H.; Park, J.K.; Jeon, H.S.; Chun, B.C. Triboelectric series and charging properties of plastics using the designed vertical-reciprocation charger. J. Electrost. 2008, 66, 578–583. [Google Scholar] [CrossRef]
- Dodbiba, G.; Shibayama, A.; Miyazaki, T.; Fujita, T. Triboelectrostatic Separation of ABS, PS and PP Plastic Mixture. Mater. Trans. 2003, 44, 161–166. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, Z.; Bai, X.; Wang, H.; Zhang, H.; Hao, J.; Wang, C. A review of the research on triboelectric separation technology. Miner. Eng. 2024, 216, 108901. [Google Scholar] [CrossRef]
- He, X.; Zhong, Z.; Ouyang, Y.; Wang, J. Investigation of tribo-electrostatic separation mechanism for thermoplastics in e-waste based on functional group distribution and surface potential. Sep. Purif. Technol. 2025, 362, 131764. [Google Scholar] [CrossRef]
- Dani, C.; Achouri, I.E.; Zeghloul, T.; Aouimeur, D.; Lungu, M.; Dascalescu, L. Triboelectric Charging and Electrostatic Separation of Granular Plastic Wastes Exposed to Long-Term Action of High Levels of Ambient Humidity. IEEE Trans. Ind. Appl. 2025, 61, 1194–1201. [Google Scholar] [CrossRef]
- Achouri, I.-E.; Csaba, D.; Thami, Z.; Mihai, L.; Dascalescu, L. Effect of ambient humidity on the tribo-electrostatic separation of granular plastic wastes. Part. Sci. Technol. 2024, 42, 908–914. [Google Scholar] [CrossRef]
- Achouri, I.E.; Richard, G.; Zeghloul, T.; Medles, K.; Dascalescu, L. New Vibrating-Table-Type Tribo-Electrostatic Separator for Selective Sorting of Granular Plastic Wastes. IEEE Trans. Ind. Appl. 2024, 60, 3537–3542. [Google Scholar] [CrossRef]
- Wang, H.; Chen, X.; Bai, Y. Application of dissolved air flotation on separation of waste plastics ABS and PS. Waste Manag. 2012, 32, 1297–1305. [Google Scholar] [CrossRef]
- Fagkaew, P.; Chawaloesphonsiya, N.; Bun, S.; Painmanakul, P. Improving the Separation of PS and ABS Plastics Using Modified Induced Air Flotation with a Mixing Device. Recycling 2022, 7, 44. [Google Scholar] [CrossRef]
- Wei, M.; Yang, T.; An, L.; Meng, X.; Tan, J.; Zhang, X. Kinetic, artificial neural network, and statistical modeling to optimize the parameters of the air flotation process to remove latex suspended solids in ABS wastewater. J. Water Process Eng. 2023, 56, 104417. [Google Scholar] [CrossRef]
- Thanh Truc, N.T.; Lee, B.-K. Sustainable and Selective Separation of PVC and ABS from a WEEE Plastic Mixture Using Microwave and/or Mild-Heat Treatment with Froth Flotation. Environ. Sci. Technol. 2016, 50, 10580–10587. [Google Scholar] [CrossRef]
- TNO Insights. Application of Dissolution to Recycle ABS Plastics in a Circular Way. 8 March 2024. Available online: https://www.tno.nl/en/newsroom/insights/2024/03/recycling-abs-plastics-with-dissolution/ (accessed on 1 March 2025).
