Abstract
Microbial fuel cells (MFCs) have shown great advantages in electricity production, heavy metal removal, and energy recovery. However, the impact and mechanism of conflicting effects of numerous electron acceptors on heavy metal removal remain unknown. The effects of different initial heavy metal concentrations, cathodic dissolved oxygen, and electrode materials on the electricity generation and heavy metal removal efficiencies of Cu(II) and Cr(VI) were investigated in this study. When the initial concentration of Cr(VI) increased from 10 mg/L to 150 mg/L, the maximum voltage, coulomb efficiency, and maximum power density declined from 99 to 44 mV, 28.63% to 18.97%, and 14.29 to 0.62 mW/m2, and the removal efficiencies of Cu(II) and Cr(VI) decreased dramatically from 98.34% and 99.92% to 67.09% and 37.06%, respectively. Under anaerobic cathodic conditions, the removal efficiency and removal rate of Cu(II) and Cr(VI) were lower than those under aerobic conditions. When the cathode electrode was titanium sheet and graphite plate, the coulomb efficiency and maximum power density increased to 38.18%, 50.71%, 33.95 mW/m2, and 62.23 mW/m2. The removal efficiency and removal rates of Cu(II) and Cr(VI) were significantly increased to 98.09%, 86.13%, and 0.47, 0.50 mg/(L h) with a graphite plate, respectively. The pH of the cathode varied considerably greater as the MFC current increased. Cu(II) and Cr(VI) were removed and reduced to elemental Cu, Cu2O, and its oxides as well as Cr(OH)3 and Cr2O3 precipitates on the cathode electrode by cathodic bioelectrochemical reduction.
1. Introduction
Large volumes of heavy metal effluent comprising chromium and copper are discharged by the electroplating, leather tanning, and textile sectors, posing harm to the ecological environment and human health by polluting groundwater, soil, and other media [1,2]. In recent decades, various methods, such as chemical precipitation, surface adsorption, and membrane filtration, have been used to treat heavy metal wastewater [3]. However, these traditional methods are prone to secondary pollution, incur high costs, and are non-sustainable. Hence, there is an urgent need to develop a sustainable method with low energy consumption and high removal efficiency for the treatment of heavy metal wastewater. Microbial fuel cells (MFCs), which can oxidize organic matter and reduce electron acceptors (usually O2) have attracted increasing attention in removing organic matter and heavy metals from wastewater in recent years [4,5].
According to some studies, MFC can remove and recover heavy metals from wastewater and generate electricity simultaneously [6,7,8]. Heavy metals with high redox potential can act as electron acceptors instead of oxygen, accepting electrons directly from the cathode. In other words, heavy metals are reduced and eliminated at the cathode by the bioelectrochemical reduction reaction [9,10]. Wang et al. found that Fe(III) can lower the cathodic diffusion internal resistance of Cr(VI) and its reduction overpotential. Fe(III) can also act as an electron mediator to transfer electrons from the cathode to Cr(VI) rather than directly from the cathode to achieve reduction [11]. However, catholyte pH had a significant effect on the reduction of heavy metals when Cu(II) and Cr(VI) were present in the cathode. At pH > 4, Cu(II) and Cr(VI) were reduced simultaneously, and the reduction efficiency of Cr(VI) and power density decreased from 63% to 18% and 4.4 to 1.1 mA/m2, respectively, when the Cu(II) concentration increased from 50 to 500 mg/L [12]. After 240 h, the reduction efficiencies as electron acceptors were 67.9% and 75.4%, respectively, with a maximum power density of 970.2 mW/m2 [13]. It was determined that when several electron acceptors are present in the cathode, the performance of electricity generation and removal efficiency, as well as the removal mechanism, are not yet obvious.
To further promote the treatment effect of MFC on heavy metals, researchers have applied various electrode materials to treat heavy metal wastewater [14]. Carbonaceous materials (such as carbon rods, carbon felt, and carbon fiber) with good electrical conductivity and physical and chemical stability are widely used as cathode materials [15]. Li et al. investigated the effects of nickel foam, stainless steel, and carbon cloth as cathode materials on the removal of Cr(VI) and Pb(II). The results revealed that nickel foam had the fastest Cr(VI) reduction rate (1.72 g/(m3 h)), followed by stainless steel ((1.47 g/(m3 h)) and carbon cloth (1.32 g/(m3 h)) [16]. To improve the electricity generation performance, the cathode material was modified with platinum, a highly active catalyst that can lower the cathodic reaction activation energy and thus increase the cathodic reaction rate [17]. Moon et al. used a platinum-supported graphite rod as the cathode, with a maximum power density of 150 mW/m2, which is three times that of a pure graphite electrode [18]. However, the effectiveness of electricity production using stainless steel, titanium sheets, and graphite plates as cathode electrodes, as well as heavy metal removal processes in wastewater, must be thoroughly investigated.
