Combined Pretreatment by Ultrasound and Struvite Precipitation of Raw Substrates: A Strategy to Overcome C/N Ratio Unbalance in Nitrogen-Rich Anaerobic Co-Digestion Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Struvite Precipitation Optimization Batch Assays
2.1.1. Substrates Pretreatment with Ultrasound
2.1.2. Struvite Chemical Precipitation
2.1.3. Statistical Analysis
2.2. Biomethanation Assays
2.2.1. Substrates and Inoculum
2.2.2. Ultrasound Pretreatment
2.2.3. Struvite Precipitation
2.2.4. Reactor Start-Up and Experimental Procedure
2.3. Analytical Procedure
- FAN = free ammonia nitrogen concentration (mg L−1);
- TAN = total ammonia nitrogen concentration (mg L−1)
- T = temperature (K)
- pH = hydrogen ionic potential
3. Discussion
3.1. Struvite Precipitation Optimization Batch Assays
3.1.1. Kinetics of the Struvite Precipitation Process
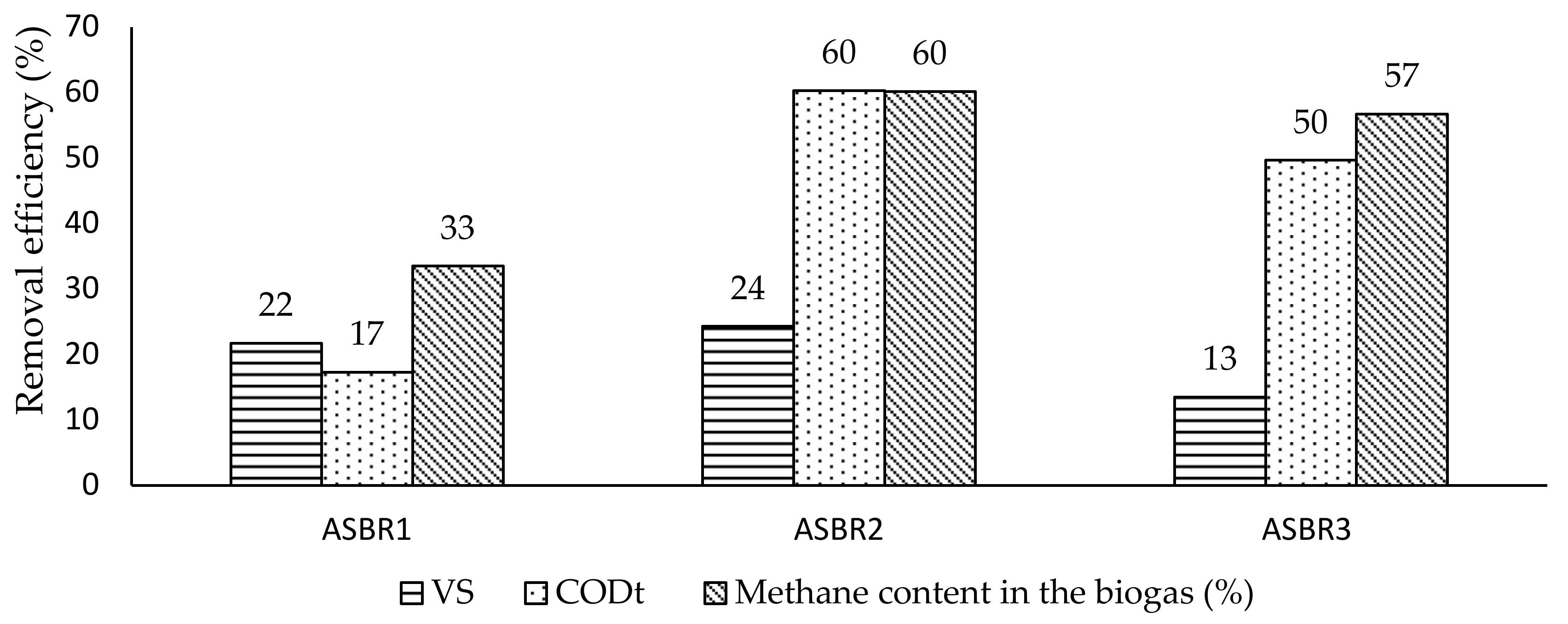
3.2. Biomethanization Assays
4. Conclusions
- the reaction time of 40 min seems to be suitable for crystallization and precipitation of struvite using a mixture of urban WWTP sludge and cattle slurry liquid fraction as substrate;
- to fulfill the stoichiometric requirements of magnesium, high amounts of marine salt are necessary, resulting in the presence of insoluble crystals, which may limit nutrient recovery processes;
- the ammonium nitrogen and orthophosphate removal efficiencies increased when the NH4+: Mg2+ stoichiometric ratio decreased from 1:1.5 to 1:3. reaching 29% and 50%, respectively;
- the application of ultrasound to the mixture of substrates, prior to the struvite precipitation process, resulted in an increase in ammonium nitrogen and orthophosphate removal efficiencies, reaching 43% and 92%, respectively, as a result of the increase in the solubilization rate of organic compounds;
- the treatment of the substrates with ultrasound (either with a EI of 109 kJ L−1 (SE of 4270 kJ TS−1) or EI of 218 kJ L−1 (SE of 8180 kJ TS−1)) resulted in an increase of 73–82% in methane content in the biogas (57–60%) and a reduction in the HRT of 25–28%;
- the treatment of the substrates with ultrasound prior to struvite precipitation enhanced ammonium nitrogen removal and, consequently, increased methane yield;
- the reduction of the energy input from 218 kJ L−1 (SE of 8180 kJ TS−1) to 109 kJ L−1 (SE of 4270 kJ TS−1) resulted in a decrease of 44% in methane yield (from 48.5 mLCH4 gVS−1 to 32.2 mLCH4 gVS−1), despite de methane content in biogas remained similar (60%);
- the combination of ultrasound (SE of 8180 kJ kgTS−1) and struvite precipitation (NH4:Mg2+ of 1:3) as pretreatment may be a feasible option to increase the efficiency of ACoD of SwS and CSLF in a context of nitrogen-rich systems, in terms of COD removal and methane production;
- the FAN/TAN ratio seems to be correlated to the methane production;
- the TAN values lower than 311 mg L−1 seems to be unfavorable to the methanogenesis;
- the reduction of the energy input from 218 kJ L−1 (SE of 8180 kJ TS−1) to 109 kJ L−1 (SE of 4270 kJ TS−1) resulted in a semi-quantitative estimative of, approximately 230 g L−1 and 100 g L−1 of struvite, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yenigun, O.; Demirel, B. Ammonia inhibition in anaerobic digestion: A review. Process Biochem. 2013, 48, 901–911. [Google Scholar] [CrossRef]
