Abstract
The objective of this study is to provide an updated account of the distribution history of two invasive molluscs, Corbicula fluminea and Dreissena polymorpha, both in Europe and worldwide. In addition to this, the study also intends to review their ecological requirements to gain a better understanding of their invasive potential and distribution dynamics. Specifically, the study focuses on updating the distribution and ecological characteristics of these freshwater bivalves in the lower sector of the Danube River and the lakes of the Danube Delta. The purpose is to better understand their invasive and distribution dynamics and to develop effective measures to limit their spread in the future. To achieve this, environmental proxies such as sediment particle size and Total Organic Carbon (TOC) concentrations were used to assess their tolerances. However, the results did not show a significant correlation between the densities of these bivalves and the analyzed environmental parameters. Despite this, the species were found in high densities and formed well-developed benthic communities in some stations. The study contributes to the understanding of the invasiveness of these bivalve species and their distribution range dynamics. Nonetheless, further investigation is required to fully comprehend the role of environmental parameters in their distribution. The study covers the period between 2010 and 2020 and focuses on the lower Danube River sector and Danube Delta.
1. Introduction
The spread of non-native invasive species in aquatic ecosystems has increased rapidly in recent decades, causing significant changes in ecology and function [1,2,3,4]. Among all the introduced species, dominating species, by density or biomass, or species bringing new traits to the ecosystem should be studied first [5,6]. Invasive bivalves play a crucial role as they can reach high densities and biomasses, affecting primary and secondary production and energy flow, and altering the community structure through ecosystem engineering. These mechanisms caused by invasive bivalves can result in significant changes to ecosystem functions and services [7,8].
This study aims to update the distribution history of two invasive molluscs, Corbicula fluminea and Dreissena polymorpha, in Europe and all over the world and to review their ecological requirements in order to understand their invasive potential and distribution dynamics. This will further serve at preparing effective measurements to limit the spread of potentially harmful species. Our new data cover the lower Danube River sector (Romania, Bulgaria, Serbia) and Danube Delta (Romania, Ukraine) between 2010 and 2020. Environmental parameters (particle size of sediments, Total Organic Carbon–TOC content) were used to examine the ecological requirements of the species. Based on the knowledge of the ecological characteristics and distribution range dynamics, we aim to contribute to the understanding of the potential invasiveness of these bivalve species.
Worldwide Historical Records of Corbicula fluminea (Müller, 1774) and Dreissena polymorpha (Pallas, 1771)
Corbicula fluminea, the Asian clam, is native to Southeast Asia and is typically found in Guangzhou, China [9]. Its native range also includes Russia, Thailand, the Philippines, China, Taiwan, Korea, Japan, Australia, and Africa [9,10,11,12]. In Southeast Asia, the clam is used for aquaculture with over 12 million tons produced per year in China [13]. The meat of the mollusc is valued for its nutritional properties and is used in pharmacology [14,15]. The Asian clam was first documented in the Columbia River in Washington (North America) in 1924 based on empty shells and in 1938 based on living individuals [16,17], and later in South America, and Europe. It was first thought to have entered the US as a food item for Chinese immigrants, but there is no evidence to support this theory [18]. Another possibility is that it entered the US with the importation of the Giant Pacific oyster Magallana gigas from Asia. Following rapid spread, it reached high densities in the US and has been found in Canada, Mexico, Panama, Argentina, Brazil, Uruguay, and Venezuela [19,20,21]. The species was first recorded in Europe near the borders of Portugal (Tagus River estuary) and in France (Dordogne River) in 1980. In 1983, it was found in the Weser River in Germany and then spread to the middle reaches of Germany in 1990 near Karlsruhe in the Upper Rhine and has since spread to most European river systems and countries, including the Norfolk Broads drainage system and the Thames and Great Ouse rivers in the UK; the Barrow, Nore, and Shannon rivers in Ireland; Austria, Bulgaria, France (Garonne, Rhone, Loire, and Seine), and Luxembourg; the delta area of the Rhine River in 1988 [22]; Poland, Portugal, and Spain by 1991; Switzerland near Basel by 1995 [23,24,25]; the River Po basin in Italy, the Elbe basin; and in 1997, downstream of the mouth of the Isar River in Germany, the Czech Republic, and Central Europe [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45]. The fast spread of the species was believed to be due to transportation by cargo ships [29,46]. After appearing in the lower reaches of the Main River in 1991, its colonization of the Danube River through the Main–Danube canal, which opened for shipping in 1992, was expected [27,47]. Juvenile specimens were recorded in the Ukrainian sector of the Danube Delta in 1995. The species was introduced into the Danube River basin at different times and sectors with at least two main introductions: one in the Lower Danube and one in the Upper Danube in 1997 [48,49]. It has also been found in some of the Danube’s tributaries, such as the Sava, Tisza, Prut, and Hungarian side arms [50,51,52,53,54]. Subfossil specimens of C. fluminea were reported in the Romanian sector of the Danube Delta in 1997, in Moldova Veche, Berzasca (Iron Gate area), and Vadu Oii. Nowadays, it inhabits the entire length of the Danube and Danube Delta [55,56,57,58,59].
Dreissena polymorpha (zebra mussel) is a Ponto-Caspian relict species native to the Black, Azov, Caspian, and Aral Sea regions [60,61,62,63]. D. polymorpha was once widespread throughout Europe but retreated during the last glaciations and became restricted to these areas. In the 18th century, its distribution expanded again due to human activities, first appearing in Rotterdam in 1826, Hamburg in 1830, and Copenhagen in 1840 [64,65]. In Great Britain, it was first recorded in the London Docks in 1824 and rapidly became widespread in the next 10 years [66]; however, its distribution stabilized after 1850. It was first recorded in Bulgaria’s Danube River by Kreglinger in 1870. Subsequently, it was found in the Danube and its tributaries, and Black Sea coastal lakes and rivers. Fossil individuals were found in Pleistocene and Holocene sediments in North-West Bulgaria and along the Danube and the Black Sea Romanian and Bulgarian shelf zone [67,68,69,70,71,72,73,74,75,76,77,78]. The zebra mussel has also been recorded in the Ovcharitsa, Zhrebchevo, and Sopot reservoirs, Lake Chepintsi, and the Pyassachnik, Ticha, and Malko Sharkovo reservoirs [79]. D. polymorpha later invaded Ireland and Spain [64,80,81,82,83]. The zebra mussel was introduced from Europe to the Laurentian Great Lakes in North America in the late 1980s [84]. It soon became a problem due to their rapid growth and tendency to attach to and foul structures, causing water access difficulties for drinking water facilities, power plants, marinas, and industries [85]. Their presence also became a nuisance for boaters, fishermen, beachgoers, and water navigators as they colonized solid structures in the water [85]. The impact of the zebra mussel invasion was revived when it was discovered in the North American Great Lakes in the 1980s and has since spread across the United States and southern Canada [86].
2. Materials and Methods
From 2010 to 2020, we collected qualitative and quantitative data on C. fluminea and D. polymorpha at 153 stations located from km 1075 of the lower Danube River down to the outer delta branches (Figure 1, Supplementary Materials S2). In total, 368 samples were analyzed (Figure 1, Supplementary Materials S1). Macrozoobenthic sampling is part of GeoEcoMar’s monitoring network for the study of biodiversity and assessment of the biological quality of watercourses on the Lower Sector of the Danube River and in the lakes of the Danube Delta.
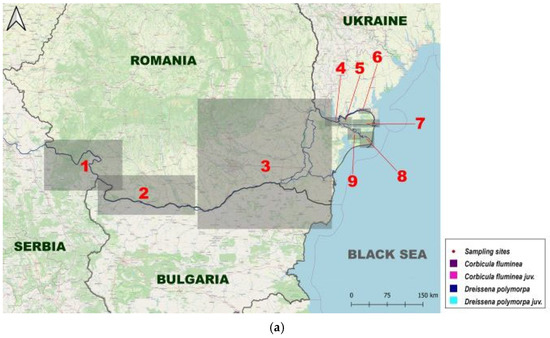
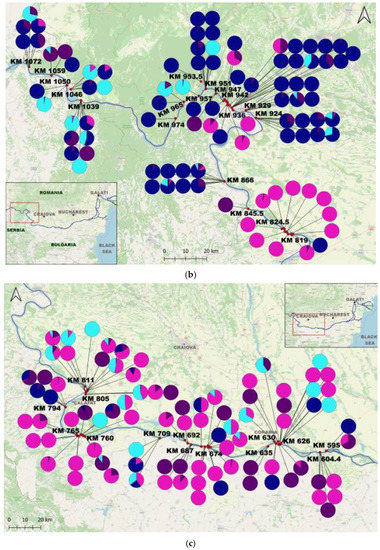
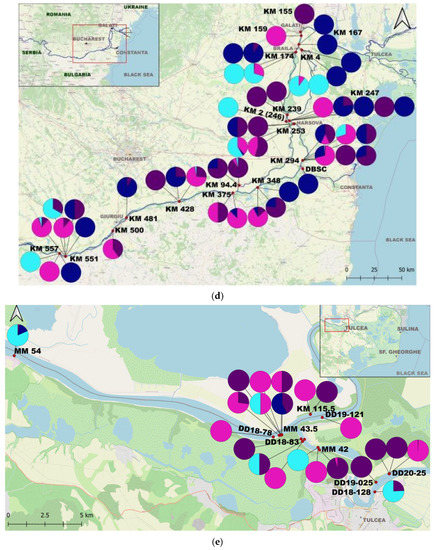
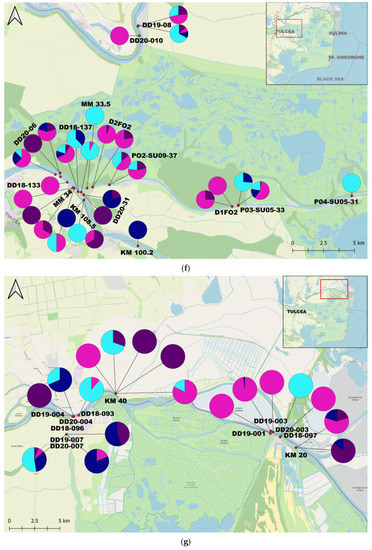
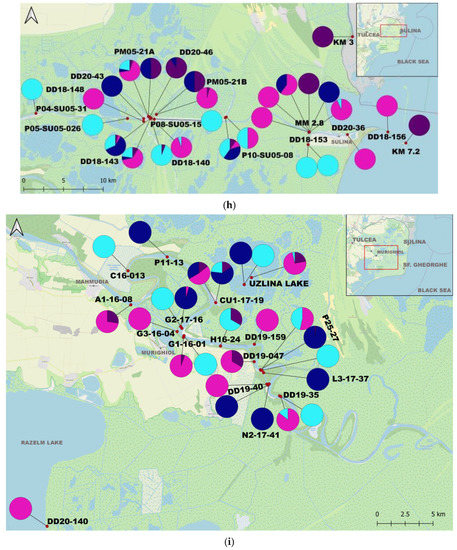
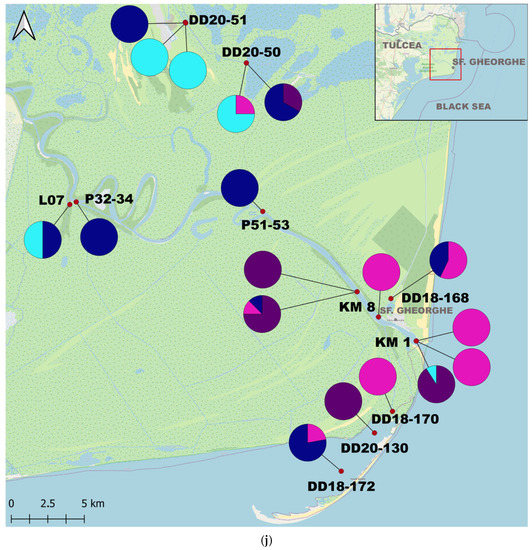
Figure 1.
