Abstract
Efficient management of N fertilizers enhances crop yields and contributes to sustainable food security. Tropical acidic soils with high Al and Fe are prone to easy loss of basic cations, such as NH4+, via leaching and erosion. Appropriate soil amendments and agronomic practices minimize the loss of fertilizer nutrients, improve soil nutrient retention, and maximize their uptake by plants. This study aimed to evaluate the effects of co-applying charcoal and sago bark ash with inorganic fertilizers on N availability, uptake, use efficiency, and dry matter production of sorghum in a tropical acid soil. The results revealed that the co-application of inorganic fertilizers with charcoal and sago bark ash increased sorghum plant height, dry matter production, N uptake and N use efficiency. The soil treated with a combination of 100% of the recommended rate of charcoal and sago bark ash (C1A1) resulted in significantly higher sorghum dry matter production, N uptake, and use efficiency compared with normal fertilization (U1). The C1A1 treatment resulted in significantly lower soil available N compared with U1. The C1A1 treatment enhanced the uptake of N by the sorghum plants, resulting in less available N in the soil after the experiment. Although the effects of co-applying charcoal and sago bark ash on soil total N were not glaring, this practice increased soil pH and total C, and reduced exchangeable acidity and Al3+. A long-term field study is recommended to confirm the effects of co-applying inorganic fertilizers with charcoal and sago bark ash on sorghum productivity, economic viability, and soil nutrient residual effects.
1. Introduction
Sorghum (Sorghum bicolor L.) is the world’s fifth most significant cereal crop after wheat, maize, rice, and barley []. Sorghum is cultivated all around the globe, including North America in the United States and Mexico, and in South America in Argentina, Peru, Nicaragua, Brazil, Uruguay, Colombia, Honduras, Venezuela, Haiti, Guatemala, and Salvador. It is also cultivated in 38 countries across Sub-Saharan Africa. In Europe, sorghum is also cultivated in Albania, Romania, France, Italy, and Spain []. Sorghum is an annual cereal crop, is adapted to withstand higher average temperatures than other cereal crops [], and is grown to provide both grain and palatable green fodder. In addition to its use as a food crop, sorghum can be used for a variety of other purposes, including dairy animal feed, novel foods, industrial uses, processed food starch, beverages, and ethanol [].
Ultisols predominate in Southeast Asian soils []. Soil pH has a strong relationship with nutrient availability to plants. Tropical Ultisols are acidic with high accumulations of Al and Fe. The relatively higher rainfall intensity in the tropics contributes to the loss of soil basic cations through leaching and erosion. This decreases the pH and fertility of Ultisols. These soils can also be inherently acidic because of the nature of their parent materials. Furthermore, crops cultivated on these acid soils are affected by other factors such as toxicities of Al and Fe, nutrient deficiencies, and root infections or diseases [,]. Low soil pH is exacerbated by the dissolution of Al ions on soil exchange sites into the soil solution. The low productivity of these types of soil is also due to the lack of available P, Ca, and Mg and rapid mineralization of organic matter [,]. If these soil constraints are not managed well, there could be spiral effects and worsening of soil conditions []. The aforementioned problems are further exacerbated when farmers use chemical fertilizers excessively in order to improve soil productivity. Liming is a common practice that promotes plant growth by increasing soil pH, reducing Al, and improving plant uptake of basic cations. However, excessive liming has negative impacts on crop and soil productivity, such as limiting the availability of N, P, Mn, Cu, and Zn []. In addition to this, liming materials are expensive, which implies a financial burden to farmers. Nitrogen fertilization has long been known to improve soil fertility and crop productivity. However, improper N fertilizer application has a negative impact on the environment, such as NO3− leaching below the root zone to contaminate ground water [] and reduction in N use efficiency (NUE) []. Excessive nitrogen use also negatively impacts plants, increasing disease incidence and susceptibility to pests []. Therefore, N availability and use efficiency need to be improved to avoid NO3− loss through leaching.
These problems can be managed or overcome in order to boost agricultural production through appropriate soil management practices. The use of charcoal and sago bark ash for soil N management is a promising approach because these materials improve NH4+ retention in soil. It is worth mentioning that Sago, particularly Metroxylon sagu, cultivated in tropical regions of Southeast Asia, is one of the most significant starch crops used as an industrial raw material []. Wahi et al. [] reported that Sarawak, in Malaysia, is currently one of the world’s largest exporters of sago products, with approximately 43 thousand tonnes exported annually. It is estimated that 0.75 tonnes of sago bark waste is produced for every tonne of sago flour produced, which is about 32,250 tonnes per year. Normally the sago bark waste is incinerated for power generation, discharged into a nearby river, or left to degrade naturally outside the sago mills []. Approximately 85% of sago bark waste is not used, creating a possibility for agricultural waste valorization. One such method of valorization is conversion into ash. However, excessive use of ash could lead to toxicity for plants because it comprises some harmful compounds including radioactive Caesium-137 and other toxic elements such as As, Cu, Co, Pb, Zn, and Ni, which can damage plants and have a negative impact on the ecosystem []. Despite this, sago bark ash could break the functional groups of charcoal because of the effect of Ca and Mg. The combination of charcoal and sago bark ash could also enhance NH4+ retention in soils []. The use of charcoal as an amendment results in nascent feedbacks because it provides a negatively charged surface to enhance nutrient retention []. When charcoal is applied to soils, it decomposes to produce organic compounds such as humic substances and other organic acids. These compounds have high affinity for Al and Fe ions. As the soil pH increases, NH4+ is adsorbed onto the negative exchange sites because of the deprotonation of the functional groups (carboxyl, phenolic, and hydroxyl) []. The use of charcoal and sago bark ash as amendments enhances soil aggregate formation, which subsequently improves NO3− retention through improved soil water holding capacity [].
However, there is a scarcity of knowledge of the combined use and application to soils of charcoal and sago bark ash, which could potentially improve the N availability, uptake, use efficiency, and dry matter production of sorghum cultivated on tropical acid soils. These agricultural wastes have large surface areas and high amounts of negatively charged sites, which can act as sites for soil cation retention. This study attempted to answer the question about whether amending chemical fertilizers with charcoal and sago bark ash can significantly improve N availability, uptake, use efficiency, and dry matter production of sorghum on tropical acid soils. The co-application of charcoal and sago bark ash as amendments are intended to provide insights on the potential of these agricultural wastes or by-products to be valorized for agronomic purposes. This would contribute significantly to sustainable crop production in tropical acid soils. Thus, the objective of this study was to evaluate the effects of amending inorganic fertilizers with charcoal and sago bark ash on N availability, uptake, use efficiency, and dry matter production of sorghum cultivated on a tropical acid soil.
