3.1. Influence of Slag Chemistry and Activator Dose in Reaction Kinetics
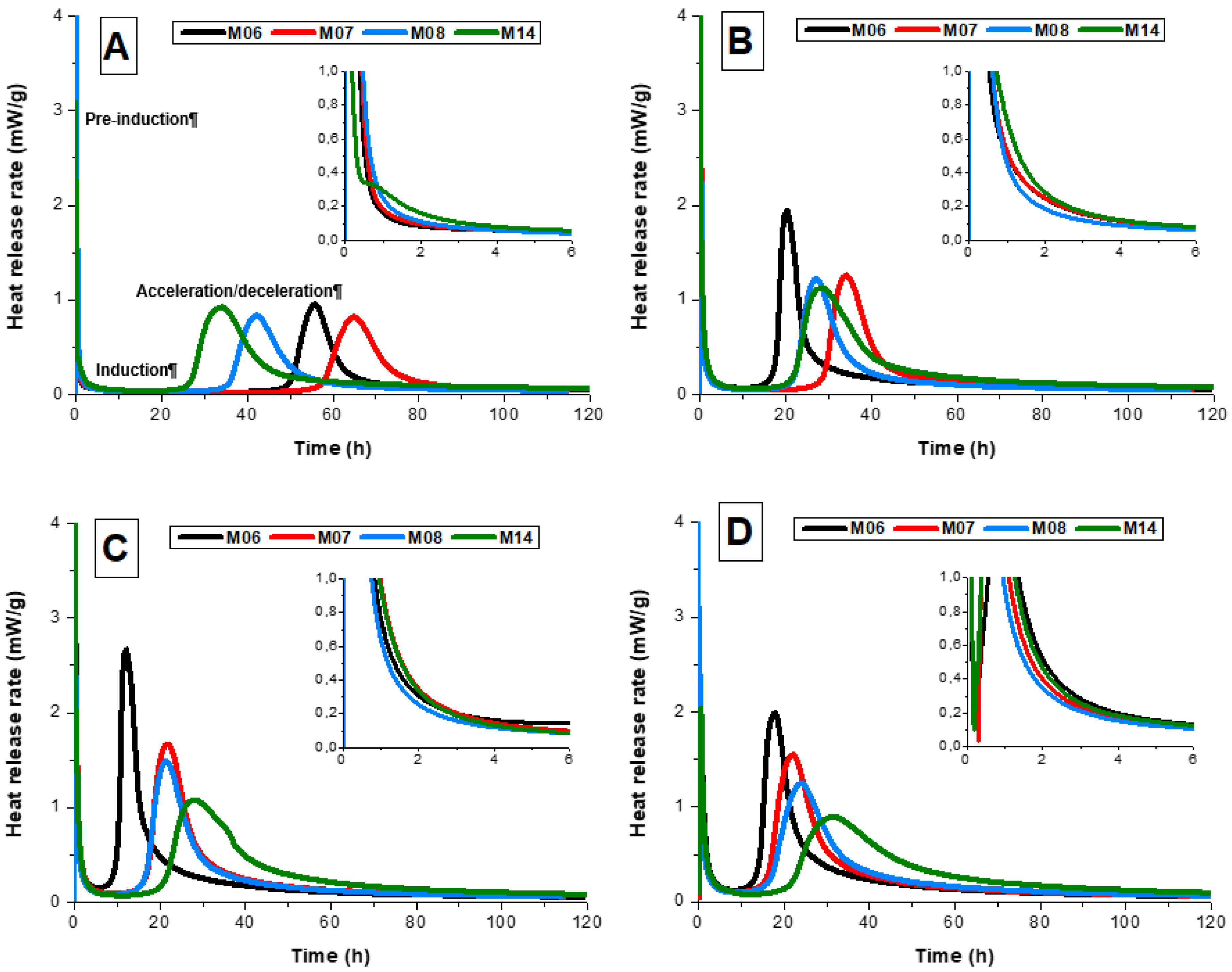
For all doses of the sodium metasilicate activator, the heat release curves (
Figure 1) exhibited a pre-induction period (first peak) during the first hour of reaction, corresponding to wetting and dissolution of the slag grains [
20], and possibly also to the adsorption of some ions onto the surface of the slag grains. The sodium metasilicate solution had a high concentration of partially-deprotonated monomeric silicate anions, which reacted rapidly with Ca
2+ as it dissolved from the surface of slag grains, to form an aluminum substituted calcium silicate hydrate (C-(A)-S-H) type gel, when the concentration of Ca
2+ reached a critical value [
11]. The identification of this pre-induction period is consistent with several previous observations of sodium silicate activated slag cements [
8,
11].
The pre-induction peak was followed by a period of low heat release and reduced reaction. The duration of this period is strongly dependent on both the slag composition and the activator dose. At the end of this period, high intensity acceleration and deceleration periods corresponding to the nucleation, growth, and precipitation of reaction products (second peak) were observed in all alkali-activated slag cements. This period took place between 7 h and 100 h after the start of the reaction (
Figure 1), depending on the slag composition and the concentration of the sodium silicate used, indicating that the reaction kinetics in alkali-activated slag cements are strongly dependent on the cement mix design.
