Abstract
Channelization is the most common hydraulic modification of urban rivers. Here, we assessed the effects of urban river morphology on benthic communities by analyzing the characteristics of benthic communities at various sites in channelized and natural rivers of the Longgang River system in southern China. We detected four Clitellata species, five Oligochaeta species, one Polychaeta species, 10 Gastropoda genera/species, two Bivalvia genera/species, two Crustacea genera/species, and 14 Insecta genera/species. Insecta and Oligochaeta were the dominant classes in the wet and dry seasons, and Chironomus plumosus was the most dominant species. The density of Clitellata was significantly lower in channelized rivers (0–0.74 ind/m2) than in natural rivers (0.61–4.85 ind/m2). The Shannon’s diversity index was significantly lower in channelized rivers (0.66–1.04) than in natural rivers (0.83–1.28) in the wet and dry season. NH3.N was positively correlated with Shannon’s diversity index, and chemical oxygen demand and river width were negatively correlated with Shannon’s diversity index. When the concentration of total phosphorus (TP) was low (<3 mg/L), it was positively correlated with Shannon’s diversity index. Our findings indicate that river channel morphology affects benthic faunal structure and diversity, but the effects varied among seasons. Minimized channelization will prevent the loss of aquatic biodiversity in subtropical urban rivers, as will preservation of natural rivers.
1. Introduction
The world is currently undergoing a rapid and dramatic process of urbanization [1]. The global urbanization rate increased from 28.3% in 1950 to 50% in 2010 [2]. Global urbanization is expected to reach 60% by 2030 [3]. Urbanization results in the transformation of natural or agricultural ecosystems into urban ecosystems [4]. Urbanization is expected to have a substantial effect on river ecosystems (e.g., “urban stream syndrome”) [5].
During the urbanization process, rivers are buried, cut, and hardened, resulting in marked decreases in the area of river water and the disappearance of floodplains [6]. The urbanization process also induces changes in river network structure, including changes in the percentage of main rivers, connectivity among rivers, and the branching ratio [7]. After rain, surface runoff rapidly increases, and the intensity and frequency of flooding increase significantly, intensifying the erosion of embankments and riverbeds, altering river morphology and habitat quality and promoting the rapid inflow of nutrients and toxic substances into river channels [8].
In normal seasons, decreases in land storage capacity lead to decreases in river flow, and this increases the difficulty of meeting the ecological water demand of rivers [9]. In addition, urbanization will greatly increase the total amount of sewage deposited into rivers, which will significantly degrade aquatic communities [10,11,12]. Despite the fact that cities account for less than 3% of the earth’s land area, the intensification of land-use change and the continuous degradation of river habitat and water quality have made urbanization one of the main causes of the loss of river biodiversity [13].
On the one hand, the composition, abundance, spatial distribution, and seasonal dynamics of river macrobenthos are sensitive to changes in the river environment [14,15]. On the other hand, compared with other organisms such as algae and fish, benthic animals have moderate growth cycles and limited dispersal abilities; they can also be easily collected, which facilitates the collection of large amounts of high-quality data [16]. Macro-benthic organisms are thus some of the most well-studied organisms in the field of urban river ecology [17].
Channelization is the most common hydraulic modification of urban rivers, and most parts or all sections are protected by cast-in-place concrete, precast concrete blocks, or masonry structures for slope protection or masonry protection [18,19]. The channelization of rivers results in the removal of stones and large woody residues from the original river bed, which increases the scouring effect of the flowing water on the river bed. These modifications decrease the availability of substrates to which benthic animals can attach, which decreases the diversity of benthic animals and leads to the extirpation of migratory species [20,21,22].
Most previous studies have focused on the effects of urban rivers and natural rivers on benthic communities, but few studies have examined the effects of river morphology on benthic communities in cities. Given that an increasing number of rivers will become a part of cities as urbanization intensifies, developing approaches to maintain the health of urban rivers requires increased attention. Studies of urban river benthos in rivers varying in morphology are important for enhancing our understanding of the effect of urbanization on river benthos.
Whether the responses of benthic communities to channelization differ between temperate rivers, which are characterized by greater seasonal variation, and subtropical urban rivers, which experience less pronounced seasonal variation, remains unclear. Addressing this will enhance our understanding of the response of urban river biodiversity to river channelization, as well as aid urban river governance and biodiversity conservation.
Here, we studied the benthic community of channelized rivers and natural rivers in a subtropical river system. Specifically, we (1) assessed the effects of urban river morphology on the benthic community, including its diversity; (2) explored the effects of river morphology on the structure and function of river ecosystems; and (3) generated new information that could be used to aid the ecological control of urban rivers and the protection of biodiversity.
2. Materials and Methods
2.1. Study Area
The main stream of the Longgang River is 35 km long, with a drainage area of 423 km2, and it is mainly located in Longgang District northeast of Shenzhen (22°35′–22°49′ N, 114°02′–114°21′ E). It originates from the northern foot of Wutong Mountain, flows through the central urban area of Longgang District, Shenzhen, and later enters Huizhou, where it joins the Dongjiang River, the mainstream of the Pearl River system [23]. The Longgang River is a typical urban river that receives subtropical rainfall. The water volume of the river is affected by the regional climate and precipitation [23]. The wet season runs from May to October, and the dry season runs from November to April [23].
The Longgang River is also the main channel for urban industrial and domestic sewage discharge, and the water environment is significantly affected by human activities [24,25,26]. To support urban development and enhance flood control, some river courses in recent years have been hardened for slope protection, and some riparian belts have been converted into wetlands or near-natural riparian belts to enhance water quality and create natural landscapes.
2.2. Study Site and Sampling Design
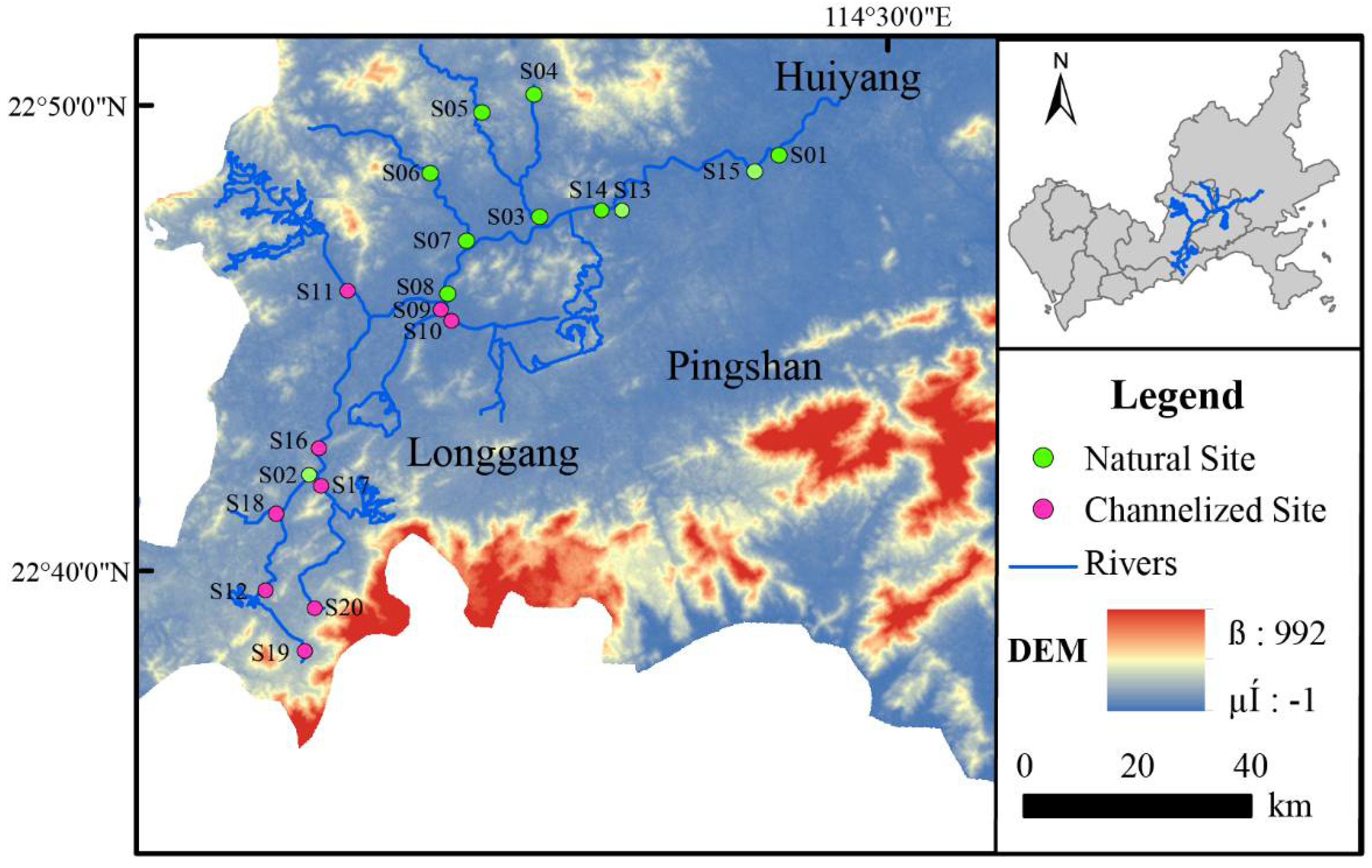
The experiments were conducted at 20 sampling sites in the Longgang River system in September (wet season) and December (dry season) of 2017 in Shenzhen, Guangdong Province, China (Figure 1). The 20 sampling points were located in the mainstream, main tributaries, and urban area of Longgang District where river water quality is regularly monitored; our sampling thus permitted a comprehensive assessment of the overall health of the Longgang River system within the urban area.
