Abstract
Today, urban greenery is at the center of attention, especially in the context of climate change. Shaped in large part by natural factors, the herb layer of public parks is a part of urban greenery that is the most sensitive to climate and soil condition changes. In this paper, we present a study intended to answer how resilient is the species composition and herb layer structure against the soil and climate condition changes in parks. To this end, we analyzed Ellenberg and Zarzycki’s ecological index numbers for species recorded in different groups in terms of historical-geographical, life forms, prevalence within the flora of Poland, and relationships with different vegetation types (phytoassociation classes) in comparison to the conditions present in parks. It was found that a large part of various species groups showed an optima and ecological tolerance spectra that went beyond the park conditions, indicating that at least some park vegetation can be expected to show resilience to changing conditions. However, changes in temperature and humidity will alter the composition and structure of the park herb layer. The direction of changes in climate and soil conditions can be decisive for herb layer transformation directions. With rising temperatures, humidity can be crucial. Poor soil moisture conditions will promote an increased share of foreign, synanthropic species, while local natural and semi-natural species will disappear. When climate change that leads to a decrease in temperatures is concerned, it is temperature and not humidity that will be the key factor in the transformation of park herb layer species compositions. The herb layer of Krakow’s parks will have the least resilience to changes in conditions within local non-synanthropic species, rare species and geophytes and to some extent also forest and meadow species.
1. Introduction
The importance of research on the synanthropic vegetation of urban green areas and their possible transformations in response to climate change or habitat changes related to the economy and investments in the city is crucial for understanding the functioning of urban green areas [1,2,3]. This better understanding allows us to moderate activities related to greenery management in order to perform practical functions and preserve natural values, such as the richness of taxa or generally understanding biodiversity [3,4,5,6]. Working on the basis of conclusions leads to reflection on the application and effectiveness of contemporary design solutions [7,8,9]. It also allows for a better assessment of urban greenery in the context of modern challenges [10,11,12,13].
Urban greenery is one of the building blocks of urban space and is currently the focus of interest for various researchers. Studies focus on various aspects of it, e.g., recreational, educational [13] or therapeutic significance [14,15,16]. Other works address cultural, historical and conservation aspects [17,18,19,20], as well as natural aspects. The structure, composition and diversity of natural elements, the processes that occur within and the factors that influence the formation of natural elements are studied [21,22,23,24]. At present, the existence of taxa of various organisms is mainly threatened by habitat loss, the appearance of introduced species, over-exploitation, pollution, and interactions between native species and natural causes [25]; the impacts of climate change on the taxa of different organisms, including plants, are also mentioned by various authors [26]. In addition, plant cover is currently being given very important tasks in response to climate change [27,28,29].
We equate the resilience of vascular plants of the herb layer of public parks with their capacity to retain species composition and structure in the face of climate and soil condition changes [30,31]. The composition and structure of urban vegetation is influenced by a variety of natural and anthropogenic factors. Climatic and soil conditions are a significant factor, but the extent and direction of plant cover shaping also depends on other factors [21,23,24,32]. In addition to global climate change, local climate change is also of essential significance. The progressive densification of development in Polish cities affects microclimatic phenomena as well as the transformation of urban greenery [33]. Soil properties are linked to climate and microclimate; but they are also influenced by various other factors [34,35,36,37].
Plant cover formation is influenced by species properties, although various authors emphasize that in the case of climate change, based on paleontological data, migration is a more effective reaction mechanism than adaptation [38,39]. There are authors who argue that, in the case of plants, migration as a process is too slow in the context of climate change [40]. Especially as there are currently many barriers that restrict migration due to the fragmentation and isolation of plant cover [41,42,43] which usually affects public parks in large cities, which in many cases are surrounded by heavily modified spaces [24,44,45,46]. However, in a study of Holocene migration, Cain (2002) [47] proved that species colonization and the reaching to their current extent are not possible when the plants’ own migration capacities are the sole factors considered. This indicates that episodic weather or climatic events allow seeds to migrate even over very long distances. In the context of the literature, it appears that both adaptation and migration are important mechanisms of plant response to climate change.
Climate change can vary in nature and severity; hence plant cover changes can vary in direction and intensity. It must be stressed here that, first of all, changes in climate and soil conditions will affect plant metabolism, causing environmental stress which will lead to adaptation and/or changes in the population parameters of individual plant species [48,49,50], including a shortening or alteration of the life cycle manifested as a change in the intensity or complete disappearance of flowering and fruiting [51,52], followed by the withdrawal of herb layer plants of public parks, which in turn may affect the overall richness and diversity of urban greenery. However, climate change will, at the same time, favor the appearance of species that have not previously been recorded [53], e.g., from the scleromorphic group [54].
Parks whose plant cover is largely deliberately arranged and designed by humans are an essential element of urban greenery. Park herb layer is among the elements that are susceptible to natural processes to the greatest extent [55]. Here, maintenance measures are mostly limited to mowing, although we can currently see the introduction of different species into the herb layer and even soil replacement [24].
Many plant cover studies explore the direct impact of climate change on its formation, their biodiversity both at the biocenotic and genetic levels, as well as the extinction and colonization processes of selected taxa [40,56,57]. However, there are a lack of studies on the impact of climate change on transformations within different plant groups that form the cover of public parks, especially herb layer plants. Some authors indicate even neglecting this type of vegetation [4]. Therefore, the aim of this study was to determine how changes in climatic and soil conditions affect herb layer composition and the groups of plant taxa in terms of historical-geographical considerations, life forms, occurrence in plant cover and sociological (phytocenological) occurrence in them, and thus changes in the richness and diversity of this urban greenery element. The related second aim was to verify whether public parks crossed by or adjacent to streams or which feature water bodies, were similar in case of herb layer plant composition and the conditions present in the parks. It was also meant to determine whether the presence of water bodies and watercourses had a significant impact on soil moisture retention in the event of climate change.
Due to the great difficulty in carrying out long-term research into vegetation composition and structure changes [58], which would also require consideration of the impact of other factors on plant cover, the reactions of different plant species or groups of plants were modeled and forecasted. This takes into account various contemporary phenomena, such as changes in plant ranges including arctic species [39,59,60,61]. Thus, this study also employed indirect methods that involved analysis of the optima of ecological tolerance ranges for the climatic and soil factors of the vascular plant species that make up the park herb layer.
2. Materials and Methods
2.1. Study Area
Krakow is a large city in the southern part of Poland and is geomorphologically diverse. Different geographical and phytogeographical regions connect here [62,63]. The city is crossed by the River Vistula’s proglacial valley that runs west along the geological formations known as the Krakow Gate [64]. The southern part of the city is bordered by the flysch Carpathian Foothills, and to the northwest are the Jurassic limestone formations of the Krakow-Częstochowa Upland, while to the north-east is the Miechów Upland (the Proszowice Plateau). This contributes to the great floristic richness of the Krakow area. Vascular plant species alone were estimated at around 1330 species [65]. Around 100 plant communities were reported to be present within Krakow [66,67]. The vegetation of Krakow is very well recognized. Many research works have been dedicated to it. The first full flora study was published in the 19th century [68], when the transformation of Krakow’s flora was already studied [69]. The vegetation of the city consists of a mosaic of different areas: among others, the exposed massif of Sikornik, Sowiniec, the Tyniec Forest, the Pychowice Meadows, the Nowa Huta Meadows, Twardowski’s Rocks, the vegetation of Krzemionki, Bodzów, Łęg, and communities along the rivers: the Vistula, Dłubnia, Rudawa, Wilga, meadow or scrub groupings in undeveloped areas [66]. Complementing the vegetation of the natural, semi-natural and synanthropic character, is landscaped greenery, with parks forming a significant portion (Figure 1).

