Abstract
Novel food refers to any type of food which was not used for human consumption before the 15 May 1997 in a specific place. This date refers to the introduction of European Union Regulation (EC) No 258/1997 which regulated the placing of novel foods or novel food ingredients on the market within the community for the first time. Then, the Regulation (EU) 2015/2283 changed the existing legislation for the categories of food belonging to novel food in order to guarantee a higher level of protection of human health and consumer interests. Algae, which are not commonly consumed by people but are considered among the most widespread foods of the future, are one of the principal food products of natural plant origin in the regulation of novel foods. However, even if algae were not well-known in the past, nowadays they are integrated into the different food cultures of the EU. This circumstance led to an analysis of the contribution of trade flows, of algae for human consumption inside and outside Europe, on the trade balance of the member countries of the European Union. Analysis of the Eurostat database was used to provide an overview of the international trade dynamics affecting the trade development of algae for human consumption in the European Union, with the aim of measuring the competitive dynamics within member countries.
1. Introduction
Since the creation of the European single market, the European Union has faced the challenges arising from the regulation of the free movement of goods [1]. The free circulation of goods is not a problem for individual products, but it concerns the relations between member States of the European Union with third countries and especially with those countries in the Mediterranean basin. This strategic role is aimed at giving importance to the development of the area, strengthening the multi-lateral relations, establishing a framework for dialogue and cooperation, as well as relations capable of establishing a new political itinerary [2,3].
Overall, the objectives that concern trade within the single market of products are to ensure consumer interests, safety, uniformity, and safety-requirements throughout the European Union [4].
In dealing with the particular field of agri-food products, the current EU legislation respects all these principles and goes even further. The principal topics of EU regulation are consumer safety and the protection of their interests in general, but no less attention is dedicated to important issues including the protection of human health. The risks need to be assessed, certain ones must be selected, and it is necessary to predict probable risks, in order to avoid them happening as well as eventually stemming their effects [5,6].
The scenario becomes even more complex when analyzing the particular category of food products called novel food. Novel food opens up a challenge that is not simple to deal with.
The term novel food refers to food that is not part of the traditional eating habits of European citizens [7]. Novel food products are foods with different characteristics in various respects and, in this context, they are a contrast to traditional food. These foods are an expression of integration of the different food cultures, so novel food is an innovative food or food produced using new technologies and new production processes. It follows that novel foods are innovative products which require a different production process from the standard EU one or derived from the application of innovative bio technologies [8].
The modern technologies applied to food can have unknown effects on health and it is therefore necessary to make use of the precautionary principle. Innovation is undoubtedly a starting point for economic progress and development. In some studies, it has been shown that the new technology investments have brought high productivity rates and rapid, positive economic growth, by demonstrating a significant effect on economic growth [9,10,11]. Currently, the theme of innovation is extremely topical because the process leads to reduced production costs and to increased profit margins [11]. The result is the commitment of the European institutions in the Europe 2020 Strategy. Europe 2020 support programs (including, for example, Horizon 2020) focus on research, development, and innovation to foster the competitiveness of European companies and achieve the objectives of economic growth [12,13].
However, the importance the theme of innovation in order to generate increasing innovation, cannot become an undisputed primary need. It is always necessary to assess whether the new products bring with them unacceptable features as they contrast with current legislation.
It is therefore necessary to verify whether the novel food opportunities provided by science and technology also create new risks [14].
The innovations, resulting from research and development (R&D) activities, must be properly regulated before placing them on the market, and must meet the demands of operators in the sector and consumers [11].
Issues of great importance, such as the health and well-being of animals, ethical profiles, consumer interests, and sector operators, should not be forgotten [8].
The definition of rules regarding these aspects is relevant from a marketing point of view. Intervening on these points has the purpose of helping firms by making the introduction of novel foods on the EU market easier [14].
The improvement of these conditions should make the procedure for placing novel foods on the European internal market easier and therefore could have an increase in the number of new products that can be imported and then sold [15].
However, the regulation process of food matters is highly complex [8]. In fact, it is necessary to understand that the legislative process is not immediate and involves multiple aspects of verification that include also those with a scientific multidisciplinary nature. Only based on results that fall outside the legal reality can the legislator make appropriate legal reasoning and then adapt an ad hoc prudent regulation. The problem of the introduction of a new law presents another feature to be protected, namely the certainty of the law, a cardinal principle of the legal system. The advancement of technologies has surely accelerated the times of innovation creation processes. It is not easy to keep up with the speed of progress, especially for a delicate and complex subject such as food. Thus, the European legislator has to deal with the needs of a market that wants to incorporate innovations. This implies a continuous updating and some changes in legislation that require, once again, a good dose of flexibility on behalf of the legislator.
Furthermore, other significant aspects concern the free circulation of goods, legal certainty and uniformity throughout Europe, sustainability, transparency, protection of scientific data and the competitive position of those who have invested in innovative projects, etc. [16,17].
The Regulation (EU) No 2015/2283 of the European Parliament and of the European Council on novel food includes cardinal principles of the current legislation on the subject of health and well-being of citizens, their social and economic interests, and food security. The regulation also includes rulings on competition, favouring the free movement of safe and wholesome food. Part of the measure refers to food traditionally consumed outside the EU. Because of this evolution, Regulation (EU) No 2015/2283 integrates novel foods with the macro-category “traditional foods from a third country”. This regulatory measure is also responsible for the regulation and guidelines of the procedures for authorizing the placing of novel food on the European Union food market. Other aims of the regulation also include combating conditions of unfair competition and promoting the free movement of safe and wholesome food.