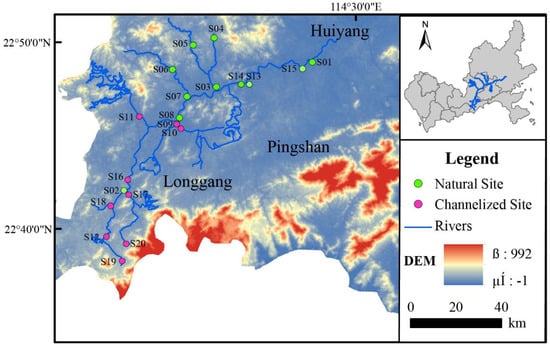
Figure 1.
Study sites in the Longgang River. Natural Site: the sites in the natural river zone; Channelized Site: the sites in the channelized river zone.
Eleven samples were taken from natural rivers, and nine sampling points were taken from channelized rivers. The natural rivers sampled had natural river banks, with natural ebb and flow areas and various aquatic vascular plants; the river banks of the channelized rivers were made of artificially solidified materials, including cement slopes or vertical embankments (Figure 1).
Benthic macroinvertebrates were collected using a Peterson sampler in the middle channel and a Surber sampler or D-shape net (0.3 mm mesh) along the littoral zone of each side. Each sample was mixed with three cores collected at each sampling site, which were taken 3–5 m away from each other and sieved through a 0.5-mm mesh. All samples retained on the sieve were fixed in 10% formaldehyde. Benthic macroinvertebrates were carefully separated from the debris and then identified to the lowest possible taxonomic level under a dissecting microscope. Different species of benthic animals at each site were counted individually. According to the sampling area, the number of species per unit area was calculated as the density of benthic animals. The benthic animals at each sampling point were weighed after blotting the surface moisture with filter paper, and the total weight of each species in each sample point was weighed using an electronic balance (accurate to 0.01g). Finally, according to the sampling area, the weight of each species per unit area was calculated as benthic biomass.
Water samples were collected for water quality analysis at the same time that benthic macroinvertebrates were sampled. Subsurface water samples (depth ~0.5 m) were collected using a bottle sampling apparatus from each site. The hydrological features of the river, such as river width (RW), flow rate, and water depth, were measured in the field. Chemical oxygen demand (COD) and total phosphorus (TP) were analyzed in the Water Quality Analysis Laboratory of the Shenzhen Ecological and Environmental Monitoring Center. Water samples were stored in ice boxes and analyzed within 24 h. TP was determined using the method of Qian and Fu (1987) [27]. A Hach DR 5000 analyzer was used to measure COD.
2.3. Data Analysis
Simpson’s diversity index [28], Shannon’s diversity index [29], and Pielou evenness index [30] were used to evaluate macrobenthic biodiversity:
where S is the number of species, ni is the total number of organisms of a particular species ‘i’, and N is the total number of organisms of all species.
Differences in the density of each benthic species between the wet and dry seasons were tested using Mann–Whitney U Tests in R (Version: 3.6.2) (https://www.r-project.org/). Differences in the density, biomass, and diversity indexes of seven macrobenthic communities between the natural rivers and channelized rivers were tested using Student’s t-tests in R (Version: 3.6.2). Differences were considered significant at p < 0.05. Similarities in macrofaunal communities between the natural rivers and channelized rivers were explored using non-metric multidimensional scaling (n-MDS), based on the Bray–Curtis similarity index in R (Version: 3.6.2). Analysis of similarity (ANOSIM) was used to test for the significance of differences in benthic communities in R (Version: 3.6.2). When necessary, data were log (x + 1) transformed to meet the assumptions of normality prior to analysis.
The relationships of macrobenthic density and the macrobenthic diversity index with environmental parameters were analyzed using the random forest model (“randomForest” package) [31] and generalized additive model (GAM) (“mgcv” package) [32,33] in R (Version: 3.6.2). IncMSE reflects the relative importance of variables; the accuracy of random forest prediction was reduced after the current variable of %IncMSE was removed. IncNodePurity indicates the cumulative contribution of each variable to the observed values at each node of the classification tree.
3. Results
3.1. The Benthic Community in the Wet and Dry Season in the Longgang River
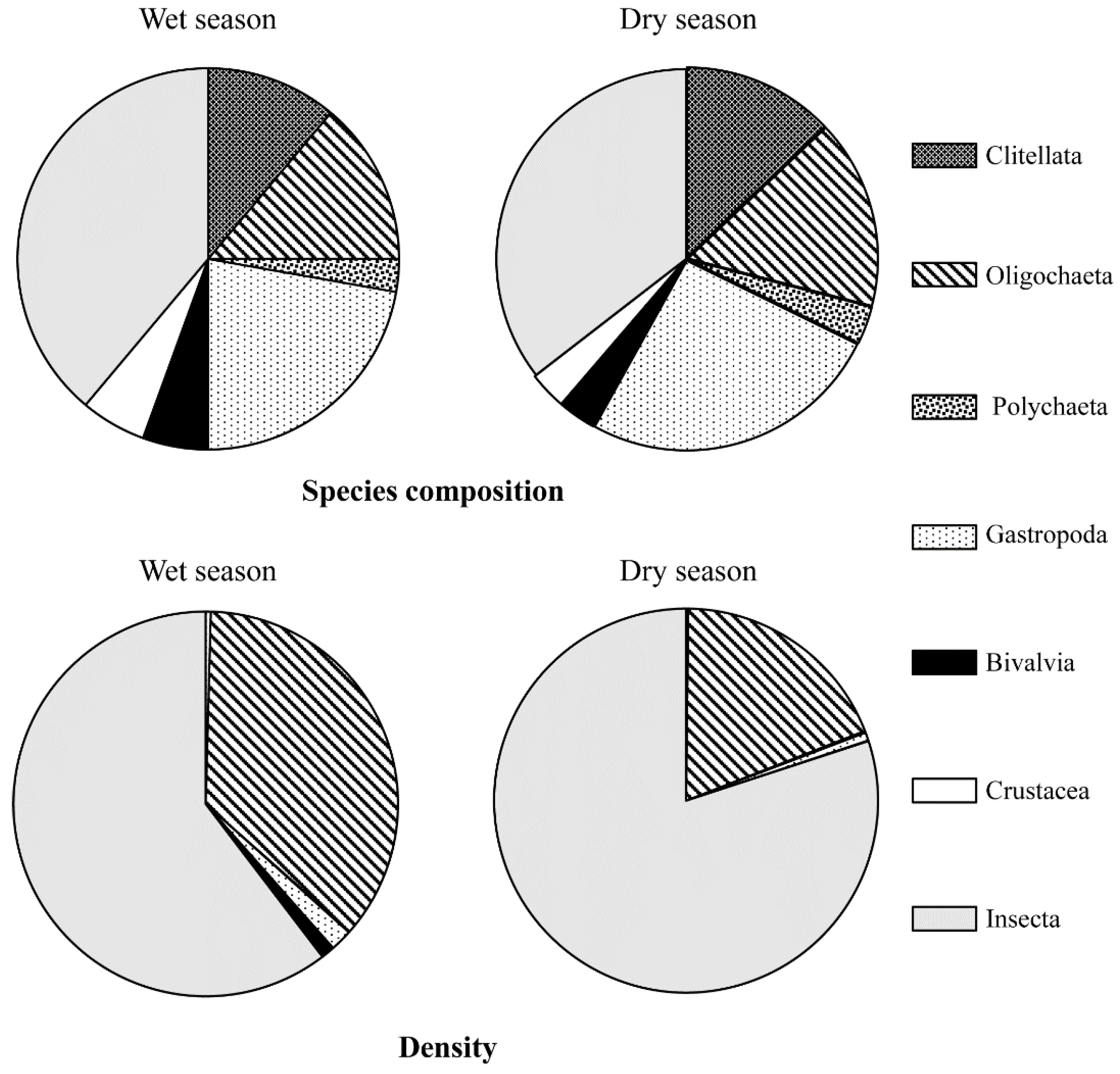
A total of 37 benthic taxa from seven classes (Clitellata, Oligochaeta, Polychaeta, Gastropoda, Bivalvia, Crustacea, and Insecta) were identified (Figure 2). The three classes of Insecta, Gastropoda, and Oligochaeta comprised 62.16% of the total number of benthic species (Figure 2, Table 1). There were four Clitellata species, five Oligochaeta species, one Polychaeta species, 10 Gastropoda genera/species, two Bivalvia genera/species, two Crustacea genera/species, and 14 Insecta genera/species (Table 1).
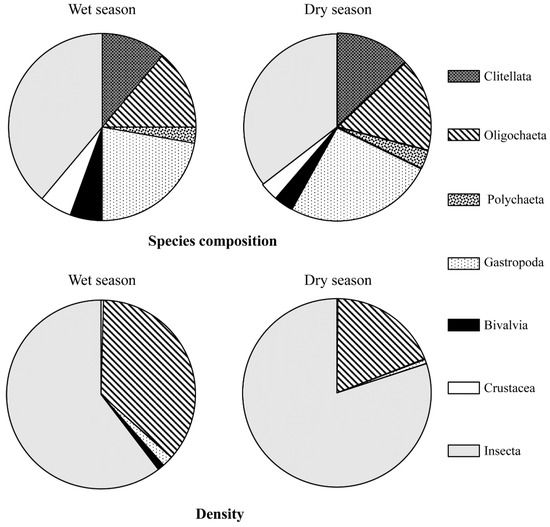
Figure 2.
Species composition and density ratio of benthic fauna in different seasons in the Longgang River.

Table 1.
Results of Mann–Whitney U Tests of benthic densities of the Longgang River in the wet and dry seasons. Density: Mean values ± SE, ind/m2. Significant p-values (<0.05) are in bold.
Season had no pronounced effect on the composition of benthic species in the Longgang River. A total of 36 and 30 benthic taxa were identified in the wet season and dry season, respectively (Figure 2). The number of Insecta, Crustacea, Gastropoda, and Bivalve species was slightly higher in the wet season than in the dry season, and no differences were observed in the number of species in the other classes between the wet season and the dry season (Figure 2).
