Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lipid Extraction Conditions
2.3. Analyses
2.4. Statistics
3. Results and Discussion
3.1. Optimum Conditions for Lipid Extraction
3.1.1. Sample-to-Solvent Ratio
3.1.2. Extraction Time
3.1.3. Repetition Number of Extractions
3.2. Compositions of Lipid Extracts
3.3. Antioxidant Properties and Applications
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ratana-arporn, P.; Chirapart, A. Nutritional evaluation of tropical green seaweeds Caulerpa lentillifera and Ulva reticulate. Nat. Sci. 2006, 40, 75–83. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, M.N.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Ueng, J.P.; Tsai, G.J. Proximate composition, total phenolic content, and antioxidant activity of seagrape (Caulerpa lentillifera). J. Food Sci. 2011, 76, C950–C958. [Google Scholar] [CrossRef]
- Sarini, A.W.; Nor’Aishah, H.; Mohd Zaini, N. Determination of antioxidant activity for seven types of macroalgae. In Proceedings of the 5th International Conference on Food Engineering and Biotechnology, IPCBEE, Penang, Malaysia, 12–14 March 2014; Volume 65, pp. 51–55. [Google Scholar]
- Sharma, B.R.; Rhyu, D.Y. Anti-diabetic effects of Caulerpa lentillifera: Stimulation of insulin secretion in pancreatic β-cells and enhancement of glucose uptake in adipocytes. Asian Pac. J. Trop. Biomed. 2014, 4, 575–580. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Rhyu, D.Y. Caulerpa lentillifera extract ameliorates insulin resistance and regulates glucose metabolism in C57BL/KsJ-db/db mice via PI3K/AKT signaling pathway in myocytes. J. Transl. Med. 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Sabirin, F.; Kazi, J.A.; Ibrahim, I.; Rashit, M.M.A. Screening of seaweeds potential against oral infections. J. Appl. Sci. Res. 2015, 11, 1–6. [Google Scholar]
