Abstract
Every year, a large amount of residual agroindustrial waste has been generated and only around 10% is in fact reused. The development of new strategies for biomass valorization is important to add value to these commodities, since biomass is an excellent alternative feedstock to obtain chemicals of interest from renewable resources. The major compound of pyrolytic treatment of lignocellulosic biomass is levoglucosan (1,6-anhydroglucopyranose), an anhydro-sugar that can be transformed into glucose and is greatly valued in the most diverse industrial sectors as a surfactant, emulsifier, or even a lubricant. In this work, levoglucosan was acylated by lipase-catalyzed transesterification in acetonitrile with great conversions and selectivities with different acyl donors such as ethyl esters of lauric, palmitic, stearic, and oleic acids prepared in situ in an integrated strategy mediated by commercial lipases Novozym435 (N435), PSIM, and the home-made biocatalyst CaLB_epoxy. As a result, all biocatalyst generated mostly monoesters, with N435 being more selective to produce lauric esters (99% at 50°C) and PSIM to produce oleic esters (97% at 55 °C) while CaLB_epoxy was more selective to produce oleic esters of levoglucosan (83% at 55°C). This is the first report in the literature on the production of high selectivity levoglucosan esters.
1. Introduction
Biotechnology has focused its attention on the development of sustainable processes. The Twelve Principles of Green Chemistry reflect the need for actions in the development of bioprocesses both at universities and in industries [1]. In this context, the availability of cheap raw materials and renewable energy is the basis for global sustainability, where strategies for the development of clean technologies for chemical processes has aimed to balance economic and environmental aspects [2]. In this trend, the application of enzymes as biocatalysts and the reuse of agroindustry waste are key-procedures in the current initiatives that are aimed at the development of sustainable chemical synthesis [3].
Every year, a substantial amount of residual lignocellulosic biomass is generated and only 10% is in fact reused. Therefore, the development of new strategies is important to add value to these commodities [4,5]. The sugarcane sector has high use of lignocellulosic biomass to obtain biofuels, such as first-generation ethanol, as well as small reuse of bagasse to obtain second- and third-generation ethanol. Its composition ranges from 40% to 50% cellulose, 20% to 30% hemicellulose, 20% to 25% lignin, and 1.5% to 3% ash [6,7,8].
Thus, new biomass valorization methods can be applied, such as pyrolysis—a process that can generate various organic compounds in short intervals of time. Among the products of rapid pyrolysis is bio-oil, a viscous mixture resulting from the oxidation of biomass polymers, of which their main component is levoglucosan (Figure 1) [9], an anhydrous sugar that can be hydrolyzed to glucose, and is used in fermentative processes to obtain various products. However, this molecule offers other chemical possibilities as it has a hydrophobic moiety and free hydroxyls [10]. Levoglucosan can be transformed into derived esters with a hydrophobic/hydrophilic balance that is of great value as surfactants, emulsifiers, or even lubricants, showing great potential of application in the most diverse industrial sectors, as our research group has observed with other esters that are produced by enzymatic routes [11].
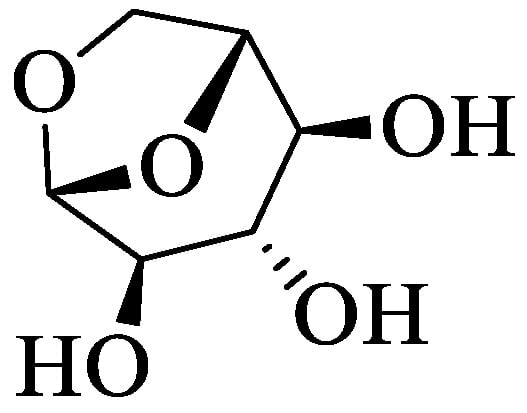
Figure 1.
Levoglucosan.
Amphiphilic esters are currently produced from alkaline glycerolizes of natural oils and fats at elevated temperatures and pressure. In addition to high energy consumption, the conditions that are used result in low productivity and the formation of polymerization byproducts, resulting in dark staining and poor product quality, which requires expensive later purification steps [12]. In this way, the use of enzymatic processes can overcome these issues and lead to a greener approach. Among the several enzymes that are currently applied in biocatalysis, lipases (triacylglycerol ester hydrolases E.C. 3.1.1.3) [13] have been outstanding for their great industrial application in the production of high added value esters due to, among other advantages, their ability to show activity both of hydrolysis and esterification, the non-requirement of cofactors, and their ability to be able to perform reactions with high regio-, enantio-, and chemo-selectivity. Lipase-catalyzed transformations play an important role in organic synthesis to fulfill the rapidly growing demand for enantiomerically pure compounds such as pharmaceuticals, agrochemicals, and natural products, mainly due to no requirement for cofactors and also the capacity of catalyzing different reactions such as asymmetric esterification, asymmetric transesterification, and asymmetric hydrolysis.
Lipase immobilization is an effective method to improve the performance of free enzymes. Compared with free enzymes, the immobilized enzymes with long-term operational stability can be easily recovered and reutilized, thus reducing the costs of catalytic processes [13]. The activity of the immobilized lipases depends on some parameters such as enzyme and support types and the protocols of immobilization. Immobilization of lipases on different supports or different protocols in the same support allows the development of systems with different stabilities and specificities, depending on the support used, the intensity of the enzyme–support interaction, and the orientation of the enzyme molecule on the support surface. The immobilization of an enzyme may produce different adverse effects on the enzyme activity because the enzyme molecule may become distorted, mainly when some multi-interactions between the enzyme and the support occur. Also, the active center may become blocked by the immobilization itself [14]. Finally, immobilization may promote diffusion problems of the substrate. These problems may have a different impact on the activity of the enzyme depending on the different immobilization strategies. Many functional groups of carriers, such as epoxy, amide, and aldehyde, have been extensively used for covalent binding of lipases [15]. Supports containing epoxy groups are characterized as having short spacer arms and being very stable at a neutral pH. They have been reported to immobilize enzymes through multipoint covalent attachment at their amino, phenolic, and thiol groups at an alkaline pH or at their carboxylic groups at a moderately acidic pH.
In this work, we performed both the esterification and transesterification of levoglucosan with different acyl donors catalyzed by the lipases Novozym 435®, PSIM®, and the home-made lipase CaLB_epoxy, aiming to evaluate conversion and selectivity for the obtained mono- and diesters in the batch processes.
2. Materials and Methods
2.1. Materials
Lipase B from Candida antarctica (CaLB, soluble form), N435 (immobilized CaLB on macroporous acrylic resin ion exchange) were purchased from Novozymes®. Lipase from Pseudomonas Cepacia was purchased from Sigma-Aldrich and 1,6-anhydroglucopyranose (levoglucosan) was purchased from Start BioScience. Epoxy resin Purolite® ECR8205F was purchased from Purolite International Limited. Aliphatic carboxylic acids, N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA), N O-Bis(trimethylsilyl)trifluoroacetamide(BSTFA), and tert-butyldimethylsilyl chloride (TBDMSCl) were purchased from Sigma-Aldrich. All other reagents that were used were of analytical grade.
2.2. Immobilization Procedures
2.2.1. Immobilization of Lipase CaLB
Epoxy resin Purolite® ECR8205F polymer was applied as support for CaLB immobilization by hydrophobic adsorption (CaLB_epoxy). For this purpose, 1 mL of the enzyme solution (0.62 U/mL of specific activity of both lipases) was diluted in 20 mL of phosphate buffer (25 mM and pH 7.0) and added to the appropriate support (3 g). The mixture was stirred for 4 h at 20°C at 180 rpm, followed by vacuum filtration. At the end, the supernatant was collected and stored for protein quantification. The solid material was gravity filtered and dried at 25°C.
2.2.2. Hydrolytic Activity Assay
The activities of free and immobilized lipases were determined as follows: 1 mL of extract or 10 mg of each supported enzyme were added to 19 mL of an emulsion that was prepared with olive oil (5% v/v) and arabic gum (10% v/v) in sodium phosphate buffer (100 mM, pH 7.0). The reactions were carried out under stirring (200 rpm) at 35°C for 30 min. The reactions were then stopped by the addition of 20 mL of acetone–ethanol mixture (1:1 v/v) and the fatty acids that were produced were extracted under agitation (200 rpm) for 10 min and titrated until end-point (pH 11.0) with NaOH solution (0.04 N). The blank assays were performed by adding the extract just after the addition of the acetone–ethanol solution to the flask. One unit of lipase activity (U) was defined as the amount of enzyme that catalyzes the release of 1 µmol of fatty acids per minute under the assay conditions. All samples were compared with the commercial preparation N435.