Insecta and Oligochaeta were the dominant classes in the wet and dry seasons (Figure 2). Insecta density was 60.39% in the wet season and 80.03% in the dry season. The Oligochaeta density was 36.12% in the wet season and 18.90% in the dry season (Figure 2).
Chironomus plumosus was the most dominant species in the wet season (484.00 ± 168.82 ind./m2) and dry season (950.67 ± 204.15 ind./m2) (Table 1). Limnodrilus hoffmeisteri, which belongs to the class Oligochaeta, was the second most dominant species in the wet season (320.33 ± 63.86 ind./m2) and dry season (211.67 ± 51.69 ind./m2) (Table 1).
Season had no pronounced effect on the density of macrobenthic species in the Longgang River (Table 1). The total density of microbenthic species was similar in the wet and dry seasons (1105.67 ± 181.45 ind./m2 and 1308.34 ± 233.16 ind./m2, respectively) (Table 1). A Mann–Whitney U Test revealed that the density of most species did not significantly differ between the wet and dry season (p > 0.05) (Table 1). However, the density of the dominant species C. plumosus significantly differed between the dry and wet seasons (Z adjusted = 2.027; p = 0.043) (Table 1).
3.2. Differences in the Benthic Community between Natural and Channelized Rivers
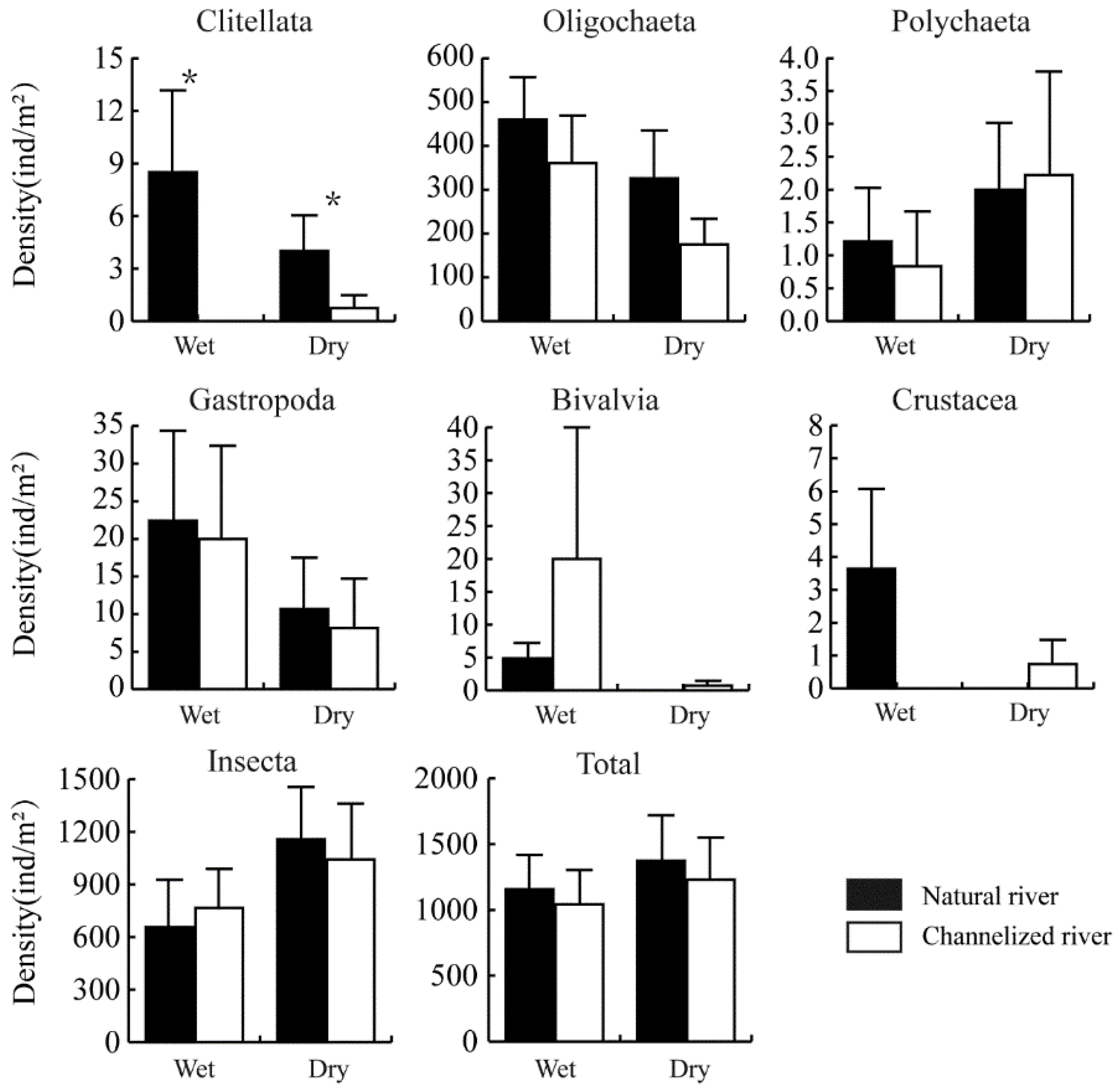
The total density of the benthic community was lower in channelized rivers than in natural rivers in both the wet and dry season; however, this difference was not significant (Figure 3). Significant differences in the density of Clitellata were found between natural and channelized rivers (Figure 3). The density of Clitellata was 8.48 ± 4.68 and 0 ind./m2 in natural and channelized rivers in the wet season (p < 0.05), respectively; the density of Clitellata was 4.00 ± 2.04 and 0.74 ± 0.74 ind./m2 in natural and channelized rivers in the dry season, respectively (p < 0.05). The density of Oligochaeta was slightly lower in channelized rivers than in natural rivers (wet season: p = 0.056; dry season: p = 0.087).
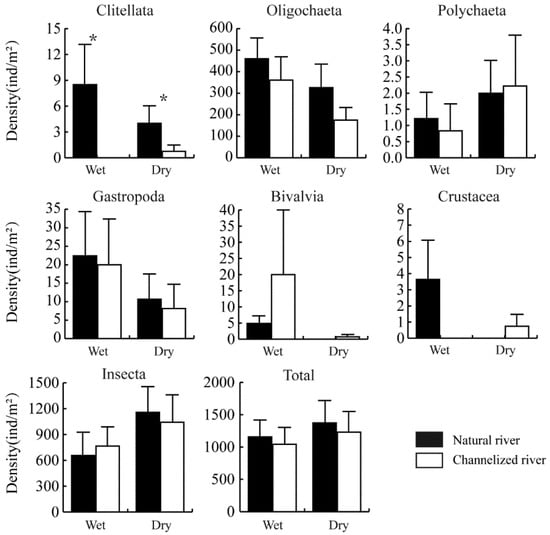
Figure 3.
Density of benthic taxa in natural and channelized rivers in the Longgang River system in different seasons (Mean + SE). The asterisk (*) represents a significant difference (p < 0.05) between the natural river and channelized river sites.
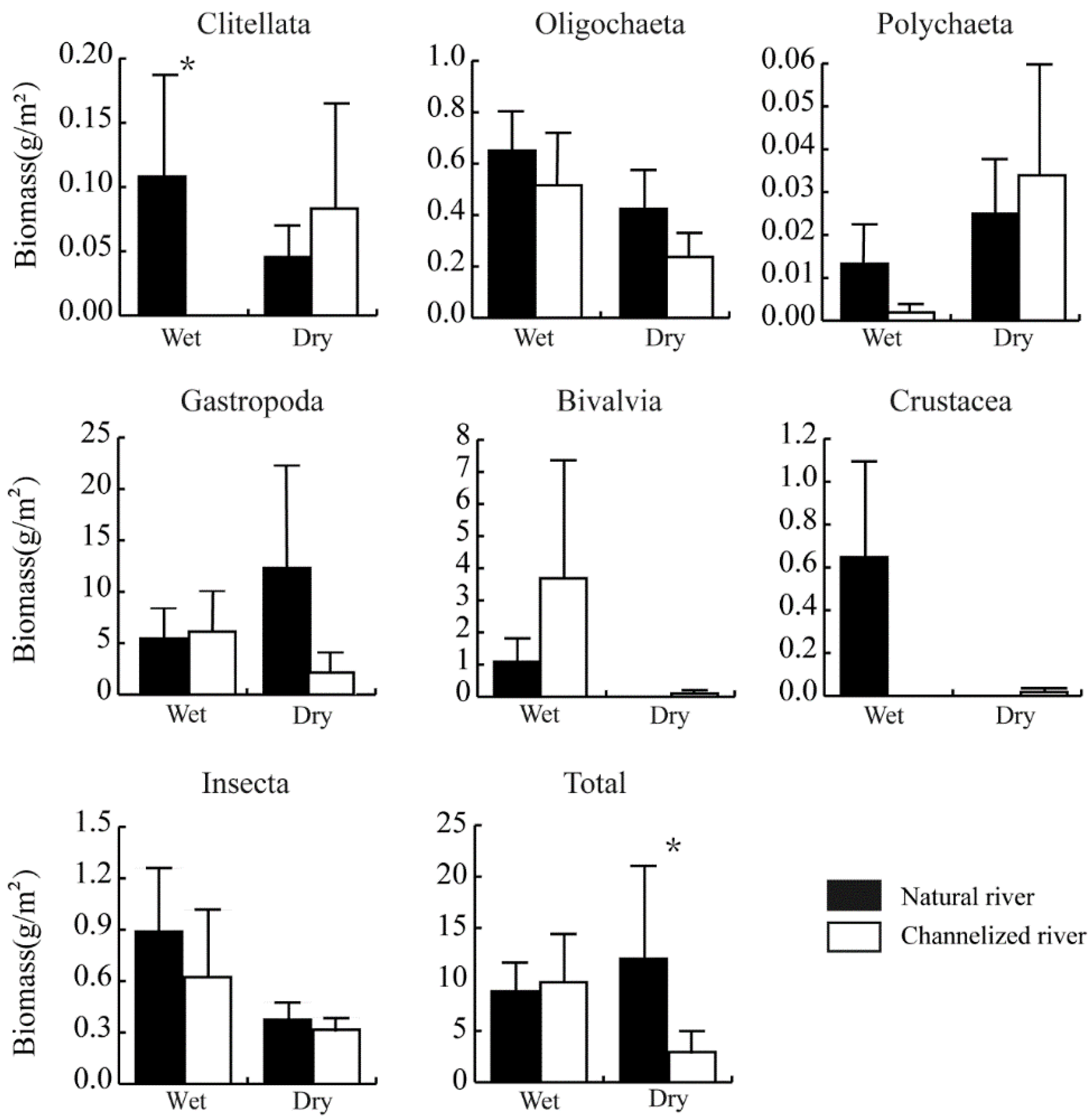
Changes in biomass were not consistent with changes in density. In the dry season, the total biomass of the benthic community was lower in channelized rivers than in natural rivers (p < 0.05). In the wet season, the total biomass of the benthic community was slightly higher in channelized rivers than in natural rivers (p = 0.164) (Figure 4). The biomass of Clitellata was 0.11 ± 0.08 and 0 g/m2 in natural and channelized rivers in the wet season, respectively, and this difference was significant. No significant differences were observed in the biomass of the other groups between natural rivers and channelized rivers.
