Abstract
Indonesia’s surimi industry is increasingly relying on small demersal fish stocks, whose biological sustainability remains critically underexamined. This study evaluates four key species—Priacanthus tayenus, Pentaprion longimanus, Upeneus sulphureus, and Nemipterus tambuloides—using the length-based spawning potential ratio (LB-SPR) method across 66,674 samples. The results reveal acute reproductive depletion, whereby the SPR values for three species fall below the 20% viability threshold, and over 70% of specimens are harvested before maturity. These patterns signal severe recruitment overfishing, with implications for ecosystem resilience and the structural stability of surimi supply chains. Given the factory-based sampling bias, the findings likely represent a worst-case scenario for the surimi-directed stock component within Indonesia’s FMA712. Strategic reforms—particularly minimum size limits, seasonal closures, and broader multisite assessments—are urgently required in order to realign fishing practices with ecological thresholds and safeguard coastal livelihoods that are dependent on this industrial value chain.
1. Introduction
Surimi is a processed food that is produced from crushed demersal fish meat; it is used to produce several products such as fish nuggets, balls, or crackers [1,2]. Due to their lucrative market value and accessibility, the industry processes demersal fish by cleaning and rinsing them to remove bioprotectants in order to achieve a high-quality product, remove any fishy smells, and prolong their shelf life during frozen storage [3,4,5,6,7,8]. These processes primarily operate with a substantial labor force, mainly in the fish-cutting phase, with an average of more than 500 workers in a single surimi plant [9,10]. As an example, there are at least 15 surimi factories in Indonesia, all of which comprise around 10,000 workers [10,11]. These laborers and manufacturers are able to help in the global consumption expansion of this product [10,12].
Demersal fish are a crucial natural resource for the surimi industry, especially in South China, Arafura, and the Java Sea [9,13,14]. The entire surimi sector in Indonesia is concentrated on Java Island, mainly focused in the Fisheries Management Area (FMA) 712, which is located in the Java Sea [9,15,16]. This trend is especially prominent in the Sunda and Sahul shelf exposure, where the demersal fishing grounds are teeming with marine life [17,18]. In contrast, demersal fishing grounds in other parts of Indonesia are relatively limited in scope, reflecting the unique concentration of fishing activities in specific regions [8,19,20].
The fish commonly used for surimi production in Indonesia come from economically low-value demersal fish [9,13,14]. These fish are crucial in marine environments and are typically found in shallow continental shelves with mud, sand, or rock substrates [21,22,23]. Accordingly, the excessive fishing activities that take place in the Java Sea impact demersal fish populations through selective extraction, the accidental capture of non-target species, and habitat alterations [24,25]. This leads to changes in biomass, species composition, and size distribution [26,27,28]. The response varies based on species traits, trophic relationships, and environmental changes [27,28,29]. Therefore, to ensure the sustainability of surimi production, it is crucial to comprehensively assess the demersal fish stock to understand the raw materials [30].
One approach that is employed in surimi processing in order to evaluate the sustainability of demersal fish types involves using the length-based spawning potential ratio (LB-SPR) [31,32,33]. The LB-SPR method incorporates fish length data and sex ratios alongside life history parameters, which are typically applied to fisheries with limited data [33,34,35]. Our study identified that many empirical studies have already applied this method to measure fish stock in the regions of the Middle East, Micronesia, West and East Africa, North America, South Asia, Asia Pacific, and Southeast Asia [30,36,37,38,39,40,41,42]. Notably, many studies have applied the LB-SPR method to measure only one fish species [26,43,44,45,46]. However, several of these studies applied this method to measure the demersal fish that were interconnected to the particular fish processing industry under study [30,34,39]. This means that the existing body of knowledge contains limited information on how this method is applied to several demersal fish species, particularly in the FMA712 location, which has become the center of the surimi industry.
Accordingly, from the perspective of the taxonomy of research gaps, the current body of knowledge implies that there are unknown areas of multidisciplinary practical knowledge, as well as population gaps [47]. This phenomenon emphasizes the necessity to conduct empirical research, using robust data sampling techniques, that practically interconnects to the related fish industry, such as surimi [30,47,48,49,50,51]. Henceforth, our study aims to address the following research questions:
- (1)
- What is the current stock status of small demersal fish species used in the surimi industry in the Java Sea fishing area?
- (2)
- How does the length-based spawning potential ratio (LB-SPR) of targeted demersal fish species indicate the sustainability of the fishing practices?
- (3)
- What are the implications of overfishing on the future availability of raw materials for Indonesia’s surimi industry and local fishing communities?
- (4)
- How can fishery management and regulations be improved to ensure the long-term sustainability of demersal fish stocks in the Java Sea?
In response to the above questions, our study performs an LB-SPR assessment of demersal fish that are utilized within the surimi industry in order to assess and understand the fish stock status. In this study, the assessed fish include Golden Threadfin Bream (Nemipterus virgatus), Big-Eyed Snapper (Priacanthus tayenustayenus), Sulfur Goatfish (Upeneus sulfurous), and Ponyfish (Pentaprion longimanus); these are used to glean insights into the sustainability of natural fish resources [31]. Concurrently, our study will apply the LB-SPR method to evaluate fishing pressures, estimate the length at first maturity, and analyze the proportion of immature fish caught in order to understand various sustainability conditions. Then, our study will reveal and highlight the overfishing phenomenon, which emphasizes multiple risks that arise as a result of the industry and ecosystems. Subsequently, our study will also propose practical recommendations for improving fishery management, including size limits and seasonal closures, and ensuring the long-term sustainability of fish stocks while supporting the surimi industry and local fishing communities.
This study contributes to a clearer understanding of the biological sustainability of small demersal fish stocks in the Java Sea, which is a critical source for Indonesia’s surimi industry. Using the length-based spawning potential ratio (LB-SPR) method on four exploited species, we provide empirical evidence of overexploitation, with SPR values for three species falling below the 20% reproductive viability threshold, as well as over 70% of individuals being harvested before reaching maturity. These findings highlight the presence of recruitment overfishing and compromised stock resilience, underscoring the urgency of reform. Based on this evidence, we recommend implementing minimum size limits that align with length-at-maturity benchmarks, as well as seasonal closures to protect spawning periods; these measures are specifically targeted to reduce immature capture and allow for stock recovery. These management strategies are not proposed generically but emerge directly from species-specific biological indicators that have been observed in the dataset. Further research should quantify socio-economic trade-offs and support the integration of these empirically grounded policies into broader sustainability frameworks and international fishery governance efforts.
2. Materials and Methods
2.1. Research Period and Study Area
This study was conducted over a one-year period, from June 2023 to June 2024, at the surimi raw material receiving facility of the SFI Corporation in East Java, Indonesia. SFI was specifically chosen as a pilot project site for biological surveys of demersal fish species destined for surimi production. The fish were sourced from Fisheries Management Area 712 (FMA712). The biological data used in this study were derived exclusively from fish entering the surimi production chain, which typically includes small- to medium-sized individuals sorted for surimi processing. Therefore, the findings presented here should be interpreted as reflecting the surimi-directed component of the catch, as opposed to the overall catch composition of the entire demersal fishery in FMA712 (Figure 1).
Figure 1.
Research location at SFI Corporation, East Java, Indonesia.
2.2. Data Collection
The primary data consist of biological observations of demersal fish, such as Golden Threadfin Bream, Big-Eyed Snapper, Sulfur Goatfish, and Ponyfish, which SFI obtained for surimi production. A pocket pull net is the standard fishing gear that is used to catch demersal fish. Each type of fish underwent random sampling, with a minimum of 700 female fish samples collected monthly. The biological parameters examined encompass length (mm), weight (g), sex, and gonad maturity stage. Length measurements were taken using a ruler, sketch, or vernier caliper with a precision of 0.01 mm (accuracy 0.005 mm), starting from the mouth’s tip to the tail. Weight measurements were conducted using precise digital scales to assess the gonad maturity stage (see Table 1) [52].

Table 1.
Gonad maturity criteria [52].
