Abstract
The interactive effects of elevated [CO2] and drought on leaf photosynthesis, physiology and yield in wheat (Triticum aestivum L.) are not well understood. This study evaluated the effects of persistent drought stress (35–45% of field water capacity) and elevated CO2 (ambient concentration + 200 μmol mol–1) on leaf photosynthesis, chlorophyll fluorescence, stress physiological indices, biomass, and grain weight (in g m−2) in two wheat cultivars (large-spike cultivar Z175 and multiple-spike cultivar Triumph) at the open-top chamber (OTC) experimental facility in North China. We found that elevated [CO2] enhanced the positive effects of drought on Fv/Fm and WUE but did not ameliorate the adverse effects of drought on PN in the two cultivars. Moreover, as a large-spike cultivar, Z175 showed enhanced photosynthesis performance and sink capacity (spike number and kernel number per spike) compared with Triumph in the grain filling stage under elevated [CO2], which helped counteract the adverse effects of drought. In contrast, although Triumph had more tillers and spikes at the current [CO2] concentration, most of them were thin and had limited photosynthesis capacity. The photosynthesis capacity of leaves on the main shoot and the spike number did not significantly increase in Triumph under elevated [CO2]. Hence, elevated [CO2] mitigated drought-induced inhibition of grain weight in Z175 plants but not in Triumph plants under persistent drought stress.
1. Introduction
Rising atmospheric CO2 concentrations ([CO2]) and droughts are predicted to impact crop growth and yield [1,2,3]. Elevated [CO2] generally reduces stomatal conductance (gs) [4,5], increases CO2 in chloroplasts, enhances the Rubisco carboxylation rate and, as a result, increases WUE and drought resistance [6,7,8]. Some studies have shown that elevated [CO2] mitigates the impact of drought stress on maize [9,10], barley [11], Arabidopsis thaliana [8], grassland species [12] and bread wheat varieties [11,13,14]. However, elevated [CO2] cannot compensate for water stress in some plants, such as soybean [3] and native and exotic riparian plants [15].
Wheat (Triticum aestivum L.) is one of world’s most important food crops. Long-term FACE (Free Air CO2 Enrichment, FACE) experiments in Arizona in the United States and in Beijing in China have shown that elevated [CO2] could increase the yield of winter wheat by 25–28% under persistent moderate drought conditions [16,17]. This yield increase is more than the 11–19% increase reported to result from irrigation conditions [16,17]. However, the results from FACE experiments in Australia showed that elevated [CO2] increased the winter wheat yield more under irrigation conditions than under drought conditions [18,19]. Therefore, the responses of plant growth to elevated [CO2] and persistent drought are very distinct between the environment, different species, soil conditions, etc.
The wheat yield in China accounts for one-fifth of the world’s total each year. Thus, variations in wheat productivity in China would cause world-wide fluctuations in wheat yield under future climates. Moreover, drought is one of the key factors limiting plant productivity in northern China and semiarid regions throughout the world. Currently, research on the interactive effects of elevated [CO2] and persistent drought on wheat in North China is lacking. The first objective of the present study is to investigate the effects of elevated [CO2] and persistent water-deficit conditions on the photosynthesis, antioxidative ability, chlorophyll fluorescence, WUE and yield of two wheat cultivars. The second aim is to investigate interspecies differences in large-spike and multi-spike wheat in response to increased [CO2] and drought stress. This study will provide insights into wheat crop adaptation and management under future climate change.
2. Materials and Methods
2.1. Experimental Design
The growth experiments were carried out within an open-top chamber facility (OTC) at the experimental station of Shanxi Agricultural University (37.42° N, 112.55° E) in Taigu, Shanxi, China. Air temperature and relative humidity inside the chambers were monitored throughout the growing season. CO2 concentrations of 400 and 600 μmol mol–1, relating to the control and elevated [CO2] level (eCO2), respectively, were generally maintained within the two growth chambers from seedling emergence to harvest (Figure 1). We did not control the CO2 concentration in the chambers when the daily minimum temperature was below zero (11 December 2013–15 February 2014 and 11 December 2014–9 February 2015). The facility operational procedures were described in Hao et al.’s [20] work. Two winter wheat cultivars, cv. Zhongmai 175 (Z175) and cv. Triumph, were selected for the experiment. Z175 was released in 2012 by the Institute of Crop Sciences, Chinese Academy of Agricultural Sciences. As a large-spike cultivar, Z175 has a thick shoot diameter, reduced tiller number, shorter plants, and higher harvest index. Triumph, a multi-spike cultivar introduced from the USA 70 years ago, has taller and thin shoots, more tillers and lower harvest indices. A total of 60 seeds of wheat were sown on 8 October 2013 and 10 October 2014 in polyethylene containers (60 cm × 40 cm × 28 cm). There were nine replicates per treatment per year.