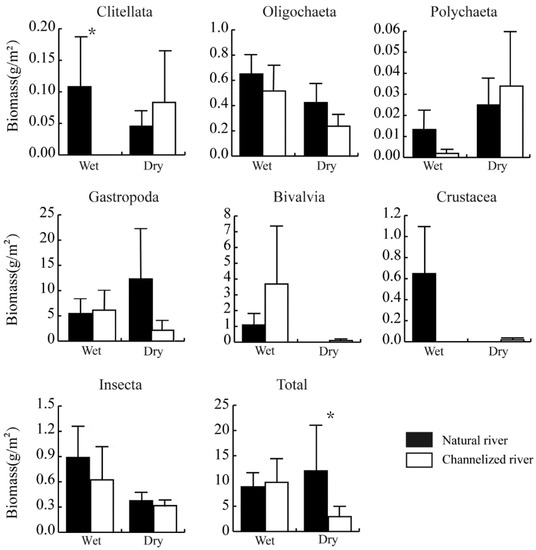
Figure 4.
Biomass of benthic fauna in natural and channelized rivers in the Longgang River system in different seasons (Mean + SE). The asterisk (*) represents a significant difference (p < 0.05) between the natural river and channelized river sites.
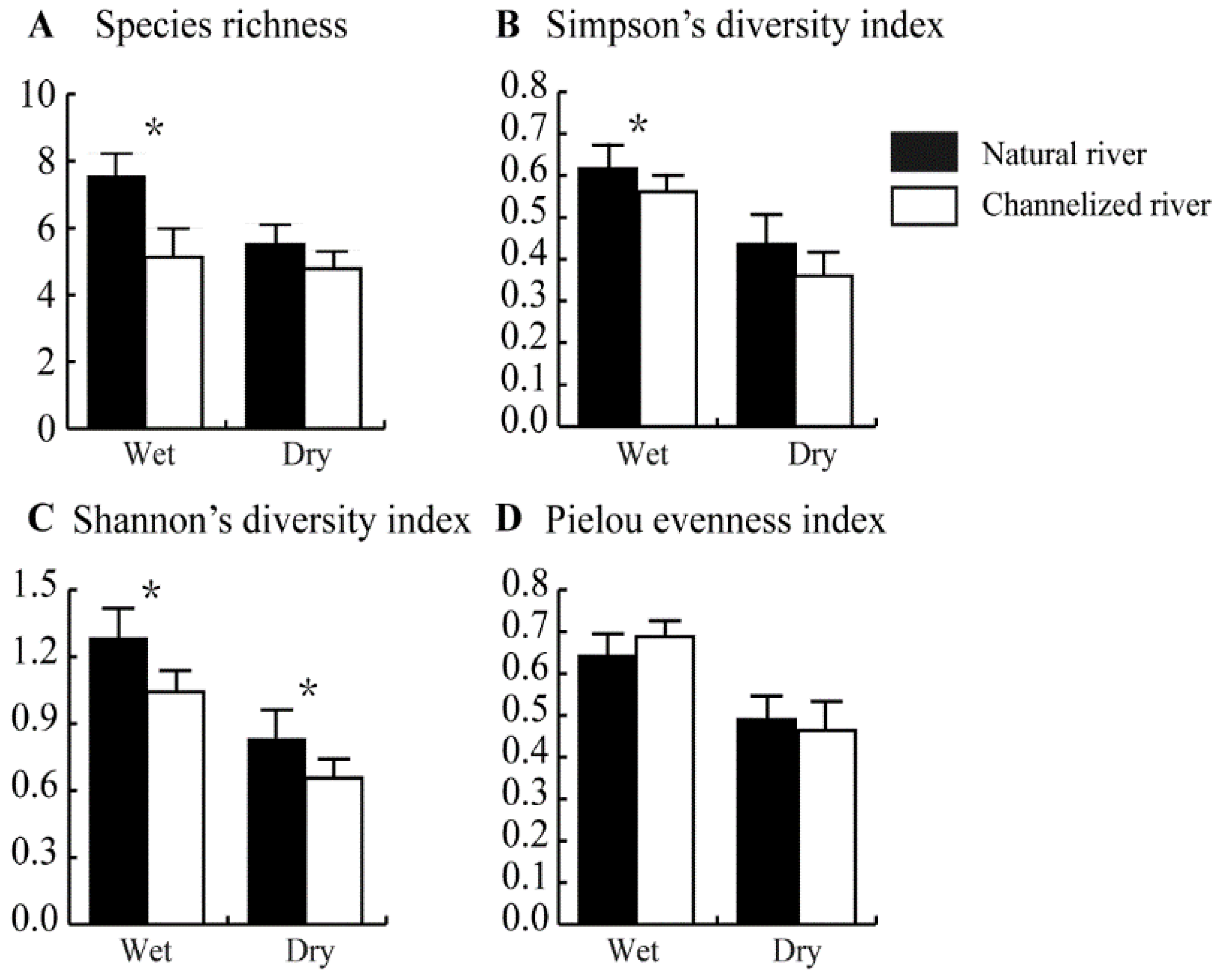
In the wet season, species richness was significantly lower in channelized rivers than in natural rivers (p < 0.05) (Figure 5A). The number of species in natural rivers and channelized rivers was 7.55 ± 0.68 and 5.13 ± 0.85, respectively (Figure 5A). In the dry season, the number of species was lower in channelized rivers (4.78 ± 0.52) than in natural rivers (5.50 ± 0.60); however, this difference was only marginally significant (p = 0.052) (Figure 5A).
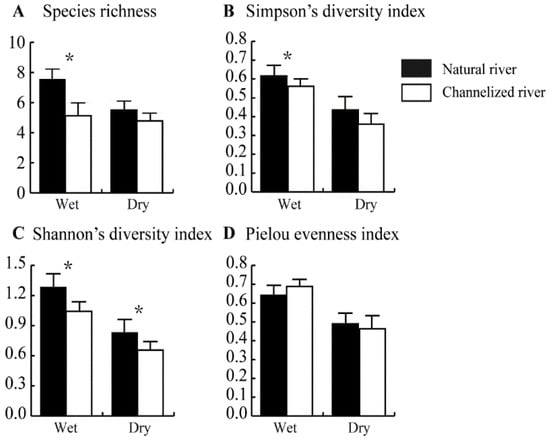
Figure 5.
Species richness, Simpson’s diversity index, Shannon’s diversity index, and Pielou evenness index of benthic fauna in natural and channelized rivers in the Longgang River system in different seasons. The asterisk (*) represents a significant difference (p < 0.05) between the natural river and channelized river sites.
Patterns of variation in Simpson’s diversity index were similar to patterns of variation in species richness. In the wet season, Simpson’s diversity index was lower in channelized rivers (0.56 ± 0.04) than in natural rivers (0.62 ± 0.06) (Figure 5B). In the dry season, Simpson’s diversity index was lower in channelized rivers (0.36 ± 0.06) than in natural rivers (0.43 ± 0.07); however, this difference was not significant (p = 0.097) (Figure 5B).
Shannon’s diversity index of the benthic community was significantly lower in channelized rivers than in natural rivers in the wet and dry seasons (p < 0.05) (Figure 5C). Shannon’s diversity index in the wet and dry seasons in channelized rivers was 1.04 ± 0.09 and 0.66 ± 0.09, respectively; Shannon’s diversity index in the wet and dry seasons in natural rivers was 1.28 ± 0.13 and 0.83 ± 0.13, respectively (Figure 5C). No differences in the Pielou evenness index of the benthic community were observed between natural rivers and channelized rivers in the wet and dry seasons (Figure 5D).
