Abstract
The latest FAO report indicates that aquaculture accounts for 51% of the global production volume of fish and seafood. However, despite the continuous growth of this activity, there is evidence of the excessive use of groundwater in its production processes, as well as pollution caused by nutrient discharges into surface waters due to the water exchange required to maintain water quality in fishponds. Given this context, the objectives of this study were as follows: (1) to review which emergent and floating plant species are used in constructed wetlands (CWs) for the bioremediation of aquaculture wastewater; (2) to identify the aquaculture species whose wastewater has been treated with CW systems; and (3) to examine the integration of CWs with recirculating aquaculture systems (RASs) for water reuse. A systematic literature review was conducted, selecting 70 scientific articles published between 2003 and 2023. The results show that the most used plant species in CW systems were Phragmites australis, Typha latifolia, Canna indica, Eichhornia crassipes, and Arundo donax, out of a total of 43 identified species. These plants treated wastewater generated by 25 aquaculture species, including Oreochromis niloticus, Litopenaeus vannamei, Ictalurus punctatus, Clarias gariepinus, Tachysurus fulvidraco, and Cyprinus carpio, However, only 40% of the reviewed studies addressed aspects related to the incorporation of RAS elements in their designs. In conclusion, the use of plants for wastewater treatment in CW systems is feasible; however, its application remains largely at the experimental scale. Evidence indicates that there are limited real-scale applications and few studies focused on the reuse of treated water for agricultural purposes. This highlights the need for future research aimed at production systems that integrate circular economy principles in this sector, through RAS–CW systems. Additionally, there is a wide variety of plant species that remain unexplored for these purposes.
1. Introduction
Aquaculture is described as the organized breeding, feeding, propagation, or protection of aquatic resources for commercial or public purposes [1]. In Mexico, according to the General Law on Sustainable Fisheries and Aquaculture, it is defined as a set of activities aimed at the controlled reproduction, pre-fattening, and fattening of aquatic fauna and flora species, carried out in facilities located in freshwater, marine, or brackish environments. These practices involve breeding or cultivation techniques for species that are suitable for commercial, ornamental, or recreational exploitation [2].
Since its origins over 4000 years ago, aquaculture has been used to produce food and other commercial products, including ornamental species, sanitary products, pharmaceuticals, and substrates for various commercial applications [3]. Today, aquaculture has evolved into a major global food-producing industry valued at USD 312.8 billion. It represents 59% of the combined fishery and aquaculture production. For the first time, aquaculture has surpassed capture fisheries, contributing 94.4 million tons, equivalent to 51% of global aquatic animal production, and reaching a record 57% of the production destined for direct human consumption [4]. These figures highlight aquaculture as a crucial sector within the global food system.
In this context, the fisheries and aquaculture industries play an essential role in the global supply of food. It is projected that aquatic animal production will increase by 10% by 2032, driven by the expansion of aquaculture [4,5]. Aquaculture’s contribution to the global supply of high-quality protein for human consumption is vital for food security and nutrition. Aquatic animal products provide 15% of the animal protein and 6% of the total protein consumed worldwide, in addition to essential nutrients such as omega-3 fatty acids, minerals, and vitamins [6]. However, these projections are alarming, as aquaculture production will need to increase to 129 million tons by 2050 to meet the growing global demand [5,6,7,8,9].
To address this challenge, the FAO’s Blue Transformation Roadmap 2022–2031 is aligned with the declarations of the Committee on Fisheries (COFI). Its goal is to lay the groundwork for ensuring sufficient supplies of aquatic foods for a growing population. This strategy considers the three dimensions of sustainability: environmental, social, and economic dimensions [10,11,12]. Equity is also a priority, with a focus on guaranteeing the availability and accessibility of safe and nutritious aquatic foods, especially for vulnerable populations [13].
From the social sustainability perspective, significant improvements are needed globally [14]. It is estimated that aquaculture directly employs 61.8 million people in primary production activities, mainly in small-scale operations. The disaggregated data by gender shows that women represent 24% of fishers and aquaculture workers, while they account for 62% of the workforce in post-harvest sectors [4]. In Mexico, there are 271,431 registered people engaged in fishing and aquaculture activities; of these, 222,744 are dedicated to fishing and aquaculture fisheries, and 48,687 are involved in controlled aquaculture systems [15]. This activity not only generates income but also provides food and employment opportunities for populations in both coastal regions and inland aquaculture areas [16,17,18]. Despite its importance, aquaculture remains concentrated in a small number of countries, while many low-income nations in Africa, Asia, Latin America, and the Caribbean have yet to fully realize its potential [4].
1.1. Problem Statement
From an environmental perspective, the rapid growth of aquaculture production systems has increased concerns about resource overexploitation and the associated environmental impacts. Despite its contribution to regional development, critical issues such as the management of pollutants from aquaculture wastewater, water scarcity, and the depletion of water resources have often been overlooked or received limited attention [19,20]. It is therefore necessary to study the inherent environmental impacts of any industry, and aquaculture is no exception. There is an ongoing debate among scientists regarding the environmental impacts of this “blue revolution,” with concerns suggesting that global aquaculture is not sustainable when analyzed through the lenses of food, energy, water, and carbon. A particular emphasis has been placed on the discharge of untreated aquaculture wastewater [14,21,22,23,24,25,26,27,28].
In some cases, aquaculture has been documented to negatively impact both marine and freshwater ecosystems by increasing disease transmission, introducing non-native species through escapes, releasing nutrient-rich waste and residues, and causing the destruction of wetlands. These factors contribute to water pollution, disrupting both the ecological balance and wildlife habitats [29,30,31].
Evidence indicates that the global sustainability pattern of aquaculture shows a significant variation between continents and countries. While most countries demonstrate social benefits and intermediate environmental costs, suggesting a balance between costs and benefits, results reveal that two-fifths of the 161 countries analyzed in 2018 achieved a high sustainability score (>50). This indicates a considerable need for improvement in aquaculture sustainability worldwide [14]. Therefore, bold and forward-looking measures are required to achieve the objectives outlined in the Declaration for Sustainable Fisheries and Aquaculture and to support the 2030 Agenda [13].
It is important to highlight that, within aquaculture operations, water use, energy costs, and feed expenses are among the most significant factors in semi-intensive and intensive systems [26,32,33,34]. Thus, innovation and research are vital to mitigate these costs and to promote the recovery and competitiveness of small- and medium-sized aquaculture enterprises (SMEs) in coastal, urban, and rural areas [10,35].
1.2. The Background on Ecotechnologies for the Treatment of Aquaculture Wastewater
The current state of the research regarding technologies that support the environmental sustainability of the aquaculture industry demonstrates the potential for implementing various systems. These include Integrated Multi-Trophic Aquaculture (IMTA), Integrated Agri-Aquaculture Systems such as aquaponics (AQ), Recirculating Aquaculture Systems (RAS), Biofloc Technology (BFT), the more recent Aquamimicry technology (AT), In-Pond Raceways Systems (IPRS), the combination of BFT and RAS (BIO-RAS), and Partitioned Aquaculture Systems (PAS) [27,36,37]. Additionally, symbiotic technologies based on plant fermentations, such as Bioaquafloc (BAF), are being developed [38,39,40].
These systems offer the advantage of water reuse through closed systems with minimal water discharge, in contrast to traditional open-flow systems that discharge untreated wastewater. This wastewater typically contains nutrient particles, primarily phosphorus and nitrogen [41,42,43]. Such nutrients can be removed through the sedimentation of suspended solids in tanks or mechanical filters, the nutrient reduction via irrigation in agricultural crops, or the use of constructed wetlands (CWs) [44,45,46,47,48,49,50,51,52].
Although wastewater treatment and reuse methods have been reported in aquaculture production, most of them require high capital investments and operational costs [53]. In this regard, constructed wetlands (CWs) represent an ecotechnology that mimics the functions of natural wetlands within a controlled environment. CW systems typically consist of a series of cells with substrates and plants, where wastewater flows through a porous medium designed for purification. These systems facilitate processes such as filtration, solids retention, nutrient removal, pathogen and microorganism elimination, and water oxygenation [49,54,55]. As such, they can be considered Nature based solutions (NbSs) [56].
Constructed wetlands can play a vital role in aquaculture, providing a low-cost alternative for treating aquaculture wastewater compared to other technologies [50,57,58,59,60,61]. However, their study has been relatively underexplored [62] and often limited to a few aquaculture species [49]. Some studies have reported remarkable removal efficiencies, with ammonia nitrogen (NH4+) removal rates of 86–98%, nitrite (NO2−) removal rates over 99%, nitrate (NO3−) removal rates between 82 and 99%, total nitrogen (TN) removal rates from 95 to 98%, phosphate removal rates from 55 to 71.2%, biochemical oxygen demand (BOD5) reductions from 25 to 55%, and suspended solids (SSs) removal rates from 47 to 86% [63,64].
The mechanisms for removing organic and inorganic compounds, as well as pollutants such as nitrogen (N) and phosphorus (P) present in aquaculture water, are carried out by plants. Through their roots, stems, and leaves, plants are capable of directly absorbing inorganic forms of nitrogen present in the pond, such as ammonium and nitrate [45]. In addition, the roots provide support for microbial communities and help capture suspended particles and pollutants, transporting nutrients to other parts of the plant, such as stems and leaves, to promote growth. The effectiveness of the contaminant removal improves when plants have more developed root systems. On the other hand, additional key processes for water purification occur within the substrate, where most of the physicochemical and biological activity takes place [54]. Within this system, microorganisms play a crucial role in nitrogen removal, as they metabolize nutrients and transform them into less harmful compounds. This process, known as microbial denitrification, includes stages such as ammonification, nitrification, and denitrification. As a result, various forms of nitrogen (NH3+, NO2−, and NO3−) can be effectively removed from the water, thus improving the quality of the aquaculture effluent [55]. These compounds are among the most critical to remove from production ponds and aquaculture wastewater [26,33,37]. Therefore, an appropriate design, vegetation selection, and substrate type are essential to improve the treatment of aquaculture wastewater and to support microbial activity within the controlled wetland environment [65,66]. Typha spp. and Phragmites australis spp. are the most commonly used species in constructed wetlands.
1.3. The Justification for the Literature Review
The treatment of wastewater is a critical and challenging issue in the aquaculture industry, as it affects surface and groundwater resources, fish production, energy consumption, and water reuse for agricultural purpose areas where there is still limited evidence [27,62]. Therefore, the appropriate selection of plant species to be used in constructed wetlands (CWs) for wastewater treatment is an essential criterion. Not all species are tolerant of aquaculture wastewater conditions; some may not survive or may fail to perform their functions adequately. Consequently, the treatment efficiency of CWs systems could be directly impacted by the selected plant species [67].
1.4. The Relevance and Practical Implications of the Research
Considering the global importance of aquaculture and its environmental impacts—particularly the pollution of water bodies—there is a pressing need worldwide to promote sustainable aquaculture practices. Constructed wetlands (CWs) represent an ecotechnology that has seen a limited application within the aquaculture sector. For this reason, focusing on the remediation of aquaculture wastewater, especially groundwater, through innovative methods is essential [68,69,70]. This study addresses a knowledge gap by synthesizing the role and significance of plant species that have proven to be effective in the bioremediation of aquaculture wastewater.
From a theoretical perspective, this review is significant because there is currently no comprehensive scientific literature that specifically focuses on the importance of plant species used in CWs for aquaculture wastewater treatment. In terms of practical application, this study is highly relevant. In Mexico alone, there are 4623 registered aquaculture farms [71]; the majority of which lack wastewater treatment systems. CWs could be integrated into these farms to mitigate environmental impacts caused by wastewater discharges, offering a low-cost alternative compared to conventional treatment technologies [33,35,53].
There is empirical evidence indicating that CWs are a viable option for sustainable aquaculture when implemented appropriately [50,57,58]. However, the number of studies and literature reviews focused on CWs in aquaculture remains limited. Based on this, the following research questions are proposed:
- RQ1. Which floating and emergent plant species have been used in constructed wetlands for bioremediation in aquaculture?
- RQ2. Which aquaculture species have had their wastewater treated with constructed wetlands?
- RQ3. Have constructed wetland systems been integrated into recirculating aquaculture systems (RAS)?
- RQ4. Is there evidence of full-scale studies or real-world applications?
Therefore, the objective of this study is to review the emergent and floating plant species used in constructed wetlands (CWs) for the bioremediation of aquaculture wastewater, to identify aquaculture species whose wastewater has been treated with CWs, and to explore the integration of CWs with recirculating aquaculture systems (RASs) for water reuse in food production and the efficient use of surface and groundwater resources.
2. Materials and Methods
2.1. The Planning of the Systematic Literature Review
The systematic literature review (SLR) was conducted with the aim of identifying and analyzing the current state of knowledge regarding the use of constructed wetlands (CWs) for the treatment of aquaculture wastewater, as well as the plant species employed in these systems. The review followed the PRISMA 2020 guidelines [72], which provide a structured and transparent framework for literature selection and analysis. (Supplementary Materials: PRISMA checklist for systematic reviews AQ-CW).
2.2. Inclusion and Exclusion Criteria
The following selection criteria were established to include only the most relevant articles capable of answering the research questions.
Inclusion criteria:
- Studies focused on the application of constructed wetland technologies—vertical flow, horizontal flow, and hybrid systems—in any configuration, used in freshwater and marine aquaculture systems.
- Peer-reviewed research articles published in scientific journals.
- Publications in any language, covering the period from 2003 to 2023, to reflect recent advances in this underexplored field and focus on studies providing relevant empirical evidence.
Exclusion criteria:
- Studies that do not report information relevant to the research questions.
- General review articles, book chapters, theses, or documents that are not original research articles.
- Articles for which the full text was not available.
- Studies published outside the defined time frame (2003–2023).
- Research not specifically addressing the application of CWs in aquaculture.
- Studies lacking information regarding the country where the research was conducted, the aquaculture species cultivated, the vegetation used, whether the system was coupled to a recirculating aquaculture system (RAS), and the study’s scale.
2.3. Study Selection and Data Collection
The SLR was carried out in five distinct stages involving the search, selection, and review of relevant studies in the field of constructed wetlands applied to aquaculture wastewater treatment.
In the first stage, Google Scholar alerts were configured using keywords in English: Constructed wetlands/Aquaculture, Plants/Bioremediation, Environmental impact/Aquaculture, and Aquaculture wastewater treatment. These alerts were reviewed daily by email for one year, due to the limited availability of information. In addition, searches were conducted in major academic databases such as ScienceDirect, Scopus, and ResearchGate using the same keywords in different combinations and permutations. Recent reviews on the topic and original research articles were also considered. The initial search yielded 10,966 articles.
