Abstract
The steel industry represents about 7% of the world’s anthropogenic CO2 emissions due to the high use of fossil fuels. The CO2-lean direct reduction of iron ore with hydrogen is considered to offer a high potential to reduce CO2 emissions, and this direct reduction of Fe2O3 powder is investigated in this research. The H2 reduction reaction kinetics and fluidization characteristics of fine and cohesive Fe2O3 particles were examined in a vibrated fluidized bed reactor. A smooth bubbling fluidization was achieved. An increase in external force due to vibration slightly increased the pressure drop. The minimum fluidization velocity was nearly independent of the operating temperature. The yield of the direct H2-driven reduction was examined and found to exceed 90%, with a maximum of 98% under the vibration of ~47 Hz with an amplitude of 0.6 mm, and operating temperatures close to 500 °C. Towards the future of direct steel ore reduction, cheap and “green” hydrogen sources need to be developed. H2 can be formed through various techniques with the catalytic decomposition of NH3 (and CH4), methanol and ethanol offering an important potential towards production cost, yield and environmental CO2 emission reductions.
1. Introduction
1.1. Background Information
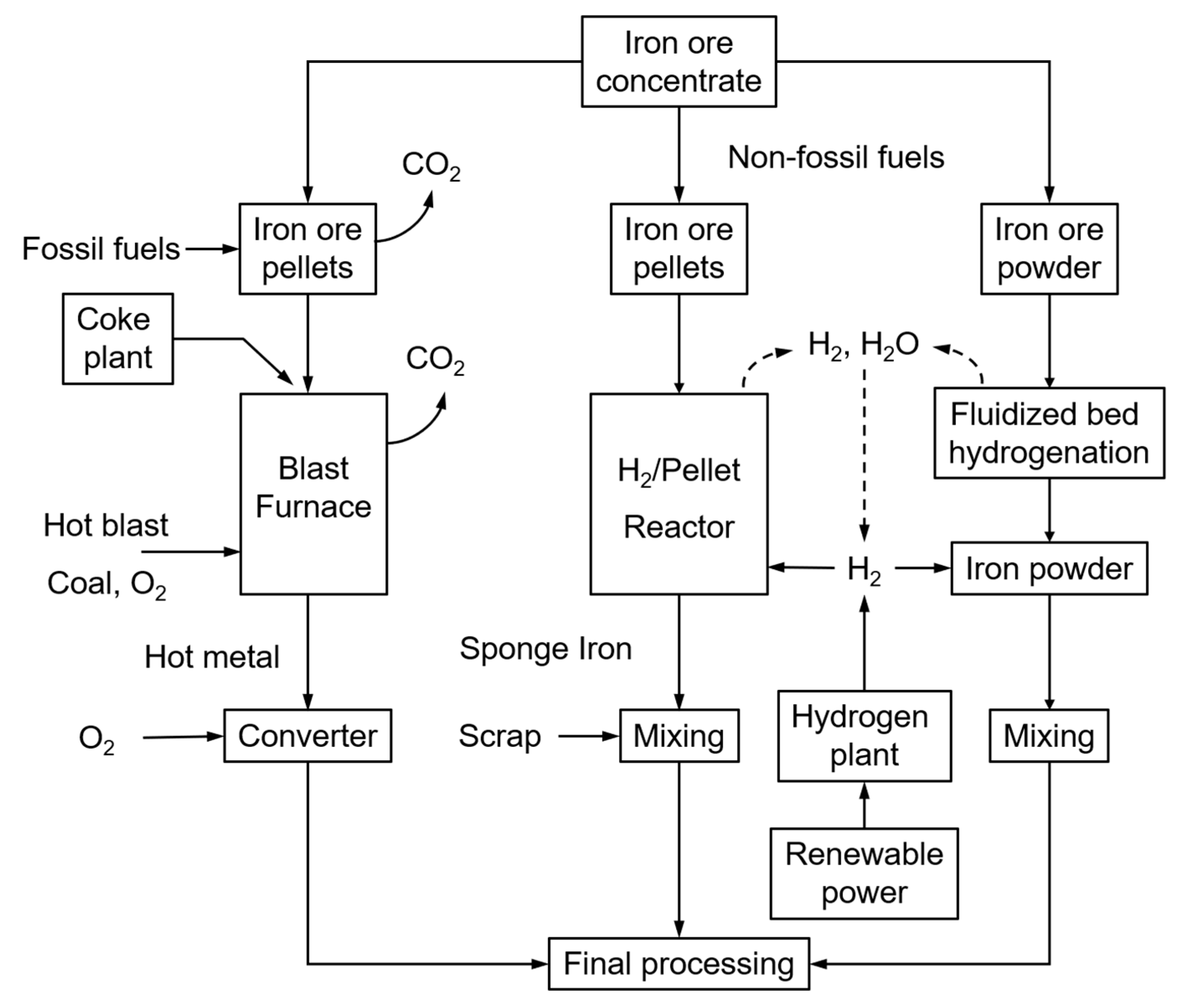
Most steel mills are currently using the blast furnace principle, fuelled by mostly coal, coke and coke oven gas. The successive processes of sinter belt, blast furnace and converter operate at extremely high temperatures, hence being energy-intensive and producing significant amounts of CO2. The steel industry represents about 7% of the world’s anthropogenic CO2 emissions. Within the objectives of COP21 (Paris, 2015), the target of steel mills is to reduce the CO2 emissions from the current (nearly) 3000 Mton/year to below 500 Mton/year. The use of renewable energy sources is, hence, a topic of major development, and ultimately the CO2-lean direct reduction of iron ore with hydrogen is considered to offer high potential. Two alternative direct H2 ore reductions (Figure 1, right-side) and the traditional fossil-fuel blast furnace operation (Figure 1, left-side) are illustrated in Figure 1 below.
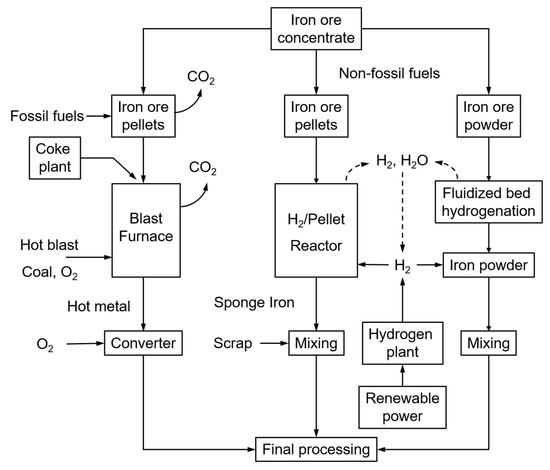
Figure 1.
Different steel-making processes: traditional, using fossil fuels (left); H2-driven (right).
Whereas the production of sponge iron from iron ore pellets is already commercially applied, the direct H2 reduction of crude iron ore powder has the advantages of omitting the sintering stage that is the major energy consumer and CO2 emission source of the iron-making process, together with the blast furnace. Sintering is indeed required to produce a sinter cake to be loaded into the blast furnace. It thermally agglomerates the mixture of iron ore fines, recycled iron-making products, fluxes, slag-forming agents and solid fuel (coke) at a temperature between 1300 and 1480 °C. Omitting the sintering operation will hence reduce the overall energy consumption and CO2 emissions by 35 to 40% [1].
The main reasons behind researching the reduction of CO2 using hydrogen are based upon limiting both the required thermal energy and the associated CO2 emissions.
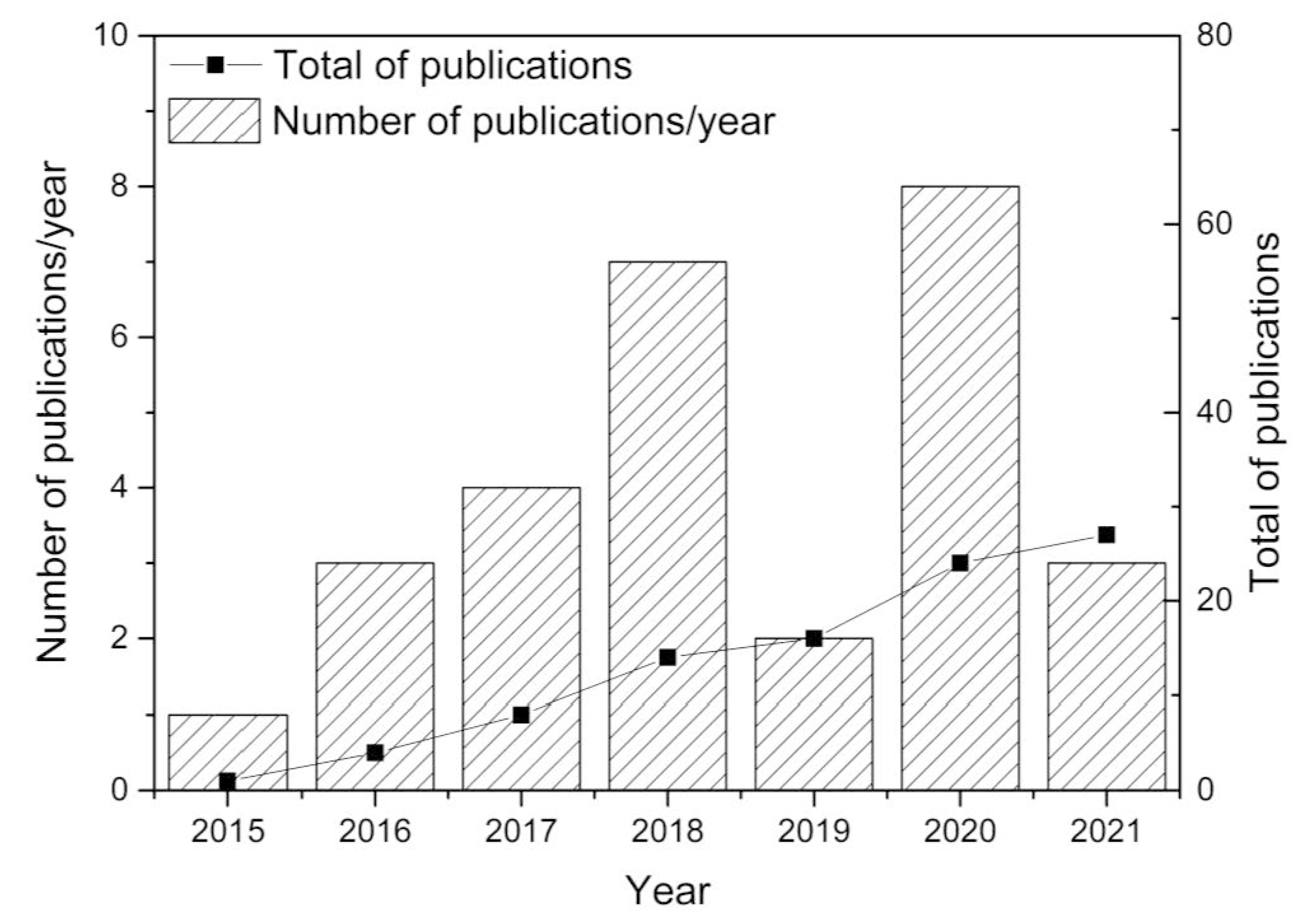
Major developments were reported since 2015, with research efforts illustrated in Figure 2. This figure was established from Web of Science statistics with appropriate keywords. Over 70% of the research dealt with the fundamentals of direct ore reduction using hydrogen and the reactors to be used (rotary furnace, plasma, fixed and fluidized beds). Additional publications tentatively focused upon process economics and environmental benefits. The fixed/moving or fluidized bed reactors represent only 7 out of 28 papers (see Table 1), stressing the need for additional research, as dealt with in the present study.