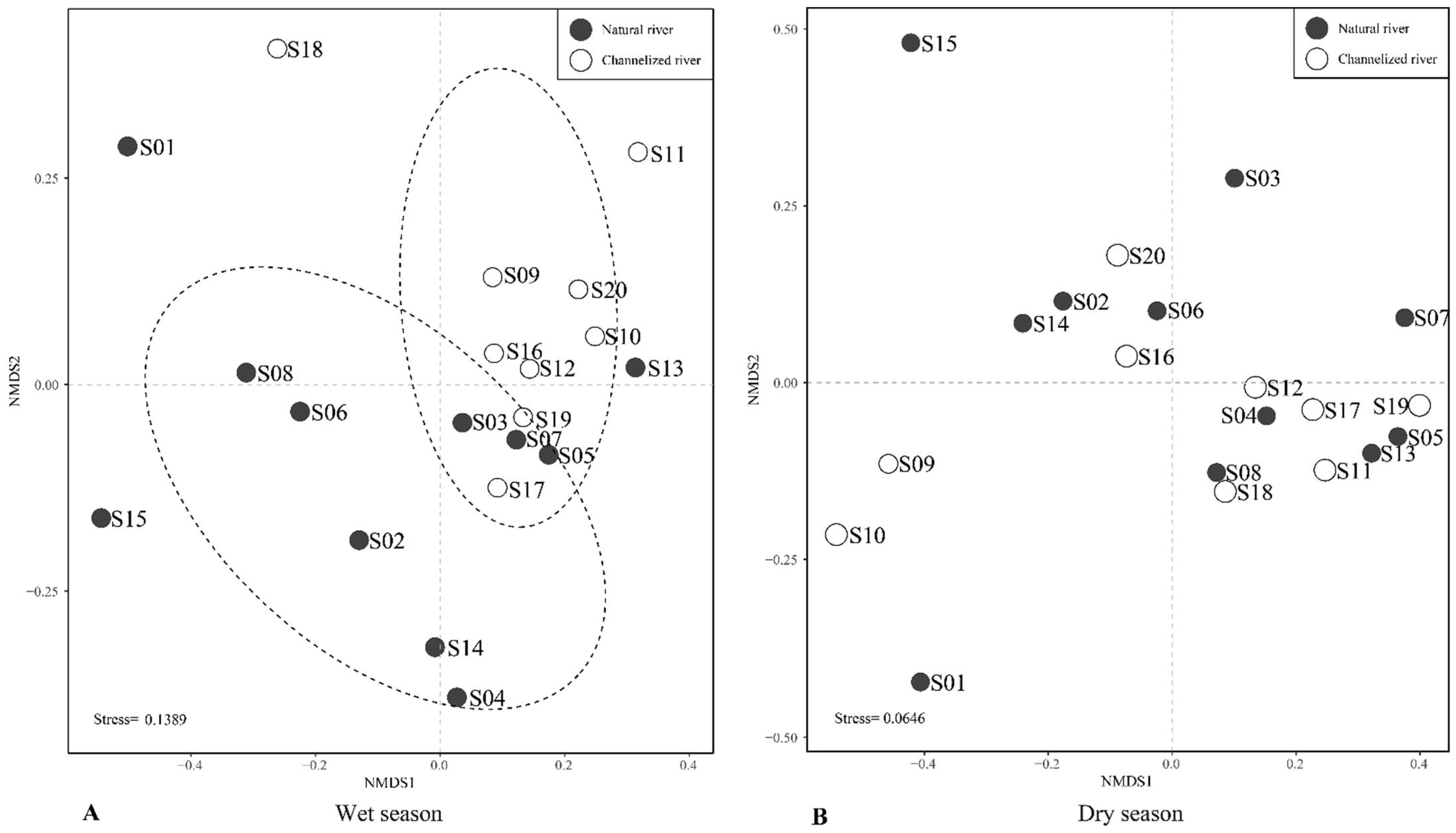
Non-metric multidimensional scaling (nMDS) ordination of the benthic community data revealed clear differences in the benthic community in the wet season in natural rivers and channelized rivers (Figure 6A). Benthic community structure in channelized rivers significantly differed from that in natural rivers (ANOSIM, p = 0.003; global tests R = 0.224). In the dry season, the nMDS results revealed no significant differences in the benthic assemblages between natural and channelized rivers (ANOSIM, p = 0.231; global tests R = 0.067, Figure 6B).
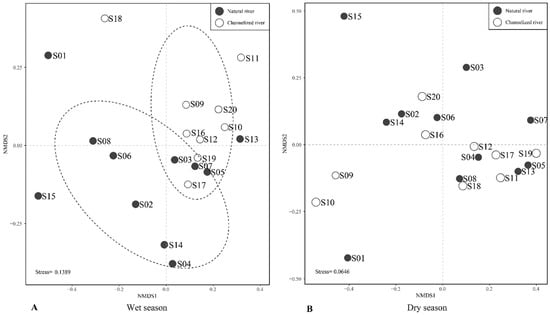
Figure 6.
A multidimensional scaling plot of benthic macroinvertebrate communities in the wet season (A) and dry season (B) in natural and channelized rivers in the Longgang River system.
3.3. Correlations between the Diversity of Benthic Communities and Environmental Parameters
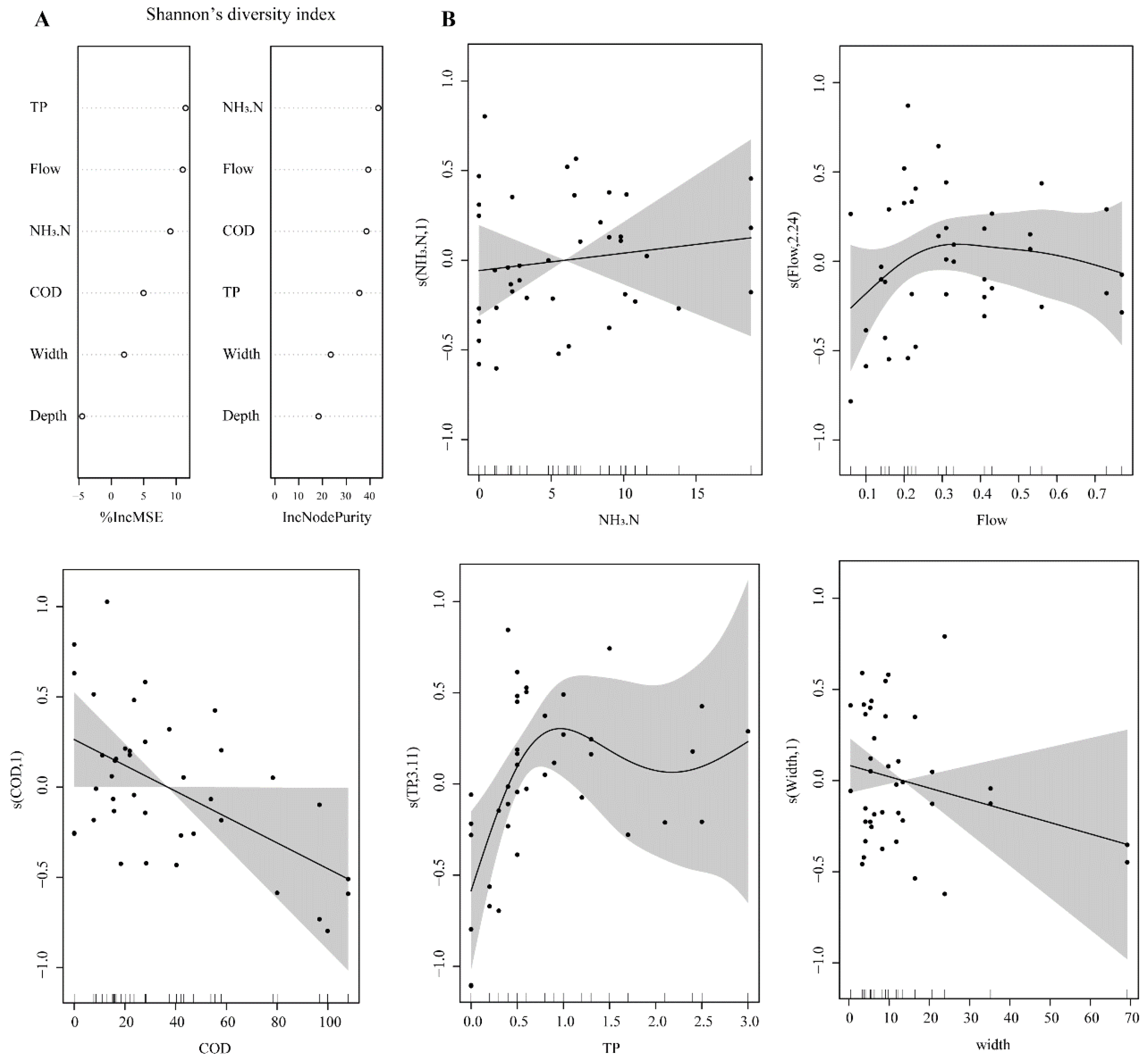
The relationships of Shannon’s diversity index and Simpson’s diversity index with environmental parameters were similar. Shannon’s diversity index was closely related to TP, flow rate, NH3.N, COD, and RW according to the random forest model (Figure 7A). The GAM model revealed that NH3.N was positively correlated with Shannon’s diversity index, and COD and RW were negatively correlated with Shannon’s diversity index (Figure 7B). When the concentration of TP was low (<3 mg/L), it was positively correlated with Shannon’s diversity index. When the flow rate was low (<0.3 m/s), it was positively correlated with Shannon’s diversity index; when the flow rate exceeded approximately 0.3 m/s, Shannon’s diversity index was negatively correlated with the flow rate (Figure 7B).
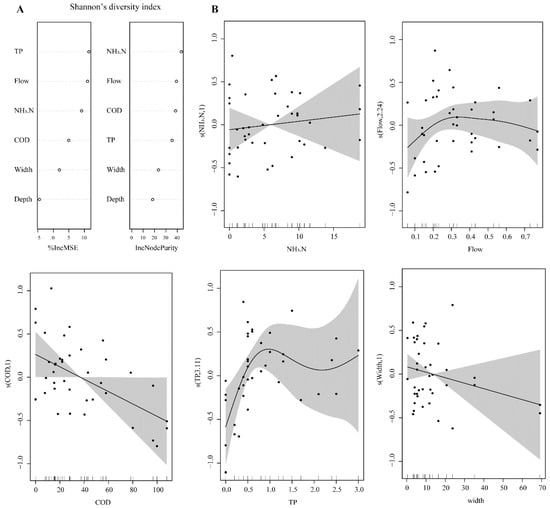
Figure 7.
