Abstract
This study compared the responses of two potato cultivars, Fianna and Lady rosetta (both determinate and indeterminate), to irrigation with various fly ash: acid mine drainage (AMD) amelioration ratios in two separate seasons. In order to calculate crude protein (CP), the nutritional composition (NC) of potato tuber samples was examined using the combustion method. The results showed that the NC of plants irrigated with AMD mixed with fly ash was significantly affected differently at harvest. In plants exposed to 75% AMD irrigation, the mean moisture content of Lady rosetta was reduced by twice more compared to Fianna. Concisely, our study has demonstrated that irrigation with AMD mixed with fly ash can promote plant growth of potato cultivars and reduce their crude protein (CP) by approximately 4% when irrigated with both 50% FA: AMD and untreated AMD (100% AMD) ratio being applied; nonetheless, it was decreased by 1% when watered with 75% FA: AMD treatment, lipids content, carbohydrates, fresh tuber weight and dry tuber weight. On average, plants from both cultivars irrigated with the 75% AMD had higher fresh tuber weight (FTW), higher dry tuber weight (DTW) and carbohydrates compared to the controls. Furthermore, Lady rosetta cultivar exhibited higher carbohydrate and crude fiber compared to the control at 75 and 50% AMD treatments, respectively. Use of AMD in agriculture is likely to reduce its devastating environmental conditions and assist in irrigation of food crops, thereby alleviating both water and food shortages. Last, all the FA-AMD-treated potatoes had Pb concentrations that were below the minimum standard limits, and this proves that FA was able to adsorb the Pb ions in the tuber samples. Importantly, fly ash reduces a metal’s concentration in AMD. However, presence of heavy metals in such potatoes needs to be explored. It is also important to relate the possible metal intake relative to the standards by World Health Organization (WHO).
1. Introduction
Acid mine drainage (AMD) water varies, with some treatment solutions, such fly ash (FA), being less expensive and capable of growing as a global byproduct of various mining operations [1]. Mines produce mine effluents in a variety of methods, such as runoff into surface and groundwater, seepage into underground water and overflow from abandoned mines. Mine effluents are both chemically toxic and radioactive (in the case of West Rand, South Africa (SA)) [2]. The fact that AMD is extremely difficult to remedy and can endure for decades, even after mine closure [3], makes it unique and particularly problematic. A range of negative effects, such as lower agricultural yields, deteriorated food quality and deterioration of soil quality, result from changes in the quantity and quality of irrigation water used in irrigated agriculture [3]. In order to enhance AMD to be utilized as irrigation water for crop production, some researchers [1,2,3] suggested use of lime, phosphorous rock, artificial wetlands and the reverse osmosis procedure. Compared to other alternative procedures, liming is quite expensive for the cost of decontaminating irrigation water supply [4]. South Africa is a water-poor country. It is, therefore, proposed that use of FA to cure AMD could help reduce water constraints in various agricultural businesses, where most staple foods are grown [5]. According to a study by Oelofse (2008), using FA in treatment of AMD may be a good alternative to liming [6]. While the rest of the world has made great progress in using environmentally friendly technology to minimize mining pollution and alleviate poverty, Africa, particularly sub-Saharan Africa, has lagged [1,2,3,4,5,6]. Even though the country’s economy is driven by a robust mining sector, SA nevertheless faces a dire water security crisis [7]. Due to the high acidity of the water entering the country’s water supply, these two phenomena have caused harm to inhabitants and ecosystems [7,8]. Residents in the area are concerned about the toxic water spilled by abandoned mines, especially those who live along the Vaal and Limpopo Rivers [8]. This alarming situation, combined with the threat that climate change poses to food security, necessitates use of less expensive, generally accessible and ecologically friendly alternatives in order to resolve the issue facing the nation. The purpose of this study is to investigate whether AMD treated with FA is appropriate for irrigation of potatoes and to examine the impact that this type of water has on the crop’s biochemical composition, physiological characteristics and rhizosphere.
1.1. Deficits of Some Techniques for Reducing Acid Mine Drainage Water’s Toxicity
Although there are many different AMD water treatment methods and processes, they all have disadvantages, such as high prices and challenges with heavy precipitation that causes insolubility [9,10]. For instance, multiple studies have employed phosphate rock to treat AMD. Phosphate is typically far more expensive than other calcium-based supplements, even though it is needed in comparable amounts [11]. However, practical use of limestone to AMD is restricted by its low solubility and propensity to create an external coating or armor of ferric hydroxide, Fe(OH)3, when applied [12,13]. On the other side, wetlands are usually recommended to lessen the number of heavy metals in contaminated water. A wetland system has three basic processes: (1) soil and substrate, (2) hydrology and (3) plants. The heavy metal removal mechanisms in wetlands are highly complex due to the interdependence of the entire process and the detrimental effects that heavy metals have on such crucial ecosystems [14].
1.2. Use of Fly Ash (FA) to Ameliorate Heavy Metal in Acid Mine Drainage
According to Yunusa et al. [15], FA can be utilized as an adsorbent to extract common colors from AMD. Use of fly ash for a variety of beneficial purposes, including waste reduction, cost-effective trash disposal and production of goods with additional value, has been researched [16]. It was demonstrated that FA could improve the physical, chemical and biological characteristics of troublesome soils while still maintaining a supply of readily available macro- and micronutrients for plant absorption. Use of fly ash in damaged soil increases plant biomass output [15,16]. FA is a good soil ameliorant and may well boost soil fertility and productivity. Elsewhere, FA was successfully used to purify water, an important source of plant nutrients (e.g., Ca, Mg, K, P, S, B, Fe, Cu and Zn), and remove heavy metals from various polluted water bodies [17,18]. There are concerns about future lime supplies, particularly that deposits may be depleted by 2050 [15]. The pH of water goes from neutral to alkaline when additional salt is added [19]. This principle is applicable in the current study, both in terms of AMD treatment and use of fly ash. Even though some heavy metals are hazardous when in solution, heavy metals can form solutions in water [20].
The above-mentioned research mentions several strategies that have been used in the past to lessen the harmful effects of AMD water. However, none of the solutions entail treating AMD with FA to evaluate its possible use in crop production. This approach has received little, if any, research and is not currently described in the literature. The water dilution strategy is predicted to reduce the detrimental effects of AMD, and the resultant water can be used to irrigate crops that are safe for human consumption and have little to no environmental impact. According to a recent study [21], AMD and fly ash were employed to reduce concentrations of metalloids. Other studies [22,23] previously discovered higher quantities of Cd, Co, Cu, Li, Hg, Sr and Zn in rice leaves, which they felt led to production of necrotic areas and significant degradation of Rubisco, particularly Rubisco LSU. Nutritional and metabolomic profile (molecular) elements of various plants have long been studied by researchers [24]. There is presently no research available that examines how heavy metals affect the metabolites in potatoes (treated or untreated with fly ash).
Potatoes (Solanum tuberosum L.) are grown for food and are the world’s fourth most significant food crop after rice, maize and wheat. Cultivation of this crop generates much economic activity all over the world. However, it is important to note that it takes a great deal of water for irrigation [25,26,27]. Even though South Africa is a water-scarce country, there are considerable areas of land dedicated to potato farming. Furthermore, in some places where additional irrigation is used for potato production, surface water is polluted by mining by-products, such as acid mine drainage and/or coal-burning fly ash. Acid mine drainage is a serious environmental issue because it contains relatively high concentrations of heavy metals (Hg, Pb, Cu, Sr and SO4), which can persist and bio-accumulate in plant cells, causing health risks to humans and animals after consumption [28]. FA, on the other hand, has been shown to reduce heavy metal concentrations in soils, such as Cd, Hg, Ni, Al and Ti [27,28]. It has been proven, for example, to reduce heavy metal toxicity in contaminated waters [29].