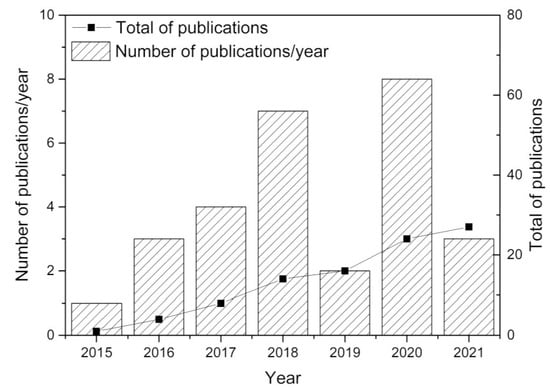
Figure 2.
Review of publications since 2015, from Web of Science, with keywords iron ore, direct reduction, hydrogen, CO2 reduction.

Table 1.
Important research contributions since 2017.
Some selected and important publications are listed in Table 1 according to their main output. Research on fixed/moving or fluidized ore reduction was separately included in the Table. The solar energy potential was not previously reported upon.
Similar research efforts on reducing the CO2 emissions from thermal processes were also presented for different fields, with a specific target of CO2 modeling, such as in a large-scale coal-fired thermal power plant [24], in integrated processing of liquified natural gas [25], in a multigeneration cascade system [26], in the pyrolysis reaction of lignocellulosic waste [27], or through the integration of rigid porous high-temperature filters in thermal processes [28], among others.
1.2. Objectives, Structure and Contribution of the Reported Research
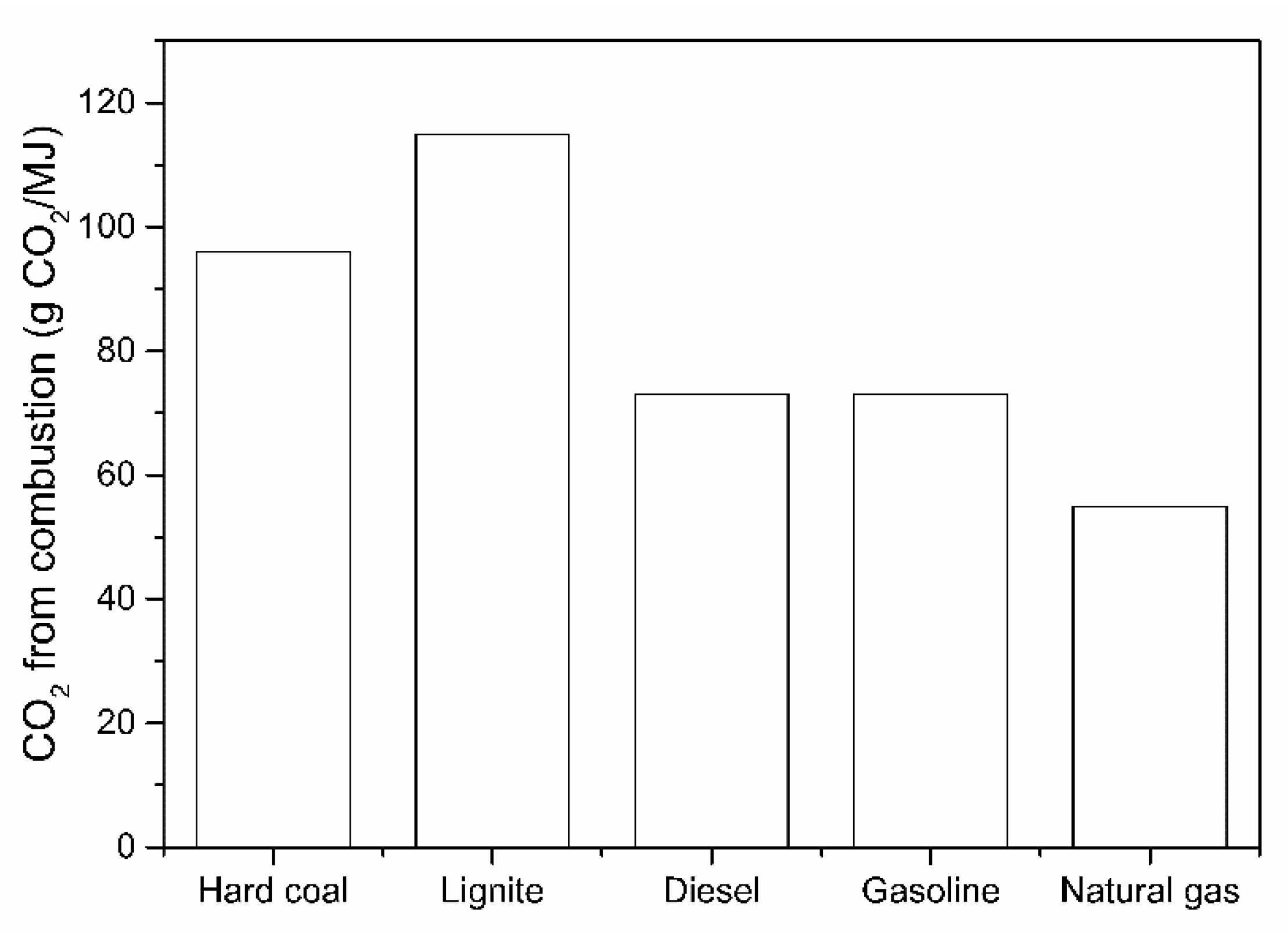
From the literature survey, it is clear that the direct H2-driven reduction of iron ore, and the considerably lower CO2 emissions, are increasingly important. The previous research developed some fundamental principles about the reaction mechanisms, reduction kinetics and CO2 reduction potential, as also dealt with in several studies about CO2 modeling. The importance of using a zero CO2 emission H2 as a fuel toward CO2 abatement in comparison with common fossil fuels is illustrated in Figure 3.
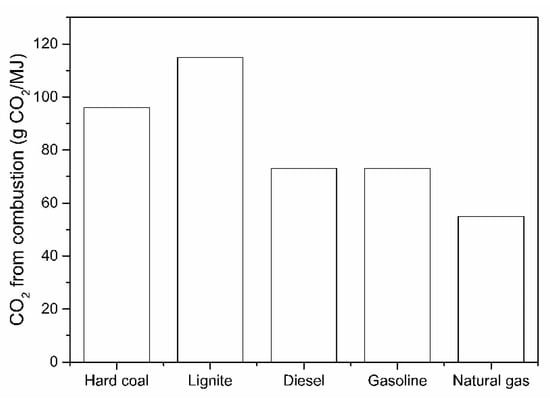
Figure 3.
CO2 emissions when burning an equivalent energy unit of fuel.
The application of fluidized bed reactors in this direct reduction principle of iron ores was only scarcely investigated. The application of sustainable solar energy was not investigated. To complete and assess the previous findings, and develop the perspective of concentrated solar energy application in the fluidized bed to direct H2 reductions of iron ore, this paper has multiple objectives.
Firstly, the properties of the iron ore powder that can be used in a fluidized bed H2-reduction reactor will be summarized.
Secondly, reaction fundamentals will be studied in both an isothermally operated macro-thermogravimetric reactor with electrical heating and in a vibrated fluidized bed reactor positioned in the cavity and focused on a concentrated solar furnace. Both the reduction yield and the fluidization reactor operation characteristics (velocities, pressure drop, temperature profiles within the reactor) will be determined. The results will confirm the potential of the direct H2 reduction of iron ore in a solar fluidized bed reactor.
Thirdly, the production of “renewable” H2 will be assessed. Although tests were performed on a lab-scale batch basis only, the economy of producing renewable H2 will be demonstrated.
Finally, all experimental findings and future developments will be summarized in the conclusions.
