Abstract
Plasma-activated water (PAW) is a novel and promising technique in the agricultural field that has the potential to improve vegetable growth and yield. The objective of this study was to determine the effect of plasma-activated water seeds treatment and growth conditions on pepper plant growth parameters and fruit quality. A factorial design of three factors (C = cultivar, GC = growth condition, and PAW = plasma activated water treatment seeds) was established, with two variants for each one: Cultivar 1 (C1) and Cultivar 2 (C2); greenhouse (G) and open field (F); PAW seeds treatment (PAW) and seeds without treatment with PAW (C). Four replicates with fifty seeds were taken for each variety. Growth and fruit quality parameters were measured in the three month period during 2021 and 2022, respectively. The significant influence of cultivar, growing condition, and PAW on fruit quality and pepper plant growth parameters were determined. The lowest values of measured parameters were obtained in the open field without PAW treatment. Pepper growth in a greenhouse from PAW-treated seeds had a higher canopy height (17.85%), weight (10.57%), number of leaves (10.5%), nodes (18.94%), and buds (37.83%). Moreover, dry matter content was higher (33.73%) as well as fruit quality: fruit weight (50.19%), diameter (24.3%), length (20.88%), and pericarp weight (49.49%). Results indicate that PAW treatment of peppers seeds can lead to production and yield improvement under different climates and growing conditions.
1. Introduction
Pepper (Capsicum annuum L.) has economic importance and is a widely produced agricultural crop with nutritionally valuable fruits. The impact of the quality of fruits is not only a matter of local but also the global economy, so the response to environmental changes is crucial for that production [1]. Production of pepper is increasing annually due to the possibility of production in most areas worldwide. Spice and sweet peppers are economically relevant and worldwide cultivated species, and the area used for production is over 1.5 million hectares [2]. Sweet pepper is a very important part of cultivation in protected areas [3]. Favorable microclimate for growth and continuous all-year production increase the overall yield in the greenhouse [4]. Ventilated greenhouse as a place for sweet pepper production can be profitable due to the longer crop season achieved with controlled growing conditions in comparison with open field production [5]. Influence of the greenhouse microenvironment results in increased fruit quality, higher crop growth, and yield [6]. Evaluation of all environmental conditions, such as temperature [7], humidity, and plant response on it [8,9], is very important in sweet pepper cultivation. Low temperature is one of the most important abiotic factors that restrict the optimal production of warm-season vegetables [10]. Additionally, climate changes in recent years have increased the number and duration of extremely high or low temperatures. Challenges for pepper producers in Croatia during the spring period are low temperatures and high humidity. Therefore, there is a need for an alternative model of safe and efficient cultivation [11]. Although greenhouse cultivation makes plants stronger to stress [12,13], the growth of sweet pepper in the greenhouse and open field is under the influence of biotic and abiotic factors [14] because global warming increases abiotic stress and impair plant growth, yield, and product quality [15]. Developing sustainable methods such as non-thermal plasma (NTP) to alleviate plant stress since plasma-generated reactive species trigger the activity of stress-responsive genes in plants [16] is necessary. Besides the fact that non-thermal or cold plasma improves resistance to abiotic stress, NTP technology is becoming increasingly popular in agriculture, in particular for seed treatment, since it improves seeds germination, growth and development of plants, and yield [17,18,19,20,21]. Plasma-activated water (PAW) or plasma-treated water (PTW) influences plant growth and development. The pathway for that is the transformation of reactive nitrogen and oxygen into ROS and NS (reactive oxygen and nitrogen species), including H2O2 and NO biomolecules [22,23,24,25,26]. Signaling molecules such as liquid ROS and NS (H2O2, NO2−, NO3−) could be nutrients for a plant. In contact with plasma and water, these species dissolve and form a plasma-activated water which is a mixture that can be, among others, a clean and sustainable alternative to nowadays widely used chemical fertilizers. Reactive species increase plant growth, but their ratio has to be optimized. Several properties were improved by using PAW, but the results are limited. Therefore, the use of PAW in agriculture demands optimization of PAW content [27].
Several authors reported the positive influence of nonthermal plasma [28,29,30,31,32,33] and plasma-activated water (PAW) [34] on the growth of pepper. However, despite studies carried out in the field of pre-sawing plasma treated seeds, reports about the effects of plasma-activated water on the morphology and phenology of resulting plants, yield, and quality of fruits are absent or very limited [35].
In this study, we set four treatments combined with two different cultivars of pepper (Bibic and Bernita) and two different growth conditions (open field and greenhouse). The combined treatments of cultivar and growing conditions were carried out during twol vegetation seasons. Additionally, we investigated the effect of PAW seed treatments on the growth and fruit quality of pepper. The objective of this study was to determine the effect of plasma-activated water seeds treatment and growth conditions on pepper plant growth parameters and fruit quality.