2.2.3. Levoglucosan Derivatization Method
In order to obtain the best resolution in the analysis of the acylation products of levoglucosan by gas chromatograph equipped with a mass spectrometry detector (GC-MS) as well as to determine the selectivity of the lipases on the position of the hydroxyl groups, we investigated silylation with BSTFA, MSTFA, and tert-butyldimethylsilane chloride.
Silylation with BSTFA and MSTFA
For the derivation of OH-levoglucosan, 20 µL of the sample were transferred to gas chromatography (GC) auto-sampling flasks containing 50 µL of pyridine and 50 µL of the derivatizing agent [14]. The flasks were purged with argon gas (Ar), sealed, and coated with Teflon. The derivative conditions were determined after the investigation of various experimental parameters including the reaction time and the temperature on the analytical responses of the compounds, TLC (Thin Layer Chromatography) monitoring [15].
Silylation with tert-butyl Dimethyl Silane Chloride
Triethylamine (5.54 mL, 39.80 mmol) at ambient temperature under argon was added to a solution of the levoglucosan compound (992.00 mg, 6.12 mmol) in dichloromethane (75 mL). The mixture was cooled to 0°C and tert-butyldimethylsilane chloride (5.40 mL, 30.6 mmol) was slowly added in the reaction medium. The ice bath was removed and the reaction was kept under stirring at room temperature for 3 h and 30 min, TLC monitoring. The mixture was evaporated under reduced pressure, the residue was diluted with hexane (20 mL), and the salt was filtered followed by washing with hexane.
2.3. Lipase-Catalyzed Acylation of Levoglucosan
2.3.1. Esterification of Long-Chain Fatty Acids
A stock solution containing the appropriate amount of fatty acid (lauric, palmitic, stearic, or oleic) and ethanol (1:1—7.50 mmol, 150 mM) in hexane was prepared and added to a 50 mL flask with the biocatalyst N435 (200 mg). The reactions were performed at 40°C during 2 h and 200 rpm of stirring on a shaker. At the end, the samples were collected and derivatized with 10 μL of MSTFA and diluted to 1.0 mL of ethyl acetate and subsequently analyzed on a gas chromatograph equipped with a mass spectrometry detector (GC-MS).
2.3.2. Transesterification of Levoglucosan
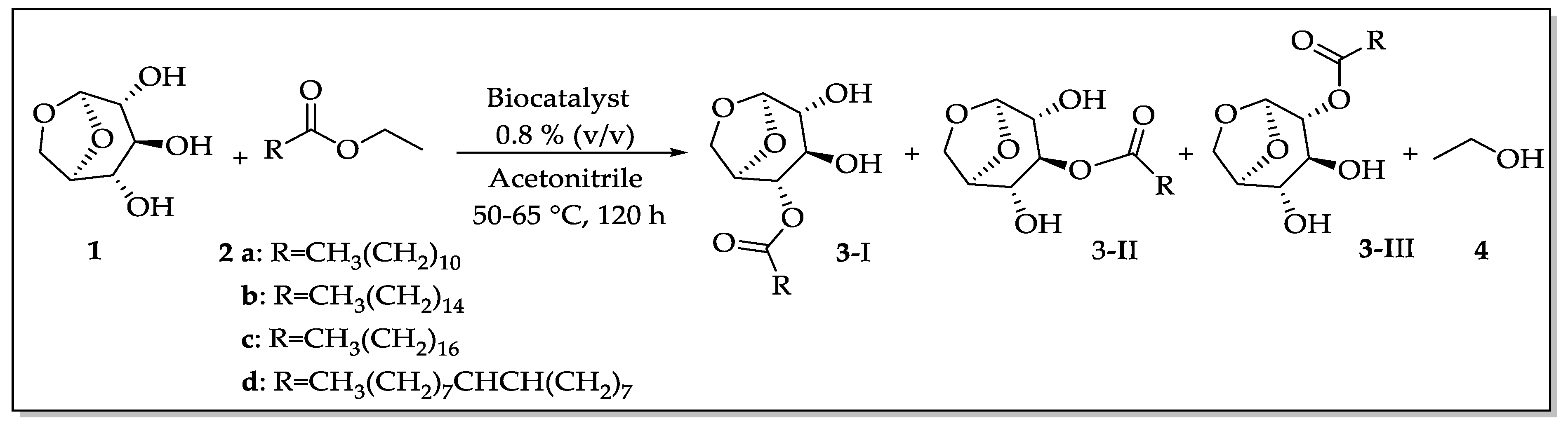
For initial screening, the esterification of levoglucosan was performed following the methodology previously described by Galleti et al., 2007 [11] with adaptations: the first step consisted of the production of the acyl donors. All transesterification reactions were performed having acyl donors as the ethyl esters of lauric, palmitic, stearic, and oleic acids (see procedure 2.3.1).
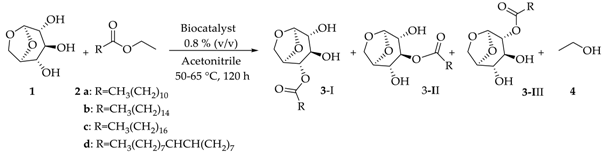
For transesterification experiments (Scheme 1), levoglucosan (1 mmol, 163 mg), and the appropriate acyl donor (2 mmol) were introduced into a vial containing 5 mL of CH3CN and the supported enzyme (40 mg, 0.8 % (v/v)). The mixture was stirred at 50–65°C for about five days, and the reaction progress was monitored by TLC and GC–MS. To stop the reaction, the biocatalysts were filtered and the products were separated by flash chromatography. The monoesters were easily separated, which were obtained as a mixture. The final products were carefully identified by NMR:1H, 13C, HMBC, HSQC,1H,1H-COSY (“Correlation Spectroscopy”) and NOEdiff (NOE “difference spectroscopy”) spectra, FTIR spectrum ν (cm−1, KBr), HRMS (High-Resolution Mass Spectrometry—for further details, see Support Information), and GC–MS analysis.
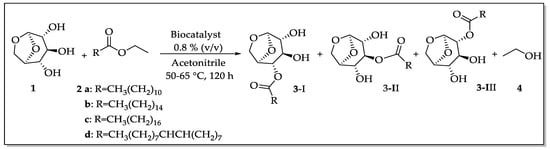
Scheme 1.
General procedure for lipase-catalyzed transesterification reaction of levoglucosan (1). Conditions: 2 ethyl esters of a: lauric, b: palmitic, c: stearic, and d: oleic acids (2.0 mmol), 3 levoglucosan monoesters aliphatic chains, and 4 ethanol.
4-O-Lauryl-1,6-anhydroglucopyranose
1H NMR (500 MHz, DMSO) δ 5.26 (d, J = 4.7 Hz, 1H, OH), 5.18 (s, 1H, H1), 5.13 (d, J = 5.1 Hz, 1H, OH), 4.43 (m, 1H, H4), 4.41 (m, 1H, H5), 3.90 (dd, J = 6.7 Hz, 1H, H6 endo), 3.52 (dd,J= 7.1 and 5.4, 1H, H6 exo), 3.37 (m, 1H, H3), 3.22 (d, J = 4.2 Hz, 1H, H2), 2.32 (t, J = 7.4 Hz, 2H, ), 1.54 (dt, J = 14.4, 7.3 Hz, 2H, 1.24 (m, 16H, ), 0.85 (t, J = 6.9 Hz, 3H, ).13C NMR (500 MHz, DMSO-d6) δ 172.28, 102.03, 76.57, 74.88, 69.47, 69.33, 65.29, 34.17, 33.98, 31.75, 29.44, 29.33, 29.16, 28.87, 28.83, 24.86, 22.55, 14.42. HRMS calculated for (M+Na)+ 367.2091, found: 367.2114. Rf: 0.29 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuric acid/Ethanol).
2-O-Lauryl-1,6-anhydroglucopyranose
1H NMR (400 MHz, DMSO-d6) δ 5.21 (s, 1H, H1), 5.18 (d, J = 6.6 Hz, 1H, OH), 5.11 (d, J = 7.0 Hz, 1H, OH), 4.57 (m, 1H, H2), 4.40 (td, J = 6.0, 1.0 Hz, 1H, H5), 3.83 (dd, J = 7.4 Hz, 0.9 Hz, 1H, H6 endo), 3.56 (dd, J = 7.3 Hz, 5.8 Hz, 1H, H6 exo), 3.35 (m, 1H, H4), 3.16 (m, 1H, H3), 2.29 (t, J = 11.7Hz, 2H, ), 1.51 (m, 2H, ), 1.24 (m, 16H, ), 0.85 (t, J = 6.8 Hz, 3H, ).13C NMR (500 MHz, DMSO-d6) δ 172.28, 102.05, 76.58, 74.89, 69.49, 69.34, 65.29, 34.17, 33.98, 31.75, 29.44, 29.33, 29.15, 28.88, 28.83, 24.86, 22.55, 14.41. HRMS calculatedfor (M+Na)+ 367.2091, found367.2114. Rf: 0.21 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuric acid/Ethanol).