- Arostegui, A.; Sarrionandia, M.; Aurrekoetxea, J.; Urrutibeascoa, I. Effect of dissolution-based recycling on the degradation and the mechanical properties of acrylonitrile–butadiene–styrene copolymer. Polym. Degrad. Stab. 2006, 91, 2768–2774. [Google Scholar] [CrossRef]
- Chen, P.; Chiang, C.H. Taguchi Method for Investigation of Ultrasonication-Assisted Dissolution of Acrylonitrile Butadiene Styrene (ABS) Rod Enclosed Within Polydimethylsiloxane (PDMS) Bulk. IEEE Access 2020, 8, 114910–114915. [Google Scholar] [CrossRef]
- Demircali, A.A.; Yilmaz, D.; Yilmaz, A.; Keskin, O.; Keshavarz, M.; Uvet, H. Enhancing mechanical properties and surface quality of FDM-printed ABS: A comprehensive study on cold acetone vapor treatment. Int. J. Adv. Manuf. Technol. 2024, 130, 4027–4039. [Google Scholar] [CrossRef]
- Titone, V.; Botta, L.; La Mantia, F.P. Mechanical Recycling of New and Challenging Polymer Systems: A Brief Overview. Macromol. Mater. Eng. 2025, 310, 2400275. [Google Scholar] [CrossRef]
- Chen, W.-T.; Nien-hwa, L.W.; Jin, K. Method of Converting Plastic Waste into Useful Stock. U.S. Patent No. 10,894,870, 19 January 2021. [Google Scholar]
- Achilias, D.S.; Giannoulis, A.; Papageorgiou, G.Z. Recycling of polymers from plastic packaging materials using the dissolution–reprecipitation technique. Polym. Bull. 2009, 63, 449–465. [Google Scholar] [CrossRef]
- Weeden, G.S., Jr.; Soepriatna, N.H.; Wang, N.-H.L. Method for Efficient Recovery of High-Purity Polycarbonates from Electronic Waste. Environ. Sci. Technol. 2015, 49, 2425–2433. [Google Scholar] [CrossRef]
- Cervantes-Reyes, A.; Núñez-Pineda, A.; Barrera-Díaz, C.; Varela-Guerrero, V.; Martínez-Barrera, G.; Cuevas-Yañez, E. Solvent effect in the polyethylene recovery from multilayer postconsumer aseptic packaging. Waste Manag. 2015, 38, 61–64. [Google Scholar] [CrossRef]
- García, M.T.; Duque, G.; Gracia, I.; de Lucas, A.; Rodríguez, J.F. Recycling extruded polystyrene by dissolution with suitable solvents. J. Mater. Cycles Waste Manag. 2009, 11, 2–5. [Google Scholar] [CrossRef]
- Mumladze, T.; Yousef, S.; Tatariants, M.; Kriūkienė, R.; Makarevicius, V.; Lukošiūtė, S.-I.; Bendikiene, R.; Denafas, G. Sustainable approach to recycling of multilayer flexible packaging using switchable hydrophilicity solvents. Green Chem. 2018, 20, 3604–3618. [Google Scholar] [CrossRef]
- Walker, T.W.; Frelka, N.; Shen, Z.; Chew, A.K.; Banick, J.; Grey, S.; Kim, M.S.; Dumesic, J.A.; Van Lehn, R.C.; Huber, G.W. Recycling of multilayer plastic packaging materials by solvent-targeted recovery and precipitation. Sci. Adv. 2020, 6, eaba7599. [Google Scholar] [CrossRef]
- Kim, T.G.; Srivastava, R.R.; Jun, M.; Kim, M.-S.; Lee, J.-C. Hydrometallurgical recycling of surface-coated metals from automobile-discarded ABS plastic waste. Waste Manag. 2018, 80, 414–422. [Google Scholar] [CrossRef]
- Yuan, W.; Teng, C.; Zhao, Y.; Huang, Q.; Wang, X.; Cai, K.; Song, Q.; Zhang, L.; Zhu, J.; Xu, L.; et al. Efficient recycling of surface-plated metals from ABS plastic waste via ammonium persulfate system. Sep. Purif. Technol. 2023, 326, 124796. [Google Scholar] [CrossRef]
- Balogun, A.F.; Baba, A.A.; Olaoluwa, D.T. Selective Extraction of Copper from Ammonia-Ammonium Sulphate Solution Using a Mixture of LIX 84-I and TBP: Parameter Determination. Mater. Circ. Econ. 2025, 7, 13. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Lu, H.; Huang, Z. Recycling of phosphorus-containing plastic based on the dual effects of switchable hydrophilicity solvents. Chemosphere 2020, 259, 127402. [Google Scholar] [CrossRef]
- Zhao, P.; Xie, J.; Gu, F.; Sharmin, N.; Hall, P.; Fu, J. Separation of mixed waste plastics via magnetic levitation. Waste Manag. 2018, 76, 46–54. [Google Scholar] [CrossRef]