2. Material and Methods
2.1. Preparing of Plasma Activated Water (PAW)
Purified water (commercially purified water of Pharmaceutical degree (Pharmacopoeia Europea, Ph. Eur. 9)) was used for making active species with a conductivity of 0.98 μS cm−1 and pH value of 6.5. The PAW was created by establishing contact of the liquid surface with the plasma plume of a kHz plasma jet. The outer and inner diameters of the quartz tube of the atmospheric pressure plasma jet were 1.5 and 1 mm, and a copper wire of 100 μm diameter served as an electrode inserted in the capillary. The sinusoidal voltage waveform of 28 kHz with 12 kV maximum voltage powered the electrode. This was performed on the grounds of preliminary optimization of the used appliance, which delivers about 15 W of power. The generated discharge was conducted from N2 (99.996% purity) with a flow rate in a capillary of 500 sccm. At the distance of 5 mm from the liquid surface, the Berzelius with 215 mL sample was placed in front of the plasma jet, which resulted in H2O2 and NO2− in the PAL. Overall treatment time was 40. Analyses of samples were conducted after treatment and a few times while storage. The QUANTOFIX test strips (nitrate/nitrite 500, 10–500 mg l−1 NO3−, 1–80 mg l−1 NO2−, nitrate/nitrite 100, 5–100 mg l−1 NO3−, 0.5–50 mg l−1 NO2−, Peroxide H2O2 25, 0.5–25 mg l−1, Peroxide H2O2 100, 0.5–25 mg l−1) were used for those analyses. The results on strips had the accurate and quantitative evaluation by QUANTOFIX Relax unit optical reader (by Macherey-Nagel, GmbHha). The calibration of nitrate/nitrite strips was conducted by NaNO3 and NH4NO3 solutions with determined concentrations. UV-VIS absorption spectroscopy was used for the verification of calibration. Monitoring the aging of samples by strips in 1 min time resolution allows determination without significant consumption of samples, and the measuring error has been less than 10%. The pH value is crucial for the aging dynamics of PAW, and it is usually achieved by using metal ion concentration, as demonstrated by Kutasi et al. [36]. Therefore, the Mg ions were used in the form of a 5 g piece of solid Mg during the plasma treatment and kept 1 h inserted in liquid. The PAW conductivity was also measured after the process and while storage with the use of a conductivity probe (Metrohm 914pH/DO/Conductometer) Pt 1000/B/O. Optical emission spectroscopy (OES) was used for monitoring each treatment. The presence of NO, and N2 was determined during the treatment and monitored in 10 min intervals, as well as peroxide, nitrate/nitrite concentrations, and pH to keep consistency. The volume of 215 mL was used during three PAW treatments, first mixed in 2000 mL bottles, and separated in 200 mL containers for the purpose of the experiment. The 15 mL was kept for aging tests within 2 weeks (Table 1). Plasma-activated water was prepared at the Institute of Physics Zagreb according to Kutasi et al. (2019) [36], Gierczik et al. (2020) [37], and Kutasi et al. (2021) [38].

Table 1.
Plasma-activated water parameters.
2.2. Experimental Site
Experiments were conducted in the climate chamber, greenhouse, and open field of the Biotechnical Department, University of Slavonski Brod, between March and July of 2021 and 2022. The greenhouse and open field are geographically located at 45°09′57″ N and 17°57′08″ E and have an altitude of 87 m. The climate of Brod-Posavina County is moderately warm and humid, with warm summers (Cfb according to Köppen) and moderately cold winters. The location is characterized by winter temperatures that can fall below 0 °C and summer temperatures that can rise above 30 °C. Greenhouse indoor temperatures and relative humidity values in 2021 and 2022 were measured daily with H560 DewPoint Pro placed 1 m above the ground in the middle of the greenhouse. Outdoor temperature and relative humidity during the experimental period were recorded by a meteorological station situated nearby the experimental field. In this experiment, a naturally ventilated passive solar greenhouse was used, and to allow air circulation on the hottest days, the greenhouse was ventilated passively through flap roof windows and lateral side panels. The greenhouse dimensions were 30 m x 8 m with a height of 8 m, and the structure consisted of polycarbonate walls and a trilaminate low-density polyethylene (LDPE) film roof (200 μm thickness) with approximately 60% photosynthetically active radiation (PAR) transmittance. It had no heating or artificial light. Average monthly (April–July 2021 and 2022, respectively) indoor greenhouse temperature (°C) and relative humidity (%) values and open field temperature (°C) and relative humidity (%) are shown in Figure 1.
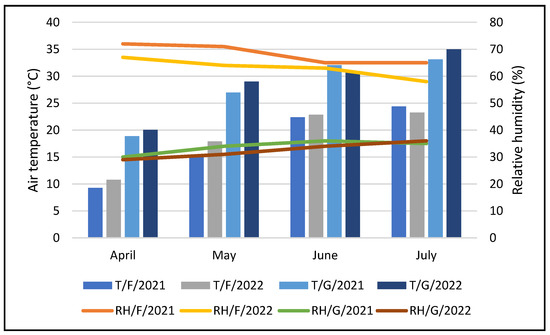
Figure 1.
Air temperature (T) and relative humidity (RH) measured in field (F) and greenhouse (G) conditions in the vegetative period (April-July) 2021 and 2022.
Four replicates (50 seeds each) for a single treatment were used. Samples of 50 seeds were placed in Petri dishes. Seed samples were divided into two groups: seeds poured with PAW and control (seeds poured with water without plasma treatment). The samples were immersed (soaked) with 20 mL of PAW and controlled water in 24 h intervals.
After imbibition, seeds were sowed in PVC containers (Pöpellmann TEKU (BP 3153/60) 53 cm × 31 cm × 5.6 cm) with 60 sowing places (volume 76 mL), filled with Potgrond P Klasmann Deilman substrate (Rp No 002). Trays with sowed seeds have been placed in controlled conditions within the growth chamber (Memmert ICH260L) with a 16/8 h photoperiod, a temperature 22/18 °C (day/night), and relative humidity of 60%.