1.3. Heavy Metal and Impacts of Acid Mine Drainage on Crop Nutritional Composition and Yield
A study by Westermann [30] observed that contamination of waterways and/or croplands with heavy metals was a well-known environmental problem that poses a major hazard to both animal and human life. As the world’s population increases, the demand for food, electricity and minerals also rises, and, in an effort to meet some of these demands, numerous pollutants are released into the environment, eventually contaminating the food chain [31]. Edible plants are the primary source of nutrition for both animals and humans, and their contamination with hazardous metals can pose serious health risks, resulting in a variety of devastating diseases. Although plants exposed to heavy metals adopt mechanisms that reduce their uptake and/or toxicity [32], these are not enough to have a significant effect, especially in soils where metal concentrations are high. Other studies confirmed that heavy metal concentrations accumulate in the plant organs in P. australis [32]. Therefore, there is a need to assess alternative strategies that can help reduce the toxicity and/or concentration of especially metals contained in irrigation water. One of these strategies includes amendment of acid mine drainage with fly ash. Thus far, there is no published study that has assessed the nutritional composition of South African potato cultivars grown under AMD ameliorated with fly ash.
2. Materials and Methods
2.1. Preparation of Samples
Acid mine effluent used in the experiments was obtained from one of South Africa’s gold-producing mines, Sibanye, in Randfontein, Gauteng Province, while the FA was collected from one of ESKOM’s electricity-generating plants, Ulula, in Mpumalanga Province. AMD was mixed with FA (FA-treated AMD) at different ratios of 1:1, 3:1 (v/w), while untreated AMD and control (tap water) were expressed by ratios 1:0 and 0:0 (Control), respectively. Solution 1:1 was made up of 50% AMD and 50% FA, 3:1 contained 75% AMD and 25% FA. Untreated AMD, that is, 1:0, contained 100% AMD, and 0:0 (Control) contained 0% FA and tap water. Each FA: AMD irrigation (FA-treated AMD) solution was mixed in a 220 L barrel. A stirrer was used to agitate the mixture for 30 min, and AMD contact time with FA was timed using a stopwatch. A benchtop pH meter (Model: ADWA-AD 1020, Hungary) was calibrated, and samples were measured for pH change during 6 spaced periods using procedures and methodologies [33]. The pH was assessed as impacted by FA at different time intervals and temperatures using one sample from each container and one tap water sample taken according to well-established sampling protocols.
2.2. Collection and Processing of Soil and Plant Material
The research was completed over the course of two growing seasons, from April to July 2018 and from September to December 2018 (2nd season). The plants were cultivated at the Florida Science campus of the University of South Africa (26°10′30″ S, 27°55′22.8″ E) in a greenhouse with a controlled average temperature of 30 °C. A growth medium was prepared using the topsoil, river sand and vermiculite at a ratio of 3:1:1, and the medium was sterilized. One bag of 20 kg of 3:2:1 (24) + Zn mixed fertilizer was mixed with the growth medium. Certified seeds of two cultivars, namely Lady rosetta (determinate) and Fianna (indeterminate), were obtained from First Potato Dynamics (Pty) Ltd. in the Western Cape Province and were stored at the National Potato Co-op (Nationale Aartappel Kantoor (NAK)). The storage facilities were in Bethal, Mpumalanga Province. All the seed tubers were first stored at 3 °C, later transferred to 15 °C after 14-days and then 30 °C intervals until the experiments were completed. The pot (20 × 20 cm) experiment was a completely randomized design with five (5) replicates per treatment. There was a 35 cm gap between each pot. There were 12 potted plants per block, totaling 48 plants per block. The experiment involved a total of 144 plants. Treatments included a control group (Treatment 1), a 50% FA: AMD ratio (Treatment 2), a 75% FA: AMD ratio (Treatment 3) and 100% AMD (untreated AMD- treatment 4). Before being subjected to the treatments, plants were thoroughly watered with tap water. Two weeks after the seedlings were established, irrigation treatments were carried out. After 120 days following planting, when the plants were mature, harvesting was carried out [34,35,36,37].
2.3. Yield and Yield Components (Number of Tubers and FTW/DTW)
Plants were harvested at the maturity growth stage, that is, 120 days after planting [34,35,36,37]. The number of tubers per plant, fresh tuber weight and dry tuber weight were all growth characteristics that were measured during harvest. The harvested tubers were rinsed with tap water, dried with paper towels and weighed to ascertain their fresh tuber weight. The dry tuber weight was determined by fresh tubers through freeze-drying tubers in a freeze dryer (Labconco, Free Zone Plus 2.5 Liter Cascade Benchtop Freeze Dry System, USA) and weighed to calculate the yield.
2.4. Moisture and Ash Content Determination
For the purpose of calculating moisture content, 2 g of each plant sample was weighed into pre-weighed crucibles, and the contents were then dried in a desiccator. Thereafter, samples were heated in an oven (Labotec Ecotherm) at 100 °C for 3 h, removed, cooled in desiccators and weighed [38].
Moisture content (%) = W2 − W3 × 100 ÷ W2 − W1
W1= Tarred weight
W2= Weight of sample and container
W3 = weight of dried samples and container
Ash content determination
In determining ash content, 2 g of each sample was weighed into a crucible and ashed at 600 °C in a muffle furnace (LM 84, Ultra-furn (SA) (PTY) Ltd., Germiston, South Africa) overnight. The sample was weighed the following day [38], and the proportion of ash was determined by deducting the ash weight from the initial weight.
Ash (dry basis) % = MASH ÷ MDRY × 100
MASH = where MASH refers to the mass of the ashed sample,
MDRY = and MDRY refer to the original masses of the dried
2.5. Protein Content Determination
Crude protein content was determined following the method by Dumas [38]. Finely ground samples were analyzed for crude protein on a LECO CN analyzer (TruMac Argon, South Africa). Samples were combusted at 1350 °C in the presence of oxygen (Hi Q), whereby the carbon and nitrogen are converted to CO2 and NOx, respectively. Both gases are measured in a thermal conductivity cell after being separated by chromatography, with argon acting as a carrier gas. By multiplying the observed nitrogen content (N) by a common protein factor (6.25) [39], the crude protein content of samples was determined.
2.6. Sample Preparation for Fiber Content Determination
The crude fiber content was determined by Weende’s method [39]. Shortly, 2 g of each powdered sample was weighed into the crucible and 1.25% sulfuric acid was added to make up to 150 mL. Samples were placed on a hot plate, followed by addition of three to five drops of n-octanol. The supernatant was boiled for 30 min and sulfuric acid was drained. The samples were then washed three times using 30 mL of hot deionized water. Thereafter, 150 mL of preheated potassium hydroxide (KOH) 1.25% and 3–5 drops of antifoam were added, the contents were boiled for 30 min, filtered and washed three times with hot deionized water.
2.7. Sample Preparation for Fat Content Determination
The fat content was determined using the Soxhlet fat extraction method [40]. Dried sample of 2 g was weighed into thimbles, followed by addition of 200 mL of petroleum ether and the contents were boiled at 40–60 °C for 6 h. After the mixture had cooled, the floatable petroleum ether was gathered and emptied into a different container for later use. The weight of the fat content in the thimbles was measured after the petroleum ether-free flask had been heated to 105 °C for an hour. The flask was ultimately taken out of the oven and placed in a desiccator to cool before being weighed.