Figure 1.
Methodology activity flow diagram. Research questions: 1. What is the share of species in parks whose optimum in terms of temperature, continentalism, and humidity factors is different from the conditions prevailing in the park; and what is the participation of species whose conditions in the park are unlike their tolerance. 2. What is the share of varied species from the four categories whose optimum in terms of temperature, continentalism and humidity factors is different from the conditions prevailing in the park; and what is the share of species whose conditions in the park are unlike their tolerance. 3. What is the relationship between habitat conditions and species composition?
Krakow lies in a temperate climate zone with continental characteristics [70]. The climate’s character is also linked to the city’s landforms and varied geomorphological formations. Krakow’s climate is subject to significant changes, both in terms of temperature, precipitation and duration of snow cover [71,72,73].
2.2. Methodology
To answer the formulated research questions according to the limitations and difficulty described in the Introduction, the methodology presented in Figure 1 was performed.
In the years 2016–2017, a survey of herbaceous vascular plants of the herb layer was performed in 41 of Krakow’s parks. Park herb layer is understood here as a compositional element that is largely subject to shaping by natural processes. Deliberate human interference is usually limited to more or less frequent mowing. In contrast to the undergrowth, the studied layer of herbs referred only to herbaceous and vascular plants and did not have to be associated with wooded areas. In some parks, such as Debnicki park, areas covered by tree stands occupy a tiny area on the outskirts and their share is negligible. Compositional elements in which the species structure was deliberately created by planting or sowing were not taken into account. At the time of the survey, the parks under study were dominated by this type of herb layer development. Deliberately designed compositional elements, if present, formed a small share of their area. Plants used in such compositional elements or typical garden varieties were either not present in the herb layer or appeared sporadically. In our opinion, this was mostly due to spontaneous spread from nearby allotment gardens rather than intentional planting or sowing. The criteria for the selection of public parks were taken from Moszkowicz and Krzeptowska-Moszkowicz (2020) [55]. A description of the characteristics of the parks and surroundings was included in Moszkowicz et al., 2021 [24].
Based on the recorded species, climatic and soil conditions relevant to answering the questions explored in the study were determined using Ellenberg and Zarzycki’s ecological index numbers [74,75,76,77].
Indicators for temperature, climate continentality and soil moisture were used for the analyses of species’ optima and tolerance ranges: T, K, Fe according to Ellenberg’s ecological index numbers, which are equated in this study with approximate and broad optima and T, K, W according to Zarzycki’s scale of ecological index numbers, which are identified with a general range of ecological tolerance. This use of particular scales stems from their character. Concerning the ecological tolerance range following Zarzycki’s indices, they present a range of values treated as an approximate scope of a species’ ecological tolerance. Ecological index numbers have certain limitations, primarily due to the methodology for their determination as well as due to the certain generality of index scales [78,79,80,81]. The range of ecological tolerance obtained using these numbers is not a precise range of ecological tolerance beyond which, according to the concepts used in the literature, an organism cannot survive. In this case, it is general enough that it does not set precise values for the conditions beyond which a given species does not occur. However, it can be expected that the species may occur outside the range defined here but in a limited manner, i.e., with lower population parameters that manifest themselves, for example, via lower abundance, or a lack of or poor flowering and fruiting. It is also important to be aware of some discrepancies in index numbers given by different authors, which is also related to the different areas from which data were obtained to determine their values [77,81,82].
The proportion of species with ecological optima and tolerance ranges that fit within the values of park conditions was then determined according to the index numbers, as well as the proportion of species with ecological optima and tolerance ranges higher and lower in relation to park conditions. As ecological index numbers have not been determined for all recorded species, for example, for agricultural crops, the analysis was therefore restricted to those species for which index numbers have been determined. There were 230 of these species. The ratio of each species group in this number is presented in Figure 2.

Figure 2.
Proportions between ratios of each plant group for the average, minimum and maximum shares in parks. Plant categories with isolated species groups; synanthropic plant taxon classes: A—non-synanthropic species, B—local synanthropic species, and C—anthropofits; prevalence of taxons in the flora of Poland: A—common species, B—frequent species, and C—rare species; life-form taxons: A—terophytes, B—haemicryptophytes, C—geophytes, and D—chamephytes; taxons of different class plant communities: A—Molinio-Arrhenatheretea, B—Stellarietea medii, C—Artemisietea vulgaris, D—Querco-Fagetea, E—Festuco-Brometea and others. Bar colors: A—light blue, B—light green, C—green, D—yellow, E—salmon.
The analysis of optima diversity and ecological tolerance was carried out for all recorded species that occur in the parks, as well as for the following isolated groups from the following herb layer plant categories:
(a) Historico-geographical (synanthropic classification): based on the literature, general groups such as non-synanthropic local species (spontaneophytes, natiphytes), synanthropic local species (apophytes), and foreign species (anthropophytes) were identified [65,83,84]. Non-synanthropic species were assumed to be native species that were not included in the list of apophytes for Poland [85];
(b) Occurrence of species in the flora of Poland: groups of common, frequent and rare species in the flora of Poland [86,87];
(c) Life forms: species groups were restricted to the four most commonly represented by parkland herb layer species: hemicryptophytes, therophytes, geophytes and chamaephytes [76,88];
(d) The phytoassociation classes most represented by species according to Matuszkiewicz (2004) [89]: Molinio-Arrhenatheretea, Stellarietea medii, Artemisietea vulgaris, Querco-Fagetea, and others, especially Festuco-Brometea and Trifolio-Geranietea.
The results have been presented in the charts attached. On the basis of the species participation whose optimum, as well as the optimum and tolerance range, were outside the values defined in specific parks, it was inferred how species number and diversity would decline when specific conditions identical to the index numbers analyzed changed. The species ratio was presented for all herb layer species, divided by diversification in their associated index numbers and the values of those numbers in parks.
The study was extended to include the classification of the parks investigated in terms of climate and soil conditions as well as their herb layer species compositions, using Ward’s agglomerative cluster analysis with Euclidean distance. Similarity in terms of floristic composition was determined using Jaccard’s (1908) [90] simple similarity coefficient as a percentage of species shared by the parks. Similarity in terms of conditions was defined as the percentage of same index values in relation to all indices.
3. Results
Based on our results, it can be concluded that climate and soil condition changes will alter the herb layer species ratios and structures of Krakow’s parks. However, the intensity and trajectory of climate change will influence the magnitude and proportions of this process in different species groups (Figure 3, Figure 4, Figure 5, Figure 6 and Figure 7).

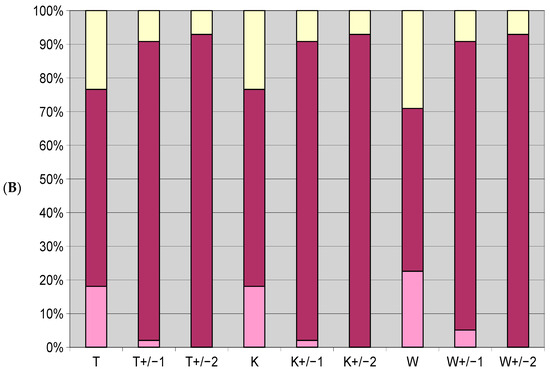
Figure 3.
Ratio of species whose optimum (A) and tolerance range (B) coincided with park conditions; blue bar (A), maroon bar (B); ratio of species for which it was higher: light blue bar (A), yellow bar (B); and for which it was lower: purple (A), pink (B). Results are presented for ecological index numbers: temperature (T), climate continentality (K), moisture (W), +/−1 indicates the range of index numbers of species increased by one higher and lower, +/−2 means the range increased by two higher and lower.


Figure 4.