After four weeks (19 April 2021 and 19 April 2022), seedlings were transplanted in pots (Pöpellmann TEKU VTG 9) 9 × 6.8 cm (0.27 L) in a greenhouse using ProLine Herb substrate (Rp No 693) suited for ecological production of pepper in small pots with added organic fertilizer Stallatico Pellettato (1800–2300 kg/ha). Six-weeks-old pepper transplants were planted on 10 May 2021 and 10 May 2022 in pots (Pöpellmann TEKU VCG 19) 19 × 14.9 cm (3 L) fulfilled with ProLine Herb Substrate 70% TerrAktiv®/coir + 30% GreenFibre® with added fertilizer. After the transplantation in pots, the plants were divided into two groups, one for a greenhouse and the other for open field cultivation (same area as the greenhouse). Irrigation was performed after sowing, when necessary, in accordance with precipitation in an open field experiment.
2.3. Experimental Design
Sweet pepper seeds: F1 seeds (Bibic (Cultivar 1) and Bernita (Cultivar 2)), produced in 2020 and 2021, respectively, were used to investigate the effect of PAW seed treatment on plant growth in the greenhouse and open fields. A factorial design of three factors (C = cultivar, GC = growth condition, and PAW = plasma-activated water treatment seeds) was established, with two variants for each one: Cultivar Bibic (C1) and Cultivar Bernita (C2); greenhouse (G) and open field (F); PAW seeds treatment (PAW) and seeds without treatment with PAW (C). Four replicates with fifty seeds were taken for each variety.
2.4. Measurements
In both years, harvests were practiced in five terms: 10 May 2021, 17 May 2021, 24 May 2021, 7 June 2021, 21 June 2021, and 10 May 2022, 17 May 2022, 24 May 2022, 7 June 2022, 21 June 2022. Plants in each replication were used to assess canopy height (cm), canopy weight (g), number of leaves, number of nodes, and number of buds. Four plants were randomly selected for each replication. Samples were transferred to the Agroecological laboratory, Biotechnical department, University of Slavonski Brod, Croatia, and stored in a cooler until used. All analyses were performed within 24 h. Mature fruits were harvested on 7 July 2021 and 7 July 2022, and then fruit weight (g), fruit diameter (cm), fruit length (cm), pericarp weight (g), and residual weight (g) (seeds, placenta, and calix) of fruits were determined. Ten mature fruits were randomly selected for each replication, and samples were transferred to the Agroecological laboratory, Biotechnical department, University of Slavonski Brod, Croatia, and stored in a cooler until used within 24 h.
Plant samples were oven dried at 70 °C for 48 h to the constant dry weight for the plant, which was weighed on an electronic scale with a precision of 0.01 g (PL3002, Mettler–Toledo International Inc., Greifensee, Switzerland) and sliding scale DIGI-MET 1226932-D (Helios Preisser, Gammertingen, Germany).
2.5. Statistical Analysis
According to the previous statements, the experiment was conducted as a three-factorial trial: two varieties of pepper (C1 and C2), treated seeds (PAW and control), and growth conditions (greenhouse and open field) in randomized blocks design with four replications. Data were analyzed using RStudio, and statistically significant differences were determined by the program MS Excell, 2019.
According to the Fisher test on the significance of variance analysis, the least significant differences (LSD) for p < 0.05 were calculated by comparing the mean values. Duncan’s test was used to determine which values have statistically significant differences precisely.
3. Results
The evaluation of measured parameters is shown in Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13. It is obvious that differences between the cultivars, growing conditions, and PAW treatment were confirmed. However, even though differences in the number of measurement parameters were evident, there was no statistical difference between parameters in each sampling date.

Table 2.
Canopy length of pepper cultivars developed under different growth conditions and seeds treatment in vegetative season 2021.

Table 3.
Canopy length of pepper cultivars development in 2022 under different growth conditions and seeds treatment in vegetative season 2022.

Table 4.
The canopy weight of pepper cultivars developed in 2021 under different growing conditions and seeds treatment in vegetative season 2021.

Table 5.
The canopy weight of pepper cultivars developed under different growing conditions and seeds treatment in vegetative season 2022.

Table 6.
The number of leaves pepper cultivars developed under different growing conditions and seed treatment in vegetative season 2021.

Table 7.
The number of leaves pepper cultivars developed under different growing conditions and seed treatment in vegetative season 2022.

Table 8.
The number of nodes pepper cultivars developed under different growing conditions and seed treatment in vegetative season 2021.

Table 9.
The number of nodes pepper cultivars developed under different growing conditions and seed treatment in vegetative season 2022.

Table 10.
The number of buds of pepper cultivars developed under different growing conditions and seed treatment in vegetative season 2021.

Table 11.
The number of buds of pepper cultivars developed under different growing conditions and seed treatment in vegetative season 2022.

Table 12.
Fruit components of pepper cultivars developed under different growing conditions and seed treatment in the vegetative season 2021.

Table 13.
Fruit components of pepper cultivars developed under different growing conditions and seed treatment in the vegetative season of 2022.
Although canopy length was higher in cultivar C1 in both years of research (Table 2 and Table 3), a statistically significant influence of cultivar was established in 2022 (Table 3). Determining the difference between cultivars regarding length of the canopy was 2.25% and 4.05% in 2021 and 2022, respectively (Table 2 and Table 3).
Also, in both years included in this research, higher canopy length in plants was determined among seeds treated with PAW. The significance of the impact of the PAW seed treatment was determined in 2021 (Table 2). Canopy length was higher than in control at 11.81% and 6.35% in 2021 and 2022, respectively (Table 2 and Table 3). In this study, the impact of growing conditions on canopy length was obvious. The influence of growing conditions was statistically significant in both research years (Table 2 and Table 3). Pepper grown in the greenhouse had a higher canopy length, 13.04%, and 6.7%, respectively. Likewise, in both years of research, interactions between cultivars and growing conditions were determined (p < 0.05) (Table 2 and Table 3).