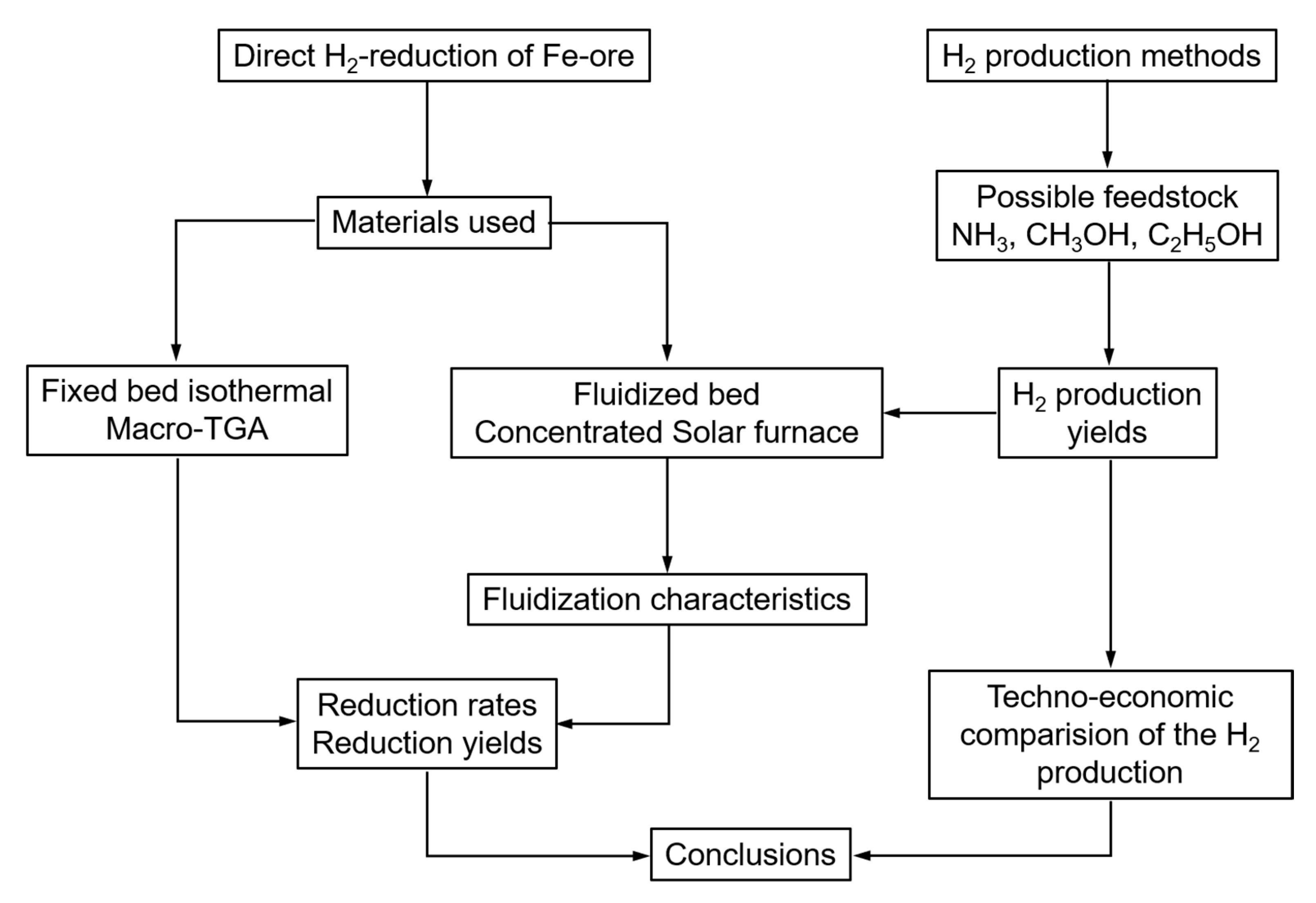
These objectives are summarized in the research framework of the paper, as presented in Figure 4.
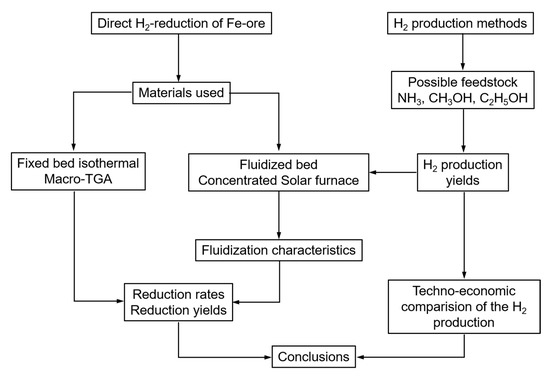
Figure 4.
Research framework.
2. Materials and Methods
2.1. Materials
The physical and chemical properties of the fine Fe2O3 particles used to examine the fluidization and hydrogen reduction are listed in Table 2. These particles belong to the Geldart group C (cohesive) particles, based on particle size, particle density and gas density [29].

Table 2.
Physical and chemical properties of fine Fe2O3 particles.
2.2. Methods and Procedures
2.2.1. Experimental Set-Ups
Two experimental rigs were used in the study: an electrically heated furnace and a solar-heated reactor.
Isothermal conversions under the H2 feed were determined by a macro-thermogravimetric analyzer (electrically-heated macro-TGA), where the weight loss under the H2 feed was monitored, according to the method described by Fernandez et al. [30]. The set-up is illustrated in Figure 5. The total heating capacity is 8 kW, and the heating rate is controlled by the thermocouple installed in the Fe2O3 ore bed.
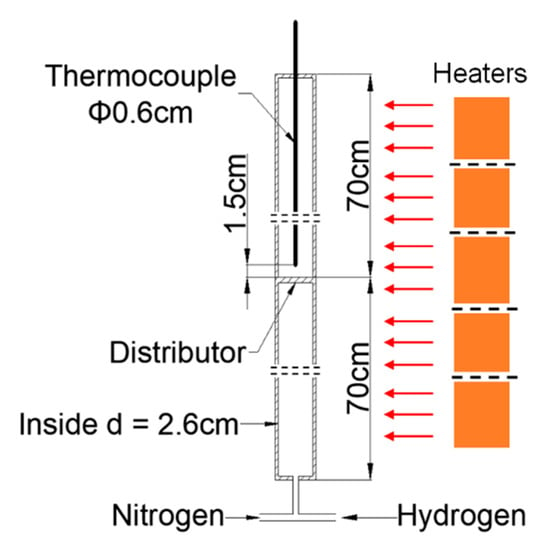
Figure 5.
Lab-scale set-up (electrical furnace).
The iron ore (170 g) was loaded inside the reactor above the distributor. The static bed height was about 16 cm. The bottom section of the electrically heated reactor served as the preheating section of the gas feed. Nitrogen was used when starting the experiment. When the reactant bed temperature reached the set point of 773 K, the N2 feed was stopped, and H2 was fed to the reactor. The time of reaction was used as a process parameter. When the required time was reached, the feed of H2 was stopped, N2 was restarted, the electric heating was stopped, and the reactor was allowed to cool. Samples of feedstock and reaction products were also analyzed for their oxygen concentration.
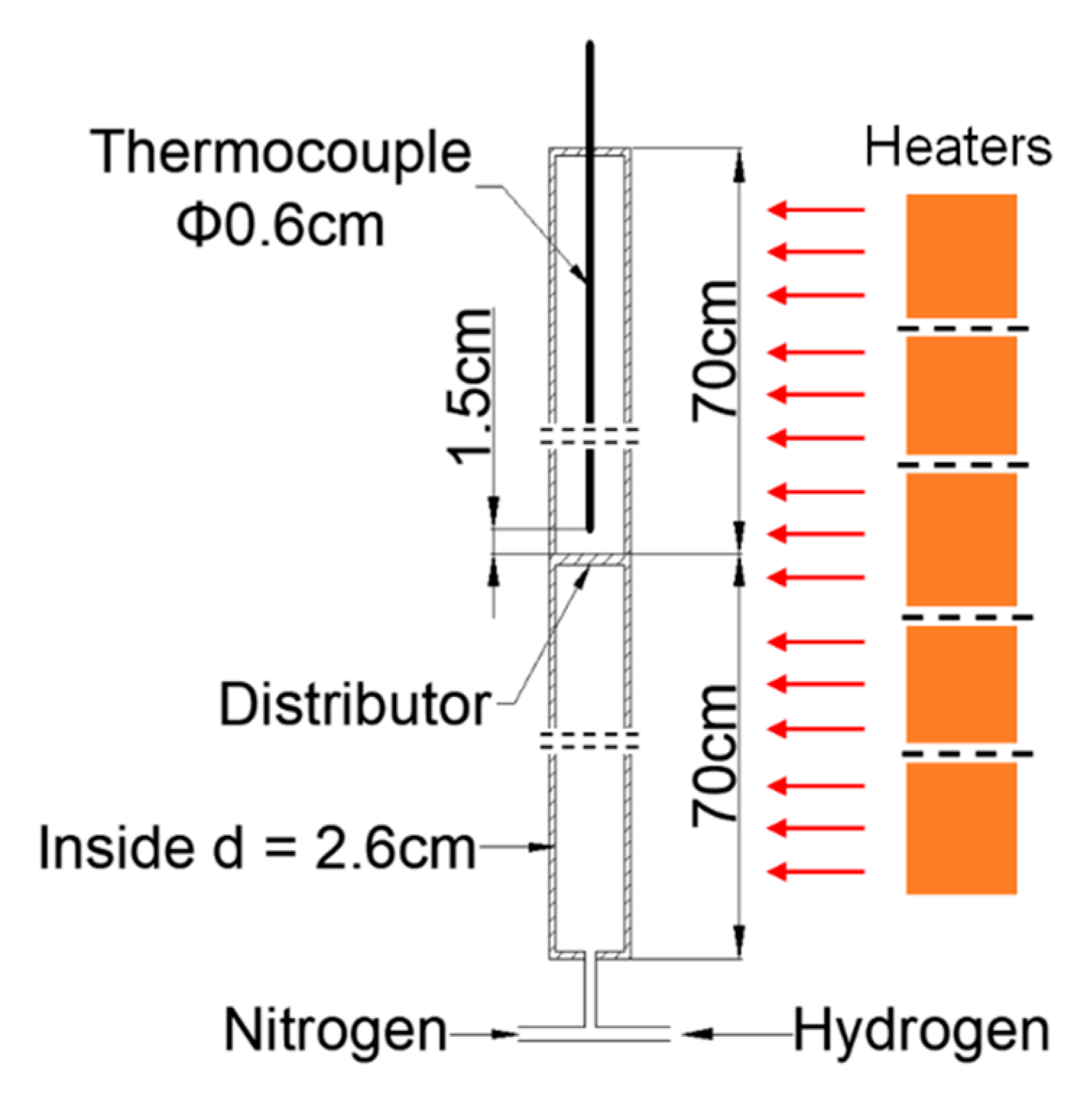
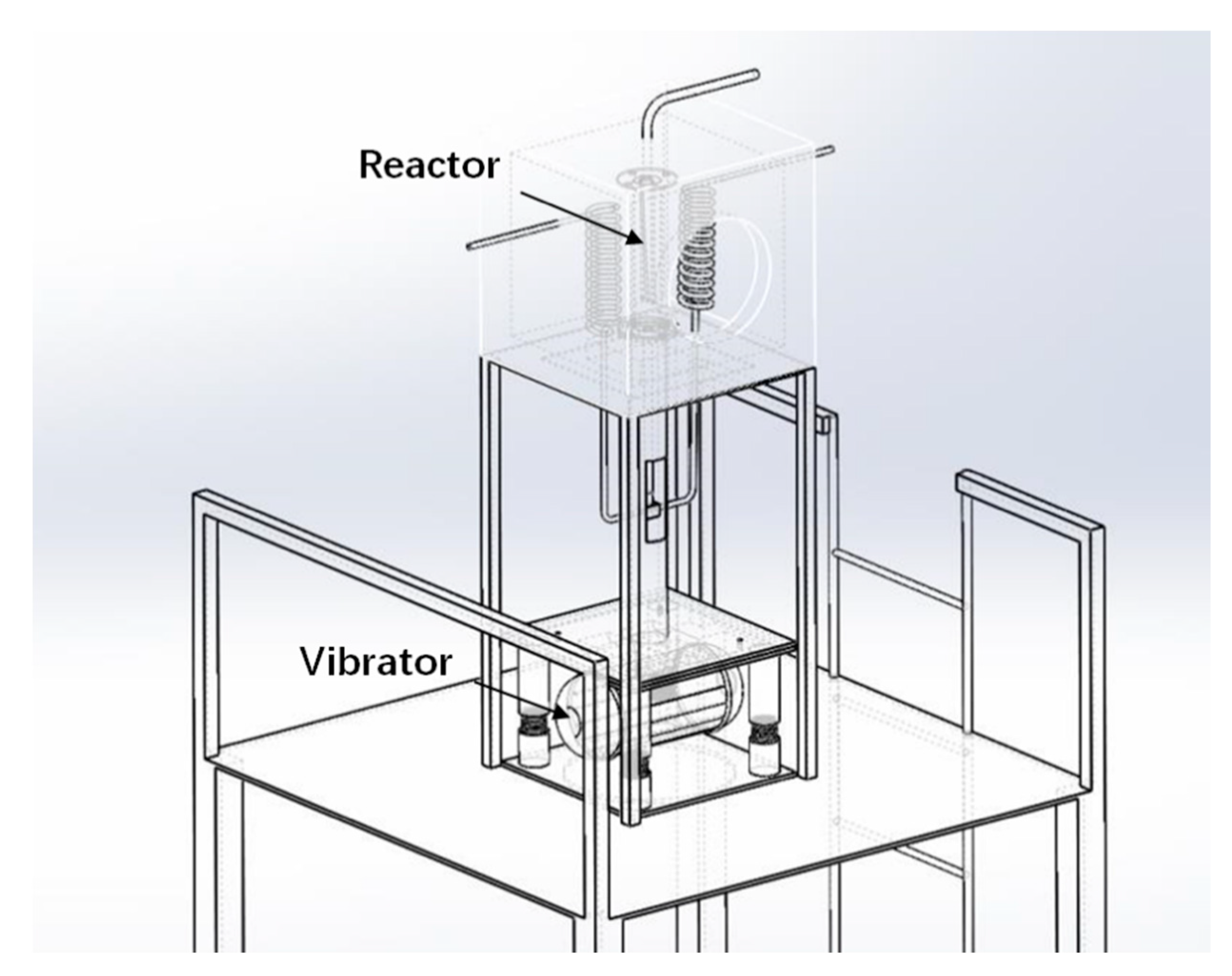
Solar H2 reductions were carried out in the solar thermal furnace, depicted in Figure 6. The maximum heat load in the cavity was ~20 kW. The vibrating fluidized bed was installed inside the 0.5 m × 0.5 m × 0.5 m cavity with an irradiation opening of 0.3 m diameter. Since we had to treat cohesive particles, a vibrating fluidized bed was used. The solar irradiation was collected by a heliostat and focused onto the cavity opening by a parabolic concentrator. The heat load could be controlled by partly defocusing the heliostat. A photograph of the experimental rig in operating conditions is shown in Figure 7.
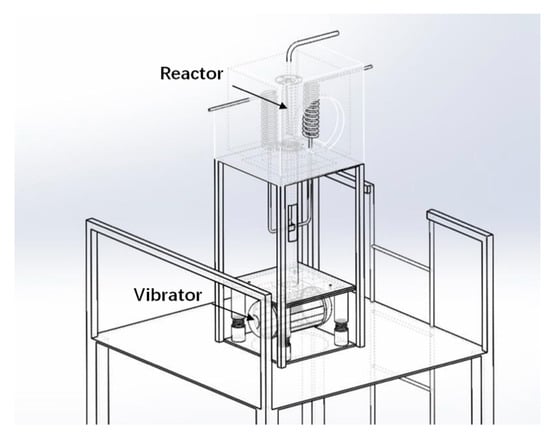
Figure 6.
Schematic of the solar receiver test unit (reactor installed in the cavity).

Figure 7.
Solar furnace in QinHuangDao (Hebei Province, China).
Table 3 lists the specifications of the solar test unit and test conditions. The internal diameter of the fluidized bed reactor was 50 mm. H2 was preheated by a coil heat exchanger also installed in the cavity and visible at the right of Figure 6. About 500 g of fine Fe2O3 particles formed the bed. A multi-orifice distributor was used to ensure a uniform gas distribution in the fluidized bed reactor [31].