2. Materials and Methods
2.1. Soil Samples Preparation and Analyses
The soil (Bekenu series, Typic Paleudults) used in this study was taken from an uncultivated secondary forest at Universiti Putra Malaysia, Bintulu Sarawak Campus (3°12′20.0″ N, 113°04′20.0″ E). The soil was sampled at a depth of 0–20 cm, after which it was air-dried, crushed, and sieved to pass through a 2 mm sieve for characterization and through a 5 mm sieve for the pot experiment. Before commencing the pot study, soil samples were analyzed for their bulk density, texture, pH, electrical conductivity, total C, total N, exchangeable NH4+, available NO3−, exchangeable acidity, exchangeable Al, exchangeable H+, and exchangeable cations using standard procedures.
2.2. Initial Characterization of Soil, Sago Bark Ash, and Charcoal
The selected physical and chemical properties of the soil, charcoal, and sago bark ash are summarized in Table 1. With the exception of soil texture, the selected physical and chemical properties of the soil (Bekenu series, Typic Paleudults) used in this study were within the ranges reported by Paramananthan []. However, the soil texture obtained was comparable to that reported previously []. The sago bark ash used in this study was obtained from Song Ngeng Sago Industries, Dalat, Sarawak, Malaysia. The charcoal was obtained from Pertama Ferroalloys Sdn Bhd, Bintulu, Sarawak, Malaysia.

Table 1.
Key physical and chemical properties of Bekenu series (Typic Paleudults), sago bark ash, and charcoal used in the pot study.
2.3. The Pot Experiment
The pot experiment was conducted in a net house located at Universiti Putra Malay-sia’s Bintulu Sarawak Campus, Malaysia. The net house was constructed with an even span design using a metal frame for the base, and a polyvinyl chloride frame for the roof. Sorghum was used as a test crop in this study. Polybags (36.5 cm in height, 18 cm in width, and 19.5 cm in diameter) were filled with 7 kg of soil. Nitrogen, P, and K fertilizers were applied for optimum growth of the test crop. The amounts of urea (46% N), Egypt Rock Phosphate (ERP) (28% P2O5), and Muriate of Potash (MOP) (60% K2O), which is also known as Potassium chloride (KCl), used were based on MARDI’s [] recommendations for maize. The amounts of urea, ERP, and MOP used were 130 kg ha−1 urea: 214 kg ha−1 ERP: 67 kg ha−1 MOP equivalent to 60 kg ha−1 N, 60 kg ha−1 P2O5, and 60 kg ha−1 K2O. The amounts of the inorganic fertilizers (urea, ERP, and MOP) were fixed at 100% of the recommended rates except for the treatment without fertilization. The fertilizers were applied on the soil surface in two equal splits at 10 and 28 days after sowing (DAS). The amount of charcoal used was based on 10 t ha−1 which is equivalent to 359.8 g per 7 kg soil for this pot study [,]. The sago bark ash used was based on 5 t ha−1, which is equivalent to 179.9 g per 7 kg soil for this pot study [,,]. The charcoal rates were varied by 25, 50, 75, and 100%. The sago bark ash rates were varied by 25, 50, and 100%. These amounts were scaled down from the standard fertilizer recommendation for maize cultivation per plant basis (based on a planting density of 27,777 plants ha−1) (Table 2 and Table 3). Currently, sorghum is not commonly planted on Malaysian Ultisols. Since this research investigates the cultivation of sorghum on these Ultisols, and sorghum is a cereal like maize, the fertilization program for maize was adopted for this study to obtain preliminary information about growing sorghum on these acidic soils.

Table 2.
Percentages of charcoal, sago bark ash, nitrogen, phosphorus, and potassium fertilizer rates used for the treatments.

Table 3.
Treatments evaluated in the pot experiment.
The soil, charcoal, and sago bark ash were mixed thoroughly, after which they were moistened with tap water at 60% water holding capacity a day before sowing. The sor-ghum seeds were soaked in water for 24 h before sowing to ensure good germination and plant establishment []. Thereafter, the seeds were sown in planting holes (1 seed per hole) at a depth of 3–4 cm per hole. The holes were then covered with loose soil. Two seeds were sown per polybag, after which they were thinned to one at 7 DAS. The volume of water used for each polybag was maintained at 50% to 60% water holding capacity throughout the pot study. The sorghum plants were checked and monitored regularly for pest attack and disease infestation throughout the experiment. The plants were monitored until harvest at 55 DAS. The treatments evaluated in this pot study are shown in Table 3.
2.4. Plant and Soil Chemical Analyses after Harvest
The sorghum plants were harvested at 55 DAS, which is the maximum reproductive stage before grain filling and physiological maturity stage of sorghum plants. This is the booting stage during which time nutrient and dry weight accumulation peaks. The plants were partitioned into leaves, stem, and roots. The roots were cleansed from soil using tap water. All the plant parts were oven dried at 60 °C until constant weight was attained, after which, their dry weights were determined using a digital weighing balance. Then, plant samples were used to determine total N using the Kjeldhal method []. For the soil, samples were collected, air-dried, ground, and sieved to pass 2 mm sieve. Then, they were analyzed for pH, exchangeable acidity, exchangeable Al, exchangeable H+, total C, CEC, total N, exchangeable NH4+, and available NO3−.Soil pH in water and KCl were determined in a 1:2.5 (soil: distilled water or KCl) using a digital pH meter []. Exchangeable acidity, exchangeable Al and exchangeable H+ were determined using acid-based titration method []. Total C was calculated as 58% of the organic matter using the loss of weight on ignition method []. Cation exchange capacity (CEC) of soil was determined using the leaching method [] followed by steam distillation []. Total N was determined using Kjeldhal method []. Exchangeable NH4+ and available NO3− were determined using the Keeney and Nelson method []. Nitrogen uptake in stem, leaves, and roots was determined by multiplying their concentrations with the dry weight of the plant parts. The N use efficiency was determined using the equation described by Dobermann []:
Uptake = Concentration × Dry weight (g)
Nutrient Use Efficiency (%) = ((uptake with fertilizer − uptake without fertilizer))/(total amount of fertilizer that had been applied) × 100
2.5. Experimental Design and Statistical Analysis
The experimental design used in the pot experiment was Completely Randomized Designed (CRD), performed in triplicate. A normality test, using Shapiro–Wilk test, was carried out before analysis to ensure the data obtained satisfies the assumptions of ANOVA. Analysis of Variance (ANOVA) was used to test treatment effects and means of treatments were compared using Tukey’s Studentized Range (HSD) test at p ≤ 0.05. The Statistical Analysis System (SAS, Cary, NC, USA) version 9.4 was used for the statistical tests.