The influence of the cultivar on the canopy weight was significant in the initial periods of measurement in both years of the study (Table 4 and Table 5). However, the impact of cultivar is not consistent. Higher canopy weight was determined in the C1 pepper cultivar in 2021 (6.61%), while in 2022, cultivar C2 had a higher canopy weight (5.61%) (Table 4 and Table 5). Plants of pepper developed from seeds treated with PAW had a higher canopy weight in both years (Table 4 and Table 5). Determined differences were 9.06% and 9.01% in 2021 and 2022, respectively. Although statistically significant influence was not determined in all sampling dates, in both years of research, a significant influence of growing conditions (greenhouse) was established (Table 4 and Table 5). Pepper plant growth in the greenhouse developed a 2.49% higher canopy weight than in the field in 2021 (Table 4). In 2022, a statistically significant interaction between cultivar, growing conditions, and PAW treatment on canopy weight was determined (p < 0.05) (Table 5).
Cultivar C1 in 2021 and 2022 developed a greater number of leaves, 3.7%, and 2.01%, respectively (Table 6 and Table 7). Moreover, seed treatment with PAW resulted in a higher number of leaves in 2022 (3.13%) (Table 7). The significant influence of PAW seed treatment on the number of leaves was determined in 2021 (Table 6). In that year, the number of leaves developed from seeds treated with PAW were 16.85% higher than the control (Table 6). In both years of the research, a significant influence of growing conditions on the number of leaves was determined (Table 6 and Table 7). In 2021 the higher number of leaves (4.24%) in the greenhouse than in the field was determined (Table 6). The interaction between the cultivar and growing conditions was determined in both years of the study (Table 6 and Table 7), and cultivar and PAW treatment, as well as growth condition and PAW treatment in 2021 (p < 0.05) (Table 6).
Cultivar C1 developed a 14.04% higher number of nodes in 2021, and C2 cultivar developed an 8.65% higher number of nodes in 2022 (Table 8 and Table 9). Pepper plants with preliminary PAW seed treatments developed a higher number of nodes, 64.29%, and 10.92%, in 2021 and 2022, respectively (Table 8 and Table 9). In the present study, the significant influence of growing conditions on the number of nodes was proven. The plants grown in the greenhouse developed a higher number of nodes, 10.94%, and 6.6%, respectively (Table 8 and Table 9). Regarding the number of nodes, the interaction between growing conditions and cultivar has been proven in 2022 (p < 0.05) (Table 9).
Cultivar C1 developed a higher number of buds (1.54%) in 2021 (Table 10), and cultivar C2 had a higher number of buds (2.92%) in 2022 (Table 11). Statistically significant impact of cultivar and preliminary PAW seed treatment was proven in both years (Table 10 and Table 11). A higher number of buds (35.54%) were developed in pepper plants from PAW seeds in 2021, and the number of buds was 10.75% higher in plants developed in 2022 (Table 10 and Table 11). The variability in the number of buds among growth conditions with different temperatures and relative humidity was statistically significant (Table 10 and Table 11). The plants grown in the greenhouse had a higher number of buds (32.13%) in 2021 and 8.44% in 2022 (Table 10 and Table 11). There was an interaction between growing conditions and cultivar in 2022 (p < 0.05) (Table 11) and growing conditions and seed treated with PAW (Table 10).
Fruit quality components were in the present study under the significant influence of cultivars in 2021 (Table 12). In the first year of research, in cultivar C1, the following higher values have been proven: fruit weight (8.84%), pericarp weight (3.27%), and residual weight (20.28%) (Table 12). However, fruit diameter and fruit length were higher in cultivar C2 (2.86% and 1.55%, respectively) (Table 12). In the second year of research, all measured parameters were higher in cultivar C2: fruit weight (13.62%), fruit diameter (6%), fruit length (9.43%), pericarp weight (12.72%), and residuals weight (17%) (Table 13). The cause for this could be stress caused by low temperatures that occasionally occurred during the cultivation period.
In both years of the research, a significant difference has been found in the fruit quality between the growing conditions (Table 12 and Table 13). Better fruit quality was found for plants grown in the greenhouse in 2021 and 2022: fruit weight (34.09% and 38.84%), fruit diameter (21.78% and 16.19%), fruit length (13.52% and 21.51%), pericarp weight (38.11% and 41.1%) and residuals weight (24.19% and 29.63%). The influence of PAW seed treatment on fruit quality was statistically significant in 2021 (Table 12). Preliminary seeds PAW treatment resulted in higher: fruit weight (17% and 23.43%), fruit diameter (4.79% and 7.6%), fruit length (6.46% and 9.57%), pericarp weight (5.68% and 24.88%) and residuals weight (38.41% and 20.77%) (Table 12 and Table 13). Moreover, in 2021 an interaction of all treatments on pericarp and residuals weight has been found (Table 12). Likewise, the interaction between growing conditions, cultivar, and PAW seed treatment were found regarding fruit weight, diameter, and fruit length (Table 12).