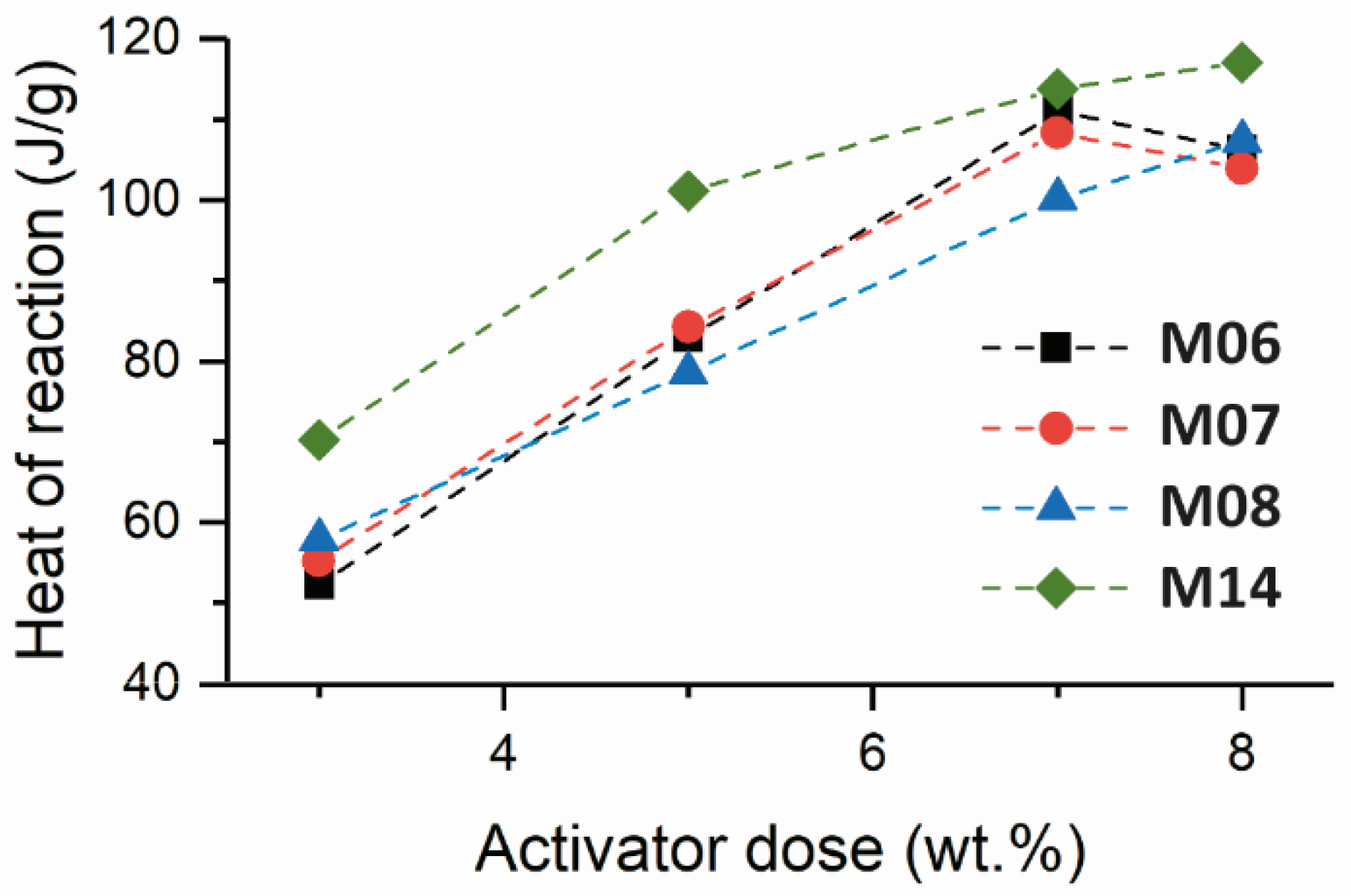
At the lowest sodium metasilicate concentration investigated (3 wt.%,
Figure 1A), sample M06 (which contained the lowest MgO content of the reaction mixes assessed) exhibited the longest induction period of all samples, lasting 38.0 h, with the maximum heat release rate in the acceleration-deceleration period (i.e., the beginning of the deceleration period) observed 55.7 h after mixing. Increased slag MgO content resulted in a reduction in the length of the induction period to 26 h and 18.6 h in samples M08 and M14, respectively, and promoted faster precipitation of the reaction products, indicated by the earlier onset of the deceleration period at 42.1 h and 34.5 h for samples M08 and M14, respectively. This is consistent with previous observations that sodium metasilicate-activated slags with higher slag MgO content reacted more rapidly than those with lower MgO content [
15].
Despite sample M07 having a higher MgO content than sample M06 (
Table 1), its induction period was more prolonged (41.7 h) and its main reaction peak in
Figure 1A was observed after 64.8 h. This behavior is likely to be related to an increased reactivity of the M06 slag, associated with a higher specific area (505.6 m
2/kg) and higher content of fine particles (d
50 of 11.2 µm) when compared to the M07 slag. It is also possible that the higher content of sulfide (represented as SO
3 in the XRF data in
Table 1, but usually present in reduced form in blast furnace slags) in M07 than M08 had some effect in retarding the reaction, considering that this was the most noticeable difference between M07 and M08, and the differences in particle size parameters were negligible. However, the presence of sulfide or other redox-active components is generally believed to increase, rather than decrease, the dissolution rates of solids [
21], so it is not very clear how such a mechanism would take place from a chemical perspective.