3. Results and Discussion
3.1. Effects of Charcoal and Sago Bark Ash on Sorghum Dry Matter Production
The treatments with charcoal and sago bark ash (C1A1, C2A3, C2A4, C3A4, and C4A4) resulted in taller plants (Figure 1) and dry matter production (leaves, stem, and roots) (Table 4, Figure 2) compared with normal fertilization (U1). Soil only (S0) resulted in the lowest sorghum plant height. The sorghum plant leaf dry matter for A1 and C1A1 was significantly higher than those of S0, U1, C1, C2A4, and C4A4. The sorghum plant stem dry matter values for C1A1 and C3A4 were the highest. The sorghum plant stem dry matter was the lowest for soil only (S0), followed by normal fertilization (U1). Treatment with 100% charcoal and 100% sago bark ash (C1A1) significantly enhanced the rooting of sorghum compared with other treatments. The sorghum plant roots’ dry matter for normal fertilization (U1) was similar to that of the charcoal only (C1) treatment.
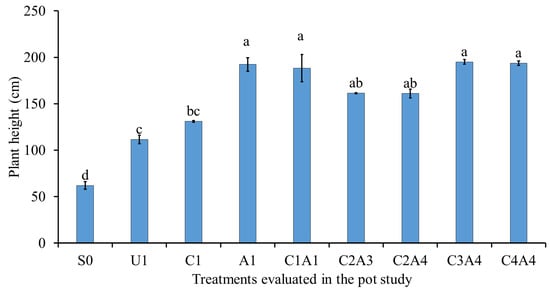
Figure 1.
The effects of treatments on sorghum plant height at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d. Bars represent the mean values ± SE of triplicates.

Table 4.
Effects of amending nitrogen, phosphorus, and potassium fertilizers with charcoal and sago bark ash on dry matter production of sorghum plants.

Figure 2.
Effects of treatments on sorghum shoot and root growth at booting stage.
Soil only resulted in the lowest sorghum plant height and dry matter production due to lack of nutrients to sustain their growth and development. The significant increase in the aboveground biomass for the higher rates of charcoal and sago bark ash suggests that this treatment combination improved the availability of nutrients for uptake by the sorghum plants [].
3.2. Effects of Charcoal and Sago Bark Ash Amendments on Sorghum Nitrogen Uptake and Use Efficiency
Values of nitrogen content in the sorghum plant parts are presented in Table 5. The effects on total N content in sorghum plant parts were similar among the soil only (S0), inorganic fertilizer with charcoal only (C1), and the combined treatments (C1A1, C2A3, C2A4, C3A4, and C4A4). The effects of C1 and A1 on N content in the sorghum leaves were significantly different. The treatment effects were similar for the N content in the stems. This finding is in agreement with that of Nguyen et al. [], who found that the amendments had no effect on N in plant parts because differences in N isotopic composition between roots and leaves reflected a fractionation during N transformation within the plant when the roots and leaves are products of the same source of N. The significant differences in leaves and roots could be attributed to how these plant parts are related to N in terms of metabolic activity. Furthermore, N is an essential component of biomolecules, nucleic acids, chlorophyll, and several secondary metabolites. The insignificant differences in stem N content are attributable to the harvesting of the plants at the maximum growth stage before reaching their reproductive stage [].

Table 5.
Effects of amending nitrogen, phosphorus, and potassium fertilizer with charcoal and sago bark ash on nitrogen content in sorghum plant parts at fifty-five days after sowing.
Nitrogen uptake in the sorghum plant leaves, stem, and roots is summarized in Table 6. The soil only treatment resulted in the lowest total N uptake, whereas the 100% charcoal and 100% sago bark ash (C1A1) combination treatment resulted in the highest total N uptake. Uptake of N in the sorghum leaves for C1A1, C2A3, C3A4, and C4A4 was significantly higher compared with the normal fertilization (U1), charcoal only (C1), and sago bark ash only (A1). For the N uptake in the stem, the combination of 75% charcoal with 50% sago bark ash (C2A3) resulted in the highest uptake. However, the effect was similar to the other combination treatments except C2A4. The 100% charcoal and 100% sago bark ash (C1A1) combination significantly increased N uptake in the roots of the sorghum plants. The effects of combining 75% charcoal with 25% and 50% sago bark ash (C2A3 and C2A4) on N uptake in the roots were comparable to those with normal fertilization (U1) and charcoal only (C1).

Table 6.
Effects of amending nitrogen, phosphorus, and potassium fertilizer with charcoal and sago bark ash on Sorghum nitrogen uptake.
Nitrogen is taken up by plants variably at different stages of their growth and development. The first of which is primary uptake phase, followed by the reduction of N to useable forms, such as assimilation into amino acids and translocation, and ending with remobilization of N to reproductive tissues []. Root growth and development are highly responsive to nutrient availability, and are regarded as a key factor in improving N use efficiency []. Root systems of cereal crops such as sorghum, rice, and maize are classified into two types: seminal roots and crown roots. This suggests that primary roots are responsible for N acquisition from deeper layers, whereas lateral roots occupy a larger volume of surface soil []. Lateral roots are known to be more susceptible to changes in N concentration in addition to biotic (mineralization, plant uptake, and immobilization) and abiotic (sorption and leaching) stresses. Low N content in the early stages of plants promotes lateral root initiation, but severe N deficit inhibits root emergence and elongation.