- Xie, J.; Lin, W.; Lyu, C.; Zhang, L.; Zhao, P.; Li, J. Total separation of multi-plastic wastes using magnetic levitation with adjustable sensitivity. Sep. Purif. Technol. 2025, 357, 130118. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, P.; Zhang, J.; Zhou, H.; Fu, J.; Turng, L.-S. Characterization of polymer materials using magnetic levitation. J. Mater. Res. 2020, 35, 1182–1189. [Google Scholar] [CrossRef]
- Xia, L.; Liu, R.; Liu, J.; Zhu, X.; Ding, A.; Cao, Q. Radial Magnetic Levitation and Its Application to Density Measurement, Separation, and Detection of Microplastics. Anal. Chem. 2023, 95, 8660–8667. [Google Scholar] [CrossRef]
- Bai, B.; Jin, H.; Zhu, S.; Wu, P.; Fan, C.; Sun, J. Experimental investigation on in-situ hydrogenation induced gasification characteristics of acrylonitrile butadiene styrene (ABS) microplastics in supercritical water. Fuel Process Technol. 2019, 192, 170–178. [Google Scholar] [CrossRef]
- Lachos-Perez, D.; Brown, A.B.; Mudhoo, A.; Martinez, J.; Timko, M.T.; Rostagno, M.A.; Forster-Carneiro, T. Applications of subcritical and supercritical water conditions for extraction, hydrolysis, gasification, and carbonization of biomass: A critical review. Biofuel Res. J. 2017, 4, 611–626. [Google Scholar] [CrossRef]
- Li, M.; Wang, W.; Yu, J. Comparison of the pyrolysis behavior of PC, ABS and PC/ABS. J. Anal. Appl. Pyrolysis 2024, 183, 106774. [Google Scholar] [CrossRef]
- Areeprasert, C.; Khaobang, C. Pyrolysis and catalytic reforming of ABS/PC and PCB using biochar and e-waste char as alternative green catalysts for oil and metal recovery. Fuel Process Technol. 2018, 182, 26–36. [Google Scholar] [CrossRef]
- Liu, G.; Liao, Y.; Ma, X. Thermal behavior of vehicle plastic blends contained acrylonitrile-butadiene-styrene (ABS) in pyrolysis using TG-FTIR. Waste Manag. 2017, 61, 315–326. [Google Scholar] [CrossRef]
- Szabo, E.; Olah, M.; Ronkay, F.; Miskolczi, N.; Blazso, M. Characterization of the liquid product recovered through pyrolysis of PMMA–ABS waste. J. Anal. Appl. Pyrolysis 2011, 92, 19–24. [Google Scholar] [CrossRef]
- Singh, G.; Brar, G.S.; Singh, R. On successive recycling of acrylonitrile butadiene styrene- melamine formaldehyde composite for sensing applications. J. Thermoplast. Compos. Mater. 2025, 38, 08927057251314438. [Google Scholar] [CrossRef]
- Rathsack, P.-H.; Scheithauer, D.; Kleeberg, J.; Gräbner, M. Chemical recycling of PC/ABS-blends by pyrolysis. J. Anal. Appl. Pyrolysis 2025, 188, 107047. [Google Scholar] [CrossRef]
- Chen, S.C.; Liao, W.H.; Hsieh, M.W. Influence of recycled ABS added to virgin polymers on the physical, mechanical properties and molding characteristics. Polym. Plast. Technol. Eng. 2011, 50, 306–311. [Google Scholar] [CrossRef]
- Sathish, T.; Sabarirajan, N.; Ravichandran, S.; Moorthy, G.M.; Dinesh Kumar, S. Novel study on improvement of plastics properties by blending of waste micro plastics into ABS plastics. Chemosphere 2022, 303, 134997. [Google Scholar] [CrossRef]
- Nam, Y.; Lee, S.; Jee, S.M.; Bang, J.; Kim, J.H.; Park, J.H. High efficiency upcycling of post-consumer acrylonitrile-butadiene-styrene via plasma-assisted mechanochemistry. Chem. Eng. J. 2024, 480, 147960. [Google Scholar] [CrossRef]
- Dorigato, A. Recycling of polymer blends. Adv. Ind. Eng. Polym. Res. 2021, 4, 53–69. [Google Scholar] [CrossRef]
- Mallick, R.; Vairakannu, P. Experimental investigation of acrylonitrile butadiene styrene plastics plasma gasification. J. Environ. Manag. 2023, 345, 118655. [Google Scholar] [CrossRef]
- Kusano, R.; Kusano, Y. Applications of Plasma Technologies in Recycling Processes. Materials 2024, 17, 1687. [Google Scholar] [CrossRef]