Map of the study area and the proportions of individuals per sample: (a) The Study Area; (b) Upstream Lower Sector of the Danube River (West); (c) Central Lower Sector of the Danube River; (d) Downstream Lower Sector of the Danube River (Est); (e) Upstream Tulcea Arm; (f) Downstream Tulcea Arm; (g) Chilia Arm; (h) Sulina Arm; (i) Upstream Sf. Gheorghe Arm; (j) Downstream Sf. Gheorghe Arm.
2.1. Sample Processing and Analysis
The sampling strategy followed the European standard [87] and employed the multi-habitat technique, which is a modified version of the AQEM method used for monitoring water bodies in Romania [88]. This multi-habitat sampling method is a European standard for surface waters and combines techniques from the AQEM/STAR methodology and other EN and ISO standards. To perform measurements and collect samples from selected sites, we used two vessels, the research vessel Istros and a small boat. A total of 368 quantitative samples of sediments were collected using a (mouth surface 420 cm2), for analysis of benthic fauna, particle sediment size, and TOC. Samples that had a depth of at least 10 cm and no signs of being disturbed (due to washing) were processed. Three replicates from each microhabitat were taken per station. On board the research vessel Istros, the samples were sieved through 500, 250, and 125 μm sieves to remove sediment, while on the small boat, these were filtered through a limnological net with a 125 μm mesh size. The samples were then preserved with a mixed solution of Rose Bengal and 4% buffered formaldehyde for later analysis of benthic organisms. In the laboratory, the sample processing and analysis followed the European standard [89]. Briefly, the samples were sorted under a Zeiss SteREO Discovery V8 microscope and a Carl Zeiss Axiostar microscope (Carl Zeiss Instruments SRL, Bucharest, Romania) and then species were identified at the lowest taxonomical level possible. The taxonomic identification was based on classical approaches found in the specialized literature [90]. All organisms in each sample were counted and the average of the three replicates per sample was calculated. The number of individuals per sample and unit area was then estimated using the appropriate multiplication factor related to the grab surface used [87]. The particle sediment size was analyzed using a laser granulometer (Malvern Mastersizer 2000E Ver.5.20, Malvern, UK) with a precision of 1% and reproducibility of over 99%. The particle size was separated into different classes (sand, silt, clay) and further classified into finer fractions, including fine sand, coarse sand, gravel, pebble, cobble, and boulder, based on the Udden–Wentworth logarithmic scale and detailed [91,92]. Debris such as detritus, aquatic vegetation, and mollusc shells were omitted during the particle size analysis. Total organic carbon (TOC) was measured using a titrimetric method [93,94].
2.2. Statistical Analysis
To describe the benthic population structure, the abundance and frequency were determined. Statistical analysis was conducted using xlSTAT 7.5.2 software [95]. Correlation between the abundance of C. fluminea and D. polymorpha and particle size of sediment and TOC concentrations were analyzed using the Pearson correlation and Principal Component Analysis (PCA). The significant principal components were identified as those with eigenvalues greater than one, according to Yang et al. (2014) [95].
2.3. Studied Species
2.3.1. Corbicula fluminea
Kingdom Animalia, Phylum Mollusca, Class Bivalvia, Subclass Autobranchia, Infraclass Heteroconchia, Subterclass Euheterodonta, Superorder Imparidentia, Order Venerida, Superfamily Cyrenoidea, Family Cyrenidae, Genus Corbicula, Species Corbicula fluminea O. F. Müller, 1774 (Figure 2).

Figure 2.
Specimen of Corbicula fluminea.
The study of C. fluminea started in 1774 with Müller, who described three species in the genus Tellina (Linne, 1758): T. fluminalis, T. fluminea, and T. fluviatilis. From that moment, many individuals of C. fluminea (Mühlfeldt, 1811) have been registered in freshwater and estuarine habitats in Southeast Asia, the Indian subcontinent, the Pacific islands, Europe, and Africa [96]. The fossil record of C. fluminea is present in Europe, North America, and Japan [10,97,98,99].
C. fluminea is a small freshwater clam with a light-colored shell characterized by distinct concentric sulcations (growth ridges) and reaching about 5 cm in length [11,100]. The dimension of the mollusc is approximately 380 mm. Its shape is either triangular or oval, with a yellowish brown or blackish color and with concentric growth lines. The teeth on the valves are heterodont, meaning there are three cardinal teeth on each valve. The internal surface of the valves in fresh specimens is white-yellow. While this species has separated sexes, some individuals are also hermaphrodites and are capable of self-fertilization. Reproduction occurs in waters with a temperature greater than 15 °C and three-month-old individuals are capable of reproduction upon reaching 8–10 mm. Hermaphrodites produce both eggs and sperm simultaneously after reaching maturity, with first, the oocyte and then the spermatozoa being released. Multiple reproductions occur during the warm season from late May until autumn. Fertilization takes place in the paleal cavity of females and both trochophore larvae (15–20 microns) and veligers (0.2 mm) develop there. Juveniles are released when they reach 1 mm, are able to produce byssus, and can feed on phytoplankton. An individual can release up to 2000 juveniles per day and an estimated 100,000 juveniles in their lifetime. It can survive for weeks out of water and in low-oxygen environments and can be found in various habitats including stagnant or flowing water with varying water quality, from oligotrophic to eutrophic, and even in well-oxygenated silty or sandy sediments. It can withstand temperatures of between 2 and 34 °C and salinities of 5% for short periods in waters with 14 psu. However, it cannot tolerate water with high organic loads [90,101].
2.3.2. Dreissena polymorpha
Kingdom Animalia, Phylum Mollusca, Class Bivalvia, Subclass Autobranchia, Infraclass Heteroconchia, Subterclass Euheterodonta, Superorder Imparidentia, Order Myida, Superfamily Dreissenoidea, Family Dreissenidae, Subfamily Dreisseninae, Genus Dreissena, Species Dreissena polymorpha Pallas, 1771 (Figure 3).

Figure 3.
Specimen of Dreissena polymorpha.
The valves of the species are relatively delicate, with prominent bumps (umbones) and striped hind parts. The dorsal side is white-green with distinct brown-black stripes—although specimens without stripes can also occur. Under the umbones on the ventral side, there is a small triangular plateau. The impression of the gills lacks a sinus.
D. polymorpha is a freshwater benthic species that forms large groups on any type of natural or artificial substrate. It lives in stagnant or slow-moving water, avoids brackish and marine waters, and can grow in both plain freshwater areas and mountain lakes. The life span of the bivalve varies from 3 to 4 years, with a longer lifespan of up to 6–9 years in colder water. It reaches sexual maturity at a length of 8–10 mm and undergoes gametogenesis when water temperatures fall below 8–18 °C. Two reproductions occur in the summer, one at the end of May and the second at the end of August. When water temperatures exceed 12 °C in spring, the simultaneous release of oocytes and spermatozoa in the water begins. Fertilization is high in D. polymorpha, and a female can lay between 104 and 1.6 × 106 eggs. The ideal temperature for fertilization is between 12 and 24 °C with a peak at 18 °C, in water with a pH of between 7.4 and 9.4, with the optimal being 8.5, and where Ca2+ ion concentration is over 12–15 mg/L [102]. The larval stage lasts from 8 days to 5 weeks and during this stage larvae are vulnerable. High larvae mortality is recorded (30–100%), notably caused by environmental factors such as predators and water turbidity, and internal factors such as egg quality and food availability (they cannot withstand periods of starvation longer than 2 weeks). Fixation of larvae occurs in the pediveligera stage and they can attach to any substrate; however, this can still result in high mortality (90–99%) due to strong currents, storms, low oxygenation, etc. The density of fixed larvae can vary from 0 to 106/m2 and they can be located anywhere from the surface to 30 m deep in water. The species can survive in water temperatures of between 3–6 °C and 31–32 °C, with a salinity range of 0.007–0.5‰ to 2–4‰, and a pH range of 7.9–9.4, requiring at least 12–15 mg/L of Ca2+. It avoids high turbidity water but can adapt in some cases, and can last up to 10 days exposed to air under conditions of 10 °C and 95% humidity, and up to 18 days under lower temperatures and high humidity [90,101].
3. Results
The two species of mollusc were found in 153 stations out of 368 samples collected in the lower Danube sector and Danube Delta. In order to better highlight the distribution of the two bivalves, we divided them into adults and juveniles, taking into account their sizes.
C. fluminea adults were found in 180 samples and juveniles in 172 samples, while adult D. polymorpha were identified in 150 samples and juvenile D. polymorpha in 105 samples. C. fluminea were commonly found in areas with flowing waters from the Danube arms such as Chilia, Sulina, and Sf. Gheorghe, and the lower course of the Danube. Conversely, D. polymorpha was present both in the main course of the Danube and the lakes of the Danube Delta, often attached to submerged plant stems or reed roots/rhizomes in lakes (Supplementary Materials S1 and S2).