In view of its importance, the first part of this paper is devoted to this new EU regulation. The second part is dedicated to the product algae. Algae is regulated among novel foods of primary origin from a third country not normally consumed by EU citizens for human consumption. In the geographical area concerned, the cultivation of algae is hardly present. Hence the importance of the discussion of extra-EU and intra-EU trade flows of the algae sector.
2. Novel Foods, Role and Perspectives. Main Elements of the Regulatory Act
Currently, the relevant legal framework on novel foods is set out in Regulation (EU) 2015/2283 of the European Parliament and of the Council, which entered into force on 1 January 2018. This regulation repealed the previous Regulation (EC) No 258/97 of the European Parliament and of the Council of 27 January 1997 concerning novel foods, novel food ingredients, and Regulation (EC) No 1852/2001, which were in force until 31 December 2017.
Novel food is defined as food that had not been consumed to a significant degree by humans in the EU before 15 May 1997, when the first Regulation on novel food came into force [8].
Novel food can be newly developed, innovative food, food produced using new technologies and production processes, as well as food which is or has been traditionally eaten outside of the EU.
Compared to the Regulation (EC) No 258/97, the definition includes a general notion of a food product that derives from the reference to Regulation (EC) No 178/2002 and incorporates its dictate. According to article 4 of Regulation (EC) No 178/2002, the procedure for the determination of novel food status expects that food business operators are those who shall verify whether the food, which they intend to place on the market within the European Union, falls within the scope of this regulation. In case there is doubt about this issue, it is required that food business operators provide the necessary information they know about the potential novel food to the member state where they first intend to place it, to demand whether or not a food falls within the scope of the regulation. Member states may consult each other and the European Commission. Food business operators are obliged to inform the European Commission of any scientific or technical information (article 25 Regulation (EC) No 178/2002) that may concern food safety on the use of the new food and any prohibitions or restrictions imposed by a third country for the novel food placed on the market. The absence of the use of algae to a significant extent for human consumption in the European Union before 15 May 1997 instead, is confirmed, in continuity with the previous Regulation (EC) 258/97 [18,19].
Through Regulation (EU) 2015/2283 on novel foods, the European Union aims to harmonize national legislation in order to overcome legal uncertainty, combat unfair competition, and promote the free movement of safe and wholesome food. In fact, this regulation brings together the key principles of current legislation on the health and well-being of citizens, their social and economic interests, and food safety.
Novel food must therefore pass approval as to its suitability in terms of food safety for human consumption before it can be placed on the market (in order to be able to be defined as novel food, it must be included in specific categories listed in article 3 Regulation (EU) 2015/2283). The European Commission is, in fact, responsible for monitoring the application of the new regulation concerning the recognition of food and its authorization of novel foods [20].
The commission authorizes and includes a novel food product if it complies with certain conditions (article 7 Regulation (EU) 2015/2283): 1. The food does not pose a safety risk to human health according to available scientific evidence; 2. The food’s intended use does not mislead the consumer, especially when the food is intended to replace another food and there is a significant change in the nutritional value; 3. When the food is intended to replace another food, it does not differ from that food in such a way that its normal consumption would be nutritionally disadvantageous for the consumer.
An innovative element from a procedural point of view is the centralization of the procedure itself. The authorization procedure is no longer the responsibility of the member states (Regulation (CE) No 258/97), but of the Commission and EFSA (European Food Safety Authority). The latter is the body responsible for the technical and scientific evaluation of the safety of products. Based on this assessment, after this process has been activated, the Commission will only be able to decide whether to include the novel foods in the Union List. This list contains all novel foods authorized to be placed on the market within the European Union as foods, in foods or used on food, in accordance with the conditions of use and labeling requirements specified therein (article 6 of Regulation (EU) 2015/2283). Registration in the Union List is an act having erga omnes effect, thus overcoming the previous system, according to which the authorization regarding the introduction of a novel food product into the market was made by a single decision.
This solution determines turning points also from a practical point of view. In this way the problems of the restrictive nature of some national authorities are avoided, also due to the reduction of the economic resources necessary to manage the dossiers relating to novel food products [21].
The procedure for authorization can start either from the Commission’s initiative or by directly applying to the Commission (article 10 Regulation (EU) 2015/2283). When the Commission receives an application for the authorization of a novel food product, the latter is made available to the Member States without delay. To deepen the theme related to the validity of a notification of a traditional food from a third country sent to the commission, it must be specified that the notification must include: (1) the applicant’s name and address; (2) the name and description of the traditional food; (3) an accurate composition of the traditional food; (4) the country or countries of origin of the traditional food; (5) documented data demonstrating the history of safe food use in a third country; (6) a proposal for the conditions of intended use and for specific labelling requirements, which do not mislead the consumer, or a verifiable justification why those elements are not necessary (article 14 Regulation (EU) 2015/2283). The applicant must communicate any information that may have an impact on the assessment of the authorization and consequently on the registration of the product in the Union List to the Commission [7].
The European institutions become competent and the centralization of the management of the procedure becomes important for several factors: the bureaucratic delays are countered and the differences in the application of the legislation are eliminated. The elimination of the latter guarantees the harmonization and homogeneity of the evaluation and therefore a homogeneity of health protection. The application for an authorization must be submitted directly online to the European Commission and must contain the scientific data regarding the substance for which they request the authorization. Specific guidelines are based on methods by the food safety Authority European Food Safety Authority (EFSA).
As far as the potential effect on human health is concerned, the European Commission asks the EFSA’s opinion. This Authority gives its opinion on the scientific aspects of the safety of novel foods (article 11 Regulation (EU) 2015/2283) and on whether the update is liable to have an effect on human health.
When the EFSA has verified the compliance with the requirements, EFSA forwards its opinion to the European Commission, to the member states and, where applicable, to the applicant (article 11 Regulation (EU) 2015/2283). Within seven months from the date of publication of the authority’s decision, the commission updates the union list, taking into account every rule required in Regulation (EU) 2015/2283.