Heat release curves for each slag activated with 5 wt.% sodium metasilicate are shown in
Figure 1B. The increased activator dose resulted in a shorter duration induction period, an earlier onset of the acceleration/deceleration peak, and more intense heat release for all samples than at the lower activator dose in
Figure 1A, indicating more rapid dissolution of the slag precursor and formation of reaction products. However, these changes did not occur to the same extent across all samples. The increased reaction kinetics observed were consistent with the higher concentration of soluble silicate supplied by the activator, which reacts with dissolved Ca
2+ ions to remove them from the aqueous phase, thus increasing the Ca
2+ concentration gradient between the pore solution and anhydrous slag grains. This drives faster dissolution of the slag particles and precipitation of mainly C-(A)-S-H type gels [
11,
22,
23], which have been reported as the main reaction product for the silicate activated slags used in this study, and others of comparable composition [
9]. Increased concentrations of soluble silicates will also reduce the degree of slag dissolution required to reach saturation in the aqueous phase relative to C-(A)-S-H, thus driving more rapid precipitation of the reaction products. Sample M06 exhibited the shortest induction period of the samples assessed, followed by sample M14 (despite its lower CaO content than the other slags), sample M08, and sample M07. This can mainly be attributed to the greater surface area of the slag used to produce sample M06 when compared with the other slags (
Table 1), driving a more rapid reaction, although differences in slag chemistry will also influence this behavior as discussed in more detail below.
Increasing the concentration of sodium metasilicate to 7 wt.% (
Figure 1C) slightly reduced the induction period for the M07 and M08 slags, compared with that observed when using 5 wt.% sodium metasilicate (
Figure 1B). Sample M06 exhibited a sharper and more intense acceleration/deceleration peak than the other samples assessed at this activator dosage, and also compared to that observed when using lower activator doses (
Figure 1A,B). Sample M14 exhibited the longest dormant period of the samples evaluated at this activator dosage as well as an acceleration/deceleration peak of lower intensity. Slag M14 had the lowest content of CaO of all the slags assessed, hence buffering the reaction for the preferential formation of C-A-S-H type phases over Mg-rich products. This behavior was also observed by Ben Haha et al. [
8], who identified the delayed reaction of high-MgO slags when compared to slags with lower MgO content when using metasilicate as the activator at a relatively high dose (10 wt.%).
It is worth noting that for sample M14, the onset of the acceleration/deceleration period occurred at approximately the same time after mixing when activated with any of the doses of sodium metasilicate studied here; this behavior was surprisingly insensitive to activator dose. However, this can be related to the importance of the release of Mg
2+ from the slag in defining the precipitation of the reaction products as this cation interacts and forms reaction products with dissolved aluminates, not with the silicate species supplied by the activator. The pH is sufficiently high to induce extensive slag glass dissolution for all activator compositions tested here (see below), so the differences in heat release peak timing are related directly to the differences in reaction product precipitation times. Thermodynamic modeling has shown that, in general, the C-(N)-A-S-H type gels forming in alkali-activated slag cements precipitate at a much lower extent of slag reaction than the other product phases such as LDHs and/or zeolites [
17]. Consequently, it is likely that this type of gel is precipitating during the first stage of the acceleration/deceleration peak identified in the heat release curves, particularly for lower-Mg slags. The latter stage of the acceleration/deceleration peak is therefore able to be associated with the precipitation of Mg-Al LDH phases.
The heat release curves for slags M06, M07, M08, and M14 activated with 8 wt.% sodium metasilicate are shown in
Figure 1D. At this activator dose, the length of the induction period and the length of the acceleration/deceleration period in each sample increases with increasing slag MgO content; the exact inverse of the relationship observed at the lowest activator dose in
Figure 1A. However, the results in
Figure 1D are consistent with previous observations of decreased reaction kinetics with increasing slag MgO content [
9,
15], which were obtained at similarly high activator doses. The relative rates of the reaction of the different slags are governed by the alkalinity and soluble silicate concentration supplied by the activator; as noted above, sample M14 reacted quickly in the presence of moderate alkali and soluble silicate concentrations (3 wt.% dose), but was not accelerated significantly in the presence of high alkali and soluble silicate concentrations.