In summary, MFC can achieve good performance in the generation of electricity as well as the removal and recovery of heavy metals. Moreover, it demonstrates the advantages of energy and resource reuse that distinguish MFC from other conventional treatment technologies. However, many issues remain in the actual treatment process, such as electron competition between oxygen and heavy metals and heavy metals in the cathode. Additionally, the removal effect, cathodic reduction rate, and mechanism are also unclear. Meanwhile, the cathodic reaction and reduction rate of heavy metals at the cathode still require further clarification under anaerobic cathodic conditions. Therefore, a dual-chamber MFC was used to treat mixed heavy metal wastewater with Cu(II) and Cr(VI). The removal characteristics of two heavy metals with and without dissolved oxygen at the cathode were investigated in this study, as were the effects of different initial heavy metal concentrations and cathode electrode materials on the performance of electricity generation and heavy metal reduction, and the products on the cathode were detected using scanning electron microscopy, linear scanning voltammetry, and electrochemical impedance measurements.
2. Materials and Methods
2.1. MFC Construction, Inoculation, and Operation
The dual-chamber MFC was made of organic glass with an effective volume of 125 cm3 for both the cathode and anode chambers and was separated using a proton-exchange membrane (PEM, NafionTM 117, Dupont, Wilmington, DE, USA) (Figure 1).
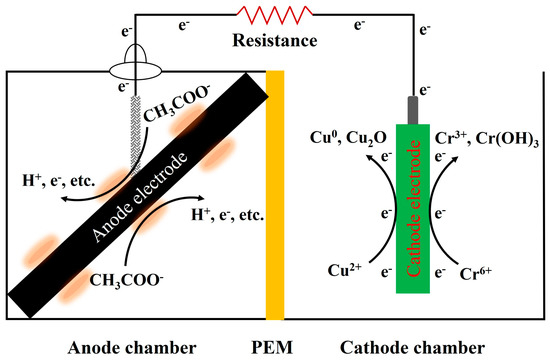
Figure 1.
The schematic diagram of dual−chamber MFC for heavy metals reduction.
Carbon felt and stainless steel were used as the anode and cathode materials, respectively. The carbon felts were placed diagonally in a square anode chamber with a surface area of 35.35 cm2. The cathode electrodes were inserted vertically into the cathode chamber with a surface area of 25.00 cm2. The electrodes were connected using copper wire with an external resistor of 200 Ω. Pretreated, concentrated anaerobic sludge from an urban wastewater plant was added to the anode chamber and supplemented with a certain amount of nutrient solution containing sodium acetate (1.0 g/L). The nutrient solution consisted of (in g/L) KCl, 0.78; NaCl, 0.58; KH2PO4, 0.68; K2HPO4 0.8; MgSO4∙7H2O, 0.1; NH4Cl, 0.28; CaCl2∙2H2O, 0.1; and 1 mL of trace elements [19,20]. Catholytes containing Cu(II) and Cr(VI) were prepared using CuCl2 and K2Cr2O7, respectively, at an initial pH of 3.
The PEM was heated in hydrogen peroxide at 80.0 °C for 1 h, soaked in distilled water for 30 min, then heated in 5.0% (w/v) dilute sulfuric acid at 80.0 °C for 1 h, and again soaked in distilled water for 30 min. The carbon felt was soaked in a 1 mol/L NaOH solution for 30 min, washed with distilled water, soaked in a 1 mol/L HCl solution for 30 min, washed with distilled water, and dried at 105 °C. Treated proton-exchange membranes and carbon felt are prepared to construct the dual-chamber MFCs.
2.2. Experiment Design
To investigate the effect of the initial heavy metal concentrations on the performance of electricity generation and removal effectiveness when multiple heavy metals are present in the catholyte, the initial concentration of Cu(II) was fixed at 100 mg/L, while that of Cr(VI) was fixed at 10, 30, 60, 100, and 150 mg/L in the catholyte and labeled as MFC-Cr10, MFC-Cr30, MFC-Cr60, MFC-Cr100, and MFC-Cr150, respectively. To further analyze the effect of cathodic dissolved oxygen on the performance of the MFC, a cathodic anaerobic group was set up and labeled as MFC-An, in which the concentrations of Cu(II) and Cr(VI) were 100 mg/L. The MFCs were set up with stainless steel sheets as the cathode electrodes and an external resistor of 200 Ω. The open circuit was the control group and was labeled as CK. Finally, the effect of the cathodic electrode material (titanium sheet and graphite plate) on the heavy metal removal efficiency was investigated and labeled MFC-Ti and MFC-Gr, respectively. The resistance coefficients of carbon felt, stainless steel, titanium sheet, and graphite plate were 0.18–0.22, 1.0 × 10−6, 6.0 × 10−7, and 1.1–1.3 × 10−7 Ω·m. The catholyte and external resistor were the same as those of MFC-Cr100. All the MFC devices were placed in a biological incubator at 30 °C.
2.3. Analytical Methods and Calculations
2.3.1. Electrical Properties of MFCs
The voltage of the MFCs was measured using a data acquisition system (DAM3055, Beijing ART Technology, Beijing, China) at 10 min intervals, and the current and power were calculated according to Ohm’s law. The polarization and power density curves of the MFCs were measured using the static method: the external resistor was adjusted from 100,000 to 10 Ω step-by-step, and the stabilized voltage across the resistor was recorded. The polarization and power density curves were plotted with the current density as the horizontal coordinate and the voltage and power density as the vertical coordinates. The internal resistance was calculated by the linear region of the polarization curve. The chemical oxygen demand (COD) of the nutrient solution containing sodium acetate from the anode chamber was analyzed by a wastewater photometer (HACH, DR 2800). The Coulombic efficiency (CE) of the anode was calculated using Equation (1).
where denotes the current (A), represents time (s), denotes the molecular weight of oxygen (32 g/mol), is the Faraday constant (96,485 C·mol−1), represents the number of transferred electrons (4 mol/mol), represents the amount of change in anode COD value (g/L), represents the effective volume of the anode (L).