4-O-Palmitoyl-1,6-anhydroglucopyranose
1H NMR (500 MHz, CDCl3) δ 5.51 (s, 1H, H1), 4.75 (m, 1H, H4), 4.59 (d, J = 6.0 Hz, 1H, H5), 4.24 (d, J = 7.1 Hz, 1H, H6 endo), 3.81 (dd, J = 7.9, 5.5 Hz, 1H, H6 exo), 3.78 (m, 1H, H3), 3.56 (m, 1H, H2), 2.39 (m, 2H, ), 1.65 (m, 2H, ), 1.28 (m, 24H, ), 0.88 (t, J = 7.0 Hz, 3H, ).13C NMR (400 MHz, CDCl3) δ 173.68, 102.21, 74.30, 72.43, 71.20, 69.58, 65.73, 34.13, 31.71, 29.64, 29.58, 29.54, 29.44, 29.39, 29.25, 29.21, 29.17, 29.10, 29.06, 24.72, 22.15, 13.96.HRMS calculated for (M+Na)+423.2717, found: 423.2717. Rf: 0.29 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuric acid/Ethanol).
2-O-Palmitoyl-1,6-anhydroglucopyranose
1H NMR (400 MHz, CDCl3) δ 5.44 (s, 1H, H1), 4.76 (m, 1H, H2), 4.58 (d, J = 5.3 Hz, 1H, H5), 4.06 (dd, J = 7.5, 0.4 Hz, 1H, H6 endo), 3.81 (dd, J = 7.5, 5.8 Hz, 1H, H6 exo), 3.63 (m, 1H, H4), 3.56 (m, 1H, H3), 2.34 (t, J = 7.5, 1.9 Hz, 2H, ), 1.60 (m, 2H, ), 1.28 (m, 24H, ), 0.88 (t, J = 6.8 Hz, 3H, ).13C NMR (400 MHz, CDCl3) δ 173.15, 101.46, 76.15, 73.75, 69.07, 68.20, 65.11, 34.50, 32.07, 29.84, 29.81, 29.74, 29.66, 29.59, 29.55, 29.51, 29.37, 29.30, 29.22, 24.99, 22.84, 14.25. HRMS calculated for (M+Na)+ 423.2717, found: 423.2898. Rf: 0.21 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuricacid/Ethanol).
4-O-Estearyl-1,6-anhydroglucopyranose
1H NMR (500 MHz, CDCl3) δ 5.50 (s, 1H, H1), 4.73 (d, J = 0.6 Hz, 1H, H4), 4.58 (d, J = 5.1 Hz, 1H, H5), 4.24 (d, J = 7.8 Hz, 1H, H6 endo), 3.80 (dd, J = 7.7, 5.5 Hz, 1H, H6 exo), 3.76 (m, 1H, H3), 3.56 (d, J = 1.3 Hz, 1H, H2), 2.39 (t, J = 7.6 Hz, 2H, CH2CH2COO), 1.66 (m, 2H, CH2CH2COO), 1.29 (m, 28H, CH3(CH2)14), 0.88 (t, J = 6.9 Hz, 3H, CH3CH2).13C NMR (500 MHz, CDCl3) δ 172.96, 102.24, 74.52, 72.68, 71.48, 70.15, 65.90, 34.41, 32.07, 29.84-29-78, 29.73, 29.66, 29.59, 29.50, 29.37, 29.22, 27.37, 27.34, 25.04, 22.83, 14.26. HRMS calculated for (M+Na)+ C24H44O6: 451.3030, found: 451.2897. Rf: 0.30 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuricacid/Ethanol).
2-O-Estearyl-1,6-anhydroglucopyranose
1H NMR (400 MHz, CDCl3) δ 5.44 (s, 1H, H1), 4.75 (m, 1H, H2), 4.59 (d, J = 1.5 Hz, 1H, H5), 4.06 (d, J = 7.4 Hz, 1H, H6 endo), 3.80 (dd, J = 7.6, 5.8 Hz, 1H, H6 exo), 3.61 (m, 1H, H4), 3.55 (m, 1H, H3), 2.34 (t, J = 7.4Hz, 2H, CH2CH2COO), 1.62 (m, 2H, CH2CH2COO), 1.29 (m, 28H, CH3(CH2)14), 0.88 (t, J = 6.8 Hz, 3H, CH3CH2).13C NMR (400 MHz, CDCl3) δ 173.05, 102.26, 76.12, 73.75, 69.01, 68.10, 65.09, 34.41, 32.07, 29.92, 29.83, 29.80, 29.74, 29.67, 29.59, 29.50, 29.48, 29.37, 29.28, 29.22, 27.34, 24.99, 22.83, 14.25. HRMS calculated for (M+Na)+ C24H44O6: 451.3030, found: 451.2978. Rf: 0.22 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuric acid/Ethanol).
4-O-Oleoyl-1,6-anhydroglucopyranose
1H NMR (500 MHz, CDCl3) δ 5.50 (s, 1H, H1), 5.34 (m, 2H, 9′ and H10′), 4.74 (m, 1H, H4), 4.58 (d, J = 4.9 Hz, 1H, H5), 4.24 (d, J = 7.7 Hz, 1H, H6 endo), 4.12 (dd, , 1H, OH), 3.81 (dd, J = 7.6, 5.7 Hz, 1H, H6 exo), 3.78 (m, 1H, H3), 3.57 (m, 1H, H2), 2.63 (m, 1H, OH), 2.39 (t, J = 7.6 Hz, 2H, CH2CH2COO), 2.05 (m, 4H, H8′ and H11′), 1.64 (m, 2H, CH2CH2COO), 1.29 (m, 20H, H4′-H7′ and H12′-H17′), 0.88 (t, J = 6.6 Hz, 3H, CH3CH2).13C NMR (500 MHz, CDCl3) δ 172.88, 130.21, 129.86, 102.27, 74.54, 72.70, 71.53, 70.17, 65.91, 34.40, 32.05, 29.92, 29.83, 29.67, 29.47, 29.46, 29.28, 29.23, 29.19, 27.35(2C), 25.04, 22.82, 14.24.HRMS calculated for (M+Na)+ C24H42O6: 449.2833, found: 449.3098. Rf: 0.30 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuric acid/Ethanol).
2-O-Oleoyl-1,6-anhydroglucopyranose
1H NMR (500 MHz, CDCl3) δ 5.44 (s, 1H, H1), 5.34 (m, 2H, 9′-H10′), 4.76 (m, 1H, H2), 4.58 (d, J = 5.4 Hz, 1H, H5), 4.06 (d, J = 7.0 Hz, 1H, H6 endo), 3.81 (dd, J = 7.4, 5.9 Hz, 1H, H6 exo), 3.61 (s, 1H, H4), 3.54 (d, J = 1.0 Hz, 1H, H3), 2.34 (t, J = 7.5Hz, 2H, CH2CH2COO), 2.00 (m, 4H, H8′ and H11′), 1.64 (m, 2H, CH2CH2COO), 1.29 (m, 20H, H4′-H7′ and H12′-H17′), 0.87 (t, J = 6.9 Hz, 3H, CH3CH2).13C NMR (500 MHz, CDCl3) δ 173.09, 130.21, 129.82, 101.44, 76.09, 73.72, 69.02, 68.11, 65.08, 34.50, 32.04, 29.90, 29.82, 29.73, 29.66, 29.46, 29.27, 29.22, 29.18, 27.37 (2C), 24.96, 22.82, 14.25.HRM calculated for (M+Na)+ C24H42O6: 449.2833, found: 449.2852. Rf: 0.22 (Eluent: Ethyl acetate/Hexane 1:1—Developer: Sulfuric acid/Ethanol).
2.4. Gas Chromatography Analysis
Helium was applied as the carrier gas in all analyses. Analyses were performed in a GC-MS (Shimadzu CG2010 –SLB-5MS capillary column 30 m, 0.25 mm ID, 0.25 um film thickness). Samples were prepared by diluting 20μL of the final product in 980 μL of ethyl acetate. The injector and detector temperatures were 250°C and the oven was maintained at 80°C for 4 min, increased to 200°C at a rate of 6°C/min for 10 min, increased to 310°C at a rate of 8°C/min, then it was held constant for 4 min, totaling 51.75 min.