% Crude fat = W1 + W2 − W3 × 100 ÷ W2
W1 = Initial thimble weight
W2 = Sample weight
W3 = Weight of sample and thimble after extract
2.8. Carbohydrate Determination
For carbohydrate content, the formula below, the arithmetic difference approach [41], was used to determine the amount of carbohydrates in test samples:
Total Carbohydrate = 100% − (% fat + % ash + % fibre + % protein)
2.9. Determination of Concentration of Mineral Nutrients in Tubers
Plant samples were digested using the reference method by the EPA 3050B. About 1 g of dried potato tuber samples was weighed and milled into a medium wall digestion tube (4IS 0 mm id × 276 mmm length). About 15 mL of concentrated nitric acid (65%) (Merck, South Africa) was added, and, after 2 h, contents were placed on a cold heating (digestion) block and heated to 100 °C until fumes reduced. Thereafter, contents were re-heated in increments of 10 °C up to a maximum of 140 °C for an hour to ensure complete digestion of plant material and as shown by development of a yellow color 41. After adding about 5 drops of AR-quality hydrogen peroxide (Chem lab supplies, Johannesburg, South Africa), the mixture was heated for 5 to 10 min, cooled and then washed with de-ionized water into a 100 °C volumetric flask. Preparation and analysis of plant samples followed [42]. The concentrations of heavy metals in plant material were recorded on a fresh- and dry weight basis. The supernatant was filtered into a storage vessel as described by [43]. The heavy metals were analyzed from potato tubers using an Inductively coupled Plasma Mass Spectrophotometer (ICAP 6300, Thermo Electron, Santa Clara, CA, USA) from the University of South Africa, Florida, South Africa. These ions are then separated and detected by the mass spectrometer following the method EPA 3050B procedures.
2.10. Mineral Analysis for FA-Treated and Untreated Acid Mine Drainage
The microwave digester system (MDS-6G, Separation Scientific, Johannesburg, South Africa), an instrument with pressure relief designed for hot acid and used for heavy metal digestion, was used for analysis of samples for heavy metal concentrations as described in [41,42,43]. The calibrated microwave unit had programmable power and a turntable minimum of 3 revolutions per minute (rpm) to ensure homogenous distribution of microwave radiation and was used for preparation of the samples for analysis. The digestion vessels were cleaned by leaching with hot hydrochloric acid (Merck, Modderfontein, South Africa) (1:1) for 2 h and thereafter rinsed with de-ionized water and weighed before use. Well shaken samples of 45 mL each were transferred into the digestion vessels and 5 mL concentrated HNO3 pipetted into each. After completion, the vessels were allowed to cool for at least 5 min in the unit before removal from the microwave. The samples were further cooled outside the microwave by letting them cool in a water bath of 25 °C. The digested material was filtered through a Whatman (No. 1) filter paper directly into acid-washed and well-rinsed plastic containers and was made up to the 30 mL mark in volumetric flasks and was analyzed. Elements Cd, Pb, Zn and Ni were determined using flame atomic absorption spectrophotometer waterbuck (ICP-MS Laser Ablation) (ICAP 6300, Thermo Electron, Santa Clara, USA).
3. Data Analysis
Analysis of variance (ANOVA) was performed on the nutritional composition data using R (R Core Team, 2017) and packages Hmisc [44], lme4 lsmeans [45]. All significant pairs of treatment means were compared using Tukey HSD test at a 5% level of significance. A t-test was also conducted to determine differences between the season in the performance (yield parameters and proximate composition) of the potato cultivar to AMD treatments. The F-test for homogeneity of variances showed significant differences for the parameters; thus, separate analysis was conducted for the seasons. Using a two-way ANOVA, all metrics (PIXI) and basic analysis were compared. The post-hoc Duncan’s Multiple Range Test and other letters were used to denote differences and differentiate the mean values at p ≤ 0.05 in cases where the treatments were distinct. Raw data collected from this study were subjected to a normality test before analysis using STATISTICA software Program version 10.
4. Results
4.1. Samples Irrigated with Treated and Untreated FA: AMD Ratios
Plants that were irrigated with 75% AMD in both Season 1 and 2 had marked differences in moisture content compared to the control. Across seasons, the mean moisture content of Lady rosetta showed a marked difference compared to Fianna (Table 1). Using a two-way ANOVA analysis, the yield component effects were significantly different. For example, AMD-treated plants exhibited significantly increased tuber yield components for each cultivar as compared to the control. Interestingly, the cultivars responded differently to the treatments in terms of tuber yield components. Specifically, Lady rosetta’s number of tubers per plant was significantly different compared with the control with the mean of (3.01–4.36) and was markedly more compared to Treatment 1 (4.75–5.93). Furthermore, the Fianna cultivar had significantly the lowest number of tubers (2.35–3.21) in comparison with the Lady rosetta cultivar (5.32–6.18). There were no significant differences in the number of tubers irrespective of the season. The interaction between treatments and the control, as indicated in Table 1, did not show any significant differences.

Table 1.
Mixed procedure for tests (type 3) of the fixed effects (AMD treatments, cultivar, season and their interactions) on proximate composition (%) and yield parameters (number of tubers (n) and average fresh tuber weight (in grams)).
Nickel (Ni) is needed by plants for absorption of iron and seed germination. However, its deficiency causes plants to produce nonviable seeds [46,47,48]. Its application through fertilizer protects crops from certain yield-limiting diseases, potentially lowering pesticide use and increasing crop production [49]. Irrigating the Fianna and Lady rosetta cultivars with 0:0 AMD resulted in a similar concentration of Ni in their tissues (0.09 mg/kg). On the other hand, watering with 1:1 AMD recorded 1.77 mg/kg and 1.68 mg/kg Ni for Fianna and Lady rosetta cultivars, respectively. Fly ash mixed with AMD seemed to have ameliorated acid mine drainage; hence, the tubers recorded the lowest amount of Ni. This was shown when the potatoes were irrigated with 3:1 FA/AMD, which resulted in accumulation of 0.01 mg/kg Ni in Fianna and 0.19 mg/kg Ni in Lady rosetta.
Comparing the moisture content in both seasons of the two cultivars to their respective controls, long-term irrigation with the various treatments had a significant impact on both (Table 1). Similarly, the treatments (0:0; 1:1, 3:1 FA-AMD, and untreated AMD) significantly affected the crude protein content of both cultivars compared to controls. In all these, Lady rosetta exhibited higher crude protein compared to Fianna for all treatments but not significant in both seasons (Table 1). In the first planting season, the highest crude protein values for both cultivars were shown when they were exposed to the 1:1 FA-AMD treatment (Table 1). This observed growth inhibition in both cultivars could be attributed possibly to phyto-accumulation of heavy metals in plant organs. In this study, potato plants were subjected to acid mine drainage treatments that, over time, inhibited nutritional composition. As a result, both cultivars produced significantly fewer tubers than their respective controls in both seasons and had lower values of fresh tuber weight and dry tuber weight (yield), especially at 50 and 100 AMD. However, both cultivars exhibited higher fresh tuber weight compared to their controls at 75% AMD (Figure 1a,e).