Table 3.
Experimental conditions.
The vibration of the fluidized bed reactor was applied by adjusting the motor inverter frequency. The vibration frequency could be adjusted up to 47.7 Hz, and the amplitude was fixed to 0.6 mm. The pressure drop was checked in real-time using a differential pressure transmitter (Rosemount Inc., Shakopee, MN, USA) through pressure drop ports just above the distributor and at the top of the reactor. Hydrogen was used as the reduction gas, whereas nitrogen was used as fluidization gas at start-up (till the desired bed temperature was obtained) and at shut-down. Nitrogen and hydrogen gas was supplied to the fluidized bed using separate mass flow controllers. After the reduction was performed for a given reaction time, nitrogen was injected again to reduce the temperature of the reactor to ambient temperature, and the products were collected for analysis.
2.2.2. Data Treatment
For both experimental set-ups, the representative reduction reaction rate is shown in Equation (1) and evaluated on the basis of the weight loss during the reduction process and confirmed by the O-content of the Fe2O3, sampled at distinct times [32,33].
3. Results and Discussion
3.1. The Fluidization Characteristics of the Fine Fe2O3 Particles
Due to the cohesiveness of the particles, fluidization with gas alone was impossible. Channels and rat holes formed in the cohesive particles. To increase the fluidity of the fine particles, fluidization must be performed with the help of an external force to decrease the interparticle Van der Waals force. The vibration causes the particles to remain non-agglomerated.
Vibrated fluidization provides a uniform distribution of the gas flow, prevents the formation of particle agglomerates, and weakens the interparticle force. When the combined forces of the vibration and the hydrodynamic shear force exerted on the particles by the gas flow (related in a fluidized bed to the bed weight) exceed the Van der Waals force, fluidization of the C-type particles is achieved. The vibration force depends upon the vibration frequency and amplitude. Equation (2) defines the effective acceleration, resulting from the effects of vibration frequency and amplitude [34,35]:
with:
g: gravitational acceleration (9.81 m/s2)
: amplitude of the vibration (m)
: frequency of the vibration (Hz; m/s)
: effective acceleration factor (m/s2)
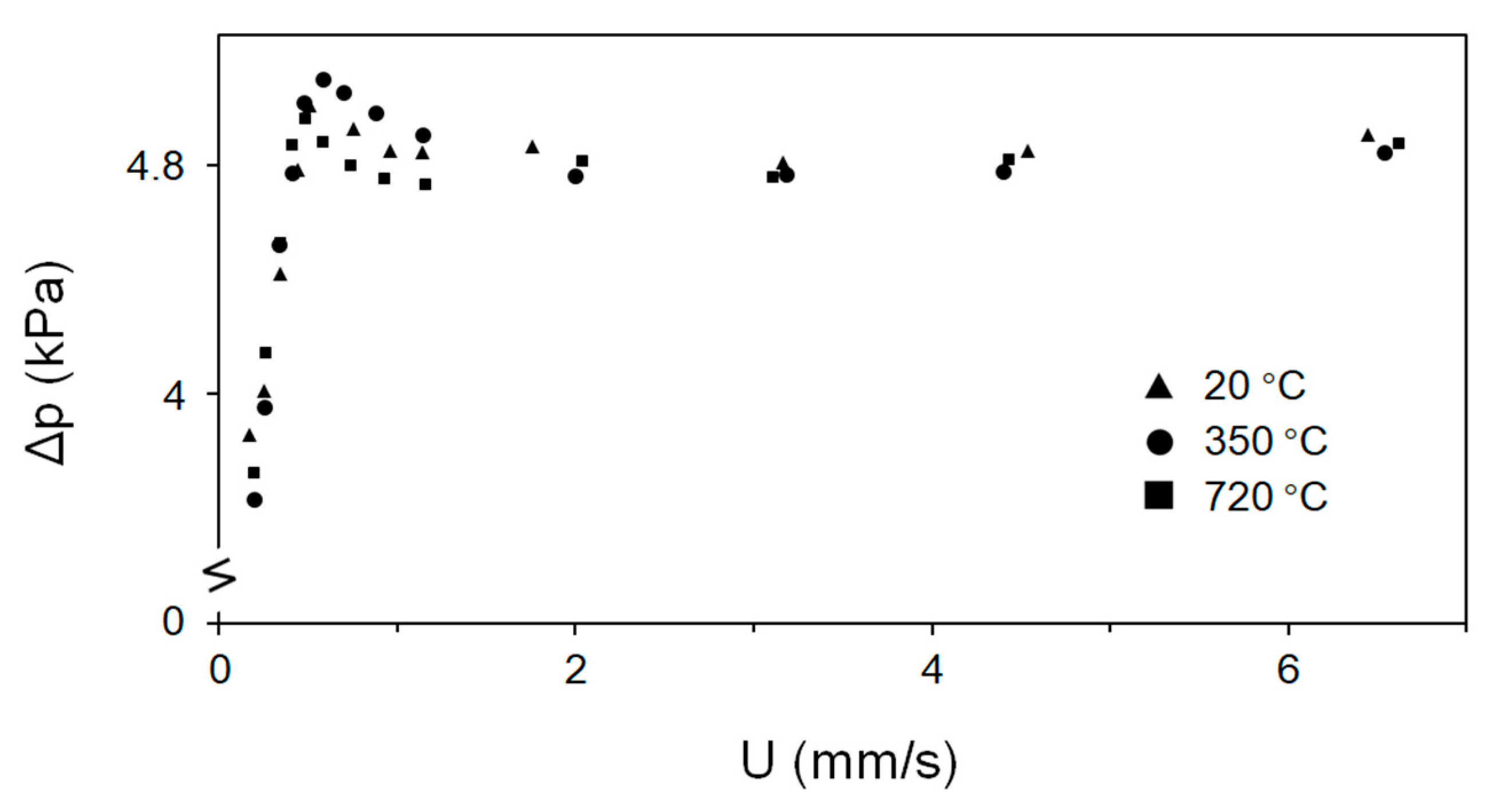
Without vibration, the fluidization characteristics showed excessive channeling. A bubbling fluidized regime was achieved when vibration was applied. Figure 8 shows pressure drop values for the 0.25 m deep bed of fine Fe2O3 particles as a function of the temperature. At reaction temperatures from 273 to 993 K, the pressure drop values at 25 Hz were 4.8 kPa, irrespective of the applied temperature. When the vibration frequency was increased, the pressure drop slightly increased by a maximum of 5%. The minimum fluidization velocity, Umf, hardly changed when the frequency and/or temperature increased, although a minimum vibration frequency of ~10 Hz was required (at the constant amplitude of 0.6 mm). The minimum fluidization velocity is around 1 mm/s.