After the experiment was completed, the cathode was tested using cyclic voltammetry at a scanning speed of 0.05 mV/s, using platinum wire as the counter electrode and Ag/AgCl as the reference electrode and the cathode as the working electrode.
2.3.2. Concentration and Morphology of Heavy Metals
The copper and chromium samples were collected and immediately filtered through a 0.45 µm filter membrane, and these concentrations in the catholyte were determined by flame atomic absorption spectrometry (Zeenit700P, Analytik Jena, Jena, Germany) according to Chinese standards (GB 7475-87 and HJ757-2015) and the recovery of chromium and copper is 85–115% and 94–106%. The morphology and elemental composition of the products on the cathode surface were analyzed by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDX) (JSM-6700F, JEOL, Akishima, Japan).
where and denote the copper or total chromium concentrations at time zero and time, respectively.
The results were analyzed using an analysis of variance (ANOVA, p = 0.05) procedure and the multiple comparisons were performed by Duncan’s new multiple range method by SAS 8.0, and all the figures were plotted by Origin 2018.
3. Results and Discussion
3.1. The Performance of Electricity Generation
The performance of MFCs in terms of electricity generation under different conditions is shown in Figure 2.
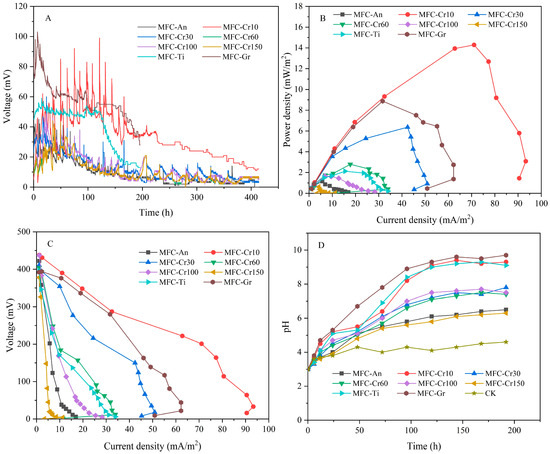
Figure 2.
The performance of MFCs in terms of electricity generation under different conditions: (A) voltage, (B) power density, (C) polarization curve, and (D) pH at the cathode.
The voltage of the MFCs gradually decreased from 99 mV to 44 mV (Figure 2A) as the initial concentration of Cr(VI) in the catholyte increased from 10 mg/L to 150 mg/L. This result indicates that an increase in Cr(VI) concentration could suppress the voltage of the MFC. For MFC-Cr10, the maximum power density was 14.29 mW/m2, which was 2.24–23.06 times higher than that of the other four groups. The maximum power density was only 0.62 mW/m2 when the Cr(VI) concentration was 150 mg/L (Figure 2B). In a typical air-cathode MFC, heavy metals with high redox potentials (e.g., Cu(II) and Cr(VI)) can act as electron acceptors in addition to O2, to obtain electrons required to complete the cathodic reaction and achieve reduction [21]. Therefore, the anaerobic cathodic condition would limit the performance of MFCs. In this study, the average voltage and maximum power density of MFC-An were 9.04 mV and 1.15 mW/m2 lower than the 13.17 mV and 1.66 mW/m2 of the MFC-Cr100. When both oxygen and copper are electron acceptors, the presence of oxygen can significantly improve the performance of electricity generation [22]. This can be explained by the result that shows that cathodic potential is higher under aerobic conditions than under anaerobic conditions. Furthermore, when paired with high anodic potential, the MFC-Cr group outperformed the MFC-An group in terms of power generation.
In addition, when the initial concentration of Cr(VI) increased from 10 to 150 mg/L, the polarization of the MFC gradually increased, and the ohmic internal resistance increased from 3.92 to 81.01 Ω, which limited electricity generation (Table 1).

Table 1.
The performance of MFCs in terms of current, coulombic efficiency, and ohmic internal resistance.
Simultaneously, Cr(VI) is reduced to Cr(III) and attached to the cathode electrode in the form of Cr(OH)3, increasing the internal cathode diffusion resistance, thereby making it more difficult for Cr(VI) to obtain electrons from the cathode electrode. The cathode efficiency would deteriorate due to the deposition of Cr(OH)3 [13]. The lack of dissolved oxygen at the cathode also leads to a loss of mass transfer; thus, the electricity generation performance decreases significantly with an increase in the initial heavy metal concentration [23]. Moreover, when the cathode was under anaerobic conditions and only heavy metals were used as electron acceptors, the performance of electricity generation was limited. The Coulomb efficiency is used to represent the ratio of electrons used to generate electricity to those supplied by organic matter. However, a considerable amount of the organic matter is consumed by microbial growth or for other reasons, which often lowers coulomb efficiency. The coulomb efficiency declined from 28.63% to 18.97% when the Cr(VI) concentration grew from 10 to 150 mg/L, whereas it was only 19.21% when the cathode was under anaerobic conditions (MFC-An). Meanwhile, we determined that the coulombic efficiency of MFC-Gr and MFC-Ti were 50.71% and 38.18%, much greater than that of MFC-Cr100 (21.41%), indicating that the coulombic efficiency may be significantly enhanced by titanium and graphite. (Table 1). This phenomenon was attributed to a lack of electron acceptors under anaerobic conditions, which reduced the metabolism of extracellular respiration of the electroactive microorganisms and allowed other non-electroactive microorganisms to obtain more electrons in competition for electron donors, resulting in a decrease in coulombic efficiency.