Figure 3 shows the effects of the treatments on N use efficiency. The normal fertilization (U1) resulted in significantly lower N use efficiency (NUE) compared with the combined treatments (C1A1, C2A3, C2A4, C3A4, and C4A4). Among the combined treatments, the 100% charcoal with 100% sago bark ash (C1A1) resulted in significantly better NUE. The effects of C2A3, C3A4, and C4A4 were similar, indicating that any of the treatments could be chosen as a good candidate for use as a soil amendment. The combination of charcoal and ash promotes absorption and subsequent use of N in different plant parts, and improves NUE. Moreover, the specific surface area and large number of functional groups in charcoal improves soil fertility and enhances NUE []. Soil type, macro- and micronutrients such as P and K in soil, and crop rotation regulate N uptake and use are reported to influence the crop requirements of N []. Mullen et al. [] opined that when the application of N exceeds the potential demand, NUE decreases. Furthermore, the N source in the root rhizosphere influences the profile of macro and micronutrients, which affects essential metabolic activities such as photosynthetic rate, plant growth, yield, and NUE []. As a result, NUE can be improved by increasing mineral uptake in tandem with N availability. A decrease in N concentration reduces N uptake and use of other mineral nutrients such as P, K, Mg, and Ca []. However, N interactions and metabolism with other nutrients differ depending on the environment, type of plant tissue, nutrient type, and genotype.
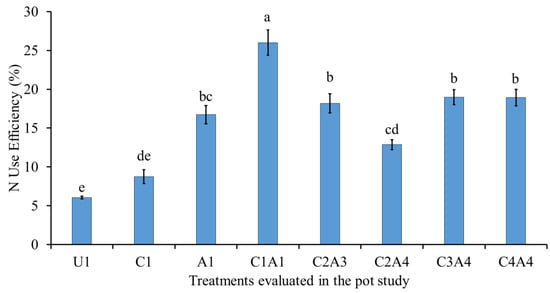
Figure 3.
Effects of treatments on nitrogen use efficiency at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d > e. Bars represent the mean values ± SE of triplicates.
3.3. Effects of Charcoal and Sago Bark Ash Amendments on Soil Nitrogen Fractions and pH at Fifty-Five Days after Sowing
The effects of the treatments on soil total N are presented in Figure 4. The normal fertilization (U1), charcoal only (C1), sago bark ash only (A1), and the combined treatments (C1A1, C2A3, C2A4, C3A4, and C4A4) had similar effects on the soil total N. The soil only resulted in the lowest total N. Despite having low amounts of N, ash can have a significant impact on the N cycle, by enhancing N mineralization in soils rich in organic matter []. Combining charcoal and ash has the potential to alter the N dynamics via several mechanisms, some of which have negative impacts []. For example, the high C:N ratio of the charcoal could limit mineralization of N, thus affecting plant N uptake [].
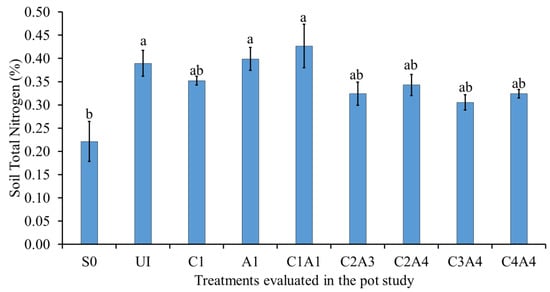
Figure 4.
Effects of treatments on soil total nitrogen at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b. Bars represent the mean values ± SE of triplicates.
Normal fertilization (U1) resulted in the highest soil exchangeable NH4+ (Figure 5). Regardless of the treatment type, the effects of C1, A1, C1A1, C2A3, C2A4, C3A4, and C4A4 on soil exchangeable NH4+ were similar. The reduction in NH4+ availability in the soils with charcoal can be explained by the contribution of bioavailable C compounds, which are able to stimulate microbial activities, resulting in soil inorganic N immobilization for a period of time. These findings are consistent with those of Deenik et al. [], who reported that soil NH4+ decreases with increasing soil microbial activity.
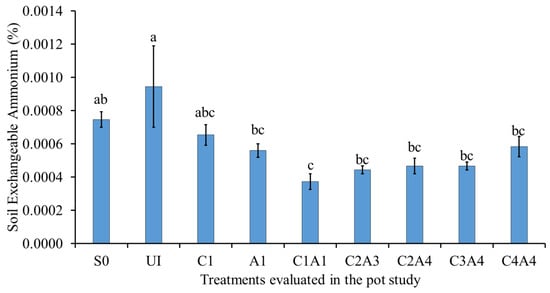
Figure 5.
Effects of treatments on soil exchangeable ammonium at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c. Bars represent the mean values ± SE of triplicates.
Figure 6 shows the effects of the treatments on soil available NO3− at 55 DAS. The soil only (S0), normal fertilization (U1), and charcoal only (C1) treatments resulted in higher available NO3− compared with those of sago bark ash only (A1) and the combined treatments (C1A1, C2A3, C2A4, C3A4, and C4A4). Nitrate in the soil decreased for the ash and combined treatments, because these amendments enhance root absorption and the use of soil NO3− and NH4+. The charcoal also adsorbs shallow inorganic N in the soil and reduces N loss, which ultimately increases plant growth [].
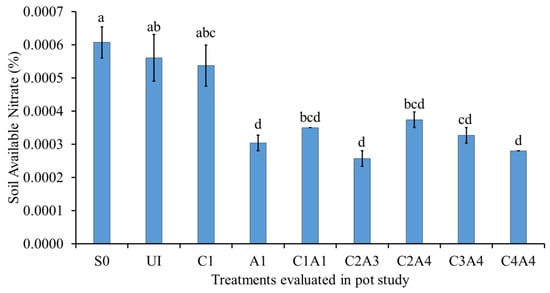
Figure 6.
Effects of treatments on available nitrate at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d. Bars represent the mean values ± SE of triplicates.
The effects of the treatments on soil pH in water and KCl were similar in pattern (Figure 7 and Figure 8). The soil only (S0), normal fertilization (U1), and charcoal only (C1) treatments resulted in significantly lower soil pH in water compared with A1, C1A1, C2A3, C2A4, C3A4, and C4A4 (Figure 7). For the soil pH in KCl, the combination of 100% charcoal with 100% sago bark ash resulted in a higher pH value compared with all of the other treatments (Figure 8). The lower soil pH of soil only (S0) was due to the soil’s inherently acidic nature (Table 1). The increase in soil pH after the addition of ash is consistent with the results of Perkiömäki et al. [], who reported that ash is alkaline and can influence soil microbial activities variably. The treatments with high ash content explains the effective liming capability compared with the treatments without ash. The high content of charcoal also contributed to an increase in soil pH. The combined use of charcoal and ash improved soil pH because of the neutralizing properties of the ash and the functional groups of the charcoal.