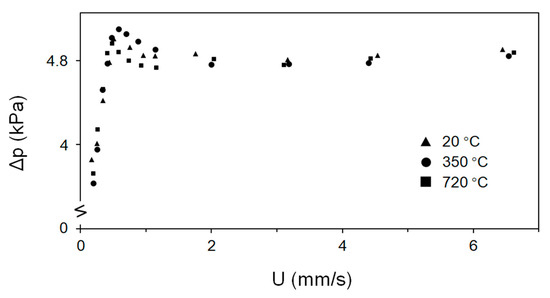
Figure 8.
Pressure drop across the bed versus superficial gas velocity.
Contrary to other Geldart particle types (A, B or D particles), fluidization has no distinct temperature-dependent tendency when vibration is applied to Geldart group C particles. At a vibration frequency of 10 Hz, the Umf value could not be determined because the pressure drop values for fine Fe2O3 particles did not exhibit a clear trend. As the external force increased, fluidization changed to the bubbling regime, with a constant pressure drop achieved in the bubbling regime.
3.2. The Fe2O3 Reduction Rate
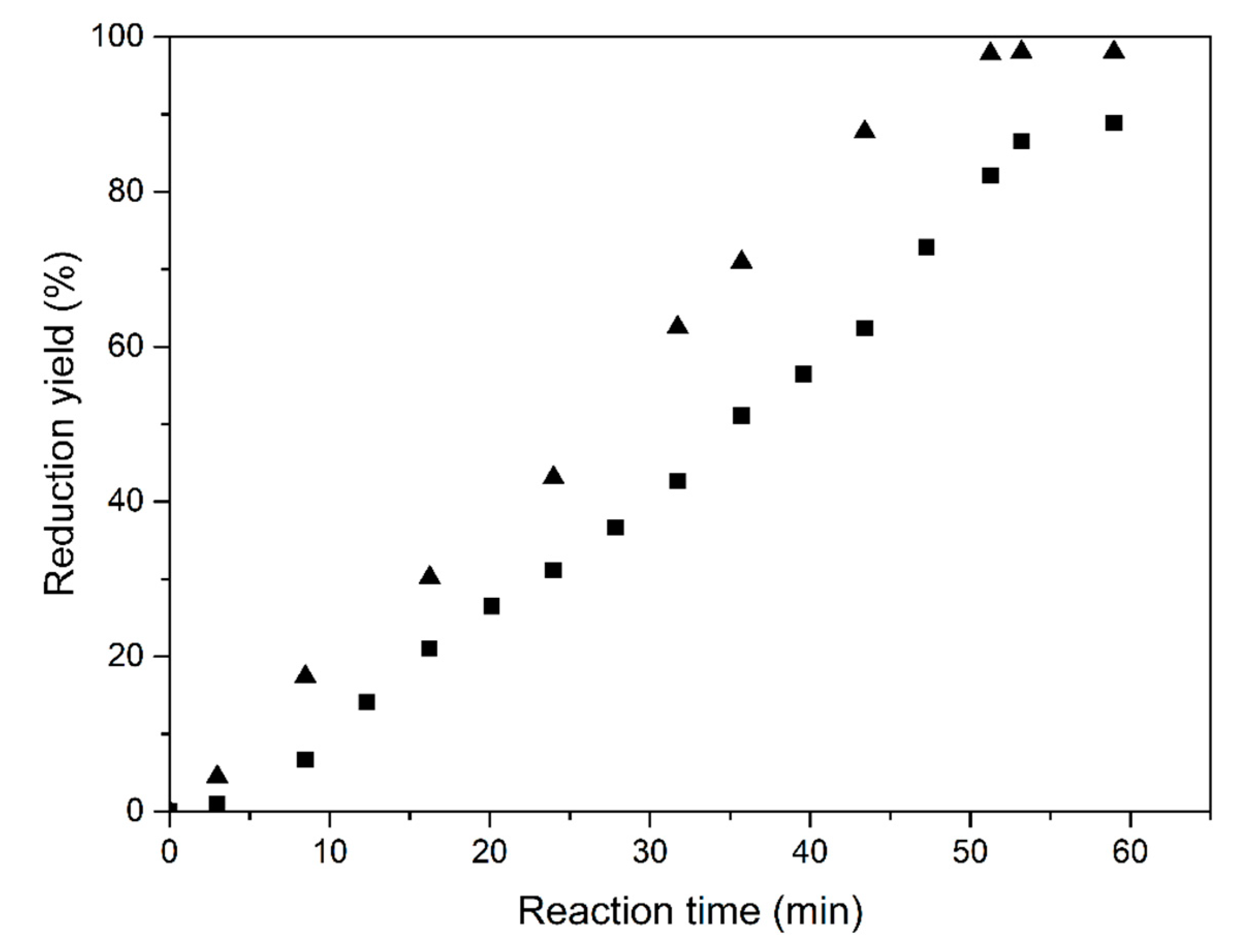
Figure 9 shows the reduction yield for the vibrated fluidized bed operation. Under the specific conditions of the test, the reaction time was about 50 min for a maximum reduction yield of about 90%. Increasing the vibration frequency to 47 Hz improved the reaction, and yields close to 98% were achieved. The superficial H2 velocity was 0.01 m/s.
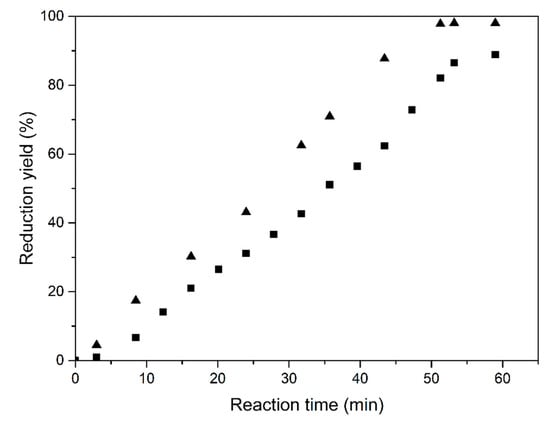
Figure 9.
Reduction of Fe2O3 at 773 K with vibration at 20 (■) and 47 Hz (▲), respectively.
Such high Fe2O3 reduction rates, achieved at the low temperature of 500 °C, demonstrate that fine iron ore particles can directly be converted into Fe without prior sintering by replacing the blast furnace with a vibro-fluidized bed and direct H2 reduction. Moreover, the application of solar heat totally eliminates the use of fossil fuels, although the fossil fuel-fired operation is also possible and can be operated at a low temperature. The potential energy savings of the direct reduction processes are considerable since neither sintering nor high-temperature blast furnace reduction is required. Since these latter processes require significant applications of fossil fuels, the CO2 savings are evident.
The remaining important issue is the production of H2, preferably by a “green” production method, as discussed below. The current H2 production methods rely mostly on steam reforming of CH4, on the gasification of coal or biomass, and on the electrolysis of water to a minor extent. Producing H2 by non-fossil fuel resources and by avoiding gasification should therefore be of primary importance.
4. Potential “Green” H2 Production Methods
4.1. NH3 Decomposition
Liquid NH3 can be considered an H2 storage medium and can be decomposed to N2 and H2. Although this decomposition is currently being tested, the literature provides sufficient background data to assess the reaction feasibility.
Electrolysis and thermo-chemical processes can be utilized for NH3 decomposition. The thermo-chemical decomposition of NH3 involves a catalyst inside a reactor using a reaction as follows:
The decomposition reaction is an endothermic thermochemical reaction that requires high temperatures, around 250–700 °C, and relatively high NH3 partial pressures [36].
Theoretically, the thermodynamic equilibrium calculation produces the single-pass conversion rate at different decomposition temperatures, as presented in Table 4. The decomposition can reach a complete reaction with a conversion rate of 99% at a temperature of at least 400 °C. Higher temperatures above 450 °C no longer strongly influence the reaction but rather its kinetics [36].

Table 4.
Equilibrium NH3 decomposition (at 1 bar) as a function of temperature, with original data from [36].
Recent studies reported various single metal catalyst, in terms of activity, in the decreasing order Ru > Ni > Rh > Co > Ir >Fe > Pt > Cr > Pd > Cu >> Te, Se, Pb [37]. However, even if Ru shows the best conversion activity, this type of catalyst is still not sufficiently active for NH3 decomposition at a temperature of 450 °C. More importantly, it will not be economically viable for large-scale applications. Multiple sources state that the Ru-based catalyst is active at a temperature of decomposition over 600 °C with a conversion rate of 97%.