In the second stage, the preliminary filtering of results was performed according to the defined inclusion and exclusion criteria, reducing the number of articles to 3588. The full texts of these articles were reviewed, and reference lists were checked to identify additional relevant studies.
During the third stage, the remaining 117 articles underwent a thorough full-text review. After applying the exclusion criteria, 27 articles were removed, resulting in a final sample of 70 potentially relevant studies.
In the fourth stage, these 70 articles were independently evaluated by the authors. By consensus, they agreed to include all 70 articles in the systematic review.
Finally, in the fifth stage, each article was analyzed using content analysis techniques and a structured content analysis guide [73] as the data collection instrument.
The systematic process of searching, selecting, and analyzing the studies is illustrated in the PRISMA flow diagram (Figure 1). The findings of each study were qualitatively synthesized and summarized in tables and graphs in accordance with the objectives of this research. A systematic literature review was conducted, selecting 70 scientific articles published between 2003 and 2023.
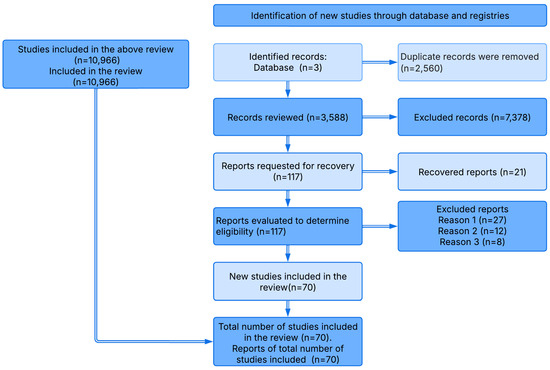
Figure 1.
PRISMA flow diagram based on [73].
3. Results and Discussion
3.1. Historical Evidence on the Treatment of Aquaculture Wastewater Using Constructed Wetlands
The application of constructed wetland (CW) systems in aquaculture was pioneered by Schwartz and Boyd [74], who implemented these systems for channel catfish (Ictalurus punctatus) production. Their CWs were planted with California bulrush (Scirpus californicus), giant cutgrass (Zizaniopsis miliacea), and maidencane (Panicum hemitomon). They concluded that a Hydraulic Residence Time (HRT) of 1 to 4 days, with Hydraulic Loading Rates (HLRs) ranging from 77 to 91 L per square meter per day (L/m2/day), was adequate. The study reported reductions in the following parameters:Total ammonia nitrogen (TAN): 1–81%; nitrite nitrogen (NO2-N): 43–98%; nitrate nitrogen (NO3-N): 51–75%; total Kjeldahl nitrogen (TKN): 45–61%; total phosphorus (TP): 59–84%; biochemical oxygen demand (BOD): 37–67%; total suspended solids (TSS): 75–87%; volatile suspended solids (VSS): 68–91%; and settleable solids (SS): 57–100%. The best performance of the CW was observed with a 4-day HRT.
Costa-Pierce [75] developed the Integrated Aquaculture–Wetland Ecosystem (AWE). This system consisted of the polyculture of aquatic plants such as Eichhornia crassipes and Ipomea aquatica, along with hybrid tilapia (Oreochromis mossambicus × O. urolepis hornorum), common carp (Cyprinus carpio), mosquitofish (Gambusia affinis), and red swamp crayfish (Procambarus clarkii). The results indicated that Eichhornia crassipes removed approximately 90% of ammonia and nitrate nitrogen, while the wetland system provided an additional 7% removal. Overall, the AWE system achieved a 97% total reduction in inorganic nitrogen concentrations in the aquaculture effluent. In addition, the system demonstrated the potential for producing aquatic food while achieving a near-complete removal of inorganic nitrogen from wastewater.
Historical evidence shows that a variety of species have been cultivated in conjunction with CW systems, including penaeid shrimp (Penaeidae), rainbow trout (Oncorhynchus mykiss), tilapia (Oreochromis spp.), and channel catfish (Ictalurus punctatus) [76,77]. These systems have been applied to both freshwater and marine environments, using water from various sources, including groundwater and surface water [78,79].
At a commercial scale, tilapia farming has been integrated with surface flow constructed wetlands (FWS) planted with Canna spp. and Scirpus sp. This system operated at a Hydraulic Loading Rate (HLR) of 3.03 m/day with an HRT of 2.9 h, supporting stocking densities greater than 35 kg/m3. The reported removal efficiencies were as follows: TSS: 90%; NO2-N: 91%; and NO3-N: 76%. The addition of a sedimentation unit significantly improved the TSS removal. This highlights the importance of considering sedimentation units in future CW systems for aquaculture wastewater treatment [79].
In another study, Zachritz and Fuller [76] designed a system that successfully supported commercial-scale tilapia production with densities over 35 kg/m3. The removal efficiencies in SSF wetlands were reported as follows: TSS: 67.2; TAN: 46%; NO2-N: 87.0%; and NO3-N: 40.6%. The authors concluded that the optimal performance of SSF wetlands, with the simultaneous removal of TAN, NO2-N, and NO3-N, was achieved when TAN loading rates were below 6.0 g/m2/day. The application of CWs for agricultural wastewater treatment, including aquaculture, has been extensively studied [80,81]. While review articles have reported on the removal efficiencies of CWs in wastewater treatment, none have provided a detailed analysis of the plant species used in these systems for aquaculture applications. In Table 1, Vymazal [49] presents a review of CW applications classified by the type of aquaculture system. That review analyzed 14 articles and focused on identifying the types of wetlands, Hydraulic Loading Rates (HLRs), Hydraulic Residence Times (HRTs), and highlighted notable removal efficiencies.

Table 1.
Cultivated species under aquaculture conditions using constructed wetlands for wastewater treatment (adapted from Vimazal [49]).
Relevant data have been reported indicating that this type of treatment is both feasible and cost-effective. This has been confirmed by Chen et al. [91] in the treatment of wastewater in Vietnam and China [92]. Even though aquaculture wastewater typically exhibits low pollutant concentrations, the large volumes involved pose significant treatment challenges. Initial findings demonstrated that intensive rainbow trout (Oncorhynchus mykiss) farms produce effluents that are 20 to 25 times more diluted than medium-strength municipal wastewater. In this context, the most important components to remove are phosphorus and nutrients such as nitrogen, which are essential factors contributing to the growth of algae and other microorganisms in aquaculture wastewater [49].
The evidence indicates that fish feed is the primary source of nitrogen (N) and phosphorus (P), contributing to over 85% of potassium (K), 70% of zinc (Zn), and at least 60% of these elements present in the effluents when water exchange rates are below 100 L of the replacement water per kg of feed input. Conversely, the presence of calcium (Ca), magnesium (Mg), sulfur (S), iron (Fe), and copper (Cu) depends more on the quality and volume of the source water [93]. Excessive concentrations of these nutrients can lead to the proliferation of harmful algal blooms in surface waters [94,95] and contribute to nutrient pollution in groundwater [96,97].
It is important to note the diversity of species studied in these systems, including Tilapia spp., Oncorhynchus mykiss, Ictalurus punctatus, and Litopenaeus vannamei. Design parameters for these systems suggest that Hydraulic Residence Times (HRTs) for nutrient removal in aquaculture wastewater can vary from as low as 0.014 days but generally do not exceed 5 days [83,84]. Hydraulic Loading Rates (HLRs), on the other hand, vary widely depending on the system configuration, the biomass load, the study period, and the age of the cultured organisms. This information suggests that, regardless of the type of the constructed wetland (CW) configuration employed, the HLR and HRT are the most critical variables to ensure a proper hydraulic performance and effective nutrient removal. However, the vegetation and substrate play fundamental roles in treatment efficiency [98]. Therefore, in the following section we analyze these components in greater detail.
3.2. Empirical Evidence on the Treatment of Aquaculture Wastewater Using Constructed Wetlands
Table 2 presents the compiled and harmonized information on the study variables reviewed in this work. In accordance with the research objectives, the table summarizes the country where each study was conducted, the aquaculture species cultivated, the type of vegetation used in the constructed wetland (CW), and the classification of the plant species (emergent or floating), as these are the most used types. The analysis also considered whether the study incorporated a recirculating aquaculture system (RAS) integrated with the wetland for water reuse purposes. Additionally, the research scale was recorded as an important variable to assess the technological maturity of CWs for aquaculture wastewater treatment [99]. This compilation provides an overview of current design trends for wetlands treating aquaculture wastewater and highlights potential areas for future research at both laboratory and commercial scales worldwide.
3.3. Research by Country on the Treatment of Aquaculture Wastewater Using Constructed Wetlands
Table 2 presents the findings of the reviewed studies, where it is evident that the application of constructed wetlands (CWs) in aquaculture is a growing field of research. Empirical investigations have been conducted in 19 countries, with China leading the number of studies, representing 33% of the total sample. This predominance may be attributed to China being the world’s largest producer of aquaculture products, contributing 36% of the total global aquaculture production [4].
In Brazil, the studies identified accounted for 15.5% of the total sample. This country’s interest in aquaculture wastewater treatment research can be associated with its position as the third-largest aquaculture producer in the Americas, following Chile and Ecuador [100]. Aquaculture represents 83.5% of Brazil’s total fish production, with Nile tilapia (Oreochromis niloticus) constituting 63.9% of the country’s aquaculture output.
The United States’ research represented 10% of the reviewed sample. Multiple factors may be driving the interest in this area, such as the country’s status as the world’s largest importer of aquaculture products [4]. The domestic supply from its 5961 fish farms remains insufficient to meet the demand. Moreover, the availability of significant research funding supports investigations into aquaculture development and wastewater management [101]. Recently, geographical areas with potential for commercial aquaculture have been identified in the U.S., based on analyses of environmental, social, and economic factors [102], reflecting a growing interest in expanding the aquaculture sector.
Additionally, 16 other countries have conducted research on the use of CWs in aquaculture. Germany, despite being a landlocked European country, accounted for 6.6% of the studies. This is noteworthy since Germany has been conducting CW research since the 1950s [103]. Excluding China, Asian countries—known for their strong presence in aquaculture—contributed 15.5% of the CW research for aquaculture wastewater treatment. This finding aligns with data indicating that Asian countries produce 70% of the world’s total aquatic animal output [13].
3.4. Aquaculture Species Reported in Constructed Wetlands Wastewater Treatment Studies
Regarding the aquaculture species reported in the reviewed literature (Table 2), there is a clear trend that reflects the most farmed species under controlled conditions worldwide. As shown in Figure 2, Nile tilapia (Oreochromis niloticus) was the most frequently studied species, appearing in 22.2% of the research reviewed. When combined with studies on blue tilapia (Oreochromis aureus) and Mozambique tilapia (Oreochromis mossambicus), tilapia species collectively represented 27.78% of the studies. As the second most important cultured fish species globally, tilapia is extremely popular among aquaculture producers; consequently, the adoption of innovative technologies for its sustainable cultivation is significant [104].

Figure 2.
Most studied aquaculture species with constructed wetlands.
The Pacific white shrimp (Litopenaeus vannamei) accounted for 16.6% of the CW studies. This may be due to L. vannamei dominating global shrimp production and being exported to numerous international markets. This species holds a leading position due to its growing global demand and competitive pricing [105]. Shrimp products, classified as high-value aquaculture commodities, are projected to reach a global production volume of 7.28 million metric tons by 2025, with a compound annual growth rate (CAGR) of 6.1% from 2020 to 2026 [106]. Therefore, the development of alternative wastewater treatment systems for shrimp aquaculture is critical for its sustainable growth.
Channel catfish (Ictalurus punctatus) represented 11.1% of the reviewed research. Native to the United States, Canada, and northeastern Mexico, this freshwater species has significant economic potential, as evidenced by the increasing global production [107]. Its favorable traits make it attractive for commercial filet production, and it remains a major aquaculture species in the United States, accounting for more than 60% of the country’s total aquaculture output [108,109]. Additionally, studies were identified involving yellow catfish (Tachysurus fulvidraco), which together accounted for 19.44% of the studies—surpassing the percentage observed for L. vannamei. This highlights the importance of developing wastewater treatment systems tailored for catfish species, which, although less economically dominant than shrimp or tilapia, are nonetheless significant for global aquaculture production.
Common carp (Cyprinus carpio) studies represented 5.56% of the reviewed literature. Despite this relatively low percentage, the global production of common carp exceeds 4.1 million metric tons [4]. Furthermore, other carp species identified in the reviewed studies include Megalobrama amblycephala, Hypophthalmichthys molitrix, Mylopharyngodon piceus, and Carassius gibelio, which collectively accounted for 11.11% of the CW research related to aquaculture wastewater treatment. This finding emphasizes their global relevance and the need for further research focused on improving wastewater management in carp aquaculture.

Table 2.
The empirical evidence of vegetation used in constructed wetlands for the treatment of aquaculture wastewater.
Table 2.