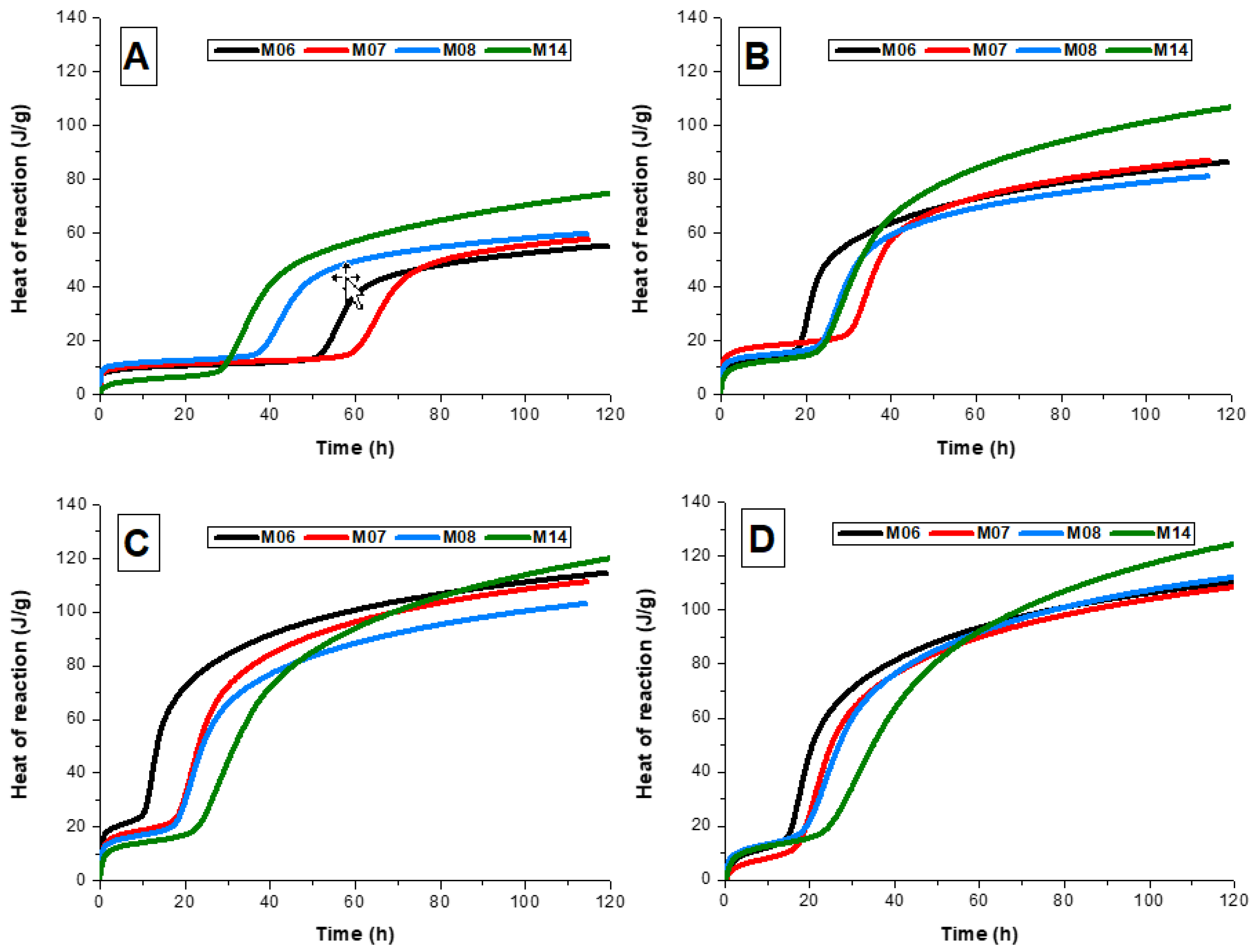
The cumulative heat release curves for all samples are shown in
Figure 2. The induction period corresponded to the relatively flat regions at the early stages of the reaction in the cumulative heat release curves. At an activator dose of 3 wt.% sodium metasilicate (
Figure 2A), increasing MgO content resulted in increased cumulative heat release during the first 100 h of reaction, indicating that increased MgO content resulted in a greater extent of reaction at this activator dosage if it is assumed that the dissolution heat of the slag glass (defined on a mass basis) is not strongly dependent on its composition and thermal history within the group of slags studied here. There is a relatively weak dependence of aluminosilicate glass and mineral enthalpy of formation on the Mg–Ca substitution [
24], and little other systematic information is available for glasses as complex as blast furnace slag, so this assumption is probably acceptable.
There was no clear systematic trend in heat release as a function of activator dose and MgO content among M06, M07, and M08 at higher alkali doses (
Figure 2B–D). A large increase in slag MgO content (i.e., sample M14) resulted in higher cumulative heat release at all activator doses, indicating that a high-MgO slag reached a higher reaction extent overall, particularly at lower activator doses. However, the cumulative heat release data show a marked dependence on the activator dose for this slag, unlike the acceleration onset time data (
Figure 1), which were relatively insensitive to activator dose.
Independent of slag chemical composition or fineness, an increase in the activator dose of up to 7 wt.% sodium metasilicate (
Figure 2A–C) showed a higher cumulative heat release at 100 h. Additionally, previous observations have shown that an increased sodium silicate modulus (SiO
2/Na
2O ratio) results in a greater cumulative heat release [
25]. Together, these trends indicate that greater availability of soluble silica in the reaction mixture up to 7 wt.% sodium metasilicate increased the extent of the reaction, most likely by accelerating the generation of conditions that are supersaturated with respect to C-A-S-H, thus increasing the driving force for slag dissolution.
