Does Maturity Change the Chemical-Bromatological Makeup of Cladodes in Spineless Forage Cactus?
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louhaichi, M.; Kumar, S.; Tiwari, S.; Islam, M.; Hassan, S.; Yadav, O.P.; Dayal, D.; Peter Moyo, H.; Dev, R.; Sarker, A. Adoption and Utilization of Cactus Pear in South Asia—Smallholder Farmers’ Perceptions. Sustainability 2018, 10, 3625. [Google Scholar] [CrossRef]
- Filho, R.R.R.; Santos, D.C.; V’eras, A.S.C.; Siqueira, M.C.B.; Novaes, L.P.; Mora-Luna, R.; Monteiro, C.C.F.; Ferreira, M.A. Can spineless forage cactus be the queen of forage crops in dryland areas? J. Arid Environ. 2021, 186, 104426. [Google Scholar] [CrossRef]
- Pinho, M.R.A.; Santos, E.M.; Oliveira, S.J.D.; Carvalho, G.G.P.D.; da Silva, T.C.; Macedo, A.J.D.S.; Correa, Y.R.; Zanine, A.D.M. Does the level of forage neutral detergent fiber affect the ruminal fermentation, digestibility and feeding behavior of goats fed cactus pear? Anim. Sci. J. 2018, 89, 1424–1431. [Google Scholar] [CrossRef]
- Parente, H.N.; Parente, M.O.M. Impacto do pastejo no ecossistema caatinga. Arq. Ciênc. Veterin’Arias. Zool. 2010, 13, 115–120. [Google Scholar]
- Njarui, D.M.G.; Gatheru, M.; Wambua, J.M.; Nguluu, S.N.; Mwangi, D.M.; Keya, G.A. Feeding management for dairy cattle in smallholder farming systems of semi-arid tropical Kenya. Livest. Res. Rural Dev. 2011, 23, 2–14. [Google Scholar]
- Acharya, P.; Biradar, C.; Louhaichi, M.; Ghosh, S.; Hassan, S.; Moyo, H.; Sarker, A. Finding a Suitable Niche for Cultivating Cactus Pear (Opuntia ficus-indica) as an Integrated Crop in Resilient Dryland Agroecosystems of India. Sustainability 2019, 11, 5897. [Google Scholar] [CrossRef]
- Cousins, A.B.; Mullendore, D.L.; Sonawane, B.V. Recent developments in mesophyll conductance in C3, C4, and crassulacean acid metabolism plants. Plant J. 2020, 101, 816–830. [Google Scholar] [CrossRef]
- Inglese, P.; Mondragon, C.; Nefzaoui, A.; Saenz, C. Crop Ecology, Cultivation and Uses of Cactus Pear; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2017. [Google Scholar]
- Cushman, J.C. Crassulacean acid metabolism. A plastic photosynthetic adaptation to arid environments. Plant Physiol. 2001, 127, 1439–1448. [Google Scholar] [CrossRef]
- Nunes, V.X.; Nunes, N.X.; Londe, L.N.; Oliveira, C.G.; Rocha, S.S. Physico-chemical characterization of prickly pear (Opunicia: Ficus indica) in the semi-arid region of Bahia State, Brazil. Afr. J. Agric. Res. 2017, 12, 3537–3541. [Google Scholar]