Within seven months the Commission also submits to the “Standing Committee on Plants, Animals, Food and Feed”, composed of representatives of the Member States and chaired by the representative of the Commission, established by article 58(1) of Regulation (EC) No 178/2002, an act authorizing the placing on the market of the novel food within the Union and updates the “Union list” (article 12 Regulation (EU) 2015/2283). By doing so, the commission also takes into account the precautionary principle together with the various specifications needed. The precautionary principle is part of general food law and occurs in cases where a possibility of harmful effects on health is identified. This includes any food types in which the novel food may be contained, doses, and other characteristics, etc. (article 7 of Regulation (EC) No 178/2002).
Thus, it is opportune to recall article 35(2), which allows for novel foods that are not included in the application of the Regulation (CE) No 258/97 and which were placed on the market before 1 January 2018.
3. Traditional Foods Originating in Third Countries
The definition of novel food also includes products that circulate in third country markets that boast a widespread consumption in the same third country of at least 25 years as a part of the customary diet of a significant number of people with a history of safe food use has been demonstrated without having created health problems or raised safety problems [7]. In this regard, the Regulation (EU) 2015/2283 intends to facilitate the placing on the European Union market of traditional foods of third countries even if those foods are derived from primary production as defined in Regulation (EC) No 178/2002 [8,19,22].
If the novel food has been traditionally consumed, the procedure is easier and faster [8]. It could be said that the European Union ratifies and allows the entry of products that, in other geographical locations of the world, boast a remarkable consumption tradition.
The regulation relies, therefore, on the historical data of non-risky use, considering it enough to allow marketing authorization. The simplification, implemented by Regulation (EU) 2015/2283, is more significant considering that authorization in this case is obtained by ratification which, unless there are any objections, is the food operator in the EU member state’s task.
So, instead of following the authorizing process for putting a novel food on the market within the Union, the applicant, intending to place a traditional food from a third country within the Union, may opt to submit a notification of that intention to the European Commission (article 14 Regulation (EU) 2015/2283). In this case, this alternative procedure is a faster, simpler way of updating the “Union list” [18].
In fact, where no duly reasoned safety objections have been submitted, the Commission can authorize and update the Union list immediately, specifying that it concerns a traditional food from a third country (article 15 Regulation (EU) 2015/2283) [20].
In the event that the Commission does not authorize the placing on the market within the Union of the traditional food concerned, the applicant has the option of submitting an application for authorization of the unauthorized food by using the notification procedure (article 16 Regulation (EU) 2015/2283) [18].
The Commission cannot always authorize the access of a traditional food from a Third Country to the European Union market. The decision not to proceed with the authorization is taken by the Commission on an autonomous basis or, where appropriate, following the opinion of the Authority (EFSA) and the Member States’ views (article 18 Regulation (EU) 2015/2283). The Commission operates independently of a specific request for consultation to determine if a particular food product falls within the definition of novel food.
The European Commission informs the applicant and the member states of the reasons why it considers that the update is not justified.
An important aspect to consider is that, although the updating of the “union list” with the novel food is not always allowed, this does not mean that the update is definitely refused. The applicant can present an application supplying, new evidence and data related to the safety objections that were made.
Where the Commission grants the authorization (already mentioned article 18), after an act of public opinion by the authority, it submits to the standing committee referred to in article 30(1) a “draft implementing act”, authorizing the placing on the market and updating the union list.
4. Confidentiality and Data Protection
If requested by the applicant, the possibility that certain information will remain confidential is guaranteed. This is, in the authors’ opinion, a key regulatory intervention. Some information requested by the commission for the evaluation of the admission of the novel food can often be the result of specific research involving an investment in terms of research. Applicants who have invested in research and development and who send an application for authorization to present a novel food to the European Commission thus have the guarantee that the evidence provided will not be made available to others. If these results, on the other hand, were made available to third parties, the latter could exploit the results of the findings [8]. This would be a noticeable disadvantage for the applicant. The work done to discover a possible innovation has given a competitive advantage to those who carried it out and it is crucial that it is protected in order not to cause problems, such as unfair competition. It is therefore a provision which aims to avoid harming the applicant’s competitive position. To obtain this protection, the applicants must specifically indicate what information they consider being of a confidential nature, accompanying the request for such protection with a verifiable reason. This confidentiality is maintained for up to three weeks after the applicants have been informed of the Commission’s position. When the three weeks have expired if the applicants have not withdrawn their request it is up to the Commission to decide what information to keep confidential [17].
All these considerations are even truer if, in the event that the request for entry of a novel food is refused, another request can still be made later, since it is certainly not a definitive refusal. Therefore, the applicant who previously submitted the application for authorization with the relevant supporting evidence, can subsequently submit another application adding, new data or other evidence to the data previously provided. If the new elements supporting the application prove decisive for the purposes of input, the commission could grant the authorization.
If the data previously provided for the authorization process were made available to third parties, the latter could simply add further evidence starting from the results disclosed by the first applicants. Third parties would not have to start a research from scratch, but they would use the results achieved by the first applicants. In the authors’ opinion, this would be unfair, and it is comforting that the legislator has regulated this aspect, protecting researchers’ efforts and avoiding market distortions and an unjust dispersion of information.
Finally, as far as data protection is concerned, the issue is important when new evidence or scientific evidence has to be made to support the application submitted by the applicant. New scientific evidence should not be used for the benefit of a subsequent application for a period of five years from the date of authorization of the novel food without the agreement of the original applicant (article 27 of Regulation (EU) 2015/2283). The data protection shall be granted by the Commission where the following conditions are met: (a) the newly developed scientific evidence or scientific data was designated as proprietary by the initial applicant at the time the first application was made; (b) the initial applicant had exclusive right of reference to the proprietary scientific evidence or scientific data at the time the first application was made; (c) the novel food could not have been assessed by the authority and authorised without the submission of the proprietary scientific evidence or scientific data by the initial applicant.