2.3. Data Analysis
The biological data of the fish were analyzed using the length-based spawning potential ratio (LB-SPR). The LB-SPR measurement used the equations developed by Jeremy Prince, which were further applied by several researchers such as Tri Ernawati and others, that incorporate the number of recruits at age 0 (Nt0) under the assumption of equilibrium conditions, along with an initial cohort size set to 1000 fish [30,53]. The following equation was used to determine the LB-SPR concerning both size and age. EPt is the reproductive output at age t; Nt is the number of individuals at age t; M is the natural mortality, which is the mean of fecundity.
The LB-SPR assessment technique utilized the size structure and spawning potential ratio (SPR) within an exploited population by considering the fishing mortality-to-natural mortality ratio (F/M), as well as two life history ratios—M/k and Lm/L∞. The natural mortality rate (M), the von Bertalanffy growth coefficient (k), the maturity measure (SoM) represented by Lm, and the asymptotic measure denoted by L∞ play significant roles in the LB-SPR assessment technique. The life history parameters of fish populations, including growth and mortality rates, were estimated using the Electronic Length Frequency Analysis-I (ELEFAN I) in the FiSAT II (FAO-ICLARM Stock Assessment Tools II. Ver. 1.2.0) package [54,55,56].
The LB-SPR analysis was conducted by utilizing growth parameters on the web-based platform barefootecologist.com.au/lbspr [57]. The LB-SPR model required various parameters as inputs, including the M/k ratio; the asymptotic mean length (L∞); the length variability at age (CVL∞), which is challenging to estimate directly without reliable length and age data so is typically assumed to be approximately 10%; and a delineation of the maturity size schedule specified in L50% and L95%, representing the sizes at which 50% and 95% of the population are mature [30].
The percentage of SPR at gonadal maturity (Lm) was determined to be an indicator of the status of the resource stock. The reference point utilized for SPR was set to 20%, while a value lower than 20% (SPR < 20%) signified the overexploitation of the resource. SPR values falling within the range of 20% to 40% (20% < SPR < 40%) suggest moderate conditions, while SPR values exceeding 40% (SPR > 40%) indicate underexploited circumstances [30].
This study’s biological sampling was conducted on fish entering the surimi production chain, which typically involves small- to medium-sized individuals sorted from the broader landings in the Java Sea. As such, the data represent the surimi-bound component of the catch, not the entire fishery. Larger fish are often directed to frozen or export markets and were not included in this analysis. Therefore, while the findings provide an important insight into overexploitation within industrial supply chains, they should be interpreted as reflecting a conservative or worst-case scenario for the demersal stock status in Fisheries Management Area 712 (FMA712), rather than a full ecosystem-wide assessment.
3. Results and Discussion
3.1. Length Frequency Distribution of Demersal Fishes
The analysis of four demersal fish species—N. tambuloides, P. longimanus, U. sulphureus, and P. tayenus—provides essential insights into their size distribution and role in the surimi industry, as shown in Figure 2. Our study measured 66,674 samples yearly, recording significant variations in total length (TL) among the species. N. tambuloides (Figure 2A) ranged from 80 to 185 mm, averaging 120.5 mm, while P. longimanus (Figure 2B) measured between 80 and 155 mm, averaging 114.3 mm. U. sulphureus (Figure 2C) ranged from 85 to 175 mm, with a mean length of 126.0 mm, and P. tayenus (Figure 2D) spanned from 75 to 210 mm, with an average of 140.1 mm. These size differences directly impact the yield and quality of surimi, with larger fish like P. tayenus potentially contributing more to production due to their broader size range and higher average length. Despite these variations, all four species consistently provided a stable raw material for surimi, ensuring steady supply across different size categories.
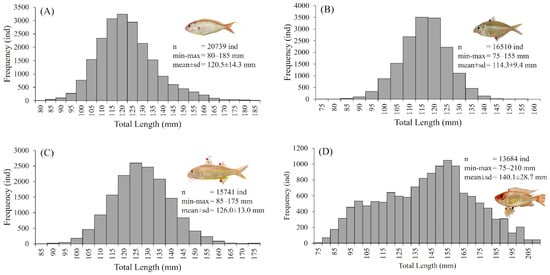
Figure 2.
The length frequency distribution of four species of demersal fish—N. tambuloides (A), P. longimanus (B), U. sulphureus (C), and P. tayenus (D)—used in the Surimi industry in Paciran, Lamongan, East Java, Indonesia. All data and fish images are original and derived from this study (Supplementary Files S1–S5).
The length frequency distributions in Figure 2 offer a detailed view of these patterns, highlighting the population structures of each species in East Java, Indonesia. This information optimizes surimi production processes and supports sustainable fishing practices by providing critical insights into the growth and reproductive rates of these fish, which are crucial for maintaining healthy stocks.
3.2. Length at First Maturity of Demersal Fishes
Figure 3 emphasizes the size estimation of female demersal fish species reaching sexual maturity by applying a logistic curve to the relationship between the proportion of mature fish and their respective length classes, explicitly determining the first maturity size (L50). At this point, 50% of the population reaches maturity. Figure 3 shows the length frequency distribution of four species—Nemipterus tambuloides (L50 at 143 mm, ~52 g), P. longimanus (120 mm, ~24 g), U. sulphureus (135 mm, ~35 g), and P. tayenus (170 mm, ~73 g). These L50 values guide fishing regulations to ensure that at least 50% of the population can reproduce before being caught.
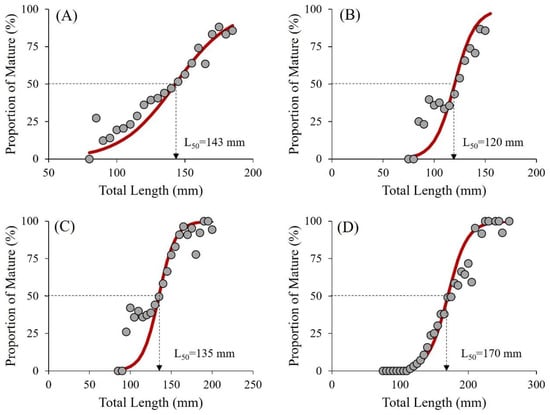
Figure 3.
The length frequency distribution of four species of demersal fish—N. tambuloides (A), P. longimanus (B), U. sulphureus (C), and P. tayenus (D)—used in the Surimi industry in Paciran, Lamongan, East Java, Indonesia. Grey dots represent the observed proportion of mature fish, while the red lines represent the estimated proportion of mature fish based on the logistic equation. See Supplementary Files S1–S5.
Panel A shows that N. tambuloides matures when 50% of the female population reaches 143 mm, while panel B indicates that P. longimanus matures earlier—at 120 mm. In panel C, U. sulphureus reaches its L50 at 135 mm; in panel D, P. tayenus reaches maturity at 170 mm, requiring the most prolonged growth period. The results were consistent with those reported in previous studies. Females of the N. tambuloides species in the Southern Gulf of Thailand reach maturity at 149 mm [58]. Other studies have reported the maturity lengths of demersal fishes caught in Tegal, Central Java, which had an L50 of 130 mm for P. longimanus, 129 mm for U. sulphureus, and 194 mm for P. tayenus [59,60]. These L50 values and the percentage of mature fish within their length classes allow fisheries to implement species-specific management practices that ensure reproductive success before fishing. By targeting these size thresholds, fisheries can maintain fish populations while supporting the surimi industry’s economic interests and ecological balance.
Table 2 highlights the concerning percentages of immature individuals across four demersal fish species, signaling potential threats to their sustainability. The N. tambuloides species shows 64.4% immature fish, most of which are caught before sexual maturity. Similarly, P. longimanus records 55.9% of immature individuals, just over half of the catch. Moreover, U. sulphureus displays 52.3% immature individuals, indicating that a significant portion of the species has not yet matured, while P. tayenus exhibits the highest percentage of immature fish at 72.1%, revealing that most individuals from this species were caught before contributing to population reproduction. Meanwhile, the mature fish represent smaller portions of the catch—N. tambuloides reaches 35.6%, P. longimanus reaches 44.1%, U. sulphureus accounts for 47.7%, and P. tayenus has only 27.9% of mature individuals. These figures indicate a heavy skew toward capturing immature fish, likely hindering population recovery and sustainability.