Dry matter content in pepper plant organs was different between cultivars, growing conditions, and seed treatment with PAW, as well as between years (Figure 2). The content of dry matter C1 was 7.82 g, and C2 had a dry matter content was 6.90 g. Comparing the dry matter of plants grown in different growing conditions, it was found that the dry matter of plants grown in the greenhouse was higher (8.10 g) than for those grown in the open field (6.62 g). On average, plants grown from the seeds treated with PAW have a dry matter of 8.93 g, and control has 5.78 g. Moreover, the difference between years has been established: in 2022, dry matter content was 8.63 g, and in 2021 it was 7.36 g (Figure 2).
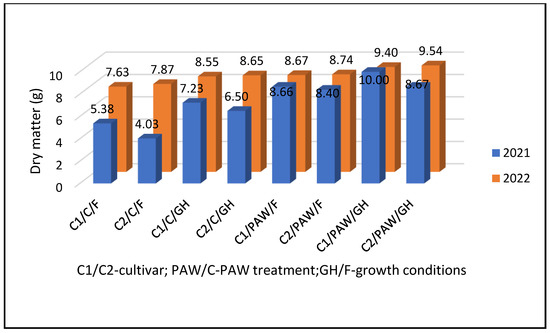
Figure 2.
Dry matter content in pepper plant organs developed under different growing conditions and seed treatment in vegetative seasons 2021 and 2022.
4. Discussion
The product quality of greenhouse vegetables [39], as well as yield and fruit weight [40], depend on plants’ genetic composition of species and cultivars (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13).
Temperature [7] and relative humidity [8] are the basic environmental factors that affect sweet pepper growth. The minimum temperature for pepper growth is about 8–12 °C [41], and the optimum temperature for pepper growth is 20–25 °C [10]. Although higher air temperature (32 °C) has improved plant height, fresh weight, dry weight, and enhanced development of leaves [7], pepper are sensitive to temperature extremes [10]. In the present study, growth conditions varied in temperature (Figure 1). A low temperature in the first hours of the day was measured in an open field (Figure 1), which could influence the low biomass and fruit quality of pepper (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13). Moreover, the spring period of 2021 had a long period of low temperatures below 0 °C, which could have an influence on low biomass of pepper plants and fruit quality. Low temperature is one of the most important abiotic factors that restrict the optimal production of warm-season vegetables [10], which can be a reason for lower values determined in the open field in comparison with production in the greenhouse in the present study (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13). The reduction in dry mass accumulation could be a result of the restriction of sucrose synthesis by low temperatures [7]. Low temperature coupled with high humidity (Figure 1) reduces the yield under open field conditions [5]. However, high night-time humidity could result in higher production of pepper biomass (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13) and fruit weight in the greenhouse [42]. Previous research [5,8,10,43] determined an increase in plant height, the number of leaves, dry weight of shoot and root, as well as fruit number and fruit size of pepper and flowering in the greenhouse in order to field conditions which are in accordance with results determined in the present study (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13) (Figure 2).
Several authors stated that PAW changes the metabolism of seeds which improves plant development [18,19,31,34]. However, the results of research on the influence of PAW on the development of pepper plants and fruits are limited. Most of the previous research investigated germination and seedlings characteristics of pepper [32]. According to provided research, nonthermal plasma improved stem length [33], shoot length, total fresh weight and leaf area, and biomass [28,30]. In the present study, higher canopy height, the number of buds, nodes, leaves, and canopy weight in plants with seeds treated with PAW were determined (Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11, Table 12 and Table 13). In the research of Nalwa et al. [29], pepper seeds exposed to plasma achieved maximum ripe fruit yield/plant. This is in accordance with the achievement in the first vegetative season in this research (Table 12). Additionally, in the present research, pepper was cultivated during two vegetative seasons. However, in the second vegetative season significant impact of PAW on fruit quality was not determined (Table 13). Moreover, in the research of Shapira et al. [35], the yield parameters of pepper (fruit yield and average mass of fruit) were not significantly different, comparing plasma treated to non-treated pepper.
The highest average of dry pepper weight under greenhouse conditions was proven [43]. Since low temperature could be the cause of the restriction of sucrose synthesis, dry weight rapidly increases under higher air temperatures [7,39]. In this research, the dry matter was increased in the greenhouse compared to the field, probably as a result of higher temperature [7] and lower humidity (Figure 2). As the authors reported, the plant’s dry weight was increased with the application of PAW [44]. In this study, the PAW treatment also increased dry matter weight in both growth conditions and vegetative seasons (Figure 1).
PAW-treated seeds developed pepper with higher growth parameters in the open field and greenhouse in both vegetative seasons. Pepper development in the field was exposed to low temperatures and high relative humidity, which caused stress to them. However, pepper produced by plasma treatments probably had a better adaptive response against stress which can be explained by enhanced total soluble sugar concentration [45]. The action of various stressors on plants usually causes the formation and accumulation of ROS at some point, which is vital for stress signaling [46]. Plasma-activated water contains ROS that acts as a mild stressor that prepares the plant for the occurrence of stronger stress [24]. This is probably the reason for the higher parameters of pepper developed from treated seeds in this research. Higher growth parameters of pepper developed in the greenhouse could be the consequence of nitrogen content in PAW. The NO3− is the main source of nitrogen for plant production of proteins and nucleic acids; therefore, it can be considered the main component of the PAW responsible for biomass increase. Another species (H2O2) can also take part in weight increase through the process of plant tissue lignification. Moreover, PAW had a positive effect on both the chlorophyll content and the photosynthetic rate. High levels of chlorophyll can be attributed to increased physiological activity and photosynthesis in plants and plant growth [47].
The response to PAW treatment depended on the plant cultivar, growing conditions as well as their interaction [17,20,21]. Higher growth parameters were determined in both vegetable seasons in PAW-developed plants. However, results concerning fruit quality were different in the vegetative season 2021 and 2022, which demand further investigation in specific conditions.