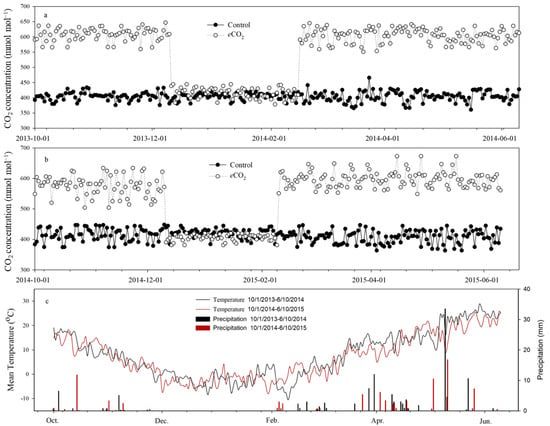
Figure 1.
The CO2 concentration (a,b) and air temperature (c) in open-top chamber of wheat growing season on 1 October 2013–1 June 2014 and 1 October 2014–1 June 2015. Control—ambient atmospheric CO2 concentration; eCO2—elevated atmospheric CO2 concentration.
Polyethylene containers were filled with clay loam soil, surface soil (0–20 cm) of nearby cropland, with a pH (1:5 soil:water) of 8.1 and contained 1.38% organic carbon (C) and 0.11% total N. Fertilizers were applied at sowing at rates of 1.73 g N and 1.31 g P per pot. The two following soil water conditions were maintained in each chamber after 1 March 2014 and 2015 with nine replications in each growth chamber: well-watered (60–80% relative water content (RWC)) or droughted (35–45% RWC). The drought and genotype treatments were randomized within each growth chamber. The soil moisture content was measured with a wet sensor (TZS-1; Zhejiang TOP Cloud-agri Technology Co., Ltd., Zhejiang, China) and maintained at the targeted moisture regimes through daily irrigation during the growth period.
2.2. Gas Exchange Measurements
Leaf photosynthetic gas exchange measurements were carried out using an open gas exchange system (LI-COR 6400; Lincoln, NE, USA) at the booting stage and grain filling stage. Healthy flag leaves of the primary tillers were selected per pot. Measurements were conducted from 09:00 to 11:30 using the methods described by Hao et al. [20]. The photosynthetic photon flux density (PPFD) was set at the saturating light level (1500 μmol m–2 s–1), and the leaf chamber temperature was constant, at 25 °C. The leaf chamber CO2 concentration was set to 400 μmol mol–1 for CK OTC and 600 μmol mol–1 for HCO2 OTC. The light-saturated net photosynthesis rate (PN), stomatal conductance (gs), and transpiration rate (E) were recorded automatically, and the water use efficiency (WUE) (WUE = PN/E) was calculated.
2.3. Chlorophyll Fluorescence
One flag leaf of the primary tillers per pot was selected for the measurement of chlorophyll fluorescence using a miniaturized pulse-amplitude modulated fluorescence analyzer (PAM-2000, Walz, Effeltrich, Germany) on the same day as the gas exchange measurements. The initial fluorescence yield of the light-adapted state (F0’), maximal fluorescence yield of the light-adapted state (Fm’), steady-state fluorescence yield (Fs), initial fluorescence of the dark-adapted state when all reaction centers were open (F0), and maximal fluorescence of the dark-adapted state when all reaction centers were closed after illumination (Fm) were measured using the methods described by Wang et al. [21]. The effective quantum yield of PSII photochemistry (ΦPSII), maximal quantum yield of PSII photochemistry (Fv/Fm), intrinsic efficiency of PSII (Fv’/Fm’), photochemical quenching coefficient (qP), and nonphotochemical quenching of variable chlorophyll fluorescence (NPQ) were determined as described by Kramer et al. [22].
2.4. Determination of Photosynthetic Pigment Content, Malondialdehyde (MDA) and Peroxidase (POD)
Flag leaves of wheat plants from three pots (out of the nine replicate pots) in each chamber were cut and immersed in liquid nitrogen and kept in a freezer at −80 °C for the analysis of the photosynthetic pigment, MDA and POD contents at the booting stage and grain filling stage. Chlorophyll a, chlorophyll b, and carotenoid contents were estimated following the protocol provided by Arnon [23]. MDA content was estimated using the thiobarbituric acid (TBA) test following the methods of Heath and Packe [24]. The activity of POD was determined as enzyme units per gram fresh weight (U/g fw) according to Sakharov and Aridilla’s method [25].