3.2. Correlating Slag Chemistry and Reaction Kinetics
A similar approach to that reported by Winnefeld et al. [
26] was adopted in this study to identify any potential correlation between the chemical composition of the slag, the initial precipitation of the reaction products, and the extent of the reaction of the slag. This considers the quality moduli for slag glasses reported by Smolczyk [
27] including the formula that is now used in the standard EN 197-1 [
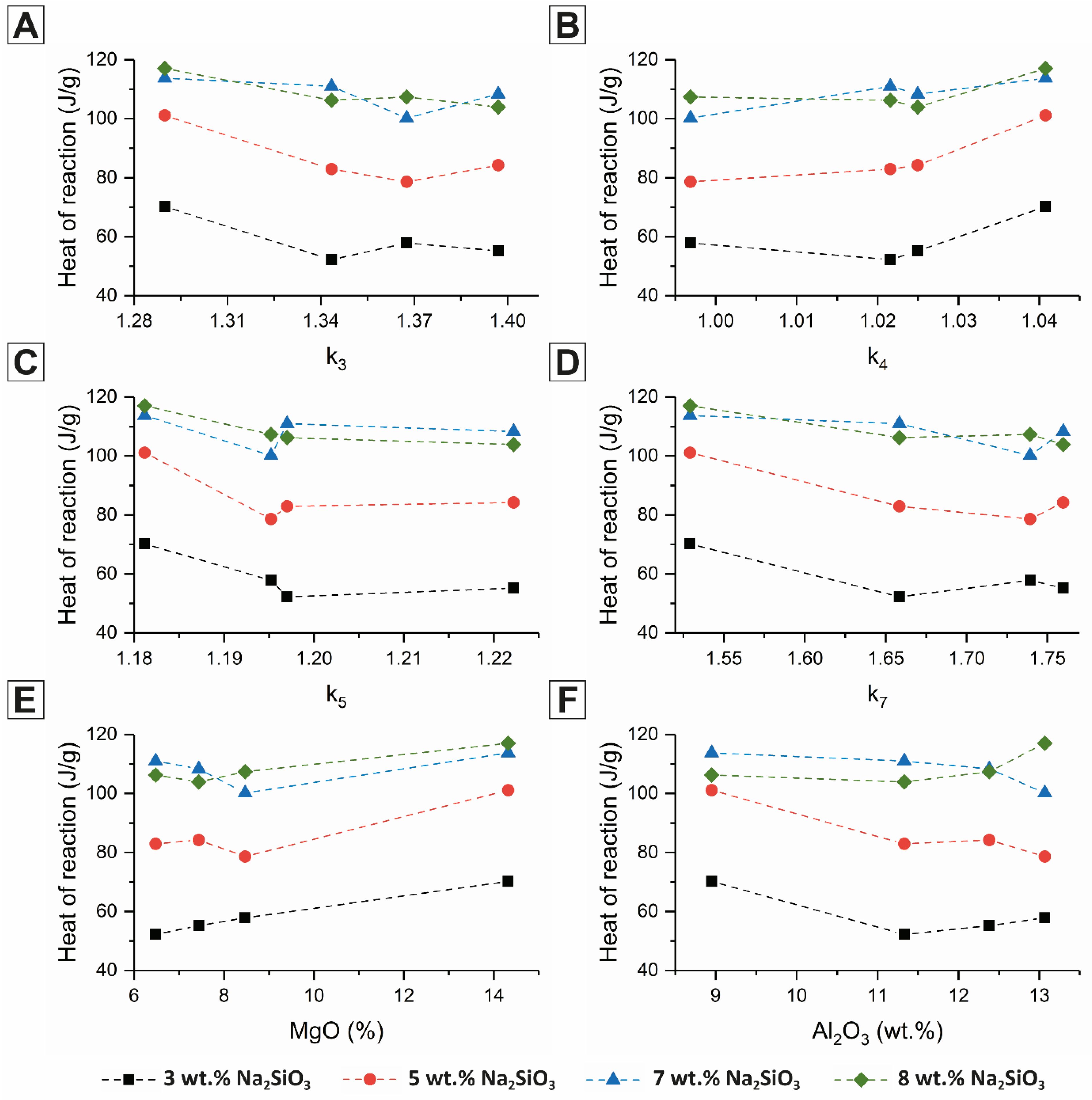
28], where each modulus is a different chemical ratio calculated according to various approaches to define the basicity of each slag according to the ratio of network modifying atoms (Ca, Mg, Al) to network forming atoms (Si, Al) (Equations (1)–(4)), where the key difference between the various formulae is the assumption that is made regarding the fraction of Al which acts as either a network modifier or a network former. The correlations between slag composition (represented by the moduli in Equations (1)–(4), and also by slag MgO and Al
2O
3 content) and the cumulative heat of reaction and maximum rate of reaction of the acceleration period, are reported in
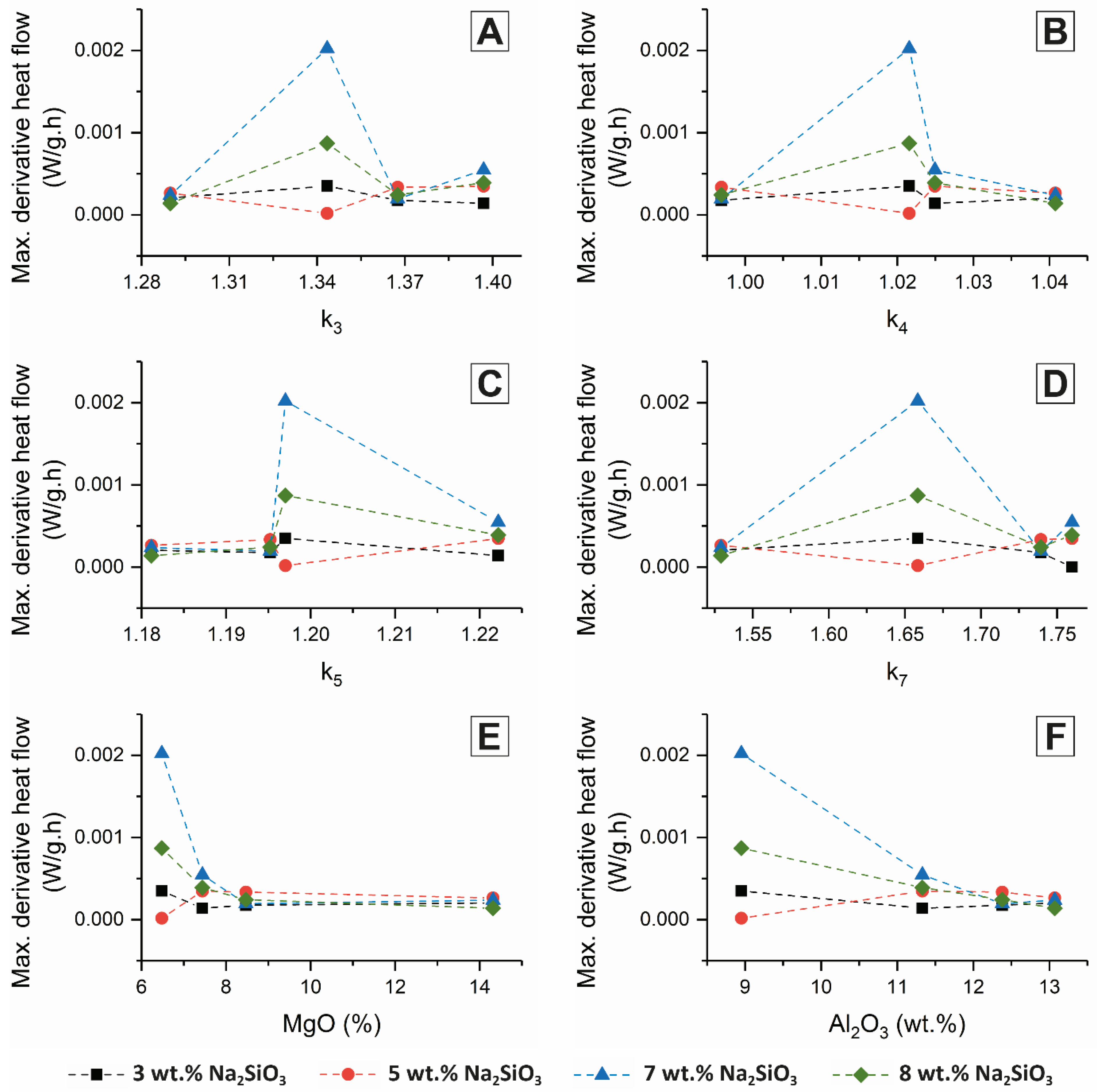
Figure 3 and
Figure 4, respectively, as a function of activator dose.
Figure 3 and
Figure 4 show that none of these slag quality coefficients were particularly effective in predicting reactivity under alkali-activation conditions; it is evident that factors other than those captured by the simple chemical moduli actually determine the extent (