Titanium and graphite have often been used as MFC electrodes because of their good electrical conductivities and stable physicochemical properties. The maximum voltage and maximum power density of MFC-Gr were 103 mV and 8.87 mW/m2, which is higher than the 48 mV and 1.66 mW/m2 of MFC-Cr100. However, the maximum voltage and power density of the MFC-Ti were 59 mV and 2.12 W/m2, respectively, and were not substantially different from those of the MFC-Cr100 (Figure 2). In terms of coulombic efficiencies, MFC-Gr and MFC-Ti were 29.30% and 16.77% higher, respectively, than MFC-Cr100 (Table 1). Graphite has a higher specific surface area than stainless steel and titanium flat, which provides sufficient reduction reaction sites. In addition, the carbon material was more effective in reducing oxygen, and the power density of MFC-Gr increased significantly. The interfacial impedance of stainless steel is 3–200 times higher than that of carbon-based electrodes, resulting in higher resistance to electron transport and higher internal resistance of MFCs [24]. In this study, the ohmic internal resistances of MFC-Gr and MFC-Ti were 6.47 Ω and 8.24 Ω, respectively, which were significantly lower than that of MFC-Cr100 at 19.45 Ω (Table 1). Porous electrode materials with larger specific surface areas perform better in terms of power production [25]. That was to say, lower chromium concentration, titanium sheet and graphite plate could significantly decrease the ohmic internal resistance of MFCs. Accordingly, the MFC-Cr10 yielded the highest current density of 93.35 mA/m2, while that of MFC-Cr150 was only 11.32 mA/m2. When the cathode electrodes were titanium sheet and graphite plate, the current density of MFC-Ti and MFC-Gr were significantly increased to 33.95 and 62.23 mA/m2, respectively, which was significantly higher than that of MFC-Cr100 (28.29 mA/m2) (Table 1).
Notably, pH is one of the fundamental factors in the electrochemical reaction. Microorganisms oxidize organic substances to generate electrons and protons () (Figure 1). The rate at which protons are transferred to the cathode is slower than that are produced at the anode and consumed at the cathode. As a result, the pH of the solution at the anode decreases, and that at the cathode increases [26]. Moreover, there is a strong positive correlation between the pH at the cathode and the current generation. The higher the current, the stronger the pH variation and alkalinity [10]. In this study, the average current of the MFC-Cr10 was 0.17 mA, which was 3.65 times and 3.28 times higher than that of the MFC-An and MFC-Cr150, respectively. The pH was increased to 5.2 and 8.2 after 24 h and 96 h and finally exceeded 9.1 after 120 h (MFC-Cr10). Therefore, pH remained slightly acidic with values of 6.5 and 6.3 at the MFC-An and MFC-Cr150, respectively. Additionally, the pH was just 7.3–7.8 and the average current was 0.061–0.066 mA, significantly lower than that of MFC-Cr10 when the concentration of Cr6+ was 30–100 mg/L. Hence, the pH of the cathode varied considerably greater as the MFC current increased.
3.2. Heavy Metals Removal in MFCs
The removal of both heavy metals was affected when Cu(II) and Cr(VI) coexisted in the catholyte. When the Cr(VI) concentration increased to 150 mg/L, the Cu(II) removal efficiency decreased from 98.34% to 67.09%, with a residual of 33.24 mg/L. This indicated that an increase in Cr(VI) concentration inhibited Cu(II) removal in the MFC. The high Cr(VI) concentration competed with Cu(II) for the surface reaction sites, and Cr(OH)3 or Cr2O3 precipitates attached to the cathode occupied the reaction sites, which exacerbated the reduction reaction. However, the removal efficiency and removal rate of Cu(II) were comparable in the MFC-Cr10, MFC-Cr30, MFC-Cr60, and MFC-Cr100 groups, with average values of 95.03% and 0.21 mg/(L h), respectively. Under anaerobic conditions, the removal efficiency and removal rates of Cu(II) were 69.17% and 0.15 mg/(L h), respectively, which were significantly lower than those of the MFC-Cr groups with dissolved oxygen. In other words, dissolved oxygen in the cathode has an extremely important positive effect on heavy metal wastewater treatment [27]. In addition, the experimental period lasted only 208 h, with Cu(II) removal efficiencies reaching 88.79% and 98.09%, respectively, when the cathode electrode materials were titanium and graphite plates. The corresponding removal rates were 0.43 and 0.47 mg/(L h), which were 2.24, 3.13, and 3.13 times higher than those of the MFC-Cr10, MFC-Cr150 and MFC-An groups, respectively (Figure 3A,C). MFC-Ti and MFC-Gr have superior electricity-generating performance, which means that they have better removal efficacy.