5. Conclusions
Contemporary agriculture requires new approaches in production related to the use of fertilizers and the application of substances that will increase the resistance of plants to the stress caused by climate change. From this point of view, the present study evaluated the performance of PAW, a new promising eco-friendly stimulant in terms of plant productivity. PAW seeds were compared to water-soaked seeds for four treatments, comprising two different cultivars of pepper and two different growth conditions. The results proved that PAW outperformed water-soaked seeds in terms of both growth conditions and cultivars. PAW treatment improved all growth parameters measured as well as fruit quality parameters compared with water-soaked treatment. In this research, PAW treatment influenced the values of measured parameters of pepper more than greenhouse conditions in regards to open fields. Therefore, it is concluded that PAW treatment is the adequate treatment for pepper seeds in terms of improving production and yield simultaneously.
PAW can be utilized when it is desired to ensure the yield of plants if they are exposed to extreme temperatures and relative humidity. Cultivation in the greenhouse increased the pepper yield compared to the open field, which would be a benefit for producers.
This study suggests that PAW is a useful technique for growing pepper plants in greenhouses and open fields; however, in future studies, more variants of PAW and cultivars should be tested to find out the best characteristics for different ranges of cultivars. It is also recommended that PAW studies should be conducted during more growing seasons in different climatic conditions for evaluation of their performance and effectiveness over time.
Author Contributions
Conceptualization, B.J.-P.; methodology, B.J.-P., K.M. and T.B.-L.; investigation, B.J.-P., R.B., N.R.F., T.B.-L., S.A. and M.J.; resources, B.J.-P.; data processing, B.J.-P. and R.B.; writing-original draft preparation, B.J.-P.; writing-review and editing, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project Adaptation of vegetables to new agrometeorological conditions in Slavonia (AVACS), KK.05.1.1.02.0004. The project was financed by the European Union from European Regional Development Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Authors express their sincere gratitude to Katica Šimunović from the University of Slavonski Brod for her advice in the statistical analysis of experimental data. Moreover, the authors express their sincere gratitude to Slobodan Milošević and Mario Rakić from the Institute of Physics for PAW preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Carmen Pinero, M.; Perez-Jimenez, M.; Lopez-Marin, J.; del Amor, F.M. Fruit Quality of Sweet Pepper as Affected by Foliar Ca Applications to Mitigate the Supply of Saline Water under a Climate Change Scenario. J. Sci. Food Agric. 2018, 98, 1071–1078. [Google Scholar] [CrossRef]
- Stagnari, F.; Campanelli, G.; Galieni, A.; Platani, C.; Bertone, A.; Ficcadenti, N. Adaptive Responses to Nitrogen and Light Supplies of a Local Varieties of Sweet Pepper from the Abruzzo Region, Southern Italy. Agronomy-Basel 2021, 11, 1343. [Google Scholar] [CrossRef]
- Mahmoud, A.M.A.; El-Eslamboly, A.A.S.A. Production and Evaluation of High Yielding Sweet Pepper Hybrids under Greenhouse Conditions. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 573–580. [Google Scholar]
- Hou, Y.; Li, A.; Li, Y.; Jin, D.; Tian, Y.; Zhang, D.; Wu, D.; Zhang, L.; Lei, W. Analysis of Microclimate Characteristics in Solar Greenhouses under Natural Ventilation. Build. Simul. 2021, 14, 1811–1821. [Google Scholar] [CrossRef]
- Singh, B.; Biwalkar, N.; Chhina, R.S. Response of Sweet Pepper (Capsicum annuum) under Varying Fertigation and Irrigation Applications Grown in Naturally Ventilated Greenhouse. J. Krishi Vigyan. 2020, 8, 236–241. [Google Scholar] [CrossRef]
- Ge, J.; Zhao, L.; Gong, X.; Lai, Z.; Traore, S.; Li, Y.; Long, H.; Zhang, L. Combined Effects of Ventilation and Irrigation on Temperature, Humidity, Tomato Yield, and Quality in the Greenhouse. Hortscience 2021, 56, 1080–1088. [Google Scholar] [CrossRef]
- Kwack, Y.; An, S.; Kim, S.K. Development of Growth Model for Grafted Hot Pepper Seedlings as Affected by Air Temperature and Light Intensity. Sustainability 2021, 13, 5895. [Google Scholar] [CrossRef]
- Chowdhury, M.; Kiraga, S.; Islam, M.N.; Ali, M.; Reza, M.N.; Lee, W.-H.; Chung, S.-O. Effects of Temperature, Relative Humidity, and Carbon Dioxide Concentration on Growth and Glucosinolate Content of Kale Grown in a Plant Factory. Foods 2021, 10, 1524. [Google Scholar] [CrossRef]