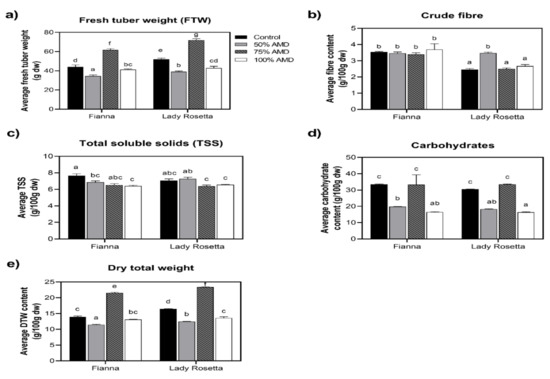
Figure 1.
(a–e) As influenced by the AMD treatment × cultivar interaction, fresh tuber weight, dry tuber weight, crude fiber and total soluble solids content, and t-test least significant difference: different letters denote that the samples are statistically different (0.05 level).
4.2. Effects of Treated and Untreated FA: AMD Ratios on Moisture Content
The moisture content of the selected potato cultivars ranged between 52.8 and 60.8% (Table 1). This has been reported in the literature, as shown by Liang et al. [50]. Moreover, values shown in other literature were based on techniques including the ‘wet basis’, which was equally adopted in this study. In this study, the moisture content was highest in Treatment 1 (control) and least in Treatment 2 (1:1 FA-AMD ratio). The significantly highest moisture content provides a suitable environment for high activities of water-soluble enzymes and co-enzymes needed for metabolic activities of plants. The crude fiber was in the range of 2.46–3.69% (Table 1). These values are dissimilar to results reported by [50,51], who found that the fiber content of Solanum indicum L. was about 1.85%. The highest fiber content was recorded in plants grown with the supply of 50% AMD (3.47%), followed by the control (3.18%), the 1:0 FA-AMD ratio (3%) and 3:1 FA-AMD ratio (2.95%), respectively. These results could be explained by the fact that different cultivars were used in the study. It is common that cultivars of the same plant species differ in moisture content. Again, it could also be a result of the stress that could have been imposed by the heavy metals that could have increased water uptake except in the control.
4.3. Effects of Treated and Untreated FA: AMD Ratios on Crude Protein
The results in Figure 1 showed that samples revealed highest crude protein content when supplied with 0:0 FA-AMD (7.20%), followed by irrigated with 73:1 FA AMD (6.76%), while the least values were recorded in potato supplied with 1:1 FA-AMD (1:0 FA-AMD (3.71%) (Table 1). The variations shown in these results relative to the published literature may have been due to different environmental conditions of sampling areas and differences in potato cultivars. For example, there are no published reports on these parameters using South African cultivars Fianna and Lady rosetta that were used in the present research. Table 1 below displays the amount of ash in the studied potato samples.
4.4. Effects of Treated and Untreated FA: AMD Ratios on Potato Crop Ash and Lipids Content
The ash content was in the range 2.51–4.82% and close to values reported by Dunbar et al. [52], where the ash content was 5.11% average for 14 different potato cultivars. In this study, the highest ash content (4.82%) was recorded when the Fianna cultivar was watered with (0:0), followed by that irrigated with 3:1 FA-AMD (3.69%), 1:1 FA-AMD (2.66%) and the least in 100% AMD (2.57%). In the current study, the highest percentage was reported in plants supported with 0:0 FA-AMD (1.59%), followed by 1:0 FA-AMD (1.43%), 1:1 FA-AMD (1.39%) and least observed was in 3:1 FA-AMD (1.34 %) (Table 1). Both cultivars’ ash contents were considerably impacted over time by the treatments used in this investigation. For example, both Treatment 4 (2.40–2.83 g) and Treatment 2 (2.47–2.90 g) were lower compared to Treatment 1 (4.54–4.96 g) and Treatment 3 (3.48–3.91 g), A similar pattern was observed in Season 2 (Table 1). Although lipids are the main energy sources, they should not include more than 30 calories per serving to prevent obesity and other linked disorders. The highest values were recorded in both cultivars, with 33.5% in Treatment 1 (control) and 16.4% and 16.5% for Lady rosetta and Fianna, respectively.
4.5. Effects of Treated and Untreated FA: AMD Ratios on Potato Crop Yield and Total Soluble Solids
When the quantity of tubers and total soluble solids for Season 1 were evaluated, the study’s findings showed no significant variations between cultivar and treatment interactions. However, there were significant differences for the number of tubers per plant and tuber fresh and dry weights (Figure 1a,e). For example, in AMD treatments, significant differences when irrigated with Treatment 3 were noted for tuber numbers per plant increase, and tuber fresh and dry weights increased when the two cultivars were compared. The results of this study demonstrate a considerable impact of AMD combined with FA on the nutritional composition of Fianna and Lady rosetta potatoes. Moreover, irrigating the potatoes with 50% and untreated AMD decreased their moisture (10%) and carbohydrate (8%) content. Nonetheless, the selected cultivars revealed no significant difference in both seasons for both cultivars when irrigated with all four FA: AMD ratios.
In Season 2, Lady rosetta had a significantly greater mean number of tubers per plant (5.32–6.18 g) than Fianna (2.35–3.21 g). The tuber fresh and dry weights of Fianna (47.8–49.0 g and 15.5–16.0 g) and Lady rosetta are not significant (47.9–49.1 g and 15.5–16.0 g), respectively (Figure 1a,e). Again, plants irrigated with the 75% AMD treatments had the highest number of tubers per plant compared to the control, which was also reflected in the tuber fresh and dry weights. The fly ash could have absorbed the heavy metals; hence, Treatment 3 improved the tuber fresh yield in both Fianna and Lady rosetta, respectively, when compared to the untreated AMD.
There were no significant differences noted for the total soluble solids when the cultivars were compared in Season 2 (Figure 2c). Moreover, in Season 2, there were no significant differences in tuber numbers per plant, tuber fresh and dry weights. For example, the 1:1 and 3:1 FA/AMD irrigation regimes revealed significant differences in tuber numbers per plant, tuber fresh and dry weights when the two cultivars were compared (Table 1). Plants treated with 75% AMD had the highest yields. In this study, because the quantities of heavy metals were not harmful during tuber bulking and development was only slowed before harvest, it is possible that treatments 2, 3 and 4 FA-AMD enhanced the tuber yield (numbers and weight per plant) of both cultivars [50].
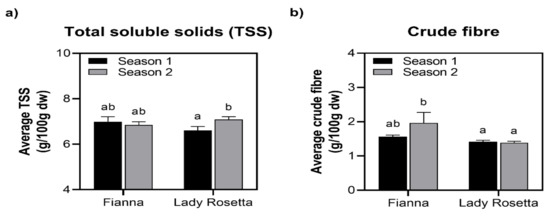
Figure 2.
(a,b) Interaction between cultivar and season as it relates to total soluble solids and crude fiber content, and t-test least significant difference indicates that samples with different letters are statistically different (0.05 level).
5. Discussion
Major and trace elements are required during the eight weeks after planting when potato plants are known to blossom and begin tuberization. In this study, the required macro-micronutrients could have been supplied by AMD and fly ash. Inevitably, the fact that the cultivars’ responses to prolonged exposure to the treatments varied in terms of growth and tuber yield could imply genetic variations in the cultivars’ capacity to absorb, translocate and accumulate heavy metals. These results are consistent with research by [51,52], which found genetic variations in accumulation of Cd and other metals in organs of two potato cultivars grown in heavy-metal-contaminated soils in Australia.