2.5. Harvesting
At maturity, wheat plants were harvested on 13 June 2014 (249 days after sowing) and 11 June 2015 (245 days after sowing). After air-drying, the spike number per square meter, number of kernels per spike, thousand seed weight, biomass and yield were determined.
2.6. Statistical Analysis
A randomized full block design was employed in a factorial arrangement with two CO2 treatments, two cultivars, and two water treatments. The standard errors were calculated using the ANOVA table’s pooled error term. Treatments were compared using Duncan’s multiple range tests at p = 0.05 in SPSS software. Percentage was used to evaluate the impact of elevated [CO2] or drought on gas exchange parameters, chlorophyll fluorescence, photosynthetic pigment content, MDA and POD concentrations, and biomass and yield. The denominator is the difference between the sum of two water statuses, two cultivars, and two growth stages under elevated [CO2] and the sum of that under atmospheric [CO2] (Control). The numerator is the sum of two water statuses, two cultivars, and two growth stages under atmospheric [CO2].
3. Results
3.1. PN and Gas Exchange Parameters
Elevated [CO2] significantly increased PN (by 20.4%) and WUE (by 65.6%) but decreased gs (by 37.3%) and E (by 30.2%) in 2015. Drought significantly decreased PN (by 45.7%), gs (by 48.9%) and E (by 41.0%) but increased WUE by 57.7%. The interaction of CO2 and drought on PN and WUE was significant (p < 0.05), but not for gs or E. Elevated [CO2] enhanced the positive effects of drought on WUE and the negative effects of drought on PN. Specifically, the decrease in PN through the eCO2 × drought interaction (−7.05 = 9.44 − 16.49) was significantly greater than that resulting from drought (−6.98 = 9.51 − 16.49) and eCO2 (4.87 = 21.35 − 16.49). The increase in WUE through the eCO2 × drought interaction (3.69 = 6.85 − 3.33) was significantly greater than that resulting from drought (1.01 = 4.34 − 3.33) and eCO2 (1.92 = 5.25 − 3.33). Cultivar, CO2 and drought had no interactive effects on PN, gs, E or WUE (Table 1). Therefore, elevated CO2 had a positive effect on increasing WUE but had little effect on improving PN. Compared with Triumph, Z175 had higher gs and PN under well-watered conditions at both the booting stage and grain filling stage. Moreover, under elevated [CO2] and drought conditions, Z175 had a higher PN than Triumph, even though the gs was low, especially at the booting stage.

Table 1.
Effects of elevated [CO2] and drought on gas exchange parameters of two wheat cultivars in 2015.
3.2. Chlorophyll Fluorescence
Elevated [CO2] had no effect on the maximal quantum yield of PSII photochemistry (Fv/Fm), intrinsic efficiency of PSII (Fv’/Fm’), effective quantum yield of PSII photochemistry (ΦPSII), photochemical quenching coefficient (qP) or nonphotochemical quenching (NPQ). Drought significantly decreased Fv’/Fm’ (by 4.60%) and ΦPSII (by 10.9%) but increased NPQ by 13.8%. CO2 and drought had positive interactive effects on Fv/Fm (p < 0.05) but no interactive effects on Fv’/Fm’, ΦPSII, qP or NPQ. The increase in Fv/Fm through the eCO2 × drought interaction (−0.0033 = 0.8081 − 0.8049) was significantly greater than that through drought (−0.0055 = 0.7994 − 0.8049) and eCO2 (−0.0010 = 0.8039 − 0.8049). Cultivar, CO2 and drought had interactive effects on Fv/Fm and Fv’/Fm’ (Table 2). Therefore, although elevated [CO2] had little effect on improving Fv/Fm in well-watered plants, it effectively increased Fv/Fm in drought-stressed plants. There was no difference between the two cultivars.

Table 2.
Effects of elevated [CO2] and drought on chlorophyll fluorescence parameters of two wheat cultivars in 2015.
3.3. Determination of Photosynthetic Pigment Content, MDA and POD Concentrations
Elevated [CO2] significantly increased the content of chlorophyll a (by 7.6%) and carotenoids (by 14.0%) but decreased that of MDA (by 37.0%) and POD (by 20.0%). Drought significantly decreased chlorophyll a (by 5.1%), chlorophyll b (by 18.6%), POD (by 11.9%), and MDA (by 42.4%). CO2 and drought had no interactive effects on chlorophyll a, chlorophyll b, carotenoid, MDA or POD. Cultivar, CO2 and drought had interactive effects on chlorophyll a and carotenoids (Table 3). Z175 had higher contents of chlorophyll a and POD than Triumph at the booting stage, especially under elevated [CO2] and drought conditions. This illustrated that more chlorophyll a and POD could be produced in Z175 than in Triumph under e[CO2] and drought.