- Edvan, R.L.; Mota, R.R.M.; Dias-Silva, T.P.; do Nascimento, R.R.; de Sousa, S.V.; da Silva, A.L.; de Araújo, M.J.; Araújo, J.S. Resilience of cactus pear genotypes in a tropical semi-arid region subject to climatic cultivation restriction. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.M.B.; de Ferreira, M.D.A.; de Albuquerque Brazil, L.H.; Araújo, P.R.B.; Verás, A.S.C.; Santos, D.C.; dos Cruz, M.A.O.M.; Melo, A.A.S.; de Oliveira, T.N.; de Souza, I.S. Substituição do milho por palma forrageira: Comportamento ingestivo de vacas mestiças em lactação. Acta Sci. Anim. Sci. 2003, 25, 347–353. [Google Scholar] [CrossRef]
- Albuquerque, I.; Araújo, G.; Santos, F.; Carvalho, G.; Santos, E.; Nobre, I.; Bezerra, L.; Silva-Júnior, J.; Silva-Filho, E.; Oliveira, R. Performance, Body Water Balance, Ingestive Behavior and Blood Metabolites in Goats Fed with Cactus Pear (Opuntia ficus-indica L. Miller) Silage Subjected to An Intermittent Water Supply. Sustainability 2020, 12, 2881. [Google Scholar] [CrossRef]
- Nefzaoui, A.; Louhaichi, M.; Ben Salem, H. Cactus as a tool to mitigate drought and to combat desertification. J. Arid Land Stud. 2014, 24, 121–124. [Google Scholar]
- Cavalcante, L.A.D.; Santos, G.R.D.A.; Silva, L.M.D.; Fagundes, J.L.; Silva, M.A.D. Respostas de genótipos de palma forrageira a diferentes densidades de cultivo. Pesqui. Agropecu. Trop. 2014, 44, 424–433. [Google Scholar] [CrossRef]
- Tegegne, F.; Kijora, C.; Peters, K.J. Study on the optimal level of cactus pear (Opuntia ficus–indica) supplementation to sheep and its contribution as source of water. Small Rumin. Res. 2007, 72, 157–164. [Google Scholar] [CrossRef]
- Misra, A.K.; Kumar, S.; Kumar, T.K.; Ahmed, S.; Palsaniya, D.R.; Ghosh, P.K.; Louhaichi, M.; Sarker, A.; Hassan, S.; Ates, S. Nutrient intake and utilization in sheep fed opuntia [Opuntia ficus-indica (L.) Mill.] in combination with conventional green and dry fodders. Range Manag. Agrofor. 2018, 39, 97–102. [Google Scholar]
- Wanderley, W.L.; Ferreira, M.D.A.; Batista, Â.M.V.; Véras, A.S.C.; Bispo, S.V.; Silva, F.M.D.; Santos, V.L.F.D. Consumo, digestibilidade e parâmetros ruminais em ovinos recebendo silagens e fenos em associação à palma forrageira. Rev. Bras. Saúde Prod. Anim. 2012, 13, 444–456. [Google Scholar] [CrossRef]