- Wang, C.Q.; Wang, H.; Bao-xin, W.; Liu, Q. Boiling treatment of ABS and PS plastics for flotation separation. Waste Manag. 2014, 34, 1206–1210. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, H.; Wang, C. Flotation separation of acrylonitrile-butadiene-styrene and polystyrene in WEEE based on oxidation of active sites. Miner. Eng. 2020, 146, 106131. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Wang, C. Surface modification and selective flotation of waste plastics for effective recycling—A review. Sep. Purif. Technol. 2019, 226, 75–94. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, H.; Wang, H.; Wang, C. Separation of hazardous polyvinyl chloride from waste plastics by flotation assisted with surface modification of ammonium persulfate: Process and mechanism. J. Hazard. Mater. 2020, 389, 121918. [Google Scholar] [CrossRef]
- Pakbar, H.; Ostad Movahed, S.; Jourabchi, S. The effect of pre-microwave irradiation on the floatation of polystyrene, polyethylene terephthalate, and polyvinylchloride using numerous traditional depressants. Prog. Rubber Plast. Recycl. Technol. 2023, 39, 307–324. [Google Scholar] [CrossRef]
- Utimura, S.K.; Chaves, A.P.; Tenório, J.A.S.; Espinosa, D.C.R. Selecting chemicals for separation of ABS and HIPS in WEEE by froth flotation. Polimeros 2019, 29, e2019017. [Google Scholar] [CrossRef]
- Fraunholcz, N. Separation of waste plastics by froth flotation—A review, part I. Miner. Eng. 2004, 17, 261–268. [Google Scholar] [CrossRef]
- Pascoe, R.D. The use of selective depressants for the separation of ABS and HIPS by froth flotation. Miner. Eng. 2005, 18, 233–237. [Google Scholar] [CrossRef]
- Wang, J.-c.; Wang, H.; Huang, L.-l.; Wang, C.-q. Surface treatment with Fenton for separation of acrylonitrile-butadiene-styrene and polyvinylchloride waste plastics by flotation. Waste Manag. 2017, 67, 20–26. [Google Scholar] [CrossRef]
- Chow, C.-F.; Wong, W.-L.; Chan, C.-W.; Chan, C.-S. Converting inert plastic waste into energetic materials: A study on the light-accelerated decomposition of plastic waste with the Fenton reaction. Waste Manag. 2018, 75, 174–180. [Google Scholar] [CrossRef]
- Li, W.; Li, Y. Selective flotation separation of polycarbonate from plastic mixtures based on Fenton treatment combined with ultrasonic. J. Mater. Cycles Waste Manag. 2022, 24, 917–926. [Google Scholar] [CrossRef]
- Bule Možar, K.; Miloloža, M.; Martinjak, V.; Radovanović-Perić, F.; Bafti, A.; Ujević Bošnjak, M.; Markić, M.; Bolanča, T.; Cvetnić, M.; Kučić Grgić, D.; et al. Evaluation of Fenton, Photo-Fenton and Fenton-like Processes in Degradation of PE, PP, and PVC Microplastics. Water 2024, 16, 673. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, J.; Jin, H.; Guo, L. Current research progress of physical and biological methods for disposing waste plastics. J. Clean. Prod. 2023, 408, 137199. [Google Scholar] [CrossRef]
- Ceretti, D.V.A.; Edeleva, M.; Cardon, L.; D’hooge, D.R. Molecular Pathways for Polymer Degradation during Conventional Processing, Additive Manufacturing, and Mechanical Recycling. Molecules 2023, 28, 2344. [Google Scholar] [CrossRef]
- Babaremu, K.; Adediji, A.; Olumba, N.; Okoya, S.; Akinlabi, E.; Oyinlola, M. Technological Advances in Mechanical Recycling Innovations and Corresponding Impacts on the Circular Economy of Plastics. Environments 2024, 11, 38. [Google Scholar] [CrossRef]
- ÇEtİN, E.; TÜRkan, O.T. Material recycling of acrylonitrile butadiene styrene (ABS) from wiring devices using mechanical recycling. Sustain. Chem. Environ. 2024, 6, 100095. [Google Scholar] [CrossRef]
- Tinz, J.; de Ancos, T.; Rohn, H. Carbon Footprint of Mechanical Recycling of Post-Industrial Plastic Waste: Study of ABS, PA66GF30, PC and POM Regrinds. Waste 2023, 1, 127–139. [Google Scholar] [CrossRef]