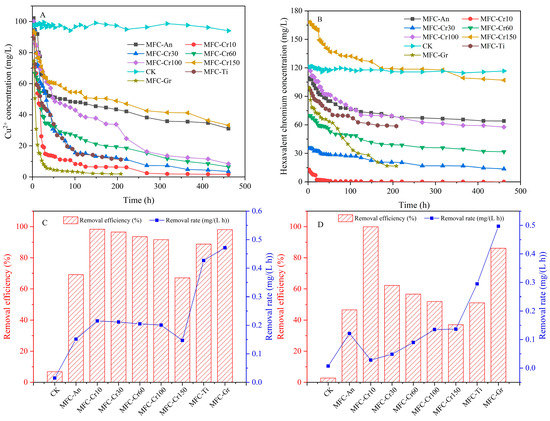
Figure 3.
The removal of heavy metals: (A) Cu concentration, (B) hexavalent chromium concentration, (C) removal efficiency and rate of Cu, and (D) removal efficiency and rate of Cr.
The removal efficiency of Cr(VI) decreased significantly from 99.92% to 37.06% when the initial concentration of Cr(Ⅵ) increased from 10 to 150 mg/L, while the removal rate increased from 0.03 to 0.14 mg/(L h). A higher initial concentration causes Cr(VI) to gain more electrons in competition with Cu(II), resulting in a significant increase in the amount of Cr(VI) removed per unit of time. However, the reduction reaction rate of Cu(II)-gaining electrons was faster than that of Cr(VI), and the removal efficiency of Cr(VI) was significantly reduced. When the cathode was in an anaerobic condition, the removal efficiency of Cr(VI) further decreased to 46.63% with a removal rate of 0.12 mg/(L h). From this perspective, the removal pattern of Cr(VI) was the same as that of Cu(II), that is, a higher initial concentration and lower removal efficiency. However, the removal rate increased with an increase in the initial concentration. Furthermore, the cathode pH increases from acidic to neutral and alkaline, which also inhibits Cr(VI) reduction, thus prolonging the reduction duration [28]. Although the removal efficiency of Cr(VI) was 51.13%, which was not significantly different from that of the MFC-Cr100, the experiment time was only 208 h, with a quicker removal rate of 0.29 mg/(L h) in the MFC-Ti group. When the electrode material was graphite, the removal efficiency and removal rate of Cr(VI) was 86.13% and 0.50 mg/(L h), which were 39.50%, 34.17%, and 4.09 and 3.67 times higher than those of the MFC-An and MFC-Cr100 groups, respectively. Cu(II), Cr(VI), and O2 can theoretically act as electron acceptors for the cathodic half-reaction (reduction reaction) when they are simultaneously in the catholyte. In an acidic solution, there are significant differences in the standard redox potentials of the three substances (, , ) [29]. Therefore, the three electron acceptors can be reduced by obtaining electrons from the cathode electrode in the order Cr(VI), O2, and Cu(II), whose reduction is thermodynamically favorable, allowing the electrons to flow spontaneously without the requirement for external power [30]. However, because the reduction reaction of Cu(II) was more rapid than that of Cr(VI) and the Cu(II) concentration was much higher than that of O2, the removal of Cu(II) was much higher than that of Cr(VI). It has been observed that Cr(VI) is reduced first because its redox potential is greater than that of V(V) when both are simultaneously present in the catholyte. When the Cr(VI) concentration gradually decreased and precipitated in the form of Cr(III), the redox potential of V(V) exceeded that of Cr(VI), and V(V) began to reduce the electron acceptor. When V(V) is completely consumed, Cr(VI) becomes the electron acceptor and continues to be reduced [13]. Therefore, both Cr(VI) and V(V) can be reduced as electron acceptors in MFC, and the competition between them will continue until one side is completely consumed. Meanwhile, Cr(OH)3 has a very small solubility product constant (6.3 × 10−31), and Cu(II) began to be reduced as an electron donor as soon as Cr(OH)3 precipitated on the cathode. However, the ohmic internal resistance significantly increases when insoluble copper reduction products, such as elemental copper or oxides, are attached to the cathode electrode [29]. In addition, the cathodic potential or voltage was not stabilized; thus, the reduction of Cu(II) and Cr(VI) was not constant, leading to a change in the electron acceptor during some periods. Moreover, competition between heavy metals with different redox potentials decreases the MFC efficiency [30]. In addition, Cr(VI) was reduced when the pH value of the cathode was below five indicating the acidic condition was beneficial to Cr(VI) reduction reaction. When the pH value of the cathode was alkaline, the reduction rate and power generation decreased significantly [31]. In a word, the Cr(VI) reduction process depended on the pH. Consequently, the removal of both Cu(II) and Cr(VI) decreased as the reaction progressed.