The empirical evidence of vegetation used in constructed wetlands for the treatment of aquaculture wastewater.
| Country | Aquaculture Species Cultivated | Vegetation | Type of Plant in the Wetlands | RAS | Study Scale | Reference |
|---|---|---|---|---|---|---|
| Taiwan | Milkfish (Chanos chanos). | Ipomoea aquatica, Paspalum vaginatum, and Phragmites australis | Emergent and Floating plants | Not included | HP | [45] |
| Taiwan | Pacific white shrimp (Litopenaeus vannamei). | Phragmites australis | Emergent plant | Included | LB | [46] |
| China | Pacific white shrimp (Litopenaeus vannamei). | Phragmites australis, smooth cordgrass (Spartina alterniflora Loisel), and scirpus (Scirpus mariqueter) | Emergent plant | Included | LB | [50] |
| China | NE | Sesuvium portulacastrum | Emergent plant | Not included | LB | [51] |
| China | Perch American (Micropterus salmoides). | Canna indica, Thalia Dealbata, and Iris germanica | Emergent plant | Not included | LB | [52] |
| China | Nile tilapia (Oreochromis niloticus). | Pontederia, Arundo, and Iris germanica. | NE | Not included | LB | [64] |
| E.E.U.U. | Channel catfish (Ictalurus punctatus). | Scirpus californicus, Zizaniopsis, miliacea, and Panicurn hemitomon | Floating plant | Included | LB | [74] |
| E.E.U.U. | Hybrid tilapia (Oreochromis mossambicus × O. urolepis hornorum common carp (Cyprinus carpio), mosquitofish (Gambusia affinis), and red swamp crayfish (Procambarus clarkii | Eichhornia crassipes and Ipomea aquatica | Floating plant | Not included | LB | [75] |
| E.E.U.U. | Nile tilapia (Oreochromis niloticus). | Canna Lillies (Canna sp.) and bulrush (Scirpus sp.) | Emergent plant | Included | LB | [76] |
| Germany | Rainbow trout (Oncorhynchus mykiss). | Phragmites australis | Emergent plant | Not included | LB | [77] |
| Australia | Rainbow trout (Oncorhynchus mykiss | Juncus kraussii | Emergent plant | Not included | LB | [78] |
| E.E.U.U. | Red tilapia (O. mossambicus O. aureus). | Typha sp. and Canna Lillies. | Emergent plant | Included | LB | [79] |
| Canada | Rainbow trout (Oncorhynchus mykiss). | Phragmites australis and Typha latifolia | Emergent plant | Not included | LB | [82] |
| Canada | Atlantic salmon (Salmo salar) | Phragmites communis and Typha latifolia | Emergent plant | Included | LB | [83] |
| E.E.U.U. | Royal salmon (Oncorhynchus tsawytscha). | NE | NE | Not included | LB | [84] |
| Germany | Rainbow trout (Oncorhynchus mykiss). | Phragmites australis | Emergent plant | Not included | LB | [86] |
| Germany | Rainbow trout (Oncorhynchus mykiss). | Phragmites communis and Phalaris arundinacea | Emergent plant | NE | LB | [87] |
| China | Channel catfish (Ictalurus punctatus). | NE | NE | Included | LB | [88] |
| China | Pacific white shrimp (Litopenaeus vannamei). | NE | NE | Included | LB | [89] |
| Vietnam | Nile tilapia (Oreochromis niloticus) and common carp (Cyprinus carpio). | Canna generalis | Emergent plant | Included | LB | [90] |
| China | Chinese carp (Ctenopharyngodon idella). | Nymphaea alba, Myriophyllum sp., and Vallisneria natans | Emergent and Floating plants | Included | LB | [110] |
| China | Giant river prawn (Macrobrachium rosenbergii). | Typha angustifolia and Canna indica | Emergent plant | Included | LB | [111] |
| Denmark | Rainbow trout (Oncorhynchus mykiss). | NE | NE | NE | LB | [112] |
| China | Channel catfish (Ictalurus punctatus), spiny barb (Spinibarbus sinensis), and yellow catfish (Pelteobagrus fulvidraco). | Canna indica, Iris tectorum, Acorus calamus, Cyperus papiro, and Thalia dealbata | Emergent plant | Included | LB | [113] |
| Israel | NE | Salicornia | Emergent plant | Included | LB | [114] |
| China | NE | Thalia dealbata, Arundo Donax, Iris versicolor, Phragmites australis, Myriophyllum spicatum, and Nymphaea alba | Emergent and Floating plants | Not included | LB | [115] |
| Finland | NE | Salicornia europaea and Phragmites australis | Emergent plant | Included | LB | [116] |
| China | Pacific white shrimp (Litopenaeus vannamei). | Phragmites australis, Spartina alterniflora Loisel, and Scirpus mariqueter | Emergent plant | Not included | LB | [117] |
| Brazil | Pacific white shrimp (Litopeneaus vannamei). | Spartina alterniflora | Floating plant | Included | LB | [118] |
| Brazil | Nile tilapia (Oreochromis niloticus). | Salvinia sp. and Eichhornia crassipes. | Floating plant | Not included | LB | [119] |
| Brazil | Nile tilapia (Oreochromis niloticus). | Eichhornia crassipes | Floating plant | Included | LB | [120] |
| Malaysia | Channel catfish (Ictalurus punctatus). | Eichhornia crassipes and Limnocharis flava | Floating plant | Not included | LB | [121] |
| Colombia | Channel catfish (Ictalurus punctatus). | Eichhornia crassipes | Floating plant | Not included | LB | [122] |
| Brazil | River shrimp (Macrobrachium amazonicum). | Eichhornia crassipes and Pistia stratiotes | Floating plant | Not included | LB | [123] |
| Brazil | Nile tilapia (Oreochromis niloticus). | Eichhornia crassipes, Pistia stratiotes, and Salvinia molesta | Floating plant | Not included | LB | [124] |
| Brazil | Nile tilapia (Oreochromis niloticus) and | NE | NE | Included | LB | [125] |
| Hungary | silver Carp (Hypophthalmichthys molitrix). Common carp (Cyprinus carpio), and sharp tooth catfish (Clarias gariepenus). | Phragmites australis, Typha angustifolia, Lemna minor, and Typha latifolia | Emergent and Floating plants | Not included | LB | [126] |
| Hungary | Sharp tooth catfish (Clarias gariepinus), Nile tilapia (Oreochromis niloticus), common carp (Cyprinus carpio), | NE | NE | Included | LB | [127] |
| Hungary | Sharp tooth catfish (Clarias gariepinus). Common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus). | Phragmites australis, Typha latifolia, Typha angustifolia, Salix viminalis, and Arundo donax | Emergent plant | Included | LB | [128] |
| China | Puffer fish (Dichotomyctere ocellatus). | Phragmites australis, Spartina alterniflora, and Scirpus mariqueter | Emergent plant | Included | LB | [129] |
| China | Channel catfish (Ictalurus punctatuss) Brema Wuchang (Megalobrama amblycephala), silver carp (Hypophthalmichthys Molitri), and black carp (Mylopharyngodon Piceus). | Canna indica, Typha latifolia, Acorus calamus, and Agave sisalana | Emergent plant | Included | ER | [130] |
| China | Chinese carp (Ctenopharyngodon idella). | Nymphaea alba L, Myriophyllum sp., and Vallisneria natans | Floating plant | Included | LB | [131] |
| Mexico | NE | Arundo donax, Medicago sativa, and Zandechia aethiopica | Emergent plant | Not included | LB | [132] |
| China | NE | Thalia dealbata, Arundo, donax, Phragmites australis, Myriophyllum spicatum, and Nymphaea alba | Emergent plant | Not included | LB | [133] |
| China | Channel catfish (Ictalurus punctatuss). | Canna indica, Typha orientalis, and Acorus calamus | Emergent plant | Included | LB | [134] |
| Italy | Sea bass (Dicentrarchus labrax), and sea bream (Sparus aurata). | Ulva Linnaeus | NE | Not included | HP | [135] |
| E.E.U.U. | Pacific white shrimp (Litopenaeus vannamei). | NE | NE | NE | LB | [136] |
| Israel | NE | Salicornia persica | NE | Included | LB | [137] |
| Germany | NE | Salicornia europaea and Tripolium pannonicum | NE | Included | LB | [138] |
| E.E.U.U. | Pacific white shrimp (Litopenaeus vannamei). | Gracilaria tikvahiae | Floating plant | Not included | LB | [139] |
| China | Milkfish (Chanos chanos). | Paspalum vaginatum, Ipomoea aquatica, and Phragmites australis | Floating plant | Not included | HP | [140] |
| Taiwan | Pacific white shrimp (Litopenaeus vannamei). | Pistia stratiotes, Typha angustifolia, Phragmites common, Canna generalis, and Cyperus alternifa | Emergent plant | Not included | LB | [141] |
| E.E.U.U. | Channel catfish (Ictalurus punctatus). | Scirpus californicus, Zizaniopsis miliacea, and Panicurn hemitomon | Emergent plant | Not included | LB | [142] |
| China | Channel catfish (Ictalurus punctatus). | NE | NE | Not included | LB | [143] |
| Hungary | NE | Phragmites Australis, Typha latifolia angustifolia, Salix viminalis, Arundo donax, and Tamarix tetrandra | Emergent plant | Not included | HP | [144] |
| Malaysia | NE | Eichhornia crassipes and Limnocharis flava | NE | Not included | LB | [145] |
| China | NE | Thalia dealbata, Canna indica, and Phragmites australis | NE | Not included | LB | [146] |
| Brazil | Nile tilapia (Oreochromis niloticus). | Eichhornia crassipes, Pistia stratiotes, and Salvínia molesta | Floating plant | Not included | LB | [147] |
| E.E.U.U. | Red tilapia (O. mossambicus x O. aureus). | Canna sp. and Scirpus sp. | Emergent plant | Included | LB | [148] |
| Thailand | Nile tilapia (Oreochromis niloticus). | NE | NE | Included | LB | [149] |
| Brazil | Tambaqui (Colossoma macropomum). | Lactuca sativa spp. | Emergent plant | Not included | LB | [150] |
| Brazil | Nile tilapia (Oreochromis niloticus). | Typha latifolia | Emergent plant | Included | LB | [151] |
| Indonesia | Pacific white shrimp (Litopenaeus vannamei). | NE | NE | Not included | LB | [152] |
| Indonesia | Claria (Clarias gariepinus). | Chrysopogon zizanioides, Cyperus alternifolius, and Eichhornia crassipes | Emergent plant | Not included | LB | [153] |
| Indonesia | Pacific white shrimp (Litopenaeus vannamei). | Chrysopogon zizanioides | Emergent plant | Not included | LB | [154] |
| Indonesia | NE | Chrysopogon zizanioides | Emergent plant | Not included | LB | [155] |
| Chile | Jaks (Carangidae). | Agarophyton chilense, Mazzaella canaliculate, and Ulva lactuca | Floating plant | Not included | LB | [156] |
| China | Nile tilapia (Oreochromis niloticus). | Chlorella sp. | Floating plant | Included | LB | [157] |
| China | Tachysurus fulvidraco and Lateo labrax japonicas. | Phragmites australis, Phalaris arundinacea, Acorus calamus, Typha latifolia, Scirpus lacustris, and Iris pseudacorus | Emergent plant | Not included | LB | [158] |
| China | NE | Phragmites australis | Emergent plant | Not included | LB | [159] |
Nomenclature: RAS: Integrated into recirculating aquaculture system, LB: Laboratory scale, HP: Pilot scale, ER: Real scale, NE: Not specified.
In total, wastewater from 25 species was studied, including some of the most popular species worldwide (Figure 3). Research has predominantly focused on the two most widely farmed species: tilapia (Oreochromis niloticus) and shrimp (Litopenaeus vannamei). However, very localized species have also been studied using constructed wetland (CW) technologies for wastewater treatment. This limited information highlights the importance of further research, as attention should not only be paid to the most popular species. Other freshwater and marine species also need to be studied, considering that in 2022 the most commercially traded aquatic animal products were finfish (65% of total value), crustaceans (23%), and mollusks and other aquatic invertebrates (11%). Salmonids were the most valuable group commercially (20% in terms of value), followed by shrimp and prawns (17%) [4]. Nevertheless, there were 448 aquaculture species farmed in 197 countries, many of which have not yet been studied [13]. In this context, only 17% of aquaculture species were found in the present study, revealing a significant knowledge gap for future research.
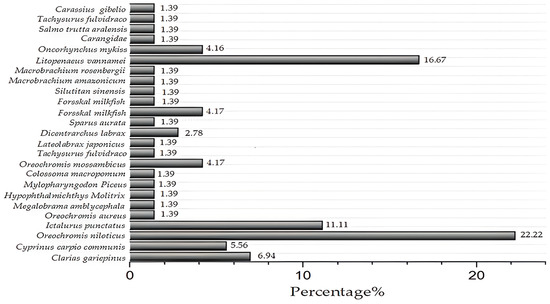
Figure 3.
Aquaculture species in constructed wetlands. (300DPI).
3.5. Plant Species Utilized in Constructed Wetlands for Aquaculture Wastewater Treatment
Existing literature reviews that compile broad information regarding the use of plants in CW for aquaculture wastewater remediation are scarce. From Scopus, 10 review articles were analyzed that explore the biological treatment of wastewater. Jin et al. [160] describes the development status and research trends in biofiltration, mentioning that this type of treatment is used as a secondary treatment, particularly for the removal of micro-contaminants through oxidation technologies and constructed wetlands on aquaculture farms. Gorito et al. [63] concluded that the removal of organic micro-contaminants using CWs is a recent area of study, requiring further research to determine the feasibility of large-scale applications. Although its potential is recognized, specific information for aquaculture remains limited.
Local context reviews, such as in Thailand, state that only 5% of aquaculture wastewater is treated in conventional plants, and only one CW system has been implemented at a real scale for domestic wastewater treatment [161]. Their national review highlights the urgent need to reuse wastewater for agricultural purposes, despite Thailand being one of Asia’s main aquaculture producers, with 1,000,181 tons [162]. On the other hand, Kurniawan et al. [163] affirm that aquaculture in Malaysia is still linked to environmental problems, despite its many benefits. They analyzed 11 articles demonstrating the effectiveness and feasibility of using CWs for the treatment of these effluents and proposed CWs as a clean production alternative using floating plants as a secondary treatment.
Wu and Song [164] emphasize that the treatment and disposal of solid aquaculture waste has attracted wide attention. They highlight the following technologies for its treatment: constructed wetlands, aerobic composting, anaerobic treatment, enzymatic or chemical hydrolysis, and aquaponics. Tom et al. [62] found that RAS technologies integrated with constructed wetlands are gaining increasing importance, as they have proven to be a viable and cost-effective method for wastewater treatment. However, they only describe the technologies without detailing essential elements in their operational characteristics, which is limited to seven studies analyzed.
Henares et al. [36] highlight CWs as a biological treatment method, describing their operation and important elements regarding vegetation, such as the use of emergent vegetation, rooted floating-leaf plants, submerged plants, and free-floating plants. Additionally, they emphasize that for proper operation, it is necessary to periodically remove part of the biomass to allow macrophytes to grow and maintain high nutrient uptake rates. They briefly analyzed removal rates and confirmed the possibility of using this biomass as fish feed. They reviewed six articles: three with emergent plants and three with free-floating plants. Henares et al. [36] agree with Gorito et al. [63] on the scarcity of scientific publications concerning the aquaculture industry, particularly regarding its global supply and demand, its environmental impacts due to effluent generation, and the treatment technologies currently available for aquaculture effluents. In terms of design, Messer et al. [165] conclude that it is vital to include basic performance parameters for wetlands, such as the influent and effluent concentrations, hydraulic retention time, Hydraulic Loading Rate, and plant species.