Table 3.
Effects of elevated [CO2] and drought on photosynthetic pigment content, POD and MDA at the booting stage and filling stage of two wheat cultivars in 2015.
3.4. Biomass and Yield
Elevated [CO2] significantly increased the main shoot diameter and biomass, spike number per square meter and kernel number per spike for two years. Elevated [CO2] had no effect on thousand seed weight and harvest index. Drought decreased the kernel number per spike and thousand seed weight by 48.4 and 11.6%, respectively. CO2 and drought had no interactive effects on spike number per square meter, kernel number per spike or thousand seed weight (Table 4). Therefore, CO2 offsets the negative impact of drought on spike number per square meter and kernel number per spike.

Table 4.
Effects of elevated [CO2] and drought on plant morphological parameters, weight, and yield component of two wheat cultivars in 2014 and 2015.
Elevated [CO2] significantly increased aboveground biomass and grain weight in g m−2 by an average of 21.8 and 31.3%, respectively, for two years. Drought decreased aboveground biomass and grain weight by 39.8 and 49.6%, respectively. CO2 and drought had a significant negative interactive effect on aboveground biomass (p < 0.05) and grain weight (p < 0.05). Specifically, the decrease in aboveground biomass through the eCO2 × drought interaction (−253.16 = 647.93 − 901.09) was significantly greater than that of the individual effects of drought (−359.97 = 541.12 − 901.09) and eCO2 (174.19 = 1075.28 − 901.09). The decrease in grain weight by the eCO2 × drought interaction (−70.46 = 132.93 − 203.38) was significantly greater than that resulting from drought (−100.36 = 103.02 − 203.38) and eCO2 (61.65 = 265.04 − 203.38). Cultivar, CO2 and drought had no interactive effects on aboveground biomass and grain weight (Figure 2 and Figure 3). Therefore, drought decreased aboveground biomass and grain weight to a greater extent under elevated CO2 than under atmospheric CO2. Nevertheless, the values of aboveground biomass and grain weight in the two cultivars (especially in Z175) were still significantly higher under elevated CO2 than under atmospheric CO2.
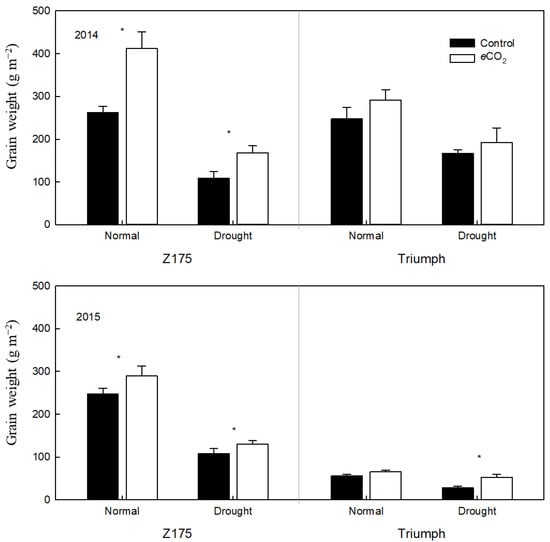
Figure 2.
Effects of elevated [CO2] and drought on the grain weight of two wheat cultivars in 2014 and 2015. Values are means of 5 replicates. Control—ambient atmospheric CO2 concentration; eCO2—elevated atmospheric CO2 concentration. * indicates significance at p ≤ 0.05.
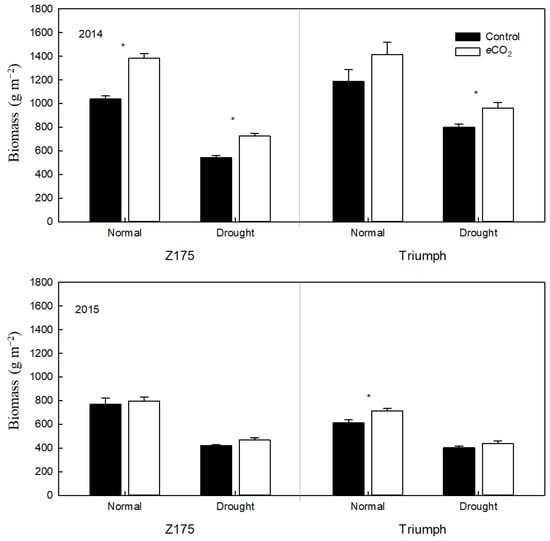
Figure 3.