3.3. Cyclic Voltammetry Analysis and Reduction Products on the Cathode Electrode
To investigate the morphology and microscopic characteristics of the reduction products on the cathode surface, SEM−EDX was used to analyze the attached substances, and cyclic voltametric scanning of the cathode was performed at the end of the experiment to determine the degree of electrochemical reaction. The electrode surfaces of all MFC groups were covered with many circular substances, whereas those of the control group were smooth and had no substance coverage (Figure 4).
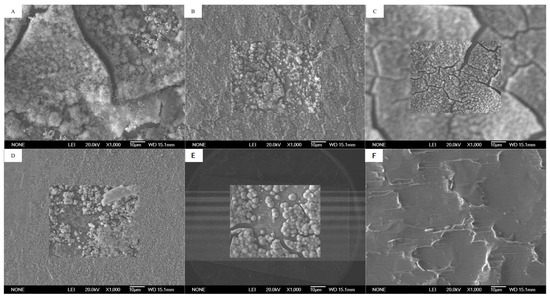
Figure 4.
SEM image (10 μm) of Cr and Cu adsorbed on the surface of cathode: (A) MFC-An, (B) MFC-Cr10, (C) MFC-Cr30, (D) MFC-Cr100, (E) MFC-Cr150, (F) CK.
Cu, Cr, and O were the main elements attached to the cathode (Table 1). The electrochemical reduction of Cu(II) and Cr(VI) was the most important process at the cathode [12,32,33]. Zhou et al. discovered that Cu(II) was reduced to elemental copper in two steps rather than directly gaining two electrons. Cu(II) gains one electron to produce Cu(I) (Cu2O), which then gains another electron to produce elemental copper [29]. In contrast, at pH > 2, Cr(VI) can directly gain electrons for reduction to Cr(III) in terms of Cr(OH)3 or Cr2O3 precipitated on the cathode electrode [31,34]. In this study, the weight percentage of chromium on the stainless steel increased from 19.04% to 24.34% as the chromium concentration increased from 10 to 150 mg/L, while that on the titanium sheet and graphite plate were even higher at 38.95% and 30.47% when the chromium concentration was 100 mg/L. The weight percentage of copper on the cathode electrode was in the range of 35.52–40.09% for all groups except on the titanium plate where the weight percentage of copper was lower at 27.79%. In addition, it was found that the weight percentage of oxygen increased with the chromium concentration from 19.42% to 29.99%, which indicated that more Cr(OH)3 and Cu2O were deposited on the cathode electrode. Therefore, it was inferred that the main products attached to the cathode were elemental Cu, Cu2O, and Cu oxides as well as Cr(OH)3 and Cr2O3. Two redox peaks were observed at potentials of −0.5 to 0.5 mV for all experimental groups. When dissolved oxygen was present in the cathode, the redox current was higher than that under anaerobic conditions (Figure 5).
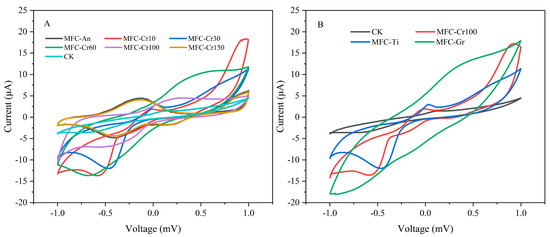
Figure 5.
Cyclic voltammograms (A) under different Cr(VI) initial concentration (10–150 mg/L) and (B) different cathode materials.
Hence, more electrons would be used for oxygen reduction than for Cu(II) and Cr(VI) reduction. Cr(OH)3 and Cr2O3 are deposited on the cathode, causing the cathodic efficiency to decrease [13]. It was observed that a higher initial Cr(VI) concentration, higher weight percentage, and higher atomic percentage (Table 2). The results demonstrated that more Cr(OH)3 or Cr2O3 was attached to the cathode, thereby leading to a decreasing trend in electricity generation as well as the removal efficiency of Cr(VI). The redox current of MFC-Gr (0.013 A) was significantly higher than that of MFC-Cr100 (0.002 A). This also explains why MFC-Gr has a higher removal efficiency and rate of Cu(II) and Cr(VI).

Table 2.
EDX energy spectrum element proportion.
4. Conclusions
In this study, the removal efficiency and electricity generation of dual-chamber MFCs were investigated under different conditions for treating heavy metal wastewater with Cu(II) and Cr(VI). The results revealed that lower chromium concentration, titanium sheet, and graphite plate could significantly decrease the ohmic internal resistance of MFCs. When the initial Cr(VI) concentration increased from 10 to 150 mg/L, the maximum voltage, coulomb efficiency, and maximum power density declined from 99 to 44 mV, 28.63% to 18.97%, and 14.29 to 0.62 mW/m2, respectively. The maximum power density under anaerobic conditions is about 1.15 mW/m2. The removal efficiencies of Cu(II) and Cr(VI) were 98.34% and 99.92%, respectively, in MFC-Cr10, which were much higher than those of MFC-Cr150 and MFC-An (67.09%, 37.06% and 69.17%, 46.63%, respectively). Titanium sheet and graphite plate greatly enhanced electricity generation and removal efficiency, with removal rates of Cu(II) and Cr(VI) of 0.43, 0.47 mg/(L h) and 0.29, 0.50 mg/(L h), respectively. Cu(II) and Cr(VI) were removed and recovered by cathodic bioelectrochemical reduction and precipitated on the cathode in the form of elemental copper, Cu2O, and oxides of copper, as well as Cr(OH)3 and Cr2O3. Therefore, it is necessary to pay attention to the interaction relationship between electron acceptors when applying MFC to treat wastewater containing multiple heavy metals.