- West, E.J.; West, R.J. Growth and survival of the invasive alga, Caulerpa taxifolia, in different salinities and temperatures: Implications for coastal lake management. Hydrobiologia 2007, 577, 87–94. [Google Scholar] [CrossRef]
- Ukabi, S.; Dubinsky, Z.; Steinberger, Y.; Israel, A. Temperature and irradiance effects on growth and photosynthesis of Caulerpa (Chlorophyta) species from the eastern Mediterranean. Aquat. Bot. 2013, 104, 106–110. [Google Scholar] [CrossRef]
- Gao, X.; Choi, H.G.; Park, S.K.; Sun, Z.M.; Nam, K.W. Assessment of optimal growth conditions for cultivation of the edible Caulerpa okamurae (Caulerpales, Chlorophyta) from Korea. J Appl. Phycol. 2019, 31, 1855–1862. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Srinorasing, T.; Chirasuwan, N.; Tamtin, M.; Bunnag, B. The potential of polysaccharide extracts from Caulerpa lentillifera waste. Int. J. Biol. Macromol. 2020, 161, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Freitas, L.C.; Barbosa, J.R.; da Costa, A.L.C.; Bezerra, F.W.F.; Pinto, R.H.H.; de Carvalho Junior, R.N. From waste to sustainable industry: How can agro-industrial wastes help in the development of new products? Resour. Conserv. Recycl. 2021, 169, 105466. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Ferri, M.; Vannini, M.; Ehrnell, M.; Eliasson, L.; Xanthakis, E.; Monari, S.; Tassoni, A. From winery waste to bioactive compounds and new polymeric biocomposites: A contribution to the circular economy concept. J. Adv. Res. 2020, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Munir, M.; Kheirkhah, H.; Baroutian, S.; Quek, S.Y.; Young, B.R. Subcritical water extraction of bioactive compounds from waste onion skin. J. Clean Prod. 2018, 183, 487–494. [Google Scholar] [CrossRef]
- Assefa, S.T.; Yang, E.Y.; Chae, S.Y.; Song, M.; Lee, J.; Cho, M.C.; Jang, S. Alpha glucosidase inhibitory activities of plants with focus on common vegetables. Plants 2020, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Xiancui, L.; Rongli, N.; Xiao, F.; Lijun, H.; Lixin, Z. Macroalgae as a source of alpha-glucosidase inhibitors. Chin. J. Oceanol. Limnol. 2005, 23, 354–356. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Zhang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-glucosidase inhibitors from plants: A natural approach to treat diabetes. Pharmacogn. Rev. 2011, 5, 19–29. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Nguyen, T.N.; Nguyen, X.H.; Huynh, N.N.T.; Min, S.B. Screening of α-glucosidase inhibitory activity of Vietnamese medicinal plants: Isolation of active principles from Oroxylum indicum. Nat. Prod. Sci. 2012, 18, 47–51. [Google Scholar]
- Telagari, M.; Hullatti, K. In-vitro α-amylase and α-glucosidase inhibitory activity of Adiantumcaudatum Linn. and Celosia argentea Linn. extracts and fractions. Indian J. Pharmacol. 2015, 47, 425–429. [Google Scholar] [PubMed]
