Abstract
This study was conducted in nine allotment garden complexes in Wrocław, West Poland (Central Europe). Soil samples were collected from each garden and analyzed for their total concentrations of Zn, Cu, Pb and Cd, alongside the percentage of organic carbon C. Contaminant levels varied widely between sites: Zn ranged from 101.1 to 3464.5 mg/kg, Cu from 24.93 to 322.45 mg/kg, Cd from 0.51 to 6.31 mg/kg, and Pb from 19.92 to 401.85 mg/kg. The highest metal contamination was found for the garden complex placed on the former impact of the Hutmen. The organic carbon content ranged from 2.12% to 7.64%, indicating substantial variability in organic matter levels across the studied sites. This variability may significantly influence the soils’ capacity to retain heavy metals. A significant positive correlation was observed between soil organic carbon and the total concentrations of Pb, Cu and Zn, suggesting that soils richer in organic matter may retain higher levels of heavy metals. These findings underscore the dual role of organic matter as both a beneficial soil component and a potential contributor to heavy metal retention in urban garden soils. Protecting and enhancing SOM in polluted soils is a beneficial strategy, remediating environmental damage while aligning with global sustainability goals.
1. Introduction
Urban allotment gardens provide a multifunctional space in modern cities, combining ecological, social, and economic roles. They support biodiversity, recreation, and urban food production, while also offering vital ecosystem services to local communities [1,2,3]. These services depend strongly on soil health, which is influenced by both beneficial and harmful soil components [1]. Soil health is the foundation of many processes that influence sustainable urban development, and the links between it and other indicators are multidirectional. Two of the most critical indicators of soil quality are soil organic matter (SOM) content and the presence of trace elements, particularly heavy metals [2]. SOM plays a fundamental role in sustaining key soil functions and regulating chemical processes, making it essential for long-term fertility and crop safety [3]. As noted by [3,4], SOM serves as an integrator of biological, chemical, and physical soil properties and is thus considered a robust soil quality indicator. Urban soil health studies are a key tool in sustainable development planning because they provide data that enables decisions to be made that improve residents’ quality of life, protect the environment and support the circular economy
The risk of contamination in urban garden soils stems from both historical and current sources [5]. Many garden plots are located on or near former industrial sites, roads with heavy traffic, or areas reclaimed from past urban uses [6]. These locations can accumulate metals such as Pb, Cd, Zn, and Cu, originating from factories, mining, or construction debris [7,8,9]. At the same time, ongoing gardening practices may contribute to contamination. The use of fertilizers and pesticides containing trace metal impurities [10] and amendments such as sewage sludge [4] can introduce additional heavy metals into the soil. Although certain metals like nickel (Ni), Zn, and Cu are essential for plant development, their excess in bioavailable forms can be toxic to both plants and humans [11]. Anthropogenic sources such as industrial emissions, compost, organic fertilizers, and waste, as well as natural inputs like rock weathering and forest fires, further compound the issue [12]. Contaminated dust from coal-fired power plants and metal processing can also settle directly on plant surfaces, increasing food chain exposure [13]. The risk of contamination is not just a health issue; it also has a direct impact on sustainable development goals. While urban gardening supports SDG 2 (Zero Hunger) and SDG 11 (Sustainable Cities), contaminated soils threaten environmental health and social equity without adequate remediation and monitoring.
The behavior and bioavailability of heavy metals in soil are governed by several interacting factors, particularly pH, fertilization, redox conditions, and SOM content. Soil acidity increases metal mobility, making contaminants more available for plant uptake. For example, Cd becomes more mobile at pH 6.5, Zn at pH 6, and Pb at pH 4.5 [14,15]. Practices like liming can reduce heavy metal mobility but may also cause unintended effects under alkaline conditions, such as the formation of mobile organo-metallic complexes [15,16]. The use of mineral fertilizers, especially those containing phosphorus, may introduce metals through contaminated raw materials [17]. SOM plays a dual role: it can bind metals into insoluble forms, limiting their movement [18,19], while also enhancing soil structure and plant tolerance to contamination [20,21]. However, the effectiveness of SOM depends on factors like humic compound composition and particle size [10,15]. Under waterlogged conditions, changing the redox potential (Eh) can further affect mobility—mobilizing elements like iron (Fe) and manganese (Mn) [22,23], or promoting immobilization through sulfide formation in soils with a high sulfate content [24].
Despite increasing interest in urban soil contamination, relatively few studies have explored the relationship between SOM and heavy metal retention in allotment gardens. Most research has focused on trace element levels in urban settings [25,26,27,28], with limited attention to how gardening practices affect soil quality [29]. However, understanding this relationship is essential for designing better management strategies for urban soils [30]. Studies show that despite frequent use, garden soils often maintain high fertility, including increased soil organic carbon SOC stocks compared to non-garden soils [31,32,33]. In fact, many urban soils exhibit deep SOM accumulation and are classified as Hortisols [34], underlining their long-term cultivation and amendment history. The specific role of SOM in modifying heavy metal bioavailability, especially in the context of urban gardening, remains underexplored.
The aim of this study was to determine the effect of SOC content compared to heavy metal pollution levels in urban allotment garden soils [35,36,37,38]. The research was conducted in nine allotment garden complexes in Wrocław (West Poland), where soils are influenced by varying degrees of urbanization and industrial legacy. These gardens are frequently used for vegetable production, and their soils are enriched with natural fertilizers to improve structure and pH [39,40,41]. We hypothesized that soils with a higher organic carbon content would show an increased retention of heavy metals (Zn, Cu, Pb, Cd), reflecting both contamination history and management practices.
2. Materials and Methods
2.1. Study Site
The selection of the nine allotment garden complexes was deliberate to encompass a wide range of environmental conditions and potential sources of soil contamination within Wrocław (Figure 1). These sites vary in proximity to industrial facilities (including the Hutmen plant, a former Polish metallurgical company involved in the processing of Cu and its alloys) [42,43,44], major transportation routes such as roads and railroads, as well as residential areas with different housing types. Additionally, historical land use was considered, including areas with past industrial activities, deposits of old rubble, and the use of potentially contaminated soil amendments like lime and compost (Further explained in Table 1).
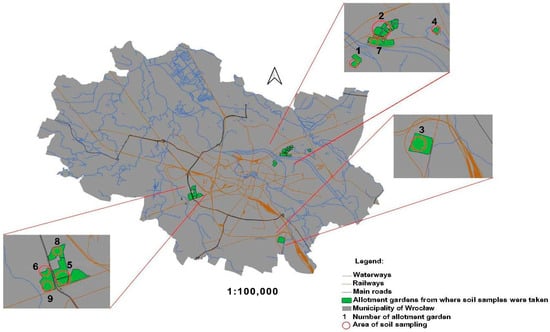
Figure 1.
Location of allotment gardens from where soil samples were taken for analysis in Wrocław city (South-West of Poland, Central Europe).

Table 1.
The characteristic of the study sites located within 9 allotment gardens.
2.2. Methods
Soil samples were collected in August 2022 from the 0–25 cm depth at sites where allotment holders grow vegetables (lettuce, red beet, onions, carrots, and spinach). Each sample weighed 1 kg. From each allotment garden, samples were taken from a minimum of three plots, with three replicates per plot. No samples were taken from pathways, recreational plots, or areas without vegetable cultivation. The collected samples were then dried and sieved.