3. Results and Discussion
Lipase-catalyzed Acylation of 1,6-anhydroglucopyranose
We started this work investigating the influence of the main reaction parameters in order to find the best conditions for the production of mono and diesters of levoglucosan from acyl donors with long carboxylic acid chain (>12C) both by lipase-catalyzed esterification and transesterification. The selectivity of commercial and home-made biocatalysts was also evaluated. Initially, the esterification of commercial levoglucosan was evaluated by applying long chain fatty acids with acetonitrile as a solvent, as described in the experimental section. As expected, none of the biocatalysts demonstrated satisfactory ester conversion (<30%—data not shown) under the conditions investigated, where lauric acid showed the best result (30% of conversion and poor selectivity). Galleti et al. (2007) [11] applied the lipase PS (Pseudomonas cepacia) on acylation of levoglucosan with several acyl donors, such as vinyl esters (acetate and laurate) and carboxylic acids (acetic and lauric) in alternative green solvents, where CH3CN and the ionic liquid [MOEMIm] [dca] were the most suitable. As a result, carboxylic acids could effectively substitute vinyl esters as acylating agents when ionic liquids were the solvent of choice. The reaction times were quite long (more than 5 days) with poor selectivity to monoesters.
Chemical strategies for the synthesis of levoglucosan esters with acyl chlorides have already been described in the 1990s [16]. In addition, just a few papers have explored the enzymatic esterification of this anhydrous sugar. However, in levoglucosan, the carbons C1 and C6 present an acetalic function and the remaining secondary hydroxyl groups are in the axial position. Therefore, levoglucosan is much less reactive than glucose or other sugars, both to chemical or enzymatic attack. This peculiar feature can be extended to the regioselective acylation of a single secondary OH function that represents a very challenging task.
The second strategy was to evaluate the performance of previously mentioned biocatalysts on the transesterification of levoglucosan using several acyl donors at different temperatures (Scheme 1), aiming to investigate the selectivity for mono- and diesters. Results are shown in Table 1.

Table 1.
Acylation of levoglucosan in CH3CN.
Analysis by TLC analysis (Rf = 0.30 and Rf = 0.21) and subsequent GC-MS confirmation demonstrated that monoesters could be obtained as major products in all reactions together with traces of diesters (<1% relative intensity could be noted after separation and analysis by high resolution mass spectrometry).
According to Table 1, better conversions could be achieved by N435 using ethyl laurate and ethyl palmitate as acyl donors at 55°C and 50°C, respectively (Entry 1 and 2). Comparing these results for those obtained by CaLB_epoxy, a change in substrate preference was observed, leading to average conversions in levoglucosan palmitate at 50°C and 55°C. Surprisingly, high conversions in levoglucosan oleate was also obtained (93%, Entry 8), which until now, is a result that has not been reported. The lipase CaLB presents a very reduced hydrophobic lid and, therefore, does not require interfacial activation to perform hydrolysis and esterification reactions. N435 is a commercial preparation containing CaLB immobilized in cationic resin by ionic adsorption, with hydrolysis activity of 256 U/g. The home-made CaLB_epoxy biocatalyst contains CaLB immobilized by hydrophobic adsorption on epoxiresin Purolite® ECR 82057, with hydrolysis activity of 419 U/g. Previous work of our group [13,14,15]. demonstrated that this derivative also showed higher esterification potential than N435 with a protein load of 11 mg/g of support against 15 mg/g of support presented by N435. In addition, the immobilization method that was applied in the new biocatalyst may have been responsible for a conformational modification of the enzyme as a product of protein-support hidrofobic interactions, making the catalytic site more suitable to receive the ethyl oleate acyl donor selectively. The use of epoxy-functionalized resins as enzyme support is advantageous because they have been considered as dual resins, since they are versatile and can generate different interactions with the enzymes to be immobilized according to changing the pH and the ionic force that is applied in the immobilization environment. These supports are very stable at a neutral pH, even under humid conditions, and can be stored for a long period. In addition, these supports can stabilize the enzyme by multipoint covalent binding, resulting in high stabilization to the derivatives [16,17,18]. The epoxy groups undergo nucleophilic attack of different groups on the surface of the enzyme such as amino, thiol, and hydroxyl, allowing intense interactions between the enzyme and the support. Epoxy groups are weakly reactive under mild conditions such as neutral pH and low ionic strength. Thus, the immobilization using this type of support must be performed in two steps: in the first, the enzyme is adsorbed onto the surface of the support [19,20]. High ionic strength is required at this stage to force the hydrophobic adsorption of the proteins on the support. After a long incubation time of the enzyme adsorbed at pH 7.0, the derivative is incubated at alkaline pH (around 10.0) to effect multi-interactions between the enzyme and the support through covalent bonding promoted by epoxy groups on the susceptible amino acids of the enzyme, giving greater stiffness to the derivative and thus, obtaining derivatives with high thermal stability. High conversions were also observed for lipase PSIM, mainly at 55°C, where similar conversions to palmitate, stearate, and oleate were demonstrated. In addition, the values were also higher than PSIM (8750.0 U/g), despite having high hydrolysis activity, and showed conversions below 75% for all the conditions that were analyzed.
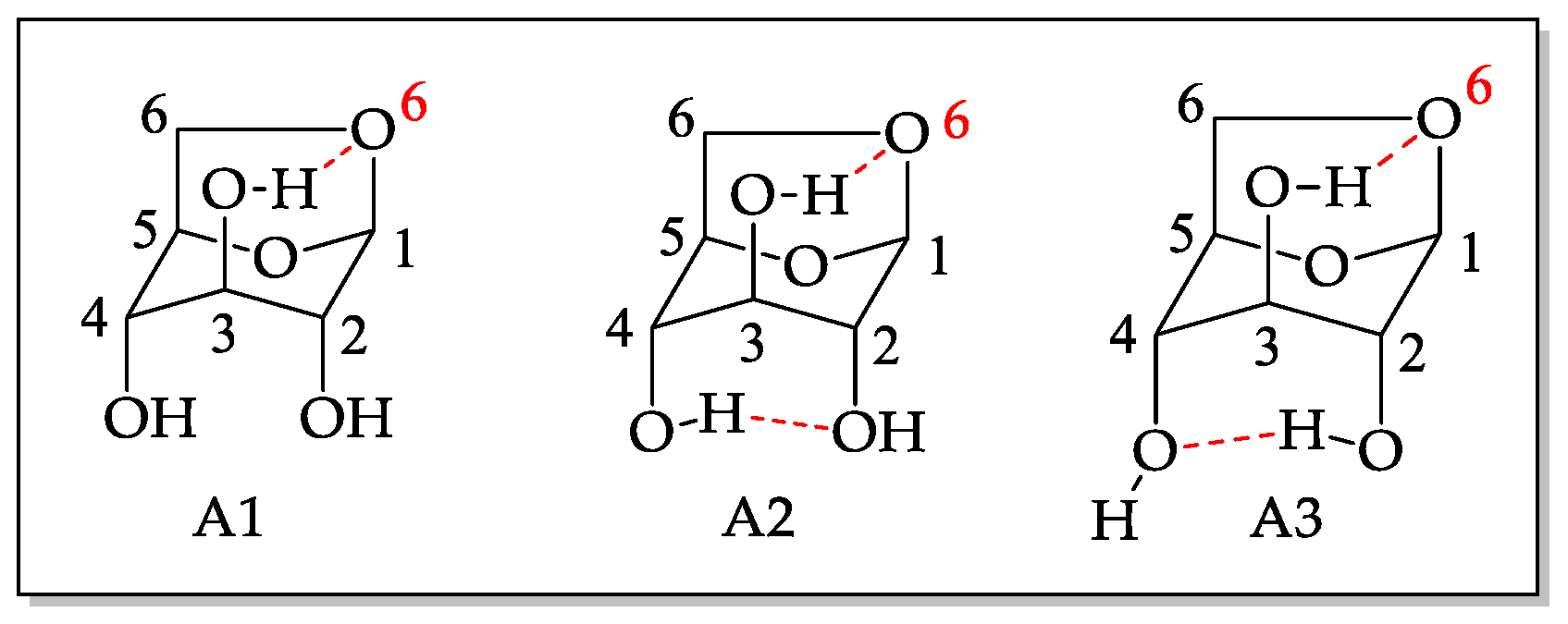
The study of levoglucosan conformation is important to understand the obtained results since the presence of three hydroxyls in the structure allows the formation of monoesters and diesters at positions C2, C3, and C4. Under the studied conditions, it was not possible to find significant diester formation. However, studies were done to evaluate lipase regioselectivity towards levoglucosan monoesters. The pyranose ring of levoglucosan can adopt two conformations: inverted chair and boat type [21,22]. In the chair conformation, the intramolecular interactions, due to hydrogen bonding occurring between the hydroxyl of C3 in the axial position and the oxygen in C6, also in the axial position, contribute to a greater stabilization of the molecule, increasing its population, but decreasing its reactivity as a nucleophile [23]. The same interaction, hydrogen donor-acceptor, can occur with the hydroxyls in C2 and C4, according to Figure 2.