- Zhang, C.; Su, H.; Baeyens, J.; Tan, T. Reviewing the anaerobic digestion of food waste for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 383–392. [Google Scholar] [CrossRef]
- Sawayama, S.; Tada, C.; Tsukahara, K.; Yagishita, T. Effect of ammonium adition on methanogenic community in a fluidized bed anaerobic digestion. J. Biosci. Bioeng. 2004, 97, 65–70. [Google Scholar] [CrossRef]
- Lauterbpcl, B.; Ortner, M.; Haider, R.; Fuchs, W. Counteracting ammonia inhibition in anaerobic digestion by removal with a hollow fiber membrane contactor. Water Resour. 2012, 46, 4861–4869. [Google Scholar] [CrossRef]
- Parameswaran, P.; Rittmann, B. Feasibility of anaerobic co-digestion of pig waste and paper sludge. Bioresour. Technol. 2012, 124, 163–168. [Google Scholar] [CrossRef]
- Astals, S.; Peces, M.; Batstone, D.; Jensen, P.; Tait, S. Characterizing and modelling free ammonia and ammonium inhibition inanaerobic systems. Water Res. 2018, 143, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.; Creamer, K. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Luostarinen, S.; Luste, S.; Sillanpaa, M. Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge rom a meat processing plant. Bioresour. Technol. 2009, 100, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Wickham, R.; Nghiem, L. Synergistic effect from anaerobic co-digestion of sewage sludge andorganic wastes. Int. Biodeterior. Biodegrad. 2017, 116, 191–197. [Google Scholar] [CrossRef]
- Elser, J.; Bennett, E. Phosphorus cycle: A broken biogeochemical cycle. Nature 2011, 478, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Stratful, I.; Scrimshaw, M.; Lester, J. Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res. 2001, 35, 4191–4199. [Google Scholar] [CrossRef]
- Romero-Guiza, M.; Astals, S.; Mata-Alvarez, J.; Chimenos, J. Feasibility of coupling anaerobic digestion and struvite precipitation in the same reactor: Evaluation of different magnesium sources. Chem. Eng. J. 2015, 270, 542–549. [Google Scholar] [CrossRef]
- Munir, M.; Li, B.; Mardon, I.; Young, B.; Baroutian, S. Integrating wet oxidation and struvit precipitation for sewage sludge treatment and phosphorus recovery. J. Clean. Prod. 2019, 232, 1043–1052. [Google Scholar] [CrossRef]
- Yoshino, M.; Yao, M.; Tsuno, H.; Somiya, I. Removal and recovery of phosphate and ammonium as struvite from supernatant in anaerobic digestion. Water Sci. Technol. 2003, 48, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Chimenos, J.; Fernandez, A.; Villalba, G.; Segarra, M.; Urrutiocoechea, A.; Artaza, B.; Espiell, F. Removal of ammonium and phosphates from wastewater resulting from the process of cochineal extraction using MgO-containing by-product. Water Res. 2003, 37, 1601–1607. [Google Scholar] [CrossRef]
- Nelson, N.; Mikkelsen, R.; Hesterberg, D. Struvite precipitation in anaerobic swine lagoon liquid: Effect of pH and Mg:P ratio and determination of rate constant. Bioresour. Technol. 2003, 89, 229–236. [Google Scholar] [CrossRef]