Emergent and Floating Plant Species Used in CW for Aquaculture Wastewater Treatment
Initial statistical data indicate that 10.06% of studies do not report the type of plant species used, corresponding to a total of 17 works. This is a notable aspect, as it represents an essential component of constructed wetlands studies. For this reason, an analysis was conducted on emergent and floating plants used in CWs and aquaculture, revealing a total of 169 plant species across 70 studies, of which 58.97% were floating plants and 41% were emergent. Figure 4 presents a Pareto diagram, showing a significant knowledge gap and potential future research lines. The data reveal frequency and percentage counts of the emergent and floating plant variables. This diagram is based on the 80/20 rule applied in quality engineering [166,167], but in this case, it indicates that 34 plant species represent 80% of the research addressed, out of the 169 plant species found. This is due to several studies using one plant or combinations of plants in their methodology, across the 70 articles analyzed.
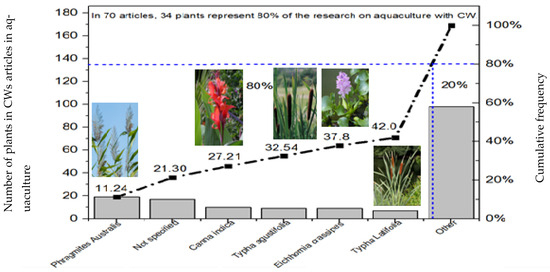
Figure 4.
Pareto chart of main plants used in constructed wetlands.
These findings suggest that researchers in this field have primarily focused on other types of wastewaters, such as industrial, municipal, and rural wastewater [168,169]. However, aquaculture wastewater is of vital importance [170]. Based on the available empirical evidence, it is recommended that wastewater treatment in aquaculture using constructed wetlands should be investigated with greater interest, due to the urgent need arising from water scarcity and the high demand for water required by this activity. Additionally, there is a knowledge gap regarding plants with potential for study and application beyond those presented here, such as ornamental plants [171], which could offer an esthetic improvement to the landscape of production farms [172].
As shown in Figure 4, Phragmites australis was identified as the most frequently studied plant species, representing 11.2% of the reviewed cases involving constructed wetlands (CWs) for aquaculture wastewater treatment. This perennial species is notable for its tolerance to a wide range of environmental conditions, primarily due to its extensive rhizome system, which can reach depths of 60 cm to 1 m. It features rigid stems with hollow internodes and varies in height from 0.5 m to 4–5 m. Its primary mode of propagation is through rhizomes, as its seed viability is very low [67].
In second place is Typha spp., widely used in CWs and accounting for 9.47% of the studies analyzed. Within this group, Typha latifolia (4.14%) and Typha angustifolia (5.33%) are the most employed species. Like Phragmites australis, these are erect perennial rhizomatous plants that can reach heights greater than 4 m. They possess an extensive rhizome system, and their leaves are flattened or slightly rounded on the underside, with spongy basal portions. Typha species prefer soils rich in organic matter and stagnant water conditions, although specific species within the genus may tolerate different water depths. T. latifolia L., commonly known as broadleaf cattail, is the most frequently used species in CWs due to its well-documented effectiveness in aquaculture wastewater treatment. Its adaptability to environments with a high organic matter content and the suitable depths found in CW systems support its widespread application [67].
The third most frequently used species was Canna indica, found in 5.92% of the reviewed studies. This perennial herb, belonging to the Cannaceae family and native to the Americas, produces ornamental flowers and has applications in landscaping, wastewater clarification, medicine, and human consumption. Canna indica is considered an ornamental plant with valuable phytoremediation attributes, such as a rapid growth, a tolerance to adverse climatic conditions, a high capacity for contaminant accumulation, and an extensive fibrous root system. Additionally, this species enhances the aerobic conditions within the CW, thereby improving the treatment efficiency [173,174].
In fourth place, Eichhornia crassipes, commonly known as water hyacinth, was utilized in 5.3% of the studies. This free-floating perennial aquatic plant, part of the Pontederiaceae family, forms dense mats on water surfaces or mud substrates. It thrives in freshwater ponds, canals, marshes, and lakes and propagates mainly through vegetative reproduction, allowing it to rapidly colonize large areas. Eichhornia crassipes is considered one of the most efficient aquatic plants for wastewater purification, as it can remove a wide range of contaminants, including heavy metals, organic substances, and nutrients such as nitrate, ammonium, and phosphorus. It is also effective in reducing total solids in municipal, industrial (e.g., textile, metallurgical, pharmaceutical, and paper mill), domestic, and sewage wastewater [175]. Its widespread use in CWs for contaminant removal is well-documented [176].
Finally, Arundo donax, commonly known as the giant reed, was reported in several studies. This grass species from the Poaceae family—believed to have originated in Asia and later spread to the Mediterranean region, North Africa, and the Americas—is one of the world’s largest herbaceous plants. Its stems can reach heights of 8 to 10 m with a diameter of 3 to 4 cm, while its roots may extend up to 5 m deep. The leaves are long and lance-shaped, measuring up to 1 m. Research has shown that its rhizome fragments maintain 100% viability even after prolonged submersion treatments. Furthermore, such flooding treatments have been associated with the highest biomass production in both shoots and roots, highlighting the giant reed’s exceptional tolerance to waterlogging. These characteristics make Arundo donax particularly suitable for the bioremediation of wastewater [177].
3.6. Constructed Wetlands Integrated into Recirculating Aquaculture Systems (RAS)
Recirculating aquaculture systems (RAS) have been developed as an intensive, efficient, safe, and environmentally friendly approach to fish production, significantly reducing water and land use [178]. These systems aim to conserve resources while maintaining a high productivity [179]. RAS ensure the optimal development of farmed species by filtering and degrading aquaculture wastewater contaminants, recycling water, and minimizing the consumption of natural resources [85]. RAS are designed as closed-loop systems with minimal water exchange, where aquaculture wastewater undergoes filtration and aeration treatments, allowing water reuse within the system. This reduces freshwater consumption and supports intensive land-based aquaculture operations, typically conducted indoors, thus providing a controlled, high-quality environment for fish survival while significantly lowering water usage [85].
The integration of compatible technologies reflects a growing trend toward innovative biological systems and technologies within aquaculture. However, such implementations often involve high costs [53]. In this context, the present study identifies research that has integrated RAS with constructed wetlands (CWs) by country, as shown in Table 3. Among the studies analyzed, 40% reported water reuse as a key objective—an essential aspect in sustainable aquaculture. Nevertheless, a major limitation of RAS–CW integrated systems lies in the need for continuous operation. The Hydraulic Loading Rates (HLRs) in CWs make it challenging to achieve uninterrupted processes [180]. Despite these operational challenges, some case studies have successfully demonstrated the application of integrated RAS–CW systems [46,48,74,83,88,89,90].

Table 3.
Studies on RAS integrated with constructed wetlands in aquaculture.
The generally accepted classification of constructed wetlands is shown in Figure 5, where three types can be observed, along with their component parts, according to the direction of the water flow [181,182,183].
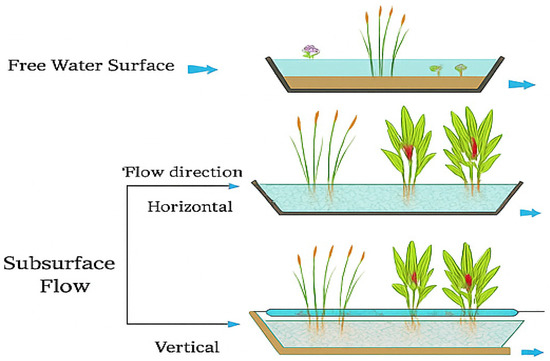
Figure 5.
Classification of constructed wetlands according to water flow and constituent elements.
In Figure 5, surface flow constructed wetlands (1) are shown. These systems consist of shallow, exposed water bodies where plants are rooted in a substrate at the bottom. These wetlands closely resemble natural ecosystems. Horizontal subsurface flow constructed wetlands (2) consist of shallow, impermeable beds filled with a porous material. This material allows efficient water passage due to its high hydraulic conductivity, promoting the formation of biofilms on its surface. Plants grow with their roots embedded in this saturated medium. Water enters from one end, flows horizontally below the surface while interacting with the substrate and plant roots, and is finally collected at the opposite end of the system. Vertical subsurface flow constructed wetlands (3), on the other hand, usually operate under unsaturated conditions. These systems include a bed approximately one meter deep, filled with porous materials such as sand or gravel. The water treatment occurs as it percolates vertically through the medium, coming into contact with plant roots, which also contribute to drainage. Water is distributed evenly over the bed surface through a network of pressurized pipes and is then collected at the bottom via perforated drainage pipes [184].
In Figure 6, three key elements are shown: a porous filter medium, microbial communities, and vegetation. The transformation of nutrients and organic matter occurs mainly due to biofilms formed by microorganisms that develop on both the porous media and within the rhizosphere. Materials used in the medium—such as soil, sand, rock, or gravel—provide a large surface area for microbial adhesion, support plant growth, and act as filters or adsorbents for pollutants present in the water.
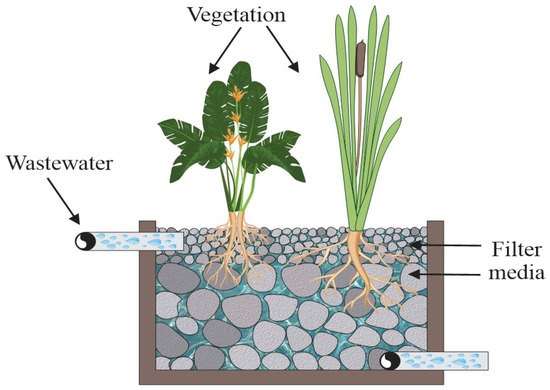
Figure 6.
Main elements of a CW: porous filter media, microbial communities and vegetation.
Plants play a crucial role: by developing roots and rhizomes, they provide a structural base for bacterial colonization and help oxygenate the zones near the roots. They also absorb nutrients such as nitrogen (N), phosphorus (P), and other essential elements primarily through their roots, which transport them internally to the stems and leaves. This process additionally supplies the carbon needed for denitrification during plant biomass decomposition, helping to retain pollutants and prevent their release from sediments.
Figure 7 shows an overview of a constructed wetland system integrated into a recirculating aquaculture system. The arrows indicate the water flow from the culture pond to the wetland system, where the water is treated and subsequently received in a treated water collection pond for its reintegration into the culture pond.

Figure 7.
A constructed wetland system integrated into a RAS–CW® aquaculture recirculation system for mesocosm-scale tilapia production [180].
The system is composed of a culture pond (1) and an overflow sedimentation tank (2), from which the wastewater is subsequently directed to a constructed wetland system. This system includes a surface flow wetland planted with Pistia stratiotes (9), Ipomoea aquatica (10), and Eichhornia crassipes (11). In addition, it contains three horizontal subsurface flow wetlands planted with Typha latifolia (13), Colocasia esculenta (14), and Ipomoea aquatica (15). The entire recirculating system is designed for the production of Oreochromis niloticus (Nile tilapia) and for cultivating plant species that may serve as complementary food sources for both tilapia and humans, such as Colocasia esculenta and Ipomoea aquatica. The removal efficiencies reported by Delfín-Portela [180] showed the following results for each plant species are as follows: Pistia stratiotes: TDS (91.94%), PO43− (100%), and BOD5 (79.57%); Ipomoea aquatica: TDS (75.05%), PO43− (100%), and BOD5 (83.33%); Eichhornia crassipes: TDS (60.70%), PO43− (87.5%), and BOD5 (45.54%); Typha latifolia: TDS (55.12%), PO43− (87.5%), and BOD5 (77.96%); and Colocasia esculenta: TDS (54.57%), PO43− (75.5%), and BOD5 (45.96%).
Another aspect that has been minimally explored in the analyzed studies is agricultural production using CW systems. However, there is evidence demonstrating the feasibility of integrating CWs with agriculture through the Wetland Culture™ method [181]. This approach is defined as a sustainable agricultural system that incorporates treatment wetlands with the dual objectives of reducing non-point source nutrient pollution and eliminating the need for synthetic fertilizers by recycling nutrients [185,186]. Additionally, Wetland Culture™ has been successfully applied in the production of maize [185].
3.7. The Research Scale of Constructed Wetlands Applied to the Aquaculture Sector
The Technology Readiness Level (TRL) is a measurement scale used to assess the maturity level of a particular technology [187]. In Mexico, the Secretariat of Science, Humanities, Technology, and Innovation (SECHTI) evaluates each project according to the criteria defined for each TRL stage. Projects are then classified based on their research progress. Following this methodology and applying the TRL scale (which ranges from levels 1 to 9), most of the selected articles correspond to TRLs 1 to 5, as they involve experimental studies conducted at laboratory-scale, microcosm, or mesocosm levels. Pilot and full-scale studies, which involve validation and demonstration in operational environments, correspond to TRLs 6 to 9, with level 9 indicating a technology fully developed and ready for commercial deployment.
Based on this classification, Table 4 summarizes the findings. For constructed wetlands applied to aquaculture wastewater treatment, studies conducted at a full scale remain limited (5.71%). The majority (91.43%) are laboratory-scale investigations (TRL 1 to 5), with only a few cases (1.43%) reported at pilot scales in relevant environments.

Table 4.
Research scale of constructed wetlands in aquaculture.
These findings highlight the need to continue developing research at all TRL stages, given the limited availability of full-scale implementation data in this field. Although CW technologies have been successfully tested at a full scale in other contexts, the results have often not been published, making it difficult to confirm their TRL 9 status in aquaculture applications. Additionally, several factors influence the operation and design of CWs in aquaculture. As emphasized by Vimazal et al. [99], advancing to relevant full-scale environments over extended operational periods is crucial. However, economic and funding constraints often limit these efforts.
3.8. Factors Which Affect Constructed Wetlands in Aquaculture
For its implementation, the first aspect to consider is the available space for its construction, which is generally substantial. Farms must allocate this space to receive and treat the wastewater [53].
Additionally, the key factors to ensure the proper functioning and effective design of a constructed wetland are summarized in Figure 8.

Figure 8.
Factors affecting CWs in aquaculture.
3.9. Impact of Plants on Aquaculture Productivity
Plants play multiple roles within the complex substrate–root–microorganism system, which facilitates the development of biofilms responsible for the biochemical transformation of pollutants [45]. In addition, plants absorb nutrients from wastewater that are necessary for their growth and development [89]. Many species have been studied for their role in phytoremediation through constructed wetlands, as presented in this research. However, there remains a wide variety of plant species, particularly in tropical regions with a high aquatic plant diversity, whose potential for pollutant removal is still unknown. The impact of integrating constructed wetlands (CWs) into aquaculture systems lies in the reduced use of water, which also leads to lower energy consumption for pumping and, consequently, a reduction in production costs. This aspect was evaluated by [33,53], who compared CW technology with other ecotechnologies and found CWs to be the most energy-efficient system.