Ratio of local non-synanthropic species (natophytes), local synanthropic species (apophytes) and foreign species (anthropophytes) whose optimum (A) and tolerance range (B) coincided with park conditions; blue bar (A), maroon bar (B); ratio of species for which it was higher: light blue bar (A), yellow bar (B); and for which it was lower: purple (A), pink (B). The results have been presented for the ecological index numbers: temperature (T), climate continentalism (K) and moisture (W).
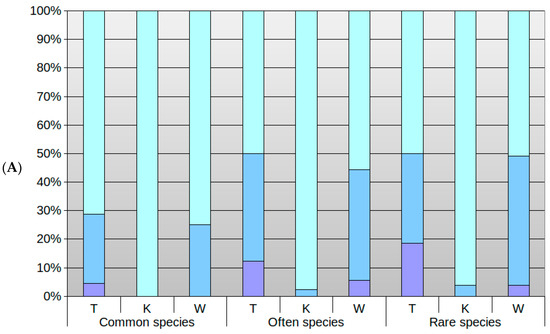
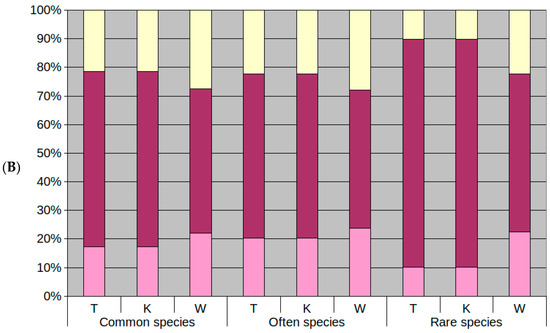
Figure 5.
Ratio of common, frequent and rare species whose optimum (A) and tolerance range (B) coincided with park conditions; blue bar (A), maroon bar (B); ratio of species for which it was higher: light blue bar (A), yellow bar (B); and for which it was lower: purple (A), pink (B). The results have been presented for the ecological index numbers: temperature (T), climate continentalism (K) and moisture (W).


Figure 6.
Ratio of species by life forms, whose optimum (A) and tolerance range (B) coincided with park conditions; blue bar (A), maroon bar (B); ratio of species for which it was higher: light blue bar (A), yellow bar (B); and for which it was lower: purple (A), pink (B). The results have been presented for the ecological index numbers: temperature (T), climate continentalism (K) and moisture (W).


Figure 7.
Ratio of species linked to various plant communities, whose optimum (A) and tolerance range (B) coincided with park conditions; blue bar (A), maroon bar (B); ratio of species for which it was higher: light blue bar (A), yellow bar (B); and for which it was lower: purple (A), pink (B). The results have been presented for the ecological index numbers: temperature (T), climate continentalism (K) and moisture (W).
3.1. Differences in Optima and Range of Ecological Tolerance against Park Conditions of All Herb Layer Plants
From all the herb layer species of Krakow’s public parks that were analyzed, the results showed (Figure 3) that the optima of most species occurred in higher temperature, warmer and more continental climates. Species whose optima for these parameters were lower for cooler and harsher climates were few, within the 8–11% range. All of these species can function in park conditions due to their wide tolerance ranges. Species whose optima overlapped with park conditions ranged from 19 to 27%. In terms of water conditions, park conditions were in the optimum of about 32% of the species, while there were more species whose optimum aligned with conditions with higher soil moisture (higher index value)—44%, but they were not as numerous as the species which preferred the remaining values, 65–70%. However, lower index values (lower moisture) displayed a higher share than in other instances—24%. It is notable that the optima of 68% of the species were in the range of +/−1, while for 75% of the species it was in the +/−2 range.
As far as the approximate range of ecological tolerance determined using Zarzycki’s ecological numbers is concerned, the dominant proportion of species (almost 80%) fell within this range in terms of temperature and climate continentality. Less than 50% was out of range in terms of moisture (Figure 3). This indicates that most species were on the edge of viability in terms of water availability. A change of 1 in the moisture index could result in 85% of the species covering the full approximate tolerance range of the park conditions. Extending the conditions range would lead to a quite small number of species at the edge of this tolerance.
Regarding Zarzycki’s index numbers, for most of the taxa analyzed, the species that had a temperature index value had the same continental value. Hence, it follows that the graphs for the ratios of species by tolerance range for T and K numbers are similar.
Based on an analysis of Figure 3, it can be concluded that most species had an optimum in the higher parameter value ranges; therefore, an increase in temperature, or a small change in climate continentality, would not have a significant impact on most species if moisture conditions were maintained or improved. In terms of changing moisture conditions, parkland herb layer species are more sensitive.
3.2. Differences in Optima and Range of Ecological Tolerance against Park Conditions of Herb Layer Plants in Groups of the Historic-Geographical Category
Considering the survival optima of herb layer species due to their historic-geographical category, the local non-synanthropic species fit best in the conditions found in the parks; more than 40% had an optimum in the conditions of the parks for temperature and moisture (Figure 4). More local synanthropic species (apophytes) had optimum values in the higher values of the factors compared to than those found in parks. Anthropophytes had the highest species ratio with higher optima, while at the same time, they had the lowest species ratio with the same index number values as those found in parks. Most of the species for non-synanthropic, and all for local synanthropic and anthropophytes, displayed values of climate continentality higher than those prevailing in the parks under study. It indicates that if climate and soil conditions change towards an increase in values, this will result in the most negative impact on local non-synanthropic species and the most beneficial impact on anthropophytes. The tolerance ranges in each group covered conditions for 40–60% of the species. The least number of species was covered by the park’s moisture conditions, which leads to the conclusion that it is a substantial factor and changes in moisture may lead to changes in population parameters and even the withdrawal of numerous species. Analysis of the tolerance ranges showed that anthropophytes were the group whose preferred moisture conditions varied from prevailing in the parks. So, their tolerance range was much broader than the index numbers were suggesting.
3.3. Differences in Optima and Range of Ecological Tolerance against Park Conditions of Herb Layer Plants in Groups of the Commonness of Taxa in the Flora of Poland
When analyzing the species of park herb layer in terms of their occurrence in the flora of Poland, it is worth noting that, similarly, the herb layer of Krakow’s public parks is dominated by common and frequent species (Figure 2). Rare species are a small group of specimens.
Considering species groups in this category, it is noticeable that most species that find the optimum in park conditions in terms of temperature are frequent plants, followed closely by common plants, and at last rare plants. In the case of moisture, the analysis found that rare plants ranked after frequent, followed by common species. Regarding climate, most species reached their optimum in a continental climate. In case of moisture, the rare taxa had the smallest proportion of species with optima in wetter conditions than those prevalent in parks. It would indicate that in terms of moisture, few of these species have a broader range of tolerance to drier conditions. In the case of frequent taxa, here we encounter species with varying optima, but with ratios more balanced than the previous group in case of moisture or temperature. Although, the majority still prefer warmer and wetter conditions. Quite a few species in this group had their optima in the park conditions. Common species had wide tolerance ranges, but most preferred warmer, more continental and wetter conditions than those estimated in the park. It means that, in the event of climatic changes leading to higher temperatures, all species groups should be able to survive without significant transformations of structure and composition, provided moisture will not decrease. A reduction of humidity in all groups will result in the disappearance of species.
In the case of rare species, very few had optima in wetter conditions and more in drier conditions than those existing in the parks, which may be due to the high variability of these conditions not allowing representatives of this group with optima to occur in wetter conditions. In addition, they also had a narrow range. A reduction in moisture would significantly affect the transformation of these species.