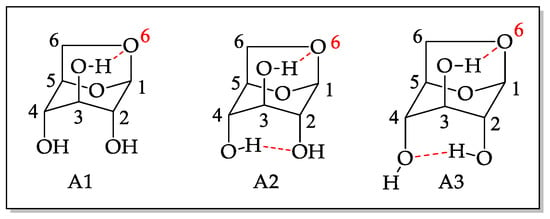
Figure 2.
Possible conformational structures for levoglucosan in the axial position [22].
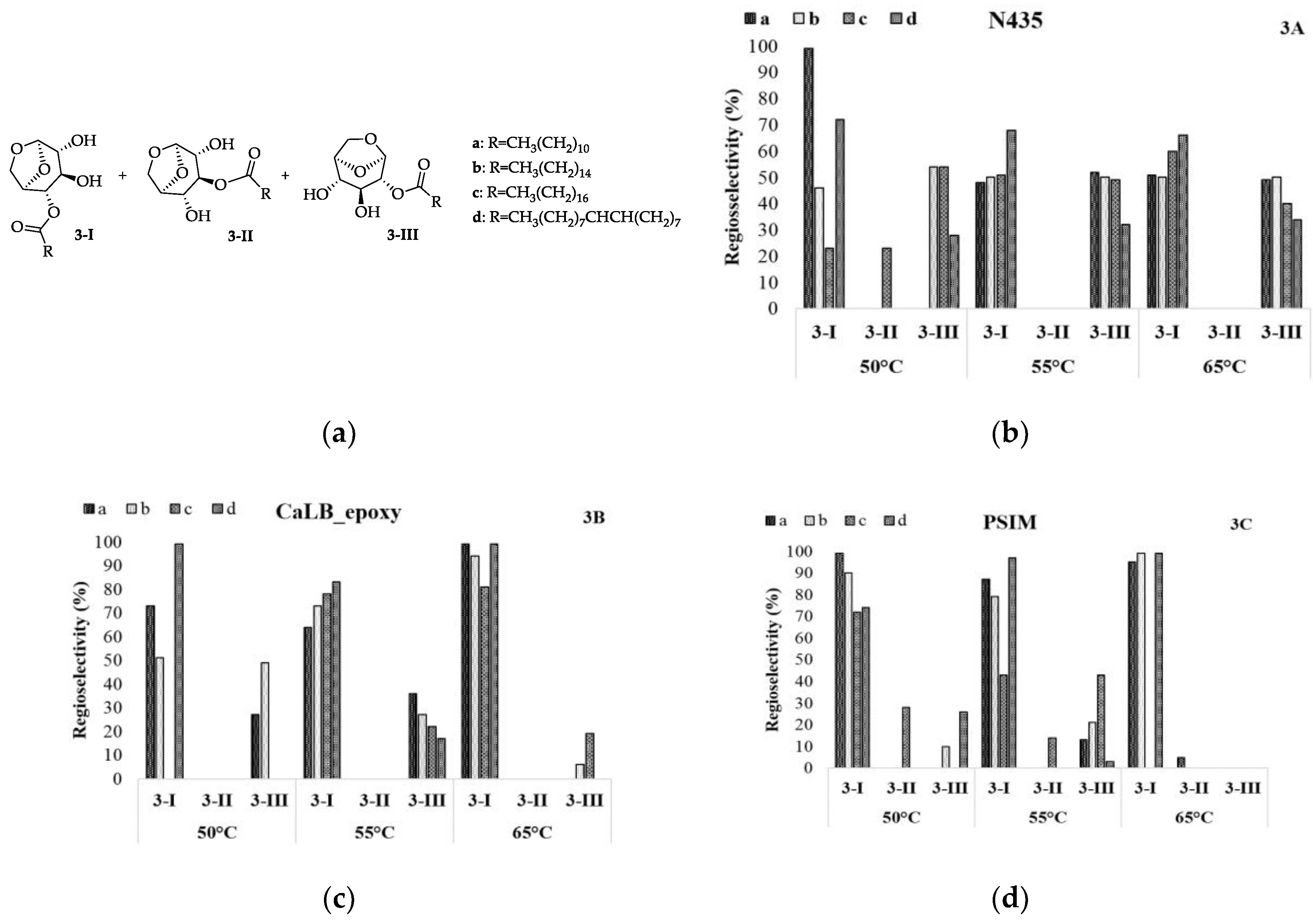
Comparing the obtained values, it is possible to observe that the regioselectivity of N435 is slightly influenced by the temperature and size of the alkyl chain, exhibiting a similar tendency for OH at C4 and C2 in all conditions that were explored, except for ethyl laurate (Figure 3B, 3-Ia- 50°C), where it is possible to observe a loss of regioselectivity at higher temperatures. Different values were obtained for CaLB_epoxy and PSIM where the preference occurred for OH-C4, as can be observed for ethyl oleate (Figure 3C, 3-Id) and ethyl laurate (Figure 3D, 3-Ia), respectively.
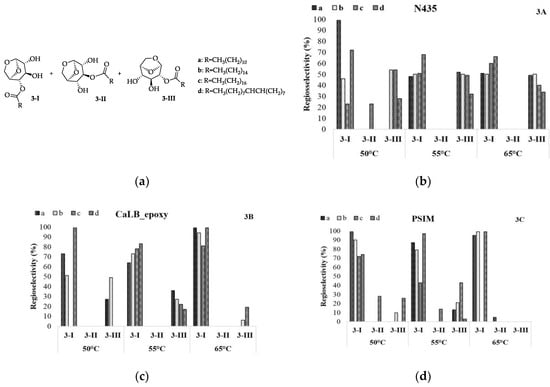
Figure 3.
Regioselectivity in the enzymatic acetylation of levoglucosan in CH3CN with ethyl aliphatic esters. Analyses were performed in a gas chromatograph equipped with a mass spectrometry detector (GC-MS).
The acyl moiety of the acyl-donor is covalently bound to the active site of the enzyme during the two transition states in the catalytic triad [24,25]. Moreover, the alcohol and the acyl binding sites are close to each other. Hence, the acyl moiety is likely to influence the regioselectivity of lipase towards the alcohol and the length and shape of the acyl part of the acyl-donor indeed influence the regioselectivity of the reaction. It was observed that regioselectivity increased with longer acyl groups for CaLB_epoxy and PSIM, as presented in Figure 3.
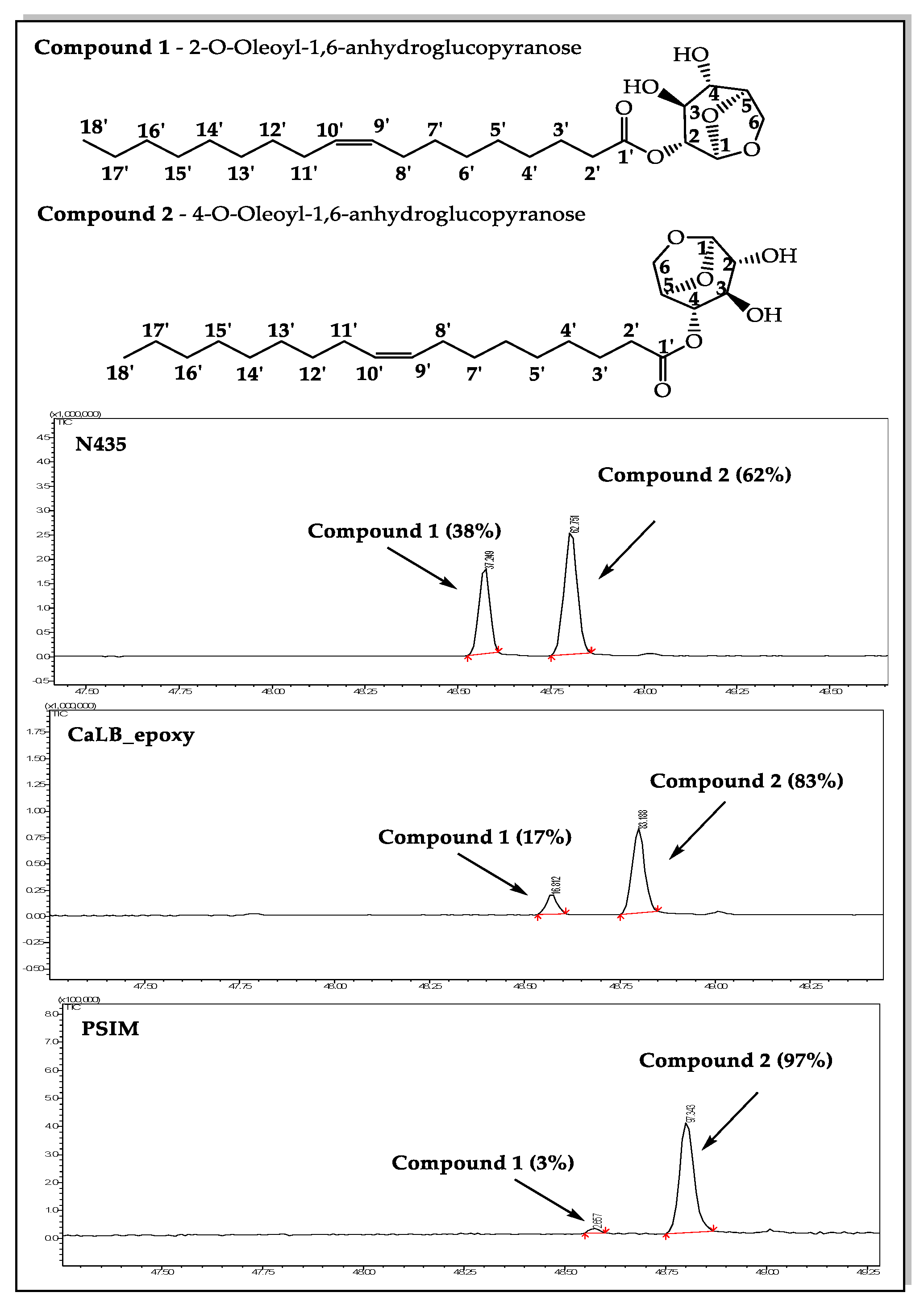
Figure 4 shows the representative chromatograms of the reaction of levoglucosan with ethyl oleate mediated by N435, CaLB_epoxy, and PSIM at 55°C in acetonitrile, where the peaks corresponding to the retention times 48.52 and 48.76 min correspond to the regioisomers obtained during the reaction.