Research and development of catalysts are done on precious metals, bimetallic materials, and metal oxides [38,39,40,41,42]. The bimetallic compound is proven to improve catalytic performance even though the manufacturing is complicated. It was also shown that metal oxides work by changing the electronic density of the active catalyst sites, and hence, are proposed as promoters and supporting materials for catalysts.
4.2. C2H5OH and CH3OH Catalytic Decomposition
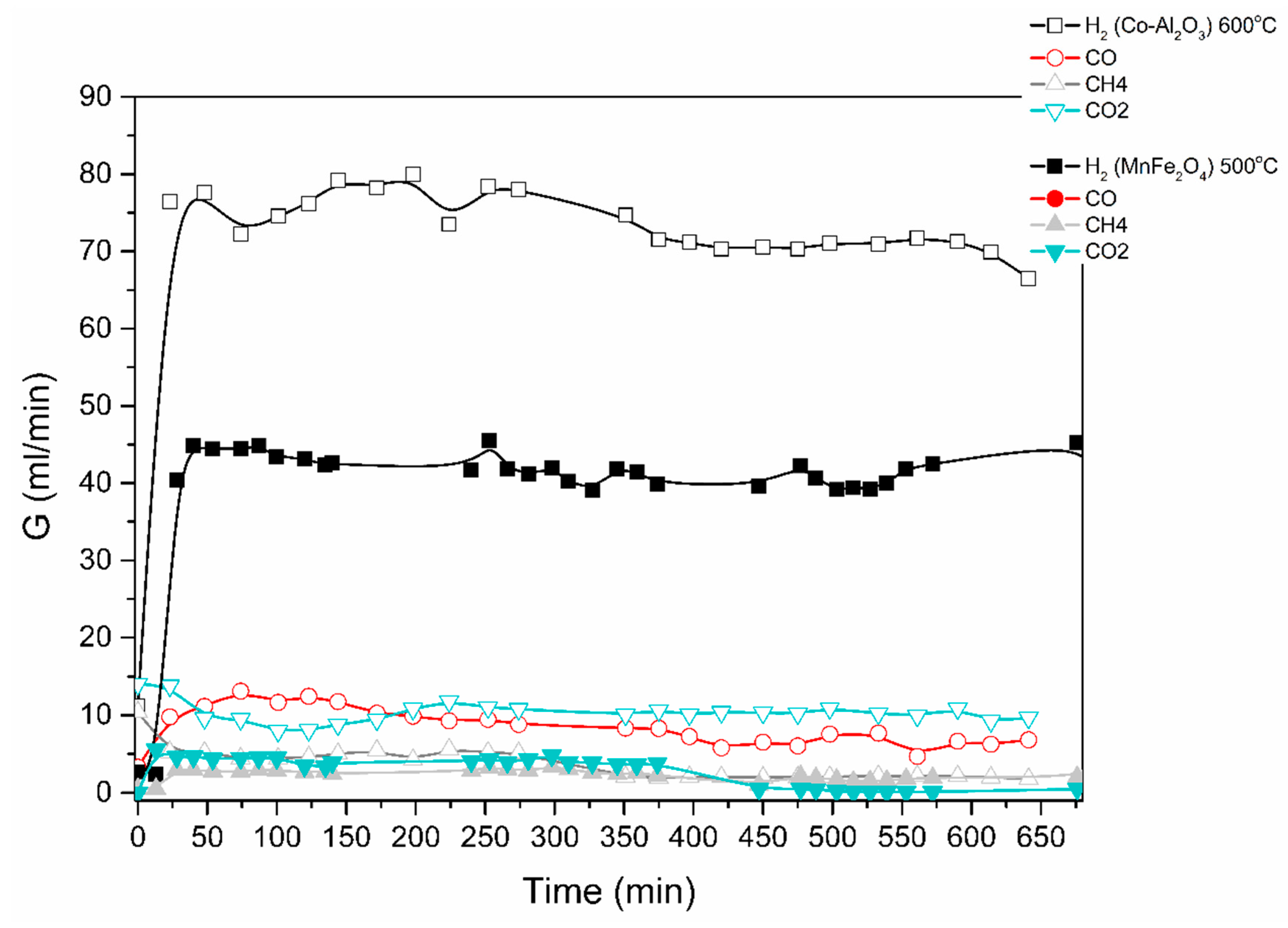
The catalytic decomposition of ethanol was studied in both the electric and solar furnaces in Figure 5, Figure 6 and Figure 7. Results are shown in Figure 10 below for an ethanol/water mixture (1 to 6 mol-ratio), at a feed rate of 0.1 mL/min of ethanol/water and using two different catalysts, i.e., MnFe2O4 and a Co-loaded Al2O3.
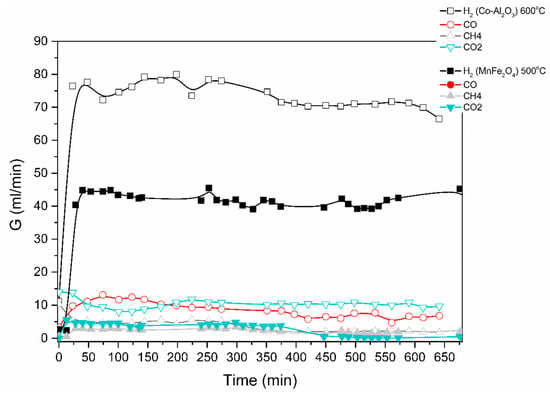
Figure 10.
Catalytic decomposition of ethanol-water (1:6) mixture using Co-Al2O3 or MnFe2O4 catalyst. Results obtained in the electric furnace at the given temperatures.
The produced gas stream was continuously monitored by a gas chromatograph. The lag time of about 25 min is due to the piping between the reactor outlet and the GC. H2, CO, CO2 and CH4 were the main products identified; acetone was also detected. The reaction mechanisms are currently under investigation.
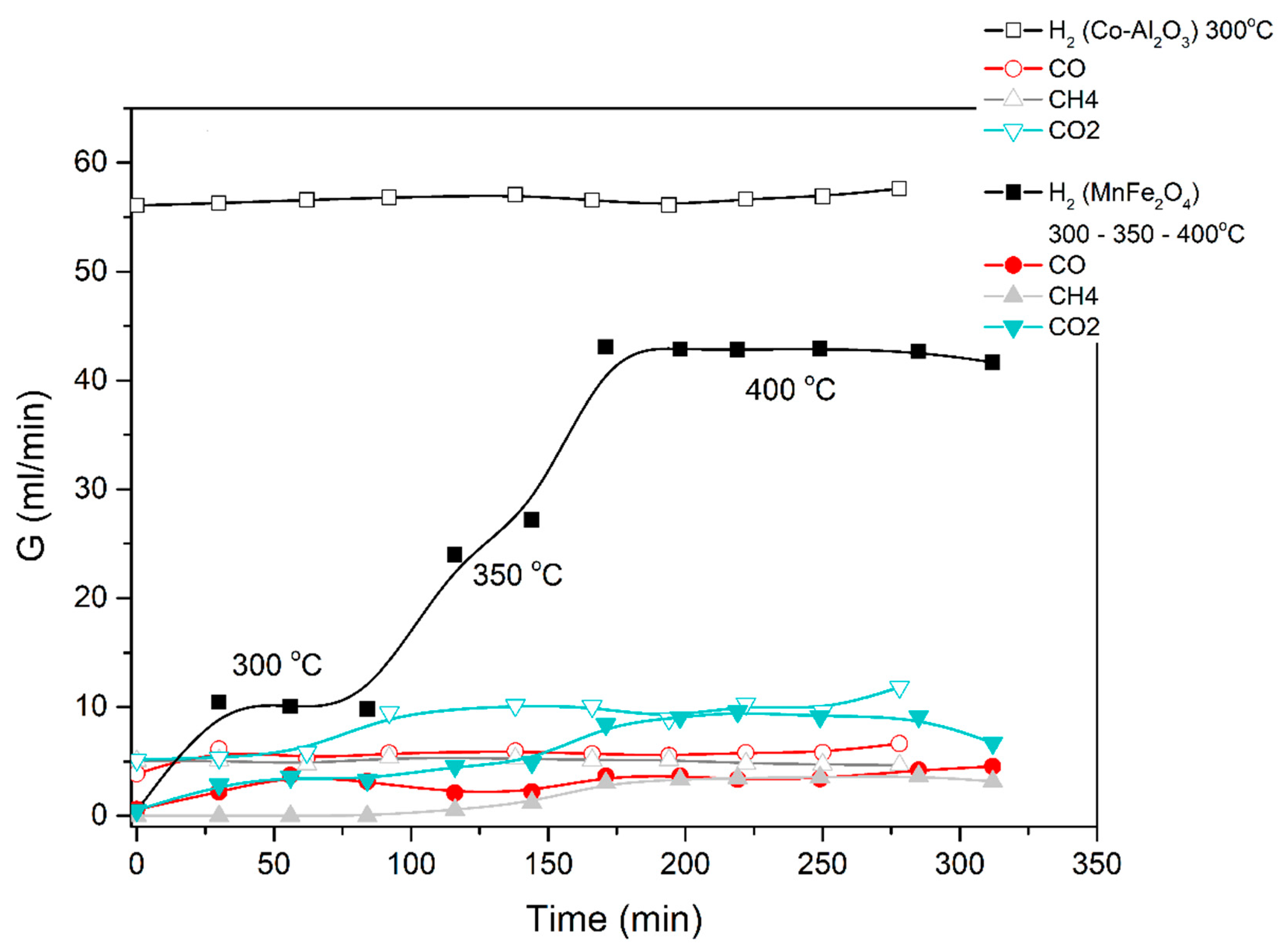
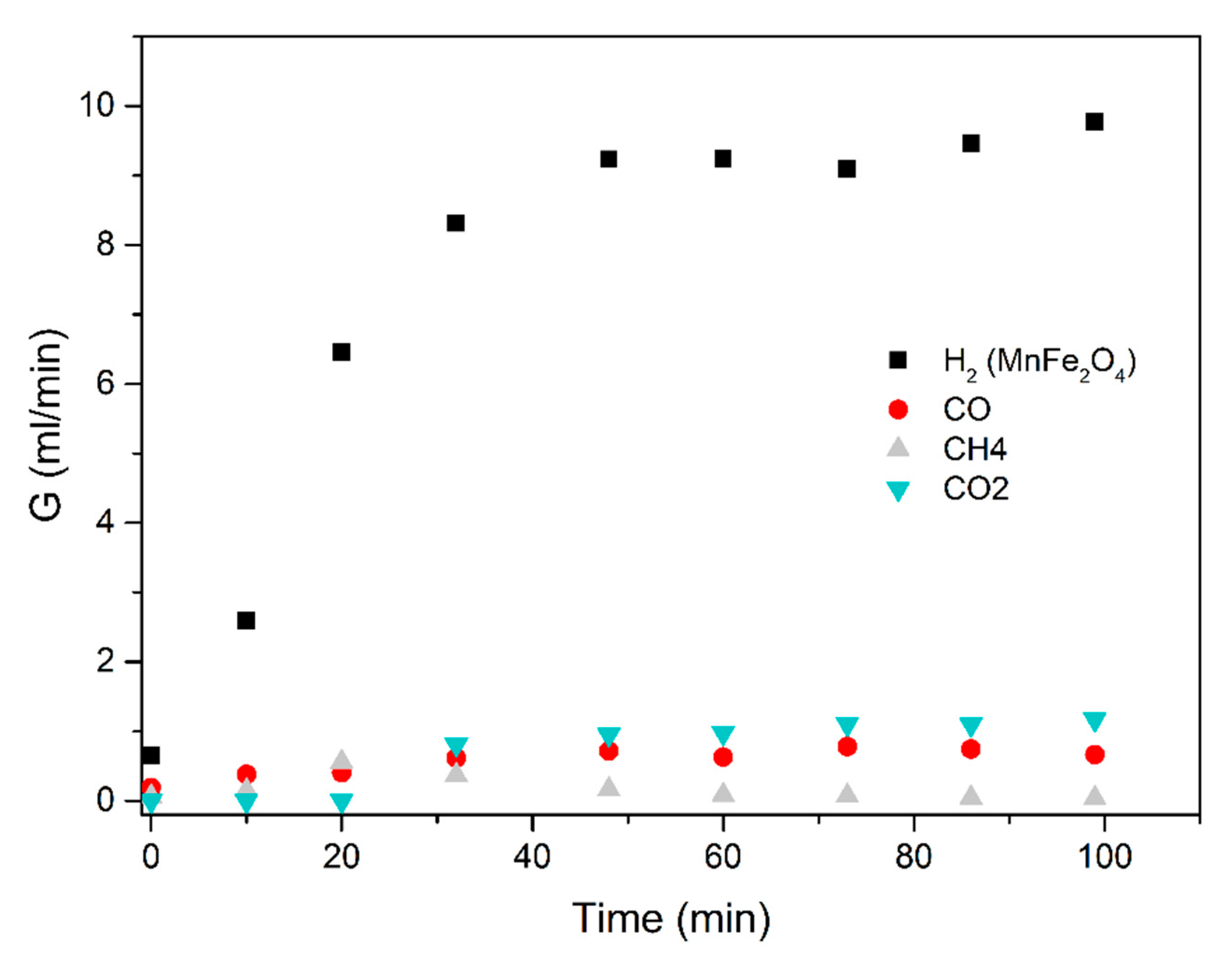
A similar procedure was applied for the steam-methanol decomposition, with results illustrated in Figure 11 and Figure 12. No coking of the catalyst was observed, and up to 5 repeat experiments for the same length of time and at successive days yield the same H2 production rates.
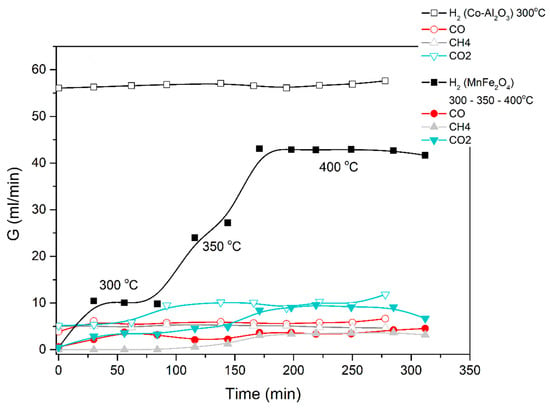
Figure 11.
Catalytic decomposition of methanol-water (1:3) mixture using Co-Al2O3 or MnFe2O4 catalyst. Results obtained in the electric furnace at the given temperatures.
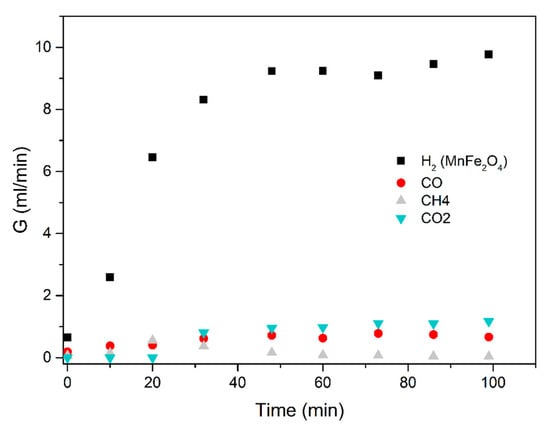
Figure 12.
Methanol-water (1:3 mixture) using the MnFe2O4 catalyst. Results obtained in the electric furnace at 300 °C.
The average results of three repeat experiments are used in the figures below. The deviation between the repeat results and the averages were below 10%, accepted as fair in view of the lag time in the reactor-to-GC piping.
The results obtained demonstrate that excellent ethanol/methanol to H2 conversions are achieved at low temperatures (300 to 500 °C is sufficient). In all the cases studied, the ratio of volumetric H2 to CO2 production was very high. Most of the feedstock carbon was detected as CO, CH4 and acetone.
4.3. Tentative Techno-Economic Comparison of Different H2 Production Technologies
From the experimental data and literature reports, the following comparison emerges (Table 5) for the levelized cost of hydrogen production (LCOH), for the GHG emission reduction potential and for the H2 yield. Current production techniques of methane steam reforming, coal and biomass gasification and water electrolysis (using a renewable electricity source from wind turbines or photovoltaics) are compared with the novel methanol, methane and ethanol decomposition reactions.