3.4. Differences in Optima and Range of Ecological Tolerance against Park Conditions of Herb Layer Plants in Groups of the Life Form Category
The vast majority of geophytes occurring in the herb layer of Krakow’s public parks had optima within the scope of the park conditions in terms of the parameters under study (Figure 6). As for the other groups, such as life forms, therophytes and chamaephytes, in particular, had optima in higher values. The hemicryptophytes mostly showed a range of tolerances covering the prevailing conditions, with a small proportion of species with optima compatible with the park conditions. Of the groups analyzed, geophytes were the most sensitive to changes in conditions due to having the narrowest tolerance ranges and also the occurrence of species with optima aligned with park conditions. An increase in temperature, however, while maintaining or increasing moisture, would benefit the therophytes and chamaephytes.
3.5. Differences in Optima and Range of Ecological Tolerance against Park Conditions of Herb Layer Plants in Groups of Different Plant Communities
Species whose optima were aligned with Krakow’s parks conditions and which were linked to synanthropic conditions (Stellarietea medii and Artemisietea vulgaris plant community classes), formed the smallest share of herb layer species. Their optima were linked to higher values of climate and soil factors. The share of species with optima aligned with the park conditions and linked with meadow communities (Molinio-Arrhenatheretea class) was greater, while the largest share of around 50% was that of species with aligned optima that formed xerothermic (Festuco-Brometea and Trifolio-Geranietea classes) and forest communities (Querco-Fagetea class). Often, the groups forming the highest shares had narrow tolerance ranges and climate and soil condition changes could potentially affect them the most, leading to their transformation. This is confirmed by the small share of xerothermic species, whose approximate ecological tolerance range fits the conditions of the parks. However, this is a very approximate range since the share of these species in terms of optima fitting into the park conditions was higher. Synanthropic species are often characterized by a wide tolerance range, but in the case of the herb layer species of Krakow’s parks there is a smaller share of species (mainly from the class Arthemisietea vulgaris) whose general range of tolerance covered the park conditions, which may indicate that for some, changes in conditions may have a negative effect, but for others it could be beneficial or irrelevant.
3.6. Relation between Site Conditions and the Plant Composition
Based on the results, it appears that adequate moisture conditions are crucial for many species found in the parks. When we analyze Figure 8, we can observe that parks with streams or small rivers in or adjacent to them were similar in terms of their herb layer composition. The exceptions were two parks whose dissimilarity in this respect stemmed from the location of these parks on the outskirts of the city. In terms of conditions (Figure 9), the public parks did not show such similarity in terms of climate and soil conditions. Even when analyzing moisture content, parks with artificial reservoirs did not show any diversification and did not differ in moisture (in areas with herb layer present) from parks without these elements. On this basis, it can be seen that while streams or watercourses could have affected the soils or moisture within the park, and therefore the species composition of the herb layer, artificial reservoirs did not appear to have a significant impact on this. They create habitat for hydrophytes and hygrophytes or plants in the bank zone, while they do not significantly affect the composition of the park herb layer.

Figure 8.
Dendrogram showing the diversity of Krakow’s parks in terms of herb layer species composition. Axis X shows park numbers. Axis Y shows bond distance. Blue circles were used to denote parks with small rivers or streams, while red circles for parks with artificial or altered reservoirs or fountains. Park numbers are given analogous to Figure 1.

Figure 9.
Dendrogram showing the variation of parks in terms of conditions defined by ecological index numbers. Park numbers on axis X are given analogous to Figure 1. Axis Y shows bond distance. Blue circles were used to denote parks with small rivers or streams, while red circles for parks with artificial or altered reservoirs. Park numbers are given analogous to Figure 1.
4. Discussion
We have found that a significant part of various species groups showed an optima and ecological tolerance spectra that went beyond the park conditions, indicating that at least some park vegetation can show resilience to changing conditions. The withdrawal of some herb layer species from public parks as a result of changes in climate and soil conditions may result in the appearance of ubiquitous and foreign species which can have negative effects, in addition to decreasing plant richness and diversity [91,92].
Our analysis show that, in the case of impacts of climate and soil conditions on shifts in public parks’ vegetation, the influence of climate features on herb layer vegetation resilience may vary [93]. The direction of these changes can cause various transformations in the herb layer composition and structure. However, the direction and magnitude of change in plant richness and overall biodiversity, according to this study, will depend on the direction of climate change.
We have found that when temperatures rise, it is not temperature but moisture changes that will have a key impact on the transformation of the park’s herb layer; this is opposed to when there is a crucial drop in temperature whereby moisture will take second place. Changes involving a reduction in rainfall and water availability with a rise in temperature will most notably cause the withdrawal of rarer local species with higher environmental requirements. For example, range shifts have been found to have shifted upwards (in terms of altitude) in common mountain species as a result of increasing temperatures and decreasing rainfall [94]. This type of plant response to the changes discussed is confirmed by studies by other authors [95], although a different behavior of plant populations cannot be excluded [96]. Similarly, local, synanthropic species may respond differently as confirmed by our study, and these diverse responses in foreign species have also been observed by other authors [97,98]. Some local taxa with the ability to survive in new conditions will be slow to colonize parks or will not colonize them at all over a period of time, due to poor migratory opportunities [40,99,100,101,102]. According to our study, climate change, which will lead to an increase in temperatures or a change in the character of the climate, without changes to water conditions or with an improvement in this regard, should not, at least initially, lead to significant change in species composition or lead to a decrease in its variety.
Our study shows that moisture conditions are essential for the maintenance of herb layer species composition and structures. Species associated with high-moisture habitats are the most vulnerable, but this factor is significant for many other species. The park herb layer will display weaker resilience to changes in this factor than to temperature. Most park herb layer species are at the edge of their moisture ecological tolerance. The importance of water conditions stands out in the analysis, particularly in the context of changing temperatures. Various authors highlight the impact of these conditions on specific plant groups. E.g. the study by Kostrakiewicz-Gierałt et al. (2022) [103] points out that fertilization and irrigation in urban parks promotes an abundance of meadow vegetation. The results of this work support the second part of this argument. In addition to synanthropic vegetation, this is the most abundant group of park herb layer species in Krakow’s public parks, composed largely of local synanthropic species. The number of species in this group with optima and ecological tolerance ranges falling into better moisture conditions than those in the parks is very high and they display considerable competitiveness. Therefore, high moisture will favor the growth of this vegetation. On the other hand, there are authors who argue that poorer moisture conditions may affect alien species more negatively than local ones [98]. We have found that resilience to changes in moisture may be greater in parks with areas crossed by poorly or unregulated rivers or streams. We have not diagnosed the direct impact of the presence of artificial reservoirs on the composition and structure of the herb layer of public parks.
Many studies indicate that climate change will favor the presence and spread of some alien species [91,104]. In contrast, studies by different authors on invasive species indicate that they respond differently to environmental change [105], so it is currently difficult to give a clear indication of how alien species will behave as a result of climate change [97]. We have found that a rise in temperature will negatively affect the share of local non-synanthropic species and support common species. Declining temperatures may favor an increase in the proportion of frequent and rare species at the expense of common species. The species that will be most sensitive to potential changes in soil conditions are specialized species with narrow tolerance ranges [106,107]. These include a significant share of local non-synanthropic species, which are often associated with specific communities, and their residual presence is associated with pre-existing vegetation and with the establishment of a niche due to a biocenotic arrangement [108,109]. In the case of the herb layer of Krakow’s public parks, these are usually rare species from Poland’s flora. The sensitivity of rare species and their greater susceptibility to climate change is also confirmed by models performed by various researchers, for example Anacker et al. (2013) [110].