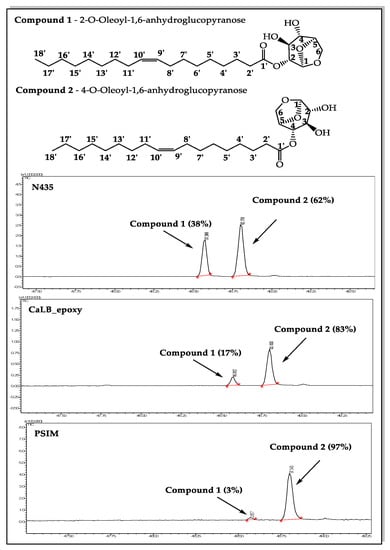
Figure 4.
Regioselectivity in the enzymatic acetylation of levoglucosan (1 mmol, 163 mg) in CH3CN (5.0 mL) with ethyl oleate (2 mmol, 619 mg) were introduced into a vial containing the supported enzyme (40 mg, 0.8% (v/v)). The mixture was stirred at 55°C for about five days and the course of the reaction was monitored by means of GC–MS.
In order to verify the regioselectivity of the reaction studied, regioisomers were isolated and characterized by ¹H NMR, 13C NMR, NOEDIFF, and Infrared (FTIR) techniques (see Support Information). In order to unequivocally determine the structure of compounds 1 and 2, 2D NMR (HMBC and HSQC) experiments were carried out. Once the structure of compounds 1 and 2 is determined, it can be established what compound is formed in the transesterification reaction. Table 2 shows the 1H NMR chemical shifts (ν, ppm) and coupling constants (JHH, Hz), and 13C chemical shifts (ν, ppm) for compounds 1 and 2.

Table 2.
13C and 1H chemical shifts (ν, ppm) in CDCl3 as solvent for compounds 1 and 2. JHH coupling constants are represented in parenthesis.
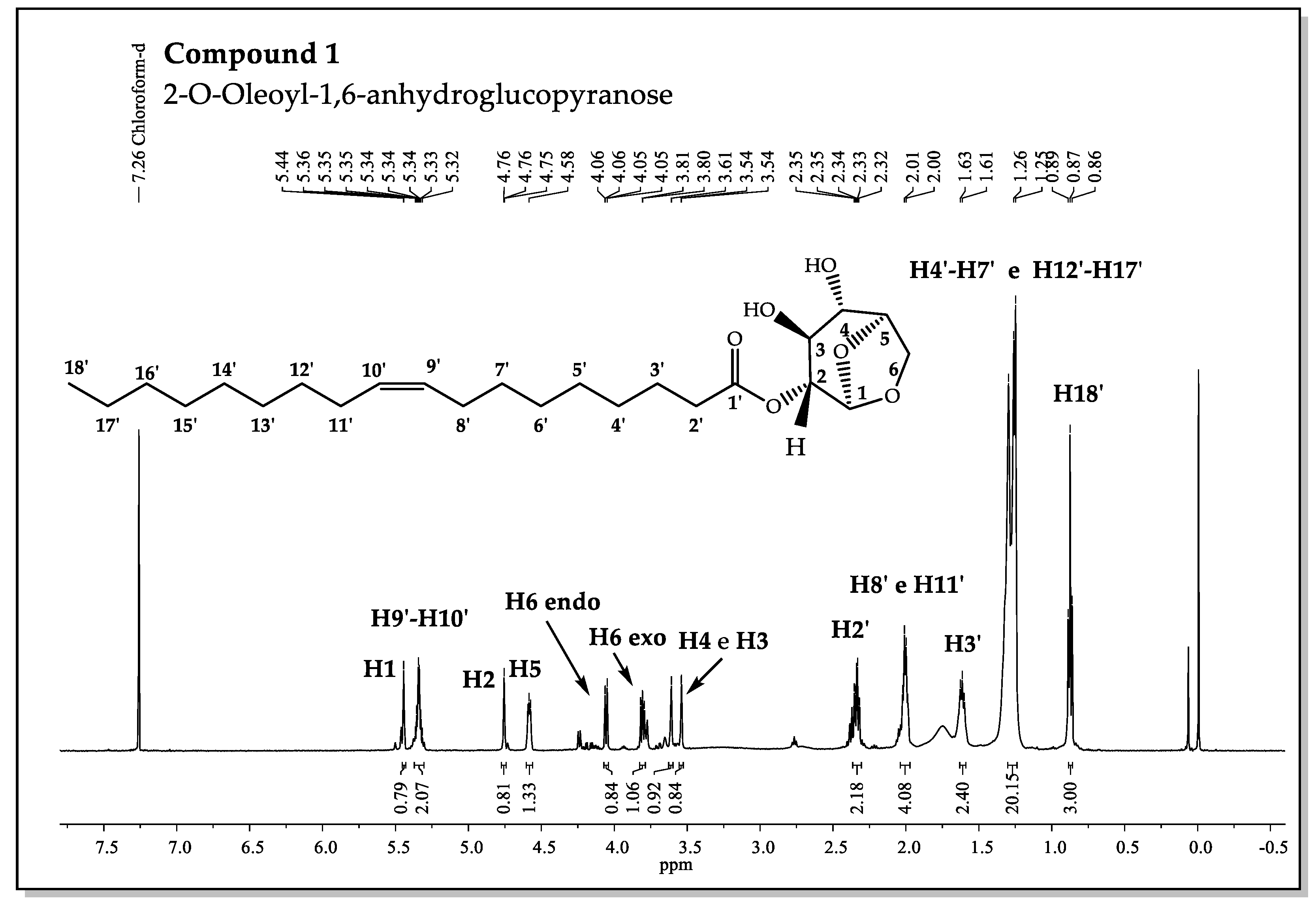
The signs CH and CH2 of compounds 1 and 2 can be more easily identified by comparing the 1H-NMR, 13C-DEPT135, and HSQC spectra, allowing the distinction between the methinics and methylenics carbon signals, and by 1H-1H-COSY, it was possible to obtain the correlations between the hydrogens that are coupled by 2-3JH,H (geminal and vicinal couplings, measurable in the 1D spectrum) and, thus, discern the multiplicity of signals observed in the 1H-NMR spectrum. The signal of the methinics carbons CH-2, CH-3, and CH-4 of the levoglucosan pyranose ring is relatively more difficult, however it has been monitored by careful consideration of spectrum correlations with coupling interaction at one and three C-H bonds [23].
The results showed that the chemical displacement of CH-4 at compound 1 appears about δH= 3.61 ppm lower than in compound 2 (δH= 4.74 ppm). Different displacements were also observed for ring CH-2, and it was not possible to observe significant differences for others. The values of the chemical displacements of the methinics carbons CH-2 and CH-4 reveal modification dependent on the substituent groups that are present in the gem-hydroxyls in C2 and C4 (Figure 5).
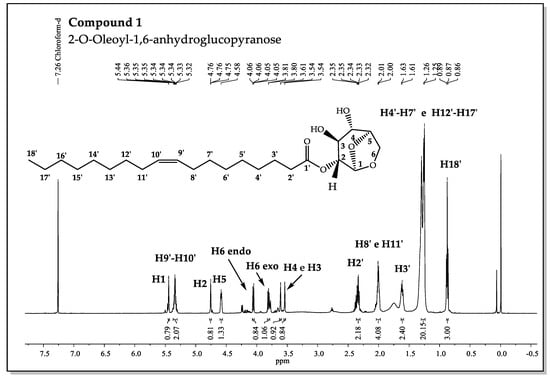
Figure 5.