The prepared soil samples were subjected to the following analyses: soil texture was determined using a combined sieving and hydrometric method. Chemical properties were assessed using standard procedures: soil pH was measured in a 1 M KCl suspension (1:2.5, v/v), and organic carbon (C org) and carbonate contents were determined using a CS-MAT 5500 analyzer (Strohlein Instruments, Kaarst, Germany). Total trace metal concentrations were determined after the digestion of the soil with aqua regia in a microwave system. The concentrations of Cd, Cu, Pb and Zn in the digested samples were determined using ICP-AES (iCAP 7400, Thermo Fisher Scientific, Waltham, MA, USA). All analyses were performed in triplicate. The quality of the results was monitored using soil reference materials (sample IDs: SRM 2709, SRM 2711, RTH 912, RTH 953), in which the certified total concentrations (“aqua regia”) of trace metals were analyzed. All analyses were performed according to current soil science standards [45].
2.3. Data Analysis
The results were statistically analyzed to determine the minimum, maximum, and mean values (including standard deviation) for total heavy metal concentrations (Zn, Cu, Pb, Cd), soil pH (measured in distilled water and 1 M KCl), carbon content [%], nitrogen content [%], and the C/N ratio. Pearson correlation coefficients were calculated to evaluate the relationships between the studied variables using Statistica software (version 13.3). Heavy metal concentrations were compared with the permissible limits established in Polish legislation [46], which specify the maximum allowable concentrations for allotment garden soils as follows: Cd—5 mg/kg, Pb—500 mg/kg, Cu—300 mg/kg, and Zn—1000 mg/kg.
OCD was calculated using the following formula [47,48]:
where
OCD = c·ρₒ·t·(1 − θ%)
- c—carbon content (g/kg),
- ρₒ—volume density (mg/m3),
- t—level thickness (m),
- θ—percentage content of the ø > 2.0 mm fraction.
The values obtained from physical and chemical analyses, trace element concentrations, and organic carbon density (OCD) were compared between the allotment garden complexes using the Kruskal–Wallis rank sum test. The analyses were performed with R Studio (version 4.4.1).
In addition, a hierarchical cluster analysis was conducted using Ward’s method (ward.D2) to identify similarities among the studied plots. The resulting dendrogram reveals how individual plots group together based on a combination of chemical and physical soil parameters. This approach enables the identification of spatial patterns and the classification of plots with comparable soil characteristics, which can be useful for environmental assessment and management decisions in allotment gardens. The cluster analysis was performed using the with R Studio (version 4.4.1)., applying the hclust() function with the Ward.D2 linkage method.
3. Results
3.1. General Characteristics of Examined Soils
The soils from the nine allotment garden complexes in Wrocław displayed varied granulometric compositions (Table 2). Complex 1 consisted primarily of sandy loam (SL). Complex 2 included sand (S), sandy clay loam (SCL), and loamy sand (LS). Complex 3 was composed of SL and SCL. Complexes 4 and 8 contained SCL. Complexes 5 and 9 had predominantly SL. Complexes 6 and 7 were mostly SL. The silt + clay content (floatable fraction < 0.02 mm) varied significantly across the garden complexes, from 5% to 33%, with the highest mean in complex 8 (26.73 ± 3.29%) and the lowest in complex 9 (15.33 ± 0.58%) (χ2 = 168.37, df = 8, p < 2.83 × 10−32; Kruskal–Wallis test).

Table 2.
Values of physical and chemical parameters of soils for nine study sites.
The soil pH (in H2O) ranged significantly from 6.65 to 8.05 (χ2 = 144.79, df = 8, p < 2.39 × 10−27). The highest pH was recorded in soils of complex 7 (7.64 ± 0.14), and the lowest in complex 3 (6.97 ± 0.22) (Table 2). The soil pH in KCl ranged significantly from 6.04 to 7.58, with the highest mean in complex 9 (7.29 ± 0.2) and the lowest in complex 1 (6.74 ± 0.12) (χ2 = 150.34, df = 8, p = 1.664 × 10−28). C content (%) varied between 2.12% and 7.64%, with the highest mean in complex 1 (4.71 ± 0.44%) and the lowest in complex 3 (2.81 ± 0.51%) (χ2 = 178.46, df = 8, p < 2.17 × 10−34). The nitrogen content (%) ranged significantly from 0.12% to 0.40%, with the highest mean in complex 1 (0.29 ± 0.04%) and the lowest in complex 3 (0.19 ± 0.04%) (χ2 = 120.95, df = 8, p < 2.11 × 10−22). The C/N ratio ranged significantly from 12.53 to 20.68, with the highest value in complex 7 (17.75 ± 1.1) and the lowest in complex 9 (14.06 ± 0.98) (χ2 = 171.42, df = 8, p < 6.51 × 10−33).
The soil physico-chemical parameters between the allotment garden complexes were compared using the Kruskal–Wallis rank sum test. The results are presented in the following format: chi-square value, degrees of freedom (df), and p-value (χ2, df, p).
The highest average OCD was recorded in garden complex 2, with a mean value of 1.77 kg/m2 and a range from 0.81 to 2.72 kg/m2 (Table 3. The lowest average OCD was found in garden complex 3, with a mean of 1.00 kg·m−2 and a range of 0.69 to 1.31 kg/m2. The remaining complexes had average OCD values ranging between 1.11 and 1.62 kg/m2. Statistical analysis using the Kruskal–Wallis test indicated significant differences in OCD stocks among the nine complexes (χ2 = 148.84; df = 8; p < 2.2 × 10−16).

Table 3.
Relative measure of soil organic carbon distribution (OCD) in nine allotment gardens complex.
3.2. Trace Elements Content of the Examined Soils
Zn and Pb had the largest share in the amount of analyzed metals, while cadmium had the smallest content (Table 4). The metal concentrations in soils collected from allotment garden complexes were ordered as follows: Cd < Pb < Cu < Zn.

Table 4.
Contents of selected heavy metals in garden soils of Wroclaw for nine study sites.
The Zn content ranged from 101.1 to 3464.5 mg/kg, with the highest mean value recorded in garden complex 5 (1380.55 ± 793.21 mg/kg) and the lowest in complex 4 (191.56 ± 55.1 mg/kg) (χ2 = 187.04, df = 8, p < 2.2 × 10−16) (Table 4). Cu content ranged from 24.93 to 322.45 mg/kg, with the highest mean concentration in garden complex 5 (213.56 ± 48.67 mg/kg) and the lowest in complex 4 (40.84 ± 12.96 mg/kg) (χ2 = 207.73, df = 8, p< 2.2 × 10−16). Pb concentrations ranged from 19.92 to 401.85 mg/kg, with the highest mean concentration in garden complex 5 (195.77 ± 35.22 mg/kg) and the lowest in complex 4 (36.7 ± 15.93 mg/kg) (χ2 = 213.62, df = 8, p = 2.2 × 10−16). The Cd content ranged from 0.51 to 6.31 mg/kg, with the highest mean concentration in complex 5 (4.81 ± 1.05 mg/kg) and the lowest in complex 4 (0.95 ± 0.11 mg/kg) (χ2 = 166.45, df = 8, p < 2.2 × 10−16). The permissible levels of selected trace elements (Zn and Cd) according to Polish standards were exceeded in some of the tested soils [46]. The highest concentrations of trace elements were found in the soils of allotment gardens in complex 5, located in the area affected by the Hutmen industrial plant.