Table 2.
Proportion of immature and mature females for four species of demersal fish.
3.3. Gonad Maturity Stage
Figure 4 shows the monthly variations in the proportion of gonad maturity stages for N. tambuloides, P. longimanus, U. sulphureus, and P. tayenus. The highest percentage of immature fish (stage I and II) was observed in August–September (76–79%) for N. tambuloides, in March (62–70%) for P. longimanus, in May (79%) for U. sulphureus, and in August–September (91–97%) for P. tayenus. Furthermore, the mature and spawning phases (stages III and IV) for all species were recorded throughout the year (Figure 4). The peak spawning periods were estimated to occur in May–June for N. tambuloides, in August–September for P. longimanus, in July–August for U. sulphureus, and in June for P. tayenus.
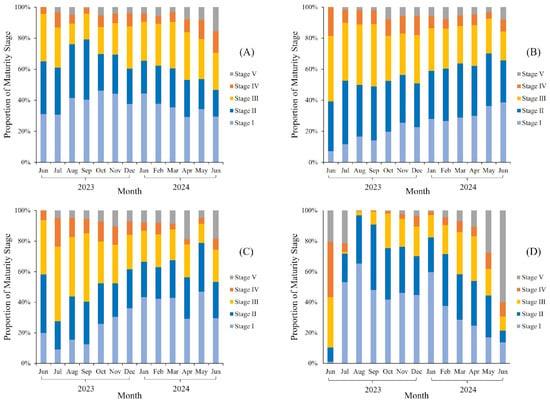
Figure 4.
Monthly percentage distribution of the gonad maturity stages of demersal fish—(A) N. tambuloides, (B) P. longimanus, (C) U. sulphureus, and (D) P. tayenus—used in the Surimi industry in Lamongan, East Java, Indonesia. See Supplementary Files S1–S5.
Our results indicate that this study is consistent with several previous studies on the same or closely related species. For example, the peak spawning of N. tambuloides in Pandeglang, Banten, occurs in June; N. japonicus in the Sunda Strait spawns occurs in May; and N. randalii in the northeastern Mediterranean spawns from March to May [61,62,63]. P. longimanus may spawn from the late dry season to the early rainy season, approximately from August to November, while U. sulphureus and Red Bigeye (P. tayenus) tend to spawn from August to October and from May to July, respectively, [64,65].
3.4. Growth and Mortality Parameter Estimates
Figure 5 shows the length–weight relationships for N. tambuloides (Figure 5A), P. longimanus (Figure 5B), U. sulphureus (Figure 5C), and P. tayenus (Figure 5D) in the surimi industry in Lamongan, East Java. Each species demonstrates a b coefficient below 3, indicating negative allometric growth (p < 0.05). Specifically, N. tambuloides (Figure 5A) records a b value of 2.78, P. longimanus (Figure 5B) has a value of 2.69, U. sulphureus (Figure 5C) registers a value of 2.81, and P. tayenus (Figure 5D) presents a value of 2.75. These values reveal that as the fish grow in length, their weight does not increase proportionately, suggesting faster length growth than weight gain.
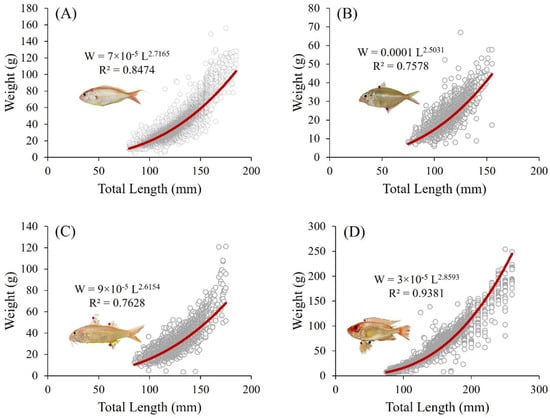
Figure 5.
Length–weight relationships for four species of demersal fish—N. tambuloides (A), P. longimanus (B), U. sulphureus (C), and P. tayenustayenus (D)—used in the Surimi industry in Paciran, Lamongan, East Java, Indonesia. All data and fish images are original and derived from this study. See Supplementary Files S1–S5.
The R2 values further illustrate the strength of the length–weight relationships for these species, with N. tambuloides (Figure 5A) showing an R2 of 0.8474, P. longimanus (Figure 5B) at 0.7578, U. sulphureus (Figure 5C) at 0.7994, and P. tayenus (Figure 5D) at 0.9381. These R2 values indicate varying degrees of correlation between length and weight, with P. tayenus (Figure 5D) showing the most vital relationship, while P. longimanus (Figure 5B) demonstrates the weakest. The relatively high R2 values for most species suggest that the length–weight model fits well, except for P. longimanus (Figure 5B), which shows a slightly weaker correlation. Understanding the growth patterns and their correlation provides critical insights for the surimi industry, helping to determine optimal harvesting sizes and strategies for each species based on their growth dynamics and weight-to-length ratios.
By integrating these insights relating to the coefficients and R2 values, fishery management can develop tailored strategies for harvesting that maximize production efficiency while ensuring sustainable fish stocks. Similar harvesting strategies, with strong relationships between length and weight, may be effective for N. tambuloides (Figure 5A) and U. sulphureus (Figure 5C). Meanwhile, given their distinct growth patterns and correlation strengths, P. longimanus (Figure 5B) and P. tayenus (Figure 5D) may require different approaches to optimize weight yield.
Table 3 presents four demersal fish species’ estimated growth and mortality parameters, highlighting their life cycles and fishing pressures. The growth rates (K) differ significantly, with N. tambuloides at 0.75, P. longimanus at 0.70, U. sulphureus at 1.10, and P. tayenus at 0.45. A higher K value indicates faster growth, making U. sulphureus more resilient to fishing pressure than P. tayenus, which grows more slowly. The asymptotic or theoretical maximum lengths (L∞) range from 162.75 mm for P. longimanus to 220.5 mm for P. tayenus. The larger size of P. tayenus pairs with its slower growth and longer maximum age of 6.46 years, signaling increased vulnerability to overfishing compared to faster-growing species like U. sulphureus, with a shorter maximum age of 2.64 years.

Table 3.
Estimated growth and mortality parameters.
Natural mortality (M) values show further variation, ranging from 0.62 for P. tayenus to 1.16 for U. sulphureus. Total mortality (Z), which combines natural and fishing mortality, peaks at 4.56 per year for U. sulphureus, revealing high fishing pressure. The exploitation rate (F/M) exceeds 0.5 for all species, with U. sulphureus and P. longimanus experiencing the highest rates at 0.74 and 0.73, respectively. Length at maturity (L50 and L95) data underline the importance of size-based fishing regulations. For instance, P. tayenus reaches 50% maturity at 170 mm and 95% maturity at 210 mm—the largest of the four species—indicating the need to protect larger fish to maintain population stability. These growth and mortality patterns emphasize the need for careful management, particularly for slower-growing species like P. tayenus, which face higher risks from overfishing.
3.5. Sustainability-Related Information in Relation to Demersal Fish Stock Assessment for the Surimi Industry
The LB-SPR assessment provided essential insights into the fishing impact on four demersal fish species in the Java Sea—N. tambuloides, P. longimanus, U. sulphureus, and P. tayenus. The results showed that the length at which 50% of the fish population becomes vulnerable to being caught (SL50) varied across the species—109.9 mm for N. tambuloides, 114.3 mm for P. longimanus, 119.4 mm for U. sulphureus, and 137.3 mm for P. tayenus (Table 4). These figures suggest that most fish are being captured before they reach maturity, which has significant implications for their ability to reproduce and sustain healthy population levels. Reinforcing this concern, the length at 95% selectivity (SL95)—the point at which most of the population becomes susceptible to capture—was also relatively low, ranging from 124.95 mm for N. tambuloides to 179.2 mm for P. tayenus (Table 4.). This indicates that current fishing practices remove fish from the population before they can meaningfully contribute to reproduction, potentially leading to long-term declines in stock if unmanaged.

Table 4.
Estimated parameters from the LB-SPR assessment for four species of demersal fish.