Our studies show that increasing temperatures may favor a rise in the share of anthropophytes, therophytes and chamaephytes. It will negatively affect the share of local non-synanthropic species and geophytes, although in their case, due to spore organs, their reaction may be slower. Various authors have observed specific responses by species that represented a particular life form that differed with respect to species with other life forms [111] or point to, for instance, hemicryptophytes as being better suited to climate changes [54] while therophytes, chamaephytes and geophytes showed a significant relationship with moisture and precipitation [112]. Based on the results obtained in this work, geophytes will show a different response to changes in soil and climate conditions in terms of life form. Most of the species that occur in Krakow’s parks find approximately optimal conditions there. As a result of environmental change, they can change growth and flowering habits very quickly [113], as most geophytes show strong phenological relationships with meteorological data [114]. However, the presence of subterranean spore and storage organs, which increase resistance to environmental stress [115], facilitates their survival in harsh conditions [51,100]. Therefore, it is uncertain whether climate change will cause the withdrawal of these species in the short term.
According to our study, changes in soil and climate conditions towards higher temperatures and reduced water availability will mainly affect the withdrawal of species associated with forest vegetation such as the Querco-Fagetea plant community class, which is confirmed by the results of other authors [54]. Other authors indicate that in certain regions in the last few decades, these communities, together with associated species, have been expanding their range at the expense of boreal conifer vegetation [116]. It is a result mainly due to eutrophication. Such changes may lead to the withdrawal of some of the synanthropic species associated with the plant community class Artemisietea vulgaris, considered by some authors to be indicators of human disturbance and impact [44] while other authors sometimes did not point to it as having such significance [117]. Based on the results of this study, it should be noted that, when synanthropic vegetation is concerned, it applies mainly to local species. However, climate change that progresses in a different direction may result in a different impact on population changes, species withdrawal processes, and colonization. The herb layer of Krakow’s parks will have the least resilience to changes in conditions within local non-synanthropic species, rare species and geophytes and to some extent also forest and meadow species.
5. Conclusions
We have found that a significant part of various species groups showed an optima and ecological tolerance spectra that went beyond the park conditions, indicating that at least some park vegetation can show resilience to changing conditions.
Moisture conditions are essential for maintenance of herb layer species composition and structures. Species associated with high-moisture habitats are the most vulnerable, but this factor is significant for many other species. The park herb layer will display weaker resilience to changes in this factor than to temperature. Most park herb layer species are at the edge of their moisture ecological tolerance.
Resilience to changes in moisture may be greater in parks with areas crossed by poorly or unregulated rivers or streams. We have not diagnosed the direct impact of the presence of artificial reservoirs on the composition and structure of the herb layer of public parks.
Increasing temperatures may favor a rise in the share of anthropophytes, therophytes, chamaephytes and common species. It will negatively affect the share of local non-synanthropic species and geophytes, although in their case, due to spore organs, their reaction may be slower.
Declining temperatures may favor an increase in the proportion of frequent and rare species at the expense of common species.
The herb layer of Krakow’s parks will have the least resilience to changes in conditions within local non-synanthropic species, rare species and geophytes and to some extent also forest and meadow species.
Author Contributions
Conceptualization, Ł.M. and I.K.-M.; methodology, Ł.M.; validation, Ł.M., I.K.-M. and M.Z.; formal analysis, Ł.M. and K.P.; investigation, Ł.M. and I.K.-M.; resources, Ł.M. and K.P.; data curation, Ł.M., I.K.-M. and K.P.; writing—original draft preparation, Ł.M.; writing—review and editing, Ł.M., I.K.-M. and M.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The study did not report any data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Winkler, J.; Matsui, Y.; Filla, J.; Vykydalová, L.; Jiroušek, M.; Vaverková, M.D. Responses of synanthropic vegetation to composting facility. Sci. Total Environ. 2023, 859, 160160. [Google Scholar] [CrossRef] [PubMed]
- Bordim, M.H.S.; Longo, R.M.; Bordim, B.S. Urban environmental sustainability: Analysis of the influence of vegetation in environmental parameters. Rev. De Gestão Ambient. E Sustentabilidade 2022, 11, 1–23. [Google Scholar] [CrossRef]
- Pickett, S.; Cadenasso, M. Linking ecological and built components of urban mosaics: An open cycle of ecological design. J. Ecol. 2008, 96, 8–12. [Google Scholar] [CrossRef]
- Bonthoux, S.; Voisin, L.; Bouché-Pillon, S.; Chollet, S. More than weeds: Spontaneous vegetation in streets as a neglected element of urban biodiversity. Landsc. Urban Plan. 2019, 185, 163–172. [Google Scholar] [CrossRef]
- Florgård, C. Preserved and remnant natural vegetation in cities: A geographically divided field of research. Landsc. Res. 2007, 32, 79–94. [Google Scholar] [CrossRef]
- Reaka-Kudla, M.L.; Wilson, D.E.; Wilson, E.O. (Eds.) Biodiversity II: Understanding and Protecting Our Biological Resources; Joseph Henry Press: Washington, DC, USA, 1996. [Google Scholar]
- Afuye, G.A.; Kalumba, A.M.; Orimoloye, I.R. Characterisation of Vegetation Response to Climate Change: A Review. Sustainability 2021, 13, 7265. [Google Scholar] [CrossRef]
- Marchionni, V.; Fatichi, S.; Tapper, N.; Walker, J.P.; Manoli, G.; Daly, E. Assessing vegetation response to irrigation strategies and soil properties in an urban reserve in southeast Australia. Landsc. Urban Plan. 2021, 215, 104198. [Google Scholar] [CrossRef]
- Seddon, N.; Smith, A.; Smith, P.; Key, I.; Chausson, A.; Girardin, C.; House, J.; Srivastava, S.; Turner, B. Getting the message right on nature-based solutions to climate change. Glob. Chang. Biol. 2021, 27, 1518–1546. [Google Scholar] [CrossRef]
- Rafiee, R.; Mahiny, A.S.; Khorasani, N. Assessment of changes in urban green spaces of Mashad city using satellite data. Int. J. Appl. Earth Obs. Geoinf. 2009, 11, 431–438. [Google Scholar] [CrossRef]
- Badach, J.; Dymnicka, M.; Baranowski, A. Urban Vegetation in Air Quality Management: A Review and Policy Framework. Sustainability 2020, 12, 1258. [Google Scholar] [CrossRef]
- Uçar, Z.; Akay, A.E.; Bilici, E. Towards green smart cities: Importance of Urban forestry and urban vegetation. International Archives of the Photogrammetry. Remote Sens. Spat. Inf. Sci.-ISPRS Arch. 2020, 2020, 399–403. [Google Scholar] [CrossRef]
- Manning, R.; More, T. Recreational Values of Public Parks. Georg. Wright Forum 2002, 19, 21–30. Available online: http://www.jstor.org/stable/43597798 (accessed on 3 November 2023).