- Carvalho, M.C.; Ferreira, M.D.A.; de Araújo Cavalcanti, C.V.; Lima, L.E.; de Silva, F.M.; da Miranda, K.F.; Chaves Véras, A.S.; de Azevedo, M.; Vieira, V.D.C.F. Associação do bagaço de cana-de-açúcar, palma forrageira e uréia com diferentes suplementos em dietas para novilhas da raça holandesa. Acta Sci. Anim. Sci. 2005, 27, 247–252. [Google Scholar] [CrossRef][Green Version]
- Trammell, M.; Walker, D. The Basics of Forage Quality. Noble News Views 2019, 37, 1–3. [Google Scholar]
- Kumar, S.; Louhaichi, M.; Dana Ram, P.; Tirumala, K.K.; Ahmad, S.; Rai, A.K.; Sarker, A.; Hassan, S.; Liguori, G.; Probir Kumar, G.; et al. Cactus Pear (Opuntia ficus-indica) Productivity, Proximal Composition and Soil Parameters as Affected by Planting Time and Agronomic Management in a Semi-Arid Region of India. Agronomy 2021, 11, 1647. [Google Scholar] [CrossRef]
- Pessoa, D.V.; Pereira de Andrade, A.; Magalhães, R.A.L.; Teodoro, A.L.; Cordeiro dos Santos, D.; Leal de Araújo, G.G.; Nunes de Medeiros, A.; Bezerra do Nascimento, D.; de Lima Valença, R.; Cardoso, D.B. Forage cactus of the genus Opuntia in different with the phenological phase: Nutritional value. J. Arid Environ. 2020, 181, 104243. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 15th ed.; Association of Official Agricultural Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Zanella, K.; Taranto, O.P. Influence of the drying operating conditions on the chemical characteristics of citric acid extracted pectins from pera sweet orange (Citrus Sinensis, L. Osbeck) albedo and flavedo. J. Food Eng. 2015, 166, 111–118. [Google Scholar] [CrossRef]
- Sniffen, C.J.; O’Connor, J.D.; Van Soest, P.J.; Fox, D.G.; Russell, J.B. A net carbohydrate and protein system for evaluating cattle diets: II. Carbohydrate and protein availability. J. Anim. Sci. 1992, 70, 3562–3577. [Google Scholar] [CrossRef]
- Lofgreen, G.P.; Meyer, J.H. A Method for Determining Total Digestible Nutrients in Grazed Forage. J. Dairy Sci. 1956, 39, 268–273. [Google Scholar] [CrossRef]
- Hall, M.B. Challenges with nonfiber carbohydrate methods. J. Anim. Sci. 2003, 81, 3226–3232. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Patil, I. Visualizations with statistical details: The ‘ggstatsplot’ approach. J. Open Source Softw. 2021, 6, 3167. [Google Scholar] [CrossRef]
- Cardoso, D.B.; Carvalho, F.F.R.; de Medeiros, G.R.; Guim, A.; Cabral, A.M.D.; Véras, R.M.L.; Santos, K.C.; dos Dantas, L.C.N.; de Oliveira Nascimento, A.G. Levels of inclusion of spineless cactus (Nopalea cochenillifera Salm Dyck) in the diet of lambs. Anim. Feed Sci. Technol. 2019, 247, 23–31. [Google Scholar] [CrossRef]