Figure 3), and particularly the rate (
Figure 4) of reaction of the slag.
Figure 3 shows that the extent of the reaction is largely insensitive to the quality coefficients within the range studied here,
k3 and
k4 are probably the closest to giving a uniform trend across all slags and activator doses, but the relationship seems relatively weak, and for
k4, the range of values spanned by this set of slags (which did differ quite significantly in composition and reactivity) was unsatisfactorily low for this to be considered as a useful parameter in the selection of slags for use in alkali-activation. Similarly, neither the MgO content or Al
2O
3 content showed a direct, monotonic correlation with either the cumulative heat release or peak heat release rate.
The correlations between the activator dosage and the cumulative heat of the reaction period are shown in
Figure 5.
For samples M06, M07, and M08, the cumulative heat of reaction after 100 h increased approximately linearly with increasing activator dosage up to 7 wt.% sodium metasilicate (
Figure 5), indicating that within this range, an increased concentration of soluble silicate in the reaction mixture promoted a greater extent of reaction. Increasing the activator dosage from 7 wt.% to 8 wt.% slightly reduced the extent of the reaction for samples M06 and M07, although it continued to increase the extent of reaction for sample M08, and to a lesser degree M14; the slag MgO content seemed to influence the reaction process at high activator doses (>7 wt.%).