The highest density of C. fluminea adults, 18,125 ind./m2, was recorded in 2010 at Mila 2.8 Sulina (D10-032) on clayey silt bottoms, and the highest density of juvenile C. fluminea, 11,289.6 ind./m2, was recorded in 2019 at Km 805 (D2-19-094) on sandy gravel bottoms (Figure 4, Supplementary Materials S1).

Figure 4.
Examples of samples, showing the high densities of C. fluminea and D. polymorpha on different substrates.
The highest density of D. polymorpha adults, 30,312.5 ind./m2, was recorded in 2010 at km 247 Downstream Harsova (D10-068) on sandy bottoms, and the highest density of D. polymorpha juveniles, 7656.25 ind./m2, was recorded in 2010 at Ceatal Sf. Gheorghe Mila 35 (D10-020) on sandy bottoms (Figure 4, Supplementary Materials S1).
It is quite challenging to find a reasonable explanation for why the population has peaked or registered a much slower colonization. There are several limiting factors that can reduce the survival and longevity of zebra mussels and Asian clams (hydrodynamic conditions that may limit the ability of individuals to colonize, exposure to wind and waves, type of suitable substrate, episodes of hypoxia, higher temperatures, fish and bird predators, etc.). Continued monitoring of D. polymorpha and C. fluminea populations should provide useful data to better understand their behavior in such particular environments.
In general, the two species appear in high densities (mussel beds), thus creating a habitat for other species of organisms, which form thriving communities characterized by high biomass, abundance, and species richness [103] (Figure 4).
The composition of the macroinvertebrate community where the two species were found is formed of species such as: Spongilla lacustris (Linnaeus, 1758), Eunapius fragilis (Leidy 1851), Oligochaeta, Hypania invalida (Grube, 1860), Manayunkia caspica (Annenkova, 1928), Anodonta cygnea (Linnaeus, 1758), Unio pictorum (Linnaeus, 1758), Viviparus viviparus (Linnaeus, 1758), Theodoxus fluviatilis (Linnaeus 1758), T. danubialis (Pfeiffer 1828), T. transversalis (Pfeiffer, 1828), Lithoglyphus naticoides (Pfeiffer 1828), Esperiana esperi (Bourguignat, 1877), E. daudebartii (Prevost, 1821), Chelicorophium curvispinum (Sars 1895), Echinogammarus tenellus behningi (Martynov, 1919), Dikerogammarus villosus (Sowinsky, 1894), Chelicorophium robustum (G.O. Sars, 1895), Gammaridae (Leach, 1813), Jaera sarsi (Valkanov, 1936), Asellus aquaticus (Linnaeus, 1758), Hydropsyche bulgaromanorum (Malicky 1977), Palingenia longicauda (Olivier, 1791), Polycentropodidae (Ulmer, 1903), Gomphus flavipes (Charpentier, 1825), and Chironomidae Larvae.
The results of statistical analysis showed no significant Pearson correlation between the densities of C. fluminea and D. polymorpha and the analyzed environmental parameters. Based on the PCA graph, we can see that these two species do not show any correlation with the two parameters considered (substrate nature and TOC) due to the adaptability of these species to environmental conditions (Table 1 and Table 2, Figure 5).

Table 1.
Correlations between the abundances of the two bivalves, particle size, and TOC content in sediments.

Table 2.
Eigenvalues, variability, and cumulative score in the PCA matrix.
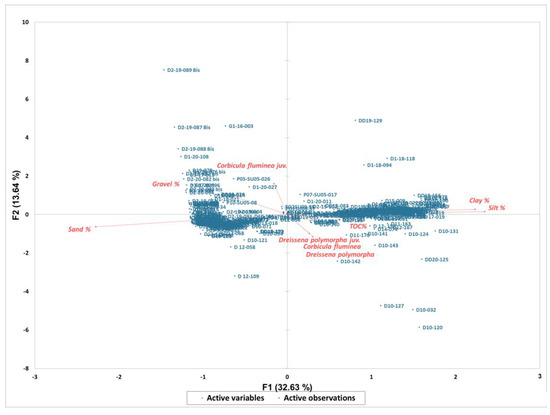
Figure 5.
Principal Component Analysis on the species abundances and particle size of sediments and TOC concentrations.
4. Discussion
4.1. Historical and Modern Distribution Range Shifts
The Danube River is an important shipping route, and, hence, a significant aquatic invasion pathway in Europe that connects the North Sea and the Black Sea through the Rhine–Main–Danube Canal, [54]. The process of C. fluminea’s colonization of Europe has undergone multiple stages since its first appearance on the continent in 1980. This process involved overcoming the isolation between large and small river basins, and spreading within occupied freshwater systems [22,24,25,28,29,30,31,33,34,35,36,38,39,40,42,43,44,45,48,49,50,51,52,53,54,55,104,105,106,107,108,109,110,111]. There have been three main stages in the distribution of C. fluminea across Europe: the first is the continental European stage, with spreading to most European countries, starting from the first discovery of the mollusc in 1980–1983 [26,27]; the second is the Danube stage, when the mollusc invaded the large basin of the Danube River in the mid-1990s [29,48]; and the third is the British stage, when colonization began later, in 1998 [31,112,113]. It is widely believed that C. fluminea entered European river systems through ship ballast water, and the initial sightings of the species in the lower reaches of navigable rivers support this theory. The species initially spread inward from the Atlantic coast and dispersed within river systems. The completion of the Main–Danube Canal in 1992 facilitated the eastward spread of the species along the Danube and into the Black Sea region. However, the first discovery of C. fluminea in the Danube Delta in Ukraine in 1995, followed by its appearance in the upper reaches and tributaries in Germany in 1997, raises the possibility of independent entry into the Danube through the canal or lower Danube ports [34,48,49]. The rate at which molluscs settle upstream is influenced by human activities such as sport fishing, aquaculture, and transportation of sand and gravel, and depends on the intensity of navigation [44]. The colonization of the Rhine, one of Europe’s largest navigable rivers, by C. fluminea took seven years, with the species first discovered in the estuary in 1988 and in the upper reaches near Basel, Switzerland in 1995 [22,24]. The average rate of migration for these molluscs is 63 km per year, with some areas experiencing a maximum rate of 276 km per year [114].
The zebra mussel was first discovered in the Caspian Sea by Pallas in 1769 and is believed to be native to the Caspian and Aral Seas, as well as the low-salinity lagoons of the Black Sea and adjacent rivers [49]. During the early 19th century, D. polymorpha spread rapidly through Europe by invading canals connected to the Danube and Dnieper basins and reached England and Prussia by 1825. The mussel continues to invade new lakes in Europe and is abundant in low-salinity (0–6 ppt) estuaries from Finland and Baltic Russia to Ireland, Britain, France, and Spain [115]. In North America, zebra mussels were transported to various lakes and river systems during the 1990s and 2000s [20,116,117]. By 2008, they were found in the upper Colorado and Arkansas drainages in Colorado, as well as a lake in Utah and a reservoir in the Monterey Bay watershed, California. The spread of zebra mussels between river basins was facilitated by canals and barges in the eastern and midwestern regions, while isolated rivers and lakes were infested through the transport of mussels on trailered boats or fishing gear [118,119,120,121]. According to our study results, C. fluminea dominate the lower Danube sector and the arms of the Danube with flowing waters while D. polymorpha were identified along the Danube course and in the lakes of the Danube Delta.
Ecological and Biological Prerequisites for Successful Invasion of D. polymorpha and C. fluminea and Implications of Their Invasiveness Potential
The rapid spread and success of zebra mussels and Asiatic clams across Europe, North America, and South America can be attributed to their biological characteristics (fast growth, early sexual maturity, high fertility, multiple reproductive methods, planktonic larvae, and ability to reach high population densities) and physiological tolerance [102,122]. The species can survive in fresh and brackish water [48,123,124,125,126] and are found in various water bodies such as rivers, lakes, reservoirs, and canals. They thrive in large water bodies and reach higher densities in flowing water [25,44]. When present in high numbers, they can displace other benthic species, compete for food resources, limit food for other species, consume many individuals of unionid mussel sperm, glochidia, and juveniles due to their high filtration rate, and transfer parasites and pathogens [102]. Their biofouling impacts on water used for power plants, drinking water systems, industrial water systems, irrigation systems, and canals have been reported in the US [127,128,129] and Europe [130]. Zebra mussels are highly reproductive filter feeders that can produce up to half of a million eggs per year. They have two life stages, a planktonic veliger larva and an adult form that attaches to hard surfaces or forms colonies by attaching to each other. Their high abundance in aquatic ecosystems can result in a significant impact, reducing plankton populations and affecting benthic invertebrates and fish [131,132,133,134,135,136,137,138,139,140,141,142,143,144,145]. The zebra mussel, D. polymorpha, has been shown to have a significant impact on aquatic ecosystems as a result of its ability to alter habitats in both an autogenic and allogenic manner [82,146,147,148,149,150,151]. This species modifies the physical and morphological properties of sediments and changes the suspended material, affecting the availability of resources in the ecosystem [138,152,153,154]. In addition, zebra mussel clumps can increase the colonizable benthic surface area, provide stress-free spaces, control the transport of particles and solutes in the near-bottom environment, alter boundary layer characteristics, increase the amount of organic material in the sediment through the deposit of feces and pseudofeces, and affect dissolved nutrient concentrations by excreting or removing seston from the water column [149,150,152,155,156,157,158,159]. The spatial and temporal distribution of C. fluminea populations may depend on a variety of factors, including the season and method of sampling, as well as abiotic factors such as substrate type [44]. C. fluminea prefers sandy silt and sandy clay sediments [44,102,160,161]. At Danube sites in Bulgaria, the highest species density was found in areas with a substrate made of coarse sand and sand with gravel. At Zagrazhden, where the highest density of juvenile specimens was observed, the substrate was coarse sand mixed with clam shells. Tributaries of the Danube with a sandy substrate, such as the Tsibritsa River, had higher population densities compared to other tributaries. In turn, in lakes and reservoirs with a substrate of sand, gravel, clay, and mud, the population density was lower than in the rivers [114]. The shells of the zebra mussel, D. polymorpha, provide a favorable environment and protection for aquatic life. This leads to shifts in the macroinvertebrate community [158,162,163,164,165]. The invasive bivalves introduce new shell substrates to the ecosystem, altering the availability and suitability of substrates for colonization by some mud/sand-dwelling benthic organisms [8]. Empty bivalve shells were found to generate a diverse range of microhabitats, attracting a macroinvertebrate community that was distinct from the natural macroinvertebrate community [166,167].