Table 5.
Techno-economic comparison (2017–2021).
From this summary table, it is clear that (i) biomass and coal gasification should be avoided because of the low H2 yield and fairly high LCOH; (ii) all other systems have a fairly similar H2 yield, although the GHG emission levels disfavor methane steam reforming and coal gasification; methane, methanol and ethanol decomposition reactions can reduce the CHG emission levels, while still achieving a competitive LCOH.
5. Conclusions
Since steel mills need to reduce their carbon footprint, the traditional high-temperature fossil-fuel-fired (>1200 °C) processes need to be avoided. The solution of the direct reduction of iron ore with hydrogen has high potential: (i) the process has a high conversion efficiency (up to 98%) at low temperatures (500–600 °C) and both the use of H2 and the significantly lower operating temperatures reduce the energy consumption and fossil-based CO2 emissions by over 75%; (ii) H2 can be generated by competitive and environmentally friendly processes, as examined in the research.
The limitations of the present research must, however, be considered.
- (i)
- Fe2O3 ore particles are of small size and cannot readily be processed by a H2 stream in a fixed or fluidized bed. A novel vibrated fluidized bed was developed and shown capable of fluidizing μm-particles at very low superficial fluidization velocities. The small-scale experiments need to be confirmed on a pilot-scale. Vibrated fluidization of the fine Fe2O3 particles caused good fluidization and yielded a nearly constant pressure drop. An increase in external force due to vibration slightly increased the pressure drop. Despite an increase in the reaction temperature, vibration produces a nearly constant minimum fluidization velocity.
- (ii)
- Although very interesting reduction reaction yields for fine Fe2O3 particles were obtained on a small-scale, it is important to investigate the production of such fine Fe2O3 feedstock on an industrial scale.
- (iii)
- The remaining issue relates to the “green” production of H2, currently based primarily on fossil fuel feedstock with a high degree of GHG emissions. Although steam reforming of ethanol and methanol offers an important potential, the methane process is preferred since its GHG emission potential is the most attractive (except for electrolysis and biomass gasification) while producing H2 at a very interesting cost. Further pilot-scale experiments are required to confirm these initial lab-scale results.
- (iv)
- To confirm the solar production potential, additional experiments will be conducted in the solar furnace as soon as the Direct Normal Irradiance values are higher (from July onwards).