3.3. Influence of Activator Dose in Phase Assemblage Evolution
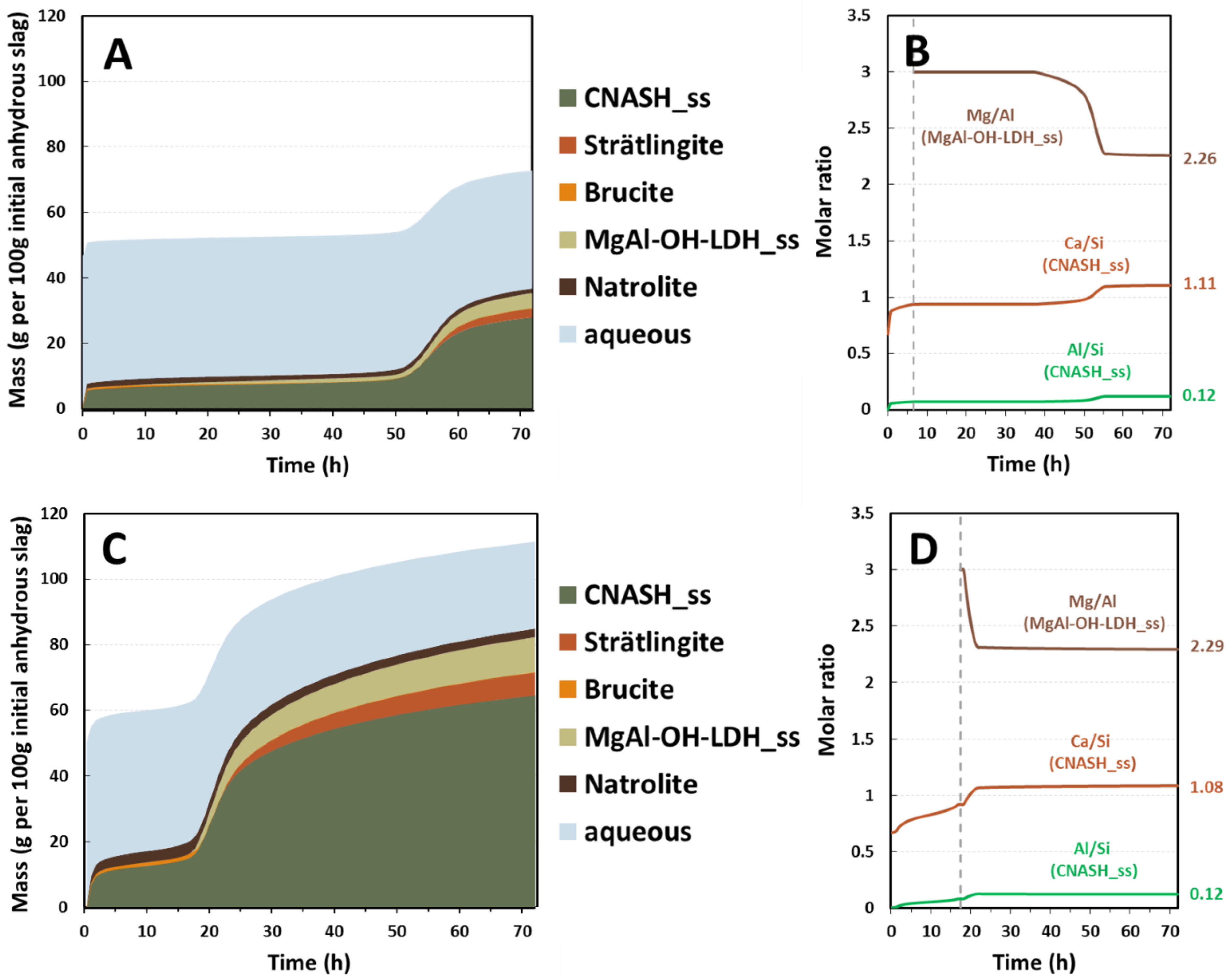
Figure 6 shows the evolution of the phase assemblage of slag M06 with two different alkali doses as a function of time. Congruent dissolution of slag was assumed as the initial alkalinity of the system would be much higher than pH 12 [
29]. The time steps used in the modeling were in accordance with the isothermal calorimetry data as discussed in
Section 3.1, while the degree of reaction at each time step was in proportion to the total heat release (
Figure 2).
The main reaction products simulated were alkali-substituted calcium aluminate silicate hydrate (C-(N)-A-S-H) gel, strätlingite, hydrotalcite-like phases, and natrolite, similar to the simulation results reported by Myers et al. [
17], which matched the experimental observations from previous studies using a subset of these slags at a single activator dose [
9,
19]. Some transient brucite formation was predicted at the higher activator dose; it is unclear whether this will happen in reality as this phase has not been reported experimentally at an early age in such binders as it may be an artifact due to the assumption of congruent slag dissolution and/or the absence of kinetic limitations on phase formation in the calculations here. The C-(N)-A-S-H gel was the main reaction product in both simulations, regardless of the degree of reaction achieved. The bulk gel chemical compositions are controlled mostly by the availability of CaO, SiO
2, and Al
2O
3 [
30,
31], which in practice relate to the chemistry of the slag used, the activator dose, and the degree of reaction achieved. When a higher dose of sodium metasilicate was used (
Figure 6C,D), the Ca/Si ratio of the simulated C-(N)-A-S-H gel was reduced due to the higher initial silica content, however this effect was less marked as the degree of reaction of the slag increased because the silica supplied by the slag became dominant. This explained the lower (<1) ratio of Ca/Si shown in
Figure 6D before reaching the highest heat of the reaction. The hydrotalcite-like phases were the main secondary phases that formed in this slag upon activation, the formation of which is closely related to the MgO content in the slag [
9] as well as the availability of Al species in the aqueous phase (
Figure 7).