5. Novel Food in the European Union: A Study of Algae Trade
5.1. Materials and Methods
In order to understand the dynamics and evolutionary picture of algae for human consumption in the EU, an analysis of international trade data was carried out with the aim of verifying the relative commercial performance of the production system of the member countries and their positioning in the international context. In order to achieve this objective, specific analysis has been carried out on the statistical data made available by Eurostat. Specifically, the analysis had the purpose of taking into account the dynamics of the intra-EU and extra-EU trade flows.
In this study, the preliminary phase was to identify the product codes of the products under investigation within the international nomenclature.
In this regard, it should be noted that international trade statistics do not have a specific code for algae for human consumption since they are included in HS 12122100 (Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether ground or not) which groups together various types of algae products. Therefore, different algae products, i.e., qualitatively different and economically uneven in price, are grouped together for the same data when trade flows are in opposite directions, i.e., imports, exports and vice versa. Although aware of the difficulties of heterogeneous data, this paper proposes an analysis of on the competitiveness of countries over algae for human consumption in the world and intra-EU scenario.
Once the code was identified, data for the four-year period 2015–2018 were collected. Subsequently, the data processing phase followed, identifying suitable indicators to adequately represent the different comparative advantage enjoyed by each country in the algae sector, in particular, the following indicators of productive specialization were used:
- -
- Intensity of specialization index (IS);
- -
- Lafay index (LFI)
- -
- Grubel–Lloyd index (GLI).
The choice of these indicators is based on the following reasons.
The intensity of specialization (IS) represents the deviation between the normalized balance of a sector and the normalized balance of all sectors [23] and is expressed with the following equation:
where Xi and Mi, in this case are exports and imports of the algae sector, respectively, while all sectors constitute agri-food. This indicator has been used to verify the degree of specialization of the sector under analysis.
The Lafay [24] index of specialization is an indicator capable of determining the specialization of a country in a given sector in relative “internal” terms, that is, with respect to the other sectors that make up the economic system of that country, or in relative “external” terms, with respect to a set of countries taken as reference [25,26]. The Lafay index (LFI), taking into account imports, makes it possible to control trade flows and, therefore, is suitable for tackling the problem of fragmentation of production. [24,27,28]. The Lafay index (LFI) is expressed by the following equation:
where X and M, in this paper, are the exports and imports of algae of the individual EU country, to and from the rest of the world, respectively, and n represents the total number of agri-food sectors. Specialization is measured through net exports, i.e., through the difference between exports and imports, which is then compared to the sum of the two. In this way, a normalized ratio is obtained, that is, a value that is a function of the percentage difference between exports and imports. The sum indicates the totality of imports and exports of agri-food with respect to which it is intended, in this specific case, to study the degree of specialization of algal products. The normalization is, therefore, obtained by “weighing” the contribution of the algae sector with its respective importance in the agri-food trade balance. According to the index, the comparative advantage of a country in the production of algae is thus measured by the deviation of the product normalized trade balance from the overall normalized trade balance (agri-food), multiplied by the share of trade (imports plus exports) of algae in total agri-food trade [27]. Positive values of the Lafay index indicate the existence of a comparative advantage, and the higher the value of the index, the greater the country’s specialisation in that sector [29]. Similarly, negative values indicate a situation of despecialisation in the sector. Further studies have considered another property of the LFI is that it can vary between −50 (full despecialisation) and +50 (full specialisation) [30].
Another indicator proposed by Grubel and Lloyd [31] measures the portion of trade between two countries within the same sectors or products. Therefore, it can be used to measure intra-sectoral trade and the simultaneous export and import of goods from the same product sector [32,33,34,35]. The Grudel–Lloyd index (GLI) can be expressed empirically:
where Xi and Mi represent respectively exports and imports between and to another country of the good i.
The GLI varies between the values 0 and 1: it assumes a zero value in the case of a sector in which the country is only an exporter or only an importer, and a value of 1 if in a sector exports are exactly the same as imports, i.e., if there is a pure exchange. To identify the type of trade, it is useful to classify the values of GLI in four groups [35,36] using the following classification into four classes:
- -
- Class 1 0.00 ≤ GLI ≤ 0.25
- -
- Class 2 0.25 < GLI ≤ 0.50
- -
- Class 3 0.50 < GLI ≤ 0.75
- -
- Class 4 0.75 < GLI ≤ 1.00.
5.2. Results
The analysis of the trade flows of “seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (hereinafter SAFF) in the countries of the European Union, starting with the Eurostat database, highlights a rather fluctuating trend in trade in the period considered (2015–2018). In particular, the trend in the quantities of imported products went from 17.159 tons in 2015 to 16.381 tons in 2018, showing substantial stability in the quantities imported. With reference, on the other hand, to exports, the products sold are of lesser entity, settling in 2018 at 6222.40 tons (Table 1).

Table 1.
Quantity of Import and export of “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in the European Union (2015–2018).
The EU countries that contribute most to the volume of products imported into the EU are, in descending order, the United Kingdom, France, Germany, Ireland and Austria. Italy has imported product quantities between 496 tones and 474 tons with a peak of 581 tons in 2016. An anomalous trend is recorded by Ireland which, from quantities that place it among the main importing countries, shows insignificant import flows with values around 10 tons. With reference to exports, on the other hand, the United Kingdom, France, Ireland, Germany, and Denmark show more significant volumes for the product analyzed than other member countries. Italy, in this context, is totally reluctant to export, demonstrating that a large part of the product is consumed internally in the country.