Effects of elevated [CO2] and drought on the aboveground biomass of two wheat cultivars in 2014 and 2015. Values are means of 5 replicates. Control—ambient atmospheric CO2 concentration; eCO2—elevated atmospheric CO2 concentration. * indicates significance at p ≤ 0.05.
4. Discussion
Elevated [CO2] has been widely reported to improve leaf WUE by increasing the photosynthesis rate and decreasing leaf transpiration (by reducing gs) [4,5]. This effect is predicted to be helpful in alleviating drought stress on crops [26,27]. Stomatal closure, however, is widely recognized as the primary limitation of carbon assimilation [28]. In the current study, elevated [CO2] increased WUE under drought by decreasing E (decreased gs) but decreased PN due to strong stomatal closure, which was in agreement with a previous study on wheat [14]. When comparing the two selected cultivars, the large-spike cultivar Z175 had a higher Pn than the multi-spike cultivar Triumph under elevated [CO2]. This helped Z175 plants assimilate more carbon than Triumph under drought stress and elevated [CO2]. Meanwhile, although elevated [CO2] increased the content of chlorophyll a and carotenoids, CO2 and drought had no interactive effects on chlorophyll a, chlorophyll b and carotenoids. Further analysis of PSII performance showed that CO2 and drought also had no interactive effects on Fv’/Fm’, ΦPSII, qP and NPQ, which was consistent with studies on soybean [3] and basil [29] but in contrast to other studies on rice [30], sugarcane [31] and Arabidopsis [8]. However, the two selected cultivars showed different response patterns in photosynthetic pigments to elevated [CO2] and drought. Thus, [CO2] and drought had positive interactive effects on chlorophyll a in Z175 and had negative effects on chlorophyll a in Triumph at the booting stage. However, the chlorophyll a in Z175 was not enough to improve PSII performance under elevated [CO2] and drought. Although we did not measure the photochemical parameters, we speculate that, rather than PSII performance (Fv’/Fm’ and ΦPSII), photosynthesis biochemical features (such as substrate and energy partitioning between rubisco carboxylation and photorespiration) possibly contribute more to carbon assimilation at low gs in Z175 under elevated [CO2] [32]. Elevated [CO2] is expected to reduce photorespiration; in contrast, however, drought has been proven to increase photorespiration. Changes in photosynthesis biochemical features would be influenced by the partitioning of substrate and photon flux between carboxylation and photorespiration and might be cultivar-dependent [27].
POD can eliminate the deleterious effects of H2O2 on an organism through the oxidation of co-substrates [33]. MDA may function as a biomarker to assess the level of oxidative stress in plants. MDA activity and content are unusually correlated with a wide range of plant physiological processes [34,35]. In the current study, elevated [CO2] significantly decreased MDA at the booting stage and the grain filling stage, and decreased POD at the booting stage but did not affect MDA or POD when combined with drought. Barickman et al. [29] and Bencze et al. [14] also reported that no significant change was observed in POD under elevated [CO2] and drought conditions. Moreover, the two selected cultivars showed different response patterns to elevated [CO2]. Z175 had more POD than Triumph, which possibly contributed to alleviating ROS damage caused by drought under elevated [CO2], especially at the booting stage. This could be another reason for the improved carbon assimilation in Z175 under elevated [CO2] and drought.
Elevated [CO2] stimulated more spikes for Z175 than for Triumph (Table 4), especially in 2014, which is consistent with previous studies [36,37]. The enhanced photosynthesis performance and sink capacity (spike number and kernel number per spike) at the grain filling stage contribute to the yield in Z175 plants under elevated [CO2], which can help counteract the adverse effects of drought. Although Triumph had more tillers and spikes at the current [CO2] concentration, most of them were thin and had limited photosynthetic capacity (Table 1 and Table 4). Both the main shoot biomass accumulation and tiller and spike number of Triumph did not significantly increase under elevated [CO2], irrespective of water status. Hence, elevated [CO2] mitigated drought-induced inhibition of grain weight in Z175 plants but not in Triumph plants under persistent drought stress. Although the response patterns of Z175 plants to elevated [CO2] and drought were the same between the two years, the magnitudes of these changes were smaller in 2015 than in 2014. According to the analysis of environmental elements in the two years, the mean daily temperature was higher (approximately 1.5–2 °C), and there were fewer precipitation days (air moisture may be lower) during March and April in 2015 than in 2014 (Figure 1). At that time, the winter wheat plants were at the elongation stage and heading stage. It was proven that plants under elevated [CO2] tend to obtain a new balance of C and N metabolism, such as a decrease in foliar photorespiration, an increase in carbohydrate assimilation and belowground partitioning, a decrease in N concentration in leaves, and an increase in N accumulation at the whole plant level [27]. The increase in air temperature and decrease in moisture may have contributed to an increase in leaf respiration, the consumption of carbohydrates [38], and a further increase in carbohydrate partitioning belowground [39] in 2015, resulting in a decrease in aboveground biomass and grain weight. Therefore, the positive effects that elevated [CO2] brought to drought-induced inhibition on grain weight in cv. Z175 diminished in the year with higher temperature and less precipitation at the key growth stages.