In addition to concerns about fish maturity, the fishing pressures exerted on these species, expressed as the F/M ratio (fishing mortality relative to natural mortality), were significantly high. P. longimanus faced the most extreme pressure, with an F/M ratio of 5.0, meaning the fishing mortality rate was five times that of the natural mortality rate. Other species also showed alarming pressure levels—N. tambuloides had an F/M ratio of 3.1, U. sulphureus had a ratio of 2.6, and P. tayenus had a ratio of 1.3. These high F/M ratios highlight unsustainable fishing practices, with mortality from fishing vastly exceeding natural death rates (Table 4). If such fishing pressures persist, the populations of these species are at risk of significant depletion, making it critical to implement effective management measures to safeguard their future.
Figure 6 illustrates the spawning potential ratio (SPR) estimates for the four assessed demersal fish species, revealing widespread reproductive depletion. N. tambuloides exhibited an SPR of just 4%, while P. longimanus and U. sulphureus showed similarly critical levels at 17% and 16%, respectively, all below the widely accepted 20% limit reference point, indicating severe overfishing and compromised stock regeneration. P. tayenus had a relatively higher SPR of 23% but still fell short of the 30% target reference point, suggesting continued vulnerability. These biologically grounded thresholds are consistent with the fisheries literature, which identifies SPR values below 20% as being indicative of recruitment overfishing, as well as SPR ≥30% as the minimum value needed to sustain long-term population viability in multispecies tropical fisheries [30,66]. Given that three species fall below the critical limit and none reach the sustainability target, stricter regulations—such as minimum size limits and seasonal closures—are urgently warranted to rebuild reproductive capacity and ensure ecological and industry resilience.
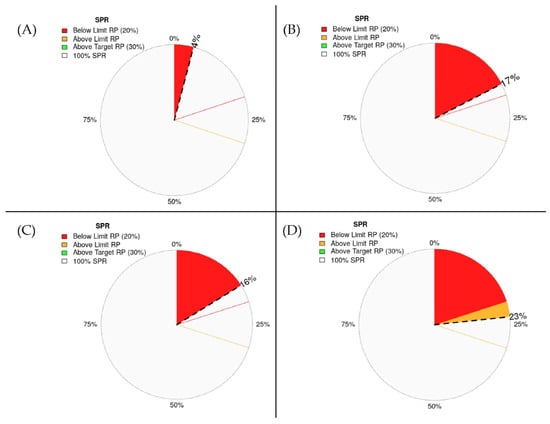
Figure 6.
The spawning potential ratio was estimated for four species of demersal fish—N. tambuloides (A), P. longimanus (B), U. sulphureus (C), P. tayenus (D). The red line indicates a limit reference point of SPR20%, while the orange line represents a target reference point of SPR30%. See Supplementary Files S1–S5.
3.6. The Implications and Recommendations of Industrial and Future Research Directions of LB-SPR Application in Relation to Demersal Fish Stock in the Java Sea, Indonesia
According to the results presented in Table 2 and Table 3, our study found that more than half of the demersal fish individuals caught—ranging from 52.3% to 72.1% across the four species—were below their respective lengths at first maturity (L50). While the LB-SPR values highlight severe overexploitation, it is important to note that these estimates are drawn exclusively from fish entering the surimi supply chain. Due to documented size-based sorting practices, these values may reflect a worst-case scenario for the exploited fraction of the population and should not be assumed to represent the entire demersal fish stock in FMA712. This finding indicates a high incidence of recruitment overfishing, as a substantial proportion of the stock is harvested before contributing to the reproductive cycle. The study also found that fishing pressures far exceeded natural mortality rates, posing a high risk of population depletion. Additionally, the estimated length at 50% selectivity for each species was consistently lower than the corresponding L50, further exacerbating reproductive loss and undermining stock resilience.
The current overfishing practices jeopardize the future of Indonesia’s surimi industry, which heavily depends on small demersal fish as raw materials, which is also suggested in the results of several existing studies [67,68,69]. If this trend continues, fish stocks will decline, leading to raw material shortages, increased costs, and reduced production [28,70,71]. The surimi industry will face significant challenges, including the need to source alternative species, which could disrupt production, decrease profitability, and harm local fishing communities [67,72,73]. This crisis could lead to widespread socio-economic impacts, particularly among the thousands of workers employed in surimi factories and fishing operations along the Java Sea [9,14,74,75].
To counteract these challenges, the industry and policymakers must enforce stricter fishing regulations, including minimum legal sizes and seasonal closures, which are grounded in the biological evidence generated by this study [76,77,78]. The LB-SPR analysis shows that more than 50% of the sampled fish across all four species were caught below their L50 maturity thresholds, while SPR values for N. tambuloides, P. longimanus, and U. sulphureus fall below the 20% reproductive viability threshold. These indicators—high proportions of immature catch and depleted spawning potential—offer a scientifically robust rationale for implementing size-based regulations to ensure reproductive opportunity before harvest. The documented temporal distribution of gonad maturity stages further supports aligning seasonal closures with peak spawning periods. Consideration must also be taken regarding fishing gear selectivity to allow for the escape of immature individuals. This study’s estimated L50 values provide a strong empirical foundation for determining species-specific minimum size limits and for developing precautionary harvest strategies. To better align industry goals with conservation needs, the authorities must reduce fishing permits, educate local fishers on sustainable practices, and offer targeted incentives for compliance [79,80]. Meanwhile, surimi producers should diversify their sourcing strategies by exploring less vulnerable species and adopt technologies that enhance traceability and resource management [81,82,83,84].
The small coverage of the study area is an essential limitation of the present study. This study conducted sampling and measurement at the factory rather than the landing stations. Fresh whole fish supplied to surimi factories are generally small- to medium-sized, while larger fish are typically sorted for export or sold to domestic buyers at higher prices. This could have led to the underestimated SPR values of demersal fishes obtained from this study of the Java Sea (FMA712). Fresh fish over 100 g are sold to frozen factories as finished products—headless, whole–gilled–scaled (WGS), or whole–gutted–gilled–scaled (WGGS)—at prices three times higher. This price difference incentivizes fishermen and suppliers to maximize sorting; for example, N. tambuloides (IDR 8300/kg for small–medium and IDR 25,500 for big size), P. longimanus (IDR 8000/kg for small–medium and IDR 13,000/kg for big size), U. sulphureus (IDR 5500/kg for small–medium and IDR 12,000/kg for big size), and P. tayenus (IDR 4500/kg for small–medium and IDR 18,500/kg for big size). For future research, scientists should investigate the broader ecological consequences of overfishing small demersal species in the Java Sea, particularly the effects on the marine food chain and local fisheries community [85,86]. Studies could also explore habitat restoration efforts and spawning area protections to enhance fish recovery [87,88,89]. Conducting a molecular assessment of the genetic diversity of species used as raw materials in the surimi industry may also be beneficial, as high diversity among tropical demersal fish with similar morphological features could lead to misidentification. Improving the LB-SPR method will provide more accurate population assessments and aid in creating better fishery management policies [90,91,92,93]. Researchers should collaborate with international organizations to adopt best practices that align with global sustainability efforts [94,95,96].
4. Conclusions
This study evaluates the sustainability of small demersal fish species that are exploited by Indonesia’s surimi industry in the Java Sea. By applying the length-based spawning potential ratio (LB-SPR) method to four key species—Nemipterus tambuloides, Pentaprion longimanus, Upeneus sulphureus, and Priacanthus tayenus—we quantify fishing pressures, assess stock status, and analyze the proportion of immature individuals in the catch. Drawing on 66,674 individual samples, we estimate critical biological parameters, including size at first maturity, and demonstrate that over 50% of the individuals are harvested before reaching reproductive viability. The analysis also reveals that SPR values for three of the four species fall below the critical 20% reference point for the surimi-bound component of the catch, signaling recruitment overfishing and biological depletion within this sector of the fishery.