Although it is crucial to be aware of the elemental concentrations in irrigation water, even when they are lower than what is recommended for irrigation water in South Africa [52], using these methods to irrigate plants over time can result in a buildup of metals in soils as well as their absorption, translocation and accumulation in plant organs. It is also critical to understand the level of heavy metals present in farmland soils, particularly soils employed in experimental settings. According to reports, heavy metal stress has a suppressive impact on plants’ general growth, which may be observed, for instance, in decreased shoot growth in plants such as potatoes and spinach [50,51,52,53], as well as a decrease in the quantity of pods and seed output in pigeon peas [53]. Exposure to excessive levels of heavy metals in plants in previous research with potatoes (Solanum tuberosum L.) [54], Brassica oleracea var. capitatae [55], Amarunthus hubridus [56], Oryza sativa [57], Triticum [58] and Brassica juncea [59] also showed a reduction in some nutritional composition and yield.
When compared to the Lady rosetta cultivar (50.8–52.0 g and 16.2–16.7 g, respectively), the tuber fresh and dry weights of the Fianna cultivar (44.8–46.0 g and 14.8–15.3 g) were significantly higher (Table 1). The differences were probably due to genetic differences in their inherent tuber sizes; that is, the Fianna cultivar generally has a bigger tuber size compared to Lady rosetta. In their study, [59] reported that tubers from different cultivars tend to exhibit varying sizes and weights, probably as a result of genetic make-up. The results of this study support that by Connel et al. [60], who reported that different irrigation regimes affect cultivars differently due to diverse genotypes. The literature shows that seasons cause differences in yields of potatoes [61].
Dietary fiber (Figure 1b) is an essential component of a healthful diet, responsible for absorption of water as well as in provision of assistance to food matter during transit in elementary systems [62,63]. In this investigation, the test samples’ protein concentration varied from 3.24 to 76.4%. Interestingly, these values are less than those reported in the literature by [64] for 14 different cultivars of Solanum indicum L.
Ash content accounts for minerals present in each food sample, which perform various physiological functions in body systems [65]. The fat content of the test potato cultivars ranged between 1.31 and 1.57%. The results were slightly higher compared to the values of [50,65], who showed 0.30% as the average lipid content of 14 potato cultivars. Nonetheless, a diet that provides one percent of its caloric energy as fat is said to be adequate in humans because some cardiovascular diseases, such as atherosclerosis, cancer and aging, require excess intake [54,65]. The carbohydrate content ranged between 16.5 and 33.5%, and the values are similar to those in Oladele and Fadare [65].
The research discovered no discernible variations in total soluble solids between the crop seasons (Figure 1c). In contrast to the control, plants irrigated with the 3:1 FA-AMD treatments had the maximum number of tubers per plant, which was evident in the fresh and dry weights of the tubers. The explanation for the difference noted in the 3:1 treatment could be that it contained fewer toxins, and, therefore, the fly ash could have been able to adsorb the heavy metals in the acid mine drainage. Such results agree with [61,62,63,64,65]. Potato tubers irrigated with 1:0 FA-AMD (6.31°Brix) exhibited the highest total soluble solids, while the least were recorded in plants irrigated with 0% AMD treatments (7.37) (Figure 2). A study [65,66,67] showed that heavy metal concentration in irrigation water significantly reduces the tuber fresh and dry weight of Fianna and Lady rosetta cultivars. Their results agreed with previous findings by Garrido et al. [68], where they agreed that acid mine effluents considerably affected tuber growth and the size thereof.
Garrido et al. [68] claim that exposure to Cd through food is a serious risk to human health because it may induce cancer, kidney tubule damage and bone fractures. The finding of this study, therefore, is significant in that Cd can be reduced to acceptable levels by Treatment 3:1 (fly ash: acid drainage). Mixing FA with AMD at a ratio of 3:1 has also been shown [66,67,68,69] to adsorb heavy metals. Other researchers [68,69,70,71] attested that, despite that Cd is a non-essential element, its ions are distributed throughout organs of potato cultivars, and it is detrimental to plant growth and human health. The purpose of this study was to determine if variations in heavy metal concentrations in tubers were caused by cultivar Fianna’s naturally higher plant absorption or by modifications in the heavy metals’ distribution throughout the plant. The concentration of mineral nutrients was often higher in Fianna tubers than Lady rosetta tubers.
Some of the selected elements, such as V, Be and Ba, were below detection in all plant organs in all fly ash acid mine drainage treatments. This is because these elements are adsorbed and are not readily available [68,69]. On the other hand, eleven other elements (Al, Pb, Co, Ni, Cd, Fe, Ca, Mg, Zn, Cu and Mo) were detected in all parts of the potatoes that were irrigated with FA-treated AMD treatments (Figure 3). When compared to the control, the AMD treatments on potato tubers significantly (P 0.01) reduced the levels of Pb, Co, Ni, Cd, Fe, B, Zn and Cu (Figure 3). According to Table 2, the treated acid mine drainage showed significant differences in the concentration of minerals such as SO42−, Na, Ca, K, Mg and Mo as they were higher.
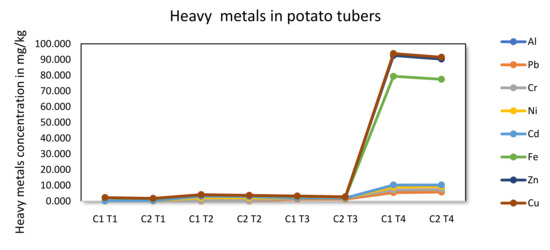
Figure 3.
Concentration of heavy metals in potato tubers (a) grown under irrigation with acid mine drainage using the Fianna (C1) and Lady rosetta (C2) cultivars. Values (mean S.E).

Table 2.
Depicts the heavy metal concentration in the FA: AMD irrigation treatments. At *** p < 0.001, values (mean S.E.) followed by similar letters in a column do not differ substantially from one another. = Not significant.
Figure 3 shows a significant increase in lead in the ameliorated and untreated AMD. Moreover, treatment 4 (1:0 FA-AMD ratio) demonstrated a significantly higher Pb concentration (0.02 mg/kg) in both cultivars compared to treatments that were ameliorated with FA and control (Figure 3), whereas Treatment 1 (0:0 AMD) displayed significantly lower Pb concentration (0.01 mg/kg for Fianna) and (0.02 mg/kg Lady rosetta) cultivars compared to treatment three (3:1 FA/AMD), with 0.00 mg/kg Pb for both cultivars, respectively. When irrigated with 0:0 AMD ratio, the test potato tubers exhibited 1.88 mg/kg and 1.33 mg/kg Fe, respectively, for Fianna and Lady rosetta (Figure 3). By contrast, 1:1 treatment ratio showed 0.77 mg/kg and 0.65 mg/kg for both cultivars, respectively. It can be said, therefore, that potatoes irrigated with 1:0 FA-AMD accumulated the highest Fe concentration (69.13 mg/kg and 67.18 mg/kg) compared to treatments that were ameliorated with FA and control (Figure 3). This is an indication that the fly ash could have absorbed Fe ions, thus reducing their availability in the potato crop.