- Yen, F.S.; Wei, J.C.C.; Lin, M.C.; Hsu, C.C.; Hwu, C.M. Long-term outcomes of adding alpha-glucosidase inhibitors in insulin-treated patients with type 2 diabetes. BMC Endocr. Disord. 2021, 21, 25. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Singh, B.; Arora, R.; Arora, S. In vitro evaluation of the α-glucosidase inhibitory potential of methanolic extracts of traditionally used antidiabetic plants. BMC Complement. Altern. Med. 2019, 19, 74. [Google Scholar] [CrossRef]
- Sato, N.; Murata, N. Membrane lipid. Meth. Enzymol. 1988, 167, 251–259. [Google Scholar]
- Lapage, G.; Roy, C.C. Improved recovery of fatty acid through direct transesterification without prior extraction of purification. J. Lipid Res. 1984, 25, 1391–1396. [Google Scholar] [CrossRef]
- Othman, R.; Amin, N.A.; Sani, M.S.A.; Fadzillah, N.A.; Jamaludin, M.A. Carotenoid and chlorophyll profiles in five species of Malaysian seaweed as potential halal active pharmaceutical ingredient (API). Int. J. Adv. Sci. Eng. Inf. Technol. 2018, 8, 1610–1616. [Google Scholar]
- Wu, X.J.; Hansen, C. Antioxidant capacity, phenolic content, and polysaccharide content of Lentinus edodes grown in whey permeate-based submerged culture. J. Food Sci. 2008, 73, M1–M8. [Google Scholar] [CrossRef] [PubMed]
- Pallab, K.; Tapan, K.B.; Tapas, K.P.; Ramen, K. Estimation of total flavonoids content (TFC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum linn. J. Drug Deliv. Ther. 2013, 3, 33–37. [Google Scholar]
- Li, X.; Zhou, A.; Han, Y. Anti-oxidation and anti-microorganism activities of purification polysaccharide from Lygodium japonicum in vitro. Carbohydr. Polym. 2006, 66, 34–42. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Zaidul, I.S.M.; Ghafoor, K.; Sahena, F.; Hakim, M.A.; Rafii, M.Y.; Abir, H.M.; Bostanudin, M.F.; Perumal, V.; Khatib, A. In vitro antioxidant and, α-glucosidase inhibitory activities and comprehensive metabolite profiling of methanol extract and its fractions from Clinacanthus nutans. BMC Complement. Altern. Med. 2017, 17, 181–191. [Google Scholar] [CrossRef]
- Li, Z.; Wang, B.; Zhang, Q.; Qu, Y.; Xu, H.; Li, G. Preparation and antioxidant property of extract and semipurified fractions of Caulerpa racemose. J. Appl. Phycol. 2012, 24, 1527–1536. [Google Scholar] [CrossRef]
- Chirasuwan, N.; Chaiklahan, R.; Kittakoop, P.; Chanasattru, W.; Ruengjitchatchawalya, M.; Tanticharoen, M.; Bunnag, B. Anti HSV–1 activity of sulphoquinovosyl diacylglycerol isolated from Spirulina platensis. Sci. Asia 2009, 35, 137–141. [Google Scholar] [CrossRef]
- Blažina, M.; Iveša, L.; Najdek, M. Caulerpa racemosa: Adaptive varieties studied by fatty acid composition (Northern Adriatic Sea, Vrsar, Croatia). Eur. J. Phycol. 2009, 44, 183–189. [Google Scholar] [CrossRef]
- Yap, W.F.; Tay, V.; Tan, S.H.; Yow, Y.Y.; Chew, J. Decoding antioxidant and antibacterial potentials of Malaysian green seaweeds: Caulerpa racemosa and Caulerpa lentillifera. Antibiotics 2019, 8, 152. [Google Scholar] [CrossRef]
- Osuna-Ruiz, I.; López-Saiz, C.M.; Burgos-Hernández, A.; Velázquez, C.; Nieves-Soto, M.; Hurtado-Oliva, M.A. Antioxidant, antimutagenic and antiproliferative activities in selected seaweed species from Sinaloa, Mexico. Pharm. Biol. 2016, 54, 2196–2210. [Google Scholar] [CrossRef]