Constructed wetland (CW) systems offer significant environmental and economic advantages. The evaluation results indicate that their construction is economically feasible for aquaculture production, particularly in regions with limited water availability. These systems provide effective water treatment at very low operational costs. The net present value (NPV) was estimated at USD 20,398.51, with an internal rate of return (IRR) of 17%. However, the costs of implementing such systems vary, largely depending on the economic resources available and the size of the farm. It is evident that as aquaculture systems shift toward more technically advanced closed-loop operations, costs increase. This helps explain why CWs are not yet widely adopted in Mexico.
In the case presented in Figure 9, the construction cost was USD 121.79 per square meter for a 900 m2 wetland system [33,53]. In general, the cost of plants is very low because many of the most studied species grow naturally across various regions of Mexico and the world. These plants can be collected with minimal investment, considering only the labor, transportation, and planting—typically taking three days to transfer and establish them in the selected wetland site [180].
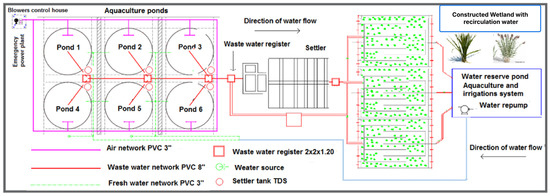
Figure 9.
An aquaculture recirculation system integrated with a full-scale constructed wetland [33,53]. Phragmites australis and Typha latifolia demonstrated removal capacities of 57% for total suspended solids (TSS), which was 90% for NO2-N (mg/L) and 68% for NO3-N (mg/L).
3.10. Challenges of Constructed Wetlands in Aquaculture
The primary limitation to a full-scale implementation is the lack of the dissemination of successful, practical experiences. Additionally, not all possible design options have been explored or adapted for different climatic regions where aquaculture is practiced. Although CWs have the potential for large-scale application, the aquaculture industry still faces several challenges, as illustrated in Figure 10. Therefore, future research should focus on the following key aspects:

Figure 10.
Challenges of constructed wetlands in aquaculture.
4. Conclusions
This research has revealed that the predominant vegetation used in constructed wetlands (CWs) includes Phragmites australis, Typha latifolia, Canna indica, Eichhornia crassipes, and Arundo donax, out of a total of 43 plant species. These plants have been applied to treat wastewater generated by the production systems of 25 aquaculture species, with Oreochromis niloticus, Litopenaeus vannamei, Ictalurus punctatus, Clarias gariepinus, Tachysurus fulvidraco, and Cyprinus carpio being the most frequently reported. The use of plants in CWs for wastewater remediation is an economical and efficient strategy; however, its application is still largely limited to experimental scales. Furthermore, there remain many plant and aquaculture species yet to be studied in this context.
The evidence indicates that CW technology has been integrated into recirculating aquaculture systems (RAS) using various designs and combinations of plant and fish species. However, most studies have focused primarily on water quality variables (environmental aspect) without considering CW–RAS integration as a productive model aligned with the principles of the circular economy.
Only a limited number of studies have reported the use of plant species that are both edible for aquaculture species and safe for human consumption. Among these are Lemna minor, Ipomoea aquatica, Pistia stratiotes, and Lactuca sativa. The use of CWs in aquaculture has shown few real-scale applications, and there is a minimal number of studies focused on water reuse for agricultural purposes.
5. Future Perspectives
This opens future research opportunities aimed at promoting responsible production and consumption practices, integrating principles of the circular economy within aquaculture farms. This study highlights a significant knowledge gap in the application of constructed wetlands within the aquaculture sector. The current limited information on both plant and aquaculture species analyzed confirms the need for further research, particularly exploring underutilized plant species to assess their adaptation to CW systems. Additionally, further investigation is required for key aquaculture species and commercially valuable plants to enable CW systems not only to recycle water but also to contribute to agricultural production, thus making the technology more appealing.
It is also necessary to determine which types of full-scale wetlands are most appropriate for mitigating pollution from aquaculture effluents before they reach receiving water bodies. Moreover, research should be expanded on the types of wetlands used in aquaculture, the contaminant removal efficiencies reported, and their integration within recirculating aquaculture systems (RAS). This will help provide useful solutions for various aquaculture practices across different regions of the country. The research field remains vast and largely unexplored.
Finally, it is essential to focus on reducing hydraulic retention times (HRTs) for water purification. Therefore, future studies should investigate the addition of carbon sources, beneficial microorganisms, and artificial aeration systems that could support reducing treatment times and enhance the efficiency of integrated RAS–CW systems.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/su17146298/s1: PRISMA checklist for systematic reviews AQ-CW.
Author Contributions
Conceptualization, E.A.B.-T., L.C.S.H. and E.D.-P.; methodology, E.A.B.-T., E.D.-P. and L.C.S.H.; validation, E.A.B.-T., L.C.S.H. and E.D.-P.; formal analysis, E.A.B.-T., L.C.S.H., E.D.-P., G.A.-C. and G.B.-D.; investigation, E.A.B.-T., E.D.-P. and G.A.-C.; resources, E.A.B.-T., L.C.S.H. and G.B.-D.; data curation, G.B.-D. and G.A.-C.; writing—original draft preparation, E.A.B.-T. and L.C.S.H.; writing—review and editing, G.A.-C., E.A.B.-T. and E.D.-P.; visualization, G.A.-C., L.C.S.H. and E.D.-P.; supervision, G.A.-C. and G.B.-D.; project administration, E.A.B.-T., L.C.S.H. and E.D.-P. funding acquisition, E.A.B.-T., L.C.S.H., E.D.-P. and G.B.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This study received external funding from the Secretaría de Ciencia, Humanidades, Tecnología e Innovación (SECHTI) through the doctoral fellowship of E.D.-P. (CVU 892099) and the postdoctoral academic position of E.A.B.-T. (CVU 774357).
Institutional Review Board Statement
Not applicable, as the study did not involve humans or animals.
Informed Consent Statement
Not applicable, as the study did not involve humans or animals.
Data Availability Statement
The raw data supporting the conclusions of this article are available from the authors upon reasonable request.
Acknowledgments
The authors would like to thank the Secretaria de Ciencia, Humanidades, Tecnología e Innovación (SECHTI) of the Mexican government for its support in the development of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2018: Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; Available online: https://openknowledge.fao.org/items/87109e17-2bb7-4d20-874b-160ac0a2b131 (accessed on 19 December 2024).
- GPAS (Ley General de Pesca y Acuacultura Sustentable). Diario Oficial de la Federación. 2018. Available online: https://www.diputados.gob.mx/LeyesBiblio/pdf/LGPAS.pdf (accessed on 19 December 2024).
- Tidwell, J.H. (Ed.) Aquaculture Production Systems; Wiley-Blackwell: Oxford, UK, 2012. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). Brief to The State of World Fisheries and Aquaculture 2024; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- White, P. Aquaculture Pollution: An Overview of Issues with a Focus on China, Vietnam, and the Philippines. 2017. Prep. Para El Banco Mund., Wash. DC, EE. UU 2017. Available online: https://acortar.link/GWB9pL (accessed on 1 March 2017).
- Hilborn, R.; Banobi, J.; Hall, S.; Pucylowski, T.; Walsworth, T. The environmental cost of animal source foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef]
- Costello, C.; Cao, L.; Gelcich, S.; Cisneros-Mata, M.Á.; Free, C.M.; Froehlich, H.E.; Golden, C.D.; Ishimura, G.; Maier, J.; Macadam-Somer, I.; et al. The future of food from the sea. Nature 2020, 588, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Tubb, C.; Seba, T. Rethinking Food and Agriculture 2020-2030: The Second Domestication of Plants and Animals, the Disruption of the Cow, and the Collapse of Industrial Livestock Farming. Ind. Biotechnol. 2021, 17, 57–72. [Google Scholar] [CrossRef]
- Boyd, C.E.; McNevin, A.A.; Davis, R.P. The contribution of fisheries and aquaculture to the global protein supply. Food Secur. 2022, 14, 805–827. [Google Scholar] [CrossRef] [PubMed]
- Valenti, W.C.; Kimpara, J.M.; Preto, B.d.L.; Moraes-Valenti, P. Indicators of sustainability to assess aquaculture systems. Ecol. Indic. 2018, 88, 402–413. [Google Scholar] [CrossRef]
- Golden, C.D.; Koehn, J.Z.; Shepon, A.; Passarelli, S.; Free, C.M.; Viana, D.F.; Matthey, H.; Eurich, J.G.; Gephart, J.A.; Fluet-Chouinard, E.; et al. Aquatic foods to nourish nations. Nature 2021, 598, 315–320. [Google Scholar] [CrossRef]
- Zhu, S.; Lyu, C. Construction and application of an evaluation index system for high-quality development in aquaculture. Aquac. Int. 2025, 33, 124. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations). The State of World Fisheries and Aquaculture 2022 Towards the Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Xu, C.; Su, G.; Brosse, S.; Zhao, K.; Zhang, M.; Xu, J. Social benefits and environmental performance of aquaculture need to improve worldwide. Commun. Earth Environ. 2024, 5, 698. [Google Scholar] [CrossRef]
- FAO (Food and Agriculture Organization of the United Nations) Fisheries & Aquaculture. Overview of the National Sector Mexico. Available online: https://www.fao.org/fishery/fr/countrysector/mx/es (accessed on 9 February 2025).
- Filipski, M.; Belton, B. Give a Man a Fishpond: Modeling the Impacts of Aquaculture in the Rural Economy. World Dev. 2018, 110, 205–223. [Google Scholar] [CrossRef]
- Custódio, M.; Villasante, S.; Calado, R.; Lillebø, A.I. Valuation of Ecosystem Services to promote sustainable aquaculture practices. Rev. Aquac. 2019, 12, 392–405. [Google Scholar] [CrossRef]
- Mair, J.; Chien, P.M.; Kelly, S.J.; Derrington, S. Social impacts of mega-events: A systematic narrative review and research agenda. J. Sustain. Tour. 2023, 31, 538–560. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Guidebook, 2nd ed.; World Aquaculture Society: Baton Rouge, LA, USA, 2012. [Google Scholar]
- Pueppke, S.G.; Nurtazin, S.; Ou, W. Water and Land as Shared Resources for Agriculture and Aquaculture: Insights from Asia. Water 2020, 12, 2787. [Google Scholar] [CrossRef]
- Edwards, P. Aquaculture environment interactions: Past, present and likely future trends. Aquaculture 2015, 447, 2–14. [Google Scholar] [CrossRef]
- Little, D.C.; Newton, R.W.; Beveridge, M.C.M. Aquaculture: A rapidly growing and significant source of sustainable food? Status, transitions and potential. Proc. Nutr. Soc. 2016, 75, 274–286. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Z.; Cheng, G.; Lu, S.; Gu, Z.; Zhu, H.; Shen, H.; Wang, J.; Chen, X. Ecological engineering in pond aquaculture: A review from the whole-process perspective in China. Rev. Aquac. 2020, 13, 1060–1076. [Google Scholar] [CrossRef]
- Jeanson, A.L.; Gotzek, D.; Mam, K.; Hecht, L.; Charvet, P.; Eckerström-Liedholm, S.; Cooke, S.J.; Pool, T.; Elliott, V.; Torres, Y. Inland Fisheries Management—Case Studies of Inland Fish. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2022; pp. 343–354. [Google Scholar] [CrossRef]
- Macusi, E.D.; Estor, D.E.P.; Borazon, E.Q.; Clapano, M.B.; Santos, M.D. Environmental and Socioeconomic Impacts of Shrimp Farming in the Philippines: A Critical Analysis Using PRISMA. Sustainability 2022, 14, 2977. [Google Scholar] [CrossRef]
- Jiang, Q.; Bhattarai, N.; Pahlow, M.; Xu, Z. Environmental sustainability and footprints of global aquaculture. Resour. Conserv. Recycl. 2022, 180, 106183. [Google Scholar] [CrossRef]
- Zimmermann, S.; Kiessling, A.; Zhang, J. The future of intensive tilapia production and the circular bioeconomy without effluents: Biofloc technology, recirculation aquaculture systems, bio-RAS, partitioned aquaculture systems and integrated multitrophic aquaculture. Rev. Aquac. 2023, 15 (Suppl. S1), 22–31. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Khanjani, M.H.; Sharifinia, M.; Miranda-Baeza, A.; Qin, J. Could Biofloc Technology (BFT) Pave the Way Toward a More Sustainable Aquaculture in Line with the Circular Economy? Aquac. Res. 2025, 2025, 1020045. [Google Scholar] [CrossRef]
- Froehlich, H.E.; Gentry, R.R.; Rust, M.B.; Grimm, D.; Halpern, B.S.; Somers, C.M. Public Perceptions of Aquaculture: Evaluating Spatiotemporal Patterns of Sentiment around the World. PLoS ONE 2017, 12, e0169281. [Google Scholar] [CrossRef]
- Kluger, L.C.; Filgueira, R.; Byron, C.J. Using media analysis to scope priorities in social carrying capacity assessments: A global perspective. Mar. Policy 2019, 99, 252–261. [Google Scholar] [CrossRef]
- Ballut-Dajud, G.A.; Herazo, L.C.S.; Fernández-Lambert, G.; Marín-Muñiz, J.L.; Méndez, M.C.L.; Betanzo-Torres, E.A. Factors Affecting Wetland Loss: A Review. Land 2022, 11, 434. [Google Scholar] [CrossRef]
- Platas-Rosado, D.; Hernández-Arzaba, J.; Gonzalez-Reynoso, L. Importancia Económica y Social del Sector Acuícola en México. Producto Agro. 2018. Available online: https://bit.ly/3S8kqqZ (accessed on 10 June 2020).
- Betanzo-Torres, E.A. La Acuacultura en México y el Uso de Tecnología Biofloc Como Alternativa Sustentable: Análisis de Adopción, Desarrollo y Comparativo Con Otras Tecnologías Para el Cultivo de Tilapia (Oreochromis Niloticus). Ph.D. Thesis, El Colegio de Veracruz, Xalapa, Mexico, 2019. [Google Scholar]
- Delfín-Portela, E.; Sandoval-Herazo, L.C.; Reyes-González, D.; Mata-Alejandro, H.; López-Méndez, M.C.; Fernández-Lambert, G.; Betanzo-Torres, E.A. Grid-Connected Solar Photovoltaic System for Nile Tilapia Farms in Southern Mexico: Techno-Economic and Environmental Evaluation. Appl. Sci. 2022, 13, 570. [Google Scholar] [CrossRef]
- Esquivel Lopez, G.; Ruelas Mojardin, L. Propuestas Para Promover el Desarrollo Sustentable en la Acuicultura Mexicana. Un análisis a Través de Los Paradigmas de la Gestión Ambiental; Centro de Estudios para el Desarrollo Rural Sostenible y la Soberanía Alimentaria. Cámara de Diputados, México, s. f. Available online: http://201.147.98.23/Ver/Documento/4692 (accessed on 17 March 2024).