1H-NMR Spectrum for 2-O-Oleoyl-1,6-anhydroglucopyranose.
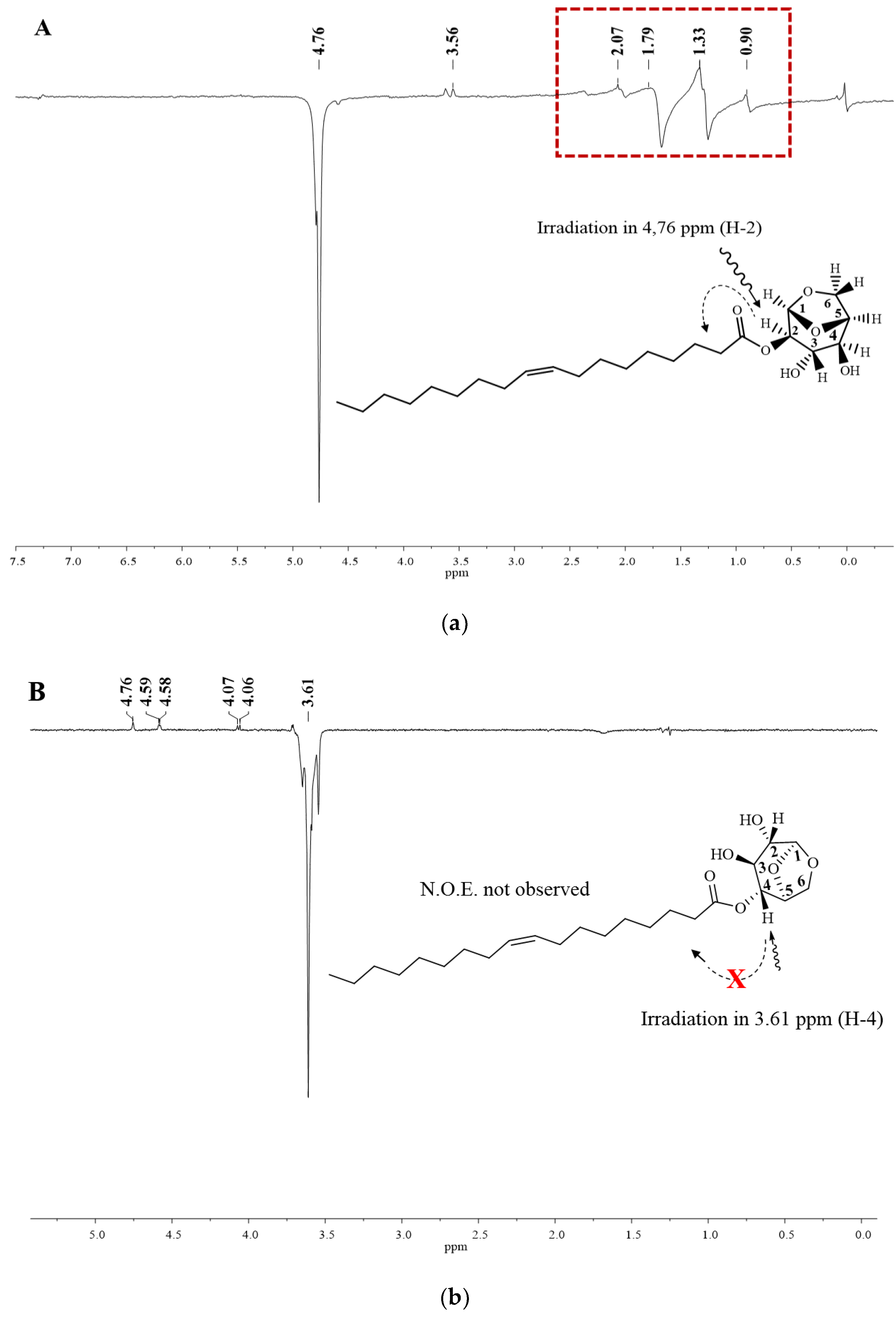
In this context, NOEdiff experiments can be used to confirm previous assignments, as well as to distinguish between levoglucosan monoester. When irradiating H2 (compound 1) at 4.76 ppm, there is an increase in the signal intensity related to hydrogens of the aliphatic ester chain by 1.35 ppm due to the Overhauser effect. When irradiating at 3.61 ppm (H4), it was not possible to observe an n.O.e (Figure 6).
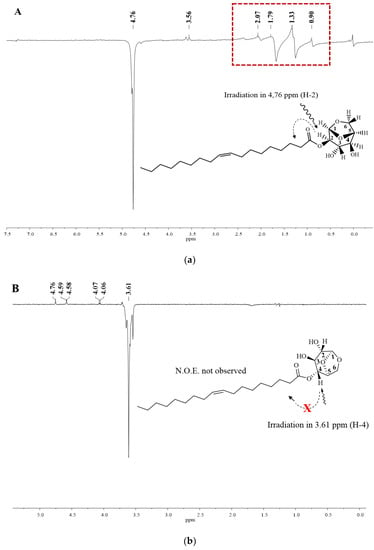
Figure 6.
Nuclear effect of the Overhauser difference spectra for irradiation in the H-2 and H-4 compound 1. Spectrum A: irradiating at 4.76 ppm (H-2) and spectrum B: irradiating at 3.61 ppm (H-4).
These experiments clearly showed that there are marked differences between reactivities on C2 and C4 hydroxyl groups and also that the type of binding and support chosen for enzyme immobilization can influence the selectivity of the enzyme so that the hydroxyl groups can be selectively transesterified, Since CaLB_epoxy and N435 have the same enzyme (CALB), but immobilized on different supports, generating different interactions between enzyme and support and consequently, different spatial conformations, revealing different preferences in the reaction under study.
Without detailed knowledge of the chemistry of the N435 support, it is not yet possible to attribute these observed differences to electronic, steric, or directed factors. To the best of our knowledge, this is the only example of this type of selective transesterification in the levoglucosan group in the presence of saturated and unsaturated long chain (≥C16) aliphatic esters using the enzymatic process.
4. Conclusions
In this work, it was possible to integrate lipase-catalyzed production of ethyl esters from different chains with the transesterification of levoglucosanam catalyzed by the lipases N435 and CalB_epoxy, where the differences of lipase interaction with the supports generated different characteristics in the biocatalysts, which resulted in differences of conversion and regioselectivity of the products formed, especially for CalB_epoxi, which showed conversions higher than 90% in monoester and regioselectivity higher than 99% for the products 2-O-Oleoyl-1,6-anhydroglucopyranose, which is first described by biocatalysi. N435 demonstrated conversions higher than 90% but lower selectivities for the monoesters 4-O-Stearyl-1,6-anhydroglucopyranose and 4-O-Stearyl -1,6-anhydroglucopyranose. Importantly, regioselectivity was completely confirmed by NMR:1H, 13C, HMBC, HSQC, 1H,1H-COSY (“Correlation Spectroscopy”) and NOEdiff (NOE “difference spectroscopy”) spectra, FTIR spectrum ν (cm-1, KBr), HRMS (High-Resolution Mass Spectrometry), and GC–MS analysis, confirming the main formation of monoesters and the correct esterification positions obtained by the studied biocatalysts, which were reported for the first time for levoglucosan esters (Show as Supplementary Materials).
Supplementary Materials
The following are available online at https://www.mdpi.com/2071-1050/11/21/6044/s1, Figure S1: Chromatogram of levoglucosan reaction medium with ethyl palmitate using N435 (derivatized) 55 °C. Peak with retention time at 9.2 for excess BSTFA; Figure S2: Chromatogram of levoglucosan reaction medium with ethyl palmitate using PSIM 56 (derivatized) 55 °C. Peak with retention time at 9.2 for excess BSTFA; Figure S3: Chromatogram of levoglucosan reaction medium with ethyl oleate using CaLB_epoxy (derivatized) 55 °C; Figure S4: Infrared spectrum of levoglucosan; Figure S5: Infrared spectrum of the transesterification reaction of levoglucosan with ethyl oleate; Figure S6: 1H-NMR spectrum for levoglucosan (DMSO); Figure S7: 1H-NMR Spectrum for 4-O-Lauryl-1,6-anhydroglucopyranose (CDCl3); Figure S8: 1H-NMR Spectrum for 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S9: 1H-NMR Spectrum for 4-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S10: 13C-NMR Spectrum for levoglucosan (DMSO); Figure S11: 13C-NMR Spectrum for 4-O-Lauryl-1,6-anhydroglucopyranose (CDCl3); Figure S12: 13C-NMR Spectrum for 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S13: 13C-NMR Spectrum for 4-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S14: 1H, 1H COSY for levoglucosan (DMSO); Figure S15: 1H, 1H COSY for 4-O-Lauryl-1,6-anhydroglucopyranose (CDCl3); Figure S16: 1H, 1H COSY for 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S17: 1H, 1H COSY for 4-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S18: HSQC for levoglucosan (DMSO); Figure S19: HSQC for 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S20: HSQC for 4-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S21: n.0.e difference spectra for irradiation in the H-2′ compound 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S22: n.0.e difference spectra for irradiation in the H-2 compound 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S23: n.0.e difference spectra for irradiation in the H-5 compound 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S24: n.0.e difference spectra for irradiation in the H-3 compound 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Figure S25: n.0.e difference spectra for irradiation in the H-4 compound 2-O-Oleoyl-1,6-anhydroglucopyranose (CDCl3); Table S1: Regioselectivity in the enzymatic acetylation of levoglucosan in CH3CN with ethyl aliphatic esters. Analyses were performed in a GC-MS.