(A) Relative importance of water environmental parameters in explaining Shannon’s diversity index according to the random forest model. The default random number was 1000. (B) Generalized additive model (GAM) plot of Shannon diversity index probability vs. the important water environmental parameters.
4. Discussion
4.1. The Benthic Community of the Longgang River, a Tropical Urban River
The dominant benthic species in the Longgang River in the wet and dry seasons were C. plumosus and L. hoffmeisteri; insect and oligochaete species were the most dominant components of the benthic community (Figure 2; Table 1). These findings are consistent with the composition of the benthic fauna in urban rivers documented in previous studies. Because of the high level of pollutants derived from human activities, the urban river benthos tends to be dominated by pollutant-tolerant species such as oligochaetes and chironomids [17]. For example, in the highly urbanized Taihu Lake Basin, the abundance of oligochaetes in the benthic community can reach 89.42% [15]. In the Secret Ravine River in California, the abundance of red Chironomus spp. in urban areas can reach 49% [34].
There were no significant differences in the composition of the benthic fauna in the Longgang River among seasons, and the peak values of benthic animals were generally consistent with the peak values of dominant species.
In urban rivers, many pollution-tolerant species without seasonal life histories often occur in these communities, and their high abundance can be maintained for long periods [35]. The composition of benthic communities in undisturbed or less disturbed natural streams often undergoes significant seasonal changes. Most sensitive groups of benthic animals have seasonal life histories, which allow them to avoid unfavorable temperatures, hydrological conditions, and competition, as well as obtain higher quality food resources [35,36,37,38]. Therefore, seasonal variation in benthic community structure in urban rivers is generally less pronounced compared with that in natural rivers [39].
Some studies have also shown that seasonal fluctuations in community density are more pronounced in channelized rivers than in natural rivers, and the peak density of the benthic fauna in urban rivers coincides with the appearance of chironomids, which are dominant species, in winter or spring [40,41]. However, most of these studies have been conducted in temperate zones, and the Longgang River is located in a subtropical region, which experiences less pronounced distinctions between seasons. Although the differences in the amount of rainfall affected the rivers in the wet and dry seasons, the main environmental conditions of the rivers, such as water temperature and food, did not differ greatly between the wet and dry seasons, and this likely explains the lack of substantial differences in the structure of the benthic fauna between seasons; differences between the wet and dry seasons were only observed for a small number of species. Given that the number of dry seasons was lower than the number of wet seasons, some taxa might show sensitivity to river flow.
The effects of urbanization on most river benthic species remain unclear, but the responses of the benthic community might differ depending on the type of river [14]. The abundance of some sensitive species is higher in urban river sections [42]. Thus, the spatial distribution of the benthic fauna in the Longgang River should be examined in future studies.
4.2. Effects of Urban River Morphology on the Benthic Community
Channel morphology had a significant effect on the density of leeches (Figure 3, Table A1), indicating that the channelization of rivers might reduce the density of leeches. Helobdella stagnalis, Glossiphonia lata, and Erpobdella octoculata were detected in natural rivers but not in channelized rivers; Glossiphonia complanata was the only leech species detected in channelized rivers in the dry season. This might be explained by the more stringent habitat requirements of leeches; the low habitat diversity in channelized rivers thus might contribute to the pronounced decrease in the density of leeches in channelized rivers relative to natural rivers.
There were no substantial differences in the abundance of dominant species of benthic animals in different sections of the Longgang River (Table A1). Consequently, differences in benthic biomass in natural and channelized rivers were not pronounced (Figure 4). Many studies have shown that coarse organic matter is low and fine organic matter is high in the exogenous organic debris from urban rivers [17,43,44]. Therefore, the proportion of tear-feeders, filter-feeders, and predators might be significantly lower in channelized rivers compared with natural rivers; by contrast, the proportion of collectors might be significantly higher in channelized rivers compared with natural rivers, and they might even become dominant in channelized rivers [45,46,47]. The aerobic decomposition of organic matter reduces the dissolved oxygen content of urban river water, which negatively affects benthic animals that prefer oxygen-rich environments and benefits oligochaetes, chironomids, leeches, and snails, which are resistant to organic pollution and hypoxia [48,49]. The results of our study are consistent with these findings and demonstrate that the water nutrient conditions of urban rivers might have a larger effect on the biomass of benthic animals in urban rivers compared with river morphology.
Channelization significantly reduced the species richness and species diversity of benthic animals in the Longgang River (Figure 5), and this effect was similar in the wet and dry seasons. This might be explained by the reduction in the heterogeneity of river habitats by hydraulic modification such as channelization, which leads to declines in benthic diversity [50]. The channelization of rivers results in the removal of stones and large woody residues from the original riverbed, which increases the scouring effect of running water on the riverbed, decreases the availability of substrates to which benthic animals can attach, and leads to the extirpation of benthic animals, including migratory species [18,21,51]. After river channelization, the lack of natural channel ebb and flow zones reduces the abundance of other organisms, including aquatic vascular plants, which reduces habitat and food diversity [18,52,53].
Channel morphology can indirectly affect the benthic fauna by affecting the water environment. Compared with channelized rivers, natural rivers have more abundant sediment silt, nutrients such as total phosphorus can be easily acquired, and the ammonia nitrogen content in the water is relatively high; this can mediate reductions in the COD content of water bodies and reduce local water flow [54,55]. The random forest model and GAM model in this study revealed that the water environmental factors most strongly affecting the diversity of benthic animals included total phosphorus, ammonia nitrogen, flow rate, and COD. Total phosphorus reflects the nutrient levels of the water body and can affect the growth of plankton. In general, total phosphorus facilitates the provisioning of food in the water body for benthic animals. The diversity of benthic animals is highest when flow rates are moderate, which is consistent with the intermediate disturbance hypothesis [56,57]. High COD concentrations reduce dissolved oxygen in water, reduce the number of hypoxia-intolerant species, and increase the number of hypoxia-tolerant species, thereby reducing the biodiversity of benthic animals; our findings indicate that ammonia nitrogen is harmful to benthic animals. Animal diversity has a slightly positive effect on benthic diversity, which might indicate that increases in ammonia nitrogen reduce the number of fish, shrimp, crabs, and other organisms, reducing the risk of predation and thus enhancing benthic biodiversity [58].
4.3. Implications for Urban River Management
The proportion of tear-feeders, filter feeders, and predators, such as snails and shellfish, is significantly lower in urban rivers compared with natural rivers, and collectors such as chironomids are dominant in urban rivers [45,59]. Patterns of benthic community structure at all sampling points in the Longgang River system were consistent with these findings. Although we are unable to evaluate the pre-urbanized benthic community, clear differences in benthic diversity were observed between channelized rivers and natural rivers. This suggests that maintaining the natural form of urban rivers that experience high levels of disturbance by humans can enhance the aquatic biodiversity of rivers. During urban construction, changes to the shape of river channels should be minimized to prevent the loss of aquatic biodiversity and river landscape diversity.
The Longgang River runs through Shenzhen, which is located in a subtropical zone. Benthic diversity was lower in channelized rivers than in natural rivers. However, the benthic diversity of the Longgang River, especially where it runs through Shenzhen (a large city), might be particularly low. Our approach provided a general snapshot of the benthic diversity of the Longgang River. Benthic diversity monitoring of various parts of the river system with different morphologies is needed to more comprehensively assess the effect of urbanization on the benthic community. Significant differences in benthic community structure were found between channelized rivers and natural rivers in the wet season; however, no significant differences in benthic community structure were observed between channelized and natural rivers in the dry season. Although variation in climate and temperature is low in tropical regions, differences in the river environment between the dry and wet seasons might alter the effects of factors such as river channel morphology on aquatic organisms. In future studies, benthic organisms should be sampled in both the wet and dry seasons.
5. Conclusions
The effect of river channel morphology on benthic fauna was explored in the Longgang River in Shenzhen, China. Benthic diversity was lower in channelized rivers than in natural rivers, and no differences in the total density and biomass of benthic animals were observed between channelized rivers and natural rivers. The density of leeches was lower in channelized rivers than in natural rivers, and the total number of leeches was lower in channelized rivers than in natural rivers. The effects of river channel morphology on benthic fauna were stronger in the wet season and weaker in the dry season. Our findings indicate that channelization should be minimized to prevent the loss of aquatic biodiversity in tropical urban rivers. Continuous monitoring efforts are needed to clarify seasonal differences in aquatic biomes.
Author Contributions
Conceptualization, W.X.; Data curation, Q.S., L.C., L.W., Y.W., Y.L. and L.X.; Formal analysis, W.X.; Investigation, L.C., L.W., Y.W., Y.L. and L.X.; Methodology, L.C., L.W., Y.W., Y.L. and L.X.; Project administration, W.X.; Writing—original draft, Q.S. and W.X.; Writing—review & editing, Q.S. and W.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shenzhen Science and Technology Program, grant number KCXFZ20201221173007020.
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Shenzhen Ecological and Environmental Monitoring Center (protocol code 20161202, 2016-12-02).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data, models, or code generated or used during the study are available from the corresponding author or the first author by request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Benthic densities of natural and channelized rivers in the Longgang River system in the wet and dry seasons. Mean values ± SE, ind./m2.
Table A1.