- Dubeux, J.C.B., Jr.; Araújo Filho, J.T.; Santos, M.V.F.; Lira, M.A.; Santos, D.C.; Pessoa, R.A.S. Adubação mineral no crescimento e composição mineral da palma forrageira-Clone IPA-201. Rev. Bras. De Ciênc. Agrár. 2010, 5, 129–135. [Google Scholar] [CrossRef]
- Santos, M.V.F.; Ferreira, M.A.; Batista, A.M.V. Valor Nutritivo e Utilização da Palma Forrageira na Alimentação de Ruminantes. In A Palma no Nordeste do Brasil, Conhecimento Atual e Novas Perspectivas de Uso, 1st ed.; Menezes, R.S.C., Simões, D.A., Sampaio, E.V.S.B., Eds.; Editora da UFPE: Recife, Brazil, 2005; pp. 143–162. [Google Scholar]
- Santos, M.V.F.; Lira, M.A.; Dubeux, J.C.B., Jr.; Ferreira, M.A.; Cunha, M.V. Palma Forrageira. In Plantas Forrageiras; Fonseca, D.M., Martuscello, J.A., Eds.; UFV: Viçosa, Brazil, 2010; pp. 459–493. [Google Scholar]
- Garcia, C.C.V.; de Mello, A.C.L.; da Cunha, M.V.; Silva, M.D.C.; Santos, M.V.F.D.; Dubeux, J.C.B., Jr.; Homem, B.G.C. Agronomic characteristics and nutritional value of cactus pear (Opuntia larreyi F.A.C) progenies. Agron. J. 2021, 19, 1–34. [Google Scholar] [CrossRef]
- Lédo, A.A.; Donato, S.L.; Aspiazu, I.; Silva, J.A.D.; Donato, P.E.; Carvalho, A.J.D. Nutrient concentration and nutritional efficiency in ‘Gigante’cactus pear submitted to different spacings and fertilizations. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 154–161. [Google Scholar] [CrossRef]
- El-Shahat, M.S.; Ragab, M.; Siliha, H.A.I.; Rabie, M.A. Physicochemical characteristics of biscuits fortified with cactus pear peel powder. Zagazig J. Agric. Res. 2017, 44, 1073–1084. [Google Scholar]
- Mayer, J.A.; Cushman, J.C. Nutritional and mineral content of prickly pear cactus: A highly water-use efficient forage, fodder and food species. J. Agron. Crop Sci. 2019, 205, 625–634. [Google Scholar] [CrossRef]
- Rivera, D.; Parish, J. Interpreting Forage and Feed Analysis Reports. In Acts of Congress, May 8 and June 30, 1914; Publication 2620; Mississippi State University: Mississippi State, MS, USA; U.S. Department of Agriculture: Washington, DC, USA, 2010. [Google Scholar]
- Grünwaldt, J.M.; Guevara, J.C.; Grünwaldt, E.G.; Martinez Carretero, E. Cacti (Opuntia sps.) as forage in Argentina dry lands. Rev. Fac. Cienc. Agrar. 2015, 47, 263–282. [Google Scholar]
- Reis, R.A.; Bertipaglia, L.M.A.; Freitas, D.; Melo, G.M.P.; Balsalobre, M.A.A. Suplementação Protéica Energética e Mineral em Sistemas de Produção de Gado de Corte nas Águas e nas Secas. In Pecuária de Corte Intensiva Nos Trópicos, 1st ed.; FEALQ: Piracicaba, Brazil, 2004; Volume 1, pp. 171–226. [Google Scholar]