- Staniewska, A. Gardens of Historic Mental Health Hospitals and Their Potential Use for Green Therapy Purposes. Land 2022, 11, 1618. [Google Scholar] [CrossRef]
- Krzeptowska-Moszkowicz, I.; Moszkowicz, Ł.; Porada, K. Evolution of the Concept of Sensory Gardens in the Generally Accessible Space of a Large City: Analysis of Multiple Cases from Kraków (Poland) Using the Therapeutic Space Attribute Rating Method. Sustainability 2021, 13, 5904. [Google Scholar] [CrossRef]
- Krzeptowska-Moszkowicz, I.; Moszkowicz, Ł.; Porada, K. What Affects the Depth of the Human–Garden Relationship in Freely Accessible Urban Sensory Gardens with Therapeutic Features in Various Users? Sustainability 2023, 15, 14420. [Google Scholar] [CrossRef]
- Taylor, H.A. Urban Public Parks, 1840–1900: Design and Meaning. Gard. Hist. 1995, 23, 201–221. [Google Scholar] [CrossRef]
- Low, S.M.; Taplin, D.; Scheld, S. Rethinking Urban Parks: Public Space and Cultural Diversity; University of Texas Press: Austin, TX, USA, 2009. [Google Scholar]
- Gawryluk, D. Historical Public Parks in Podlasie and their Current Day Modernisation. Form. Urban Green Areas 2015, 1, 74–80. [Google Scholar]
- Bobek, W.; Łakomy, K.; Hodor, K. Contemporary processes and the selection of materials in historical urban greenery areas on example from Cracow and Warsaw. IOP Conf. Ser. Mater. Sci. Eng. 2018, 471, 092018. [Google Scholar] [CrossRef]
- Bianco, P.M.; Fanelli, G.; Tescarollo, P.; Pignatti, S. Ruderalization in a Roman Parks as a Result of Changing Managemant. Urban Habitats 2003, 1, 87–104. [Google Scholar]
- LaPaix, R.; Freedman, B. Vegetation Structure and Composition within Urban Parks of Halifax Regional Municipality, Nova Scotia, Canada. Landsc. Urban Plan. 2010, 98, 124–135. [Google Scholar] [CrossRef]
- Concepción, E.D.; Obrist, M.K.; Moretti, M.; Altermatt, F.; Baur, B.; Nobis, M.P. Impacts of urban sprawl on species richness of plants, butterflies, gastropods and birds: Not only built-up area matters. Urban Ecosyst. 2016, 19, 225–242. [Google Scholar] [CrossRef]
- Moszkowicz, Ł.; Krzeptowska-Moszkowicz, I.; Porada, K. Relationship between parameters of public parks and their surroundings and the richness, diversity and species composition of vascular herbaceous plants on the example of Krakow in Central Europe. Landsc. Online 2021, 94, 1–16. [Google Scholar] [CrossRef]
- Venter, O.; Brodeur, N.N.; Nemiroff, L.; Belland, B.; Dolinsek, I.J.; Grant, J.W. Threats to endangered species in Canada. Bioscience 2006, 56, 903–910. [Google Scholar] [CrossRef]
- Woo-Durand, C.; Matte, J.M.; Cuddihy, G.; McGourdji, C.L.; Venter, O.; Grant, J.W. Increasing importance of climate change and other threats to at-risk species in Canada. Environ. Rev. 2020, 28, 449–456. [Google Scholar] [CrossRef]
- Szopińska, E.; Kazak, J.; Kempa, O.; Rubaszek, J. Spatial Form of Greenery in Strategic Environmental Management in the Context of Urban Adaptation to Climate Change. Pol. J. Environ. Stud. 2019, 28, 2845–2856. [Google Scholar] [CrossRef]
- Apostolopoulou, D.; Tsoka, S. Climate change and built environment—The role of urban greenery as a mitigation strategy in Greek urban areas. IOP Conf. Ser. Earth Environ. Sci. 2021, 899, 012018. [Google Scholar] [CrossRef]
- Hedblom, M.; Prevot, A.C.; Grégorie, A. Science fiction blockbuster movies—A problem or a path to urban greenery? Urban For. Urban Green. 2022, 74, 127661. [Google Scholar] [CrossRef]
- MacGillivray, C.W.; Grime, J.P.; The Integrated Screening Programme (ISP) Team. Testing predictions of the resistance and resilience of vegetation subjected to extreme events. Funct. Ecol. 1995, 9, 640–649. [Google Scholar] [CrossRef]
- Steel, Z.L.; Foster, D.; Coppoletta, M.; Lydersen, J.M.; Stephens, S.L.; Paudel, A.; Collins, B.M. Ecological resilience and vegetation transition in the face of two successive large wildfires. J. Ecol. 2021, 109, 3340–3355. [Google Scholar] [CrossRef]
- Czochański, J.; Wiśniewski, P. River valleys as ecological corridors—Structure, function and importance in the conservation of natural resources. Ecol. Quest. 2018, 29, 77–87. [Google Scholar] [CrossRef]
- Szczerek, E. The Problem of Densification of Large-Panel Housing Estates upon the Example of Cracow. Land 2021, 10, 1359. [Google Scholar] [CrossRef]
- Hilgard, E.W. A Report on the Relations of Soil to Climate; Bulletin, U.S. Department of Agriculture, Weather Bureau: Washington, DC, USA, 1892; Volume 3, pp. 1–59.
- Eagleson, P.S. Climate, soil, and vegetation: 6. Dynamics of the annual water balance. Water Resour. Res. 1978, 14, 749–764. [Google Scholar] [CrossRef]
- Sevink, J. Soil development in the coastal dunes and its relation to climate. Landscape Ecol. 1991, 6, 49–56. [Google Scholar] [CrossRef]
- Sanchis, M.P.S.; Torri, D.; Borselli, L.; Poesen, J. Climate effects on soil erodibility. Earth Surf. Process. Landf. 2008, 33, 1082–1097. [Google Scholar] [CrossRef]
- Huntley, B. How Plants Respond to Climate Change: Migration Rates, Individualism and the Consequences for Plant Communities. Ann. Bot. 1991, 67, 15–22. Available online: http://www.jstor.org/stable/42758387 (accessed on 17 November 2023). [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Jump, A.S.; Peñuelas, J. Running to stand still: Adaptation and the response of plants to rapid climate change. Ecol. Lett. 2005, 8, 1010–1020. [Google Scholar] [CrossRef]
- Wilson, E.O.; MacArthur, R.H. The Theory of Island Biogeography; Princeton University Press: Princeton, NJ, USA, 1967; Volume 1. [Google Scholar]
- Honnay, O.; Endels, P.; Vereecken, H.; Hermy, M. The role of patch area and habitat diversity in explaining native plant species richness in disturbed suburban forest patches in northern Belgium. Divers. Distrib. 1999, 5, 129–141. [Google Scholar] [CrossRef]
- Couvreur, M.; Christiaen, B.; Verheyen, K.; Hermy, M. Large herbivores as mobile links between isolated nature reserves through adhesive seed dispersal. Appl. Veg. Sci. 2004, 7, 229–236. [Google Scholar] [CrossRef]
- Kowarik, I. Some responses of flora and vegetation to urbanization in Central Europe. In Urban Ecology: Plants and Plant Communities in Urban Environments; Sukopp, H., Hejny, S., Kowarik, I., Eds.; SPB Academic Publishing: Amsterdam, The Netherlands, 1990; pp. 45–74. [Google Scholar]
- Ranta, P.; Viljanen, V. Vascular plants along an urban-rural gradient in the city of Tampere, Finland. Urban Ecosyst. 2011, 14, 361–376. [Google Scholar] [CrossRef]
- Czortek, P.; Pielech, R. Surrounding landscape influences functional diversity of plant species in urban parks. Urban For. Urban Green. 2020, 47, 126525. [Google Scholar] [CrossRef]