Author Contributions
Conceptualization, H.W.; Data curation, Y.M.; Methodology, C.J.; Software, W.D.; Supervision, H.W.; Validation, J.L. and H.L.; Visualization, Z.W. and H.M.; Writing—original draft, H.W. and Y.L.; Writing—review & editing, D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (42107030, 52070156), Natural Science Basic Research Program of Shaanxi Province (2021JM-329, 2020JQ-617), Natural Science Foundation of Shaanxi Provincial Department of Education (20JK0783), Qinchuangyuan Project for the Team of Scientists and Engineers in Shaanxi Province of China (2022KXJ-115), and the APC was funded by 2107030 and 52070156.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Liu, S.-H.; Huang, W.-J.; Lin, C.-W.; Zhu, T.-J. Enhanced copper removal and bioelectricity generation in sediment microbial fuel cells through biostimulation and bioaugmentation. J. Clean. Prod. 2022, 350, 131458. [Google Scholar] [CrossRef]
- Matsena, M.T.; Chirwa, E.M.N. Hexavalent chromium-reducing microbial fuel cell modeling using integrated Monod kinetics and Butler-Volmer equation. Fuel 2022, 312, 122834. [Google Scholar] [CrossRef]
- Rajendran, S.; Priya, T.A.K.; Khoo, K.S.; Hoang, T.K.A.; Ng, H.-S.; Munawaroh, H.S.H.; Karaman, C.; Orooji, Y.; Show, P.L. A critical review on various remediation approaches for heavy metal contaminants removal from contaminated soils. Chemosphere 2022, 287, 132369. [Google Scholar] [CrossRef]
- Yu, Y.; Ali, J.; Yang, Y.; Kuang, P.; Zhang, W.; Lu, Y.; Li, Y. Synchronous Cr(VI) Remediation and Energy Production Using Microbial Fuel Cell from a Subsurface Environment: A Review. Energies 2022, 15, 1989. [Google Scholar] [CrossRef]
- Yu, H.; Zhao, Q.; Liu, X.; Meng, F.; Ruan, L.; Sun, T.; Liu, W.; Zhu, Y.; Li, W.; Meng, F.; et al. Enhanced chromium recovery and simultaneous sludge degradation in a novel bioelectrochemical system assembled with bio/abio-cathodes. Sep. Purif. Technol. 2020, 250, 117229. [Google Scholar] [CrossRef]
- Wang, H.; Ren, Z.J. Bioelectrochemical metal recovery from wastewater: A review. Water Res. 2014, 66, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, B.; Garlapati, V.K.; Sharma, S.; Bhadra, S.; Maddirala, S.; Varsha, K.M.; Motru, V.; Goswami, P.; Sevda, S.; Aminabhavi, T.M. Bioelectrochemical systems-based metal recovery: Resource, conservation and recycling of metallic industrial effluents. Environ. Res. 2022, 204, 112346. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.-M.; Ong, S.-A.; Ho, L.-N.; Wong, Y.-S.; Thung, W.-E.; Teoh, T.-P. The reaction of wastewater treatment and power generation of single chamber microbial fuel cell against substrate concentration and anode distributions. J. Environ. Health Sci. Eng. 2020, 18, 793–807. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, B.; Li, J.; Lv, Q.; Wang, S.; Gu, Q. Enhanced vanadium (V) reduction and bioelectricity generation in microbial fuel cells with biocathode. J. Power Sources 2017, 359, 379–383. [Google Scholar] [CrossRef]
- Wang, H.; Long, X.; Zhang, J.; Cao, X.; Liu, S.; Li, X. Relationship between bioelectrochemical copper migration, reduction and electricity in a three-chamber microbial fuel cell. Chemosphere 2020, 241, 125097. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, L.; Pan, Y.; Quan, X.; Li Puma, G. Impact of Fe(III) as an effective electron-shuttle mediator for enhanced Cr(VI) reduction in microbial fuel cells: Reduction of diffusional resistances and cathode overpotentials. J. Hazard. Mater. 2017, 321, 896–906. [Google Scholar] [CrossRef]
- Gangadharan, P.; Nambi, I.M. The performance of Cu(2+)as dissolved cathodic electron-shuttle mediator for Cr(6+)reduction in the microbial fuel cell. Sustain. Environ. Res. 2020, 30, 19. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, C.; Ni, J.; Zhang, J.; Huang, W. Simultaneous reduction of vanadium (V) and chromium (VI) with enhanced energy recovery based on microbial fuel cell technology. J. Power Sources 2012, 204, 34–39. [Google Scholar] [CrossRef]
- Mier, A.A.; Olvera-Vargas, H.; Mejía-López, M.; Longoria, A.; Verea, L.; Sebastian, P.J.; Arias, D.M. A review of recent advances in electrode materials for emerging bioelectrochemical systems: From biofilm-bearing anodes to specialized cathodes. Chemosphere 2021, 283, 131138. [Google Scholar] [CrossRef] [PubMed]