The cluster analysis revealed three distinct groups of plots based on the similarity of their soil properties (Figure 2). However, the cluster differentiation does not appear to be fully related to location or the indicated sources of historical contamination. Allotment garden complexes previously influenced by the Hutmen smelter (plots 5, 6, 8, and 9) are found in two different clusters. Notably, cluster 5 stands out as having the highest concentrations of all four analyzed metals.
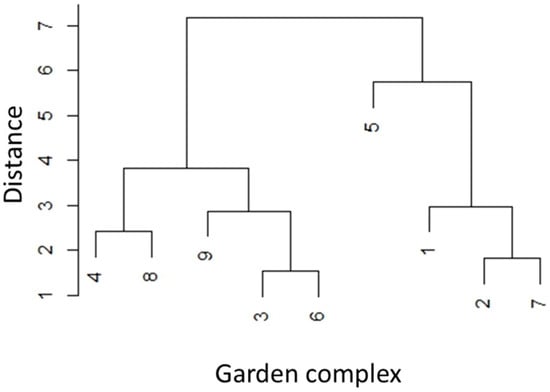
Figure 2.
Cluster analysis of soil physicochemical properties and heavy metal content in urban allotment gardens. Note: History and localization of allotment gardens in Wrocław: 1—Main contamination source: foreign substances introduced into soil (e.g., contaminated lime or compost). 2, 4, 7—Past industrial activities possibly contributing to contamination of nearby areas. 3—Possible contamination from old rubble found in depressions. 5, 6, 8, 9—Main contamination source: Hutmen, mainly emitting copper (Cu) [40,41].
3.3. Correlation Between Selected Heavy Metals and Carbon Content in Soil
Significant positive correlations were found between the heavy metal concentrations and organic carbon content based on Pearson’s correlation analysis (Table 5). Strong correlations were also identified for copper–carbon (r = 0.6031) and lead–carbon (r = 0.7245) (Figure 3B,C). Zinc and carbon showed a moderate correlation (r = 0.3912) (Figure 3A), while cadmium and carbon exhibited a weak and statistically insignificant correlation (r = 0.1860, p = 0.056) (Figure 3D).

Table 5.
The results of the correlation between the Zn, Cu, Pb and Cd [mg/kg] and C [%] content.
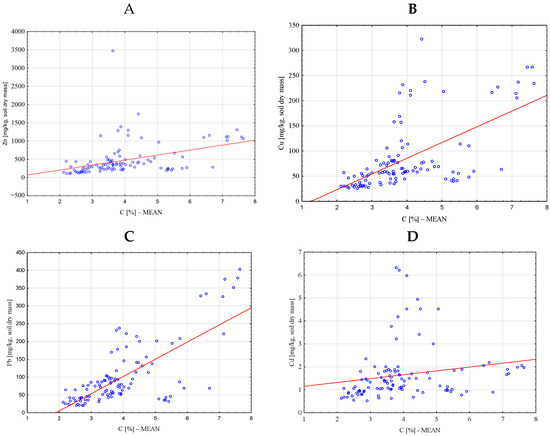
Figure 3.
Correlations between the concentrations of selected heavy metals (mg/kg soil dry mass)—Zn (A), Cu (B), Pb (C), Cd (D)—and the organic carbon content (%) in the studied soils.
4. Discussion
This study revealed substantial variability in the total heavy metal concentrations and SOC content across nine urban allotment garden soils in Wrocław (Poland). The concentrations of Zn, Cu, Pb, and Cd remained within the permissible limits established by Polish environmental standards. The highest levels were found in gardens near former industrial sites, especially Hutmen, confirming the persistent impact of historical contamination, as also reported by [43,44]. The SOC content ranged from 2.12% to 7.64%, reflecting differences in site-specific management and organic amendments.
A key finding from the study is the positive correlation between SOC content and the total concentrations of Zn, Cu, and Pb. This relationship suggests that soils with higher organic carbon levels tend to retain and accumulate greater amounts of these heavy metals, likely due to the complexation and adsorption properties of SOM, which enhance metal retention and reduce their mobility. This aligns with previous studies indicating that SOM acts as a strong sorbent, forming organo-metallic complexes that immobilize metals and reduce their mobility [39,41]. More specifically, increased SOM has been shown to reduce the extractability and bioavailability of metals such as Pb by forming stable organic–metal complexes, thus minimizing plant uptake and associated health risks [45,46].
According to the authors of the publication [37], soil horizons that are rich in humus OC contain compounds that have a high capacity to bind metals, such as humic and fulvic acids. These substances form stable complexes with trace metal cations such as Cu2+, Zn2+ and Fe3+, causing these cations to accumulate in this layer. In the context of the correlation between SOM and the trace element content in soils, numerous researchers have identified that SOM not only influences the retention and availability of trace elements but also affects their chemical speciation and potential toxicity. This accumulation can be attributed to the chelating properties of humic substances that effectively bind with metal ions, reducing their leachability. According to [36,37], the SOM content and pH are the main factors that control the bio-availability and chemical behavior of heavy metals. The authors also suggest that the higher concentration of trace elements in organic matter-rich soil horizons is due to the presence of soil organisms such as fungi, bacteria, algae and earthworms. These organisms often concentrate trace elements in their bodies or move them around within the soil microenvironment. This increases the concentration of metals in the organic horizon, the zone of greatest biological activity [37]. SOC acts as both a buffer and a mediator for heavy metal behavior in soils. Its presence can significantly reduce the risk of plants taking up heavy metals and groundwater leaching, especially when enhanced by strategic amendments like compost, manure, or biochar. This immobilizing effect was further emphasized by [49,50,51], who found that organic amendments such as compost or manure can significantly reduce the phytoavailability of metals like Cd and Pb, protecting plant roots by locking metals within the soil matrix. At the same time, [50] emphasizes the dual role of SOM in polluted soils. While it reduces metal phytoavailability through immobilization, organic amendments themselves—especially those derived from urban or industrial waste—can contain elevated levels of heavy metals like Cd, Pb, and Zn. SOM can indeed be a source of heavy metals in soil, particularly when it originates from contaminated or anthropogenic sources such as sewage sludge, composts, or industrial waste residues. While SOM is widely used to improve soil health and immobilize metals, it can also act as a vector for introducing new contaminants if not properly managed [51]. The dynamics of trace elements in soils were also evident in agricultural settings. Ref. [52] observed strong correlations between organic carbon and labile forms of trace metals such as cobalt, suggesting that organic fertilizers can influence both the stability and potential mobility of these elements. There is a well-established positive correlation between SOM and the total trace element content across diverse soil types and management systems. This relationship is governed by the chemical characteristics of SOM, including its cation exchange capacity and ability to form organo-metallic complexes, which enhance the retention and control the bioavailability of trace elements in soil matrices.