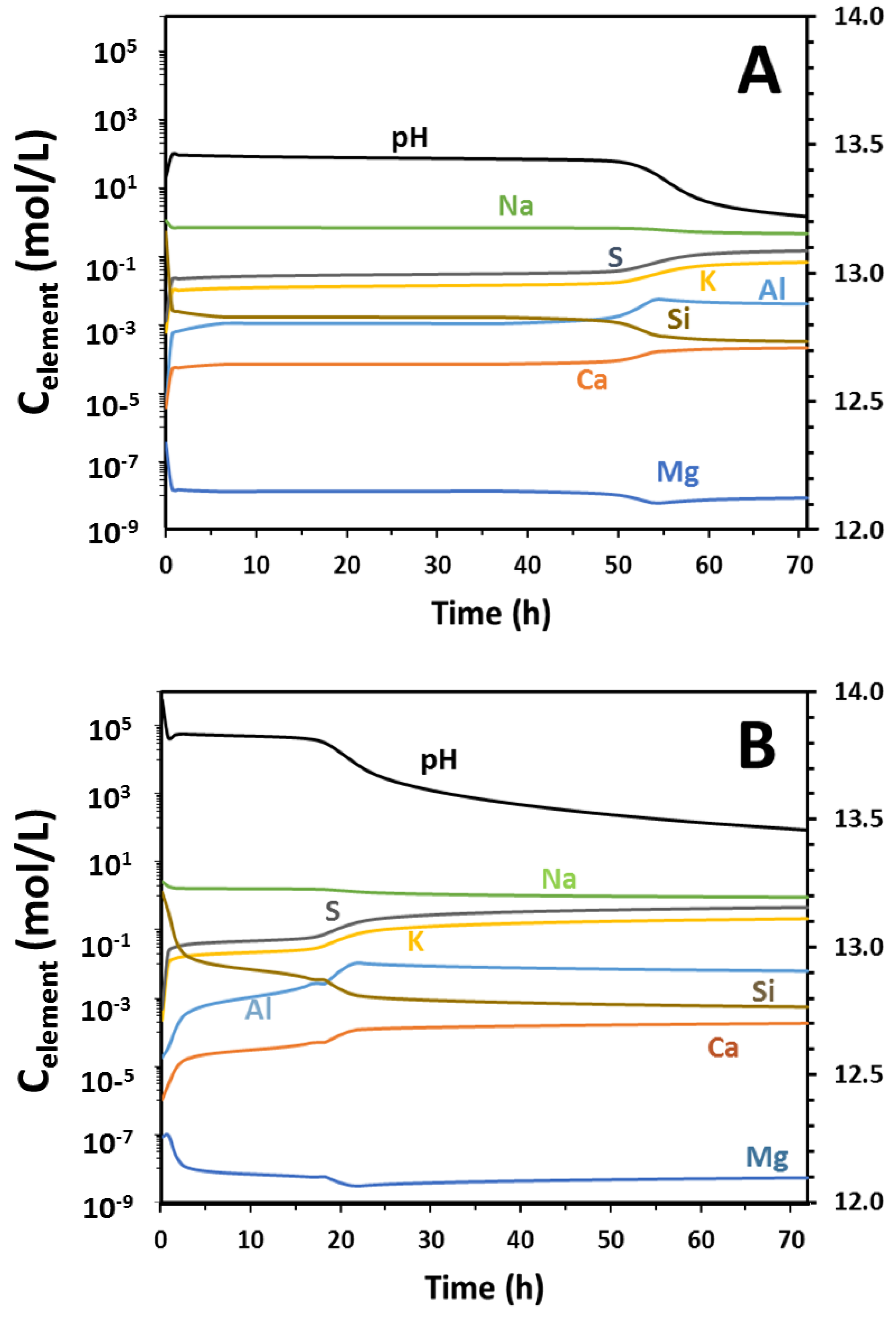
Figure 7 shows the compositions of the aqueous constituents shown in
Figure 6;
Figure 7A corresponds to
Figure 6A, and
Figure 7B to
Figure 6C. In each case, the pH was above 13 but decreased with time; the pH of these alkali-activated slag binders in the fresh state was within the same range as the pH developed by the hydration of plain Portland cement, and so should not be viewed as unusually or inappropriately high for the general usage of alkali-activated slag as a cement. The concentrations of dissolved ions in this thermodynamic simulation are determined by equilibrium with the precipitated solid phase assemblage at each point in time, so after the initial rapid reaction (which removes much of the Si from solution), these concentrations change only slowly until there is a shift in the makeup of the phase assemblage that then generates a rapid re-equilibration of the pore solution (e.g., at 52 h in
Figure 7A, just before 20 h in
Figure 7B). It is notable that the dissolved sulfur concentrations remain high throughout the reaction process; this element is present in reduced form (HS
−), which is not readily incorporated into any of the solid phases within the database used, and so remains free in solution.