These findings confirm that demersal stocks in the Java Sea face systemic reproductive pressure, threatening ecosystem integrity, coastal livelihoods, and the long-term viability of the surimi value chain. Analysis through the length-based spawning potential ratio (LB-SPR) provides a strong empirical basis for targeted policy interventions in data-limited fisheries. The LB-SPR results show a large proportion of individuals harvested below L50 thresholds, as well as SPR values far below replacement levels, clearly supporting precautionary harvest strategies and gear selectivity standards to reduce immature catches. The evidence calls for the urgent adoption of biologically grounded measures, including species-specific minimum size limits and seasonal closures aligned with spawning periods. Effective implementation requires adaptive enforcement, site-specific gear validation, and socio-economic feasibility assessments, especially for small-scale fishers. These measures should be underpinned by coordinated multi-level governance and inclusive stakeholder engagement, involving fishers’ associations, industry actors, and local communities.
In conclusion, this study calls for multisite assessments across broader fishery management areas to validate stock-specific patterns and guide policy precision. Parallel research should investigate the socio-economic consequences of overfishing and assess trade-offs in regulatory implementation. Aligning Indonesia’s fishery governance with international sustainability norms—such as SDG 14 and the FAO Code of Conduct for Responsible Fisheries—will be essential to ensure domestic food security, safeguard marine ecosystems, and sustain global market competitiveness.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17114827/s1, File S1: Normality & Homogeneity Test; File S2: Kurisi (Nemipterus tambuloides) SPR Raw Calculation; File S3: Kapasan (Pentaprion longimanus) SPR Raw Calculation; File S4: Kuniran (Upeneus sulphureus) SPR Raw Calculation; & File S5: Swanggi (Priacanthus tayenus) SPR Raw Calculation.
Author Contributions
Conceptualization: K.C.N.; methodology: K.C.N., S.B., M.M., Z.A. and N.Z.; validation: M.M. and N.Z.; formal analysis: K.C.N.; investigation: K.C.N.; resources: K.C.N.; data curation: S.B., M.M., Z.A. and N.Z.; writing—original draft preparation: K.C.N.; writing—review and editing: K.C.N., M.M. and N.Z.; visualization: K.C.N.; supervision: S.B., M.M., Z.A. and N.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
This article does not contain any studies involving human or animal participants.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Bagus Satria, Ayu Ervinia, Fitri Dewi, Maulana Agung Wibowo, and the team from SFI Corporation, who helped in the writing and research of this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sonu, S.C. Surimi, 13th ed.; NOAA technical memorandum NMFS-SWR; National Marine Fisheries Service: Terminal Island, CA, USA, 1986. [Google Scholar]
- Park, J.W.; Graves, D.; Draves, R.; Yongsawatdigul, J. Manufacture of Surimi. In Surimi and Surimi Seafood, 3rd ed.; Park, J.W., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 55–96. ISBN 978-0-429-11273-7. [Google Scholar]
- Okazaki, E.; Kimura, I. Frozen Surimi and Surimi-Based Products. In Seafood Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 209–235. ISBN 978-1-118-34617-4. [Google Scholar]
- Shi, H.; Zhang, M.; Liu, X.-C.; Yao, X.; Wang, W.; Zheng, J.; Tomasevic, I.; Sun, W. Improved Qualities of Salt-Reduced Tilapia Surimi by Adding Konjac Glucomannan: Insight into the Edible Traits, Gel Properties and Anti-Freezing Ability. Food Hydrocoll. 2024, 153, 109971. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Xavier, K.A.M.; Nayak, B.B.; Dhanapal, K.; Balange, A.K. Instrumental Quality Attributes of Single Washed Surimi Gels of Tilapia: Effect of Different Washing Media. LWT 2017, 86, 385–392. [Google Scholar] [CrossRef]
- de Oliveira, D.L.; Grassi, T.L.M.; Santo, E.F.E.; Cavazzana, J.F.; Marcos, M.T.S.; Ponsano, E.H.G. Washings and Cryoprotectants for the Production of Tilapia Surimi. Food Sci. Technol. 2017, 37, 432–436. [Google Scholar] [CrossRef]
- Yin, T.; Park, J.W. Comprehensive Review: By-Products from Surimi Production and Better Utilization. Food Sci. Biotechnol. 2023, 32, 1957–1980. [Google Scholar] [CrossRef] [PubMed]
- Dimarchopoulou, D.; Wibisono, E.; Saul, S.; Carvalho, P.; Nugraha, A.; Mous, P.J.; Humphries, A.T. Combining Catch-Based Indicators Suggests Overexploitation and Poor Status of Indonesia’s Deep Demersal Fish Stocks. Fish. Res. 2023, 268, 106854. [Google Scholar] [CrossRef]
- Sihono, S.; Purnomo, A.; Wibowo, S.; Dewi, F. Current (2021) Status of Surimi Industry in Indonesia and Possible Solutions: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 919, 012036. [Google Scholar] [CrossRef]
- Hikmayani, Y.; Apriliani, T.; Adi, T.R. Alternative Solutions for the Sustainability of the Surimi Industry in Indonesia. Bul. Ilm. Mar. Sos. Ekon. Kelaut. Dan Perikanan 2017, 3, 41–51. [Google Scholar] [CrossRef]
- Indonesian Fishery Producers, Processing, and Marketing Association Focus Group Discussion: Building a Sustainable Surimi Industry and Surimi Based Products. Available online: https://ap5i-indonesia-seafood.com/indoap5i/2022/03/15/fgd-membangun-industri-surimi-dan-produk-olahan-surimi-based-products-yang-berkelanjutan-14-maret-2022/ (accessed on 7 October 2024).
- FAOSTAT. Food Balance Sheets 2010–2022—Global, Regional and Country Trends. Version: 91; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Leadbitter, D.; Guenneugues, P.; Park, J. The Production of Surimi and Surimi Seafood from Tropical Fish—A Landscape View of the Industry, 1st ed.; Report to the Certification and Rating Collaboration; Certification and Ratings Collaboration: Vancouver, BC, Canada, 2020. [Google Scholar]
- Pangsorn, S.; Laong-manee, P.; Siriraksophon, S. Status of Surimi Industry in the Southeast Asia; Surimi Industry in Southeast Asia; TD/RES 118; Training Department, Southeast Asian Fisheries Development Center: Bangkok, Thailand, 2007. [Google Scholar]
- Prayitno, M.; Setiawan, H.; Jatmiko, I.; Arif Rahman, M.; Wiadnya, D. Spawning Potential Ratio (SPR) of Sulphur Goatfish (Upeneus sulphureus): Biological Basis for Demersal Fishery Management in Java Sea. IOP Conf. Ser. Earth Environ. Sci. 2020, 441, 012141. [Google Scholar] [CrossRef]
- Ministry of Marine Affairs and Fisheries of Indonesia. Analysis of Key Performance Indicators of the Marine and Fisheries Sector for the Period 2019–2023; Data, Statistics, and Information Center of the Indonesian Ministry of Marine Affairs and Fisheries: Jakarta, Indonesia, 2024; Volume 2, ISBN 2829-7245.