- der Ploeg, A.; Heuvelink, E. The Influence of Temperature on Growth and Development of Chrysanthemum Cultivars: A Review. J. Hortic. Sci. Biotechnol. 2006, 81, 174–182. [Google Scholar] [CrossRef]
- Angmo, P.; Phuntsog, N.; Namgail, D.; Chaurasia, O.P.; Stobdan, T. Effect of Shading and High Temperature Amplitude in Greenhouse on Growth, Photosynthesis, Yield and Phenolic Contents of Tomato (Lycopersicum esculentum Mill.). Physiol. Mol. Biol. Plants 2021, 27, 1539–1546. [Google Scholar] [CrossRef]
- Kang, M.H.; Jeon, S.S.; Shin, S.M.; Veerana, M.; Ji, S.-H.; Uhm, H.-S.; Choi, E.-H.; Shin, J.H.; Park, G. Dynamics of Nitric Oxide Level in Liquids Treated with Microwave Plasma-Generated Gas and Their Effects on Spinach Development. Sci. Rep. 2019, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Maher, A.; Kamel, E.; Enrico, F.; Atif, I.; Abdelkader, M. An Intelligent System for the Climate Control and Energy Savings in Agricultural Greenhouses. Energy Effic. 2016, 9, 1241–1255. [Google Scholar] [CrossRef]
- Thomaier, S.; Specht, K.; Henckel, D.; Dierich, A.; Siebert, R.; Freisinger, U.B.; Sawicka, M. Farming in and on Urban Buildings: Present Practice and Specific Novelties of Zero-Acreage Farming (ZFarming). Renew. Agric. Food Syst. 2015, 30, 43–54. [Google Scholar] [CrossRef]
- Mussa, A.; Shinichi, K. Effect of Planting Space and Shoot Pruning on the Occurrence of Thrips, Fruit Yield and Quality Traits of Sweet Pepper (Capsicum annum L.) under Greenhouse Conditions. J. Entomol. Zool. Stud. 2019, 7, 787–792. [Google Scholar]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of Combined Abiotic and Biotic Stresses on Plant Growth and Avenues for Crop Improvement by Exploiting Physio-Morphological Traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed]
- Susmita, C.; Kumar, S.P.J.; Chintagunta, A.D.; Lichtfouse, E.; Naik, B.; Ramya, P.; Kumari, K.; Kumar, S. Non-Thermal Plasmas for Disease Control and Abiotic Stress Management in Plants. Environ. Chem. Lett. 2022, 20, 2135–2164. [Google Scholar] [CrossRef]
- Staric, P.; Vogel-Mikus, K.; Mozetic, M.; Junkar, I. Effects of Nonthermal Plasma on Morphology, Genetics and Physiology of Seeds: A Review. Plants 2020, 9, 1736. [Google Scholar] [CrossRef]
- Puac, N.; Gherardi, M.; Shiratani, M. Plasma Agriculture: A Rapidly Emerging Field. Plasma Process. Polym. 2018, 15, 1700174. [Google Scholar] [CrossRef]
- Puac, N.; Skoro, N.; Spasic, K.; Zivkovic, S.; Milutinovic, M.; Malovic, G.; Petrovic, Z.L. Activity of Catalase Enzyme in Paulownia Tomentosa Seeds during the Process of Germination after Treatments with Low Pressure Plasma and Plasma Activated Water. Plasma Process. Polym. 2018, 15, 1700082. [Google Scholar] [CrossRef]
- Attri, P.; Ishikawa, K.; Okumura, T.; Koga, K.; Shiratani, M. Plasma Agriculture from Laboratory to Farm: A Review. Processes 2020, 8, 1002. [Google Scholar] [CrossRef]
- Ranieri, P.; Sponsel, N.; Kizer, J.; Rojas-Pierce, M.; Hernandez, R.; Gatiboni, L.; Grunden, A.; Stapelmann, K. Plasma Agriculture: Review from the Perspective of the Plant and Its Ecosystem. Plasma Process. Polym. 2021, 18, 2000162. [Google Scholar] [CrossRef]
- Kocira, S.; Perez-Piza, M.C.; Bohata, A.; Bartos, P.; Szparaga, A. Cold Plasma as a Potential Activator of Plant Biostimulants. Sustainability 2022, 14, 495. [Google Scholar] [CrossRef]
- Graves, D.B.; Bakken, L.B.; Jensen, M.B.; Ingels, R. Plasma Activated Organic Fertilizer. Plasma Chem. Plasma Process. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Adhikari, B.; Adhikari, M.; Ghimire, B.; Park, G.; Choi, E.H. Cold Atmospheric Plasma-Activated Water Irrigation Induces Defense Hormone and Gene Expression in Tomato Seedlings. Sci. Rep. 2019, 9, 16080. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The Potential of Cold Plasma for Safe and Sustainable Food Production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef]
- Ingels, R.; Graves, D.B. Improving the Efficiency of Organic Fertilizer and Nitrogen Use via Air Plasma and Distributed Renew-Able Energy. Plasma Med. 2015, 5, 257–270. [Google Scholar] [CrossRef]
- Kucerova, K.; Henselova, M.; Slovakova, L.; Bacovcinova, M.; Hensel, K. Effect of Plasma Activated Water, Hydrogen Peroxide, and Nitrates on Lettuce Growth and Its Physiological Parameters. Appl. Sci. 2021, 11, 1985. [Google Scholar] [CrossRef]
- Safari, N.; Iranbakhsh, A.; Oraghi Ardebili, Z. Non-Thermal Plasma Modified Growth and Differentiation Process of Capsicum annuum PP805 Godiva in in Vitro Conditions. Plasma Sci. Technol. 2017, 19, 055501. [Google Scholar] [CrossRef]