- Tanna, B.; Choudhary, B.; Mishra, A. Metabolite profiling, antioxidant, scavenging and anti-proliferative activities of selected tropical green seaweeds reveal the nutraceutical potential of Caulerpa spp. Algal Res. 2018, 36, 96–105. [Google Scholar] [CrossRef]
- Sharma, B.R.; Kim, H.J.; Kim, M.S.; Park, C.M.; Rhyu, D.Y. Caulerpa okmurae extract inhibits adipogenesis in 3T3–L1 adipocytes and prevents high–fat diet–induced obesity in C57BL/6 mice. Nutr. Res. 2017, 47, 44–52. [Google Scholar] [CrossRef]
- Rahman, S.M.; Neaz, S.; Alam, M.M.; Nur, J. Hypolipidemic activity of ethanolic extract of Caulerpa recemosa. BIRDEM Med. J. 2019, 9, 197–201. [Google Scholar] [CrossRef]

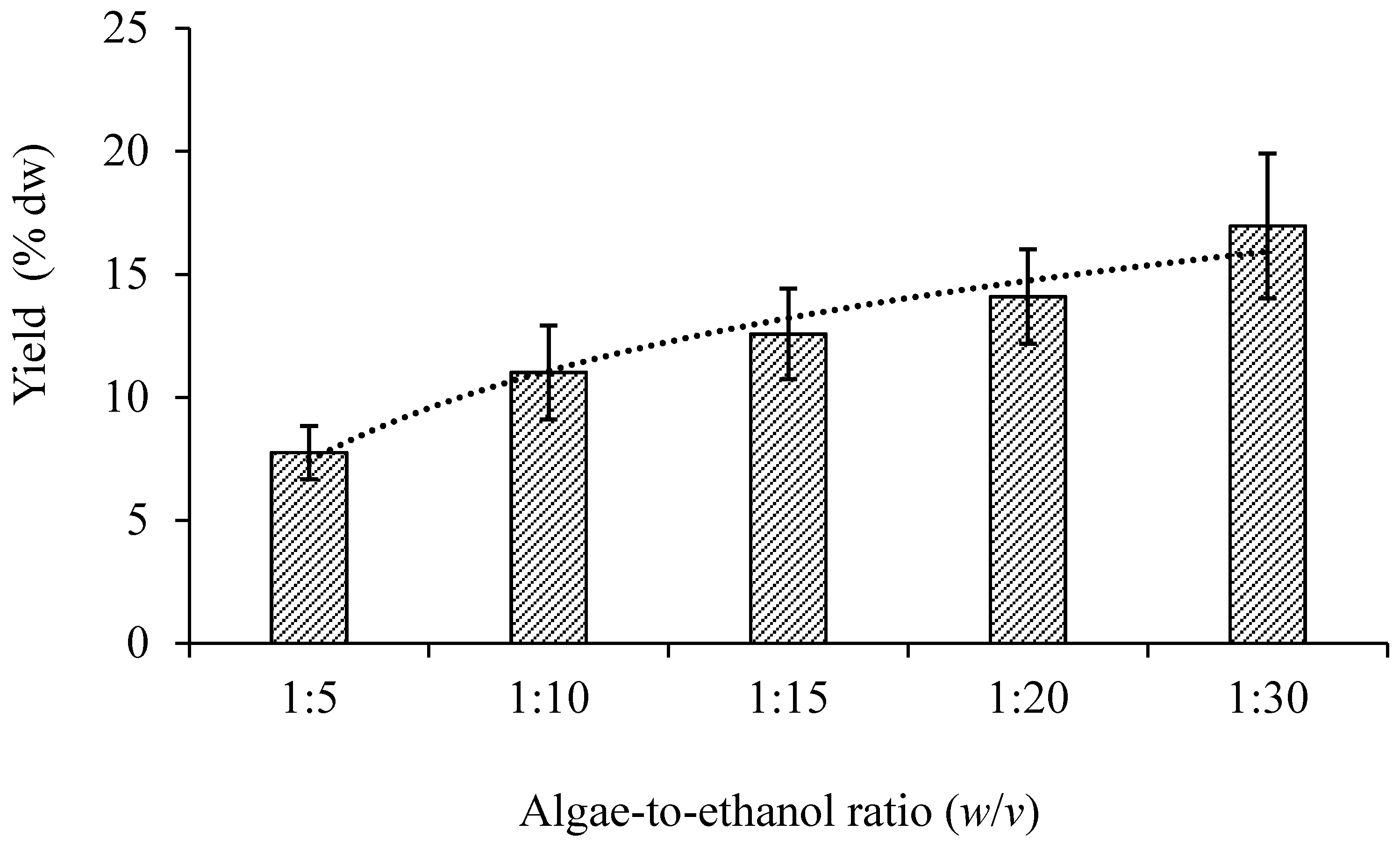
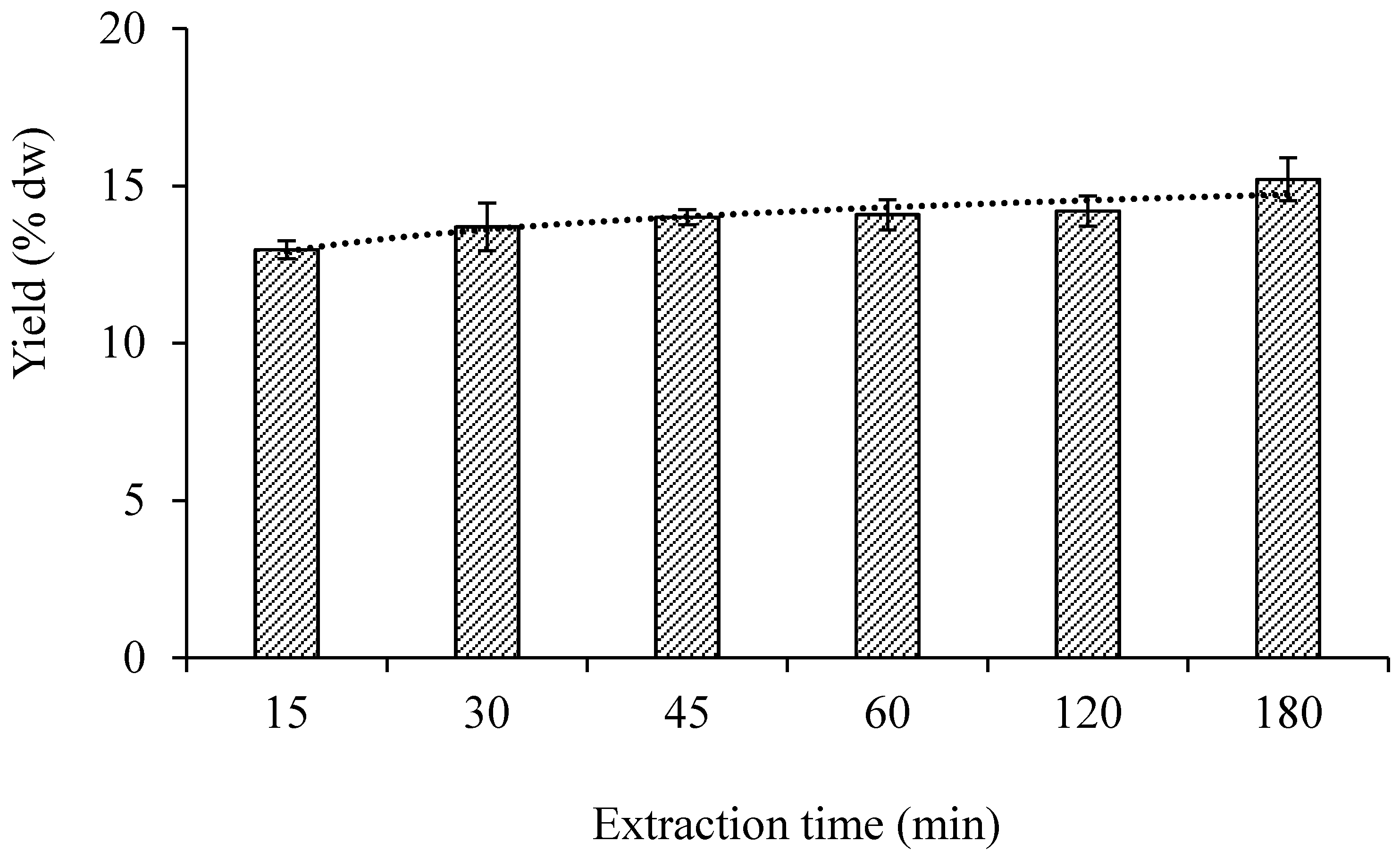
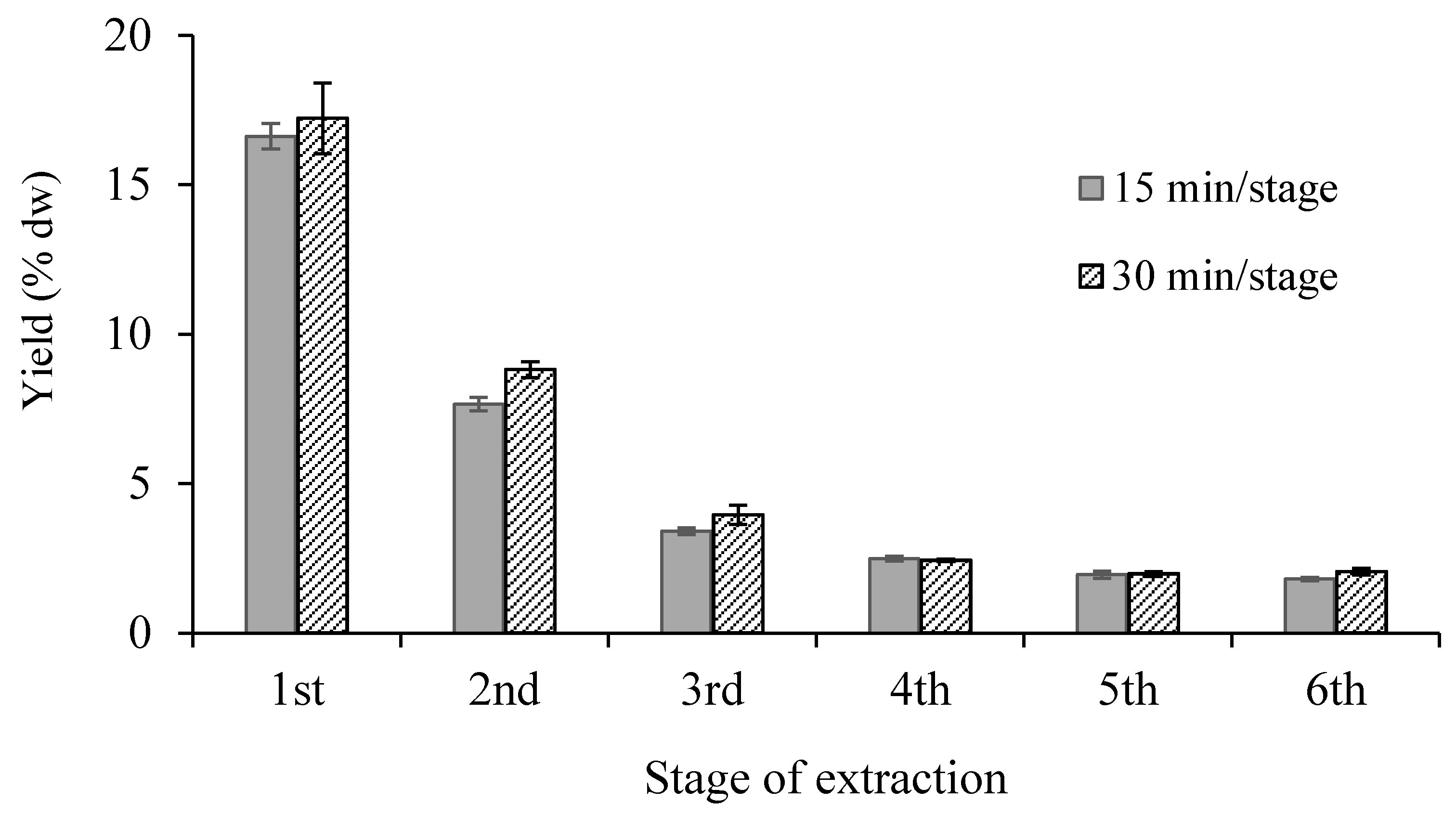
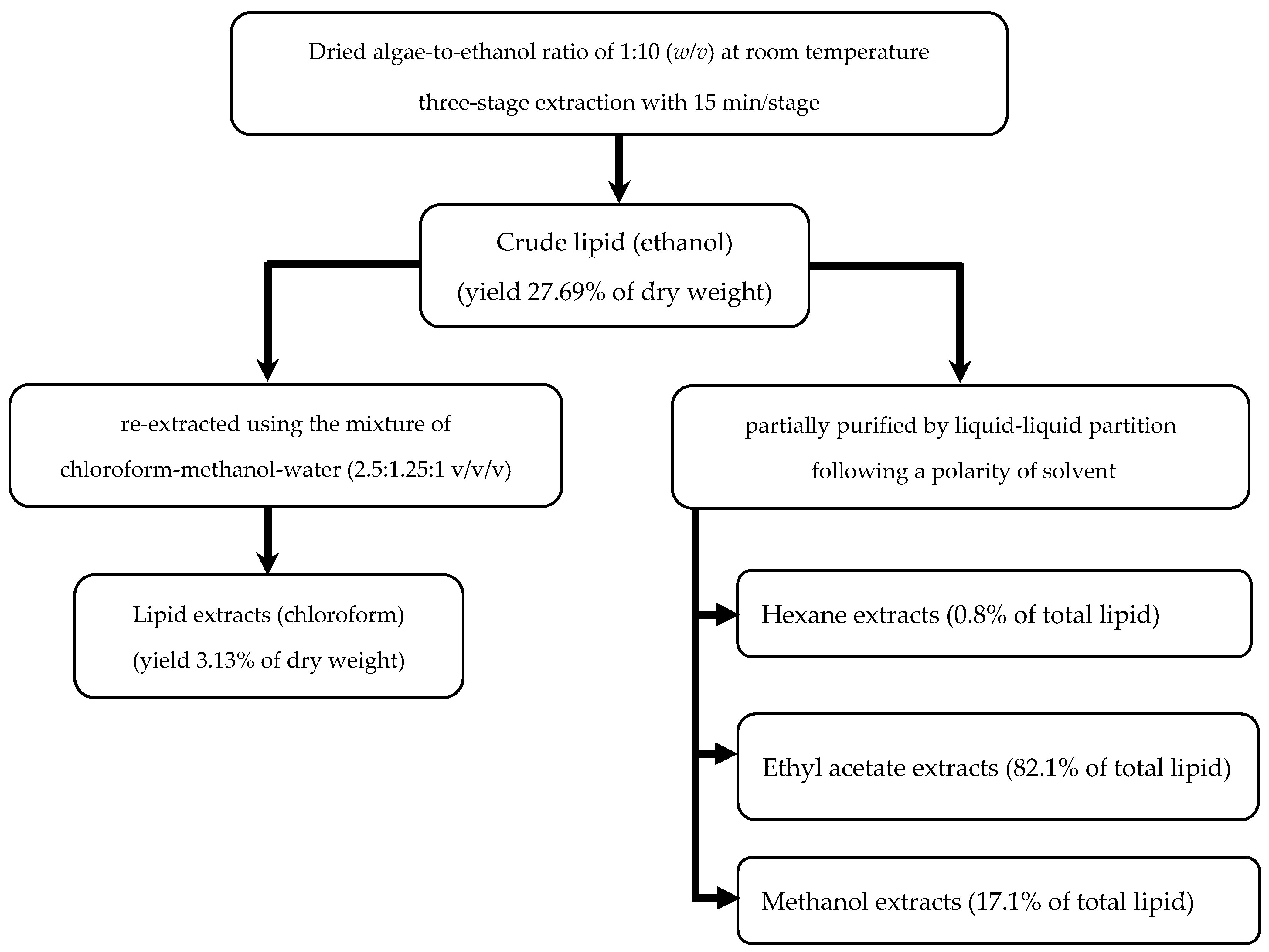
| Number of Stage | Total Time of Extraction (min) | Total Volume of Ethanol (mL) * | Total Yield (% dw) | |||
|---|---|---|---|---|---|---|
| 15 | 30 | 15 | 30 | 15 | 30 | |
| 1 | 15 | 30 | 150 | 150 | 16.62 | 17.23 |
| 2 | 30 | 60 | 300 | 300 | 24.28 | 26.04 |
| 3 | 45 | 90 | 450 | 450 | 27.69 | 30.00 |
| 4 | 60 | 600 | 30.18 | |||
| Compositions | Value |
|---|---|
| Chlorophyll a (µg/mg of extract) | 1.77 ± 0.25 |
| Chlorophyll b (µg/mg of extract) | 0.91 ± 0.09 |
| Carotenoids (µg/mg of extract) | 0.70 ± 0.09 |
| Total fatty acid (TFA; % weight of lipid extract) | 58.60 ± 4.95 |
| Fatty acid compositions (% mol.) | |
| Myristic acid (14:0) | 4.89 ± 0.36 |
| Palmitic acid (16:0) | 74.48 ± 3.24 |
| Palmitoleic acid (16:1n7) | 6.82 ± 0.38 |
| Stearic acid (18:0) | 2.50 ± 0.28 |
| Vaccenic acid (18:1n7) | 3.03 ± 0.28 |