Benthic densities of natural and channelized rivers in the Longgang River system in the wet and dry seasons. Mean values ± SE, ind./m2.
| Wet Season | Dry Season | ||||
|---|---|---|---|---|---|
| Natural Area | Channelized Area | Natural Area | Channelized Area | ||
| Clitellata | Glossiphonia complanata | 0.61 ± 0.61 | 0.00 ± 0.00 | 0.61 ± 0.61 | 0.74 ± 0.74 |
| Helobdella stagnalis | 1.21 ± 0.81 | 0.00 ± 0.00 | 1.21 ± 1.21 | 0.00 ± 0.00 | |
| Glossiphonia lata | 1.82 ± 0.94 | 0.00 ± 0.00 | 0.61 ± 0.61 | 0.00 ± 0.00 | |
| Erpobdella octoculata | 4.85 ± 4.23 | 0.00 ± 0.00 | 1.21 ± 0.81 | 0.00 ± 0.00 | |
| Oligochaeta | Rhyacodrilus sinicus | 6.06 ± 3.86 | 2.22 ± 2.22 | 3.03 ± 2.08 | 0.00 ± 0.00 |
| Limnodrilus hoffmeisteri | 387.88 ± 91.38 | 237.78 ± 84.96 | 258.79 ± 83.89 | 154.07 ± 50.97 | |
| Branchiura sowerbyi | 60.00 ± 29.23 | 80.74 ± 38.27 | 41.82 ± 20.60 | 20.74 ± 9.46 | |
| Limnodrilus grandisetosus | 1.21 ± 1.21 | 2.96 ± 2.96 | 1.21 ± 1.21 | 0.00 ± 0.00 | |
| Teneridrilus mastix | 4.85 ± 3.72 | 1.48 ± 1.48 | 1.21 ± 1.21 | 0.74 ± 0.74 | |
| Polychaeta | Nephtys oligobranchia | 1.21 ± 0.81 | 0.74 ± 0.74 | 1.82 ± 0.94 | 2.22 ± 1.57 |
| Gastropoda | Semisulcospira libertina | 1.82 ± 1.82 | 0.74 ± 0.74 | 1.21 ± 1.21 | 4.44 ± 4.44 |
| Pomacea canaliculata | 0.61 ± 0.61 | 0.00 ± 0.00 | 1.22 ± 0.61 | 0.00 ± 0.00 | |
| Parafossarulus eximius | 3.64 ± 3.64 | 8.89 ± 8.89 | 0.00 ± 0.00 | 0.74 ± 0.74 | |
| Bellamya purificata | 0.61 ± 0.61 | 2.22 ± 2.22 | 1.21 ± 0.81 | 0.00 ± 0.00 | |
| Melanoides tuberculata | 13.94 ± 8.74 | 3.70 ± 3.70 | 6.06 ± 5.43 | 1.48 ± 1.48 | |
| Bellamya aeruginosa | 0.61 ± 0.61 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Gyraulus convexiusculus | 0.61 ± 0.61 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Radix swinhoei | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.74 ± 0.74 | |
| Tarebia granifera | 0.61 ± 0.61 | 2.22 ± 1.57 | 0.00 ± 0.00 | 0.74 ± 0.74 | |
| Bivalvia | Corbicula fluminea | 2.42 ± 1.86 | 8.15 ± 8.15 | 0.00 ± 0.00 | 0.74 ± 0.74 |
| Limnoperna fortunei | 2.42 ± 1.86 | 9.63 ± 9.63 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Crustacea | Exopalaemon modestus | 3.03 ± 2.08 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.74 ± 0.74 |
| Palaemonetes sinensis | 0.61 ± 0.61 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Insecta | Orthocladius rivulorum | 33.33 ± 14.13 | 0.00 ± 0.00 | 16.36 ± 11.06 | 0.00 ± 0.00 |
| Propsilocerus akamusi | 41.82 ± 21.98 | 45.93 ± 32.35 | 12.12 ± 12.12 | 0.00 ± 0.00 | |
| Procladius choreus | 22.42 ± 17.76 | 8.89 ± 8.89 | 0.00 ± 0.00 | 6.67 ± 6.67 | |
| Orthetrum sp. | 1.21 ± 1.21 | 0.00 ± 0.00 | 0.61 ± 0.61 | 0.00 ± 0.00 | |
| Pericoma sp. | 0.61 ± 0.61 | 0.00 ± 0.00 | 0.61 ± 0.61 | 0.74 ± 0.74 | |
| Labrogomphus sp. | 2.42 ± 1.86 | 0.74 ± 0.74 | 0.61 ± 0.61 | 2.96 ± 1.61 | |
| Microchironomus tener | 3.64 ± 3.64 | 24.44 ± 24.44 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Dicrotendipes tritomus | 8.49 ± 7.84 | 80.00 ± 63.75 | 70.91 ± 70.91 | 38.52 ± 27.37 | |
| Baetis sp. | 7.27 ± 4.88 | 0.74 ± 0.74 | 0.61 ± 0.61 | 1.48 ± 0.98 | |
| Copera sp. | 7.88 ± 4.48 | 0.74 ± 0.74 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Chaoborus sp. | 13.33 ± 13.33 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| Chironomus plumosus | 465.45 ± 280.53 | 506.67 ± 173.40 | 917.58 ± 277.09 | 991.11 ± 319.84 | |
| Tanypus chinensis | 3.03 ± 2.08 | 13.33 ± 13.33 | 14.55 ± 14.55 | 0.00 ± 0.00 | |
| Glyptotendipes barbipes | 45.45 ± 24.60 | 0.00 ± 0.00 | 17.58 ± 14.05 | 0.00 ± 0.00 | |
| Total | 1156.98 ± 262.04 | 1042.96 ± 260.71 | 1372.73 ± 345.53 | 1229.63 ± 321.10 | |
References
- Chen, M.; Zhang, H.; Liu, W.; Zhang, W. The global pattern of urbanization and economic growth: Evidence from the last three decades. PLoS ONE 2014, 9, e103799. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; He, Q.; Liu, X.; Zhuang, Y.; Hong, S. Global urbanization research from 1991 to 2009: A systematic research review. Landsc. Urban Plan. 2012, 104, 299–309. [Google Scholar] [CrossRef]
- Zhang, X.Q. The trends, promises and challenges of urbanisation in the world. Habitat. Int. 2016, 54, 241–252. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, J.; Xu, Z.; Wang, X.; Meersmans, J. Ecosystem services supply and demand response to urbanization: A case study of the Pearl River Delta, China. Ecosyst. Serv. 2021, 49, 101274. [Google Scholar] [CrossRef]
- Booth, D.B.; Roy, A.H.; Smith, B.; Capps, K.A. Global perspectives on the urban stream syndrome. Freshw. Sci. 2016, 35, 412–420. [Google Scholar] [CrossRef]
- Chen, F.; Yuan, W.; Wang, L.; Ding, J.; Li, C.; Wang, B. Consideration of river governance based on the concept of urban spatial resilience. IOP Conf. Ser. Earth Environ. Sci. 2022, 983, 12087. [Google Scholar] [CrossRef]
- Fan, X.; Cui, B.; Zhao, H.; Zhang, Z. The Changes of Wetland Network Pattern Associated with Water Quality in the Pearl River Delta, China. CLEAN–Soil Air Water 2012, 40, 1064–1075. [Google Scholar] [CrossRef]
- Douglas, I.; Kobold, M.; Lawson, N.; Pasche, E.; White, I. Characterisation of Urban Streams and Urban Flooding; CRC Press: Boca Raton, FL, USA, 2007; pp. 41–70. ISBN 0429224346. [Google Scholar]
- Luo, Z.; Shao, Q.; Zuo, Q.; Cui, Y. Impact of land use and urbanization on river water quality and ecology in a dam dominated basin. J. Hydrol. 2020, 584, 124655. [Google Scholar] [CrossRef]
- Qin, H.; Su, Q.; Khu, S.; Tang, N. Water quality changes during rapid urbanization in the Shenzhen River Catchment: An integrated view of socio-economic and infrastructure development. Sustainability 2014, 6, 7433–7451. [Google Scholar] [CrossRef]
- Almeida, C.A.; Quintar, S.; González, P.; Mallea, M.A. Influence of urbanization and tourist activities on the water quality of the Potrero de los Funes River (San Luis–Argentina). Environ. Monit. Assess. 2007, 133, 459–465. [Google Scholar] [CrossRef]
- Luo, K.; Hu, X.; He, Q.; Wu, Z.; Cheng, H.; Hu, Z.; Mazumder, A. Impacts of rapid urbanization on the water quality and macroinvertebrate communities of streams: A case study in Liangjiang New Area, China. Sci. Total Environ. 2018, 621, 1601–1614. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, R.I.; Weber, K.; Padowski, J.; Flörke, M.; Schneider, C.; Green, P.A.; Gleeson, T.; Eckman, S.; Lehner, B.; Balk, D.; et al. Water on an urban planet: Urbanization and the reach of urban water infrastructure. Glob. Environ. Chang. 2014, 27, 96–105. [Google Scholar] [CrossRef]
- Roy, A.H.; Rosemond, A.D.; Paul, M.J.; Leigh, D.S.; Wallace, J.B. Stream macroinvertebrate response to catchment urbanisation (Georgia, U.S.A.). Freshw. Biol. 2003, 48, 329–346. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Cheng, L.; Cai, Y.; Yin, H.; Gao, J.; Gao, Y. Macroinvertebrate assemblages in streams and rivers of a highly developed region (Lake Taihu Basin, China). Aquat. Biol. 2014, 23, 15–28. [Google Scholar] [CrossRef]
- Purcell, A.H.; Bressler, D.W.; Paul, M.J.; Barbour, M.T.; Rankin, E.T.; Carter, J.L.; Resh, V.H. Assessment tools for urban catchments: Developing biological indicators based on benthic macroinvertebrates 1. JAWRA J. Am. Water Resour. Assoc. 2009, 45, 306–319. [Google Scholar] [CrossRef]
- Walsh, C.J.; Roy, A.H.; Feminella, J.W.; Cottingham, P.D.; Groffman, P.M.; Morgan, R.P. The urban stream syndrome: Current knowledge and the search for a cure. J. N. Am. Benthol. Soc. 2005, 24, 706–723. [Google Scholar] [CrossRef]
- Ramírez, A.; Engman, A.; Rosas, K.G.; Perez-Reyes, O.; Martinó-Cardona, D.M. Urban impacts on tropical island streams: Some key aspects influencing ecosystem response. Urban Ecosyst. 2012, 15, 315–325. [Google Scholar] [CrossRef]
- Anderson, M.G.; Steinberg, M.; Tontar, C. A New Urban Revival: Floodplain Acquisition in Lawrence, Massachusetts. In Times Are Changing—Flood Mitigation Technology: Proceedings of the 22nd Annual Conference, 18–22 May 1998; Natural Hazards Research and Applications Information Center, University of Colorado: Boulder, CO, USA, 1998; p. 201. [Google Scholar]