Author Contributions
Conceptualization, J.B. and Y.D.; methodology, S.L., H.Z. and R.D.; validation, all authors; formal analysis, J.B., H.Z. and Y.D.; investigation, S.L., J.N. and Y.D.; resources, J.B.; data curation, all authors; writing—original draft preparation, J.B. and Y.D.; writing—review and editing, all authors; project administration, J.B.; funding acquisition, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Beijing Advanced Innovation Centre for Soft Matter Science and Engineering of the Beijing University of Chemical Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request to the corresponding author. The data are not publicly available until the Ph.D. of Yimin Deng has been released by KULeuven (expected 2022).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, P.; Ryberg, M.; Yang, Y.; Feng, K.; Kara, S.; Hauschild, M.; Chen, W.-Q. Efficiency stagnation in global steel production urges joint supply- and demand-side mitigation efforts. Nat. Commun. 2021, 12, 2066. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Fan, D.-Q.; Abdelghany, A. Design of Novel Flash Ironmaking Reactors for Greatly Reduced Energy Consumption and CO2 Emissions. Metals 2021, 11, 332. [Google Scholar] [CrossRef]
- Abolpour, B.; Afsahi, M.M.; Azizkarimi, M. Hydrogen reduction of magnetite concentrate particles. Miner. Process. Extr. Metall. 2021, 130, 59–72. [Google Scholar] [CrossRef]
- Murakami, T.; Wakabayashi, H.; Maruoka, D.; Kasai, E. Effect of Hydrogen Concentration in Reducing Gas on the Changes in Mineral Phases during Reduction of Iron Ore Sinter. ISIJ Int. 2020, 60, 2678–2685. [Google Scholar] [CrossRef]
- Patisson, F.; Mirgaux, O. Hydrogen Ironmaking: How It Works. Metals 2020, 10, 922. [Google Scholar] [CrossRef]
- Zhang, A.; Monaghan, B.J.; Longbottom, R.J.; Nusheh, M.; Bumby, C.W. Reduction Kinetics of Oxidized New Zealand Ironsand Pellets in H2 at Temperatures up to 1443 K. Metall. Mater. Trans. B 2020, 51, 492–504. [Google Scholar] [CrossRef]
- Bai, M.; Long, H.; Li, L.; Liu, D.; Ren, S.-B.; Zhao, C.-F.; Cheng, J. Kinetics of iron ore pellets reduced by H2N2 under non-isothermal condition. Int. J. Hydrogen Energy 2018, 43, 15586–15592. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, J.; Qin, B.; Dong, Y.; Lu, Y.; Li, Y.; Hao, W.; Zhang, Y. Reduction kinetics of hematite ore fines with H2 in a rotary drum reactor. Powder Technol. 2018, 332, 18–26. [Google Scholar] [CrossRef]
- Bai, M.-H.; Long, H.; Ren, S.-B.; Liu, D.; Zhao, C.-F. Reduction Behavior and Kinetics of Iron Ore Pellets under H2–N2 Atmosphere. ISIJ Int. 2018, 58, 1034–1041. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Q.; Qie, Y.; Liu, X.; Lan, C.; Li, J.; Liu, S. Effect of hydrogen addition on reduction behavior of iron oxides in gas-injection blast furnace. Thermochim. Acta 2017, 648, 79–90. [Google Scholar] [CrossRef]
- Elzohiery, M.; Sohn, H.Y.; Mohassab, Y. Kinetics of Hydrogen Reduction of Magnetite Concentrate Particles in Solid State Relevant to Flash Ironmaking. Steel Res. Int. 2017, 88, 1600133. [Google Scholar] [CrossRef]
- Du, W.; Yang, S.; Pan, F.; Shangguan, J.; Lu, J.; Liu, S.; Fan, H. Hydrogen Reduction of Hematite Ore Fines to Magnetite Ore Fines at Low Temperatures. J. Chem. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Sohn, H.Y.; Mohassab, Y.; Elzohiery, M.; Fan, D.-Q.; Abdelghany, A. Status of the Development of Flash Ironmaking Technology. In Applications of Process Engineering Principles in Materials Processing, Energy and Environmental Technologies; The Minerals, Metals & Materials Series; Wang, S., Free, M., Alam, S., Zhang, M., Taylor, P., Eds.; Springer: Cham, Switzerland, 2017; pp. 15–23. [Google Scholar] [CrossRef]
- Htet, T.T.; Yan, Z.; Spooner, S.; Degirmenci, V.; Meijer, K.; Li, Z. Gasification and physical-chemical characteristics of carbonaceous materials in relation to HIsarna ironmaking process. Fuel 2021, 289, 119890. [Google Scholar] [CrossRef]
- Bhaskar, A.; Assadi, M.; Nikpey Somehsaraei, H. Decarbonization of the Iron and Steel Industry with Direct Reduction of Iron Ore with Green Hydrogen. Energies 2020, 13, 758. [Google Scholar] [CrossRef] [Green Version]
- Vogl, V.; Åhman, M.; Nilsson, L.J. Assessment of hydrogen direct reduction for fossil-free steelmaking. J. Clean. Prod. 2018, 203, 736–745. [Google Scholar] [CrossRef]
- He, K.; Zheng, Z.; Chen, H.; Hao, W. Reduction Behaviors of Hematite to Metallic Iron by Hydrogen at Low Temperatures. In Energy Technology 2021: Carbon Dioxide Management and Other Technologies; Springer: Cham, Switzerland, 2021; pp. 111–122. [Google Scholar]
- Spreitzer, D.; Schenk, J. Fluidization behavior and reducibility of iron ore fines during hydrogen-induced fluidized bed reduction. Particuology 2020, 52, 36–46. [Google Scholar] [CrossRef]
- Knop, K.; Kubiak, H. Process engineering and cost efficiency of steelmaking by means of hydrogen-based direct reduction. Stahl und Eisen 1996, 116, 55–64. [Google Scholar]
- Hamadeh, H.; Mirgaux, O.; Patisson, F. Detailed Modeling of the Direct Reduction of Iron Ore in a Shaft Furnace. Materials 2018, 11, 1865. [Google Scholar] [CrossRef] [Green Version]
- Ranzani da Costa, A.; Wagner, D.; Patisson, F. Modelling a new, low CO2 emissions, hydrogen steelmaking process. J. Clean. Prod. 2013, 46, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Lazou, A.; van der Eijk, C.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. On the Direct Reduction Phenomena of Bauxite Ore Using H2 Gas in a Fixed Bed Reactor. J. Sustain. Metall. 2020, 6, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Moldenhauer, P.; Linderholm, C.; Rydén, M.; Lyngfelt, A. Avoiding CO2 capture effort and cost for negative CO2 emissions using industrial waste in chemical-looping combustion/gasification of biomass. Mitig. Adapt. Strateg. Glob. Chang. 2020, 25, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Hoseinzadeh, S.; Stephan Heyns, P. Advanced Energy, Exergy, and Environmental (3E) Analyses and Optimization of a Coal-Fired 400 MW Thermal Power Plant. J. Energy Resour. Technol. 2021, 143, 082106. [Google Scholar] [CrossRef]
- Tjahjono, T.; Ehyaei, M.A.; Ahmadi, A.; Hoseinzadeh, S.; Memon, S. Thermo-Economic Analysis on Integrated CO2, Organic Rankine Cycles, and NaClO Plant Using Liquefied Natural Gas. Energies 2021, 14, 2849. [Google Scholar] [CrossRef]
- Mahmoudan, A.; Samadof, P.; Hosseinzadeh, S.; Garcia, D.A. A multigeneration cascade system using ground-source energy with cold recovery: 3E analyses and multi-objective optimization. Energy 2021, 233, 121185. [Google Scholar] [CrossRef]
- Torres, E.; Rodriguez-Ortiz, L.A.; Zalazar, D.; Echegaray, M.; Rodriguez, R.; Zhang, H.; Mazza, G. 4-E (environmental, economic, energetic and exergetic) analysis of slow pyrolysis of lignocellulosic waste. Renew. Energy 2020, 162, 296–307. [Google Scholar] [CrossRef]
- Li, S.; Baeyens, J.; Dewil, R.; Appels, L.; Zhang, H.; Deng, Y. Advances in rigid porous high temperature filters. Renew. Sustain. Energy Rev. 2021, 139, 110713. [Google Scholar] [CrossRef]
- Kong, W.; Tan, T.; Baeyens, J.; Flamant, G.; Zhang, H. Bubbling and Slugging of Geldart Group A Powders in Small Diameter Columns. Ind. Eng. Chem. Res. 2017, 56, 4136–4144. [Google Scholar] [CrossRef]
- Fernandez, A.; Soria, J.; Rodriguez, R.; Baeyens, J.; Mazza, G. Macro-TGA steam-assisted gasification of lignocellulosic wastes. J. Environ. Manag. 2019, 233, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Geldart, D.; Baeyens, J. The design of distributors for gas-fluidized beds. Powder Technol. 1985, 42, 67–78. [Google Scholar] [CrossRef]
- Haitao, W.; Sohn, H.Y. Reduction of Magnetite Concentrate Particles by H2+CO at 1673 K. ISIJ Int. 2015, 55, 706–708. [Google Scholar]
- Zhang, B.; Wang, Z.; Gong, X.; Guo, Z. Characterization of Precipitated Carbon by XPS and Its Prevention Mechanism of Sticking during Reduction of Fe2O3 Particles in the Fluidized Bed. ISIJ Int. 2013, 53, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-Y.; Huang, A.-N.; Kuo, H.-P. Analysis of interparticle forces and particle-wall interactions by powder bed pressure drops at incipient fluidization. Powder Technol. 2018, 325, 64–68. [Google Scholar] [CrossRef]
- Wank, J.R.; George, S.M.; Weimer, A.W. Vibro-fluidization of fine boron nitride powder at low pressure. Powder Technol. 2001, 121, 195–204. [Google Scholar] [CrossRef]
- Al-Qodah, Z.; Al-Busoul, M.; Al-Hassan, M. Hydro-thermal behavior of magnetically stabilized fluidized beds. Powder Technol. 2001, 115, 58–67. [Google Scholar] [CrossRef]
- Lucentini, I.; García Colli, G.; Luzi, C.D.; Serrano, I.; Martínez, O.M.; Llorca, J. Catalytic ammonia decomposition over Ni-Ru supported on CeO2 for hydrogen production: Effect of metal loading and kinetic analysis. Appl. Catal. B Environ. 2021, 286, 119896. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Zhao, S.; Zhang, Y. FeCe nanocomposite with high iron content as efficient catalyst for generation of COx-free hydrogen via ammonia decomposition. J. Rare Earths 2020, 38, 1053–1059. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Cha, J.; Kwak, Y.; Park, Y.; Jo, Y.S.; Jeong, H.; Sohn, H.; Yoon, C.W.; Kim, Y.; Kim, K.-B.; et al. Top-Down Syntheses of Nickel-Based Structured Catalysts for Hydrogen Production from Ammonia. ACS Appl. Mater. Interfaces 2021, 13, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Carter, J.H.; Dummer, N.F.; Richards, N.; Morgan, D.J.; Golunski, S.E.; Hutchings, G.J. Ammonia Decomposition Enhancement by Cs-Promoted Fe/Al2O3 Catalysts. Catal. Lett. 2020, 150, 3369–3376. [Google Scholar] [CrossRef]
- Ye, T.-N.; Lu, Y.; Kobayashi, Y.; Li, J.; Park, S.-W.; Sasase, M.; Kitano, M.; Hosono, H. Efficient Ammonia Synthesis over Phase-Separated Nickel-Based Intermetallic Catalysts. J. Phys. Chem. C 2020, 124, 28589–28595. [Google Scholar] [CrossRef]
- Yu, Y.; Gan, Y.-M.; Huang, C.; Lu, Z.-H.; Wang, X.; Zhang, R.; Feng, G. Ni/La2O3 and Ni/MgO–La2O3 catalysts for the decomposition of NH3 into hydrogen. Int. J. Hydrogen Energy 2020, 45, 16528–16539. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).