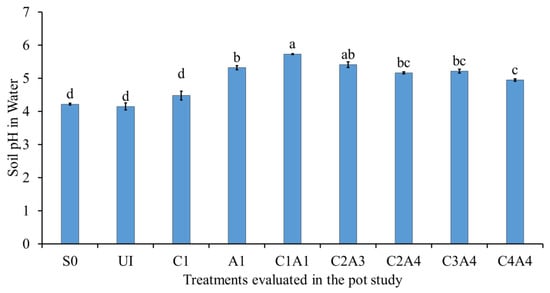
Figure 7.
Effects of treatments on soil pH in water at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d. Bars represent the mean values ± SE of triplicates.
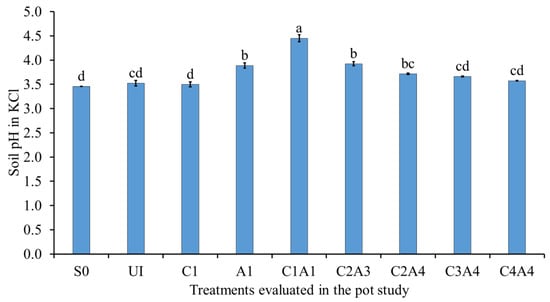
Figure 8.
Effects of treatments on soil pH in potassium chloride at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d. Bars represent the mean values ± SE of triplicates.
3.4. Effects of Charcoal and Sago Bark Ash Amendments on Selected Soil Chemical Properties at Fifty-Five Days after Sowing
The reduction in soil exchangeable acidity, Al3+, and H+ are related to the increase in the soil pH (Figure 9, Figure 10 and Figure 11). The soil only (S0) treatment resulted in the highest soil exchangeable acidity, followed by normal fertilization (U1) (Figure 9). The co-application of inorganic fertilizers with the amendments resulted in lower soil exchangeable acidity. The normal fertilization (U1) treatment resulted in no significant difference in soil exchangeable Al3+ compared with the soil only (S0) treatment (Figure 10). The use of inorganic fertilizers with amendments such as charcoal alone (C1) or in combination decreased exchangeable Al. The co-application of inorganic fertilizers with 100% sago bark ash (A1), 100% charcoal with 100% sago bark ash (C1A1), and 75% charcoal with 50% sago bark ash (C2A3) also resulted in negligible amount of soil exchangeable Al3+. When compared with the soil only, the combination of both amendments (C1A1, C2A4, C3A4, and C4A4) resulted in lower soil exchangeable H+.
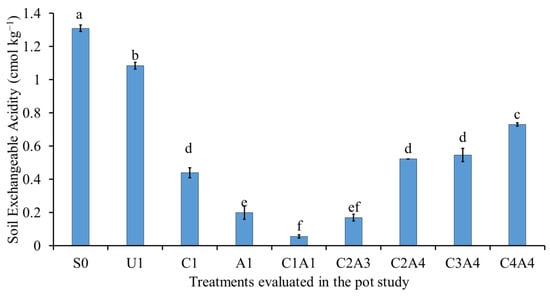
Figure 9.
Effects of treatments on soil exchangeable acidity at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d > e > f. Bars represent the mean values ± SE of triplicates.
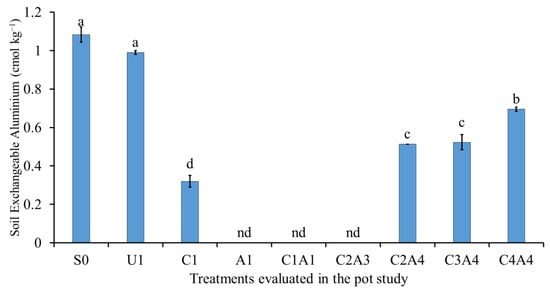
Figure 10.
Effects of treatments on soil exchangeable aluminum at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d. Bars represent the mean values ± SE of triplicates. Note: (nd = not determined).
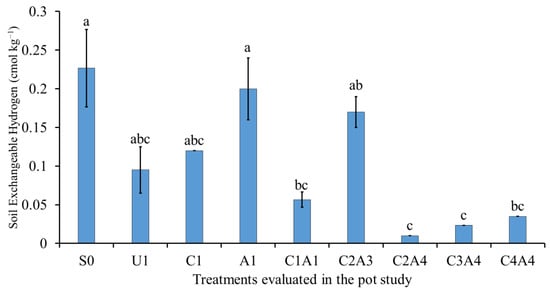
Figure 11.
Effects of treatments on soil exchangeable hydrogen at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c. Bars represent the mean values ± SE of triplicates.
These findings are similar to those of Nigussie et al. [], who reported that the increase in soil pH for treatments with charcoal was due to its high surface area and porous structure, which increase soil CEC and binds Al and Fe to soil exchange sites. This explains why the soil only and normal fertilization treatments demonstrated higher acidity compared to the treatments with ash and charcoal amendments. Vithanage et al. [] opined that the reduction in exchangeable Al3+ and H+ could be attributed to the formation of Al complexes by oxidized organic functional groups, such as phenolic and carboxylic groups, on the surface area of charcoal.
Figure 12 shows the effects of the treatments on soil electrical conductivity (EC). The soil only (S0), co-application of inorganic fertilizer with charcoal only (C1), and the treatments with 25% sago bark ash (C2A4, C3A4, and C4A4) resulted in lower soil EC compared to treatment with U1, A1, and C1A1, because the application of charcoal had minimal effects towards soil EC. This observation corroborates the findings of Jones and Quilliam [] and Lucchini et al. [], who reported similar effects of wood ash and charcoal on soil EC and pH. The results show that wood ash had a significantly greater effect on these two soil properties when compared to charcoal.
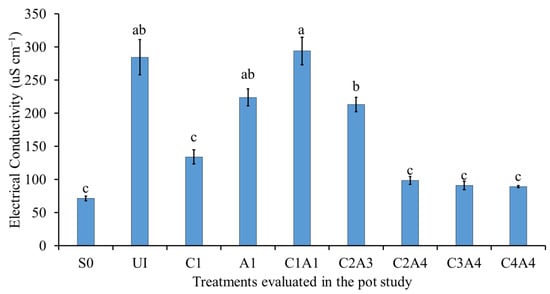
Figure 12.