5. Conclusions
Elevated [CO2] significantly increased chlorophyll a and carotenoids, decreased gs and E, and, consequently, increased PN and WUE in well-watered wheat plants. However, when plants were under drought stress, elevated [CO2] only improved WUE and Fv/Fm and did not ameliorate the adverse effects of drought on PN. Moreover, Z175 showed more improved photosynthesis performance and sink capacity (spike number and kernel number per spike) than Triumph at the grain filling stage under elevated [CO2]. Elevated [CO2] partially mitigated drought-induced inhibition of the grain weight of Z175 plants but not of Triumph plants. Therefore, to fully utilize the fertilization effect of future elevated [CO2] on growth and yield in wheat, we may cultivate wheat cultivars that can adapt to drought stress in semi-arid regions.
Author Contributions
Data curation, D.Z. and W.L.; Writing—original draft, Q.Y. and Y.Z.; Writing—review & editing, P.L.; Project administration, X.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministerial and Provincial Co-Innovation Centre for Endemic Crops Production with High-quality and Efficiency in Loess Plateau, grant number SBGJXTZX-27, Youth Talents Support Program of Shanxi Agricultural University, grant number BJRC201602, National Natural Science Foundation of China, grant number 31971773 and Fundamental Research Program of Shanxi Province, grant number 202103021224152.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
[CO2]—atmospheric CO2 concentration; E—transpiration rate; HCO2—high atmospheric CO2 concentration; ETR—electron transport rate; F0—minimal fluorescence yield of the dark-adapted state; F0’—minimal fluorescence yield of the light-adapted state; Fm– maximal fluorescence yield of the dark-adapted state; Fm’—maximal fluorescence yield of the light-adapted state; FM—fresh mass; Fs—steady-state fluorescence yield; Fv/Fm—maximal quantum yield of PSII photochemistry; Fv’/Fm’—intrinsic efficiency of PSII; gs–stomatal conductance; MDA–malondialdehyde; NPQ– nonphotochemical quenching; PN—net photosynthetic rate; POD—peroxidase; qP—photochemical quenching coefficient; ROS—reactive oxygen species; WUE—water-use efficiency (=PN/E); ΦPSII—effective quantum yield of PSII photochemistry.
References
- IPCC. Summary for policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T., Qin, D., Plattner, G.K., Tignor, M., Allen, S., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P., Eds.; Cambridge University Press: New York, NY, USA, 2013; pp. 281–304. [Google Scholar]
- Friedlingstein, P.; O’sullivan, M.; Jones, M.W.; Andrew, R.M.; Hauck, J.; Olsen, A.; Peters, G.P.; Peters, W.; Pongratz, J.; Sitch, S.; et al. Global Carbon Budget. Earth Syst. Sci. Data 2020, 12, 3269–3340. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Qiao, Y.; Wang, Y.; Cai, Z.; Dong, B.; Shi, C.; Liu, Y.; Li, X.; Liu, M. Effects of elevated CO2 on the growth, seed yield, and water use efficiency of soybean (Glycine max (L.) Merr.) under drought stress. Agric. Water Manag. 2013, 129, 105–112. [Google Scholar] [CrossRef]
- Hao, X.Y.; Han, X.; Lam, S.K.; Wheeler, T.; Ju, H.; Wang, H.R.; Li, Y.C.; Lin, E.D. Effects of fully open-air [CO2] elevation on leaf ultrastructure, photosynthesis and yield of two soybean cultivars. Photosynthetica 2012, 50, 362–370. [Google Scholar] [CrossRef]
- Deryng, D.; Elliott, J.; Folberth, C.; Müller, C.; Pugh, T.A.M.; Boote, K.J.; Conway, D.; Ruane, A.C.; Gerten, D.; Jones, J.W.; et al. Regional disparities in the beneficial effects of rising CO2 concentrations on crop water productivity. Nat. Clim. Chang. 2016, 6, 786–790. [Google Scholar] [CrossRef]