- Pullammanappallil, P.; Mohan, G. Recovery of Nutrients from Water and Wastewater by Precipitation as Struvite. U.S. Patent US20160185633A1, 30 June 2016. [Google Scholar]
- Ruiz-Hermando, M.; Simyn, F.; Labanda, J.; Llorens, J. Effect of ultrasound, thermal and alkali treatments on the rheological profile and water distribution of waste activated sludge. Chem. Eng. J. 2014, 255, 14–22. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Kang, X. Short chain fatty acids accumulation and microbial community succession during ultrasonic-Pretreated sludge anaerobic fermentation process: Effect of alkaline adjustment. Int. Biodeterior. Biodegrad. J. 2014, 94, 128–133. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.; Appels, L.; Dewil, R. Ultrasonic Treatment of Waste Sludge: A Review on Mechanisms and Applications. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1200–1288. [Google Scholar] [CrossRef]
- Kim, D.; Lee, J. Ultrasonic sludge disintegration for enhanced methane production. Bioprecess Biosyst. Eng. 2012, 35, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Deepanraj, B.; Sivasubramanian, V.; Jayaraj, S. Effect of substrate pretreatment on biogas production through anaerobic digestion offood waste. Int. J. Hydrogen Energy 2017, 42, 26522–26528. [Google Scholar] [CrossRef]
- Uysal, A.; Yilmazel, Y.; Demirer, G. The determination of fertilizer quality of the formed struvite from effluentof a sewage sludge anaerobic digester. J. Hazard. Mater. 2010, 181, 248–254. [Google Scholar] [CrossRef]
- Ometto, F.; Quiroga, G.; Pšenička, P.; Whitton, R.; Jefferson, B.; Villa, R. Impacts of microalgae pretreatments for improved anaerobic digestion: Thermal treatment, thermal hydrolysis, ultrasound and enzymatic hydrolysis. Water Res. 2014, 65, 350–361. [Google Scholar] [CrossRef]
- APHA. Standard Methods for Examination of Water and Wastewater, 18th ed.; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Ogunwande, G.; Osunade, J.; Adekalu, K.; Ogunjimi, L. Nitrogen loss in chicken litter compost as affected by carbon to nitrogen ratio and turning frequency. Bioresour. Technol. 2008, 99, 7495–7503. [Google Scholar] [CrossRef] [PubMed]
- Watteau, F.; Geneviève, V. Characterization of organic matter microstructure dynamics during co-composting of sewage sludge, barks and green waste. Bioresour. Technol. 2011, 102, 9313–9317. [Google Scholar] [CrossRef] [PubMed]
- Koster, I. Characteristics of the pH-influenced adaptation of methanogenic sludge to ammonium toxicity. J. Chem. Technol. Biotechnol. 1986, 36, 445–455. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Battistoni, P.; De Angelos, A.; Pavan, P.; Prisciandaro, M.; Cecchi, F. Phosphorus removal from a real anaerobic supernatant by struvite crystallization. Water Res. 2001, 35, 2167–2178. [Google Scholar] [CrossRef]
- Pullmmanappallil, P.; Chynoweth, D.; Gerasimos, L.; Svoronos, S. Stable performance of anaerobic digestion in the presence of a high concentration of propionic acid. Bioresour. Technol. 2001, 78, 165–169. [Google Scholar] [CrossRef]
- Idelovitch, E.; Michail, M. Nitrogen removal by free ammonia stripping from high pH ponds. J. Water Pollut. Control Fed. 1981, 53, 1391–1401. Available online: https://www.jstor.org/stable/25041502 (accessed on 1 September 2020).