- Silva, V.L.; Costa, L.S.; Bastos, M.P.V.; Facuri, L.M.A.M.; Rego Júnior, N.O.; Silva, M.V. Caracterização físico-química e bioquímica do farelo de palma forrageira re-donda (Opuntia ficus) utilizado na alimentação de ruminantes. Pubvet 2011, 5, 1–13. [Google Scholar]
- Rodriguez-Felix, A.; Cantwell, M. Developmental changes in the composition and quality of prickly pear cactus cladodes (nopalitos). Plant Foods Hum. Nutr. 1988, 38, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Bispo, S.V.; Ferreira, M.A.; Veras, A.S.C.; Batista, A.M.V.; Pessoa, R.A.S.; Bleuel, M.P. Palma forrageira em substituicaoao feno de capim-elefante. Efeito sobre consumo, digestibilidade e caracterısticas de fermentacao ruminal em ovinos. Rev. Bras. Zootec. 2007, 36, 1902–1909. [Google Scholar] [CrossRef]
- Vieira, E.L.; Batista, A.M.V.; Guim, A.; Carvalho, F.F.; Nascimento, A.C.; Araujo, R.F.S.; Mustafa, A.F. Effects of hay inclusion on intake, in vivo nutrient utilization and ruminal fermentation of goats fed spineless cactus (Opuntia ficus-indica Mill) based diets. Anim. Feed Sci. Technol. 2008, 141, 199–208. [Google Scholar] [CrossRef]
- Velásquez, P.A.T.; Berchielli, T.T.; Reis, R.A.; Rivera, A.R.; Dian, P.H.M.; Texeira, I.A.M. Composição química, fracionamento de carboidratos e proteínas e digestibilidade in vitro de forrageiras tropicais em diferentes idades de corte. Rev. Bras. Zootec. 2010, 39, 1206–1213. [Google Scholar] [CrossRef]
- Santos, M.V.F.; Souza, T.C.; Mendoza, P.V.; Santos, D.C.; Cunha, M.V.; Silva, M.C.; Dubeux, J.C.B., Jr.; Lira, M.A.; Mello, A.C.L.; Ferreira, R.L.C. Chemical Composition and Spine Occurrence in Cactus Pear Genotypes. In Proceedings of the IX International Congress on Cactus Pear and Cochineal: CAM Crops for a Hotter and Drier World, Coquimbo, Chile, 26 March 2017; pp. 213–219. [Google Scholar]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Moore, K.J.; Jung, H.G. Lignin and fiber digestion. J. Range Manag. 2001, 54, 420–430. [Google Scholar] [CrossRef]
- Hall, M.B.; Akinyode, A. Cottonseed Hulls Working with a Novel Fiber Source. In Proceedings of the Annual Florida Ruminant Nutrition Symposium, Gainesville, FL, USA, 3–5 February 2000; pp. 179–186. [Google Scholar]