In terms of value, SAFF’s imports in the EU, as Table 2 documents, range from 55.3 million euros in 2015 to 61.6 million euros in 2018, with the United Kingdom, France, Italy and Germany accounting for the lion’s share. A similar situation is also shown in terms of exports, where The Netherlands and Denmark have a significant weight, while Italy shows economic similarity with previous observation in terms of quantity (Tables in Supplementary Materials, Table S1, Table S2).

Table 2.
Value of Import and export of “seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether ground or not” (HS 12122100) in European Union (2015–2018).
On examining the analyses carried out, a deficit in the trade balance of the SAFF emerges in the EU countries, as reported in Table 3, as most of the member countries are strongly in deficit, due to the absence of domestic production. Therefore, they are forced to import, in some cases, substantial quantities of SAFF. Few countries, on the other hand, show a positive trade balance for SAFFs, such as Denmark, the Netherlands and Ireland, which probably demonstrates the activity of handling the processed product that is re-exported to the same EU countries. Overall, the trade balance has improved over the period considered in terms of both quantity and value.

Table 3.
Trade balance of import and export of “seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether ground or not” (HS 12122100) in the European Union (2015–2018).
Interesting results emerged from the subdivision of the total commercial data between “EU 28” and “Extra-EU 28”. The analysis carried out, again on the basis of Eurostat data, show that, as far as imports are concerned, internal movements averaged 73% on the European level, while the remaining share of imports was due to non-EU countries. Compared to the average figure, there are some member countries where the share of “Extra-EU Import 28” is more significant, e.g., Cyprus, France, Latvia, Netherlands, and Romania (Figure 1).
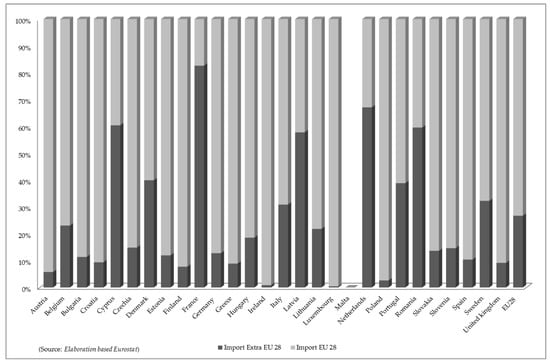
Figure 1.
Proportional distribution of imported quantities of “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in the European Union (2015–2018).
Intra-Community exports prevail in most of the member countries with the exception of Croatia, Finland, Ireland, Lithuania, Poland, and Slovenia (Figure 2) which have trade relations with non-EU countries. Overall, the average quantity of EU product exported to the various EU countries is 70%, while 30% is destined for non-EU countries.
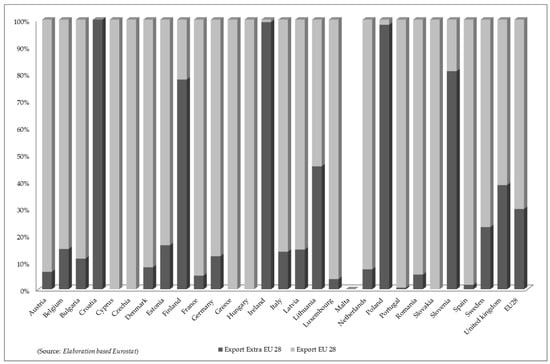
Figure 2.
Proportional distribution of exported of “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in European Union (2015–2018).
In order to measure the importance of SAFF’s trade, the indicators included in the methodology and summarised in Figure 3 have been developed. More specifically, an examination of the analyses carried out shows that the intensity of the specialisation indicator has negative values, demonstrating that in the European Union there are imbalances for the product analysed (SAFF) in the trade structure for the sector analysed.
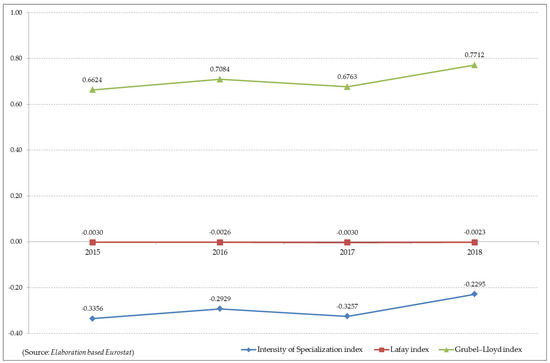
Figure 3.
Dynamics of the production specialization indicators of the “seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether ground or not” (HS 12122100) in European Union.
Through a differentiated analysis of data of individual member countries, some of them (Denmark, the United Kingdom, Sweden, Portugal, the Netherlands, Ireland, and Latvia) present positive values, proving a certain vocation for export (Tables in Supplementary Materials, Table S3, Table S4, Table S5, Table S6).
With reference to the LFI indicator, the no-processing carried out confirm the aforementioned trend, i.e., imports are higher than exports and from this trend a strong dependence on foreign countries to carry out the industrial processes of manipulation that allow the products made from algae to achieve significant export flows (Figure 3).
With regard to the GLI indicator, considering that the index assumes values between 0 and 1, the analysis carried out confirms that in the European Union the average flows are not balanced (Figure 3). It is possible to highlight a different situation within the single member states (Figure 4), with trade activities ranging from weak to strong. The GLI 0.00 to 0.25 implies that the imports are at least seven times the exports, and GLI 0.25 to 0.50 implies that the imports are three to seven times the exports. Similarly, the GLI 0.50 to 0.75 implies that the exports are 1.67 to 3.00 times the imports, and GLI 0.75 to 1.00 implies that the exports are no more than 1.67 times the imports (Tables in Supplementary Materials, Table S7, Table S8, Table S9).