- Dong, L.; Zhi, W.; Li, J.; Li, W. Fine Regeneration of ABS and PS Plastics from Waste Refrigerators through Mechanical Physical Recycling. ACS Sustain. Resour. Manag. 2024, 1, 908–915. [Google Scholar] [CrossRef]
- Singh, P.; Katiyar, P.; Singh, H. Impact of compatibilization on polypropylene (PP) and acrylonitrile butadiene styrene (ABS) blend: A review. Mater. Today Proc. 2023, 78, 189–197. [Google Scholar] [CrossRef]
- Luna, C.B.B.; Siqueira, D.D.; Araújo, E.M.; do Nascimento, E.P.; da Costa Agra de Melo, J.B. Evaluation of the SEBS copolymer in the compatibility of PP/ABS blends through mechanical, thermal, thermomechanical properties, and morphology. Polym. Adv. Technol. 2022, 33, 111–124. [Google Scholar] [CrossRef]
- Pê, F.R.; dos Santos Filho, E.A.; de Souza, M.F.; Dias, R.A.; Severo, A.M.C.; do Nascimento, E.P.; Wellen, R.M.R.; Araújo, E.M.; Luna, C.B.B. Toward improving the compatibility of the polypropylene (PP)/acrylonitrile–butadiene–styrene (ABS) blends through the incorporation of SEP and SEBS copolymers. Polym. Bull. 2024, 81, 1–28. [Google Scholar] [CrossRef]
- Bonda, S.; Mohanty, S.; Nayak, S.K. Influence of compatibilizer on mechanical, morphological and rheological properties of PP/ABS blends. Iran. Polym. J. 2014, 23, 415–425. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Liao, Q.; Xu, Z. Environmentally-friendly technology for rapid on-line recycling of acrylonitrile-butadiene-styrene, polystyrene and polypropylene using near-infrared spectroscopy. J. Clean. Prod. 2019, 213, 838–844. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Song, J.; He, M.; Song, J.; Xia, K. Recycling of acrylonitrile–butadiene–styrene (ABS) copolymers from waste electrical and electronic equipment (WEEE), through using an epoxy-based chain extender. Polym. Degrad. Stab. 2015, 112, 167–174. [Google Scholar] [CrossRef]
- Brennan, L.B.; Isaac, D.H.; Arnold, J.C. Recycling of acrylonitrile–butadiene–styrene and high-impact polystyrene from waste computer equipment. J. Appl. Polym. Sci. 2002, 86, 572–578. [Google Scholar] [CrossRef]
- Feng, J.; Yuan, Q.; Sun, X. Improving the properties of ABS by blending with PP and using PP-g-PS as a compatibilizer. Polym.-Plast. Technol. Mater. 2021, 60, 798–806. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Gopalakrishna, K.; Vivek, C.S. Plating on acrylonitrile–butadiene–styrene (ABS) plastic: A review. J. Mater. Sci. 2016, 51, 3657–3674. [Google Scholar] [CrossRef]
- Vazquez, Y.V.; Barbosa, S.E. Process Window for Direct Recycling of Acrylonitrile-Butadiene-Styrene and High-Impact Polystyrene from Electrical and Electronic Equipment Waste. Waste Manag. 2017, 59, 403–408. [Google Scholar] [CrossRef]
- Teixeira, F.d.S.M.; Peres, A.C.d.C.; Pacheco, E.B.A.V. Mechanical recycling of acrylonitrile-butadiene-styrene copolymer and high impact polystyrene from waste electrical and electronic equipment to comply with the circular economy. Front. Sustain. 2023, 4, 1203457. [Google Scholar] [CrossRef]
- Liu, X.; Bertilsson, H. Recycling of ABS and ABS/PC blends. J. Appl. Polym. Sci. 1999, 74, 510–515. [Google Scholar] [CrossRef]
- Signoret, C.; Girard, P.; Guen, A.L.; Caro-Bretelle, A.-S.; Lopez-Cuesta, J.-M.; Ienny, P.; Perrin, D. Degradation of Styrenic Plastics during Recycling: Accommodation of PP within ABS after WEEE Plastics Imperfect Sorting. Polymers 2021, 13, 1439. [Google Scholar] [CrossRef]
- Garcia, D.; Balart, R.; Sánchez, L.; López, J. Compatibility of recycled PVC/ABS blends. Effect of previous degradation. Polym. Eng. Sci. 2007, 47, 789–796. [Google Scholar] [CrossRef]
- Zhang, L.-X.; Zhou, C.; Sun, S.-L.; Ren, L.; Ma, X.-L.; Zhang, M.-Y.; Zhang, H.-X. Study of compatibility, morphology structure and mechanical properties of CPVC/ABS blends. J. Appl. Polym. Sci. 2010, 116, 3448–3454. [Google Scholar] [CrossRef]