- Ramos, A.O.; Ferreira, M.A.; Véras, A.S.C.; Costa, S.B.M.; Conceição, M.G.; Silva, E.C.; Salla, L.E.; Souza, A.R.D.L. Diferentes fontes de fibra em dietas a base de palma forrageira na alimentação de ovinos. Rev. Bras. Saúde Prod. Anim. 2013, 14, 648–659. [Google Scholar] [CrossRef]
- Muller, M.E.; Prado, I.N. Metabolismo da pectina em animais ruminantes, uma revisão. Varia Sci. 2005, 4, 45–56. [Google Scholar]
- Li, X. Plant cell wall chemistry: Implications for ruminant utilisation. J. Appl. Anim. Nutr. 2021, 9, 31–56. [Google Scholar] [CrossRef]
- Menezes, D.R.; Araújo, G.G.L.D.; Socorro, E.P.D.; Oliveira, R.L.; Bagaldo, A.R.; Silva, T.M.; Pereira, L.G.R. Níveis de uréia em dietas contendo co-produto de vitivinícolas e palma forrageira para ovinos Santa Inês. Arq. Bras. Med. Vet. Zootec. 2009, 61, 662–667. [Google Scholar] [CrossRef]
- dos Santos Sá, W.C.C.; Santos, E.M.; de Oliveira, J.S.; Perazzo, A.F. Production of Spineless Cactus in Brazilian Semiarid. In New Perspectives in Forage Crops; IntechOpen: London, UK, 2018; pp. 25–50. [Google Scholar]
- Delmer, D.P.; Amor, Y. Cellulose biosynthesis. Plant Cell 1995, 7, 987–1000. [Google Scholar]
- Chesson, A. Nutritional significance and nutritive value of plant Polysaccharides. In Feedstuff Evaluation; Wiseman, T., Cole, D.T.A., Eds.; Butterworths: New York, NY, USA, 1990; pp. 179–195. [Google Scholar]
- Wedig, C.L.; Jaster, E.H.; Moore, K.J.; Merchen, N.R. Rumen turnover and digestion of normal and brown midrib sorghum x sudan-grass hybrid silages in dairy cattle. J. Dairy Sci. 1987, 70, 1220–1227. [Google Scholar] [CrossRef]
- Minson, D.J. Influence of lignin and silicon on a summative system for assessing the organic matter digestibility of panicum. Aust. J. Agric. Res. 1971, 22, 89–598. [Google Scholar] [CrossRef]
- Costa, R.C.; Beltr~ao Filho, E.M.; Medeiros, A.N.; Givisiez, P.E.N.; Queiroga, R.C.R.E.; Melo, A.A.S. Effects of increasing levels of cactus pear (Opuntia ficus-indica L. Miller) in the diet of dairy goats and its contribution as a source of water. Small Rumin. Res. 2009, 82, 62–65. [Google Scholar] [CrossRef]

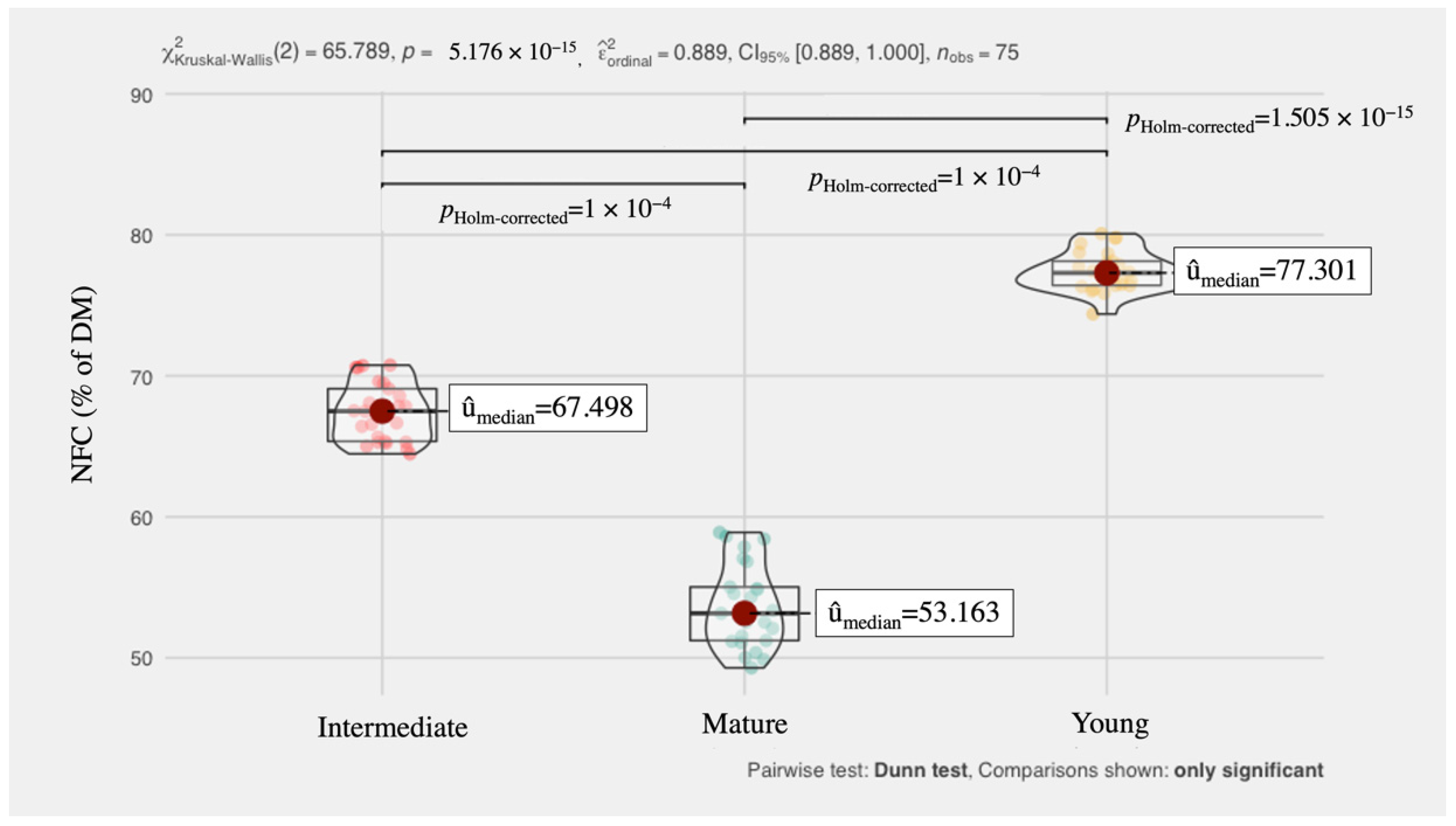
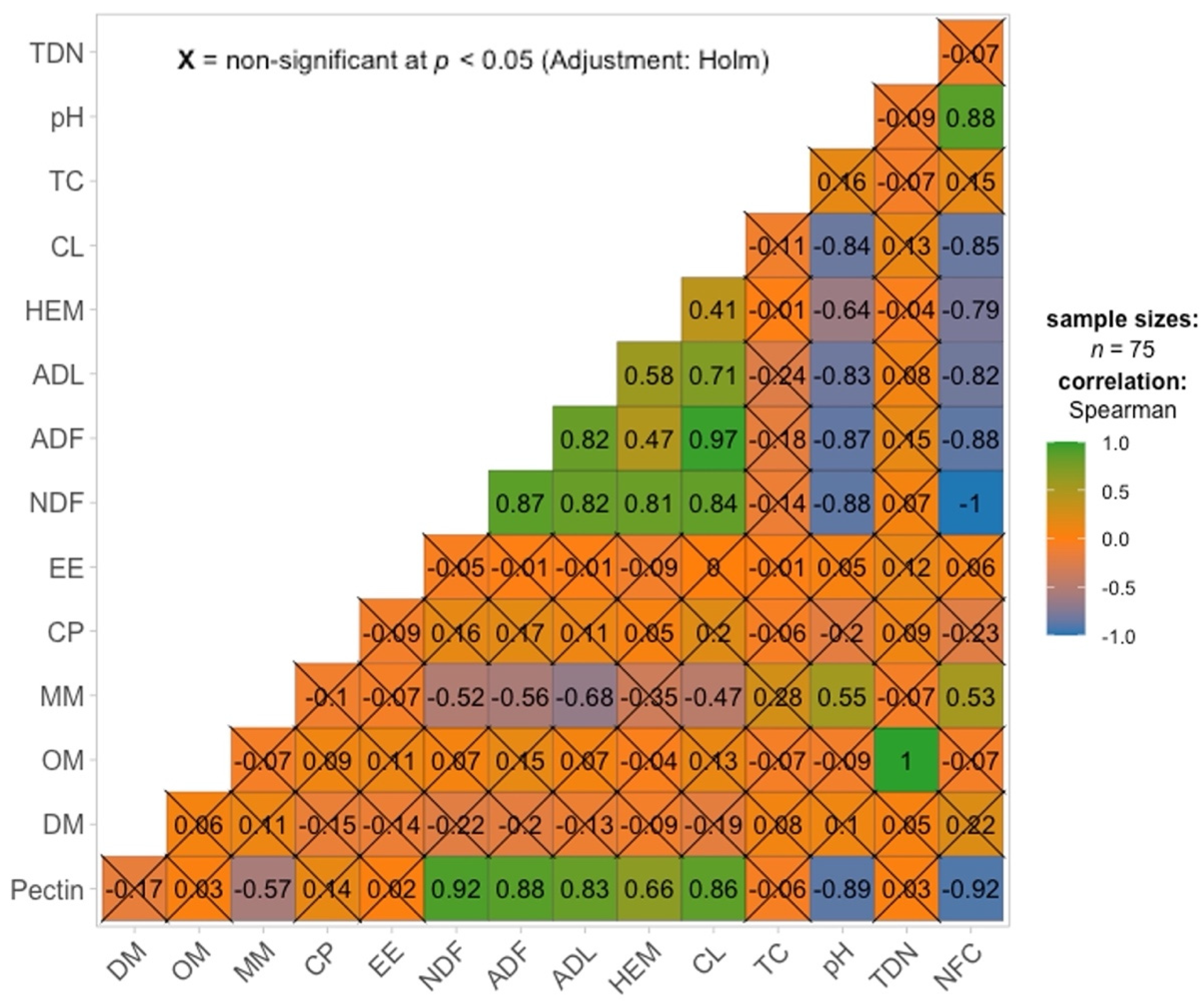
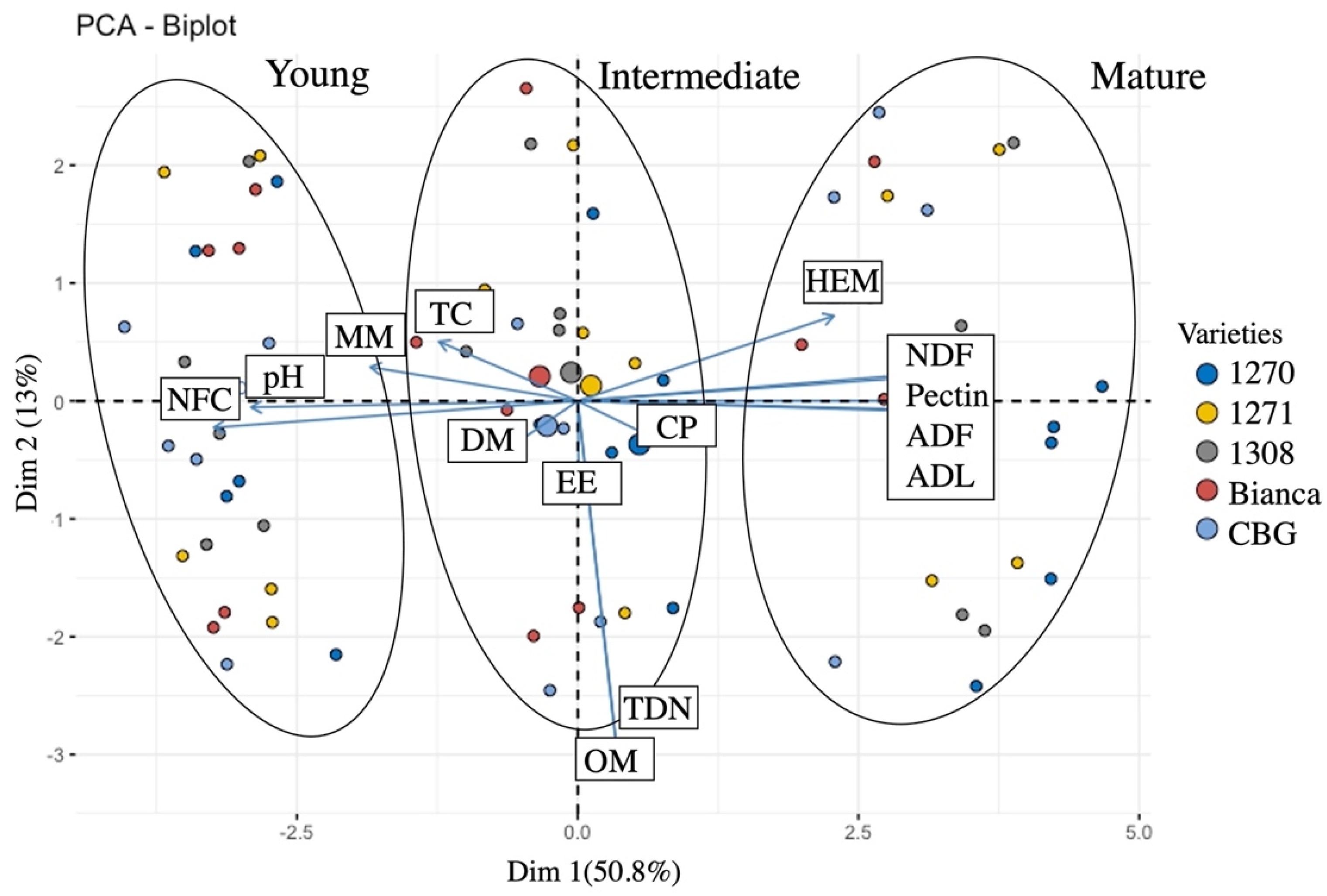
| Parameters (% Except pH) | Phase | Mean | SE | 95% Confidence Interval | Median | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| DM | Young | 13.72 | 0.08 | 13.56 | 13.87 | 13.77 | 13.02 | 14.29 |
| Intermediate | 13.53 | 0.07 | 13.38 | 13.68 | 13.46 | 12.84 | 14.26 | |
| Mature | 13.55 | 0.08 | 13.40 | 13.70 | 13.61 | 12.86 | 14.14 | |