- Cain, M.C.; Damman, H.; Muir, A. Seed dispersal and the Holocene migration of woodland herbs. Ecol. Monogr. 1998, 68, 325–347. [Google Scholar] [CrossRef]
- Chaudhry, S.; Sidhu, G.P.S. Climate change regulated abiotic stress mechanisms in plants: A comprehensive review. Plant Cell Rep. 2021, 41, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Waring, G.L.; Cobb, N.S. The impact of plant stress on herbivore population dynamics. Insect-Plant Interact. 1992, 4, 167–226. [Google Scholar]
- Ahmad, P.; Prasad, M.N.V. (Eds.) Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Springer Science & Business Media: New York, NY, USA, 2011. [Google Scholar]
- Grime, J.P. Plant Strategies and Vegetation Processes Vegetation Processes; John Wiley & Sons, Limited: Chichester, UK, 1979. [Google Scholar]
- Sandvik, S.M.; Totland, Ø. Short-term effects of simulated environmental changes on phenology, reproduction, and growth in the late-flowering snowbed herb Saxifraga stellaris L. Ecoscience 2000, 7, 201–213. [Google Scholar] [CrossRef]
- Shuman, B.; Newby, P.; Huang, Y.; Webb, T. Evidence for the close climatic control of New England vegetation history. Ecology 2004, 85, 1297–1310. [Google Scholar] [CrossRef]
- Van Der Veken, S.; Bossuyt, B.; Hermy, M. Climate gradients explain changes in plant community composition of the forest understory: An extrapolation after climate warming. Belg. J. Bot. 2004, 137, 55–69. [Google Scholar]
- Moszkowicz, Ł.; Krzeptowska-Moszkowicz, I. Impact of the public parks location in the city on the richness and diversity of herbaceous vascular plants on the example of Krakow Southern Poland. Plants Urban Areas Landsc. 2020, 98–103. [Google Scholar] [CrossRef]
- Hamrick, J.L. Response of forest trees to global environmental changes. For. Ecol. Manag. 2004, 197, 323–335. [Google Scholar] [CrossRef]
- Breshears, D.; Huxman, T.; Adams, H.; Zou, C.; Davison, J. Vegetation synchronously leans upslope as climate warms. Proc. Natl. Acad. Sci. USA 2008, 105, 11591–11592. [Google Scholar] [CrossRef]
- Kapfer, J.; Hédl, R.; Jurasinski, G.; Kopecký, M.; Schei, F.H.; Grytnes, J.A. Resurveying historical vegetation data—Opportunities and challenges. Appl. Veg. Sci. 2017, 20, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Williams, S.E.; Shoo, L.P.; Isaac, J.L.; Hoffmann, A.A.; Langham, G. Towards an integrated framework for assessing the vulnerability of species to climate change. PLoS Biol. 2008, 6, e3252008. [Google Scholar] [CrossRef] [PubMed]
- Moritz, C.; Agudo, R. The Future of Species Under Climate Change: Resilience or Decline? Science 2013, 341, 504–508. [Google Scholar] [CrossRef] [PubMed]
- Kondracki, J. Geografia Regionalna Polski. Wydanie 2 Poprawione; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2000. [Google Scholar]
- Kornaś, J.; Medwecka-Kornaś, A. Szata roślinna Krakowa. Folia Geogr. Ser. Geogr.-Phys. 1974, 8, 153–169. [Google Scholar]
- Wójcik, A. Budowa geologiczna.—W. In Atlas Kampusu 600-Lecia Odnowienia Uniwersytetu Jagiellońskiego; Jędrychowski, I., Ed.; Instytut Geografi i i Gospodarki Przestrzennej UJ: Kraków, Poland, 2007. [Google Scholar]
- Trzcińska-Tacik, H. Flora synantropijna Krakowa. Zesz. Nauk. UJ Kraków Rozpr. 1979, 32, 1–249. [Google Scholar]
- Dubiel, E. Mapa roślinności rzeczywistej miasta Krakowa [Map of actual vegetation of the city of Cracow]. Zesz. Nauk. UJ Prace Bot. 1991, 22, 121–133. [Google Scholar]
- Dubiel, E.; Szwagrzyk, J. (Eds.) Atlas Roślinności Rzeczywistej Krakowa; Urząd Miasta Krakowa, Wydział Kształtowania Środowiska: Kraków, Poland, 2008. [Google Scholar]
- Berdau, F. Flora Cracoviensis; Typis, C. R. Universitatis Jagiellonicae: Cracoviae, Poland, 1859; s. viii + 448. [Google Scholar]
- Raciborski, M. Zmiany zaszłe we florze okolic Krakowa w ciągu ostatnich lat dwudziestu pięciu pod względem roślin dziko rosnących. Spraw. Komis. Fizjogr. 1884, 18, 99–126. [Google Scholar]
- Hess, M. Klimat Krakowa. Folia Geographica. Ser. Geogr.-Phys. 1974, 8, 45–102. [Google Scholar]
- Matuszko, D. (Ed.) Klimat Krakowa w XX Wieku; Instytut Geografii i Gospodarki Przestrzennej Uniwersytetu Jagiellońskiego: Kraków, Poland, 2007; pp. 1–251. [Google Scholar]
- Bokwa, A. Wieloletnie Zmiany Struktury Mezoklimatu Miasta na Przykładzie Krakowa; Instytut Geografii i Gospodarki Przestrzennej Uniwersytetu Jagiellońskiego: Kraków, Poland, 2010; pp. 1–258. [Google Scholar]
- Piotrowicz, K. Sezonowa i Wieloletnia Zmienność Typów Pogody w Krakowie; Instytut Geografii i Gospodarki Przestrzennej Uniwersytetu Jagiellońskiego: Kraków, Poland, 2010; pp. 1–314. [Google Scholar]
- Ellenberg, H. Zeigerwerte der Gefeaßpflanzen Mitteleuropas; Scripta Geobotanica: Göttingen, Germany, 1974; Volume 9, pp. 1–97. [Google Scholar]
- Ellenberg, H. Zeigerwerte der Gefeaßpflanzen Mitteleuropas. 2; Auflage. Scripta Geobotanica: Göttingen, Germany, 1979; Volume 9, pp. 1–122. [Google Scholar]
- Frank, D.; Klotz, S. Biologisch-Ökologische Daten zur Flora der DDR (Biological-Ecological Data on the Flora of the GDR); Wissenschaftliche Beiträge der Martin-Luther-Universität Halle-Wittenberg: Halle, Germany, 1988; pp. 1–168. [Google Scholar]
- Zarzycki, K.; Trzcinska-Tacik, H.; Rózanski, W.; Szalag, Z.; Wolek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plants of Vascular Plants of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002. [Google Scholar]
- Persson, S. Ecological Indicator Values as an Aid in the Interpretation of Ordination Diagrams. J. Ecol. 1981, 69, 71–84. [Google Scholar] [CrossRef]
- Ter Braak, C.J.F.; Gremmen, N.J.M. Ecological amplitudes of plant species and the internal consistency of Ellenberg’s indicator values for moisture. Vegetatio 1987, 69, 79–87. [Google Scholar] [CrossRef]
- Berg, C.; Welk, E.; Jaeger, E. Revising Ellenberg’s indicator values for continentality based on global vascular plant species distribution. Appl. Veg. Sci. 2017, 20, 482–493. [Google Scholar] [CrossRef]
- Roo-Zielińska, E. Porównanie europejskich skal ekologicznych liczb wskaźnikowych w ocenie środowiska fizycznogeograficznego na podstawie charakterystycznych gatunków roślin wrzosowisk i ubogich muraw bliźniczkowych z klasy Nardo-Callunetea= A comparison of European scales of ecological indicator values in assessing the natural environment on the basis of species characteristic for heaths and poor grasslands of class Nardo-Callunetea. Przegląd Geogr. 2018, 90, 403–434. [Google Scholar] [CrossRef]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulissen, D. Zeigerwerte von Pflanzen in Mitteleuropa; Scripta Geobotanica: Göttingen, Germany, 1991; Volume 18, pp. 1–248. [Google Scholar]
- Kornaś, J. Geograficzno-historyczna klasyfikacja roślin synantropijnych. Mat. Zakł. Fitosoc. Stos. UW 1968, 25, 33–41. [Google Scholar]