- Hosseinpour, P.M.; Morshedian, J.; Barikani, M.; Azizi, H.; Pakdaman, A.S. Morphological, mechanical, and rheological studies of PVC/ABS blends in the presence of maleic anhydride. J. Vinyl Addit. Technol. 2010, 16, 127–134. [Google Scholar] [CrossRef]
- Mahanta, D.; Dayanidhi, S.A.; Mohanty, S.; Nayak, S.K. Mechanical, thermal, and morphological properties of recycled polycarbonate/recycled poly(acrylonitrile-butadiene-styrene) blend nanocomposites. Polym. Compos. 2012, 33, 2114–2124. [Google Scholar] [CrossRef]
- Jin, D.W.; Shon, K.H.; Jeong, H.M.; Kim, B.K. Compatibility enhancement of ABS/polycarbonate blends. J. Appl. Polym. Sci. 1998, 69, 533–542. [Google Scholar] [CrossRef]
- Nishino, K.; Shindo, Y.; Takayama, T.; Ito, H. Improvement of impact strength and hydrolytic stability of PC/ABS blend using reactive polymer. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Andrzejewski, J.; Danielak, A.; Piasecki, A.; Islam, A.; Szostak, M. Biocarbon-based sustainable reinforcing system for technical polymers. The structure-properties correlation between polycarbonate (PC) and polybutylene terephthalate (PBT)-based blends containing acrylonitrile-butadiene-styrene (ABS). Sustain. Mater. Technol. 2023, 36, e00612. [Google Scholar] [CrossRef]
- Zhang, B.B.; Chen, Y.; Wang, F.; Hong, R.Y. Surface modification of carbon black for the reinforcement of polycarbonate/acrylonitrile–butadiene–styrene blends. Appl. Surf. Sci. 2015, 351, 280–288. [Google Scholar] [CrossRef]
- Murakami, O. Technology for recycling plastic materials. Environ. Technol. Ed. 2001, 96, 6–9. [Google Scholar]
- Rodríguez-Reyna, S.L.; Mata, C.; Díaz-Aguilera, J.H.; Acevedo-Parra, H.R.; Tapia, F. Mechanical properties optimization for PLA, ABS and Nylon + CF manufactured by 3D FDM printing. Mater. Today Commun. 2022, 33, 104774. [Google Scholar] [CrossRef]
- Yankin, A.; Alipov, Y.; Temirgali, A.; Serik, G.; Danenova, S.; Talamona, D.; Perveen, A. Optimization of Printing Parameters to Enhance Tensile Properties of ABS and Nylon Produced by Fused Filament Fabrication. Polymers 2023, 15, 3043. [Google Scholar] [CrossRef]
- Kudva, R.A.; Keskkula, H.; Paul, D.R. Properties of compatibilized nylon 6/ABS blends: Part I. Effect of ABS type. Polymer 2000, 41, 225–237. [Google Scholar] [CrossRef]
- Venkatesh, R.; Jerold John Britto, J.; Amudhan, K.; Anbumalar, V.; Prabhakaran, R.; Thiyanesh Sakthi, R. Experimental investigation of mechanical properties on CF reinforced PLA, ABS and Nylon composite part. Mater. Today Proc. 2023, 76, 647–653. [Google Scholar] [CrossRef]
- Luna, C.B.B.; do Nascimento, E.P.; Siqueira, D.D.; Soares, B.G.; Agrawal, P.; de Mélo, T.J.A.; Araújo, E.M. Tailoring Nylon 6/Acrylonitrile-Butadiene-Styrene Nanocomposites for Application against Electromagnetic Interference: Evaluation of the Mechanical, Thermal and Electrical Behavior, and the Electromagnetic Shielding Efficiency. Int. J. Mol. Sci. 2022, 23, 9020. [Google Scholar] [CrossRef] [PubMed]
- Lay, M.; Thajudin, N.L.N.; Hamid, Z.A.A.; Rusli, A.; Abdullah, M.K.; Shuib, R.K. Comparison of physical and mechanical properties of PLA, ABS and nylon 6 fabricated using fused deposition modeling and injection molding. Compos. Part B Eng. 2019, 176, 107341. [Google Scholar] [CrossRef]
- Peterson, A.M. Review of acrylonitrile butadiene styrene in fused filament fabrication: A plastics engineering-focused perspective. Addit. Manuf. 2019, 27, 363–371. [Google Scholar] [CrossRef]
- Bhaskar, R.; Butt, J.; Shirvani, H. Investigating the Properties of ABS-Based Plastic Composites Manufactured by Composite Plastic Manufacturing. J. Manuf. Mater. Process. 2022, 6, 163. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).