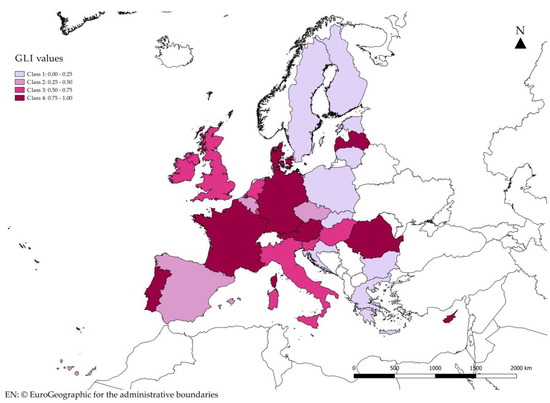
Figure 4.
The classification of GLI values.
In this study, the “external” Lafay index was also used in order to identify the specialisation in the algae sector of the different EU Member States. Figure 5 shows the distribution of the “external” LFI of the algae sector in the Member States in ascending order. Thanks to the LFI, it can be said that there are eleven member states specialising in the algae sector, in ascending order: Bulgaria, Lithuania, Latvia, Belgium, United Kingdom, Sweden, Ireland, Spain, Germany, Denmark, and the Netherlands.
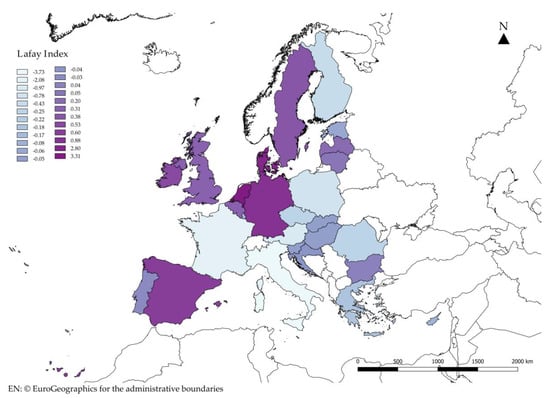
Figure 5.
Lafay index for the sector “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in European Union.
On the whole, the analysis carried out shows that the EU is highly deficient of algae and this is due to an absent internal production probably linked to the low degree of innovation in the production systems that leads to a very low level of production. Moreover, the analysis of the data shows the level of weakness of the EU in general and of the individual countries in particular, with some exceptions to be attributed in the first place to the propensity to export in general and to the possibility of acting only as a transit country for EU imports. The indicators identified highlight the level of weakness of the EU system vis-à-vis the SAFFs, which is largely due to the lack of raw materials to be used in technological production processes.
6. Discussion and Conclusions
The quality of agri-food production and the economic viability of the markets are closely linked to information. This situation does not always safeguard the correct nature of the information and conscious choices of the consumers. The economic viability of productive markets helps to create distortions in procedure capable of impeding the correct functioning according to economic theory and causes misunderstandings and asymmetry in the information given [2].
The evolution of food habits can offer new marketing frontiers for food business operators interested in novel food. However, food in the European Union historically has its own specific, deeply rooted traditions, in particular, in member states bordering the Mediterranean Sea.
The adaptation and therefore the consumption of novel food in the EU will probably occur more gradually, i.e., later than in other member countries which do not have their own typical cuisine and are instead more open to innovation and experimentation in the food sector.
In fact, the characteristic normative trait promoted by the European Union, while emphasizing the sense of curiosity for the eating habits and lifestyles of other populations or influenced by science and technology, is projected on an innovative food scenario offering a possible solution to the future problem of the alleged lack of food.
With the introduction of the regulation of novel foods, the European Union tried to establish a certain balance between free circulation of new food products and consumer protection considering the principle of precaution. Until 2013, there were only 70 applications for new food products that were authorised and registered in the “Union’s list”. Having considered the various problems, the unforeseeable development of science and technology, and needing to bear in mind the regulations, a reform was considered opportune. The reform saw the introduction of the Regulation 2015/2283 in order to protect consumers and favour the competitivity of the domestic market [7].
Despite this, it is clear to the European legislator that, in third countries, there are different laws relating to safety. The levels of safety and protection standards required do not necessarily coincide and therefore there is a risk that the levels accepted in third countries will be lower than those in Union. Lawmakers of third countries, among other things, look at the physical predispositions and habits of local consumers. This decision shows considerable flexibility on behalf of the European legislator.
In the case of foods traded in third countries that do not have a history of consumption longer than 25 years and foods that are the result of innovation, a simple ratification is not enough, and so the law sets a different path to obtain an authorization. This path is long and complex. When innovation is the discussion subject, especially in the food sector, the variables that involve safety and human health protection are more uncertain and fuzzier, if not in some cases perhaps unknown. To broaden the theme related to the validity of a notification of a traditional food from a third country sent to the commission it must be specified that the notification must include: (1) the applicant’s name and address; (2) the name and description of the traditional food; (3) an accurate composition of the traditional food; (4) the country or countries of origin of the traditional food; (5) documented data demonstrating the history of safe food use in a third country; (6) a proposal for the conditions of intended use and for specific labelling requirements, which do not mislead the consumer, or a verifiable justification why those elements are not necessary (article 14 Regulation (EU) 2015/2283).
For this reason, a difficult struggle exists for the European legislator to deal with who must balance the following conflicting needs: on the one hand, to incorporate at the legal level, technological innovations and, on the other, to balance the risks that this may entail, especially in a very sensitive field represented by the food industry [17,21].
The novel food regulation can be a serious, albeit unintended, non-tariff trade barrier to imports from the developing world into the EU. There is a tendency for traders and exporters to redirect their marketing strategies to these markets on a preferential basis [8].