- Henares, M.N.P.; Medeiros, M.V.; Camargo, A.F.M. Overview of strategies that contribute to the environmental sustainability of pond aquaculture: Rearing systems, residue treatment, and environmental assessment tools. Rev. Aquac. 2019, 12, 453–470. [Google Scholar] [CrossRef]
- Laktuka, K.; Kalnbalkite, A.; Sniega, L.; Logins, K.; Lauka, D. Towards the Sustainable Intensification of Aquaculture: Exploring Possible Ways Forward. Sustainability 2023, 15, 16952. [Google Scholar] [CrossRef]
- Nunes, L.J.L.; da Silva Campos, C.V.F.; da Silva, S.M.B.C.; Gálvez, A.O.; Brito, L.O.; dos Santos, J.F. The culture of Nile tilapia (Oreochromis niloticus) juvenile at different culture technologies: Autotrophic, bioflocs and Synbiotic. Aquaculture 2024, 588, 740912. [Google Scholar] [CrossRef]
- Pimentel, O.A.L.F.; Wasielesky, W.; da Silva, N.P.; Borges, L.D.V.; Krummenauer, D. Fertilizing synbiotic system with different vegetable brans: Effects on nitrification, plankton composition, and growth of Penaeus vannamei in the nursery phase. Aquac. Int. 2024, 32, 6407–6429. [Google Scholar] [CrossRef]
- Celdran-Sabater, D. Que es la tecnologia BAF. Available online: https://www.bioaquafloc.com/que-es-la-tecnologia-baf/ (accessed on 9 December 2024).
- Spencer, B.E. Environmental impacts of aquaculture. Aquaculture 2002, 203, 397–398. [Google Scholar] [CrossRef]
- Martinez-Porchas, M.; Martinez-Cordova, L.R. World Aquaculture: Environmental Impacts and Troubleshooting Alternatives. Sci. World J. 2012, 2012, 389623. [Google Scholar] [CrossRef]
- Herath, S.S.; Satoh, S. Environmental impacts of nitrogen and phosphorus from aquaculture. In Feed and Feeding Practices in Aquaculture; Elsevier: Amsterdam, The Netherlands, 2022; pp. 427–444. [Google Scholar] [CrossRef]
- Brix, H. How ‘Green’ Are Aquaculture, Constructed Wetlands and Conventional Wastewater Treatment Systems? Water Sci. Technol. 1999, 40, 45–50. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Jing, S.-R.; Lee, D.-Y.; Wang, T.-W. Nutrient removal from aquaculture wastewater using a constructed wetlands system. Aquaculture 2002, 209, 169–184. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Jing, S.-R.; Lee, D.-Y. The potential use of constructed wetlands in a recirculating aquaculture system for shrimp culture. Environ. Pollut. 2003, 123, 107–113. [Google Scholar] [CrossRef]
- Lin, Y.-F.; Jing, S.-R.; Lee, D.-Y.; Chang, Y.-F.; Chen, Y.-M.; Shih, K.-C. Performance of a constructed wetland treating intensive shrimp aquaculture wastewater under high hydraulic loading rate. Environ. Pollut. 2005, 134, 411–421. [Google Scholar] [CrossRef]
- Guo, H.; Liao, L.; Zheng, Z.; Xu, J.; Wei, Q.; Chen, P.; Wang, K. Evaluating the Effects of Aquaculture Wastewater Irrigation with Fertilizer Reduction on Greenhouse Tomato Production, Economic Benefits and Soil Nitrogen Characteristics. Phyton 2023, 92, 3291–3304. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed wetlands for treatment of industrial wastewaters: A review. Ecol. Eng. 2014, 73, 724–751. [Google Scholar] [CrossRef]
- Tepe, Y.; Temel, F.A. Treatment of Effluents from Fish and Shrimp Aquaculture in Constructed Wetlands. In Constructed Wetlands for Industrial Wastewater Treatment; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 105–125. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; He, W.; Liu, H.; Chen, J.; Wei, X.; Mu, J. Sesuvium portulacastrum-Mediated Removal of Nitrogen and Phosphorus Affected by Sulfadiazine in Aquaculture Wastewater. Antibiotics 2022, 11, 68. [Google Scholar] [CrossRef]
- Li, H.; Huo, Y.; He, X.; Yao, L.; Zhang, H.; Cui, Y.; Xiao, H.; Xie, W.; Zhang, D.; Wang, Y.; et al. A male germ-cell-specific ribosome controls male fertility. Nature 2022, 612, 725–731. [Google Scholar] [CrossRef]
- Betanzo-Torres, E.A.; Piñar-Álvarez, M.d.l.Á.; Sierra-Carmona, C.G.; Santamaria, L.E.G.; Loeza-Mejía, C.-I.; Marín-Muñiz, J.L.; Sandoval Herazo, L.C. Proposal of Ecotechnologies for Tilapia (Oreochromis niloticus) Production in Mexico: Economic, Environmental, and Social Implications. Sustainability 2021, 13, 6853. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, M.; Chen, J.; Cui, H.; Zhu, S.; Jin, T.; Qu, K.; Cui, Z. Purification of seawater aquaculture using constructed wetlands in a recirculating aquaculture system for Pacific white shrimp (Litopenaeus vannamei) and analysis of microbial community structure. J. Water Process Eng. 2024, 66, 105959. [Google Scholar] [CrossRef]
- Hijosa-Valsero, M.; Sidrach-Cardona, R.; Pedescoll, A.; Sánchez, O.; Bécares, E. Role of Bacterial Diversity on PPCPs Removal in Constructed Wetlands. In Constructed Wetlands for Industrial Wastewater Treatment; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 405–426. [Google Scholar] [CrossRef]
- Le Gouvello, R.; Brugere, C.; Simard, F. Aquaculture and Nature-Based Solutions. In Aquaculture and Nature-Based Solutions; IUCN Commission on Ecosystem Management C: Gland, Switzerland, 2022. [Google Scholar]
- Castine, S.; McKinnon, A.; Paul, N.; Trott, L.; de Nys, R. Wastewater treatment for land-based aquaculture: Improvements and value-adding alternatives in model systems from Australia. Aquac. Environ. Interact. 2013, 4, 285–300. [Google Scholar] [CrossRef]
- Omotade, I.F.; Alatise, M.O.; Olanrewaju, O.O. Recycling of aquaculture wastewater using charcoal based constructed wetlands. Int. J. Phytoremediation 2019, 21, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Haydar, S.; Anis, M.; Afaq, M. Performance evaluation of hybrid constructed wetlands for the treatment of municipal wastewater in developing countries. Chin. J. Chem. Eng. 2020, 28, 1717–1724. [Google Scholar] [CrossRef]
- Uusheimo, S.; Huotari, J.; Tulonen, T.; Aalto, S.L.; Rissanen, A.J.; Arvola, L. High Nitrogen Removal in a Constructed Wetland Receiving Treated Wastewater in a Cold Climate. Environ. Sci. Technol. 2018, 52, 13343–13350. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, A.; Shukla, B.K.; Bansal, V. Sustainable design of textile industry effluent treatment plant with constructed wetland. Mater. Today 2022, 61, 537–542. [Google Scholar] [CrossRef]
- Tom, A.P.; Jayakumar, J.S.; Biju, M.; Somarajan, J.; Ibrahim, M.A. Aquaculture wastewater treatment technologies and their sustainability: A review. Energy Nexus 2021, 4, 100022. [Google Scholar] [CrossRef]
- Gorito, A.M.; Lado Ribeiro, A.R.; Pereira, M.F.R.; Almeida, C.M.R.; Silva, A.M.T. Advanced oxidation technologies and constructed wetlands in aquaculture farms: What do we know so far about micropollutant removal? Environ. Res. 2022, 204, 111955. [Google Scholar] [CrossRef]
- Zhang, S.; Li, G.; Chang, J.; Li, X. Aerated Enhanced Treatment of Aquaculture Effluent by Three-stage, Subsurface-Flow Constructed Wetlands under a High Loading Rate. Pol. J. Environ. 2014, 23, 1821–1830. [Google Scholar]
- Sochacki, A.; Yadav, A.K.; Srivastava, P.; Kumar, N.; Fitch, M.W.; Mohanty, A. Constructed Wetlands for Metals: Removal Mechanism and Analytical Challenges. In Constructed Wetlands for Industrial Wastewater Treatment; John Wiley & Sons, Ltd.: Chichester, UK, 2018; pp. 223–247. [Google Scholar] [CrossRef]
- Gorito, A.M.; Ribeiro, A.R.; Almeida, C.M.R.; Silva, A.M.T. A review on the application of constructed wetlands for the removal of priority substances and contaminants of emerging concern listed in recently launched EU legislation. Environ. Pollut. 2017, 227, 428–443. [Google Scholar] [CrossRef]
- Vymazal, J. Emergent plants used in free water surface constructed wetlands: A review. Ecol. Eng. 2013, 61, 582–592. [Google Scholar] [CrossRef]
- Nasr-Allah, A. Tilapia production using climate smart aquaculture system in Egypt, in-pond raceway system (IPRS). In Proceedings of the 4th International Conference on Sustainable Development of Livestock Production Systems “Smart and Precise Farming”, Alexandria, Egypt, 19 November 2019; p. 3. Available online: https://hdl.handle.net/20.500.12348/4078 (accessed on 17 January 2025).
- Zimmermann, S. Sistemas Acuícolas sin Efluentes. In Proceedings of the II CIA 2020—Congreso Internacional de Acuicultura, Cajamarca, Peru, 26 November 2020; Available online: https://www.regioncajamarca.gob.pe/media/portal/KJDIG/documento/8531/BROCHURE_II_CIA_2020_FINAL.pdf (accessed on 22 January 2024).
- Bertolini, R.; Sabbag, O.; Zimmermann, S.; Hein, G. Tecnologias Avaliadas: Dados Económicos de Diferentes Tecnologias de Produção Intensiva de Tilápias. Cartilha de custos. Ministério da Agricultura, Pecuária e Abastecimento. 2021. Available online: https://www.gov.br/agricultura/pt-br/assuntos/aquicultura-e-pesca/informativos/cartilha-de-custos-tilapia (accessed on 22 January 2024).
- CONAPESCA (Comisión Nacional de Acuacultura y Pesca). El Portal Electrónico del Gobierno. Available online: https://www.gob.mx/conapesca (accessed on 10 February 2025).
- Hernández Sampieri, R.; Fernandez Collado, C.; Baptista Lucio, M.d.p. Metodología de la Investigacion; McGraw-Hill: México City, México, 2010; Available online: https://bit.ly/4jvcxbm (accessed on 15 May 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 327, n71. [Google Scholar] [CrossRef]
- Schwartz, M.E.; Boyd, C.E. Constructed Wetlands for Treatment of Channel Catfish Pond Effluents. Progress. Fish-Culturist 1995, 57, 255–266. [Google Scholar] [CrossRef]
- Costa-Pierce, B.A. Preliminary investigation of an integrated aquaculture–wetland ecosystem using tertiary-treated municipal wastewater in Los Angeles County, California. Ecol. Eng. 1998, 10, 341–354. [Google Scholar] [CrossRef]
- Zachritz, W.H.; Fuller, J.W. Performance of an artificial wetlands filter treating facultative lagoon effluent at Carville, Louisiana. Water Environ. Res. 1993, 65, 46–52. [Google Scholar] [CrossRef]
- Schulz, C.; Gelbrecht, J.; Rennert, B. Treatment of rainbow trout farm effluents in constructed wetland with emergent plants and subsurface horizontal water flow. Aquaculture 2003, 217, 207–221. [Google Scholar] [CrossRef]
- Lymbery, A.J.; Doupé, R.G.; Bennett, T.; Starcevich, M.R. Efficacy of a subsurface-flow wetland using the estuarine sedge Juncus kraussii to treat effluent from inland saline aquaculture. Aquac. Eng. 2006, 34, 1–7. [Google Scholar] [CrossRef]
- Zachritz, W.H.; Hanson, A.T.; Sauceda, J.A.; Fitzsimmons, K.M. Evaluation of submerged surface flow (SSF) constructed wetlands for recirculating tilapia production systems. Aquac. Eng. 2008, 39, 16–23. [Google Scholar] [CrossRef]
- Vymazal, J. Constructed Wetlands for Wastewater Treatment: Five Decades of Experience. Environ. Sci. Technol. 2011, 45, 61–69. [Google Scholar] [CrossRef]
- Cargnin, J.M.R.; João, J.J. Removal of nutrients from aquaculture residual water: A review. Ambiente Agua Interdiscip. J. Appl. Sci. 2021, 16, e2747. [Google Scholar] [CrossRef]
- Comeau, Y.; Brisson, J.; Réville, J.P.; Forget, C.; Drizo, A. Phosphorus removal from trout farm effluents by constructed wetlands. Water Sci. Technol. 2001, 44, 55–60. [Google Scholar] [CrossRef]
- Naylor, S.; Brisson, J.; Labelle, M.A.; Drizo, A.; Comeau, Y. Treatment of freshwater fish farm effluent using constructed wetlands: The role of plants and substrate. Water Sci. Technol. 2003, 48, 215–222. [Google Scholar] [CrossRef]
- Michael, J.H. Nutrients in salmon hatchery wastewater and its removal through the use of a wetland constructed to treat off-line settling pond effluent. Aquaculture 2003, 226, 213–225. [Google Scholar] [CrossRef]
- Li, G.; Wu, Z.; Cheng, S.; Liang, W.; He, F.; Fu, G.; Zhong, F. Application of constructed wetlands on wastewater treatment for aquaculture ponds. Wuhan Univ. J. Nat. Sci. 2007, 12, 1131–1135. [Google Scholar] [CrossRef]
- Sindilariu, P.-D.; Wolter, C.; Reiter, R. Constructed wetlands as a treatment method for effluents from intensive trout farms. Aquaculture 2008, 277, 179–184. [Google Scholar] [CrossRef]
- Sindilariu, P.-D.; Schulz, C.; Reiter, R. Treatment of flow-through trout aquaculture effluents in a constructed wetland. Aquaculture 2007, 270, 92–104. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, G.; Liu, J.; Zhu, Y.; Xu, J. Performance of a constructed wetland in treating brackish wastewater from commercial recirculating and super-intensive shrimp grow out systems. Bioresour. Technol. 2011, 102, 9416–9424. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhou, Q.H.; Xu, D.; He, F.; Cheng, S.; Liang, W.; Wu, Z. Vertical-flow constructed wetlands applied in a recirculating aquaculture system for channel catfish culture: Effects on water quality and zooplankton. J. Environ. 2010, 277, 1063–1070. [Google Scholar]
- Konnerup, D.; Trang, N.T.D.; Brix, H. Treatment of fishpond water by recirculating horizontal and vertical flow constructed wetlands in the tropics. Aquaculture 2011, 313, 57–64. [Google Scholar] [CrossRef]
- Chen, J.; Guo, F.; Wu, F.; Bryan, B.A. Costs and benefits of constructed wetlands for meeting new water quality standards from China’s wastewater treatment plants. Resour. Conserv. Recycl. 2023, 199, 107248. [Google Scholar] [CrossRef]
- Akyürek, A.; Ağdağ, O.N. Comparison of constructed wetlands and package type sequencing batch biological treatment plants in rural areas in terms of efficiency and cost in a full-scale example. Ecol. Eng. 2024, 201, 107190. [Google Scholar] [CrossRef]
- Tellbüscher, A.A.; Gebauer, R.; Mráz, J. Nutrients revisited: Review and meta-data analysis of nutrient inputs into freshwater aquaculture systems. Aquaculture 2025, 595, 741633. [Google Scholar] [CrossRef]
- Deng, Y.Y.; Zou, M.Y.; Liu, W.; Lian, Y.L.; Guo, Q.M.; Zhang, X.M. Antibiotic removal and microbial response mechanism in constructed wetlands treating aquaculture wastewater containing veterinary drugs. J. Clean. Prod. 2023, 394, 136271. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, S.; Ma, Z.; Li, Y.; Huang, D.; Zhang, J. Study on the purification of aquaculture tailwater under Sulfamethoxazole stress using algae-bacteria biofilms: Nutrient removal efficiency, microbial community, and ARGs. Process Saf. Environ. Prot. 2024, 191, 1432–1444. [Google Scholar] [CrossRef]
- Rocha, C.; Ibanhez, J.; Leote, C. Benthic nitrate biogeochemistry affected by tidal modulation of Submarine Groundwater Discharge (SGD) through a sandy beach face, Ria Formosa, Southwestern Iberia. Mar. Chem. 2009, 115, 43–58. [Google Scholar] [CrossRef]
- Zidan, A.A.; Wu, Z.; Wang, Y.; Chen, Y.; Liu, J. Nutrient distribution and nitrate processing in a mangrove tidal creek affected by submarine groundwater discharge (SGD). Mar. Pollut. Bull. 2025, 212, 117575. [Google Scholar] [CrossRef]
- Saeed, T.; Muntaha, S.; Rashid, M.; Sun, G.; Hasnat, A. Industrial wastewater treatment in constructed wetlands packed with construction materials and agricultural by-products. J. Clean. Prod. 2018, 189, 442–453. [Google Scholar] [CrossRef]
- Vymazal, J.; Zhao, Y.; Mander, Ü. Recent research challenges in constructed wetlands for wastewater treatment: A review. Ecol. Eng. 2021, 169, 106318. [Google Scholar] [CrossRef]
- Dolores-Salinas, E.; Miret-Pastor, L. Environmental certifications in Brazilian aquaculture. Aquac. Int. 2024, 32, 8609–8630. [Google Scholar] [CrossRef]
- USDA (U.S. Department of Agriculture). USDA Announces More than $2 Million to Strengthen Specialty Crop Sector and Expand Crop Storage for Producers. USA. 2024. Available online: https://bit.ly/3GECw1i (accessed on 24 June 2024).