The weak and statistically non-significant correlation between SOC and Cd (p = 0.056) suggests that Cd exhibits different behavior, likely due to its higher mobility as previously reported in studies [53,54,55,56]. The distinct behavior of Cd in relation to SOM in soils requires an in-depth discussion of this phenomenon.
Cd in soils originates from pedogenic release from parent materials and anthropogenic inputs, including atmospheric deposition, agricultural amendments (e.g., phosphate fertilizers, manure), biosolids, and industrial wastes [24]. In addition to industrial sources, cadmium Cd accumulates in agricultural and garden soils due to the long-term use of phosphate fertilizers. Other contributing factors include irrigation with contaminated wastewater and the improper disposal of Cd containing products [57]. Numerous references provide strong evidence that SOM significantly influences the behavior of Cd through sorption, retention, and mobility, and its effects can vary depending on environmental factors such as pH, redox potential, and microbial activity. Refs. [57,58] state that the uptake of Cd by plants depends on the mobility of Cd in soil, which is in turn substantially influenced by SOM. Mobile Cd generally includes dissolved and weakly adsorbed Cd. SOC absorbs cadmium (Cd2+) via functional groups such as hydroxyls, carboxyls and phenolics. Cd2+ forms inner-sphere complexes with the surface oxygen atoms of humus or oxyhydroxides [59,60,61,62,63]. The addition of SOM to soil may increase the Cd adsorption capacity of the soil [63]. The addition of SOC can directly or indirectly change soil pH [60,62], which is the key factor influencing Cd partitioning in soil [63,64,65,66]. Soil Cd is usually found to be bound to SOM, Fe/Al/Mn oxyhydroxides, and, to a lesser extent, clay minerals [63,64,65,66,67]. When analyzing the behavior of Cd in contaminated soils (including urban soils), it is important to consider the competition between metals in soils. Multiple heavy metals can compete for the same sorption sites, altering ion retention and causing antagonistic, synergistic or additive effects that modify speciation and availability. Cd is generally more affected by competition than Pb due to its greater mobility [68,69,70,71].
The accumulation of trace elements in soils can be attributed to the chelating properties of humic substances that effectively bind with metal ions, reducing their leachability. Moreover, [72] reported that the quantity and quality of SOM in soils and sediments directly affect the potential toxicity and mobility of trace elements. Their findings further reinforce that not just the amount but the composition of SOM (e.g., fulvic vs. humic acids) matters for trace element dynamics. Organic compounds in the soil that may form complexes with metal ions may be grouped into three main classes: (1) naturally occurring molecules derived from soil biota and with a known structure and chemical properties (polysaccharides, amino acids, polyphenols, and aliphatic acids), (2) xenobiotic organic compounds originating from human, agricultural, industrial, and urban activities, and (3) humic substances—comprising humic acids, fulvic acids, and humin—that bind trace elements (such as Cu, Fe, Cd, Zn, V, Ni) primarily via carboxylic and phenolic groups [73].
The mobility of trace elements in soils depends on their partitioning between solid and liquid phases, regulated by physicochemical and biological processes [74,75]. Trace elements may be incorporated into mineral lattices, adsorbed onto Fe/Mn oxides or carbonates, or precipitated as carbonates [73]. The chemical speciation of these elements is critical, as they interact with organic and inorganic ligands (e.g., hydroxyl, carbonate, sulfate, chloride, dissolved organic matter (SOM) and chelators), with ligand concentrations increasing at higher pH. Humic acids can enhance the adsorption of Cd, Co, Cu and Zn even at low pH; however, at a high pH, they may reduce precipitation via metal–humate formation. The stability of metal–SOM complexes varies by metal and pH (e.g., Cu complexes dissociate at low pH), with particulate SOM enhancing adsorption through its high CEC. Meanwhile, soluble SOM fractions may increase TE mobility. Low-molecular-weight compounds, such as fulvic acids, can remain in solution and mobilize bound metals [67,73,76,77,78,79].
An important issue is the dual role of SOM in urban garden soils: enhancing soil fertility and structure, while simultaneously influencing heavy metal dynamics. SOM contributes to soil productivity through multiple properties such as texture, nutrient availability, pH, and microbial activity [43]. The SOC content of urban garden soils—ranging from 2.12% to 7.64% in these study soils—was strongly influenced by site-specific management practices, including the addition of composts and commercial substrates, as previously described by [44]. Composting, while beneficial for SOM accumulation and fertility, may influence the solubility of certain metals. Ref. [54] reported that composting can increase metal availability depending on the nature of organic inputs, while [55] showed that soluble organic fractions enhance metal mobility through complexation. The authors of [56,57] further emphasized the need to monitor metal speciation in amended soils, especially those treated with sewage sludge or organic waste, as these can alter the solid-solution partitioning of metals and affect their environmental impact.
Several studies suggest that the application of organic amendments, such as compost and biosolids, over several decades can significantly increase the SOM and potentially introduce heavy metals if the inputs are not strictly regulated. For instance, Ref. [80] found that applying compost to urban soils increases the content of SOM, but this can also affect the mobility and accumulation of heavy metals, depending on the source and frequency of the amendment. The link between elevated SOM and urban legacy contamination is reinforced by the work of [81], who demonstrated that SOM levels in remediated urban soils frequently exceeded those in native soils, particularly in cases where legacy contamination necessitated barrier or amendment strategies. In agricultural contexts, applying organic waste such as compost or sewage sludge to soil can improve its quality, but it can also introduce trace metals that may accumulate, particularly if applied at high rates [8]. Another field study found that, even two years after the application of compost, trace metals remained relatively immobile, although their total concentrations in the soil increased. References [82,83] found that the soil metal concentrations in urban allotment gardens correlated with both the age of cultivation and SOM, suggesting a combined influence of past inputs (e.g., fertilizers and compost) and historical pollution. Thus, high SOM in urban soils often reflects a dual legacy: anthropogenic enrichment through amendment practices, and long-term accumulation from industrial or atmospheric sources. Both of these processes can increase the metal-holding capacity of urban soils, meaning that SOM is a key, yet complex, indicator of the area’s contamination history. In geological settings, organic-rich sediments from ancient anoxic environments often exhibit co-enrichment of Mo, U and V, suggesting that SOM acts as a geochemical trap during diagenesis [84].
Materials such as sewage sludge, manure, industrial waste and phosphate fertilizers often contain high concentrations of metals such as Cd, Cu, Zn and Pb. Once these materials are applied to soil, the metals can accumulate and persist over time, particularly in the topsoil layers. Some studies have consistently observed higher metal concentrations in urban soils that align with historical industrial or municipal waste use rather than natural soil processes. This enrichment exhibits a spatial pattern and is strongly associated with land use and anthropogenic sources [73]. Applying organic waste such as compost or sewage sludge to soil can improve its quality, but it can also introduce trace metals that may accumulate, particularly if applied at high rates [85]. Another field study found that, even two years after the application of compost, trace metals remained relatively immobile, although their total concentrations in the soil increased. References [73,85] discuss how metal mobility and accumulation patterns can vary significantly depending on the form of external input—soluble versus particulate-bound—further supporting the idea that observed concentrations can often be attributed to the nature of the materials applied.