- Evans, J.R.; Clarke, V.C. The nitrogen cost of photosynthesis. J. Exp. Bot. 2019, 70, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Zhong, Y.; Shangguan, Z. Contrasting responses of leaf stomatal characteristics to climate change: A considerable challenge to predict carbon and water cycles. Glob. Chang. Biol. 2017, 23, 3781–3793. [Google Scholar] [CrossRef] [PubMed]
- Zinta, G.; AbdElgawad, H.; Domagalska, M.A.; Vergauwen, L.; Knapen, D.; Nijs, I.; Janssens, I.A.; Beemster, G.T.; Asard, H. Physiological, biochemical, and genome-wide transcriptional analysis reveals that elevated CO2 mitigates the impact of combined heat wave and drought stress in Arabidopsis thaliana at multiple organizational levels. Glob. Chang. Biol. 2014, 20, 3670–3685. [Google Scholar] [CrossRef]
- Kadam, N.N.; Xiao, G.; Melgar, R.J.; Bahuguna, R.N.; Quinones, C.; Tamilselvan, A.; Prasad, P.V.V.; Jagadish, K.S. Agronomic and physiological responses to high temperature drought: And elevated CO2 in cereals. Adv. Agron. 2014, 127, 111–156. [Google Scholar]
- Zong, Y.Z.; Shangguan, Z.P. Increased sink capacity enhances C and N assimilation under drought and elevated CO2conditions in maize. Integr. Agric. 2016, 15, 2775–2785. [Google Scholar] [CrossRef]
- Robredo, A.; Pérez-López, U.; Miranda-Apodaca, J.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. Elevated CO2 reduces the drought effect on nitrogen metabolism in barley plants during drought and subsequent recovery. Environ. Exp. Bot. 2011, 71, 399–408. [Google Scholar] [CrossRef]
- Miranda-Apodaca, J.; Pérez-López, U.; Lacuesta, M.; Mena-Petite, A.; Muñoz-Rueda, A. The type of competition modulates the ecophysiological response of grassland species to elevated CO2 and drought. Plant Biol. 2014, 17, 298–310. [Google Scholar] [CrossRef]
- Wall, G.W.; Garcia, R.L.; Kimball, B.A.; Hunsaker, D.J.; Pinter, P.J., Jr.; Long, S.P.; Osborne, C.P.; Hendrix, D.L.; Wechsung, F.; Wechsung, G.; et al. Interactive effects of elevated carbon dioxide and drought on wheat. Agron. J. 2006, 98, 354–381. [Google Scholar] [CrossRef]
- Bencze, S.; Bamberger, Z.; Janda, T.; Balla, K.; Varga, B.; Bedő, Z.; Veisz, O. Physiological response of wheat varieties to elevated atmospheric CO2 and low water supply levels. Photosynthetica 2014, 52, 71–82. [Google Scholar] [CrossRef]
- Perry, L.G.; Shafroth, P.B.; Blumenthal, D.M.; Morgan, J.A.; LeCain, D.R. Elevated CO2 does not offset greater water stress predicted under climate change formative and exotic riparian plants. New Phytol. 2013, 197, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef]
- Shuai, J.; Hui, J. Interactive effects of elevated carbon dioxide and water on the growth and development of winter wheat. J. Agrometeorol. 2013, 34, 31–37. [Google Scholar]
- Fitzgerald, G.J.; Tausz, M.; O’Leary, G.; Mollah, M.R.; Tausz-Posch, S.; Seneweera, S.; Mock, I.; Löw, M.; Partington, D.L.; McNeil, D.; et al. Elevated atmospheric [CO2] can dramatically increase wheat yields in semi-arid environments and buffer against heat waves. Glob. Chang. Biol. 2016, 22, 2269–2284. [Google Scholar] [CrossRef] [PubMed]
- Houshmandfar, A.; Fitzgerald, G.J.; O’Leary, G.; Tausz-Posch, S.; Fletcher, A.; Tausz, M. The relationship between transpiration and nutrient uptake in wheat changes under elevated atmospheric CO2. Physiol. Plant. 2017, 163, 516–529. [Google Scholar] [CrossRef]
- Hao, X.Y.; Li, P.; Li, H.Y.; Zong, Y.Z.; Zhang, B.; Zhao, J.Z.; Han, Y.H. Elevated [CO2] increased photosynthesis and yield without decreasing stomatal conductance in broomcorn millet. Photosynthetica 2017, 55, 176–183. [Google Scholar] [CrossRef]
- Wang, A.; Lam, S.K.; Hao, X.; Li, F.Y.; Zong, Y.; Wang, H.; Li, P. Elevated CO2 reduces the adverse effects of drought stress on a high-yielding soybean (Glycine max (L.) Merr.) cultivar by increasing water use efficiency. Plant Physiol. Bioch. 2018, 132, 660–665. [Google Scholar] [CrossRef]