Our study found that the dominant macroinvertebrates were amphipods, corophidae, gammaridae, gastropods, oligocheta, polychaeta, and insect larvae (trichoptera, chironomidae, etc.). C. fluminea registered high densities where the substrate was clayey silt or sandy gravel bottoms, and D. polymorpha preferred sandy bottoms. Different taxonomic groups may be attracted to empty shells for various reasons. Empty shells can provide a hard surface for the growth of amphipods, found in high abundance individuals, such as Corophium curvispinum that live in sediment tubes that are attached to the empty bivalve shells of Dreissena or Corbicula. The shells also offer a hard surface and shelter for the tubes of polychaetes and trichopteres, which are fixed to the valves of Dreissena or Corbicula. Gastropods, leeches, several crustacean species, and other organisms also find attachment sites on the hard shells’ surface.
4.2. The Species Populations’ Dynamics and Trends
The density of the zebra mussel, D. polymorpha, and the Asian clam, C. fluminea, has been reported in various North American and European rivers. In North America, the maximum density of C. fluminea can range from 10,000 to 20,000 ind./m2 and can dominate the benthic community [168]. The highest recorded density was 131,000 ind./m2 in California [33,99]. In Europe, C. fluminea has also been observed to attain high densities, such as 736 ind./m2 in the Hungarian stretch of the Danube River, 5000 ind./m2 in the Vén-Duna side arm of the Danube River in Hungary (km 1480–1483), and up to 9636 ind./m2 in the Barrow River in Ireland [53,54,150]. Also, high densities were registered in an artificial channel near the Elbe River, 1045–4224 ind./m2 [169]. In 2004, a maximum density of 1680 ind./m2 was estimated in the Ukrainian section of the river (km 39) according to Lyashenko and Makovskii (2011) [111]. The densities in other European rivers have been estimated as the following: up to a maximum of 1500 ind./m2 in the Upper Rhine River (2003–2005) [111], 2120 ind./m2 in the Mero River basin (Calicia–Northwest on the Iberian) [42], up to 2500 ind/m2 in the Chet River, Norfolk (UK), dominated by small individuals (2 to 9 mm in length) [168], and up to 9636 ind./m2 in the Barrow River in Ireland [113]. In 2003, the river Altrhein (7 km downstream of Basel) had 200–600 clams ind./m2. In Basel, the density of C. fluminea ranged from 5 to 200 ind./m2 and at upstream locations from 1 to 20 ind./m2. In the Canal de Huningue, densities of 10–50 ind./m2 were recorded. C. fluminea was most abundant (up to 600 clams ind./m2) on sandy substrates near the banks of the river. D. polymorpha has been present and dominant in Lake Balaton since the 1930s, with densities reaching 220,000 ind./m2 [170,171]. In the Szczecin Lagoon in Germany, the species forms large aggregations with a density of up to 10,000 ind./m2 [170]. A large and stable population of D. polymorpha was found in the 1950s in the Szczecin Lagoon with an average density of 15,400 ind./m2 [171]. In the eastern Gulf of Finland, in the Neva estuary, D. polymorpha registered up to 3.000 ind./m2 [172]. D. polymorpha also registered high densities in the Danube River at 1821–1825 km of 188–1322 ind./m2 [173].
In our study, the highest density of C. fluminea adults, 18,125 ind./m2, was recorded in 2010 at Mila 2.8 Sulina (D10-032) on clayey silt bottoms, and the highest density of juveniles, 11,289.6 ind./m2, was recorded in 2019 at Km 805 (D2-19-094) on sandy gravel bottoms. D. polymorpha adults recorded their highest density, 30,312.5 ind./m2, in 2010 at km 247 Downstream Harsova (D10-068) on sandy bottoms, and D. polymorpha juveniles recorded their highest density, 7656.25 ind./m2, in 2010 at Ceatal Sf. Gheorghe Mila 35 (D10-020) on sandy bottoms.
The density of both species can vary depending on environmental factors such as bottom structure, slope, and water conditions. In favorable conditions, these two species can quickly become the dominant species, with harmful consequences for the native species and the functioning of the ecosystem [174].
Even if these two species are found in great abundance on certain types of substrates, they can be found in all types of sediment, thus making them the two alien invasive species one of the biggest threats to biodiversity worldwide.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su15118526/s1. Supplementary Materials S1. The distribution of the freshwater bivalves Corbicula fluminea and Dreissena polymorpha on the Lower Danube River and Danube Delta (abundance, granulometry of sediments, carbon organic total); Supplementary Materials S2. The distribution of the freshwater bivalves Corbicula fluminea and Dreissena polymorpha on the Lower Danube River and Danube Delta (Coordinates).
Author Contributions
Conceptualization, A.B.P., A.S. and I.C.; methodology, A.B.P., S.K. and C.G.; software, A.B.P. and S.K.; validation, A.B.P., A.E. and A.S.; formal analysis, A.B.P. and S.K.; investigation, A.B.P., C.G., A.S. and I.C.; resources, A.B.P. and A.E..; data curation, A.B.P., A.S. and I.C.; writing—original draft preparation, A.B.P.; writing—review and editing, A.B.P., A.S. and I.C.; visualization, A.B.P., A.S. and I.C.; supervision, A.B.P., A.S., A.E. and I.C.; project administration, A.B.P., A.S. and I.C.; funding acquisition, A.B.P. and A.E. All authors have read and agreed to the published version of the manuscript.
Funding
The collection: analysis and interpretation of data received funding from the Romanian Ministry of Research in the framework of the CORE Programme NUCLEU, research projects: PN 09-41-03-05, PN 19-20-02-04 from 2010 to 2019 “Danube River: Evolution of morphological, Sedimentological, Geoecological and Estimating Anthropogenic Pollution”, in 2019–PN19-20-04-01 “Multidisciplinary Research regarding the effects produced by the anthropic interventions on the Danube Delta and the coastal area and the possibilities of rehabilitation of the environment”, in 2016, 2017–PN-16-45-05-03 “The complex qualitative and quantitative assessment of the ecological changes caused by the rectification of the Sf. Gheorghe meanders and the elaboration of a program of environmental rehabilitation measures”, from 2017 to 2019–PN 09-41-03-04, PN 19-20-02-03 “Assessment of the Ecological Status of the Aquatic Ecosystems in the Danube Delta and the Razelm-Sinoe Lagoon Complex, based on the temporal and spatial analysis of the hydromorphological, physico-chemical and biological indicators” and other important projects: DANUBIUS RI (sectorial project S7/2013), DANS (project funded through the Research, Development and Innovation Program for River, Delta sea Systems-DANUBIUS, Contract no. 4/7.05.2018), carried out by the GeoEcoMar. This work was also part of the project “Analysis of the potential for sustainable use of vegetation specific to the Danube-Delta system Danube-Black Sea–D3MN” POC/78/1/2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the Granulometry Department and Naliana Lupascu for helping to improve the paper, by providing the granulometry data and TOC data. We would also like to thank two anonymous reviewers for their helpful comments on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lodge, D.M.; Stein, R.A.; Brown, K.M.; Covish, A.P.; Bronmark, C.; Garvey, J.E.; Klosiewki, S.P. Predicting impact of freshwater exotic species on native biodiversity: Challenges on spatial scaling. Aust. J. Ecol. 1998, 23, 53–67. [Google Scholar] [CrossRef]
- Ricciardi, A.; MacIsaac, H.J. Recent mass invasion of the North American Great Lakes by Ponto-Caspian species. Trends Ecol. Evol. 2000, 15, 62–65. [Google Scholar] [CrossRef]
- Kolar, C.S.; Lodge, D.M. Ecological predictions and risk assessment for alien fishes in North America. Science 2002, 298, 1233–1236. [Google Scholar] [CrossRef]
- Strayer, D.L. Alien species in fresh waters: Ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 2010, 55 (Suppl. S1), 152–174. [Google Scholar] [CrossRef]
- Simberloff, D. How common are invasion-induced ecosystem impacts? Biol. Invasions 2011, 13, 1255–1268. [Google Scholar] [CrossRef]
- Sousa, R.; Morais, P.; Dias, E.; Antunes, C. Biological invasions and ecosystem functioning: Time to merge. Biol. Invasions 2011, 13, 1055–1058. [Google Scholar] [CrossRef]
- Vaughn, C.C.; Hakenkamp, C.C. The functional role of burrowing bivalves in freshwater ecosystems. Freshw. Biol. 2001, 46, 1431–1446. [Google Scholar] [CrossRef]
- Sousa, R.; Gutierrez, J.L.; Aldridge, D.C. Nonindigenous invasive bivalves as ecosystem engineers. Biol. Invasions 2009, 11, 2367–2385. [Google Scholar] [CrossRef]
- Araujo, R.; Moreno, D.; Ramos, M.A. The Asiatic clam Corbicula fluminea (Müller, 1774) (Bivalvia: Corbiculidae) in Europe. Am. Malacol. Bull. 1993, 10, 39–49. [Google Scholar]
- Zhadin, V. Mollusks of the Fresh and Brackish Waters of the USSR; Academy of Sciences of the USSR: Moscow, Russia; Leningrad, Russia, 1952; 376p. (In Russian) [Google Scholar]
- Morton, B. Corbicula in Asia—An Updated Synthesis. In American Malacological Bulletin, Special Edition; American Malacological Union: Buffalo, NY, USA, 1986; pp. 113–124. [Google Scholar]
- DAISIE (Delivering Alien Invasive Species Inventories to Europe). Handbook of Alien Species in Europe; Springer: Dordrecht, The Netherlands, 2009; pp. 269–374. [Google Scholar]
- Chen, H.; Zha, J.; Liang, X.; Bu, J.; Wang, M.; Wang, Z. Sequencing and de novo assembly of the Asian clam (Corbicula fluminea) transcriptome using the Illumina GAIIx method. PLoS ONE 2013, 8, e79516. [Google Scholar] [CrossRef] [PubMed]
- Chijimatsu, T.; Umeki, M.; Kataoka, Y.; Kobayashi, S.; Yamada, K.; Oda, H.; Mochizuki, S. Lipid components prepared from a freshwater Clam (Corbicula fluminea) extract ameliorate hypercholesterolaemia in rats fed highcholesterol diet. Food Chem. 2013, 136, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Chen, S.; Ye, X.; Zhong, J.; Wu, N.; Dong, S.; Yang, B.; Liu, D. Antioxidant and anti-tumor activity of a polysaccharide from freshwater clam, Corbicula fluminea. Foods Funct. 2013, 4, 539–548. [Google Scholar] [CrossRef]
- Burch, J.W. Checklist of West American molluscs, family Corbiculidae. Conchol. Club South. Calif. Minutes 1944, 36, 1–18. [Google Scholar]
- Counts, C.L., III. Corbicula fluminea (Bivalvia: Sphaeriacea) in British Columbia. Nautika 1995, 95, 12–13. [Google Scholar]
- Hanna, G.D. Introduced mollusks of western North America. Calif. Acad. Sci. Occas. Pap. 1966, 48, 1–108. [Google Scholar]
- McMahon, R.F. The occurrence and spread of the introduced Asiatic freshwater clam, Corbicula fluminea (Müller) in North America: 1924–1982. Nautilus 1982, 96, 134–141. [Google Scholar]
- Foster, A.M.; Fuller, P.; Benson, A.; Constant, S.; Raikow, D.; Larson, J.; Fusaro, A. Corbicula fluminea; USGS Nonindigenous Aquatic Species Database: Gainesville, FL, USA, 2013. Available online: http://nas.er.usgs.gov/queries/FactSheet.aspx?speciesID=92 (accessed on 27 February 2023).