- Sarr, A.-C.; Sepulchre, P.; Husson, L. Impact of the Sunda Shelf on the Climate of the Maritime Continent. J. Geophys. Res. Atmos. 2019, 124, 2574–2588. [Google Scholar] [CrossRef]
- Saintilan, N.; Horton, B.; Törnqvist, T.E.; Ashe, E.L.; Khan, N.S.; Schuerch, M.; Perry, C.; Kopp, R.E.; Garner, G.G.; Murray, N.; et al. Widespread Retreat of Coastal Habitat Is Likely at Warming Levels above 1.5 °C. Nature 2023, 621, 112–119. [Google Scholar] [CrossRef]
- Dimarchopoulou, D.; Mous, P.J.; Firmana, E.; Wibisono, E.; Coro, G.; Humphries, A.T. Exploring the Status of the Indonesian Deep Demersal Fishery Using Length-Based Stock Assessments. Fish. Res. 2021, 243, 106089. [Google Scholar] [CrossRef]
- Matrutty, D.D.; Waileruny, W.; Noija, D. Fishing Ground Distribution of Deep Sea Demersal Fish in South Coast of Ambon, Indonesia. Aquac. Aquar. Conserv. Legis. 2017, 10, 25–31. [Google Scholar]
- Rajan, P.T. Chapter 11—Marine Fishery Resources and Species Diversity of Tropical Waters. In Biodiversity and Climate Change Adaptation in Tropical Islands; Sivaperuman, C., Velmurugan, A., Singh, A.K., Jaisankar, I., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 323–354. ISBN 978-0-12-813064-3. [Google Scholar]
- Brander, K. Demersal Species Fisheries. In Encyclopedia of Ocean Sciences; Steele, J.H., Ed.; Academic Press: Oxford, UK, 2001; pp. 718–725. ISBN 978-0-12-227430-5. [Google Scholar]
- Khedkar, G.D.; Jadhao, B.V.; Chavan, N.V.; Khedkar, C.D. Demersal Species of Tropical Climates. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; FISH; Academic Press: Oxford, UK, 2003; pp. 2438–2442. ISBN 978-0-12-227055-0. [Google Scholar]
- Suharsono, A.; Mustofa, A.; Hizbulloh, L.; Irschlinger, T.; Tolvanen, S. Supporting Marine Fishing Sustainably: A Review of Central and Provincial Government Support for Marine Fisheries in Indonesia, 1st ed.; IISD, GSI, WWF, and Marine Change: Manitoba, MB, Canada, 2021; ISBN CC BY-NC-SA 4.0. [Google Scholar]
- Atmaja, S.B.; Sadhotomo, B.; Nugroho, D. Overfishing on purse seine semi industry fisheries in the Java Sea and management implications. J. Kebijak. Perikan. Indones. 2017, 3, 51–60. [Google Scholar] [CrossRef]
- Silberschneider, V.; Gray, C.A.; Stewart, J. Age, Growth, Maturity and the Overfishing of the Iconic Sciaenid, Argyrosomus japonicus, in South-Eastern, Australia. Fish. Res. 2009, 95, 220–229. [Google Scholar] [CrossRef]
- Pham, C.-V.; Wang, H.-C.; Chen, S.-H.; Lee, J.-M. The Threshold Effect of Overfishing on Global Fishery Outputs: International Evidence from a Sustainable Fishery Perspective. Fishes 2023, 8, 71. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Tai, T.C. End Overfishing and Increase the Resilience of the Ocean to Climate Change. Front. Mar. Sci. 2020, 7, 2739–2744. [Google Scholar] [CrossRef]
- Durant, J.M.; Holt, R.E.; Langangen, Ø. Large Biomass Reduction Effect on the Relative Role of Climate, Fishing, and Recruitment on Fish Population Dynamics. Sci. Rep. 2024, 14, 8995. [Google Scholar] [CrossRef]
- Prince, J.; Victor, S.; Kloulchad, V.; Hordyk, A. Length Based SPR Assessment of Eleven Indo-Pacific Coral Reef Fish Populations in Palau. Fish. Res. 2015, 171, 42–58. [Google Scholar] [CrossRef]
- Hordyk, A.; Ono, K.; Valencia, S.; Loneragan, N.; Prince, J. A Novel Length-Based Empirical Estimation Method of Spawning Potential Ratio (SPR), and Tests of Its Performance, for Small-Scale, Data-Poor Fisheries. ICES J. Mar. Sci. 2015, 72, 217–231. [Google Scholar] [CrossRef]
- Hommik, K.; Fitzgerald, C.J.; Kelly, F.; Shephard, S. Dome-Shaped Selectivity in LB-SPR: Length-Based Assessment of Data-Limited Inland Fish Stocks Sampled with Gillnets. Fish. Res. 2020, 229, 105574. [Google Scholar] [CrossRef]
- Yonvitner; Kurnia, R.; Boer, M. Length Based-Spawning Potential Ratio (LB-SPR), on Exploited Demersal Stock (Priachantus tayenus) in Small Scale Fisheries, Sunda Strait. IOP Conf. Ser. Earth Environ. Sci. 2021, 744, 012103. [Google Scholar] [CrossRef]
- Yonvitner; Kurnia, R.; Boer, M. Life History and Length Based-Spawning Potential Ratio (LB-SPR) of Exploited Demersal Fish Stock (Upeneus Sp) in Sunda Strait. IOP Conf. Ser. Earth Environ. Sci. 2021, 718, 012074. [Google Scholar] [CrossRef]
- Medeiros-Leal, W.; Santos, R.; Peixoto, U.I.; Casal-Ribeiro, M.; Novoa-Pabon, A.; Sigler, M.F.; Pinho, M. Performance of Length-Based Assessment in Predicting Small-Scale Multispecies Fishery Sustainability. Rev. Fish Biol. Fish. 2023, 33, 819–852. [Google Scholar] [CrossRef]
- Yokie, A.A. An Assessment of the Sardinella Maderensis Stock of Liberia Coastal Waters Using the Length Based Spawning Potential Ratio (Lbspr). In Proceedings of the GRÓ Fisheries Training Program Under the Auspices of UNESCO, Hafnarfjörður, Iceland, 1 January 2020; GRO-FTP: Reykjavík, Iceland, 2019; pp. 1–22. [Google Scholar]
- Kibona, O.M.; Jonasson, J.P. Application of Length-Based Spawning Potential Ratio Method and Analysis of the Structure of the Electronic Catch Assessment Survey in Marine Waters of Mainland, Tanzania. In Proceedings of the GRÓ Fisheries Training Program Under the Auspices of UNESCO, Hafnarfjörður, Iceland, 1 January 2020; GRO-FTP: Reykjavík, Iceland, 2019; pp. 1–40. [Google Scholar]
- Sigler, M.F.; Lunsford, C.R. Effects of Individual Quotas on Catching Efficiency and Spawning Potential in the Alaska Sablefish Fishery. Can. J. Fish. Aquat. Sci. 2001, 58, 1300–1312. [Google Scholar] [CrossRef]
- Valinassab, T.; Adjeer, M.; Sedghi, N.; Kamali, E. Monitoring of demersal resources by Swept Area Method within the Persian Gulf and Oman Sea. J. Anim. Environ. 2010, 2, 45. [Google Scholar]
- Ashida, H.; Suzuki, N.; Tanabe, T.; Suzuki, N.; Aonuma, Y. Reproductive Condition, Batch Fecundity, and Spawning Fraction of Large Pacific Bluefin Tuna Thunnus orientalis Landed at Ishigaki Island, Okinawa, Japan. Environ. Biol. Fish. 2015, 98, 1173–1183. [Google Scholar] [CrossRef]
- Prince, J.; Creech, S.; Madduppa, H.; Hordyk, A. Length Based Assessment of Spawning Potential Ratio in Data-Poor Fisheries for Blue Swimming Crab (Portunus Spp.) in Sri Lanka and Indonesia: Implications for Sustainable Management. Reg. Stud. Mar. Sci. 2020, 36, 101309. [Google Scholar] [CrossRef]
- Ernawati, T.; Budiarti, T. Life History and Length Base Spawning Potential Ratio (LBSPR) of Malabar Snapper Lutjanus malabaricus (Bloch & Schneider, 1801) in Western of South Sulawesi, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 404, 012023. [Google Scholar] [CrossRef]
- Aulia, I.; Rahmawati, A.; Syauqi, M.; Wahyuningsih, M.; Raimahua, S.; Akmalia, W.; Khalifa, M. Age Structure, Growth, and Mortality of Blue Swimming Crab (Portunus pelagicus Linnaeus,1758) in Banten Bay Waters. J. Biodjati 2023, 8, 69–80. [Google Scholar] [CrossRef]
- White, D.B.; Palmer, S.M. Age, Growth, and Reproduction of the Red Snapper, Lutjanus campechanus, from the Atlantic Waters of the Southeastern US. Bull. Mar. Sci. 2004, 75, 335–360. [Google Scholar]
- Zedta, R.R.; Madduppa, H. Exploitation Level of Yellowfin Tuna (Thunnus albacares) Resources in Indian Ocean Using Spawning Potential Ratio Analysis Approach. J. Penelit. Perikan. Indones. 2021, 27, 33–41. [Google Scholar]
- Taylor, R.G.; Whittington, J.A.; Grier, H.J.; Crabtree, R.E. Age, Growth, Maturation, and Protandric Sex Reversal in Common Snook, Centropomus Undecimalis, from the East and West Coasts of South Florida. Fish. Bull. 2000, 98, 612–624. [Google Scholar]
- Miles, D.A. A Taxonomy of Research Gaps: Identifying and Defining the Seven Research Gaps. In Proceedings of the Doctoral Workshop: Finding Research Gaps-Research Methods and Strategies, Dallas, TX, USA, 16 August 2017; pp. 1–15. [Google Scholar]
- Goodyear, C. Spawning Stock Biomass per Recruit in Fisheries Management: Foundation and Current Use. In Canadian Special Publication of Fisheries and Aquatic Sciences; Canadian Science Publishing: Ottawa, ON, Canada, 1993; Volume 120, pp. 67–81. ISBN 978-0-660-14956-1. Available online: https://www.researchgate.net/publication/251832024_Spawning_Stock_Biomass_per_Recruit_in_Fisheries_Management_Foundation_and_Current_Use/citation/download (accessed on 7 October 2024).