- Kuba, T.; Smolders, G.; Van Loosdrecht, M.; Heijnen, J. Biological phosphorus removal from wastewater by anaerobic-anoxic sequencing batch reactor. Water Sci. Technol. 1993, 27, 241–252. [Google Scholar] [CrossRef]
- Pinatha, Y.; Polprasert, C.; Englande, A.J., Jr. Product and cost perspectives of phosphorus recovery from human urine using solid waste ash and sea salt addition—A case of Thailand. Sci. Total Environ. 2020, 713, 136514. [Google Scholar] [CrossRef]
- Bougrier, C.; Carrere, H.; Delgenes, J. Solubilization of waste-activated sludge by ultrasonic treatment. Chem. Eng. J. 2005, 106, 163–169. [Google Scholar] [CrossRef]
- Salsabil, M.; Prorot, A.; Casellas, M.; Dagot, C. Pretreatment of activated sludge: Effect of sonication on aerobic and anaerobic digestibility. Chem. Eng. J. 2009, 148, 327–335. [Google Scholar] [CrossRef]
- Hidalgo, D.; Corona, F.; Martín-Marroquín, J.; del Álamo, J.; Aguado, A. Resource recovery from anaerobic digestate: Struvite crystallisation versus ammonia stripping. Desalination Water Treat. 2013, 57, 2626–2632. [Google Scholar] [CrossRef]
- Jia, G.; Zhang, H.; Krampe, J.; Muster, T. Applying a chemical equilibrium model for optimizing struvite precipitation for ammonium recovery from anaerobic digester effluent. J. Clean. Prod. 2017, 147, 297–305. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, Z.; Liu, J.; Tang, B.; Ye, J.; Zhang, L. Optimization of struvite crystallization to recover nutrients from raw swine wastewater. Desalination Water Treat. 2015, 56, 3106–3112. [Google Scholar] [CrossRef]