The current study’s findings showed a substantial difference between the concentration of Cu in tissues of potatoes produced using treated and untreated acid mine drainage (Figure 3). Especially, irrigation with 1:0 FA-AMD recorded a Cu concentration of 1.22 mg/kg and 1.23 mg/kg for the respective cultivars compared to treatments that were ameliorated with FA and control (Figure 3). On the other hand, potatoes supplied with 0:0 FA-AMD displayed significantly lower Cu: 0.00 mg/kg for the Fianna and 0.00 mg/kg for Lady rosetta cultivars compared to watering with 3:1 FA-AMD, which resulted in 0.03 mg/kg Cu in Fianna and 0.02 mg/kg in Lady rosetta. All the FA-treated AMD-irrigated potatoes had significantly higher Cu levels compared to the control (Figure 3). The CODEX general standard limits for food pollutants and toxins in food and feed (CODEX STAN 193-1995), which indicate a maximum 0.3 mg/kg for potato, were met by these heavy metal concentrations [70,71,72,73,74]. The WHO and FAO recommend that the concentration of Cu in food should not be above 30 mg/kg, while that of the NAFDAC is 20 mg/kg for food. Table 2 shows that fly ash was able to ameliorate the copper ions in the 100% AMD, thus rendering the potatoes safe to consume.
6. Conclusions
In conclusion, according to this study, long-term irrigation with AMD combined with fly ash can stunt the growth of potato cultivars and lower their levels of crude protein (CP), lipids, carbs, fresh tuber weight and dried tuber weight. Furthermore, genetic variation in cultivar response to the different AMD and fly ash treatments was also noted in this study. In comparison to cultivars treated to a 1:1 FA-AMD treatment, those exposed to a 3:1 FA-AMD treatment had superior plant development and fewer total soluble solids in the tubers. Although some heavy metals are essential for plant growth, it is important to stress that the presence of Pb, Cd, Ni and Hg in the treatments was of major concern, especially their accumulation in potato tubers. These heavy metals are considered major environmental contaminants with serious health hazard implications in humans.
Most of the published studies to date have focused on the soil and environmental conditions that influence heavy metal accumulation in potatoes. Meanwhile, different agronomic strategies for reducing heavy metal concentrations in potato tubers, such as enhancing irrigation water quality, avoiding saline and acidic soil, lowering the level of heavy metals in fertilizers and improving zinc deficiency, have been suggested for future research. The mechanisms that limit cellular uptake of Cd, Zn, Pb and Ni, as well as their subsequent transit and allocation within the potato plant, have, to an extent, been ignored. Understanding these pathways may help researchers to create more effective methods for lowering tuber heavy metal concentrations, especially when it comes to breeding and creating potato cultivars with low tuber heavy metal accumulation. It was the main purpose of this project to describe the basic features of Cd, Zn, Pb and Ni uptake and translocation and to uncover the reasons behind genotypic differences in heavy metal accumulation in tubers. Last, all the FA-AMD-treated potatoes had Pb concentrations below the minimum standard limits, and this proves that FA was able to adsorb the Pb ions in the tuber samples. Importantly, fly ash reduces a metal’s concentration in AMD.
Author Contributions
M.V.R. is the main author and she initiated the project. R.M. assisted with the experimental design as well as data collection. N.I.M. assisted with the laboratory assays and analysis of the data. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author of this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Masindi, V.; Foteinis, S.; Renforth, P.; Ndiritu, J.; Maree, J.P.; Tekere, M.; Chatzisymeon, E. Challenges and avenues for acid mine drainage treatment, beneficiation, and valorisation in circular economy: A review. Ecol Eng. 2022, 183, 106740. [Google Scholar] [CrossRef]
- Masood, N.; Hudsone-Edwards, K.; Farooqi, A. True cost of coal: Coal mining industry and its associated environmental impacts on water resource development. J. Sustain. Min. 2020, 19, 35–149. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States–An overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Van Huis, A. Insects as food and feed, a new emerging agricultural sector: A review. J. Insects Food Feed. 2020, 6, 27–44. [Google Scholar] [CrossRef]
- Genty, T.; Bussière, B.; Potvin, R.; Benzaazoua, M.; Zagury, G.J. Dissolution of calcitic marble and dolomitic rock in high iron concentrated acid mine drainage: Application to anoxic limestone drains. J. Environ. Earth Sci. 2012, 66, 2387–2401. [Google Scholar] [CrossRef]
- Payus, C.; Ann Huey, L.; Adnan, F.; Besse Rimba, A.; Mohan, G.; Kumar Chapagain, S.; Roder, G.; Gasparatos, A.; Fukushi, K. Impact of extreme drought climate on water security in North Borneo: Case study of Sabah. Water 2020, 12, 1135. [Google Scholar] [CrossRef]
- Matabane, D.L. Identification Determination of Potentially Toxic Elements in Water and Sediments from Blood and Mokolo rivers in Limpopo Province, South Africa. Ph.D. Thesis, University of Limpopo, Mankweng, South Africa, 2019. [Google Scholar]
- Chen, G.; Ye, Y.; Yao, N.; Hu, N.; Zhang, J.; Huang, Y. A critical review of prevention, treatment, reuse, and resource recovery from acid mine drainage. J. Clean. Prod. 2021, 329, 129666. [Google Scholar] [CrossRef]
- Kumar, M.; Nandi, M.; Pakshirajan, K. Recent advances in heavy metal recovery from wastewater by biogenic sulfide precipitation. J. Environ. Manag. 2021, 278, 111555. [Google Scholar] [CrossRef]
- Abrams, S.A.; Atkinson, S.A. Calcium, magnesium, phosphorus and vitamin D fortification of complementary foods. J. Nutri. 2003, 133, 2994S–2999S. [Google Scholar] [CrossRef]
- Ziemkiewicz, P.F.; McDonald, L.M. Acid mine drainage formation, control and treatment: Approaches and strategies. J. Extr Ind. Soc. 2019, 6, 241–249. [Google Scholar]
- Mbamba, C.K. Using froth Flotation to Mitigate Acid Rock Drainage Risks While Recovering Valuable Coal from Ultrafine Colliery Wastes. Master’s Thesis, University of Cape Town, Cape Town, South Africa, 2011. [Google Scholar]
- Rai, G.K.; Bhat, B.A.; Mushtaq, M.; Tariq, L.; Rai, P.K.; Basu, U.; Dar, A.A.; Islam, S.T.; Dar, T.U.; Bhat, J.A. Insights into decontamination of soils by phytoremediation: A detailed account on heavy metal toxicity and mitigation strategies. J. Physiol. Plant. 2021, 173, 287–304. [Google Scholar] [CrossRef]