- Gabriel, W.L.; Sissenwine, M.P.; Overholtz, W.J. Analysis of Spawning Stock Biomass per Recruit: An Example for Georges Bank Haddock. N. Am. J. Fish. Manag. 1989, 9, 383–391. [Google Scholar] [CrossRef]
- Ault, J.S.; Smith, S.G.; Luo, J.; Monaco, M.E.; Appeldoorn, R.S. Length-Based Assessment of Sustainability Benchmarks for Coral Reef Fishes in Puerto Rico. Environ. Conserv. 2008, 35, 221–231. [Google Scholar] [CrossRef]
- Arocha, F.; Bárrios, A. Sex Ratios, Spawning Seasonality, Sexual Maturity, and Fecundity of White Marlin (Tetrapturus albidus) from the Western Central Atlantic. Fish. Res. 2009, 95, 98–111. [Google Scholar] [CrossRef]
- Malau, A.E.S.; Tallo, I.; Soewarlan, L.C. Gonadal Maturity Level of Kurisi Fish (Nemipterus bathybius) in Kupang Bay. J. Techno-Fish 2022, 6, 144–158. [Google Scholar]
- Ernawati, T.; Kembaren, D.D. Bambang Sumiono Population Parameters of Painted Spiny Lobster (Panulirus versicolor) in Northern Sikka and Adjacent Waters. BAWAL 2014, 6, 169–175. Available online: https://core.ac.uk/outputs/267084903/?utm_source=pdf&utm_medium=banner&utm_campaign=pdf-decoration-v1 (accessed on 7 October 2024).
- Pauly, D.; David, N. ELEFAN I, a BASIC Program for the Objective Extraction of Growth Parameters from Length-Frequency Data. Berichte Der Dtsch. Wiss. Komm. Für Meeresforsch. 1981, 28, 205–211. [Google Scholar]
- Gayanilo Jr, F.; Sparre, P.; Pauly, D. FAO-ICLARM Stock Assessment Tools II: FiSAT II: User’s Guide; Computerized Information Series: Fisheries; Rev. Version; WorldFish Center, FAO Computerized Information Series: Rome, Italy, 2005; Volume Revised Version, ISBN 978-92-5-104640-1. [Google Scholar]
- Pauly, D.; Greenberg, A. ELEFAN in R: A New Tool for Length-Frequency Analysis, 3rd ed.; Fisheries Centre Research Reports; Fisheries Centre, University of Britisch Columbia: Vancouver, BC, Canada, 2013; ISBN 1198-6727. [Google Scholar]
- Cinner, J.S. The Barefoot Ecologist’s Toolbox 2020. R Statistical Computing; Open-Source Software Packages. Research Council Centre of Excellence for Coral Reef Studies. Available online: http://barefootecologist.com.au/. (accessed on 28 March 2025).
- Paul, M.; Pradit, S.; Hajisamae, S.; Perngmak, P.; Hoque, S. Size and Growth Variation at Maturity of Six Nemipterus Species in the South China Sea. Russ. J. Agric. Socio-Econ. Sci. 2016, 59, 156–164. [Google Scholar] [CrossRef]
- Oktaviani, D.; Faizah, R.; Nugroho, D. Biological Aspects of Longfin Mojarra (Pentaprion longimanus, Cantor 1849) in North Coast of Central Java, Indonesia. Biodiversitas J. Biol. Divers. 2018, 19, 683–689. [Google Scholar] [CrossRef]
- Nugroho, D.; Patria, M.P.; Supriatna, J.; Adrianto, L. Biological Characteristics on Three Demersal Fish Landed in Tegal, North Coast of Central Java, Indonesia. Biodiversitas J. Biol. Divers. 2016, 17, 679–686. [Google Scholar] [CrossRef]
- Brojo, M.; Sari, R.P. Reproductive Biology of Fivelined Threadfin Bream (Nemipterus tambuloides Blkr.) What Landed in Place of Fish Auction Labuan, Pandeglang. J. Iktiologi Indones. 2002, 2, 9–13. [Google Scholar]
- Hidayat, T.M. Fish Stock Assessment of Threadfin Bream (Nemipterus japonicus, Bloch 1791) Resources in Banten Bay. Bachelor’s Thesis, IPB University, Bogor, Indonesia, 2015. Available online: http://repository.ipb.ac.id/handle/123456789/75890 (accessed on 28 March 2025).
- Demirci, S.; Demirci, A.; Şimşek, E. Spawning Season and Size at Maturity of a Migrated Fish, Randall’s Threadfin Bream (Nemipterus randalli) in Iskenderun Bay, Northeastern Mediterranean, Turkey. Fresenius Environ. Bull. 2018, 27, 503–507. [Google Scholar]
- Akter, M.; Sharifuzzaman, S.M.; Shan, X.; Rashed-Un-Nabi, M. Reproduction, Growth, Mortality and Yield of the Goatfish Upeneus sulphureus in Northern Bay of Bengal, Bangladesh. J. Ichthyol. 2020, 60, 441–452. [Google Scholar] [CrossRef]
- Prihatiningsih; Taufik, M.; Chodrijah, U. Some Biological Stock Indicators of Red Bigeye (Priacanthus macracanthus Cuvier, 1829) in Palabuhanratu Waters, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 674, 012005. [Google Scholar] [CrossRef]
- Hordyk, A.R.; Ono, K.; Prince, J.D.; Walters, C.J. A Simple Length-Structured Model Based on Life History Ratios and Incorporating Size-Dependent Selectivity: Application to Spawning Potential Ratios for Data-Poor Stocks. Can. J. Fish. Aquat. Sci. 2016, 73, 1787–1799. [Google Scholar] [CrossRef]
- Warren, C.; Steenbergen, D.J. Fisheries Decline, Local Livelihoods and Conflicted Governance: An Indonesian Case. Ocean. Coast. Manag. 2021, 202, 105498. [Google Scholar] [CrossRef]
- Muawanah, U.; Pomeroy, R.S.; Marlessy, C. Revisiting Fish Wars: Conflict and Collaboration over Fisheries in Indonesia. Coast. Manag. 2012, 40, 279–288. [Google Scholar] [CrossRef]
- World Bank. World Bank Oceans for Prosperity: Reforms for a Blue Economy in Indonesia; World Bank: Washington, DC, USA, 2021; pp. 1–41. [Google Scholar]
- Teh, L.S.; Cheung, W.W.; Christensen, V.; Sumaila, U. Can We Meet the Target? Status and Future Trends for Fisheries Sustainability. Curr. Opin. Environ. Sustain. 2017, 29, 118–130. [Google Scholar] [CrossRef]
- Dahlet, L.I.; Himes-Cornell, A.; Metzner, R. Fisheries Conflicts as Drivers of Social Transformation. Curr. Opin. Environ. Sustain. 2021, 53, 9–19. [Google Scholar] [CrossRef]
- Okeke-Ogbuafor, N.; Gray, T. Is Community-Based Management of Small-Scale Fisheries in Sierra Leone the Answer to Their Problems? World Dev. Perspect. 2021, 21, 100292. [Google Scholar] [CrossRef]
- Sari, I.; Ichsan, M.; White, A.; Raup, S.A.; Wisudo, S.H. Monitoring Small-Scale Fisheries Catches in Indonesia through a Fishing Logbook System: Challenges and Strategies. Mar. Policy 2021, 134, 104770. [Google Scholar] [CrossRef]
- Ye, Y.; Gutierrez, N.L. Ending Fishery Overexploitation by Expanding from Local Successes to Globalized Solutions. Nat. Ecol. Evol. 2017, 1, 0179. [Google Scholar] [CrossRef]