- Divya, D.; Gopinath, L.R.; Christy, P. A review on current aspects and diverse prospects for enhancing biogas production in sustainable means. Renew. Sustain. Energy Rev. 2015, 42, 590–699. [Google Scholar] [CrossRef]
- Mao, C.; Zhang, T.; Wang, X.; Feng, Y.; Ren, G.; Tang, G. Process performance and methane production optimizing of anaerobic co-digestion of swine manure and corn straw. Sci. Rep. 2017, 7, 9379. [Google Scholar] [CrossRef] [PubMed]
- Nutongkaew, T.; Prasertsan, P.; Sompong, O.; Chanthong, S.; Suyotha, W. Improved Methane Production Using Lignocellulolytic Enzymes from Trichoderma koningiopsis TM3 through Co-digestion of Palm Oil Mill Effluent and Oil Palm Trunk Residues. Waste Biomass Valorization 2020, 11, 5123–5136. [Google Scholar] [CrossRef]
- Marti, N.; Pastor, L.; Bouzas, A.; Ferrer, J.; Seco, A. Phosphorus recovery by struvite crystallization in WWTPs: Influence of the sludge treatment line operation. Water Res. 2010, 44, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016, 107, 143–156. [Google Scholar] [CrossRef]
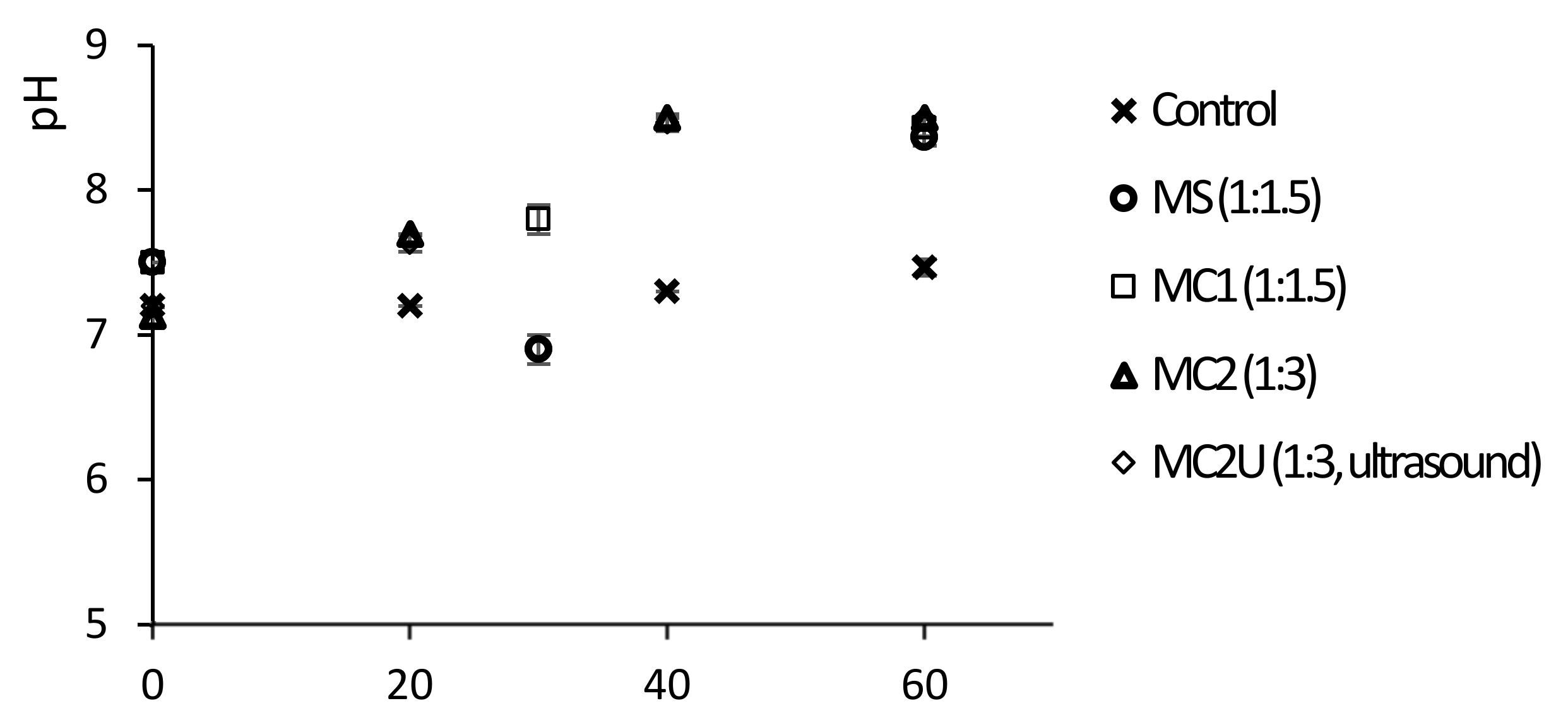
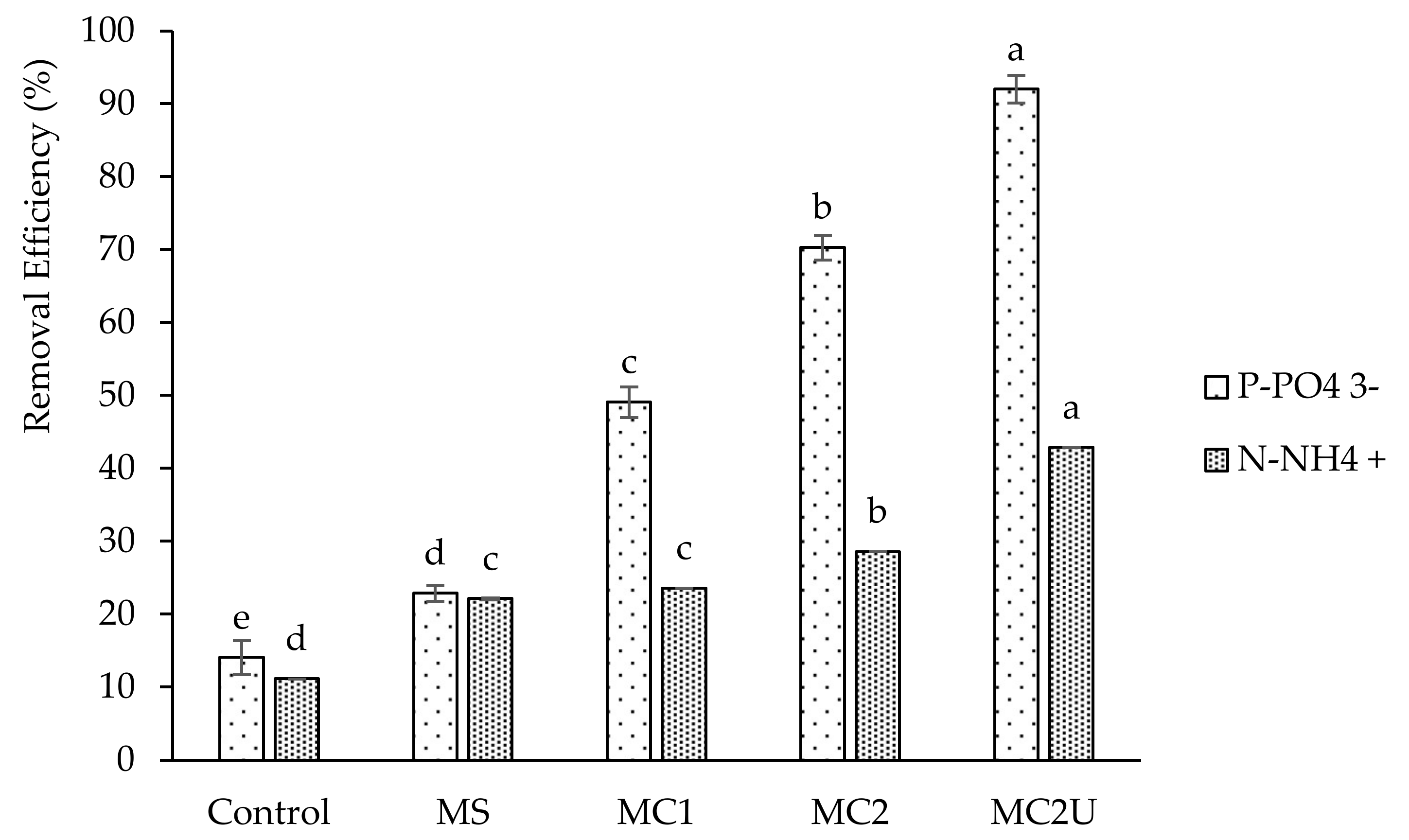
| Assays Description | |
|---|---|
| Control | - |
| MS | marine salt as source of magnesium using a NH4+:Mg2+ stoichiometric proportion of 1:1.5 |
| MC1 | MgCl2 as source of magnesium using a NH4+:Mg2+ stoichiometric proportion of 1:1.5 |
| MC2 | MgCl2 as source of magnesium using a NH4+:Mg2+ stoichiometric proportion of 1:3 |
| MC2U | ultrasound pretreatment with an energy input (EI) of 218 kJ L−1 followed by struvite precipitation using MgCl2 as magnesium source and a NH4+:Mg2+ stoichiometric proportion of 1:3 |
| Reactor | Pretreatment Conditions |
|---|---|