SOM primarily contributes to pollutant sorption and immobilization through exchange sorption, complexation and chelation. The extent to which it limits the mobility of trace elements depends on its properties and degree of transformation [72,74], with it acting as both a stabilizing agent and a long-term sink for metals from atmospheric deposition, runoff, and industrial waste [85,86,87,88].
High humus stocks in soils maintain their productive functions and determine carbon sequestration. Carbon sequestration is a method of reducing atmospheric CO2 emissions through the fixation and long-term storage of carbon in the soil, which helps to mitigate the effects of global warming. References [89,90] noted that the C/N ratios in the accumulation horizons of vegetable garden soils were higher than in the surrounding plow layers, ranging between 15 and 30. These elevated humus stocks reflect both greater organic inputs and the long-term buildup of stable SOM. Moreover, SOM in soil—especially at neutral or alkaline pH—has a high sorption capacity, further favoring the immobilization of heavy metals.
The limitations of our study include its focus solely on total metal concentrations without fractionation or bioavailability assessment, and the geographically limited sampling within Wrocław. Additionally, exploring the impact of different organic amendment types (e.g., sewage sludge, green waste composts) on metal behavior is vital, given their varied effects on metal mobility shown in [51,53,57].
5. Conclusions
This manuscript aims to clarify the relationship between SOM and heavy metal contamination in urban allotment gardens. We analyzed the concentrations of selected heavy metals (Pb, Cu, Zn, Cd) and the SOM content in soils from nine allotment garden complexes in Wrocław, examining their distribution and variability. Although a higher SOM content correlated with the total concentrations of metals such as Pb, Cu, and Zn, some of these concentrations exceeded Polish legal limits. In this study, we did not assess metal mobility or bioavailability, focusing instead on total metal concentrations and their association with SOM. The observed correlations indicate that higher SOM may coincide with elevated metal accumulation, but due to the immobilization of elements by SOM, the environmental risk is limited. However, the implications for environmental risk and plant uptake require further investigation.
This study highlights the importance of continuous monitoring and integrated soil management practices in addressing contamination and maintaining soil health in urban gardens. Sustainable urban agriculture depends on balancing soil quality with contamination control to ensure safe food production.
Author Contributions
Conceptualization: D.G., I.G. and K.S.; methodology: D.G., I.G. and K.S.; software: D.G.; validation: D.G., I.G. and K.S.; formal analysis: D.G.; investigation: D.G.; resources: D.G.; data curation: D.G., I.G., M.Z. and K.S.; writing—original draft preparation: D.G., I.G. and K.S.; writing—review and editing: D.G., I.G. and K.S.; visualization: D.G. and M.Z.; supervision: K.S. and I.G.; project administration: D.G.; funding acquisition: D.G. and K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Wroclaw University of Environmental and Life Sciences (Poland) as the Ph.D. research programme “Bon doktoranta SD UPWr” no. N020/0004/22. The APC was funded by Wroclaw University of Environmental and Life Sciences, Institute of Soil Science, Plant Nutrition and Environmental Protection.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
We would like to thank the management of the Family Allotment Gardens “Lepsze Jutro”, “Wytchnienie”, “Złocień”, “Spółdzielca”, “Jarzębina”, “Radość”, “Nowy Kanał”, “Malina”, “Oświata” as well as the allotment holders for making the research areas available.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Breuste, J.H.; Artmann, M. Allotment Gardens Contribute to Urban Ecosystem Service: Case Study Salzburg, Austria. J. Urban Plan. Dev. 2015, 141, 05014023. [Google Scholar] [CrossRef]
- Cabral, I.; Costa, S.; Weiland, U.; Bonn, A. Urban Gardens as Multifunctional Nature-Based Solutions for Societal Goals in a Changing Climate. In Nature-Based Solutions to Climate Change Adaptation in Urban Areas; Kabisch, N., Korn, H., Stadler, J., Bonn, A., Eds.; Springer: Cham, Switzerland, 2017; pp. 267–284. [Google Scholar] [CrossRef]
- Melon, M.; Dzieduszyński, T.; Gawryszewska, B.; Lasocki, M.; Hoppa, A.; Przybysz, A.; Sikorski, P. Urban Allotment Gardens with Turf Reduce Biodiversity and Provide Limited Regulatory Ecosystem Services. Sustainability 2025, 17, 6216. [Google Scholar] [CrossRef]
- Salomon, M.J.; Watts-Williams, S.J.; McLaughlin, M.J.; Cavagnaro, T.R. Urban Soil Health: A City-Wide Survey of Chemical and Biological Properties of Urban Agriculture Soils. J. Clean. Prod. 2020, 275, 122900. [Google Scholar] [CrossRef]
- Tresch, S.; Moretti, M.; Le Bayon, R.-C.; Mäder, P.; Zanetta, A.; Frey, D.; Fliessbach, A. A Gardener’s Influence on Urban Soil Quality. Front. Environ. Sci. 2018, 6, 25. [Google Scholar] [CrossRef]
- Ouédraogo, R.A.; Chartin, C.; Kambiré, F.C.; van Wesemael, B.; Delvaux, B.; Milogo, H.; Bielders, C.L. Short and Long-Term Impact of Urban Gardening on Soil Organic Carbon Fractions in Lixisols (Burkina Faso). Geoderma 2020, 362, 114110. [Google Scholar] [CrossRef]
- Doran, J.W.; Zeiss, M.R. Soil Health and Sustainability: Managing the Biotic Component of Soil Quality. Appl. Soil Ecol. 2000, 15, 3–11. Available online: https://digitalcommons.unl.edu/agronomyfacpub/15 (accessed on 25 June 2025). [CrossRef]
- Joimel, S.; Cortet, J.; Consalès, J.N.; Branchu, P.; Haudin, C.S.; Morel, J.L.; Schwartz, C. Contribution of chemical inputs on the trace elements concentrations of surface soils in urban allotment gardens. J. Soils Sediments 2021, 21, 328–337. [Google Scholar] [CrossRef]
- Paul, E.A. The Nature and Dynamics of Soil Organic Matter: Plant Inputs, Microbial Transformations, and Organic Matter Sta-bilization. Soil Biol. Biochem. 2016, 98, 109–126. [Google Scholar] [CrossRef]
- Kabala, C.; Chodak, T.; Szerszen, L.; Karczewska, A.; Szopka, K.; Fratczak, U. Factors Influencing the Concentration of Heavy Metals in Soils of Allotment Gardens in the City of Wroclaw, Poland. Fresenius Environ. Bull. 2009, 18, 1277–1282. [Google Scholar]
- Mitchell, R.G.; Spliethoff, H.M.; Ribaudo, L.N.; Lopp, D.M.; Shayler, H.A.; Marquez-Bravo, L.G.; Lambert, V.T.; Ferenz, G.S.; Russell-Anelli, J.M.; Stone, E.B.; et al. Lead (Pb) and Other Metals in New York City Community Garden Soils: Factors Influencing Contaminant Distributions. Environ. Pollut. 2014, 187, 162–169. [Google Scholar] [CrossRef]
- Delbecque, N.; Verdoodt, A. Spatial Patterns of Heavy Metal Contamination by Urbanization. J. Environ. Qual. 2016, 45, 9–17. [Google Scholar] [CrossRef]