Author Contributions
M.A.d.N. and L.E.G. were responsible for the execution of all experiments of this work. E.M.B. and F.C.L.A. contributed to the structural elucidation and determination of the regioselectivity of levoglucosan esters. R.A.C.L. was responsible for the guidance in the analytical experiments as well as in the purchase and supply of the analytical reagents. R.O.M.A.d.S. and R.W. contributed to the structural elucidation of the obtained compounds, biocatalyst production and manuscript writing, besides coordinating the research. I.I.J. is the general research coordinator and scientific advisor.
Funding
This research received no external funding.
Acknowledgments
The authors thank CAPES, CNPq, and FAPERJ for financial support. LIA CNRS France-Brazil “Energy & Environment” is also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Morais, A.R.; Bogel-Lukasik, R. Green chemistry and the biorefinery concept. Sustain. Chem. Process. 2013, 1, 18. [Google Scholar] [CrossRef]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: Productively. Green Chem. 2005, 7, 761. [Google Scholar] [CrossRef]
- Turner, M.K. Biocatalysis in organic chemistry (Part II): Present and future. Trends Biotechnol. 1995, 13, 253–258. [Google Scholar] [CrossRef]
- Octave, S.; Thomas, D. Biorefinery: Toward an industrial metabolism. Biochimie 2009, 91, 659–664. [Google Scholar] [CrossRef]
- Sánchez, C. Lignocellulosic residues: Biodegradation and bioconversion by fungi. Biotechnol. Adv. 2009, 27, 185–194. [Google Scholar] [CrossRef]
- Ortiz, A.L.; Segura, F.N.; Jabalera, R.S.; Paula, M.M.D.; Del Campo, E.A.; Gutiérrez, J.S.; Bretado, M.E.; Collins-Martínez, V.; Bretado, M.A.E. Low temperature sugar cane bagasse pyrolysis for the production of high purity hydrogen through steam reforming and CO2 capture. Int. J. Hydrog. Energy 2013, 38, 12580–12588. [Google Scholar] [CrossRef]
- Hoi, L.W.S.; Martincigh, B.S. Sugar cane plant fibres: Separation and characterisation. Ind. Crop. Prod. 2013, 47, 1–12. [Google Scholar]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Rocha, I.M.; Galvão, T.L.P.; Sapei, E.; Da Silva, M.D.; Da Silva, M.A.V.R. Levoglucosan: A Calorimetric, Thermodynamic, Spectroscopic, and Computational Investigation. J. Chem. Eng. Data 2013, 58, 1813–1821. [Google Scholar] [CrossRef]
- Junior, I.I.; Flores, M.C.; Sutili, F.K.; Leite, S.G.F.; Leandro, L.S.; Leal, I.C.R.; De Souza, R.O. Lipase-catalyzed monostearin synthesis under continuous flow conditions. Org. Process Res. Dev. 2012, 16, 1098–1101. [Google Scholar] [CrossRef]
- Galletti, P.; Moretti, F.; Samori’, C.; Tagliavini, E. Enzymatic acylation of levoglucosan in acetonitrile and ionic liquids. Green Chem. 2007, 9, 987–991. [Google Scholar] [CrossRef]
- Manzocco, L.; Calligaris, S.; Da Pieve, S.; Marzona, S.; Nicoli, M.C. Effect of monoglyceride-oil–water gels on white bread properties. Food Res. Int. 2012, 49, 778–782. [Google Scholar] [CrossRef]
- Do Nascimento, M.A.; Gotardo, L.E.; Leão, R.A.C.; De Castro, A.M.; De Souza, R.O.; Itabaiana, I. Enhanced Productivity in Glycerol Carbonate Synthesis under Continuous Flow Conditions: Combination of Immobilized Lipases from Porcine Pancreas and Candida antarctica (CALB) on Epoxy Resins. ACS Omega 2019, 4, 860–869. [Google Scholar] [CrossRef]
- De Souza, S.P.; De Almeida, R.A.D.; Garcia, G.G.; Leão, R.A.C.; Bassut, J.; De Souza, R.O.M.A.; Itabaiana, I. Immobilization of lipase B from Candida antarctica on epoxy-functionalized silica: Characterization and improving biocatalytic parameters. J. Chem. Technol. Biotechnol. 2017, 93, 105–111. [Google Scholar] [CrossRef]
- Aguillón, A.R.; Avelar, M.N.; Gotardo, L.E.; De Souza, S.P.; Leão, Raquel, A.C.; Itabaiana, I.; Miranda Leandro, S.M.; De Souza, R.O.M.A. Immobilized lipase screening towards continuous-flow kinetic resolution of (±)-1,2-propanediol. Mol. Cat. 2019, 467, 128–134. [Google Scholar] [CrossRef]
- Fathi, Z.; Doustkhah, E.; Rostamnia, S.; Darvishi, F.; Ghodsi, A.; Ide, Y. Interaction of Yarrowia lipolytica lipase with dithiocarbamate modified magnetic carbon Fe 3 O 4 @C-NHCS 2 H core-shell nanoparticles. Int. J. Boil. Macromol. 2018, 117, 218–224. [Google Scholar] [CrossRef]
- Babaki, M.; Yousefi, M.; Habibi, Z.; Brask, J.; Mohammadi, M. Preparation of highly reusable biocatalysts by immobilization of lipases on epoxy-functionalized silica for production of biodiesel from canola oil. Biochem. Eng. J. 2015, 101, 23–31. [Google Scholar] [CrossRef]
- Zdráhal, Z.; Oliveira, J.; Vermeylen, R.; Claeys, M.; Maenhaut, W. Improved Method for Quantifying Levoglucosan and Related Monosaccharide Anhydrides in Atmospheric Aerosols and Application to Samples from Urban and Tropical Locations. Environ. Sci. Technol. 2002, 36, 747–753. [Google Scholar] [CrossRef]
- Schummer, C.; Delhomme, O.; Appenzeller, B.; Wennig, R.; Millet, M. Comparison of MTBSTFA and BSTFA in derivatization reactions of polar compounds prior to GC/MS analysis. Talanta 2009, 77, 1473–1482. [Google Scholar] [CrossRef]
- Trost, B.M. The Atom Economy—A search for synthetic efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- Mateo, C.; Torres, R.T.R.; Fernandez-Lorente, G.; Ortiz, C.; Fuentes, M.; Hidalgo, A.; López-Gallego, F.; Abian, O.; Palomo, J.M.; Betancor, L.; et al. Epoxy-Amino Groups: A New Tool for Improved Immobilization of Proteins by the Epoxy Method. Biomacromolecules 2003, 4, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Boissièere-Junot, N.; Tellier, C.; Rabiller, C. On the Regioselective Acylation of 1,6-Anhydro-β- d—and l -Hexopyranoses Catalysed by Lipases: Structural Basis and Synthetic Applications. J. Carbohydr. Chem. 1998, 17, 99–115. [Google Scholar] [CrossRef]
- Rotticci, D. Understanding and Engineering the Enantioselectivity of Candida Antarctica Lipase B towards Sec–Alcohols. Ph.D. Thesis, Royal Institute of Technology, Department of Chemistry, Organic Chemistry, Stockholm, Sweden, 2000. [Google Scholar]
- Uriarte, I.; Écija, P.; Lozada-Garcia, R.; Çarçabal, P.; Cocinero, E.J. Investigating the Conformation of the Bridged Monosaccharide Levoglucosan. ChemPhysChem 2018, 19, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Hunter, B.K.; Hall, L.D.; Sanders, J.K.M. The Conformation of Vinblastine in Solution as Determined by N.O.E. Difference Spectroscopy. J. Chem. Soc. Perkin Trans. 1983, 1, 657–665. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).