- CABI. Corbicula fluminea. In Invasive Species Compendium; CAB International: Wallingford, UK, 2013; Available online: www.cabi.org/isc (accessed on 4 January 2023).
- Bij de Vaate, A.; Greijdanus-Klaas, M. The Asiatic clam, Corbicula fluminea Muller, 1774 (Pelecypoda: Corbiculidae), a new immigrant in the Netherlands. Bull. Zool. Mus. 1990, 12, 173–178. [Google Scholar]
- Rey, P.; Ortlepp, J.; Küry, D. Wirbellose Neozoen im Hochrhein. Ausbreitung und ökologische Bedeutung. Schr. Umw. BUWAL Bern 2004, 380, 1–88. [Google Scholar]
- Schmidlin, S.; Bauer, B. Distribution and substrate preference of the invasive clam Corbicula fluminea in the river Rhine in the region of Basel (Switzerland, Germany, France). Aquat. Sci. 2007, 69, 153–161. [Google Scholar] [CrossRef]
- Schmidlin, S.; Schmera, D.; Ursenbacher, S.; Bauer, B. Separate introductions but lack of genetic variability in the invasive clam Corbicula spp. in Swiss lakes. Aquat. Invasions 2012, 7, 73–80. [Google Scholar] [CrossRef]
- Mouthon, J. Sur la presence en France et en Portugal de Corbicula (Bivalvi and Corbiculidae) onginaire d’Asia. Basterie 1981, 45, 109–116. [Google Scholar]
- Kinzelbach, R. Die Körbchenmuscheln Corbicula fluminalis, Corbicula fluminea und Corbicula fluviatilis in Europa (Bivalvia: Corbiculidae). Mainz. Nat. Arch. 1991, 29, 215–228. [Google Scholar]
- Grabow, K.; Martens, A. Vorkomen von Corbicula fluminea (O. F. Müller, 1774) und “C. fluminalis” (O. F. Müller, 1774) im östlichen Mittellandkanal (Bivalvia: Corbiculidae). Mitt. Der Dtsch. Malakozool. Ges. 1995, 56–57, 19–23. [Google Scholar]
- Tittizer, T.; Taxacher, M. Erstnachweis von Corbicula fluminea/fluminalis (Müller, 1774) (Corbiculidae, Mollusca) in der Donau. Lauterbornia 1997, 31, 103–107. [Google Scholar]
- Brauckmann, C.; Brauckmann, B.; Groning, E. Zur Ausbreitung der Korbchenmuschel Corbicula in Mitteleuropa. Jahresber. Nat. Ver. Wupp. 1999, 52, 221–228. [Google Scholar]
- Howlett, D.; Baker, R. Corbicula fluminea (Müller): New to UK. J. Conchol. 1999, 36, 83. [Google Scholar]
- Beran, L. First record of Corbicula fluminea (Mollusca: Bivalvia) in the Czech Republic. Acta Soc. Zool. Bohem. 2000, 64, 1–2. [Google Scholar]
- Beran, L. Spreading expansion of Corbicula fluminea (Mollusca: Bivalvia) in the Czech Republic. Heldia 2006, 65–66, 187–192. [Google Scholar]
- Vincent, T.; Brancotte, V. Répartition actuelle et modes de progression de Corbicula spp. en France. Bull. Soc. Zool. Fr. 2002, 127, 241–252. [Google Scholar]
- Willing, M.J. Sphaerium solidum and Corbicula fluminea: Two rare bivalve molluscs in the River Great Ouse system in Cambridgeshire. Nat. Cambs. 2007, 49, 39–49. [Google Scholar]
- Cianfanelli, S.; Lori, E.; Bodon, M. Non-indigenous freshwater mollusks and their distribution in Italy. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats. Invading Nature; Springer Series in Invasion, Ecology; Gherardi, F., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 103–121. [Google Scholar]
- Ayres, C. A new record of Asian clam Corbicula fluminea (Müller, 1774) in Galicia (Iberian Peninsula)—Ribeiras do Louro e Gándaras de Budiño wetland. Aquat. Invasions 2008, 3, 439–440. [Google Scholar] [CrossRef]
- Pérez-Quintero, J. Revision of the distribution of Corbicula fluminea (Müller, 1774) in the Iberian Peninsula. Aquat. Invasions 2008, 3, 355–358. [Google Scholar] [CrossRef]
- Elliott, P.; zu Ermgassen, P.S.E. The Asian clam (Corbicula fluminea) in the River Thames, London, England. Aquat. Invasions 2008, 3, 54–60. [Google Scholar] [CrossRef]
- Morais, P.; Teodósio, J.; Reis, J.; Chícharo, M.A.; Chícharo, L. The Asian clam Corbicula fluminea (Müller, 1774) in the Guadiana River Basin (southwestern Iberian Peninsula): Setting the record straight. Aquat. Invasions 2009, 4, 681–684. [Google Scholar] [CrossRef]
- Fabbri, R.; Landi, L. New records of exotic mussels, decapod, crustaceans and fishes from Emilia-Romagna and first record of Corbicula fluminea (O.F. Müller, 1774) in Italy. Quad. Studi E Not. Stor. Nat. Della Romagna 1999, 12, 9–20. [Google Scholar]
- Lois, S. New records of Corbicula fluminea (Müller, 1774) in Galicia (Northwest of the Iberian Peninsula): Mero, Sil and Deva rivers. Aquat. Invasions 2010, 5 (Suppl. S1), S17–S20. [Google Scholar] [CrossRef]
- Marescaux, J.; Pigneur, L.M.; Van Doninck, K. New records of Corbicula clams in French rivers. Aquat. Invasions 2010, 5 (Suppl. S1), S35–S39. [Google Scholar] [CrossRef]
- Lucy, F.E.; Karatayev, A.Y.; Burlakova, L.E. Predictions for the spread, population density, and impacts of Corbicula fluminea in Ireland. Aquat. Invasions 2012, 7, 465–474. [Google Scholar] [CrossRef]
- Kamburska, L.; Lauceri, R.; Beltrami, M.; Boggero, A.; Cardeccia, A.; Guarneri, I.; Manca, M.; Riccardi, N. Establishment of Corbicula fluminea (O.F. Müller, 1774) in Lake Maggiore: A spatial approach to trace the invasion dynamics. BioInvasions Rec. 2013, 2, 105–117. [Google Scholar] [CrossRef]
- Leuven, R.S.E.W.; van der Velde, G.; Baijens, I.; Snijders, J.; van der Zwart, C.; Lenders, H.H.R.; Bij De Vaate, A. The river Rhine: A global highway for dispersal of aquatic invasive species. Biol. Invasions 2009, 11, 1998–2008. [Google Scholar] [CrossRef]
- Tittizer, T.; Leuchs, H.; Banning, M. Das Makrozoobenthos der Donau im Abschnitt Kehlheim-Jochenstein (Donau- km 2414–2202). Limnol. Aktuell Stuttg. 1994, 2, 173–188. [Google Scholar]
- Alexandrov, B.; Boltachev, A.; Kharchenko, T.; Lyashenko, A.; Son, M.; Tsarenko, P.; Zhukinsky, V. Trends of aquatic alien species invasions in Ukraine. Aquat. Invasions 2007, 2, 215–242. [Google Scholar] [CrossRef]
- Son, M.O. Invasive mollusks (Mollusca, Bivalvia, Gastropoda) in the Danube Delta. Vestn. Zool. 2007, 41, 213–218. (In Russian) [Google Scholar]
- Paunović, M. Qualitative composition of the macroinvertebrate communities in the Serbian sector of the Sava River. Int. Assoc. Danub. Res. 2004, 35, 349–354. [Google Scholar]
- Paunović, M.; Csányi, B.; Knežević, S.; Simić, V.; Nenadić, D.; Jakovčev-Todorović, D.; Stojanović, B.; Cakić, P. Distribution of Asian clams Corbicula fluminea (Müller, 1774) and C. fluminalis (Müller, 1774) in Serbia. Aquat. Invasions 2007, 2, 99–106. [Google Scholar] [CrossRef]
- Munjiu, O.; Shubernetski, I. First record of Asian clam Corbicula fluminea (Müller, 1774) in the Republic of Moldova. Aquat. Invasions 2010, 5 (Suppl. S1), S67–S70. [Google Scholar] [CrossRef]
- Bódis, E.; Nosek, J.; Oertel, N.; Tóth, B.; Fehér, Z. A comparative study of two Corbicula morphs (Bivalvia, Corbiculidae) inhabiting River Danube. Int. Rev. Hydrobiol. 2011, 96, 257–273. [Google Scholar] [CrossRef]
- Bódis, E.; Sipkay, C.; Tóth, B.; Oertel, N.; Nosek, J.; Hornung, E. Spatial and temporal variation in biomass and size structure of Corbicula fluminea in Danube River catchment, Hungary. Biologia 2012, 67, 739–750. [Google Scholar] [CrossRef]
- Bij de Vaate, A.; Hulea, O. Range extension of the Asiatic clam Corbicula fluminea (Müller 1774) in the River Danube: First record from Romania. Lauterbornia 2000, 38, 23–26. [Google Scholar]
- Skolka, M.; Gomoiu, M.T. Alien invertebrate species in Romanian waters. Ovidius University Annals of Natural Sciences, Biology. Ecol. Ser. 2001, 5, 51–55. [Google Scholar]
- Popa, O.P. Studiul Unor Specii de Moluste Bivalve Strãine în Fauna României (Faunisticã, Biologie si Diversitate Geneticã a Populatiilor). Ph.D. Thesis, Universitatea din Bucuresti, Soala Doctoralã a Facultãtii de Biologie, Bucuresti, Romania, 2008. (In Romanian). [Google Scholar]