- NOOA (National Oceanic and Atmospheric Administration). Identification of Areas of Opportunity for Aquaculture in California and the Gulf of Mexico. USA. 2024. Available online: https://bit.ly/4cTRH2Q (accessed on 17 November 2024).
- Vymazal, J. The Historical Development of Constructed Wetlands for Wastewater Treatment. Land 2022, 11, 174. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sharifinia, M.; Hajirezaee, S. Recent progress towards the application of biofloc technology for tilapia farming. Aquaculture 2022, 552, 738021. [Google Scholar] [CrossRef]
- Citarasu, T.; Babu, M.M.; Yilmaz, E. Alternative medications in shrimp health management for improved production. Aquaculture 2022, 561, 738695. [Google Scholar] [CrossRef]
- Widiasa, I.N.; Susanto, H.; Ting, Y.P.; Suantika, G.; Steven, S.; Khoiruddin, K.; Wenten, I.G. Membrane-based recirculating aquaculture system: Opportunities and challenges shrimp farming. Aquaculture 2023, 579, 740224. [Google Scholar] [CrossRef]
- Lara-Rivera, A.L.; Parra-Bracamonte, G.M.; Sifuentes-Rincón, A.M.; Gojón-Báez, H.H.; Rodríguez-González, H.; Montelongo-Alfaro, I.O. Channel catfish (Ictalurus punctatus Rafinesque, 1818): Current status and problematic situation in Mexico. Lat. Am. J. Aquat. Res. 2017, 43, 424–434. [Google Scholar] [CrossRef]
- Zhong, L.; Song, C.; Chen, X.; Deng, W.; Xiao, Y.; Wang, M.; Qin, Q.; Luan, S.; Kong, J.; Bian, W. Channel catfish in China: Historical aspects, current status, and problems. Aquaculture 2016, 465, 367–373. [Google Scholar] [CrossRef]
- Abass, N.Y.; Su, B.; Perera, D.A.; Qin, Z.; Li, H.; Alsaqufi, A.; Elaswad, A.; Ye, Z.; Dong, S.; Dunham, R.A. Effects of family and promoter on growth performance of ccGH cDNA transgenic channel catfish, Ictalurus punctatus, grown in a trough culture system. Aquaculture 2021, 536, 736468. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.Y.; Tao, L.; Li, X.; Song, J.; Zhang, C.; Zhu, J.Q. Purification performance and production of a recirculating pond aquaculture system based on paddy field. Adv. J. Food Sci. Technol. 2012, 4, 383–388. [Google Scholar]
- Mahmood, T.; Zhang, J.; Zhang, G. Assessment of Constructed Wetland in Nutrient Reduction, in the Commercial Scale Experiment Ponds of Freshwater Prawn Macrobrachium rosenbergii. Bull. Environ. Contam. Toxicol. 2015, 96, 361–368. [Google Scholar] [CrossRef]
- Dalsgaard, J.; von Ahnen, M.; Naas, C.; Pedersen, P. Nutrient removal in a constructed wetland treating aquaculture effluent at short hydraulic retention time. Aquac. Environ. Interact. 2018, 10, 329–343. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Li, G.; Wu, H.-B.; Liu, X.-G.; Yao, Y.-H.; Tao, L.; Liu, H. An integrated recirculating aquaculture system (RAS) for land-based fish farming: The effects on water quality and fish production. Aquac. Eng. 2011, 45, 93–102. [Google Scholar] [CrossRef]
- Shpigel, M.; Ben-Ezra, D.; Shauli, L.; Sagi, M.; Ventura, Y.; Samocha, T.; Lee, J.J. Constructed wetland with Salicornia as a biofilter for mariculture effluents. Aquaculture 2013, 412–413, 52–63. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Li, G.; Li, X.; Tao, L. Four stage hybrid constructed wetlands treating low strength aquaculture wastewater with and without artificial aeration. Environ. Prot. Eng. 2015, 41, 31–42. [Google Scholar] [CrossRef]
- Webb, J.M.; Quintã, R.; Papadimitriou, S.; Norman, L.; Rigby, M.; Thomas, D.N.; Le Vay, L. The effect of halophyte planting density on the efficiency of constructed wetlands for the treatment of wastewater from marine aquaculture. Ecol. Eng. 2013, 61, 145–153. [Google Scholar] [CrossRef]
- Pham, T.T.H.; Cochevelou, V.; Dinh, H.D.K.; Breider, F.; Rossi, P. Implementation of a constructed wetland for the sustainable treatment of inland shrimp farming water. J. Environ. Manag. 2021, 279, 111782. [Google Scholar] [CrossRef]
- Sousa, W.T.Z.; Panitz, C.M.N.; Thomaz, S.M. Performance of pilot-scale vertical flow constructed wetlands with and without the emergent macrophyte Spartina alterniflora treating mariculture effluent. Braz. Arch. Biol. Technol. 2011, 54, 405–413. [Google Scholar] [CrossRef]
- Bitar, A.L.; Tauk-Tornisielo, S.M.; Oliveira Santos, A.A.; Malagutti, E.N.; Silva, I.M. Tratamento de efluentes de pesque-pague em sistema construído de áreas alagadas. Holos Enviroment 2009, 9, 2. [Google Scholar] [CrossRef]
- Osti, J.A.S.; Carmo, C.F.D.; Cerqueira, M.A.S.; Giamas, M.T.D.; Peixoto, A.C.; Vaz-Dos-Santos, A.M.; Mercante, C.T.J. Nitrogen and phosphorus removal from fish farming effluents using artificial floating islands colonized by Eichhornia crassipes. Aquac. Rep. 2020, 17, 100324. [Google Scholar] [CrossRef]
- Kamarudzaman, A.N.; Gana, A.D.A.; Jalil, M.F.A.; Aziz, R.A. Landfill Leachate Treatment Using SSF-FWS Constructed Wetland Planted with Limnocharis flava and Eihhornia crassipes under Different Hydraulic Loading Rate. Key Eng. Mater. 2013, 594–595, 344–349. [Google Scholar] [CrossRef]
- Díaz, C.A.; Atencio, G.V.; Pardo, C.S. Assessment of an artificial free-flow wetland system with water hyacinth (Eichhornia crassipes) for treating fish farming effluents. Rev. Colomb. Cienc. Pecu. 2014, 27, 202–210. [Google Scholar] [CrossRef]
- Henry-Silva, G.G.; Camargo, A.F.M. Tratamento de efluentes de carcinicultura por macrófitas aquáticas flutuantes. Rev. Bras. Zootec. 2008, 37, 181–188. [Google Scholar] [CrossRef]
- Henry-Silva, G.G.; Camargo, A.F.M. Efficiency of aquatic macrophytes to treat Nile tilapia pond effluents. Sci. Agricola 2006, 63, 433–438. [Google Scholar] [CrossRef]
- Pereira, J.S.; Mercante, C.T.J.; Lombardi, J.V.; Vaz-Dos-Santos, A.M.; Carmo, C.F.D.; Osti, J.A.S. Eutrophization process in a system used for rearing the Nile tilapia (Oreochromis niloticus), São Paulo State, Brazil. Acta Limnol. Bras. 2013, 24, 387–396. [Google Scholar] [CrossRef]
- Kerepeczki, E.; Gal, D.; Szabo, P.; Pekar, F. Preliminary investigations on the nutrient removal efficiency of a wetland-type ecosystem. Hydrobiologia 2003, 506, 665–670. [Google Scholar]
- Gál, D.; Pekár, F.; Kerepeczki, É.; Váradi, L. Experiments on the operation of a combined aquaculture-algae system. Aquac. Int. 2007, 15, 173–180. [Google Scholar] [CrossRef]
- Gál, D.; Kerepeczk, É.; Kosáros, T.; Pekár, F. The waste nutrients reutilisation capacity of combined pond aquaculture systems. Analele Universitatii din Oradea, Fascicula: Ecotoxicologie, Zootehnie si Tehnologii de Industrie Alimentara. Analele Univ. Din Oradea 2009, 2, 307–316. [Google Scholar]
- Xu, J.; Shi, Y.; Zhang, G.; Liu, J.; Zhu, Y. Effect of hydraulic loading rate on the efficiency of effluent treatment in a recirculating puffer aquaculture system coupled with constructed wetlands. J. Ocean Univ. China 2013, 13, 146–152. [Google Scholar] [CrossRef]
- Galaviz, M.A.A.; Díaz, J.A.Á.; Sandoval, P.H. Evaluación de un humedal artificial piloto para el tratamiento de aguas residuales domesticas—Fitorremediacíon con Eichhornia crassipes. Ra Ximhai 2024, 20, 17–37. [Google Scholar] [CrossRef]
- Rannap, R.; Kaart, M.M.; Kaart, T.; Kill, K.; Uuemaa, E.; Mander, Ü.; Kasak, K. Constructed wetlands as potential breeding sites for amphibians in agricultural landscapes: A case study. Ecol. Eng. 2020, 158, 106077. [Google Scholar] [CrossRef]
- Ramírez-Carrillo, H.; Luna-Pabello, V.; Arredondo-Figueroa, J. Evaluation of an intermittent artificial vertical flow wetland, to obtain good quality water for aquaculture. Rev. Mex. Ing. Quimica 2009, 8, 93–99. [Google Scholar]
- Tee, H.-C.; Lim, P.-E.; Seng, C.-E.; Nawi, M.-A.M. Newly developed baffled subsurface-flow constructed wetland for the enhancement of nitrogen removal. Bioresour. Technol. 2012, 104, 235–242. [Google Scholar] [CrossRef]
- Zhong, F.; Liang, W.; Yu, T.; Cheng, S.P.; He, F.; Wu, Z.B. Removal efficiency and balance of nitrogen in a recirculating aquaculture system integrated with constructed wetlands. J. Environ. Sci. Health A. 2011, 46, 789–794. [Google Scholar] [CrossRef]
- Porrello, S.; Lenzi, M.; Persia, E.; Tomassetti, P.; Finoia, M.G. Reduction of aquaculture wastewater eutrophication by Phyto treatment ponds system. Aquaculture 2003, 219, 515–529. [Google Scholar] [CrossRef]
- Fleckenstein, L.J.; Tierney, T.W.; Fisk, J.C.; Ray, A.J. The effects of different solids and biological filters in intensive pacific white shrimp (Litopenaeus vannamei) production systems. Aqua. Eng. 2020, 91, 102120. [Google Scholar] [CrossRef]
- Ma, X.; Song, X.; Li, X.; Fu, S.; Li, M.; Liu, Y. Characterization of Microbial Communities in Pilot-Scale Constructed Wetlands with Salicornia for Treatment of Marine Aquaculture Effluents. Archaea 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Buhmann, A.; Papenbrock, J. Biofiltering of aquaculture effluents by halophytic plants: Basic principles, current uses and future perspectives. Environ. Exp. Bot. 2013, 92, 122–133. [Google Scholar]
- Kinne, P.N.; Samocha, T.M.; Jones, E.R.; Browdy, C.L. Characterization of Intensive Shrimp Pond Effluent and Preliminary Studies on Biofiltration. North Am. J. Aquac. 2001, 63, 25–33. [Google Scholar] [CrossRef]
- Hu, J.; Hu, R.; Qi, D.; Lu, X. Study on treatment of aquaculture wastewater using a hybrid constructed wetland. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd International Conference on Energy Materials and Environment Engineering, Bangkok, Thailand, 10–12 March 2017; Volume 61, p. 012015. [Google Scholar] [CrossRef]
- Lin, Y.; Jing, S.; Lee, D.; Chang, Y.; Sui, H. Constructed Wetlands for Water Pollution Management of Aquaculture Farms Conducting Earthen Pond Culture. Water Environ. Res. 2010, 82, 759–768. [Google Scholar] [CrossRef]
- Schwartz, M.F.; Boyd, C.E. Channel Catfish Pond Effluents. Progress. Fish Cult. 1994, 56, 273–281. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Z.-H.; Zhao, J.-G.; Gu, B.-H. Contaminant Removal of Domestic Wastewater by Constructed Wetlands: Effects of Plant Species. J. Integr. Plant Biol. 2007, 49, 437–446. [Google Scholar] [CrossRef]
- Kerepeczki, É.; Gál, D.; Kosáros, T.; Hegedűs, R.; Gyalog, G.; Pekár, F. Natural water treatment method for intensive aquaculture effluent purification. Stud. Univ. Vasile Goldis Ser. Stiintele Vietii 2011, 21, 827–837. [Google Scholar]
- Iamchaturapatr, J.; Yi, S.W.; Rhee, J.S. Nutrient removals by 21 aquatic plants for vertical free surface-flow (VFS) constructed wetland. Ecol. Eng. 2007, 29, 287–293. [Google Scholar] [CrossRef]
- Zhang, S.; Ban, Y.; Xu, Z.; Cheng, J.; Li, M. Comparative evaluation of influencing factors on aquaculture wastewater treatment by various constructed wetlands. Ecol. Eng. 2016, 93, 221–225. [Google Scholar] [CrossRef]
- de Vasconcelos, V.M.; de Morais, E.R.C.; Faustino, S.J.B.; Hernandez, M.C.R.; Gaudêncio, H.R.d.S.C.; de Melo, R.R.; Junior, A.P.B. Floating aquatic macrophytes for the treatment of aquaculture effluents. Environ. Sci. Pollut. Res. 2020, 28, 2600–2607. [Google Scholar] [CrossRef]
- Al-Hafedh, Y.S.; Alam, A.; Alam, M.A. Performance of plastic biofilter media with different configuration in a water recirculation system for the culture of Nile tilapia (Oreochromis niloticus). Aquac. Eng. 2003, 29, 139–154. [Google Scholar] [CrossRef]
- Nootong, K.; Nurit, S.; Powtongsook, S. Control of Inorganic Nitrogen and Suspended Solids Concentrations in a Land-Based Recirculating Aquaculture System. Eng. J. 2013, 17, 49–60. [Google Scholar] [CrossRef][Green Version]
- De Farias Lima, J.; Bastos, A.M.; Duarte, S.S.; dos Santos, U.R.A. Are artificial semi-dry wetlands efficient in wastewater treatment from different fish densities and for lettuce production? Int. J. Environ. Sci. Technol. 2021, 19, 8329–8340. [Google Scholar] [CrossRef]
- Teixeira, D.L.; Souza, A.; Moura, G.d.S.; Júnior, M.C.R.L. Reuse of aquaculture wastewater treated in constructed wetlands. Rev. Eng. Na Agric. Reveng 2021, 29, 347–354. [Google Scholar] [CrossRef]
- Raharjo, S.; Suprihatin, N.S.I.; Riani, E. The Benefits of Constructed Wetlands Application in a Vannamei Shrimp (Litopenaeus vannamei) 2015. Cultivation System with a Mesohaline Condition. Available online: https://acortar.link/AjxcAD (accessed on 7 May 2025).