- Nakano, D.; Nagayama, S.; Kawaguchi, Y.; Nakamura, F. River restoration for macroinvertebrate communities in lowland rivers: Insights from restorations of the Shibetsu River, north Japan. Landsc. Ecol. Eng. 2008, 4, 63–68. [Google Scholar] [CrossRef]
- Garcia, X.; Schnauder, I.; Pusch, M.T. Complex hydromorphology of meanders can support benthic invertebrate diversity in rivers. Hydrobiologia 2012, 685, 49–68. [Google Scholar] [CrossRef]
- Zika, U.; Peter, A. The introduction of woody debris into a channelized stream: Effect on trout populations and habitat. River Res. Appl. 2002, 18, 355–366. [Google Scholar] [CrossRef]
- Wang, S.; Wang, T.; Lin, H.; Stewart, S.D.; Cheng, G.; Li, W.; Yang, F.; Huang, W.; Chen, Z.; Xie, S. Impacts of environmental factors on the food web structure, energy flows, and system attributes along a subtropical urban river in southern China. Sci. Total Environ. 2021, 794, 148673. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Wang, H.; Xia, B.; Jiang, C.; Wu, W.; Li, Z.; Li, S.; Su, H.; Bai, Z.; Xu, S. Pollution Characterization and Comprehensive Water Quality Assessment of Rain-source River: A Case Study of the Longgang River in Shenzhen. Huan Jing Ke Xue = Huanjing Kexue 2022, 43, 782–794. [Google Scholar]
- Yang, Y.; Hu, M.; Lu, W.; Xue, L.; Lin, X.; Liu, E. Occurrence, distribution, and risk assessment of PPCPs in water and sediments of Longgang River in Shenzhen City, south China. Desalin. Water Treat. 2020, 189, 196–206. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X. Integrated river basin management in rapidly urbanizing areas: A case of Shenzhen, China. Front. Environ. Sci. Eng. China 2011, 5, 243–254. [Google Scholar] [CrossRef]
- Qian, J.L.; Fu, L.M. Using persulfate oxidation method for the determination of total nitrogen and total phosphorus in water. Environ. Sci. 1987, 8, 81–84. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Gomez-Rubio, V. Generalized Additive Models: An Introduction with R (2nd Edition). J. Stat. Softw. 2018, 86, 1–5. [Google Scholar] [CrossRef]
- Wood, S.N.; Pya, N.; Säfken, B. Smoothing parameter and model selection for general smooth models. J. Am. Stat. Assoc. 2016, 111, 1548–1563. [Google Scholar] [CrossRef]
- Line, D.E. Effect of development on water quality for seven streams in North Carolina. Environ. Monit. Assess. 2013, 185, 6277–6289. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, D.M.; Hawkins, C.P. Land use and the structure of western US stream invertebrate assemblages: Predictive models and ecological traits. J. N. Am. Benthol. Soc. 2008, 27, 986–999. [Google Scholar] [CrossRef]
- Chinnayakanahalli, K.J.; Hawkins, C.P.; Tarboton, D.G.; Hill, R.A. Natural flow regime, temperature and the composition and richness of invertebrate assemblages in streams of the western United States. Freshw. Biol. 2011, 56, 1248–1265. [Google Scholar] [CrossRef]
- Murphy, J.F.; Giller, P.S. Seasonal dynamics of macroinvertebrate assemblages in the benthos and associated with detritus packs in two low-order streams with different riparian vegetation. Freshw. Biol. 2000, 43, 617–631. [Google Scholar] [CrossRef]
- Johnson, R.C.; Carreiro, M.M.; Jin, H.; Jack, J.D. Within-year temporal variation and life-cycle seasonality affect stream macroinvertebrate community structure and biotic metrics. Ecol. Indic. 2012, 13, 206–214. [Google Scholar] [CrossRef]
- Helms, B.S.; Schoonover, J.E.; Feminella, J.W. Seasonal variability of landuse impacts on macroinvertebrate assemblages in streams of western Georgia, USA. J. N. Am. Benthol. Soc. 2009, 28, 991–1006. [Google Scholar] [CrossRef]
- Ortiz, J.D.; Puig, M.A. Point source effects on density, biomass and diversity of benthic macroinvertebrates in a Mediterranean stream. River Res. Appl. 2007, 23, 155–170. [Google Scholar] [CrossRef]
- Shieh, S.; Kondratieff, B.C.; Ward, J.V. Longitudinal changes in benthic organic matter and macroinvertebrates in a polluted Colorado plains stream. Hydrobiologia 1999, 411, 191–209. [Google Scholar] [CrossRef]
- Angradi, T.R.; Bolgrien, D.W.; Jicha, T.M.; Moffett, M.F. Macroinvertebrate assemblage response to urbanization in three mid-continent USA great rivers. Fund. Appl. Limnol. 2010, 176, 183–198. [Google Scholar] [CrossRef]
- Miserendino, M.L.; Masi, C.I. The effects of land use on environmental features and functional organization of macroinvertebrate communities in Patagonian low order streams. Ecol. Indic. 2010, 10, 311–319. [Google Scholar] [CrossRef]
- Gadawski, P.; Riss, H.W.; Płóciennik, M.; Meyer, E.I. City Channel Chironomids-Benthic Diversity in Urban Conditions. River Res. Appl. 2016, 32, 1978–1988. [Google Scholar] [CrossRef]
- Stepenuck, K.F.; Crunkilton, R.L.; Wang, L. Impacts of urban landuse on macroinvertebrate communities in southeastern Wisconsin streams 1. JAWRA J. Am. Water Resour. Assoc. 2002, 38, 1041–1051. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.D.; Lu, J. Urban River Pollution Control and Remediation. Procedia Environ. Sci. 2012, 13, 1856–1862. [Google Scholar] [CrossRef]
- Li, F.; Bae, M.; Kwon, Y.; Chung, N.; Hwang, S.; Park, S.; Park, H.; Kong, D.S.; Park, Y. Ecological exergy as an indicator of land-use impacts on functional guilds in river ecosystems. Ecol. Model. 2013, 252, 53–62. [Google Scholar] [CrossRef]
- Brönmark, C.; Hansson, L. The Biology of Lakes and Ponds; Oxford University Press: Oxford, UK, 2017; ISBN 0191022543. [Google Scholar]
- Usseglio Polatera, P.; Beisel, J.N. Longitudinal changes in macroinvertebrate assemblages in the Meuse River: Anthropogenic effects versus natural change. River Res. Appl. 2002, 18, 197–211. [Google Scholar] [CrossRef]
- Habersack, H.; Hein, T.; Stanica, A.; Liska, I.; Mair, R.; Jäger, E.; Hauer, C.; Bradley, C. Challenges of river basin management: Current status of, and prospects for, the River Danube from a river engineering perspective. Sci. Total Environ. 2016, 543, 828–845. [Google Scholar] [CrossRef]
- Nakano, D.; Nagayama, S.; Kawaguchi, Y.; Nakamura, F. Significance of the stable foundations provided and created by large wood for benthic fauna in the Shibetsu River, Japan. Ecol. Eng. 2018, 120, 249–259. [Google Scholar] [CrossRef]
- Whitcraft, C.R.; Levin, L.A. Regulation of benthic algal and animal communities by salt marsh plants: Impact of shading. Ecology 2007, 88, 904–917. [Google Scholar] [CrossRef]
- Giller, P.S.; Giller, P. The Biology of Streams and Rivers; Oxford University Press: Oxford, UK, 1998; ISBN 0198549776. [Google Scholar]
- Al-Shami, S.A.; Rawi, C.S.M.; Ahmad, A.H.; Hamid, S.A.; Nor, S.A.M. Influence of agricultural, industrial, and anthropogenic stresses on the distribution and diversity of macroinvertebrates in Juru River Basin, Penang, Malaysia. Ecotoxicol. Environ. Saf. 2011, 74, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, J.; Shi, W.; Wang, M.; Chen, K.; Wang, L. Priority pollutants in water and sediments of a river for control basing on benthic macroinvertebrate community structure. Water 2019, 11, 1267. [Google Scholar] [CrossRef]
- Townsend, C.R.; Scarsbrook, M.R.; Dolédec, S. The intermediate disturbance hypothesis, refugia, and biodiversity in streams. Limnol. Oceanogr. 1997, 42, 938–949. [Google Scholar] [CrossRef]
- Eötvös, C.B.; Lövei, G.L.; Magura, T. Predation pressure on sentinel insect prey along a riverside urbanization gradient in Hungary. Insects 2020, 11, 97. [Google Scholar] [CrossRef]
- Peterson, C.H.; Summerson, H.C.; Thomson, E.; Lenihan, H.S.; Grabowski, J.; Manning, L.; Micheli, F.; Johnson, G. Synthesis of linkages between benthic and fish communities as a key to protecting essential fish habitat. Bull. Mar. Sci. 2000, 66, 759–774. [Google Scholar]
- Wallace, J.B.; Webster, J.R. The role of macroinvertebrates in stream ecosystem function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).