Effects of treatments on soil electrical conductivity at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c. Bars represent the mean values ± SE of triplicates.
Among the treatments, the soil treated with charcoal (C1, C1A1, C2A3, C2A4, C3A4, and C4A4) demonstrated enhanced soil total C (Figure 13). The increase in soil total C is attributed to the relatively high C content of the treatments. The soil total C was significantly lower in S0, U1, and A1. The absence of C in these three treatments explains the lower soil total C content. With decreasing amount of charcoal, soil total C decreased. Charcoal has highly recalcitrant C molecules, which are poor decomposable C substrates, resulting in lower C mineralization of native soil C, causing deceleration of C cycling in the soil []. However, this is debatable, because charcoal particles promote litter decomposition as a result of higher microbial activity [].
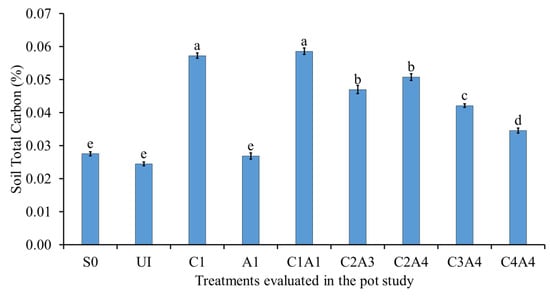
Figure 13.
Effects of treatments on soil total carbon at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c > d > e. Bars represent the mean values ± SE of triplicates.
The results for the soil cation exchange capacity (CEC) for the various treatments are presented in Figure 14. The normal fertilization (U1) resulted in the lowest soil CEC. The effects of the soil only (S0), C1, C2A4, C3A4, and C4A4 treatments on the soil CEC were similar. These results suggest that the addition of charcoal and sago bark ash had no significant effects on soil CEC because CEC is partly not affected by initial soil pH []. Conversely, Glaser et al. [] and Yuan et al. [] reported that the addition of charcoal to acidic soils increased soil CEC and the amounts of exchangeable base cations.
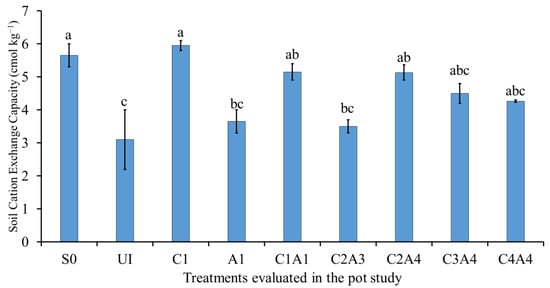
Figure 14.
Effects of treatments on soil exchangeable cation exchange capacity at fifty-five days after sowing. Means with different letter(s) indicate significant differences between treatments according to Tukey’s HSD test at p ≤ 0.05, and α 0.05 that is a > b > c. Bars represent the mean values ± SE of triplicates.
4. Conclusions
Amending inorganic fertilizers with charcoal and sago bark ash increases sorghum plant height, dry matter production, N uptake, and N use efficiency. This is due to the unique properties of charcoal, which has a significant number of functional groups that are capable of retaining NH4+ for sorghum plant uptake. As a result, the applied N was used efficiently because the combined charcoal and sago bark ash treatments reduce the potential for NO3− leaching losses. Although the effects of co-application of charcoal and sago bark ash on soil total N were not glaring, this practice increased soil pH, total C, and reduced exchangeable acidity and Al3+. A field study is recommended to further assess the effects of amending inorganic fertilizers with charcoal and sago bark ash on sorghum productivity, economic viability, and soil nutrient residual effects. Fourier transform infrared (FTIR) spectral analysis and analysis of the element composition of the amendments using EDX-S is necessary to identify the functional groups and chemical composition of the charcoal and sago bark ash.
Author Contributions
Conceptualization, N.H.H., O.H.A., L.O., H.Y.C. and M.B.J.; methodology, N.H.H., P.D.J., P.P. and L.O.; validation, O.H.A., L.O. and H.Y.C.; formal analysis, O.H.A. and M.B.J.; investigation, N.H.H.; resources, O.H.A., L.O., H.Y.C. and N.H.H.; data curation, N.H.H., P.D.J. and P.P.; writing—original draft preparation, N.H.H.; writing—review and editing, O.H.A., M.B.J. and A.A.M.; visualization, N.H.H., P.D.J., P.P. and A.A.M.; supervision, O.H.A., L.O. and H.Y.C.; project administration, O.H.A., M.B.J. and A.A.M.; funding acquisition, O.H.A., M.B.J. and A.A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Higher Education, Malaysia with grant number [FRGS/1/2016/WAB01/UPM/02/2].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The authors acknowledge the support of the Ministry of Higher Education, Malaysia for financial assistance and the collaboration with, and facilities provided by Universiti Putra Malaysia (Malaysia), Universiti Malaysia Sabah (Malaysia), Universiti Malaysia Kelantan (Malaysia), Management and Science University (Malaysia), and Universiti Islam Sultan Sharif Ali (Brunei Darussalam).
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. Managing Living Soils; Global Soil Partnership International Technical Workshop: Rome, Italy, 2012; pp. 20–25. Available online: http://www.fao.org/documents/card/en/c/d018fe5b-59af-454e-8a27-8c75b671ba37/ (accessed on 15 October 2021).