| ASBR1 | ACoD without pretreatment of substrates |
| ASBR2 | ACoD with ultrasound and struvite pretreatment: Energy Input of 218 kJ L−1 and NH4+:Mg2+ of 1:3) |
| ASBR3 | ACoD with ultrasound and struvite pretreatment: Energy Input of 109 kJ L−1 and NH4+:Mg2+ of 1:3) |
| TS (g L−1) | VS (g L−1) | tCOD (g L−1) | sCOD (g L−1) | PO43−-P (g L−1) | NH4+-N (g L−1) | C/N | pH |
|---|---|---|---|---|---|---|---|
| 20 ± 1 | 16 ± 2 | 16.3 ± 0.25 | 2.04 ± 0.45 | 0.37 ± 0.01 | 0.67 ± 0.00 | 4 ± 0.1 | 7.5 ± 0.1 |
| TAN (mg L−1) | Orthophosphate (mg L−1) | ||
|---|---|---|---|
| Control | Ti | 630 ± 10 | 480 ± 16 |
| Tf | 560 ± 12 | 406 ± 11 | |
| MS | Ti | 630 ± 6 | 433 ± 9 |
| Tas | 491 ± 13 | 334 ± 22 | |
| MC1 | Ti | 636 ± 11 | 467 ± 5 |
| Tas | 486 ± 8 | 318 ± 19 | |
| MC2 | Ti | 490 ± 13 | 348 ± 11 |
| Tas | 350±11 | 175 ± 4 | |
| MC2U | Ti | 710 ± 98 | 389 ± 3 |
| Tau | 980 ± 68 | 719 ± 3 | |
| Tas | 560 ± 23 | 58 ± 7 |
| Parameter | SwS | CSLF | Inoculum |
|---|---|---|---|
| TS (g L−1) | 9.7–24.0 | 13.8–26.1 | 14.0–17.2 |
| VS (g L−1) | 7.3–20.1 | 8.0–19 | 10.4–13.6 |
| TOC (g L−1) | 4.0–11.2 | 10.8 | 5.8–7.5 |
| tCOD (g L−1) | 24.8–47.7 | 8.8–32.0 | 9.6–21.9 |
| sCOD (g L−1) | 2.6–6.6 | 2.6–9.1 | 0.4–1.0 |
| TKN (g L−1) | 3.2–3.9 | 3.5–3.6 | 3.0–5.0 |
| TAN (mg L−1) | 952 | 840–1317 | 854–1513 |
| FAN (mg L−1) | 0.04–13.29 | 7–28 | 18.74–33.18 |
| PO43−-P (mg L−1) | 224–728 | 205–634 | 854–1513 |
| pH | 5.2–7.1 | 6.9 | 7.3 |
| Biomethanation Experiment | CODs (mg L−1) | TAN (mg L−1) | FAN (mg L−1) | PO43− (mg L−1) | ST (g L−1) | VS (g L−1) | SS (g L−1) | |
|---|---|---|---|---|---|---|---|---|