| Average | 13.60 | 0.04 | 13.51 | 13.69 | 13.64 | 12.84 | 14.29 | |
| OM | Young | 66.33 | 0.59 | 65.18 | 67.48 | 66.79 | 61.72 | 70.20 |
| Intermediate | 66.62 | 0.53 | 65.57 | 67.66 | 66.09 | 62.05 | 71.05 | |
| Mature | 66.82 | 0.55 | 65.73 | 67.90 | 66.83 | 62.39 | 71.04 | |
| Average | 66.59 | 0.32 | 65.96 | 67.21 | 66.76 | 61.72 | 71.05 | |
| MM *# | Young | 12.54 | 0.45 | 11.66 | 13.41 | 13.28 | 8.19 | 14.59 |
| Intermediate | 11.29 | 0.44 | 10.43 | 12.16 | 12.35 | 6.79 | 12.62 | |
| Mature | 9.79 | 0.40 | 9.00 | 10.58 | 9.79 | 6.32 | 12.54 | |
| Average | 11.21 | 0.28 | 10.66 | 11.75 | 12.14 | 6.32 | 14.59 | |
| CP | Young | 4.05 | 0.09 | 3.87 | 4.22 | 4.08 | 3.34 | 5.11 |
| Intermediate | 4.20 | 0.10 | 4.01 | 4.40 | 4.09 | 3.43 | 5.11 | |
| Mature | 4.25 | 0.09 | 4.07 | 4.43 | 4.21 | 3.36 | 5.10 | |
| Average | 4.17 | 0.05 | 4.06 | 4.27 | 4.11 | 3.34 | 5.11 | |
| EE | Young | 1.32 | 0.01 | 1.29 | 1.34 | 1.32 | 1.23 | 1.43 |
| Intermediate | 1.30 | 0.02 | 1.27 | 1.33 | 1.27 | 1.21 | 1.43 | |
| Mature | 1.31 | 0.01 | 1.29 | 1.34 | 1.30 | 1.22 | 1.43 | |
| Average | 1.31 | 0.01 | 1.29 | 1.33 | 1.29 | 1.21 | 1.43 | |
| NDF * | Young | 15.64 | 0.24 | 15.18 | 16.11 | 15.78 | 13.47 | 17.42 |
| Intermediate | 24.54 | 0.44 | 23.68 | 25.40 | 24.65 | 20.92 | 27.90 | |
| Mature | 37.21 | 0.57 | 36.10 | 38.32 | 37.50 | 31.85 | 40.90 | |
| Average | 25.80 | 1.06 | 23.73 | 27.87 | 24.65 | 13.47 | 40.90 | |
| ADF * | Young | 11.27 | 0.21 | 10.85 | 11.69 | 11.30 | 9.49 | 12.75 |
| Intermediate | 17.37 | 0.61 | 16.16 | 18.57 | 17.04 | 11.53 | 21.49 | |
| Mature | 26.71 | 0.15 | 26.42 | 27.01 | 26.51 | 25.50 | 27.95 | |
| Average | 18.45 | 0.77 | 16.94 | 19.96 | 17.04 | 9.49 | 27.95 | |
| ADL * | Young | 0.82 | 0.05 | 0.72 | 0.91 | 0.87 | 0.47 | 1.21 |
| Intermediate | 2.35 | 0.08 | 2.19 | 2.51 | 2.48 | 1.79 | 2.95 | |
| Mature | 3.32 | 0.15 | 3.03 | 3.61 | 3.56 | 1.94 | 4.19 | |
| Average | 2.16 | 0.13 | 1.90 | 2.42 | 2.18 | 0.47 | 4.19 | |
| pH * | Young | 0.47 | 0.00 | 0.47 | 0.48 | 0.47 | 0.45 | 0.50 |
| Intermediate | 0.44 | 0.00 | 0.44 | 0.44 | 0.44 | 0.43 | 0.45 | |
| Mature | 0.42 | 0.00 | 0.41 | 0.42 | 0.42 | 0.40 | 0.43 | |
| Average | 2.16 | 0.13 | 1.90 | 2.42 | 2.18 | 0.47 | 4.19 | |
| Pectin * | Young | 0.44 | 0.00 | 0.44 | 0.45 | 0.44 | 0.40 | 0.50 |
| Intermediate | 11.60 | 0.07 | 11.48 | 11.73 | 11.68 | 11.08 | 12.05 | |
| Mature | 14.97 | 0.04 | 14.89 | 15.05 | 14.93 | 14.65 | 15.30 | |