- Belon, E.; Boisson, M.; Deportes, I.Z.; Eglin, T.K.; Feix, I.; Bispo, A.O.; Galsomies, L.; Leblond, S.; Guellier, C.R. An Inventory of Trace Elements Inputs to French Agricultural Soils. Sci. Total Environ. 2012, 439, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy Metals in Agricultural Soils: Sources, Influencing Factors, and Remediation Strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Agrawal, S.B.; Singh, A.; Sharma, R.K.; Agrawal, M. Bioaccumulation of Heavy Metals in Vegetables: A Threat to Human Health. Terr. Aquat. Environ. Toxicol. 2007, 1, 13–23. [Google Scholar]
- Verkleij, J.A.C. The Effects of Heavy Metals Stress on Higher Plants and Their Use as Biomonitors. In Plants as Bioindicators: Indicators of Heavy Metals in the Terrestrial Environment; Markert, B., Ed.; VCH: New York, NY, USA, 1993; pp. 415–424. [Google Scholar]
- Sady, W.; Smoleń, S. Wpływ czynników glebowo-nawozowych na akumulację metali ciężkich w roślinach. Rocz. Akad. Rol. W Poznaniu. Ogrod. 2004, 37, 269–277. [Google Scholar]
- Kabata-Pendias, A.; Piotrowska, M.; Witek, T. Ocena jakości i możliwości rolniczego użytkowania gleb zanieczyszczonych metalami ciężkimi. IUNG Ser. P 1993, 53, 5–14. [Google Scholar]
- Fidos, M.J.; Rutkowska, B. Accumulation of Selected Trace Elements in Soil and Roadside Trees–Case Study. Soil Sci. Annu. 2023, 74, 163082. [Google Scholar] [CrossRef]
- Gorlach, E.; Gambus, F. Nawozy Fosforowe i Wieloskładnikowe jako Źródło Zanieczyszczenia Gleby Metalami Ciężkimi. Zesz. Probl. Post. Nauk Roln. 1997, 448a, 139–146. [Google Scholar]
- Mehes-Smith, M.; Nkongolo, K.; Cholew, E. Coping Mechanisms of Plants to Metal Contaminated Soil. In Environmental Change and Sustainability; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Hołtra, A.; Zamorska-Wojdyła, D. The Input of Trace Elements from the Motor Transport into Urban Soils of Wrocław, Poland. Sci. Total Environ. 2018, 631–632, 1163–1174. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace Elements in the Soil–Plant Interface: Phytoavailability, Translocation, and Phytoremediation–A Review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Cambier, P.; Michaud, A.; Paradelo, R.; Germain, M.; Mercier, V.; Guérin-Lebourg, A.; Revallier, A.; Houot, S. Trace Metal Availability in Soil Horizons Amended with Various Urban Waste Composts During 17 Years–Monitoring and Modelling. Sci. Total Environ. 2019, 651, 2961–2974. [Google Scholar] [CrossRef] [PubMed]
- Krysiak, A.; Karczewska, A. Wpływ Zawodnienia na Mobilność Arzenu w Glebach Rejonu Dawnego Górnictwa Złota i Arsenu w Złotym Stoku. Rocz. Glebozn. 2011, 62, 240–248. [Google Scholar]
- Zhang, Z.; Furman, A. Soil Redox Dynamics under Dynamic Hydrologic Regimes–A Review. Sci. Total Environ. 2021, 763, 143026. [Google Scholar] [CrossRef]
- Gebski, M. Czynniki Glebowe oraz Nawozowe Wpływające na Przyswajanie Metali Ciężkich przez Rośliny. Postęp. Nauk Roln. 1998, 45, 3–16. [Google Scholar]
- Wu, Q.; Congreves, K.A. Soil Health Benefits Associated with Urban Horticulture. Sci. Total Environ. 2024, 912, 168852. [Google Scholar] [CrossRef]
- Rossini-Oliva, S.; Nuñez, R.L. Is It Healthy Urban Agriculture? Human Exposure to Potentially Toxic Elements in Urban Gardens from Andalusia, Spain. Environ. Sci. Pollut. Res. 2024, 31, 36626–36642. [Google Scholar] [CrossRef]
- Byers, H.L.; McHenry, L.J.; Grundl, T.J. Increased Risk for Lead Exposure in Children Through Consumption of Produce Grown in Urban Soils. Sci. Total Environ. 2020, 743, 140414. [Google Scholar] [CrossRef]
- Souri, M.K.; Hatamian, M.; Tesfamariam, T. Plant Growth Stage Influences Heavy Metal Accumulation in Leafy Vegetables of Garden Cress and Sweet Basil. Chem. Biol. Technol. Agric. 2019, 6, 25. [Google Scholar] [CrossRef]
- Home, R.; Lewis, O.; Bauer, N.; Fliessbach, A.; Frey, D.; Lichtsteiner, S.; Moretti, M.; Tresch, S.; Young, C.; Zanetta, A.; et al. Effects of garden management practices, by different types of gardeners, on human wellbeing and ecological and soil sustainability in Swiss cities. Urban Ecosyst. 2019, 22, 189–199. [Google Scholar] [CrossRef]
- Jean-Soro, L.; Le Guern, C.; Bechet, B.; Lebeau, T.; Ringeard, M.F. Origin of trace elements in an urban garden in Nantes, France. J. Soils Sediments 2015, 15, 1802–1812. [Google Scholar] [CrossRef]
- Heděnec, P.; Nilsson, L.O.; Zheng, H.; Gundersen, P.; Schmidt, I.K.; Rousk, J.; Vesterdal, L. Mycorrhizal Association of Common European Tree Species Shapes Biomass and Metabolic Activity of Bacterial and Fungal Communities in Soil. Soil Biol. Biochem. 2020, 149, 107933. [Google Scholar] [CrossRef]
- Edmondson, J.L.; Davies, Z.G.; Gaston, K.J.; Leake, J.R. Urban Cultivation in Allotments Maintains Soil Qualities Adversely Affected by Conventional Agriculture. J. Appl. Ecol. 2014, 51, 880–889. [Google Scholar] [CrossRef]
- Vasenev, V.I.; Stoorvogel, J.J.; Vasenev, I.I. Urban Soil Organic Carbon and Its Spatial Heterogeneity in Comparison with Natural and Agricultural Areas in the Moscow Region. Catena 2013, 107, 96–102. [Google Scholar] [CrossRef]
- Burghardt, W.; Heintz, D.; Hocke, N. Soil Fertility Characteristics and Organic Carbon Stock in Soils of Vegetable Gardens Compared with Surrounding Arable Land at the Center of the Urban and Industrial Area of Ruhr, Germany. Eurasian Soil Sci. 2018, 51, 1067–1079. [Google Scholar] [CrossRef]
- Hernandez-Soriano, M.C.; Jimenez-Lopez, J.C. Effects of Soil Water Content and Organic Matter Addition on the Speciation and Bioavailability of Heavy Metals. Sci. Total Environ. 2012, 423, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Plak, A.; Bis, M.; Lata, L.; Melke, J.; Mojak, J. The Assessment of Heavy Metals Content in Total and Bioavailable Forms in the Soils Surrounding Cementownia Chełm S.A. in Chełm, Poland. Pol. J. Soil Sci. 2016, 49, 15–28. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar] [CrossRef]
- Li, Y.; Gong, X.; Xiong, J.; Sun, Y.; Shu, Y.; Niu, D.; Lin, Y.; Wu, L.; Zhang, R. Different Dissolved Organic Matters Regulate the Bioavailability of Heavy Metals and Rhizosphere Microbial Activity in a Plant-Wetland Soil System. J. Environ. Chem. Eng. 2021, 9, 106823. [Google Scholar] [CrossRef]
- Makuch-Pietraś, I.; Wójcikowska-Kapusta, A. Differences in the Content of Zn Fractions in the Profiles of Soils from Allotment and Domestic Gardens in South-Eastern Poland. Land 2021, 10, 886. [Google Scholar] [CrossRef]
- Chodak, T.; Szerszeń, L.; Bogacz, A.; Gałka, B.; Kabała, C.; Kaszubkiewicz, J. Badania Monitoringowe Skażenia Gleb i Roślin na Obszarach Szczególnej Ochrony Środowiska Położonych na Terenie Miasta Wrocławia; Akademia Rolnicza we Wrocławiu: Wro-cław, Poland, 2001. [Google Scholar]
- Eko.org.pl. Gleby We Wrocławiu. Available online: http://eko.org.pl/wroclaw/srodowisko/gleby1.html (accessed on 6 July 2025).