- Li, C.; Quan, Q.; Gan, Y.; Dong, J.; Fang, J.; Wang, L.; Liu, J. Effects of heavy metals on microbial communities in sediments and establishment of bioindicators based on microbial taxa and function for environmental monitoring and management. J. Sci. Total Environ. 2020, 749, 141555. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Ukhurebor, K.E.; Onyancha, R.B.; Osibote, O.A.; Darmokoesoemo, H.; Kusuma, H.S. Fly ash-based adsorbent for adsorption of heavy metals and dyes from aqueous solution: A review. J. Mater. 2021, 14, 2751–2774. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Emenike, E.C.; Iwuozor, K.O.; Okoro, H.K.; Ige, O.O. Acid mine drainage: The footprint of the Nigeria mining industry. J. Chem. Africa. 2022, 5, 1907–1920. [Google Scholar] [CrossRef]
- Shaheen, S.M.; Hooda, P.S.; Tsadilas, C.D. Opportunities and challenges in the use of coal fly ash for soil improvements. A review. J. Environ. Manag. 2014, 145, 249–267. [Google Scholar] [CrossRef]
- Ukwattage, N.L.; Ranjith, P.G.; Bouazza, M. The use of coal combustion fly ash as a soil amendment in agricultural lands (with comments on its potential to improve food security and sequester carbon). Fuel 2013, 109, 400–408. [Google Scholar] [CrossRef]
- Nemutanzhela, M.V.; Modise, D.M.; Siyoko, K.J.; Kanu, S.A. Assessment of Growth, Tuber Elemental Composition, Stomatal Conductance, and Chlorophyll Content of Two Potato Cultivars Under Irrigation with Fly Ash-Treated Acid Mine Drainage. Am. J. Potato Res. 2017, 94, 367–378. [Google Scholar] [CrossRef]
- Shi, Y.L.; Yang, W.; Ren, M.E. Hydrological characteristics of the Changing and its relation to sediment transport to the sea. Cont. Shelf Res. 1985, 4, 5–15. [Google Scholar]
- Chatterjee, C.; Gopal, R.; Dube, B.K. Impact of iron stress on biomass, yield, metabolism, and quality of potato (Solanum tuberosum L.). Sci. Hortic. 2006, 108, 1–6. [Google Scholar] [CrossRef]
- Farooq, M.; Anwar, F.; Rashid, U. Appraisal of heavy metal contents in different vegetables grown in the vicinity of an industrial area. Pak. J. Bot. 2008, 40, 2099–2106. [Google Scholar]
- Frossard, E.; Bucher, M.; Mächler, F.; Mozafar, A.; Hurrell, R. Potential for increasing the content and bioavailability of Fe, Zn, and Ca in plants for human nutrition. J. Sci. Food Agric. 2000, 80, 861–879. [Google Scholar] [CrossRef]
- Nandy, M.; Bhattacharya, S. Energy Issues in India and South Africa. J. Infrastruct. 2009, 7, 69–90. [Google Scholar]
- Da Silva, H.C.; De Souza, L.A.; Dos Santos, H.F.; De Almeida, W.B. Determination of Anticancer Zn (II)–Rutin Complex Structures in Solution through Density Functional Theory Calculations of 1H NMR and UV–VIS Spectra. Am. Chem. Soc. Omega 2021, 5, 3030–3042. [Google Scholar] [CrossRef] [PubMed]
- Bansal, S.K.; Trehan, S.P. Effect of potassium on yield and processing quality attributes of potato. Karnataka J. Agric. Sci. 2011, 24, 48–54. [Google Scholar]
- Elfnesh, F.; Tekalign, T.; Solomon, W. Processing quality of improved potato (Solanum tuberosum L.) cultivars as influenced by growing environment and blanching. Afr. J. Food. Sci. 2011, 5, 324–332. [Google Scholar]
- Balistrieri, L.S.; Seal, R.R., II; Piatak, N.M.; Paul, B. Assessing the concentration, speciation, and toxicity of dissolved metals during mixing of acid-mine drainage and ambient river water downstream of the Elizabeth Copper Mine, Vermont, USA. Appl. Geochem. 2007, 22, 930–952. [Google Scholar] [CrossRef]
- Harmanescu, M.; Alda, L.M.; Bordean, D.M.; Gogoasa, I.; Gergen, I. Heavy metals health risk assessment for population via consumption of vegetables grown in old mining area; a case study: Banat County, Romania. Chem. Cent. J. 2011, 5, 64. [Google Scholar] [CrossRef]
- Westermann, D.T. Nutritional requirements of potatoes. Am. J. Potato Res. 2005, 82, 301–307. [Google Scholar] [CrossRef]
- Antonious, G.F.; Snyder, J.C. Accumulation of heavy metals in plants and potential phytoremediation of lead by potato, Solanum tuberosum L. J. Environ. Sci. Health 2007, 42, 811–816. [Google Scholar] [CrossRef]
- Onder, S.; Caliskan, M.E.; Onder, D.; Caliskan, S. Different irrigation methods, and water stress effects on potato yield and yield components. Agric. Water Manag. 2005, 73, 73–86. [Google Scholar] [CrossRef]
- Lerna, A. Influence of harvest date on nitrate contents of three potato varieties for off-season production. J. Food Compos. Anal. 2009, 22, 551–555. [Google Scholar]
- Araujoa, G.C.L.; Gonzaleza, M.H.; Ferreira, A.G.; Nogueiraa, A.A.; No’bregab, J.A. Effect of acid concentration on closed-vessel microwave-assisted digestion of plant materials. Spectrochim. Acta. 2002, 57, 2121–2132. [Google Scholar] [CrossRef]
- Maier, N.A.; McLaughlin, M.J.; Heap, M.; Butt, M.; Smart, M.K. Effect of nitrogen source and calcitic lime on soil pH and potato yield, leaf chemical composition, and tuber cadmium concentrations. J. Plant Nutr. 2002, 25, 523–544. [Google Scholar] [CrossRef]
- Ogbonna, O.; Jimoh, W.L.; Awagu, E.F.; Bamishaiye, E.I. Determination of some trace elements in water samples within Kano Metropolis. Adv. Appl. Sci. Res. 2011, 2, 62–68. [Google Scholar]
- Dumas, J.B.A. Organic analysis methods. Anal. Chem. Phy. J. 1831, 247, 198–213. [Google Scholar]
- Thompson, M.; Owen, L.; Wilkinson, K.; Wood, R.; Damant, A. A comparison of the Kjeldahl and Dumas methods for the determination of protein in foods, using data from a proficiency testing scheme. Analyst 2002, 127, 666–1668. [Google Scholar] [CrossRef]
- Onwuka, G.I. Food Analysis and Instrumentation: Theory and Practice; Naphthali Prints Lagos: Lagos, Nigeria, 2005; pp. 1–219. [Google Scholar]
- Pearson, D. The Chemical Analysis of Foods, 7th ed; Churchill Livingstone: Edinburgh, Scotland; London, UK; New York, NY, USA, 1976; Volume 30, pp. 387–497. [Google Scholar]
- Bizzi, C.A.; de Moraes Flores, É.M.; Picoloto, R.S.; Barin, J.S.; Nóbrega, J.A. Microwave-assisted digestion in closed vessels: Effect of pressurization with oxygen on digestion process with diluted nitric acid. Anal. Methods. 2010, 2, 734–738. [Google Scholar] [CrossRef]
- Pan, X.Y.; Li, J.Y.; Deng, K.Y.; Xu, R.K.; Shen, R.F. Four-year effects of soil acidity amelioration on the yields of canola seeds and sweet potato and N fertilizer efficiency in an ultisol. Field Crops Res. 2019, 237, 1–11. [Google Scholar] [CrossRef]
- Galan, E.; Gómez-Ariza, J.L.; González, I.; Fernández-Caliani, J.C.; Morales, E.; Giráldez, I. Heavy metal partitioning in river sediments severely polluted by acid mine drainage in the Iberian Pyrite Belt. J. Appl. Geochem. 2003, 18, 409–421. [Google Scholar] [CrossRef]