- Ayilu, R.K.; Fabinyi, M.; Barclay, K.; Bawa, M.A. Blue Economy: Industrialisation and Coastal Fishing Livelihoods in Ghana. Rev. Fish Biol. Fish. 2023, 33, 801–818. [Google Scholar] [CrossRef] [PubMed]
- Stacey, N.; Gibson, E.; Loneragan, N.R.; Warren, C.; Wiryawan, B.; Adhuri, D.S.; Steenbergen, D.J.; Fitriana, R. Developing Sustainable Small-Scale Fisheries Livelihoods in Indonesia: Trends, Enabling and Constraining Factors, and Future Opportunities. Mar. Policy 2021, 132, 104654. [Google Scholar] [CrossRef]
- Melnychuk, M.C.; Kurota, H.; Mace, P.M.; Pons, M.; Minto, C.; Osio, G.C.; Jensen, O.P.; de Moor, C.L.; Parma, A.M.; Richard Little, L.; et al. Identifying Management Actions That Promote Sustainable Fisheries. Nat. Sustain. 2021, 4, 440–449. [Google Scholar] [CrossRef]
- Long, T.; Widjaja, S.; Wirajuda, H.; Juwana, S. Approaches to Combatting Illegal, Unreported and Unregulated Fishing. Nat. Food 2020, 1, 389–391. [Google Scholar] [CrossRef]
- Latuconsina, H. Dissemination of the Impact of Overfishing and Mitigation Efforts Through the Development of Marine Protected Areas. Agrikan J. Agribisnis Perikan. 2023, 16, 200–208. [Google Scholar]
- Tolentino-Zondervan, F.; Zondervan, N.A. Sustainable Fishery Management Trends in Philippine Fisheries. Ocean. Coast. Manag. 2022, 223, 106149. [Google Scholar] [CrossRef]
- Nugroho, K.C.; Zulbainarni, N.; Asikin, Z.; Budijanto, S.; Marimin, M. Toward a Sustainable Surimi Industry: Comprehensive Review and Future Research Directions of Demersal Fish Stock Assessment Techniques. Sustainability 2024, 16, 7759. [Google Scholar] [CrossRef]
- Martín-Sánchez, A.M.; Navarro, C.; Pérez-Álvarez, J.A.; Kuri, V. Alternatives for Efficient and Sustainable Production of Surimi: A Review. Compr. Rev. Food Sci. Food Saf. 2009, 8, 359–374. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Xavier, M.; Nayak, B.B.; Apang, T.; Balange, A.K. Quality Characteristics of Tilapia Surimi: Effect of Single Washing Cycle and Different Washing Media. J. Aquat. Food Prod. Technol. 2018, 27, 643–655. [Google Scholar] [CrossRef]
- Nurhayati, T.; Trilaksani, W.; Ramadhan, W.; Ichsani, S. The Role of Pepsin in Improving the Quality of Surimi of Red Tilapia (Orechromisniloticus). Curr. Res. Nutr. Food Sci. 2022, 10, 584–594. [Google Scholar]
- Herbert-Read, J.E.; Thornton, A.; Amon, D.J.; Birchenough, S.N.R.; Côté, I.M.; Dias, M.P.; Godley, B.J.; Keith, S.A.; McKinley, E.; Peck, L.S.; et al. A Global Horizon Scan of Issues Impacting Marine and Coastal Biodiversity Conservation. Nat. Ecol. Evol. 2022, 6, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Danovaro, R.; Fanelli, E.; Aguzzi, J.; Billett, D.; Carugati, L.; Corinaldesi, C.; Dell’Anno, A.; Gjerde, K.; Jamieson, A.J.; Kark, S.; et al. Ecological Variables for Developing a Global Deep-Ocean Monitoring and Conservation Strategy. Nat. Ecol. Evol. 2020, 4, 181–192. [Google Scholar] [CrossRef]
- Geist, J.; Hawkins, S.J. Habitat Recovery and Restoration in Aquatic Ecosystems: Current Progress and Future Challenges. Aquat. Conserv. Mar. Freshw. Ecosyst. 2016, 26, 942–962. [Google Scholar] [CrossRef]
- Taylor, M.D.; Chick, R.C.; Lorenzen, K.; Agnalt, A.-L.; Leber, K.M.; Blankenship, H.L.; Haegen, G.V.; Loneragan, N.R. Fisheries Enhancement and Restoration in a Changing World. Fish. Res. 2017, 186, 407–412. [Google Scholar] [CrossRef]
- Duarte, C.M.; Agusti, S.; Barbier, E.; Britten, G.L.; Castilla, J.C.; Gattuso, J.-P.; Fulweiler, R.W.; Hughes, T.P.; Knowlton, N.; Lovelock, C.E.; et al. Rebuilding Marine Life. Nature 2020, 580, 39–51. [Google Scholar] [CrossRef]
- Taylor, J.J.; Rytwinski, T.; Bennett, J.R.; Smokorowski, K.E.; Lapointe, N.W.R.; Janusz, R.; Clarke, K.; Tonn, B.; Walsh, J.C.; Cooke, S.J. The Effectiveness of Spawning Habitat Creation or Enhancement for Substrate-Spawning Temperate Fish: A Systematic Review. Environ. Evid. 2019, 8, 19. [Google Scholar] [CrossRef]
- Cousido-Rocha, M.; Cerviño, S.; Alonso-Fernández, A.; Gil, J.; Herraiz, I.G.; Rincón, M.M.; Ramos, F.; Rodríguez-Cabello, C.; Sampedro, P.; Vila, Y.; et al. Applying Length-Based Assessment Methods to Fishery Resources in the Bay of Biscay and Iberian Coast Ecoregion: Stock Status and Parameter Sensitivity. Fish. Res. 2022, 248, 106197. [Google Scholar] [CrossRef]
- Coscino, C.L.; Bellquist, L.; Harford, W.J.; Semmens, B.X. Influence of Life History Characteristics on Data-Limited Stock Status Assertions and Minimum Size Limit Evaluations Using Length-Based Spawning Potential Ratio (LBSPR). Fish. Res. 2024, 276, 107036. [Google Scholar] [CrossRef]
- Lauden, H.N.; Xu, X.; Lyu, S.; Lin, K.; Chen, N.; Wang, X. Assessment of the Fish Stock Status Using LBSPR with Its Implications on Fisheries Management: A Case Study of Nemipterus virgatus, Priacanthus macracanthus, and Saurida undosquamis in the Northern South China Sea. J. Appl. Ichthyol. 2024, 2024, 6808795. [Google Scholar] [CrossRef]
- Nyboer, E.A.; Reid, A.J.; Jeanson, A.L.; Kelly, R.; Mackay, M.; House, J.; Arnold, S.M.; Simonin, P.W.; Sedanza, M.G.C.; Rice, E.D.; et al. Goals, Challenges, and next Steps in Transdisciplinary Fisheries Research: Perspectives and Experiences from Early-Career Researchers. Rev. Fish Biol. Fish. 2023, 33, 349–374. [Google Scholar] [CrossRef] [PubMed]
- Garcia Lozano, A.J.; Decker Sparks, J.L.; Durgana, D.P.; Farthing, C.M.; Fitzpatrick, J.; Krough-Poulsen, B.; McDonald, G.; McDonald, S.; Ota, Y.; Sarto, N.; et al. Decent Work in Fisheries: Current Trends and Key Considerations for Future Research and Policy. Mar. Policy 2022, 136, 104922. [Google Scholar] [CrossRef]
- Oanta, G.A. International Organizations and Deep-Sea Fisheries: Current Status and Future Prospects. Mar. Policy 2018, 87, 51–59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).