According to Hermann [8], current regulations have discouraged investments in supply chains and has limited the growth of the market. This also negatively affects income generation and rural poverty alleviation in developing countries.
Serious concern exists over the development of activities promoting food export chains on the topic of food safety concerning food types that could be included in the list of novel food. Moreover, recent studies have shown how European consumers might be willing to pay for these “new” products and more variable diets. Production and export of these products would generate income for poor farmers in developing countries. In addition, this would benefit the conservation of biodiversity [17,21].
In the member states, a certain assertion has been made of some species of algae (Nori, Spiruline, etc) for human consumption. What promotes consumption, is a demand expressed by consumers who are aware that algae are of natural origin and maintain a much lower environmental impact than the production of ingredients of animal origin. Algae are environmentally sustainable and also bio-economically sustainable because the cultivation of algae does not use agricultural land and ensures noteworthy employment levels.
On an international level, the study of commercial competitivity is an excellent instrument for understanding the behaviour of the market for a certain product. Recent studies show that the European Union is not very competitive in relation to honey exports and is strongly oriented towards imports. In this context, Italy is at a notable disadvantage in terms of the sales of honey compared to countries which produce larger quantities or highly specialised products [37].
Besides the analysis of competition, interesting results can be drawn from production analyses, conservation status, and market trends of the European eel in order to contribute to the discussion on the sustainability of eel populations [38].
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/12/2/555/s1. Table S1. Value of Imports of the different agri-food sectors in the European Union (2015–2018); Table S2. Relative importance of Import “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in the European Union on the total agricultural and agri-food sector (2015–2018); Table S3. Value of Export Extra EU of the different agri-food sectors in the European Union (2015–2018); Table S4. Value of Export Intra EU of the different agri-food sectors in the European Union (2015–2018); Table S5. Value of Export of the different agri-food sectors in the European Union (2015–2018); Table S6. Relative importance of Export “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in the European Union on the total agricultural and agri-food sector (2015–2018); Table S7. Intensity of Specialization index of sector “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in European Union (2015–2018); Table S8. Internal Lafay index of sector “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in European Union (2015–2018); Table S9. Grubel-Lloyd index of sector “Seaweeds and other algae; fit for human consumption, fresh, chilled, frozen or dried, whether or not ground” (HS 12122100) in European Union (2015–2018).
Author Contributions
Funding
This work was financially supported through the project “Economic assessments of the sustainability of agri-food systems” by UNICT 2016–2018 “Piano per la Ricerca. Linea di intervento 2-Seconda annualità P7/WP2 (5A722192141)”. Project leader: Gaetano Chinnici.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pappalardo, G.; Allegra, V.; Bucca, M.; Zarbà, A.S. Euro-Mediterranean agri-food trade. Contribution to the development of the Barcelona Process: The Union for the Mediterranean. Econ. Agro-Aliment. 2013, 15, 37–72. [Google Scholar]
- Prestamburgo, S.; Sgroi, F. Agro-food markets’ functional efficiency, products’ quality and information’s role. Qual. Access Success 2018, 19, 145–149. [Google Scholar]
- Sgroi, F.; Piraino, F.; Donia, E. Determinants of ready-to-eat products purchase intentions: An empirical study among the Italian consumers. HortScience 2018, 53, 656–660. [Google Scholar] [CrossRef]
- Allegra, V.; Zarbà, C.; La Via, G.; Zarbà, A.S. Why the new orange juice consumption model favors global trade and growth in orange production. Br. Food J. 2019, 121, 1954–1968. [Google Scholar] [CrossRef]
- Zarbà, A.S.; Di Vita, G.; Allegra, V. Strategy development for Mediterranean pot plants: A stakeholder analysis. Qual. Access Success 2013, 14, 52–58. [Google Scholar]
- Allegra, V.; Zarbà, A.S.; Zarbà, C. Recent overview of the agri-food commercial economy of Italy with the rest of the world. Connections with the national economic system. Qual. Access Success 2019, 20, 13–20. [Google Scholar]
- Volpato, A. La riforma del regolamento sui Novel Food: Alla ricerca di un impossibile equilibrio? Riv. Di Dirit. Aliment. 2015, 4, 26–43. [Google Scholar]
- Hermann, M. The impact of the European Novel Food Regulation on trade and food innovation based on traditional plant foods from developing countries. Food Policy 2009, 3, 499–507. [Google Scholar] [CrossRef]
- Mehmet, A. Technological Progress, Innovation and Economic Growth; the Case of Turkey. Procedia Soc. Behav. Sci. 2015, 195, 776–782. [Google Scholar]
- Donia, E.; Mineo, A.M.; Sgroi, F. A methodological approach for assessing businness investments in renewable resources from a circular economy perspective. Land Use Policy 2018, 76, 823–827. [Google Scholar] [CrossRef]
- Testa, R.; Di Trapani, A.M.; Sgroi, F.; Tudisca, S. Economic analysis of process innovations in the management of olive farms. Am. J. Appl. Sci. 2014, 11, 1486–1491. [Google Scholar] [CrossRef]
- Zarbà, C.; Bracco, S.; Zarbà, A.S. The progress and competitiveness of SMEs from SBA up to Horizon 2020: A new approach of innovation and technological advancement. Qual. Access Success 2014, 15, 202–206. [Google Scholar]
- Raheem, D.; Shishaev, M.; Dikovitsky, V. Food System Digitalization as a Means to Promote Food and Nutrition Security in the Barents Region. Agriculture 2019, 9, 168. [Google Scholar] [CrossRef]
- Neuwirth, R.J. “Novel food for thought” on law and policymaking in the global creative economy. Eur. J. Law Econ. 2014, 37, 13–50. [Google Scholar] [CrossRef]
- Allegra, V.; Bracco, S.; Zarbà, A.S. Evolutionary trends of the agro-food enterprises and related atmospheric emission: The case of Italy. Qual. Access Success 2018, 19, 13–18. [Google Scholar]
- Zarbà, C.; Allegra, V.; Zarbà, A.S.; Zocco, G. Wild leafy plants market survey in Sicily: From local culture to food sustainability. Aims Agric. Food 2019, 4, 534–546. [Google Scholar] [CrossRef]
- Craddock, N. The EU Novel Food Regulation. Impact on the Potential Export of Exotic Traditional Foods to the EU: Suggestions for Revision. Discussion Paper Prepared for UNCTAD and CBI, in Cooperation with GTZ, GFU and IPGRI CBI by Neville Craddock Associates (United Kingdom), November 2005. Available online: http://www.underutilized-species.org/Documents/PUBLICATIONS/cbi_unctad_paper_on_eu_nfr.pdf (accessed on 11 January 2020).