- Aguinaga, O.E.; Wakelin, J.F.; White, K.N.; Dean, A.P.; Pittman, J.K. The association of microbial activity with Fe, S and trace element distribution in sediment cores within a natural wetland polluted by acid mine drainage. Chemosphere 2019, 231, 432–441. [Google Scholar] [CrossRef]
- Kuznetsova, A.; Brockhoff, P.B.; Christensen, R.H. lmerTest package: Tests in linear mixed-effects models. J. Stat. Softw. 2017, 82, 1–26. [Google Scholar]
- Bolker, B. Dealing with quasi-models in R. Compare 2017, 1, 45–59. [Google Scholar]
- Vadapalli, V.K.; Klink, M.J.; Etchebers, O.; Petrik, L.F.; Gitari, W.; White, R.A.; Key, D.; Iwuoha, E. Neutralization of acid mine drainage using fly ash, and strength development of the resulting solid residues. South Afr. J. Sci. 2008, 104, 317–322. [Google Scholar]
- Vadapalli, V.R.; Gitari, M.W.; Petrik, L.F.; Etchebers, O.; Ellendt, A. Integrated acid mine drainage management using fly ash. J. Environ. Sci. Health 2012, 47, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.H.; Welch, R.M.; Cary, E.E.; Checkai, R.T. Micronutrients: Beneficial effects of nickel on plant growth. J. Plant Nutr. 1987, 10, 2125–2135. [Google Scholar] [CrossRef]
- Chen, C.; Huang, D.; Liu, J. Functions and toxicity of nickel in plants: Recent advances and prospects. Clean Soil Air Water 2009, 37, 304–313. [Google Scholar] [CrossRef]
- Liang, M.U.; Zhou, T.H.; Zhang, R.F.; Sun, Q.H.; Xu, Y.W. Nutritional evaluation of different cultivars of potatoes (Solanum tuberosum L.) from China by grey relational analysis (GRA) and its application in potato steamed bread making. J. Integr. Agric. 2019, 18, 231–245. [Google Scholar]
- Aberoumand, A. Assay of Nutritional Potential of the Fruits of Solanum indicum L. in Iran. J. Agric. Technol. 2012, 8, 923–929. [Google Scholar]
- Dunbar, K.R.; Mclaughlin, M.J.; Reid, R.J. The uptake and partitioning of cadmium in two cultivars of potato (Solanum tuberosum L). J. Exp. Bot. 2003, 54, 349–354. [Google Scholar] [CrossRef]
- McLaughlin, M.J.; Williams, C.M.J.; McKay, A.J.K.G.; Kirkham, R.J.K.G.; Gunton, J.J.K.G.; Jackson, K.J.; Thompson, R.; Dowling, B.; Partington, D.; Smart, M.K.; et al. Effect of cultivar on uptake of cadmium by potato tubers. Aust. J. Agric. Res. 1994, 45, 1483–1495. [Google Scholar] [CrossRef]
- Zheng, N.; Wang, Q.; Zhang, X.; Zheng, D.; Zhang, Z.; Zhang, S. Population health risk due to dietary intake of heavy metals in the industrial area of Huludao city, China. Sci. Total Environ. 2007, 387, 96–104. [Google Scholar] [CrossRef]
- Pandey, N.; Sharma, C.P. Effect of heavy metals Co2+, Ni2+, and Cd2+ on growth and metabolism of cabbage. Plant Sci. 2002, 163, 753–758. [Google Scholar] [CrossRef]
- Akubugwo, I.E.; Obasi, N.A.; Chinyere, G.C.; Ugbogu, A.E. Nutritional and chemical value of Amaranthus hybridus L. leaves from Afikpo, Nigeria. Afr. J. Biotechnol. 2007, 6, 2833–2839. [Google Scholar] [CrossRef]
- Moya, J.; Ros, R.; Picazo, I. Influence of cadmium and nickel on growth, net photosynthesis, and carbohydrate distribution in rice plants. Photosynth. Res. 1993, 36, 75–80. [Google Scholar] [CrossRef]
- Öncel, I.; Keleş, Y.; Üstün, A. Interactive effects of temperature and heavy metal stress on the growth and some biochemical compounds in wheat seedlings. Environ. Pollut. 2000, 107, 315–320. [Google Scholar] [CrossRef]
- John, R.; Ahmad, P.; Gadgil, K.; Sharma, S. Heavy metal toxicity: Effect on plant growth, biochemical parameters, and metal accumulation by Brassica juncea L. Int. J. Plant Prod. 2009, 3, 65–76. [Google Scholar]
- Connel, B.S.; Cox, M.; Singer, I. Nickel and Chromium. In Disorders of Minerals Metabolism; Brunner, F., Coburn, J.W., Eds.; Academic Press: Cambridge, MA, USA, 1984; pp. 472–532. [Google Scholar]
- Akan, J.C.; Kolo, B.G.; Yikala, B.S.; Ogugbuaja, V.O. Determination of Some Heavy Metals in Vegetable. Samples from Biu Local Government Area, Borno State, North-Eastern Nigeria. J. Environ. Monit. Anal. 2013, 1, 40–46. [Google Scholar] [CrossRef]
- Lahlou, O.; Ouattar, S.; Ledent, J.F. The effect of drought and cultivar on growth parameters, yield, and yield components of potato. Agronomie 2003, 23, 257–268. [Google Scholar] [CrossRef]
- DeBuchananne, D.A.; Lawson, V.F. Effect of plant population and harvest timing on yield and chipping quality of Atlantic and Norchip potatoes at two Iowa locations. Am. Potato J. 1991, 68, 287–297. [Google Scholar] [CrossRef]
- Champ, M.; Langkilde, A.M.; Brouns, F.; Kettlitz, B.; Collet, Y.L.B. Advances in dietary fiber characterization. 1. Definition of dietary fiber, physiological relevance, health benefits, and analytical aspects. Nutr. Res. Rev. 2003, 16, 71–82. [Google Scholar] [CrossRef]
- Oladele, A.T.; Fadare, O.O. Heavy metals and proximate composition of forest leafy vegetables in oil-producing areas of Nigeria. EJESM 2015, 8, 451–463. [Google Scholar] [CrossRef]
- Antia, B.S.; Akpan, E.J.; Okon, P.A.; Umoren, I.U. Nutritive and anti-nutritive evaluation of sweet potatoes (Ipomoea batatas) leaves. Pak. J. Nutri. 2006, 5, 166–168. [Google Scholar] [CrossRef]
- Ceballos, H.; Sánchez, T.; Chávez, A.L.; Iglesias, C.; Debouck, D.; Mafla, G.; Tohme, J. Variation in crude protein content in cassava (Manihot esculenta Crantz) roots. J. Food. Compos. Anal. 2006, 19, 589–593. [Google Scholar] [CrossRef]
- Garrido, A.E.; Condori, J.; Strosnider, W.H.; Nairn, R.W. Acid mine drainage impacts irrigation water resources, agricultural soils, and potatoes in Potosi, Bolivia. JASMR 2009, 30, 486–499. [Google Scholar] [CrossRef]
- Greger, M.; Brammer, E.; Lindberg, S.; Larsson, G.; Idestam-Almquist, J. Uptake and physiological effects of cadmium in sugar beet (Beta vulgaris) related to mineral provision. J. Exp. Bot. 1991, 42, 729–737. [Google Scholar] [CrossRef]
- Ashrafzadeh, S.; Gaw, S.; Genet, R.; Glover, C.N.; Leung, D.W. Natural variation in correlations between cadmium and micronutrients in potato tubers. J. Food Comp. Anal. 2017, 59, 55–60. [Google Scholar] [CrossRef]
- Hamid, N.; Bukhari, N.; Jawaid, F. Physiological responses of Phaseolus vulgaris to different lead concentrations. Pak. J. Bot. 2010, 42, 239–246. [Google Scholar]
- Musyoka, N.M.; Petrik, L.F.; Hums, E. Ultrasonic assisted synthesis of zeolite A from coal fly ash using mine waters (acid mine drainage and circumneutral mine water) as a substitute for ultra-pure water. In Proceedings of the International Mineral Water Association, Aachen, Germany, 4–11 September 2011; pp. 423–428. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).