- Kramer, D.M.; Johnson, G.; Kiirats, O.; Edwards, G.E. New fluorescence parameters for the determination of QARedox state and excitation energy fluxes. Photosynth. Res. 2004, 79, 2092–2118. [Google Scholar] [CrossRef] [PubMed]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Heath, R.L.; Packer, L. Photo peroxidation in isolated chloroplast. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Sakharov, I.Y.; Aridilla, G.B. Variation of peroxidase activity in cacao beans during their ripening, fermentation and drying. Food Chem. 1999, 65, 51–54. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Cornic, G. Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ. 2002, 25, 275–294. [Google Scholar] [CrossRef] [PubMed]
- Shanker, A.K.; Gunnapaneni, D.; Bhanu, D.; Vanaja, M.; Lakshmi, N.J.; Yadav, S.K.; Prabhakar, M.; Singh, V.K. Elevated CO2 and water stress in combination in plants: Brothers in arms or partners in crime? Biology 2022, 11, 1330. [Google Scholar] [CrossRef]
- Misson, L.; Limousin, J.-M.; Rodriguez, R.; Letts, M.G. Leaf physiological responses to extreme droughts in Mediterranean Quercus ilex forest. Plant Cell Environ. 2010, 33, 1898–1910. [Google Scholar] [CrossRef]
- Barickman, T.C.; Adhikari, B.; Sehgal, A.; Walne, C.H.; Reddy, K.R.; Gao, W. Drought and Elevated CO2 Impacts Photosynthesis and Biochemicals of Basil (Ocimum basilicum L.). Stresses 2021, 1, 223–237. [Google Scholar] [CrossRef]
- Baker, J.; Hartwell Allen, L.; Boote, K.; Pickering, N. Rice responses to drought under carbon dioxide enrichment. 1. Growth and yield. Glob. Chang. Biol. 1997, 3, 119–128. [Google Scholar] [CrossRef]
- Joseph, C.V.V.; Leon, H.A.J. Growth at elevated CO2 delays the adverse effects of drought stress on leaf photosynthesis of the C4 sugarcane. Plant Physiol. 2009, 166, 107–116. [Google Scholar]
- Yu-Zheng, Z.; Han-Qing, Z.; Ping, L.; Dong-Sheng, Z.; Xing-Yu, H.; Zhi-Qiang, G. Leaf nitrogen have a better relationship with photosynthesis performance across wheat species under elevated CO2 and drought. Plant Physiol. Bioch. 2021, 166, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Schwanz, P.; Polle, A. Differential stress responses of antioxidative systems to drought in pedunculate oak (Quercus robur) and maritime pine (Pinuspinaster) grown under high CO2 concentrations. Exp. Bot. 2001, 52, 133–143. [Google Scholar]
- Kravić, N.; Marković, K.; Anđelković, V.; Šukalović, V.H.-T.; Babić, V.; Vuletić, M. Growth, proline accumulation and peroxidase activity in maize seedlings under osmotic stress. Acta Physiol. Plant. 2012, 35, 233–239. [Google Scholar] [CrossRef]
- Ba Rnes, J.D.; Ollerenshaw, J.H.; Whitfield, C.P. Effects of elevated CO2 and/or O3 on growth, development and physiology of wheat (Triticum aestivum L.). Glob. Chang. Biol. 2010, 1, 129–142. [Google Scholar] [CrossRef]
- Balaguer, L.; Barnes, J.D.; Panicucci, A.; Borland, A.M. Production and utilization of assimilates in wheat (Triticum aestivum L.) leaves exposed to elevated O3 and/or CO2. New Phytol. 1995, 129, 557–568. [Google Scholar] [CrossRef]
- Collins, A.D.; Ryan, M.G.; Adams, H.D.; Dickman, L.T.; Garcia-Forner, N.; Grossiord, C.; Powers, H.H.; Sevanto, S.; McDowell, N.G. Foliar respiration is related to photosynthetic, growth and carbohydrate response to experimental drought and elevated temperature. Plant Cell Environ. 2021, 44, 3853–3865. [Google Scholar] [CrossRef]
- Drake, J.E.; Tjoelker, M.G.; Aspinwall, M.J.; Reich, P.B.; Pfautsch, S.; Barton, C.V. The partitioning of gross primary production for young Eucalyptus tereticornis trees under experimental warming and altered water availability. New Phytol. 2019, 222, 1298–1312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).