- Tan, K.H. Soil Sampling, Preparation, and Analysis, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar] [CrossRef]
- Ministry of the Environment. Regulation of the Minister of the Environment of 1 September 2016 on the Method of Assessing Soil Surface Contamination. Dz.U. 2016, 1395. Available online: http://isap.sejm.gov.pl (accessed on 1 October 2024).
- Poeplau, C.; Vos, C.; Don, A. Soil organic carbon stocks are systematically overestimated by misuse of the parameters bulk density and stone content. SOIL 2017, 3, 61–66. [Google Scholar] [CrossRef]
- United States Department of Agriculture (USDA). Soil Carbon Stock Monitoring (CEMA 221); USDA-NRCS: Washington, DC, USA, 2023. Available online: https://www.nrcs.usda.gov/sites/default/files/2024-02/FY24_CEMA_221_Soil%20Carbon%20Stock%20Monitoring_10-2023_0.pdf (accessed on 5 August 2025).
- Lošák, T.; Hlušek, J.; Filipčík, R.; Pospíšilová, L.; Maňásek, J.; Prokeš, K.; Buňka, F.; Kráčmar, S.; Martensson, A.; Orosz, F. Effect of nitrogen fertilization on metabolisms of essential and non-essential amino acids in field-grown grain maize (Zea mays L.). Plant Soil Environ. 2010, 56, 574–579. [Google Scholar] [CrossRef]
- Szynkowska, M.I.; Pawlaczyk, A.; Maćkiewicz, E. Bioaccumulation and Biomagnification of Trace Elements in the Environment. In Trace Elements in the Environment: Biogeochemistry, Biotechnology and Bioremediation; Chojnacka, K., Saeid, A., Eds.; Wiley: Hoboken, NJ, USA, 2018; pp. 61–104. [Google Scholar] [CrossRef]
- Sun, H.; Tan, C.; Huang, D. Effects of Soil Organic Matter on the Accumulation, Availability and Morphology of Soil Heavy Metals. J. Nat. Sci. Hunan Norm. Univ. 2011, 34, 82–87. [Google Scholar]
- Unrine, J.M.; Hopkins, W.A.; Romanek, C.S.; Jackson, B.P. Bioaccumulation of Trace Elements in Amphibians: Influence of Trophic Position and Carbon Source. Environ. Pollut. 2007, 149, 182–192. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowska-Malina, J. Functions of Organic Matter in Polluted Soils: The Effect of Organic Amendments on Phytoavailability of Heavy Metals. Appl. Soil Ecol. 2018, 123, 542–545. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, M.K.; Idris, A.M.; Phoungthong, K.; Habib, M.A.; Mustafa, R.A. Geochemical Speciation and Bioaccumu-lation of Trace Elements in Different Tissues of Pumpkin in the Abandoned Soils: Health Hazard Perspective in a Developing Country. Toxin Rev. 2021, 41, 1124–1138. [Google Scholar] [CrossRef]
- Karabcová, H.; Pospíšilová, L.; Fiala, K.; Škarpa, P.; Bjelková, M. Effect of Organic Fertilizers on Soil Organic Carbon and Risk Trace Elements Content in Soil under Permanent Grassland. Soil Water Res. 2015, 10, 228–235. [Google Scholar] [CrossRef]
- Pinto, A.P.; Vilar, M.T.; Pinto, F.C.; Mota, A.M. Organic Matter Influence in Cadmium Uptake by Sorghum. J. Plant Nutr. 2005, 27, 2175–2188. [Google Scholar] [CrossRef]
- Pandit, T.K.; Naik, S.K.; Patra, P.K.; Das, D.K. Influence of Lime and Organic Matter on the Mobility of Cadmium in Cadmi-um-Contaminated Soil in Relation to Nutrition of Spinach. Soil Sediment Contam. 2012, 21, 419–433. [Google Scholar] [CrossRef]
- Filipović, L.; Romić, M.; Romić, D.; Filipović, V.; Ondrašek, G. Organic Matter and Salinity Modify Cadmium Soil (Phy-to)availability. Ecotoxicol. Environ. Saf. 2018, 147, 824–831. [Google Scholar] [CrossRef]
- Ingelmo, F.; Molina, M.J.; Soriano, M.D.; Gallardo, A.; Lapeña, L. Influence of Organic Matter Transformations on the Bioa-vailability of Heavy Metals in a Sludge-Based Compost. J. Environ. Manag. 2012, 95, S104–S109. [Google Scholar] [CrossRef]
- Kubier, A.; Wilkin, R.T.; Pichler, T. Cadmium in Soils and Groundwater: A Review. Appl. Geochem. 2019, 108, 104388. [Google Scholar] [CrossRef]
- Bondar, Y.; Chrastný, V.; Šípková, A.; Pecková, E. Fabrication of Nanocomposite Zeolite Granules for Cadmium Stabilization in Contaminated Soils. Environ. Pollut. Bioavailab. 2025, 37, 2522282. [Google Scholar] [CrossRef]
- Yuan, C.; Li, Q.; Sun, Z.; Sun, H. Effects of Natural Organic Matter on Cadmium Mobility in Paddy Soil: A Review. J. Environ. Sci. 2021, 104, 204–215. [Google Scholar] [CrossRef]
- Smolders, E.; Mertens, J. Cadmium. In Heavy Metals in Soils: Environmental Pollution; Alloway, B., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 22, pp. 283–311. [Google Scholar] [CrossRef]
- Yuan, C.; Li, F.; Cao, W.; Yang, Z.; Hu, M.; Sun, W. Cadmium solubility in paddy soil amended with organic matter, sulfate, and iron oxide in alternative watering conditions. J. Hazard. Mater. 2019, 378, 120672. [Google Scholar] [CrossRef]
- Smolders, E.; Mertens, J. Cadmium. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 283–311. [Google Scholar]
- Christensen, T.H. Cadmium soil sorption at low concentrations: I. Effect of time, cadmium load, pH, and calcium. Water Air Soil Pollut. 1984, 21, 105–114. [Google Scholar] [CrossRef]
- Degryse, F.; Smolders, E.; Parker, D.R. Partitioning of metals (Cd, Co, Cu, Ni, Pb, Zn) in soils: Concepts, methodologies, prediction and applications—A review. Eur. J. Soil Sci. 2009, 60, 590–612. [Google Scholar] [CrossRef]
- Sauvé, S.; Hendershot, W.; Allen, H.E. Solid-solution partitioning of metals in contaminated soils: Dependence on pH, total metal burden, and organic matter. Environ. Sci. Technol. 2000, 34, 1125–1131. [Google Scholar] [CrossRef]