- Rusli, S.F.N.; Abu Bakar, M.H.; Loh, K.S.; Mastar, M.S. Review of high-performance biocathode using stainless steel and carbon-based materials in Microbial Fuel Cell for electricity and water treatment. Int. J. Hydrog. Energy 2019, 44, 30772–30787. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Xu, Y. Performance of Pb(II) reduction on different cathodes of microbial electrolysis cell driven by Cr(VI)-reduced microbial fuel cell. J. Power Sources 2019, 418, 1–10. [Google Scholar] [CrossRef]
- Watanabe, K. Recent Developments in Microbial Fuel Cell Technologies for Sustainable Bioenergy. J. Biosci. Bioeng. 2008, 106, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Chang, I.S.; Kim, B.H. Continuous electricity production from artificial wastewater using a mediator-less microbial fuel cell. Bioresour. Technol. 2006, 97, 621–627. [Google Scholar] [CrossRef]
- Tandukar, M.; Huber, S.J.; Onodera, T.; Pavlostathis, S.G. Biological chromium(VI) reduction in the cathode of a microbial fuel cell. Environ. Sci. Technol. 2009, 43, 8159–8165. [Google Scholar] [CrossRef] [PubMed]
- Rabaey, I.; Ossieur, W.; Verhaege, M.; Verstraete, W. Continuous microbial fuel cells convert carbohydrates to electricity. Water Sci. Technol. 2005, 52, 515–523. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.; Liu, F.; Chen, Z. Mechanisms and challenges of microbial fuel cells for soil heavy metal(loid)s remediation. Sci Total Environ. 2021, 756, 143865. [Google Scholar] [CrossRef]
- Heijne, A.T.; Liu, F.; Weijden, R.v.d.; Weijma, J.; Buisman, C.J.N.; Hamelers, H.V.M. Copper Recovery Combined with Electricity Production in a Microbial Fuel Cell. Environ. Sci. Technol. 2010, 44, 4376–4381. [Google Scholar] [CrossRef]
- Oh, S.-E.; Logan, B.E. Proton exchange membrane and electrode surface areas as factors that affect power generation in microbial fuel cells. Appl. Microbiol. Biotechnol. 2006, 70, 162–169. [Google Scholar] [CrossRef]
- Ouitrakul, S.; Sriyudthsak, M.; Charojrochkul, S.; Kakizono, T. Impedance analysis of bio-fuel cell electrodes. Biosens. Bioelectron. 2007, 23, 721–727. [Google Scholar] [CrossRef]
- Wang, J.; Song, X.; Wang, Y.; Abayneh, B.; Ding, Y.; Yan, D.; Bai, J. Microbial community structure of different electrode materials in constructed wetland incorporating microbial fuel cell. Bioresour. Technol. 2016, 221, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Gil, G.-C.; Chang, I.-S.; Kim, B.H.; Kim, M.; Jang, J.-K.; Park, H.S.; Kim, H.J. Operational parameters affecting the performannce of a mediator-less microbial fuel cell. Biosens. Bioelectron. 2003, 18, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yuan, Y.; Li, F.-b.; Feng, C.-h. In-situ Cr(VI) reduction with electrogenerated hydrogen peroxide driven by iron-reducing bacteria. Bioresour. Technol. 2011, 102, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Cecconet, D.; Callegari, A.; Capodaglio, A.G. Bioelectrochemical Systems for Removal of Selected Metals and Perchlorate from Groundwater: A Review. Energies 2018, 11, 2643. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S. Efficacy of Cu(II) as an electron-shuttle mediator for improved bioelectricity generation and Cr(VI) reduction in microbial fuel cells. Bioresour. Technol. 2019, 273, 122–129. [Google Scholar] [CrossRef]
- Hidayat, A.R.P.; Widyanto, A.R.; Asranudin, A.; Ediati, R.; Sulistiono, D.O.; Putro, H.S.; Sugiarso, D.; Prasetyoko, D.; Purnomo, A.S.; Bahruji, H.; et al. Recent development of double chamber microbial fuel cell for hexavalent chromium waste removal. J. Environ. Chem. Eng. 2022, 10, 107505. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Lei, L. Electricity production during the treatment of real electroplating wastewater containing Cr6+ using microbial fuel cell. Process Biochem. 2008, 43, 1352–1358. [Google Scholar] [CrossRef]
- Wu, X.; Ren, X.; Owens, G.; Brunetti, G.; Zhou, J.; Yong, X.; Wei, P.; Jia, H. A Facultative Electroactive Chromium(VI)-Reducing Bacterium Aerobically Isolated From a Biocathode Microbial Fuel Cell. Front. Microbiol. 2018, 9, 2883. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Huang, L.; Zhou, P.; Quan, X.; Li Puma, G. Correlation between circuital current, Cu(II) reduction and cellular electron transfer in EAB isolated from Cu(II)-reduced biocathodes of microbial fuel cells. Bioelectrochemistry 2017, 114, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Daghio, M.; Tofalos, A.E.; Franzetti, A.; Mastorgio, A.F.; Saponaro, S.; Sezenna, E. Microbial Assisted Hexavalent Chromium Removal in Bioelectrochemical Systems. Water 2020, 12, 466. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).