| Average | 11.84 | 0.29 | 11.27 | 12.40 | 11.68 | 8.23 | 15.30 | |
| Parameters (%) | Phase | Mean | SE | 95% Confidence Interval | Median | Minimum | Maximum | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| TC *# | Young | 71.69 | 1.10 | 69.53 | 73.85 | 69.86 | 63.85 | 82.08 |
| Intermediate | 71.76 | 1.09 | 69.61 | 73.91 | 70.41 | 61.94 | 80.68 | |
| Mature | 66.49 | 2.30 | 61.97 | 71.01 | 70.18 | 50.36 | 82.48 | |
| Average | 69.98 | 0.95 | 68.1 | 71.86 | 70.36 | 50.36 | 82.5 | |
| HEM * | Young | 4.38 | 0.35 | 3.68 | 5.07 | 4.33 | 0.96 | 7.93 |
| Intermediate | 7.18 | 0.72 | 5.76 | 8.59 | 7.53 | 1.56 | 15.26 | |
| Mature | 10.5 | 0.58 | 9.36 | 11.64 | 10.66 | 5.16 | 14.87 | |
| Average | 7.35 | 0.43 | 6.49 | 8.21 | 7.15 | 0.96 | 15.3 | |
| CL * | Young | 10.45 | 0.22 | 10 | 10.9 | 10.48 | 8.5 | 12.25 |
| Intermediate | 15.02 | 0.60 | 13.82 | 16.21 | 14.9 | 9.66 | 19.46 | |
| Mature | 23.39 | 0.18 | 23.02 | 23.76 | 23.63 | 21.91 | 25.22 | |
| Average | 16.29 | 0.66 | 14.99 | 17.58 | 14.9 | 8.5 | 25.2 | |
| TDN | Young | 44.81 | 0.79 | 43.26 | 46.36 | 45.36 | 38.71 | 50.06 |
| Intermediate | 45.16 | 0.72 | 43.74 | 46.58 | 44.34 | 39.16 | 51.38 | |
| Mature | 45.45 | 0.74 | 43.98 | 46.92 | 45.39 | 39.55 | 51.31 | |
| Average | 45.14 | 0.43 | 44.29 | 45.99 | 45.26 | 38.71 | 51.4 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naorem, A.; Louhaichi, M.; Hassan, S.; Sarker, A.; Udayana, S.K.; Jayaraman, S.; Patel, S. Does Maturity Change the Chemical-Bromatological Makeup of Cladodes in Spineless Forage Cactus? Sustainability 2022, 14, 11411. https://doi.org/10.3390/su141811411
Naorem A, Louhaichi M, Hassan S, Sarker A, Udayana SK, Jayaraman S, Patel S. Does Maturity Change the Chemical-Bromatological Makeup of Cladodes in Spineless Forage Cactus? Sustainability. 2022; 14(18):11411. https://doi.org/10.3390/su141811411
Chicago/Turabian StyleNaorem, Anandkumar, Mounir Louhaichi, Sawsan Hassan, Ashutosh Sarker, Shiva Kumar Udayana, Somasundaram Jayaraman, and Sachin Patel. 2022. "Does Maturity Change the Chemical-Bromatological Makeup of Cladodes in Spineless Forage Cactus?" Sustainability 14, no. 18: 11411. https://doi.org/10.3390/su141811411
APA StyleNaorem, A., Louhaichi, M., Hassan, S., Sarker, A., Udayana, S. K., Jayaraman, S., & Patel, S. (2022). Does Maturity Change the Chemical-Bromatological Makeup of Cladodes in Spineless Forage Cactus? Sustainability, 14(18), 11411. https://doi.org/10.3390/su141811411