| ASBR1: Control | 1280.0 ± 122 | 311.6 ± 5 | 8.6 ± 0.1 | 481 ± 5.4 | 16.0 ± 1.4 | 9.9 ± 0.1 | 13.2 ± 4.5 | |
| ASBR2: US + SP | TBU | 2240.0 ± 80 | 700.3 ± 99 | 2.5 ± 0.3 | 589 ± 3.6 | 26.6 ± 1.1 | 19.9 ± 1.0 | 9.6 ± 0.6 |
| TAU | 3920.0 ± 113 | 735.4 ± 49 | 2.6 ± 0.2 | 611 ± 32.2 | 29.3 ± 1.6 | 21.9 ± 1.4 | 9.3 ± 0.1 | |
| TAS | 4480.0 ± 93 | 560.3 ± 99 | - | 557 ± 2.9 | 38.4 ± 0.1 | 30.8 ± 0.1 | 8.8 ± 0.4 | |
| ASBR3: US/2 + SP | TBU | 2240.0 ± 86 | 595.3 ± 49 | - | 252.0 ± 3.6 | 25.5 ± 0.9 | 18.6 ± 0.5 | 25.8 ± 0.1 |
| TAU | 3205.0 ± 99 | 630.3 ± 99 | - | 360 ± 10.9 | 21.2 ± 0.1 | 15.6 ± 0.1 | 20.3 ± 0.8 | |
| TAS | - | 490.2 ± 89 | - | 209 ± 2.2 | 39.3 ± 0.1 | 24.7 ± 0.1 | 18.1 ± 0.1 |
| Parameter | ASBR1 | ASBR2 | ASBR3 |
|---|---|---|---|
| pH | 7.4 ± 0.1 | 7.8 ± 0.1 | 7.7 ± 0.1 |
| TS (g L−1) | 16.0 ± 1 | 34.1 ± 0.8 | 31.2 ± 0.5 |
| VS (g L−1) | 10.0 ± 0.1 | 22.3 ± 0.8 | 19.0 ± 0.5 |
| OM (%) | 62.4 ± 0.1 | 65.3 ± 0.1 | 62.0 ± 0.1 |
| tCOD (mg L−1) | 24,128 ± 269 | 38,495 ± 2102 | 32,013 ± 336 |
| sCOD (mg L−1) | 1280.0 ± 0.1 | 1600.0 ± 101 | 1440.0 ± 226 |
| TKN (mg L−1) | 1225.6 ± 0.1 | 1435.7 ± 42 | 1295.6 ± 36 |
| TAN (mg L−1) | 311.7 ± 5 | 420.2 ± 26 | 490.2 ± 89 |
| FAN (mg L−1) | 8.6 ± 0.1 | 27.8 ± 0.1 | 26.1 ± 5 |
| FAN/TAN (%) | 2.7 ± 0.1 | 6.6 ± 0.1 | 5.3 ± 0.1 |
| PO43−-P (mg L−1) | 481.0 ± 5 | 526.2 ± 0.1 | 203.3 ± 2.9 |
| C/N | 5.0 ± 0.1 | 8.6 ± 0.1 | 8.1 ± 0.1 |
| OLR (kgCOD m−3 d −1) | 0.2 ± 0.1 | 7.0 ± 0.1 | 0.6 ± 0.1 |
| OLR (gVS L−1 d−1) | 0.15 ± 0.1 | 0.47 ± 0.1 | 0.38 ± 0.1 |
| Methane content in biogas (%) | 33 | 60 | 57 |
| Maximum observed biodegradation rate rate (g L−1 d−1) | 0.17 | 0.53 | 0.64 |