- Chmiel, J. Zróżnicowanie Przestrzenne Flory Jako Podstawa Ochrony Przyrody w Krajobrazie Rolniczym; Bogucki Wydawnictwo Naukowe: Poznañ, Poland, 2006; pp. 1–250. [Google Scholar]
- Zając, A.; Zając, M. A tentative list of segetal and ruderal apophytes in Poland.—Prowizoryczna lista apofitów segetalnych i ruderalnych w Polsce. Zesz. Nauk. Uniw. Jagiellońskiego. Pr. Bot. 1992, 24, 7–23. [Google Scholar]
- Zając, A.; Zając, M. Atlas Rozmieszczenia Roślin Naczyniowych w Polsce: Dodatek/Distribution Atlas of Vascular Plants in Poland: Appendix; Instytut Botaniki Uniwersytetu Jagiellońskiego: Kraków, Poland, 2019. [Google Scholar]
- Zarzycki, K.; Mirek, Z. Red list of plants and fungi in Poland. In Czerwona Lista Roślin i Grzybów Polski; Wojewoda, W., Szeląg, Z., Eds.; Instytut Botaniki im. W. Szafera PAN: Kraków, Poland, 2006. [Google Scholar]
- Raunkiær, C. Planterigets Livsformer og deres Betydning for Geografien; Gyldendalske Boghandel-Nordisk Forlag: København and Kristiania, Denmark, 1907; pp. 1–132. [Google Scholar]
- Matuszkiewicz, W. Przewodnik do Oznaczania Zbiorowisk Roślinnych Polski; PWN: Warszawa, Poland, 2004. [Google Scholar]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Haeuser, E.; Dawson, W.; van Kleunen, M. The effects of climate warming and disturbance on the colonization potential of ornamental alien plant species. J. Ecol. 2017, 105, 1698–1708. [Google Scholar] [CrossRef]
- Turbelin, A.; Catford, J.A. Invasive plants and climate change. In Climate Change; Elsevier: Amsterdam, The Netherlands, 2021; pp. 515–539. [Google Scholar] [CrossRef]
- Chelli, S.; Wellstein, C.; Campetella, G.; Canullo, R.; Tonin, R.; Zerbe, S.; Gerdol, R. Climate change response of vegetation across climatic zones in Italy. Clim. Res. 2017, 71, 249–262. [Google Scholar] [CrossRef]
- Brusca, R.C.; Wiens, J.F.; Meyer, W.M.; Eble, J.; Franklin, K.; Overpeck, J.T.; Moore, W. Dramatic response to climate change in the Southwest: Robert Whittaker’s 1963 Arizona Mountain plant transect revisited. Ecol. Evol. 2013, 3, 3307–3319. [Google Scholar] [CrossRef]
- Sætersdal, M.; Birks, H.J.B. A comparative ecological study of Norwegian mountain plants in relation to possible future climatic change. J. Biogeogr. 1997, 24, 127–152. [Google Scholar] [CrossRef]
- Parmesan, C.; Hanley, M.E. Plants and climate change: Complexities and surprises. Ann. Bot. 2015, 116, 849–864. [Google Scholar] [CrossRef] [PubMed]
- Thuiller, W.; Richardson, D.M.; Midgley, G.F. Will Climate Change Promote Alien Plant Invasions? In Biological Invasions; Nentwig, W., Ed.; Ecological Studies 193; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar] [CrossRef]
- Liu, Y.; Oduor, A.M.O.; Zhang, Z.; Manea, A.; Tooth, I.M.; Leishman, M.R.; Xu, X.; van Kleunen, M. Do invasive alien plants benefit more from global environmental change than native plants? Glob. Chang. Biol. 2017, 23, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Matlack, G.R. Plant species migration in mixed-history forest landscape in eastern North America. Ecology 1994, 75, 1491–1502. [Google Scholar] [CrossRef]
- Brunet, J.; Oheimb, G. Migration of vascular plants to secondary woodlands in souther Sweden. J. Ecol. 1998, 86, 429–438. [Google Scholar] [CrossRef]
- Orczewska, A.; Ferens, M. Migration of herb layer species into the poorest post-agricultural pine woods adjacent to ancient Pine forests. Pol. J. Ecol. 2011, 59, 75–85. [Google Scholar]
- Moszkowicz, L. Distribution of vascular plant species in woodland patches of Ojców National Park (southern Poland) in relation to seed dispersal. Acta Soc. Bot. Pol. 2016, 85, 3484. [Google Scholar] [CrossRef][Green Version]
- Kostrakiewicz-Gierałt, K.; Gmyrek, K.; Pliszko, A. The Effect of the Distance from a Path on Abiotic Conditions and Vascular Plant Species in the Undergrowth of Urban Forests and Parks. Int. J. Environ. Res. Public Health 2022, 19, 5621. [Google Scholar] [CrossRef]
- Krzeptowska-Moszkowicz, I.; Moszkowicz, Ł. Selected problems of Ailanthus altissima (Mill.) Swingle presence in urban spaces: The case of the city centre of Kraków. In Proceedings of the Plants Urban Areas Landscape International Scientific Conference, Nitra, Slovakia, 14–15 May 2014. [Google Scholar] [CrossRef]
- Lamsal, P.; Kumar, L.; Aryal, A.; Atreya, K. Invasive alien plant species dynamics in the Himalayan region under climate change. Ambio 2018, 47, 697–710. [Google Scholar] [CrossRef]
- Peterken, G.F. Natural Woodland. Ecology and Conservation in Northern Temperate Regions; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Kim, J.D.; Park, G.E.; Lim, J.-H.; Yun, C.W. Vegetation Type Classification and Endemic-Rare Plants Investigation in Forest Vegetation Area Distributed by Vulnerable Species to Climate Change, Mt. Jiri. J. Korean Soc. For. Sci. 2018, 107, 113–125. [Google Scholar] [CrossRef]
- Austin, M.P. Continuum Concept, Ordination Methods, and Niche Theory. Annu. Rev. Ecol. Syst. 1985, 16, 39–61. Available online: http://www.jstor.org/stable/2097042 (accessed on 9 December 2021). [CrossRef]
- Silvertown, J. Plant coexistence and the niche. Trends Ecol. Evol. 2004, 19, 605–611. [Google Scholar] [CrossRef]
- Anacker, B.L.; Gogol-Prokurat, M.; Leidholm, K.; Schoenig, S. Climate change vulnerability assessment of rare plants in California. Madroño 2013, 60, 193–210. [Google Scholar] [CrossRef]
- Chapin, F.S., III; Shaver, G.R.; Giblin, A.E.; Nadelhoffer, K.J.; Laundre, J.A. Responses of arctic tundra to experimental and observed changes in climate. Ecology 1995, 76, 694–711. [Google Scholar] [CrossRef]
- Schmiedel, U.; Dengler, J.; Etzold, S. Vegetation dynamics of endemic-rich quartz fields in the Succulent Karoo, South Africa, in response to recent climatic trends. J. Veg. Sci. 2012, 23, 292–303. [Google Scholar] [CrossRef]
- Halevy, A.H. Recent advances in control of flowering and growth habit of geophytes. Acta Hortic. 1990, 266, 35–42. [Google Scholar] [CrossRef]
- Eppich, B.; Dede, L.; Ferenczy, A.; Ágnes, G.; Horváth, L.; Isépy, I.; Priszter, S.; Hufnagel, L. Climatic effects on the phenology of geophytes. Appl. Ecol. Environ. Res. 2009, 7, 253–266. [Google Scholar] [CrossRef]
- Borochov, A.; Spiegelstein, H.; Weiss, D. Dormancy and storage of geophytes. Acta Hortic. 1997, 430, 405–410. [Google Scholar] [CrossRef]
- Laiviņš, M. Environmental changes related dynamics of the number of sites of rare indigenous. Balt. For. 1990, 3, 9–18. [Google Scholar]
- Hill, M.O.; Roy, D.B.; Thompson, K. Hemeroby, urbanity and ruderality: Bioindicators of disturbance and human impact. J. Appl. Ecol. 2002, 39, 708–720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).