- Bonora, G.I. Novel Foods nel Reg. (UE) n. 2015/2283 e gli insetti: Una possibile evoluzione dei costumi alimentari? Riv. Di Dirit. Aliment. 2016, 1, 42–54. [Google Scholar]
- Van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; FAO Forestry Paper 171; FAO: Rome, Italy, 2013. [Google Scholar]
- Lähteenmäki-Uutela, A. European novel food legislation as a restriction to trade. Presented at the seminar Pro-poor Development in Low-income Countries, Montpellier, France, 25–27 October 2007. [Google Scholar]
- Harrison-Dunn, A. UK FSA Closes Door on Novel Food Applications as Austerity Bites. Available online: https://www.nutraingredients.com/Article/2014/04/18/UK-novel-food-agency-closes-doors-to-new-applications (accessed on 28 October 2019).
- Smil, V. Worldwide transformation of diets, burdens of meat production and opportunities for novel food proteins. Enzym. Microb. Technol. 2002, 30, 305–311. [Google Scholar] [CrossRef]
- Subioli, G. Un’applicazione degli indici di Sophistication al Commercio Agroalimentare. Ph.D. Thesis, Università degli Studi della Tuscia, Viterbo, Italy, 2010. [Google Scholar]
- Lafay, G. The Measurement of Revealed Comparative Advantages; Dagenais, M., Ed.; International Trade Modeling, Chapman & Hall: London, UK, 1992. [Google Scholar]
- Boffa, F.; Bolatto, S.; Zanetti, G. Specializzazione Produttiva e Crescita: Un’analisi Mediante Indicatori; Working Paper Ceris-Cnr; n. 1/2009; Research Institute on Sustainable Economic Growth: Moncalieri, Italy, 2009. [Google Scholar]
- Fanti, J. L’internazionalizzazione e le Specializzazioni Commerciali delle regioni Italiane nel Settore Agroalimentare. Ph.D. Thesis, Alma Mater Studiorum Università di Bologna, Bologna, Italy, 2015. [Google Scholar] [CrossRef]
- Desai, F.P. Trends in Fragmentation of Production: A Comparative Study of Asia and Latin America. Procedia Soc. Behav. Sci. 2012, 37, 217–229. [Google Scholar] [CrossRef]
- Ferrarini, B.; Scaramozzino, P. Production complexity, adaptability and economic growth. Struct. Chang. Econ. Dyn. 2016, 37, 52–61. [Google Scholar] [CrossRef]
- Borin, A.; Lamieri, M. Misurare la qualità dei beni nel commercio internazionale. In Eppur si Muove. Come Cambia L’export Italiano; Lanza, A., Quintieri, B., Eds.; Rubbettino: Soveria Mannelli, Italy, 2007. [Google Scholar]
- Marconi, D.; Rolli, V. Comparative Advantage Patterns and Domestic Determinants in Emerging Countries; Research paper no. 2008/81; World Institute for Development Economic Research: Helsinki, Finland, 2008. [Google Scholar]
- Grubel, H.; Lloyd, P.J. Intra Industry Trade: The Theory and Measurement of International Trade in Differentiated Products; Halsted Press: New York, NY, USA, 1975. [Google Scholar]
- Grubel, H.G.; Lloyd, P. The Empirical Measurement of Intra-Industry Trade. Econ. Rec. 1971, 47, 494–517. [Google Scholar] [CrossRef]
- Zarbà, A.S.; Allegra, V.; Pappalardo, G. Current scenarios in the Romanian agri-food trade inside the Eu. Qual. Access Success 2013, 14, 85–92. [Google Scholar]
- Allegra, V.; Zarbà, C.; Zarbà, A.S. Propulsive competitive scenarios for a new qualification of almond sector. Qual. Access Success 2016, 17, 99–105. [Google Scholar]
- Hoang, V. The Dynamics of Agricultural Intra-Industry Trade: A Comprehensive Case Study in Vietnam. Struct. Chang. Econ. Dyn. 2019, 49, 74–82. [Google Scholar] [CrossRef]
- Qasmi, B.A.; Fausti, S.W. NAFTA Intra-industry Trade in Agricultural Food Products. Agribusiness 2001, 17, 217–229. [Google Scholar] [CrossRef]
- Pippinnato, L.; di Vita, G.; Brun, F. Trade and comparative advantage analysis of the EU honey sector with a focus on the Italian market. Qual. Access Success 2019, 20, 485–492. [Google Scholar]
- Violi, L.; Falcone, G.; De Luca, A.I.; Chies, L. Sustainability of European eel population: A statistical survey on production, conservation status and market trends. Qual. Access Success 2015, 16, 83–90. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).