- Raharjo, S.; Fitriyah Irmawati, E.S.; Manaf, M. Constructed Wetland with Flow Water Surface Type for Elimination of Aquaculture Wastewater from Catfish (Clarias gariepinus, Var). In Proceedings of the IOP Conference Series: Earth and Environmental Science, Proceedings of the 4th International Seminar on Sciences, Bogor, Indonesia, 19–20 October 2017; Volume 187, p. 012061. [Google Scholar] [CrossRef]
- Raharjo, S.; Suprihatin, S.; Indrasti, N.S.; Riani, E.; Supriyadi, S.; Hardanu, W. lahan basah buatan sebagai media pengolahan air limbah budidaya udang vaname (litopenaeus vannamaei) bersalinitas rendah (Constructed Wetland for Remediation of Brackish Wastewater from White Shrimp (Litopenaeus vannamaei) Cultivation). J. Mns. Dan Lingkung. 2015, 22, 201. [Google Scholar] [CrossRef][Green Version]
- Raharjo, S. kemampuan lahan basah buatan dan fitoremediasi rumput akar wangi (chrysopogon zizanioides, l) dalam mengendalikan air limbah budidaya udang vaname (litopenaeus vannamaei). In Proceedings of the Indonesian Clean Technology Meeting, Jakarta, Indonesia, 12 April 2017; pp. 64–71. [Google Scholar]
- Ramos, R.; Gallardo, S. Capacidad de biofiltración de nutrientes y crecimiento de macroalgas utilizando efluentes generados en el cultivo del pez dorado Seriola lalandi (Perciformes: Carangidae). Rev. Biol. Mar. Oceanogr. 2021, 56, 13–21. [Google Scholar] [CrossRef]
- Sri-uam, P.; Donnuea, S.; Powtongsook, S.; Pavasant, P. Integrated Multi-Trophic Recirculating Aquaculture System for Nile Tilapia (Oreochlomis niloticus). Sustainability 2016, 8, 592. [Google Scholar] [CrossRef]
- Liu, J.; Yi, N.-K.; Wang, S.; Lu, L.-J.; Huang, X.-F. Impact of plant species on spatial distribution of metabolic potential and functional diversity of microbial communities in a constructed wetland treating aquaculture wastewater. Ecol. Eng. 2016, 94, 564–573. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiao, L.; Qin, H.; Li, F. Effect of Environmental Stress on the Nutrient Stoichiometry of the Clonal Plant Phragmites australis in Inland Riparian Wetlands of Northwest China. Front. Plant Sci. 2022, 12, 705319. [Google Scholar] [CrossRef]
- Jin, L.; Sun, X.; Ren, H.; Huang, H. Biological filtration for wastewater treatment in the 21st century: A data-driven analysis of hotspots, challenges and prospects. Sci. Total Environ. 2023, 855, 158951. [Google Scholar] [CrossRef]
- Kanchanapiya, P.; Tantisattayakul, T. Wastewater reclamation trends in Thailand. Water Sci. Technol. 2022, 86, 2878–2911. [Google Scholar] [CrossRef]
- World Bank Open Data. World Bank Open Data. Available online: https://bit.ly/3YlmMqe (accessed on 19 November 2024).
- Kurniawan, S.B.; Ahmad, A.; Rahim, N.F.M.; Said, N.S.M.; Alnawajha, M.M.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Ismail, N.; Hasan, H.A. Aquaculture in Malaysia: Water-related environmental challenges and opportunities for cleaner production. Environ. Technol. Innov. 2021, 24, 101913. [Google Scholar] [CrossRef]
- Wu, Y.; Song, K. Source, Treatment, and Disposal of Aquaculture Solid Waste: A Review. J. Environ. Eng. 2021, 147, 03120012. [Google Scholar] [CrossRef]
- Messer, T.L.; Moore, L.T.; Nelson, N.; Ahiablame, L.; Bean, E.Z.; Boles, C.; Cook, S.L.; Hall, S.G.; McMaine, J.; Schlea, D. Constructed Wetlands for Water Quality Improvement: A Synthesis on Nutrient Reduction from Agricultural Effluents. Trans. ASABE 2021, 64, 625–639. [Google Scholar] [CrossRef]
- Dosantos, P.S.; Bouchet, A.; Mariñas-Collado, I.; Montes, S. OPSBC: A method to sort Pareto-optimal sets of solutions in multi-objective problems. Expert Syst. Appl. 2024, 250, 123803. [Google Scholar] [CrossRef]
- Nurjanah, D.A.; Kusminah, I.L.; Rachmat, A.N.; Nabella, N. Analisis Penentuan Komponen Kritis Small Excavator Menggunakan Metode FMEA dan Diagram Pareto. J. Saf. Health Environ. Eng. 2024, 1, 7–15. [Google Scholar] [CrossRef]
- Sandoval Herazo, L.C.; Marín-Muñiz, J.L.; Alvarado-Lassman, A.; Zurita, F.; Marín-Peña, O.; Sandoval-Herazo, M. Full-Scale Constructed Wetlands Planted with Ornamental Species and PET as a Substitute for Filter Media for Municipal Wastewater Treatment: An Experience in a Mexican Rural Community. Water 2023, 15, 2280. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; Sandoval Herazo, L.C.; López-Méndez, M.C.; Sandoval-Herazo, M.; Meléndez-Armenta, R.Á.; González-Moreno, H.R.; Zamora, S. Treatment Wetlands in Mexico for Control of Wastewater Contaminants: A Review of Experiences during the Last Twenty-Two Years. Processes 2023, 11, 359. [Google Scholar] [CrossRef]
- Li, K.; Jiang, R.; Qiu, J.; Liu, J.; Shao, L.; Zhang, J.; Liu, Q.; Jiang, Z.; Wang, H.; He, W.; et al. How to control pollution from tailwater in large scale aquaculture in China: A review. Aquaculture 2024, 590, 741085. [Google Scholar] [CrossRef]
- Zitácuaro-Contreras, I.; Vidal-Álvarez, M.; Hernández y Orduña, M.G.; Zamora-Castro, S.A.; Betanzo-Torres, E.A.; Marín-Muñíz, J.L.; Sandoval-Herazo, L.C. Environmental, Economic, and Social Potentialities of Ornamental Vegetation Cultivated in Constructed Wetlands of Mexico. Sustainability 2021, 13, 6267. [Google Scholar] [CrossRef]
- Sandoval, L.; Zamora-Castro, S.; Vidal-Álvarez, M.; Marín-Muñiz, J. Role of Wetland Plants and Use of Ornamental Flowering Plants in Constructed Wetlands for Wastewater Treatment: A Review. Appl. Sci. 2019, 9, 685. [Google Scholar] [CrossRef]
- Liu, X.; Wang, Y.; Liu, H.; Zhang, Y.; Zhou, Q.; Wen, X.; Guo, W.; Zhang, Z. A systematic review on aquaculture wastewater: Pollutants, impacts, and treatment technology. Environ. Res. 2024, 262, 119793. [Google Scholar] [CrossRef] [PubMed]
- Di Luca, G.A.; de Las Mercedes Mufarrege, M.; Hadad, H.R.; Maine, M.A.; Nocetti, E.; Campagnoli, M.A. Floating treatment wetlands with Canna indica for the removal of Cr (III) and Cr (VI) from water: A comprehensive study. Sci. Total Environ. 2024, 940, 173642. [Google Scholar] [CrossRef]
- Dhir, B. Role of Wetlands. In Phytoremediation: Role of Aquatic Plants in Environmental Clean-Up; Springer: Pune, India, 2013; pp. 65–93. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. Water hyacinth (Eichhornia crassipes) for organic contaminants removal in water—A review. J. Hazard. Mater. Adv. 2022, 7, 100092. [Google Scholar] [CrossRef]
- Lino, G.; Espigul, P.; Nogués, S.; Serrat, X. Arundo donax L. growth potential under different abiotic stress. Heliyon 2023, 9, e15521. [Google Scholar] [CrossRef]
- Badiola, M.; Mendiola, D.; Bostock, J. Recirculating Aquaculture Systems (RAS) analysis: Main issues on management and future challenges. Aquac. Eng. 2012, 51, 26–35. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Delfin-Portela, E. Diseño y Evaluación de un Sistema Integrado de Recirculación Acuícola Con Humedales Construidos Como Filtro Biológico (RAS-CW) Con un Enfoque de Economía Circular. Ph.D. Thesis, Instituto Tecnológico Superior de Misantla, Misantla, Mexico, 2024. [Google Scholar]
- Kadlec, R.H.; Wallace, S. Treatment Wetlands; Taylor and Francis Group: Florida, FL, USA, 2008. [Google Scholar]
- Kadlec, R.H.; Knight, R.L. Treatment Wetlands; Lewis Publishers Inc: Ann Arbor, MI, USA, 1996; pp. 68–80. [Google Scholar]
- Hernández, M.E. Ecological engineering for controlling water pollution in Latin America En. In Ecological Engineering for Controlling Water Pollution in Latin America in Ecological Dimensions for Sustainable Socio-Economic Development; Yañez-Arancibia, A., Yáñez-Arancibia, A., Dávalos-Sotelo, R., Day, J.W., Reyes, E., Eds.; WIT Press: Southampton, UK, 2013. [Google Scholar]
- Marín-Muñiz, J.L.; Ortega-Pineda, G.; Zitácuaro-Contreras, I.; Vidal-Álvarez, M.; Martínez-Aguilar, K.E.; Álvarez-Hernández, L.M.; Zamora-Castro, S. Removal of Nitrogen, Phosphates, and Chemical Oxygen Demand from Community Wastewater by Using Treatment Wetlands Planted with Ornamental Plants in Different Mineral Filter Media. Nitrogen 2024, 5, 903–914. [Google Scholar] [CrossRef]
- Boutin, K.D.; Mitsch, W.J.; Everham, E.; Bakshi, B.R.; Zhang, L. An evaluation of corn production within a Wetlaculture™ system at Buckeye Lake, Ohio. Ecol. Eng. 2021, 171, 106366. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Jiang, B.; Miller, S.K.; Boutin, K.D.; Zhang, L.; Wilson, A.; Bakshi, B.R. Wetlaculture: Solving Harmful Algal Blooms with a Sustainable Wetland/Agricultural Landscape. In Engineering and Ecosystems; Springer International Publishing: Cham, Switzerland, 2023; pp. 333–354. [Google Scholar] [CrossRef]
- NASA (National Aeronautics and Space Administration). Technology Readiness Levels. 2023. Available online: https://bit.ly/4cZWLCY (accessed on 19 November 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).