- Deb, U.K.; Bantilan, M.C.S.; Hash, C.T.; Ndjeunga, J. Adoption of improved sorghum cultivars. In Sorghum Genetic Enhancement: Research Process, Dissemination and Impacts; International Crops Research Institute for the Semi-Arid Tropics: Patancheru, India, 2004; pp. 181–198. [Google Scholar]
- Hall, A.J.; Bandyopadhyay, R.; Chandrashekar, A.; Shewry, P.R. Technical and institutional options for sorghum grain mold management and the potential for impact on the poor: Overview and recommendations. In Technical and Institutional Options for Sorghum Grain Mold Management: Proceeding of an International Consultation; International Crops Research Institute for the Semi-Arid Tropic: Patancheru, India, 2000; pp. 18–19. [Google Scholar]
- Taylor, J.R.; Schober, T.J.; Bean, S.R. Novel food and non-food uses for sorghum and millets. J. Cereal Sci. 2006, 44, 252–271. [Google Scholar] [CrossRef]
- Rabileh, M.A.; Shamshuddin, J.; Panhwar, Q.A.; Rosenani, A.B.; Anuar, A.R. Effects of biochar and/or dolomitic limestone application on the properties of Ultisol cropped to maize under glasshouse conditions. Can. J. Soil Sci. 2015, 95, 37–47. [Google Scholar] [CrossRef]
- Arshad, M.A.; Soon, Y.K.; Azooz, R.H.; Lupwayi, N.Z.; Chang, S.X. Soil and crop response to wood ash and lime application in acidic soils. Agron. J. 2012, 104, 715–721. [Google Scholar] [CrossRef]
- Kunito, T.; Isomura, I.; Sumi, H.; Park, H.D.; Toda, H.; Otsuka, S.; Nagaoka, K.; Saeki, K.; Senoo, K. Aluminum and acidity suppress microbial activity and biomass in acidic forest soils. Soil Biol. Biochem. 2016, 97, 23–30. [Google Scholar] [CrossRef]
- Tiessen, H.; Cuevas, E.; Chacon, P. The Role of Soil Organic Matter in Sustaining Soil Fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors Controlling Humification and Mineralization of Soil Organic Matter in the Tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, V.C. Ameliorating soil acidity of tropical Oxisols by liming for sustainable crop production. Adv. Agron. 2008, 99, 345–399. [Google Scholar]
- Hussain, B.; Ashraf, M.N.; Abbas, A.; Li, J.; Farooq, M. Cadmium stress in paddy fields: Effects of soil conditions and remediation strategies. Sci. Total Environ. 2021, 754, 142188. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Malhi, S.S. Interactions of nitrogen with other nutrients and water: Effect on crop yield and quality, nutrient use efficiency, carbon sequestration, and environmental pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar]
- Li, Z.; Xu, B.; Du, T.; Ma, Y.; Tian, X.; Wang, F.; Wang, W. Excessive nitrogen fertilization favors the colonization, survival, and development of Sogatella furcifera via bottom-up effects. Plants 2021, 10, 875. [Google Scholar] [CrossRef]
- Nabeya, K.; Nakamura, S.; Nakamura, T.; Fujii, A.; Watanabe, M.; Nakajima, T.; Nitta, Y.; Goto, Y. Growth behavior of sago palm (Metroxylon sagu Rottb.) from transplantation to trunk formation. Plant Prod. Sci. 2015, 18, 209–217. [Google Scholar] [CrossRef]
- Wahi, R.; Abdullah, L.C.; Mobarekeh, M.N.; Ngaini, Z.; Yaw, T.C.S. Utilization of esterified sago bark fibre waste for removal of oil from palm oil mill effluent. J. Environ. Chem. Eng. 2017, 5, 170–177. [Google Scholar] [CrossRef]
- Perkiömäki, J. Wood Ash Use in Coniferous Forests: A Soil Microbiological Study into the Potential Risk of Cadmium Release; Finnish Forest Research Institute: Vantaa, Finland, 2004. [Google Scholar]
- Wang, Q.; Liu, C.; Huang, D.; Dong, Q.; Li, P.; Ma, F. High-efficient utilization and uptake of N contribute to higher NUE of ‘Qinguan’apple under drought and N-deficient conditions compared with ‘Honeycrisp’. Tree Physiol. 2019, 39, 1880–1895. [Google Scholar] [CrossRef] [PubMed]
- Paramisparam, P.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y.; Johan, P.D.; Hamidi, N.H. Co-application of charcoal and wood ash to improve potassium availability in tropical mineral acid soils. Agronomy 2021, 11, 2081. [Google Scholar] [CrossRef]
- Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y. Combined use of charcoal, sago bark ash, and urea mitigate soil acidity and aluminium toxicity. Agronomy 2021, 11, 1799. [Google Scholar] [CrossRef]
- Pitman, R.M. Wood Ash Use in Forestry—A Review of the Environmental Impacts. For. An Int. J. For. Res. 2006, 79, 563–588. [Google Scholar] [CrossRef]
- Paramananthan, S. Soils of Malaysia: Their Characteristics and Identification; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2000; Volume 1. [Google Scholar]
- Soil Survey Staff. Soil Survey Field and Laboratory Methods Manual; Soil survey investigations report no. 51, Version 2.0; Burt, R., Staff, S.S., Eds.; U.S. Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014.
- Malaysian Agricultural Research and Development Institute (MARDI). Jagung Manis Baru, Masmadu [New Sweet Corn, Masmadu]; Malaysian Agricultural Research and Development: Serdang, Malaysia, 1993; pp. 3–5.
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The effect of biochars on maize (Zea mays) germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Ndor, E.; Dauda, S.N.; Azagaku, E.D. Response of maize varieties (Zea mays) to biochar amended soil in Lafia, Nigeria. J. Exp. Agric. Int. 2015, 5, 525–531. [Google Scholar] [CrossRef]
- Mandre, M.; Pärn, H.; Ots, K. Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For. Ecol. Manag. 2006, 223, 349–357. [Google Scholar] [CrossRef]
- Ozolinčius, R.; Buožytė, R.; Varnagirytė-Kabašinskienė, I. Wood ash and nitrogen influence on ground vegetation cover and chemical composition. Biomass Bioenergy 2007, 31, 710–716. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Ch'ng, H.; Ahmed, O.; Majid, N. Improving phosphorus availability, nutrient uptake and dry matter production of zea mays l. on a tropical acid soil using poultry manure biochar and pineapple leaves compost. Exp. Agric. 2016, 52, 447–465. [Google Scholar] [CrossRef]
- Bremner, J.M. Total nitrogen. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 1149–1178. [Google Scholar]
- Peech, M. Hydrogen-ion activity. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 914–926. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Applications; Longman Scientific & Technical: Essex, UK, 1994. [Google Scholar]
- Chefetz, B.; Hatcher, P.G.; Hadar, Y.; Chen, Y. Chemical and biological characterization of organic matter during composting of municipal solid waste. Am. Soc. Agron. Crop. Sci. Soc. Am. Soil Sci. Soc. Am. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Cotennie, A. Soil and plant testing as a basis of fertilizer recommendation. In FAO Soil Bulletin; FAO: Rome, Italy, 1980; Volume 38, p. 118. [Google Scholar]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effects of the application of charred bark of Acacia mangium on the yield of maize, cowpea and peanut, and soil chemical properties in South Sumatra, Indonesia. Soil Sci. Plant Nutr. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Keeney, D.R.; Nelson, D.W. Nitrogen—inorganic forms. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1983, 9, 643–698. [Google Scholar]
- Dobermann, A.R. Nitrogen use efficiency-state of the art. Agron. Fac. Publ. 2005, 316. Available online: https://digitalcommons.unl.edu/agronomyfacpub/316 (accessed on 1 September 2022).