- Catianis, I.; Secrieru, D.; Pojar, I.; Grosu, D.; Scrieciu, A.; Pavel, A.B.; Vasiliu, D. Water Quality, Sediment Characteristics and Benthic Status of the Razim-Sinoie Lagoon System, Romania. Open Geosci. 2018, 10, 12–33. [Google Scholar] [CrossRef]
- Ferreira-Rodriguez, N.; Pavel, A.B.; Cogalniceanu, D. Integrating expert opinion and traditional ecological knowledge in invasive alien species management: Corbicula in Eastern Europe as a model. Biol. Invasions 2021, 23, 1087–1099. [Google Scholar] [CrossRef]
- Golikov, A.; Starobogatov, Y. Zoogeographical characteristics of the gastropod mollusks of Black and Azov Seas. In Biological Studies of the Black Sea and Its Fishery Resources; Nauka Publ.: Moscow, Russia, 1968; pp. 70–83. (In Russian) [Google Scholar]
- Skarlato, O.; Starobogatov, Y. Class of Bivalve Mollusks—Bivalvia. In The Key for the Fauna of the Black and Azov Seas; Mordukhay-Boltovskoy, F., Ed.; Naukova Dumka Publ.: Sydney, Australia, 1972; pp. 178–249. (In Russian) [Google Scholar]
- Valkanov, A.; Petrova, V.; Roshdestvenski, A.; Marinov, T.; Naidenow, W. Black Sea lakes. In The Black Sea; Valkanov, A., Marinov, H., Danov, H., Vladev, P., Eds.; G. Bakalov Publ.: Varna, Bulgaria, 1978; pp. 262–283. (In Bulgarian) [Google Scholar]
- Marinov, T. The Zoobenthos from the Bulgarian Sector of the Black Sea; Publishing House of the Bulgarian Academy of Sciences: Sofia, Bulgaria, 1990; 195p. (In Bulgarian) [Google Scholar]
- Kerney, M.P.; Morton, B.S. The distribution of Dreissena polymorpha in Britain. J. Conchol. 1970, 27, 97–100. [Google Scholar]
- Olenin, S.; Orlova, M.; Minchin, D. Dreissena polymorpha (Pallas, 1771). In Case Histories on Introduced Species: Their General Biology, Distribution, Range Expansion and Impact; Gollasch, S., Minchin, D., Rosenthal, H., Voigt, M., Eds.; Logos-Verlag: Berlin, Germany, 1999; pp. 37–42. [Google Scholar]
- Aldridge, D.C.; Elliot, P.; Moggridge, G.D. The recent and rapid spread of the zebra mussel (Dreissena polymorpha) in Great Britain. Biol. Conserv. 2004, 119, 253–261. [Google Scholar] [CrossRef]
- Kobelt, W.; Haas, F. Iconographie der Land Süsswasser-Mollusken mit vorzüglicher Berücksichtigung der europäischen noch nicht abgebildeten Arten von E. A. Rossmässler. Neue Folge. Siebzehnter Band Wiesb. 1911, 1–60, 451–480. [Google Scholar]
- Wohlberedt, O. Zur Molluskenfauna von Bulgarien. Abh. Ber. Nat. Ges. Görlitz 1911, 27, 167–234. [Google Scholar]
- Hesse, P. Zur Kenntnis der Molluskenfauna von Ostrumelien. III. Nachr. Dtsch. Malakozool. Ges. 1914, 46, 49–58. [Google Scholar]
- Drensky, P. Synopsis and distribution of freshwater Mollusca in Bulgaria. Annuaire de l’Universite de Sofia. Fac. Phys. Math. 1947, 43, 33–54. (In Bulgarian) [Google Scholar]
- Petrbok, J. The freshwater molluscs of the lakes of Varna and of Gebedže. Arbeiten aus der Biologischen Meeresstation in Varna. Bulgarien 1947, 13, 71–75. (In Bulgarian) [Google Scholar]
- Valkanov, A. Katalog unserer Schwarzmeerfauna. Arbeiten aus der Biologischen Meeresstation in Varna. Bulgarien 1957, 19, 1–62. (In Bulgarian) [Google Scholar]
- Russev, B.; Yaneva, I.; Detcheva, R.; Karapetkova, M. Zusammensetzung der Hydrofauna. In Limnologie der Bulgarischen Donauzuflüsse; Russev, B., Ed.; Knizhen Tigar Publ.: Sofia, Bulgaria, 1994; pp. 130–174. (In Bulgarian) [Google Scholar]
- Angelov, A. Mollusca: Gastropoda et Bivalvia aquae dulcis. In Catalogus Faunae Bulgaricae, 4; Pensoft: Sofia, Bulgaria, 2000; 57p. [Google Scholar]
- Shopov, V. Distribution of Upper Quaternary Mollusca Communities in the Outer Zone of the South Bulgarian Black Sea Shelf; Geologia Balcanica, BAS Publ.: Sofia, Bulgaria, 1979; Volume 9, pp. 51–66. [Google Scholar]
- Shopov, V. Biostratigraphy of the Upper Quaternary Sediments from the South-West Parts of the Black Sea; Geologica Balcanica, BAS Publ.: Sofia, Bulgaria, 1984; Volume 14, pp. 17–38. (In Russian) [Google Scholar]
- Hrischev, H.; Shopov, V. Marine Pleistocen in the Bay of Burgas and the Problem of Relations between Uzunlar and Karangat Beds; Geologica Balcanica, BAS Publ.: Sofia, Bulgaria, 1979; Volume 9, pp. 69–84. (In Russian) [Google Scholar]
- Liutzkanov, D. Morphology, Taxonomy and Ecology of Fossil and Recent Dreissena polymorpha Pall. in Bulgaria. Ph.D. Thesis, Institute of Zoology, Bulgarian Academy of Sciences, Sofia, Bulgaria, 1981; 253p. (In Bulgarian). [Google Scholar]
- Hubenov, Z. Dreissena (Bivalvia: Dreissenidae)—Systematics, autochthonous and anthropogenic areas. Acta Zool. Bulg. 2005, 57, 259–268. [Google Scholar]
- Kerney, M.P. Atlas of the Land and Freshwater Molluscs of Britain and Ireland; Harley Books: Colchester, UK, 1999. [Google Scholar]
- Araujo, R.; Alvarez, R.M. El mejillo’n cebra en el Ebro: Un grave caso de riesgo ambiental en Arago’n. Nat. Aragonesa 2001, 8, 39–46. [Google Scholar]
- Minchin, D.; Lucy, F.; Sullivan, M. Zebra mussel: Impacts and spread. In Invasive Aquatic Species of Europe: Distribution, Impact and Management; Leppäkoski, E., Gollasch, S., Olenin, S., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; pp. 135–146. [Google Scholar]
- Pollux, B.; Minchin, D.; van der Velde, G.; van Alen, T.; Moon-van der Staay, S.Y.; Hackstein, J. Zebra mussels (Dreissena polymorpha) in Ireland, AFLP-fingerprinting and boat traffic both indicate an origin from Britain. Freshw. Biol. 2003, 48, 1127–1139. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Muncaster, B.W.; Mackie, G.L. Ecological and genetic studies on Dreissena polymorpha (Pallas): A new mollusc in the Great Lakes. Can. J. Fish. Aquat. Sci. 1989, 46, 1491–1587. [Google Scholar] [CrossRef]
- Nalepa, T.F.; Schloesser, D.W. (Eds.) Zebra Mussels Biology, Impacts, and Control, 2nd ed.; Lewis Publishers, CRC Press: Boca Raton, FL, USA, 2013; 815p, ISBN 9780429110863. [Google Scholar]
- MacIsaac, H.J. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. Am. Zool. 1996, 36, 287–299. [Google Scholar] [CrossRef]
- SR EN ISO 10870; Water Quality—Guidelines for the Selection of Sampling Methods and DEVICES for Benthic Macroinvertebrates in Fresh Waters. ISS: Belgrade, Serbia, 2012.
- SR EN ISO 16150; Water Quality—Guidance on Pro-Rata Multi-Habitat Sampling of Benthic Macro-Invertebrates from Wade Able Rivers. ISS: Belgrade, Serbia, 2012.
- SR EN ISO 5661–1; Water Quality. Part 1, General Guidelines for Establishing Sampling Programs and Techniques. ISS: Belgrade, Serbia, 2008.
- Godeanu, S. Continental waters, overview. In The Diversity of the Living World. Illustrated Identification Manual of the Flora and Fauna of Romania; Godeanu, S.P., Ed.; Bucura Mond Publishing: Bucharest, Romania, 2002; pp. 1–24. ISBN 973-98248-5-4. [Google Scholar]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Verdonschot, P.F.M. Beken in Beeld; IBN: Wageningen, The Netherlands; RIZA: Leystad, The Netherlands; Ministerie van Verkeer Waterstaat: Den Haag, The Netherlands, 1999; 25p. (In Dutch)
- Black, C.A. Methods of Soil Analysis, Part I; American Society of Agronomy: Madison, WI, USA, 1965; 1572p. [Google Scholar]
- Gaudette, H.; Flight, W.; Toner, L.; Folger, D. An inexpensive titration method for the determination of organic carbon in recent sediments. J. Sediment. Petrol. 1974, 44, 249–253. [Google Scholar]
- Addinsoft. XLSTAT Statistical and Data Analysis Solution; Addinsoft: New York, NY, USA, 2020; Available online: https://www.xlstat.com (accessed on 1 February 2023).