- Nalwa, C.; Thakur, A.K.; Vikram, A.; Rane, R.; Vaid, A. Effect of Cold Plasma Treatment and Priming in Bell Pepper (Capsicum annuum L.). Int. J. Bio-Resour. Stress Manag. 2017, 8, 535–538. [Google Scholar] [CrossRef]
- Iranbakhsh, A.; Ardebili, Z.O.; Ardebili, N.O.; Ghoranneviss, M.; Safari, N. Cold Plasma Relieved Toxicity Signs of Nano Zinc Oxide in Capsicum annuum Cayenne via Modifying Growth, Differentiation, and Physiology. Acta Physiol. Plant. 2018, 40, 154. [Google Scholar] [CrossRef]
- Stepanova, V.; Slavicek, P.; Kelar, J.; Prasil, J.; Smekal, M.; Stupavska, M.; Jurmanova, J.; Cernak, M. Atmospheric Pressure Plasma Treatment of Agricultural Seeds of Cucumber (Cucumis sativus L.) and Pepper (Capsicum annuum L.) with Effect on Reduction of Diseases and Germination Improvement. Plasma Process. Polym. 2018, 15, 1700076. [Google Scholar] [CrossRef]
- Thisawech, M.; Saritnum, O.; Sarapirom, S.; Prakrajang, K.; Phakham, W. Effects of Plasma Technique and Gamma Irradiation on Seed Germination and Seedling Growth of Chili Pepper. Chiang Mai J. Sci. 2020, 47, 73–82. [Google Scholar]
- Kasih, T.P.; Purwondho, R.; Danil, D.; Radjagukguk, R.; Bagaskara, A. Germination Enhancement of Green Bell Pepper (Capsicum annuum L.) by Using Non Thermal Argon Plasma. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 12131. [Google Scholar]
- Sivachandiran, L.; Khacef, A. Enhanced Seed Germination and Plant Growth by Atmospheric Pressure Cold Air Plasma: Combined Effect of Seed and Water Treatment. RSC Adv. 2017, 7, 1822–1832. [Google Scholar] [CrossRef]
- Shapira, Y.; Bormashenko, E.; Drori, E. Pre-Germination Plasma Treatment of Seeds Does Not Alter Cotyledon DNA Structure, nor Phenotype and Phenology of Tomato and Pepper Plants. Biochem. Biophys. Res. Commun. 2019, 519, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Kutasi, K.; Popovic, D.; Krstulovic, N.; Milosevic, S. Tuning the Composition of Plasma-Activated Water by a Surface-Wave Microwave Discharge and a KHz Plasma Jet. Plasma Sources Sci. Technol. 2019, 28, 095010. [Google Scholar] [CrossRef]
- Gierczik, K.; Vukusic, T.; Kovacs, L.; Szekely, A.; Szalai, G.; Milosevic, S.; Kocsy, G.; Kutasi, K.; Galiba, G. Plasma-Activated Water to Improve the Stress Tolerance of Barley. Plasma Process. Polym. 2020, 17, 1900123. [Google Scholar] [CrossRef]
- Kutasi, K.; Krstulovic, N.; Jurov, A.; Salamon, K.; Popovic, D.; Milosevic, S. Controlling: The Composition of Plasma-Activated Water by Cu Ions. Plasma Sources Sci. Technol. 2021, 30, 045015. [Google Scholar] [CrossRef]
- Gruda, N.; Savvas, D.; Colla, G.; Rouphael, Y. Impacts of Genetic Material and Current Technologies on Product Quality of Selected Greenhouse Vegetables—A Review. Eur. J. Hortic. Sci. 2018, 83, 319–328. [Google Scholar] [CrossRef]
- Gungor, F.; Yildirim, E. Effect of different growing media on quality, growth and yield of pepper (Capsicum annuum L.) under greenhouse conditions. Pakistan J. Bot. 2013, 45, 1605–1608. [Google Scholar]
- Ropokis, A.; Ntatsi, G.; Kittas, C.; Katsoulas, N.; Savvas, D. Effects of Temperature and Grafting on Yield, Nutrient Uptake, and Water Use Efficiency of a Hydroponic Sweet Pepper Crop. Agronomy-Basel 2019, 9, 110. [Google Scholar] [CrossRef]
- Bakker, J.C. The Effects of Air Humidity on Growth and Fruit Production of Sweet Pepper (Capsicum annuum L.). J. Hortic. Sci. 1989, 64, 41–46. [Google Scholar] [CrossRef]
- Khaitov, B.; Yun, H.J.; Lee, Y.; Ruziev, F.; Le, T.H.; Umurzokov, M.; Bo, A.B.; Cho, K.M.; Park, K.W. Impact of Organic Manure on Growth, Nutrient Content and Yield of Chilli Pepper under Various Temperature Environments. Int. J. Environ. Res. Public Health 2019, 16, 3031. [Google Scholar] [CrossRef] [PubMed]
- Rathore, V.; Tiwari, B.S.; Nema, S.K. Treatment of Pea Seeds with Plasma Activated Water to Enhance Germination, Plant Growth, and Plant Composition. Plasma Chem. Plasma Process. 2022, 42, 109–129. [Google Scholar] [CrossRef]
- Rashid, M.; Rashid, M.M.; Reza, M.A.; Talukder, M.R. Combined Effects of Air Plasma Seed Treatment and Foliar Application of Plasma Activated Water on Enhanced Paddy Plant Growth and Yield. Plasma Chem. Plasma Process. 2021, 41, 1081–1099. [Google Scholar] [CrossRef]
- Holubova, L.; Kyzek, S.; Durovcova, I.; Fabova, J.; Horvathova, E.; Sevcovicova, A.; Galova, E. Non-Thermal Plasma–A New Green Priming Agent for Plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, K.; Henselova, M.; Slovakova, L.; Hensel, K. Effects of Plasma Activated Water on Wheat: Germination, Growth Parameters, Photosynthetic Pigments, Soluble Protein Content, and Antioxidant Enzymes Activity. Plasma Process. Polym. 2019, 16, 1800131. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).