| Maximum observed methane production rate (mL g−1 d−1) | 77 | 643 | 743 |
| YCH4/VS (mLCH4 gVS−1) | 26.5 | 48.5 | 32.2 |
| HRT (d) | 66 | 47 | 49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coura, R.D.; Rodrigues, A.C.; Alonso, J.M.; Ferraz, A.I.; Brito, L.M.; Abrantes, J.C.C.; Brito, A.G. Combined Pretreatment by Ultrasound and Struvite Precipitation of Raw Substrates: A Strategy to Overcome C/N Ratio Unbalance in Nitrogen-Rich Anaerobic Co-Digestion Systems. Sustainability 2021, 13, 2175. https://doi.org/10.3390/su13042175
Coura RD, Rodrigues AC, Alonso JM, Ferraz AI, Brito LM, Abrantes JCC, Brito AG. Combined Pretreatment by Ultrasound and Struvite Precipitation of Raw Substrates: A Strategy to Overcome C/N Ratio Unbalance in Nitrogen-Rich Anaerobic Co-Digestion Systems. Sustainability. 2021; 13(4):2175. https://doi.org/10.3390/su13042175
Chicago/Turabian StyleCoura, Renata D’arc, Ana Cristina Rodrigues, Joaquim Mamede Alonso, Ana Isabel Ferraz, Luis Miguel Brito, João Carlos Castro Abrantes, and António Guerreiro Brito. 2021. "Combined Pretreatment by Ultrasound and Struvite Precipitation of Raw Substrates: A Strategy to Overcome C/N Ratio Unbalance in Nitrogen-Rich Anaerobic Co-Digestion Systems" Sustainability 13, no. 4: 2175. https://doi.org/10.3390/su13042175
APA StyleCoura, R. D., Rodrigues, A. C., Alonso, J. M., Ferraz, A. I., Brito, L. M., Abrantes, J. C. C., & Brito, A. G. (2021). Combined Pretreatment by Ultrasound and Struvite Precipitation of Raw Substrates: A Strategy to Overcome C/N Ratio Unbalance in Nitrogen-Rich Anaerobic Co-Digestion Systems. Sustainability, 13(4), 2175. https://doi.org/10.3390/su13042175