- Yang, Y.; Liu, Z.; Chen, F.; Wu, S.; Zhang, L.; Kang, M.; Li, J. Assessment of trace element contamination in sediment cores from the Pearl River and estuary, South China: Geochemical and multivariate analysis approaches. Environ. Monit. Assess. 2014, 186, 8089–8107. [Google Scholar] [CrossRef]
- McMahon, R. Ecology of an invasive pest bivalve, Corbicula. In The Mollusca; Russell-Hunter, W., Ed.; Academic Press: New York, NY, USA, 1983; pp. 505–561. [Google Scholar]
- Linstow, O.V. Beitrag zur Geschichte und Verbreitung von Corbicula fluminalis. Arch. Für Molluskenkd. 1922, 54, 113–144. [Google Scholar]
- Ellis, A.E. British Freshwater Bivalva Mollusca. In Synopses of the British Fauna (New Series) Vol. 11, Linnean Society of London; Academic Press: London, UK, 1978; 109p. [Google Scholar]
- Britton, J.C.; Morton, B. Corbicula in North América: The evidence reviewed and evaluated. In Proceedings of the First International Corbicula Symposium 1977, Fort Worth, TX, USA, 13–15 October 1977; Britton, J.C., Ed.; Texas Christian University: Fort Worth, TX, USA, 1979; pp. 250–287. [Google Scholar]
- Woodward, S.L.; Quinn, J.A. Encyclopedia of Invasive Species; ABC-CLIO, LLC.: Goleta, CA, USA, 2011; 764p. [Google Scholar]
- Birnbaum, C. NOBANIS—Invasive Alien Species Fact Sheet—Dreissena polymorpha—From: Online Database of the European Network on Invasive Alien Species—NOBANIS. 2011. Available online: www.nobanis.org (accessed on 1 January 2023).
- Sousa, R.; Antunes, C.; Guilhermino, L. Ecology of the invasive Asian clam Corbicula fluminea (Müller, 1774) in aquatic ecosystems: An overview. Ann. Limnol. Int. J. Limnol. 2008, 44, 85–94. [Google Scholar] [CrossRef]
- Zaiko, A.; Daunys, D.; Olenin, S. Habitat engineering by the invasive zebra mussel Dreissena polymorpha (Pallas) in a boreal coastal lagoon: Impact on biodiversity. Helgol. Mar Res. 2009, 63, 85–94. [Google Scholar] [CrossRef]
- Bij de Vaate, A. Colonization of the German part of the River Rhine by the Asiatic clam, Corbicula fluminea Muller, 1774 (Pelecypoda, Corbiculidae). Bull. Zool. Mus. 1991, 13, 13–16. [Google Scholar]
- Csányi, B. Spreading invaders along the Danubian highway: First record of Corbicula fluminea (O.F. Müller 1774) and C. fluminalis (O.F. Müller 1774) in Hungary (Mollusca, Bivalvia). Folia Hist. Nat. Musei Matra. 1999, 23, 343–345. [Google Scholar]
- Fischer, W.; Schultz, P. Erstnachweis Corbicula cf. Fluminea (O.F. Müller 1774) (Mollusca: Bivalvia: Corbiculidae) aus Österreich, sowie ein Nachweis von lebenden Microcolpia daudebartii acicularis (Ferussac 1821) (Mollusca: Gastropoda: Melanopsidae) aus Bad Deutsch-Altenburg (NÖ, Österreich). Club Conchyl. Inf. 1999, 31, 23–26. [Google Scholar]
- Hubenov, Z. Corbiculidae—A new family for the Bulgarian recent malacofauna (Mollusca, Bivalvia). Acta Zool. Bulg. 2001, 53, 61–66. [Google Scholar]
- Vrabec, V.; Čejka, T.; Šporka, F.; Hamerlík, L.; Král, D. First record of Corbicula fluminea (Mollusca, Bivalvia) from Slovakia with a note about its dispersion in Central Europe. Biologia 2003, 58, 942–952. [Google Scholar]
- Lyashenko, A.V.; Sinitzina, O.O.; Voloshkevich, E.V. Exotic benthic invertebrates in the water bodies of the lower reaches of the Danube. Hydrobiol. Zhurnal 2005, 41, 46–56. (In Russian) [Google Scholar]
- Popa, O.P.; Murariu, D. Freshwater bivalve molluscs invasive in Romania. In Biological Invasions: Towards a Synthesis; Pyšek, P., Pergl, J., Eds.; Institute of Ecology of the TU: Berlin, Germany, 2009; pp. 123–133. [Google Scholar]
- Lyashenko, A.V.; Makovskii, V.V. Molluscs of genus Corbicula in the Ukrainian section of the Danube. Hydrobiol. Zhurnal 2011, 47, 43–52. (In Russian) [Google Scholar]
- Sweeney, P. First record of Asian clam Corbicula fluminea (Müller, 1774) in Ireland. Ir. Nat. J. 2009, 30, 147–148. [Google Scholar]
- Caffrey, J.; Evers, S.; Millane, M.; Moran, H. Current status of Ireland’s newest invasive species—The Asian clam Corbicula fluminea (Müller, 1774). Aquat. Invasions 2011, 6, 291–299. [Google Scholar] [CrossRef]
- Hubenov, Z.; Trichkova, T.; Kenderov, L.; Kozuharov, D. Distribution of Corbicula fluminea (Mollusca: Corbiculidae) over an Eleven-Year Period of its Invasion in Bulgaria. Acta Zool. Bulg. 2013, 65, 315–326. [Google Scholar]
- Strayer, D.L.; Smith, L.C. Distribution of the zebra mussel (Dreissena polymorpha) in estuaries and brackish waters. In Zebra Mussels: Biology, Impacts, and Control; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 715–726. [Google Scholar]
- Ludyanskiy, M.L.; McDonald, D.; MacNeill, D. Impact of the zebra mussel, a bivalve invader. BioScience 1993, 43, 533–544. [Google Scholar] [CrossRef]
- Ram, J.L.; Karim, A.S.; Banno, F.; Kashian, D.R. Invading the invaders: Reproductive and other mechanisms mediating the displacement of zebra mussels by quagga mussels. Int. J. Invertebr. Reprod. Dev. 2011, 56, 21–32. [Google Scholar] [CrossRef]
- Carlton, J.T. Dispersal Mechanisms of the Zebra Mussel (Dreissena polymorpha). In Zebra Mussels: Biology, Impacts, and Control; Lewis Publishers: Boca Raton, FL, USA, 1993; pp. 677–704. [Google Scholar]
- Johnson, L.E.; Ricciardi, A.; Carlton, J.T. Overland dispersal of aquatic invasive species: A risk assessment of transient recreational boating. Ecol. Appl. 2001, 11, 1789–1799. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Burlakova, L.E.; Mastitsky, S.E.; Padilla, D.K.; Mills, E.L. Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. J. Shellfish. Res. 2011, 30, 923–931. [Google Scholar] [CrossRef]
- Kelly, N.E.; Wantola, K.; Weisz, E.; Yan, N.D. Recreational boats as a vector of secondary spread for aquatic invasive species and native crustacean zooplankton. Biol. Invasions 2012, 15, 509–519. [Google Scholar] [CrossRef]
- McMahon, R.F. Evolutionary and physiological adaptations of aquatic invasive animals: R selection versus resistance. Can. J. Fish. Aquat. Sci. 2002, 59, 1235–1244. [Google Scholar] [CrossRef]
- Sousa, R.; Rufino, M.; Gaspar, M.; Antunes, C.; Guilhermino, L. Abiotic impacts on spatial and temporal distribution of Corbicula fluminea (Müller, 1774) in the River Minho Estuary, Portugal. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 98–110. [Google Scholar] [CrossRef]
- van de Velde, S.; Jorissen, E.L.; Neubauer, T.A.; Radan, S.; Pavel, A.B.; Stoica, M.; Van Baak, C.G.C.; Gandara, A.M.; Popa, L.; de Stigter, H.; et al. A conservation palaeobiological approach to assess faunal response of threatened biota under natural and anthropogenic environmental change. Biogeosciences 2019, 16, 2423–2442. [Google Scholar] [CrossRef]
- Gogaladze, A.; Raes, N.; Biesmeijer, J.C.; Ionescu, C.; Pavel, A.B.; Son, M.O.; Gozak, N.; Anistratenko, V.V.; Wesselingh, F.P. Social network analysis and the implications for Pontocaspian biodiversity conservation in Romania and Ukraine: A comparative study. PLoS ONE 2020, 15, e0221833. [Google Scholar] [CrossRef]
- Gogaladze, A.; Son, M.O.; Lattuada, M.; Anistratenko, V.V.; Syomin, V.L.; Pavel, A.B.; Popa, O.P.; Popa, L.O.; ter Poorten, J.J.; Biesmeijer, J.C.; et al. Decline of unique Pontocaspian biodiversity in the Black Sea Basin: A review. Ecol. Evol. 2021, 11, 12923–12947. [Google Scholar] [CrossRef]
- Ingram, W.M. Asiatic clams as potential pests in California water supplies. J. Am. Water Work. Assoc. 1959, 51, 363–370. [Google Scholar] [CrossRef]
- Isom, B.G.; Bowman, C.F.; Johnson, J.T.; Rodgers, E.B. Controlling Corbicula (Asiatic clam) in complex power plant and industrial water systems. In American Malacological Bulletin, Special Edition; American Malacological Union: Buffalo, NY, USA, 1986; Volume 2, pp. 95–98. [Google Scholar]
- Prokopovich, N.P.; Hebert, D.J. Sedimentation in the Delta-Mendota Canal. J. Am. Water Work. Assoc. 1965, 57, 375–382. [Google Scholar] [CrossRef]
- Rosa, I.C.; Pereira, J.L.; Gomes, J.; Saraiva, P.M.; Gonçalves, F.; Costa, R. The Asian clam Corbicula fluminea in the European freshwater-dependent industry: A latent threat or a friendly enemy? Ecol. Econ. 2011, 70, 1805–1813. [Google Scholar] [CrossRef]
- Clarke, K.B. The infestation of waterworks by Dreissena polymorpha, a freshwater mussel. J. Inst. Water Eng. 1952, 6, 370–379. [Google Scholar]
- Mackie, G.L. Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 1991, 219, 251–268. [Google Scholar] [CrossRef]
- MacIsaac, H.J.; Lonnee, C.J.; Leach, J.H. Suppression of microzooplankton by zebra mussels: Importance of mussel size. Freshw. Biol. 1995, 34, 379–387. [Google Scholar] [CrossRef]
- Ricciardi, A.; Whoriskey, F.G.; Rasmussen, J.B. Impact of the Dreissena invasion on native unionid bivalves in the upper St. Lawrence River. Can. J. Fish. Aquat. Sci. 1996, 53, 1434–1444. [Google Scholar] [CrossRef]
- Schloesser, D.W.; Nalepa, T.F.; Mackie, G.L. Zebra mussel infestation of unionid bivalves (Unionidae) in North America. Am. Zool. 1996, 36, 300–310. [Google Scholar] [CrossRef]