- Sauvé, S.; Norvell, W.A.; McBride, M.; Hendershot, W. Speciation and complexation of cadmium in extracted soil solutions. Environ. Sci. Technol. 2000, 34, 291–296. [Google Scholar] [CrossRef]
- Bur, T.; Crouau, Y.; Bianco, A.; Gandois, L.; Probst, A. Toxicity of Pb and of Pb/Cd combination on the springtail Folsomia candida in natural soils: Reproduction, growth and bioaccumulation as indicators. Sci. Total Environ. 2012, 414, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X.; Dai, S.; Zhou, J.; Liu, D.; Hu, Q.; Bai, J.; Zhao, L.; Nazir, N. Impact of different industrial activities on heavy metals in floodplain soil and ecological risk assessment based on bioavailability: A case study from the Middle Yellow River Basin, northern China. Environ. Res. 2023, 235, 116695. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, L.; Zhong, R.; Bao, M.; Guo, H.; Xie, Z. Binding characteristics of humic substances with Cu and Zn in response to inorganic mineral additives during swine manure composting. J. Environ. Manag. 2022, 305, 114387. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Wang, Y.; Wang, Y.; Xu, J.; Liu, X. Competitive adsorption of lead and cadmium on soil aggregate at mi-cro-interfaces: Multi-surface modeling and spectroscopic studies. J. Hazard. Mater. 2023, 448, 130915. [Google Scholar] [CrossRef]
- Baran, A.; Mierzwa-Hersztek, M.; Gondek, K.; Tarnawski, M.; Szara, M.; Gorczyca, O.; Koniarz, T. The influence of the quantity and quality of sediment organic matter on the potential mobility and toxicity of trace elements in bottom sediment. Environ. Geochem. Health 2019, 41, 2893–2910. [Google Scholar] [CrossRef]
- Carrillo-González, R.; Šimůnek, J.; Sauvé, S.; Adriano, D. Mechanisms and pathways of trace element mobility in soils. Adv. Agron. 2006, 91, 111–178. [Google Scholar] [CrossRef]
- Adriano, D.C. Trace Elements in Terrestrial Environments: Biogeochemistry, Bioavailability and Risks of Metals, 2nd ed.; Springer: New York, NY, USA, 2001; p. 867. [Google Scholar] [CrossRef]
- Gao, Y.; Kan, A.T.; Tomson, M.B. Critical evaluation of desorption phenomena of heavy metals from natural sediments. Environ. Sci. Technol. 2003, 37, 5566–5573. [Google Scholar] [CrossRef] [PubMed]
- Bataillard, P.; Cambier, P.; Picot, C. Short-term transformations of lead and cadmium compounds in soil after contamination. Eur. J. Soil Sci. 2003, 54, 365–376. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1992. [Google Scholar]
- Chen, Y.; Gat, P.; Frimmel, F.H.; Abbt-Braun, G. Metal binding by humic substances and dissolved organic matter derived from compost. In Soil and Water Pollution Monitoring, Protection and Remediation; Twardowska, I., Allen, H.E., Häggblom, M.M., Stefaniak, S., Eds.; NATO Science Series; Springer: Dordrecht, The Netherlands, 2006; Volume 69, pp. 287–298. [Google Scholar] [CrossRef]
- Shi, W.; Lü, C.; He, J.; En, H.; Gao, M.; Zhao, B.; Zhou, B.; Zhou, H.; Liu, H.; Zhang, Y. Nature differences of humic acids fractions induced by extracted sequence as explanatory factors for binding characteristics of heavy metals. Ecotoxicol. Environ. Saf. 2018, 154, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Trammell, T.L.E.; Day, S.; Pouyat, R.V.; Rosier, C.; Scharenbroch, B.; Yesilonis, I. Drivers of urban soil carbon dynamics. In Urban Soils, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017; Chapter 4; p. 28. [Google Scholar] [CrossRef]
- Moskal, B.T.; Berthrong, S.T. Novel soil barrier systems potentially protect urban growing beds from legacy soil contamination and improve soil health. Urban Agric. Reg. Food Syst. 2018, 3, 180003. [Google Scholar] [CrossRef]
- Fathy, D.; Wagreich, M.; Fathi, E.; Ahmed, M.S.; Leila, M.; Sami, M. Maastrichtian anoxia and its influence on organic matter and trace metal patterns in the southern Tethys realm of Egypt during greenhouse variability. ACS Omega 2023, 8, 19603–19612. [Google Scholar] [CrossRef]
- Wong, C.S.C.; Li, X.; Thornton, I. Urban environmental geochemistry of trace metals. Environ. Pollut. 2006, 142, 1–16. [Google Scholar] [CrossRef]
- Alvarenga, P.; Palma, P.; Mourinha, C.; Farto, M.; Dôres, J.; Patanita, M.; Cunha-Queda, C.; Natal-da-Luz, T.; Renaud, M.; Sousa, J.P. Recycling organic wastes to agricultural land as a way to improve its quality: A field study to evaluate benefits and risks. Waste Manag. 2017, 61, 582–592. [Google Scholar] [CrossRef]
- Clemente, R.; Hartley, W.; Riby, P.; Dickinson, N.M.; Lepp, N.W. Trace element mobility in a contaminated soil two years after field-amendment with a greenwaste compost mulch. Environ. Pollut. 2010, 158, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Haque, E.; Thorne, P.S.; Nghiem, A.A.; Yip, C.S.; Bostick, B.C. Lead (Pb) concentrations and speciation in residential soils from an urban community impacted by multiple legacy sources. J. Hazard. Mater. 2021, 416, 125886. [Google Scholar] [CrossRef]
- Salomon, M.J.; Cavagnaro, T.R. Healthy soils: The backbone of productive, safe and sustainable urban agriculture. J. Clean. Prod. 2022, 341, 130808. [Google Scholar] [CrossRef]
- Dobson, M.C.; Edmondson, J.L.; Warren, P.H. Urban food cultivation in the United Kingdom: Quantifying loss of allotment land and identifying potential for restoration. Landsc. Urban Plan. 2020, 199, 103803. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).