| Oleic acid (18:1n9) | 2.92 ± 0.39 |
| Linoleic acid (18:2) | 2.69 ± 0.38 |
| Linoleic acid (18:3) | 0.93 ± 0.13 |
| Arachidonic acid (20:4) | 0.95 ± 0.09 |
| Eicosapentaenoic acid (20:5) | 1.01 ± 0.34 |
| Saturated fatty acids (SFA) | 82.56 ± 2.37 |
| Monounsaturated fatty acids (MFA) | 12.68 ± 0.44 |
| Polyunsaturated fatty acids (PFA) | 3.09 ± 0.69 |
| Characteristic | Crude Lipid | Purified Lipid |
|---|---|---|
| Total phenolic (mg GAE/g sample) | 2.07 ± 0.34 | 17.46 ± 2.24 |
| Total flavonoids (mg QE/g sample) | 5.40 ± 0.76 | 55.48 ± 7.78 |
| IC50 ABTS (mg/mL) | 1.67 ± 0.09 | 0.21 ± 0.06 |
| IC50 DPPH (mg/mL) | 3.55 ± 0.53 | 0.43 ± 0.11 |
| IC50 α-glucosidase (mg/mL) | 8.97 ± 0.19 | Not determined |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinorasing, T.; Chirasuwan, N.; Bunnag, B.; Chaiklahan, R. Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy. Sustainability 2021, 13, 4491. https://doi.org/10.3390/su13084491
Srinorasing T, Chirasuwan N, Bunnag B, Chaiklahan R. Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy. Sustainability. 2021; 13(8):4491. https://doi.org/10.3390/su13084491
Chicago/Turabian StyleSrinorasing, Thanyarat, Nattayaporn Chirasuwan, Boosya Bunnag, and Ratana Chaiklahan. 2021. "Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy" Sustainability 13, no. 8: 4491. https://doi.org/10.3390/su13084491
APA StyleSrinorasing, T., Chirasuwan, N., Bunnag, B., & Chaiklahan, R. (2021). Lipid Extracts from Caulerpa lentillifera Waste: An Alternative Product in a Circular Economy. Sustainability, 13(8), 4491. https://doi.org/10.3390/su13084491