- Nalepa, T.F.; Hartson, D.J.; Gostenik, G.W.; Fanslow, D.L.; Lang, G.A. Changes in the freshwater mussel community of Lake St. Clair: From Unionidae to Dreissena polymorpha in eight years. J. Great Lakes Res. 1996, 22, 354–369. [Google Scholar] [CrossRef]
- Nalepa, T.F.; Hartson, D.J.; Fanslow, D.L.; Lang, G.A. Recent population changes in freshwater mussels (Bivalvia: Unionidae) and zebra mussels (Dreissena polymorpha) in Lake St. Clair, USA. Am. Malacol. Bull. 2001, 16, 141–145. [Google Scholar]
- Karatayev, A.Y.; Burlakova, L.E.; Padilla, D.K. The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in eastern Europe. J. Shellfish Res. 1997, 16, 187–203. [Google Scholar]
- Caraco, N.F.; Cole, J.J.; Raymond, P.A.; Strayer, D.L.; Pace, M.L.; Findlay, S.E.G.; Fischer, D.T. Zebra mussel invasion in a large, turbid river: Phytoplankton response to increased grazing. Ecology 1997, 78, 588–602. [Google Scholar] [CrossRef]
- Bastviken, D.T.E.; Caraco, N.F.; Cole, J.J. Experimental measurements of zebra mussel (Dreissena polymorpha) impact on phytoplankton community composition. Freshw. Biol. 1998, 39, 375–386. [Google Scholar] [CrossRef]
- Pace, M.L.; Findlay, S.E.G.; Fischer, D. Effects of an invasive bivalve on the zooplankton community of the Hudson River. Freshw. Biol. 1998, 39, 103–116. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Padilla, D.K. The impact of Dreissena polymorpha (Pallas) invasion on unionid bivalves. Int. Rev. Hydrobiol. 2000, 85, 529–541. [Google Scholar] [CrossRef]
- Erben, R.; Lajtner, J.; Lucic, A.; Maguire, I.; Klobucar, G.I.V. Attachment of the zebra mussel on the artificial substrates in the reservoir Dubrava (River Drava, Croatia). Int. Assoc. Danub. Res. 2000, 33, 225–231. [Google Scholar]
- Strayer, D.L.; Hattala, K.; Kahnle, A. Effects of an invasive bivalve (Dreissena polymorpha) on fish populations in the Hudson River estuary. Can. J. Fish. Aquat. Sci. 2004, 61, 924–941. [Google Scholar] [CrossRef]
- Maguire, M.; Grey, J. Determination of zooplankton dietary shift following a zebra mussel invasion, as indicated by stable isotope analysis. Freshw. Biol. 2006, 51, 1310–1319. [Google Scholar] [CrossRef]
- Jones, C.G.; Lawton, J.H.; Shachak, M. Organisms as ecosystem engineers. Oikos 1994, 69, 373–386. [Google Scholar] [CrossRef]
- Nalepa, T.F.; Fahnenstiel, G.L. Dreissena polymorpha in the Saginaw Bay, Lake Huron Ecosystem: Overview and perspective. J. Great Lakes Res. 1995, 21, 411–416. [Google Scholar] [CrossRef]
- Olenin, S. Comparative study of the south-eastern Baltic coastal zone and the Curonian Lagoon bottom communities. In Proceedings of the 13th Baltic Marine Biologists Symposium; Andrushaitis, A., Ed.; Institute of Aquatic Ecology, University of Latvia: Riga, Latvia, 1997; pp. 151–159. [Google Scholar]
- Stewart, T.W.; Miner, J.G.; Lowe, R.L. Quantifying mechanisms for zebra mussel eVects on benthic macroinvertebrates: Organic matter production and shell-generated habitat. J. N. Am. Benthol. Soc. 1998, 17, 81–94. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Burlakova, L.E.; Padilla, D.K. Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In Invasive Aquatic Species of Europe: Distribution, Impacts and Management; Leppakoski, E., Gollasch, S., Olenin, S., Eds.; Springer: Dordrecht, The Netherlands, 2002; pp. 433–446. [Google Scholar]
- Vanderploeg, H.A.; Nalepa, T.F.; Jude, D.J.; Mills, E.L.; Holeck, K.T.; Liebig, J.R.; Grigorovich, I.A.; Ojaveer, H. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Can. J. Fish. Aquat Sci. 2002, 59, 1209–1228. [Google Scholar]
- Bially, A.; MacIsaac, H.J. Fouling mussels (Dreissena spp.) colonize soft sediments in Lake Erie and facilitate benthic invertebrates. Freshw. Biol. 2000, 43, 85–97. [Google Scholar] [CrossRef]
- Beekey, M.A.; McCabe, D.J.; Marsden, J.E. Zebra mussel colonisation of soft sediments facilitates invertebrate communities. Freshw. Biol. 2004, 49, 535–545. [Google Scholar] [CrossRef]
- Hecky, R.E.; Smith, R.E.H.; Barton, D.R.; Guilford, S.J.; Taylor, W.D.; Charlton, M.N.; Howell, T. The nearshore phosphorus shunt: A consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Can. J. Fish. Aquat Sci. 2004, 61, 1285–1293. [Google Scholar] [CrossRef]
- Karatayev, A.Y.; Lyakhnovich, V.P.; Afanasiev, S.A.; Burlakova, L.E.; Zakutsky, V.P.; Lyakhov, S.M.; Miroshnichenko, M.P.; Moroz, T.G.; Nekrasova, M.Y.; Skalskaya, I.A.; et al. The place of species in ecosystem. In Freshwater Zebra Mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae); Starobogatov, J.I., Ed.; Systematics, Ecology, Practical Meaning; Nauka Press: Moscow, Russia, 1994; pp. 206–221. (In Russian) [Google Scholar]
- Botts, P.; Silver, B.; Patterson, A.; Schloesser, D.W. Zebra mussel effects on benthic invertebrates: Physical or biotic? J. N. Am. Benthol. Soc. 1996, 15, 179–184. [Google Scholar] [CrossRef]
- Gutierrez, J.L.; Jones, C.G.; Strayer, D.L.; Iribame, O.O. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos 2003, 101, 79–90. [Google Scholar] [CrossRef]
- Beekey, M.A.; McCabe, D.J.; Marsden, J.E. Zebra mussels affect benthic predator foraging success and habitat choice on soft sediments. Oecologia 2004, 141, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Karatayev, A.Y.; Howells, R.G.; Burlakova, L.E.; Sewell, B.D. History of spread and current distribution of Corbicula fluminea (Müller) in Texas. J. Shellfish. Res. 2005, 24, 553–559. [Google Scholar]
- Pacioglu, O.; Duţu, L.; Duţu, F.; Pavel, A.B. Habitat preferences and trophic interactions of the benthic invertebrate communities inhabiting depositional and erosional banks of a meander from Danube Delta (Romania). Glob. Ecol. Conserv. 2022, 38, e02213. [Google Scholar] [CrossRef]
- Pacioglu, O.; Duţu, F.; Pavel, A.B.; Duţu, L.T. The influence of hydrology and sediment grain-size on the spatial distribution of macroinvertebrate communities in two submerged dunes from the Danube Delta (Romania). Limnetica 2022, 41, 85–100. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Z.; Karatayev, A.V. Invasive mussels induce com-munity changes by increasing habitat complexity. Hydrobiologia 2012, 685, 121–134. [Google Scholar] [CrossRef]
- Dieterich, A.; Mörtl, M.; Eckmann, R. The effects of zebra mussels (Dreissena polymorpha) on the foraging success of Eurasian perch (Perca fluviatilis) and ruffe (Gymnocephalus cernuus). Int. Rev. Hydrobiol. 2004, 89, 229–237. [Google Scholar] [CrossRef]
- Mayer, C.M.; Rudstam, L.G.; Mills, E.L.; Cardiff, S.G.; Bloom, C.A. Zebra mussels (Dreissena polymorpha), habitat alteration, and yellow perch (Perca flavescens) foraging: System-wide effects and behavioural mechanisms. Can. J. Fish. Aquat. Sci. 2001, 58, 2459–2467. [Google Scholar] [CrossRef]
- Ricciardi, A.; Whoriskey, F.G.; Rasmussen, J.B. The role of the zebra mussel (Dreissena polymorpha) in structuring macroinvertebrate communities on hard substrata. Can. J. Fish. Aquat. Sci. 1997, 54, 2596–2608. [Google Scholar] [CrossRef]
- Nosek, J.; Oertel, N.; Bódis, E.; Tóth, B. A bentikus szervesanyag és a makroger-inctelen társulások tér- és id”obeli változása a Duna Kismaros (1688 fkm) és Göd (1668 fkm) közötti szakaszán (Spatial and temporal changes of benthic organicmatter and macroinvertebrate communities in the Kismaros (1688 rkm)-Göd (1668 rkm) section of the River Danube). Acta Biol. Debrecina Suppl. Oecol. Hung. 2009, 20, 165–179. [Google Scholar]
- Pavel, A.B.; Menabit, S.; Pop, I.C.; Stanescu, I.; Lupascu, N. The spatio-temporal distribution of the Ponto-Caspian polychaete in the Lower Sector of the Danube River and in Danube Delta. Glob. Ecol. Conserv. 2021, 28, e01623. [Google Scholar] [CrossRef]
- Aldridge, D.C.; Müller, S.J. The Asiatic clam, Corbicula fluminea, in Britain: Current status and potential impacts. J. Conchol. 2001, 37, 177–183. [Google Scholar]
- Beran, L. Současný stav invaze a neobvyklá lokalita korbikuly asijské [The current state of the invasion and the unusual habitat of the Asian clam]. Živa 2013, 1, 25. (In Czech) [Google Scholar]
- Sebestye´n, O. Colonization of two new fauna elements of Pontus-origin (Dreissena polymorpha Pall. and Corophium curvispinum G.O. Sars forma devium Wundsch) in Lake Balaton. Verh. Int. Ver. Theor. Angew. Limnol. 1938, 8, 169–181. [Google Scholar]
- Balogh, C.; Musko, I.B.; G-To´th, L.; Nagy, L. Quantitative trends of zebra mussels in Lake Balaton (Hungary) in 2003–2005 at different water levels. Hydrobiologia 2008, 613, 57–69. [Google Scholar] [CrossRef]
- Orlova, M.I.; Panov, V.E. Establishment of the zebra mussel, Dreissena polymorpha (Pallas), in the Neva Estuary (Gulf of Finland, Baltic Sea): Distribution, population structure and possible impact on local unionid bivalves. Hydrobiologia 2004, 514, 207–217. [Google Scholar] [CrossRef]
- Sporka, F.; Nagy, S. The macrozoobenthos of parapotamon-type side arms of the Danube River in Slovakia and its response to flowing conditions. Biol. Bratisl. 1998, 53/5, 633–643. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).