- Nguyen, T.T.N.; Wallace, H.M.; Xu, C.Y.; Xu, Z.; Farrar, M.B.; Joseph, S.; Van Zwieten, L.; Bai, S.H. Short-term effects of organo-mineral biochar and organic fertilisers on nitrogen cycling, plant photosynthesis, and nitrogen use efficiency. J. Soils Sediments 2017, 17, 2763–2774. [Google Scholar] [CrossRef]
- Hasbullah, N.A. Use of Clinoptilolite Zeolite to Improve Efficiency of Phosphorus Use in Acid Soils. Ph.D. Thesis, University Putra Malaysia, Serdang, Malaysia, 2016. [Google Scholar]
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Li, S.; Li, D.; Li, J.; Li, G.; Zhang, B. Evaluation of Humic Substances during Co-Composting of Sewage Sludge and Corn Stalk under Different Aeration Rates. Bioresour. Technol. 2017, 245, 1299–1302. [Google Scholar] [CrossRef]
- Mandal, V.K.; Sharma, N.; Raghuram, N. Molecular targets for improvement of crop nitrogen use efficiency: Current and emerging options. In Engineering Nitrogen Utilization in Crop. Plants; Springer: Cham, Switzerland, 2018; pp. 77–93. [Google Scholar]
- Ismaili, K.; Ismaili, M.; Ibijbijen, J. The use of 13C and 15N based isotopic techniques for assessing soil C and N changes under conservation agriculture. Eur. J. Agron. 2015, 64, 1–7. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Aflakpui, G. Spatial patterns of water and nitrogen response within corn production fields. Agric. Sci. 2012, 73–96. [Google Scholar] [CrossRef][Green Version]
- Mullen, R.W.; Freeman, K.W.; Raun, W.R.; Johnson, G.V.; Stone, M.L.; Solie, J.B. Identifying an in-season response index and the potential to increase wheat yield with nitrogen. Agron. J. 2003, 95, 347–351. [Google Scholar] [CrossRef]
- Xiong, Q.; Hu, J.; Wei, H.; Zhang, H.; Zhu, J. Relationship between Plant Roots, Rhizosphere Microorganisms, and Nitrogen and Its Special Focus on Rice. Agriculture 2021, 11, 234. [Google Scholar] [CrossRef]
- Waters, B.M.; Sankaran, R.P. Moving micronutrients from the soil to the seeds: Genes and physiological processes from a biofortification perspective. Plant Sci. 2011, 180, 562–574. [Google Scholar] [CrossRef]
- Saarsalmi, A.; Smolander, A.; Kukkola, M.; Arola, M. Effect of wood ash and nitrogen fertilization on soil chemical properties, soil microbial processes, and stand growth in two coniferous stands in Finland. Plant Soil 2010, 331, 329–340. [Google Scholar] [CrossRef]
- Clough, T.J.; Condron, L.M. Biochar and the nitrogen cycle: Introduction. J. Environ. Qual. 2010, 39, 1218–1223. [Google Scholar] [CrossRef]
- Steiner, C.; Glaser, B.; Geraldes Teixeira, W.; Lehmann, J.; Blum, W.E.; Zech, W. Nitrogen retention and plant uptake on a highly weathered central Amazonian Ferralsol amended with compost and charcoal. J. Plant Nutr. Soil Sci. 2008, 171, 893–899. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal volatile matter content influences plant growth and soil nitrogen transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Xia, H.; Riaz, M.; Zhang, M.; Liu, B.; El-Desouki, Z.; Jiang, C. Biochar increases nitrogen use efficiency of maize by relieving aluminum toxicity and improving soil quality in acidic soil. Ecotoxicol. Environ. Saf. 2020, 196, 110531. [Google Scholar] [CrossRef] [PubMed]
- Perkiömäki, J.; Levula, T.; Fritze, H. A reciprocal decomposition experiment of Scots pine needles 19 yr after wood ash fertilization. Soil Biol. Biochem. 2004, 36, 731–734. [Google Scholar] [CrossRef]
- Nigussie, A.; Kissi, E.; Misganaw, M.; Ambaw, G. Effect of biochar application on soil properties and nutrient uptake of lettuces (Lactuca sativa) grown in chromium polluted soils. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 369–376. [Google Scholar]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon 2017, 113, 219–230. [Google Scholar] [CrossRef]
- Jones, D.L.; Quilliam, R.S. Metal contaminated biochar and wood ash negatively affect plant growth and soil quality after land application. J. Hazard. Mater. 2014, 276, 362–370. [Google Scholar] [CrossRef]
- Lucchini, P.; Quilliam, R.S.; DeLuca, T.H.; Vamerali, T.; Jones, D.L. Increased bioavailability of metals in two contrasting agricultural soils treated with waste wood-derived biochar and ash. Environ. Sci. Pollut. Res. 2014, 21, 3230–3240. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.; Neves, E.G.; et al. Black carbon affects the cycling of non-black carbon in soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.-C.; Zackrisson, O. Fire-Derived Charcoal Causes Loss of Forest Humus. Science 2008, 320, 629. [Google Scholar] [CrossRef]
- Martinsen, V.; Alling, V.; Nurida, N.L.; Mulder, J.; Hale, S.E.; Ritz, C.; Rutherford, D.W.; Heikens, A.; Breedveld, G.D.; Cornelissen, G. pH effects of the addition of three biochars to acidic Indonesian mineral soils. Soil Sci. Plant Nutr. 2015, 61, 821–834. [Google Scholar] [CrossRef]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating physical and chemical properties of highly weathered soils in the tropics with charcoal—A review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Yuan, J.H.; Xu, R.K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).