Abstract
Objective: The aim of this study was to systematically map evidence to inform best practices for sexual and reproductive healthcare delivered via telehealth (TeleSRH) in United States-based Title X-funded clinics. Methods: We searched three databases (2017–2025) for studies evaluating effectiveness, harms, patient and provider experiences, barriers/facilitators, and engagement strategies encompassing TeleSRH for sexually transmitted infections (STIs), contraceptive care/family planning (CC/FP), and sexual wellness, in countries with a human development index of ≥0.8. Results: From 5963 references and 436 articles, we included 142 eligible publications. TeleSRH use declined since the COVID-19 pandemic’s peak but remains higher than pre-pandemic. Evidence comes mostly from poor-quality studies. TeleSRH increases access and adherence to STI prevention (e.g., pre-exposure prophylaxis for HIV). Tele-follow-up may safely facilitate HIV care continuity. For CC/FP, TeleSRH is comparable to in-person care for patient satisfaction and uptake; patients are less likely to select long-acting reversible contraception but post-initiation tele-follow-up may increase its continuation rates. Vasectomy completion rates may be similar between pre-procedural counseling via telehealth versus in-person. TeleSRH’s potential benefits might include reduced travel time, wait times, no-show rates, and clinic human resource burden (via tele-triaging) and increased preventative screening rates for STIs and non-communicable diseases, prescription refill rates, ability to receive confidential care in preferred settings, and rural/marginalized community outreach. Implementation challenges span technological and capital constraints, provider availability, staff capability building, restrictive policies, language incompatibility, and patient mistrust. Supplementing synchronous TeleSRH with asynchronous communication (e.g., mobile application) may improve continued patient engagement. Conclusions: Preventive, diagnostic, and therapeutic TeleSRH can be effective, with high patient acceptability; however, effectiveness and adoption hinge on contextual factors outlined in this review.
1. Introduction
Sexual and reproductive healthcare (SRH) is a cornerstone of preventive health, encompassing a wide range of services including sexually transmitted infection (STI) prevention and treatment, contraceptive counseling, family planning, and the promotion of sexual well-being [1]. Access to timely, high-quality SRH services is essential for individual health and public health outcomes alike [1,2]. However, long-standing barriers such as geographic isolation, provider shortages, transportation challenges, stigma, and cost continue to limit access, particularly among underserved populations in the United States [1,2,3].
Telehealth has emerged as a promising strategy to expand access to SRH services, particularly within Title X-funded settings that serve individuals regardless of ability to pay [4,5]. With the COVID-19 pandemic catalyzing widespread adoption of virtual care models, SRH services rapidly adapted to incorporate telehealth via synchronous modalities such as video and telephone and asynchronous modalities such as patient portals and mobile applications [4,6]. Telehealth offers potential advantages, including increased privacy, convenience, and reach, especially for populations facing structural barriers to in-person care [7,8,9,10,11,12,13,14]. Despite its promise, questions persist about its effectiveness, acceptability, and equity [4]. Furthermore, the existing literature varies in scope, populations studied, and the definitions of SRH services employed, making it challenging for clinicians, healthcare administrators, and policymakers to synthesize best practices and identify implementation gaps [4,5,6,7]. To date, there is limited comprehensive mapping of the landscape of telehealth applications for SRH that focuses on a wider range of services covered by Title X-funded clinics, such as services for STI, contraceptive care, family planning, and sexual well-being.
Previously, a systematic review evaluated telehealth services for women’s preventive health services, which included STI prevention and family planning services, in studies published between July 2016 and March 2022 [4]. This review found only two randomized controlled trials and five non-randomized studies which met their eligibility criteria. However, Title X-funded clinics cover a wider range of services (e.g., post-diagnostic management and treatment of STI, sexual well-being, etc.) and cater to a wider consumer base apart from only biologically female patients. Implementation of telehealth or any modifications to clinic workflows would require considering its impact on all patient populations served by the clinic. Therefore, there is need for a comprehensive evidence review spanning all services provided in such clinics and for all patient populations served in such clinics.
This review aims to identify and describe the current research on sexual and reproductive health services provided via telehealth in developed countries, for the intended target audience being Title-X funded clinics. For this evidence map, we operationalized “sexual and reproductive healthcare” as the subset of services that align most closely with the U.S. Title X program and with SRH domains most commonly delivered through telehealth in clinical practice. These include family planning/contraception counseling and provision, STI prevention, testing, and management, including HIV prevention and care, and sexual wellness services (e.g., management of sexual dysfunction, libido concerns, or partner communication). Broader gynecologic conditions such as menstrual disorders, dysmenorrhea, pelvic pain, and other non-SRH gynecologic diagnoses were excluded because their evaluation and management typically rely on in-person diagnostic procedures and fall outside the primary scope of Title X-funded care. Our search strategy was therefore tailored to focus on telehealth-enabled SRH services most relevant to U.S. public health and clinical delivery contexts.
We restrict this mapping review to countries with a human development index of 0.8 or more. This threshold corresponds to very-high-HDI settings, which more closely resemble U.S. Title X clinical environments in terms of digital infrastructure, reimbursement policies, workforce capacity, and patient population characteristics. Including lower-HDI settings would have introduced heterogeneity in telehealth feasibility, regulation, and access that may not be generalizable to U.S. Title X clinics. While studies from high-HDI countries (HDI 0.7–0.79; example, Mexico, Peru, Colombia, Ecuador, etc.) may also offer relevant insights, they were excluded to maintain fidelity to the review’s implementation-focused purpose for U.S. Title X-funded settings. We highlight this as an area for expansion in future evidence reviews. This review may be used as a resource for implementation of telehealth in healthcare settings and for the development of practice protocols, policy recommendations, and other related work. The second purpose of this review is to identify research gaps and inform future research on sexual and reproductive health delivered via telehealth.
Key Questions
Five key questions were identified to guide this mapping review. The literature reviewed in this report is organized around these questions. The five key questions are as follows:
- How have effectiveness, harms, and acceptability of telehealth methods for SRH been studied?
- What are patient experiences, patient preferences, and patient choice in the context of telehealth utilization?
- What are provider experiences and preferences in the context of telehealth utilization?
- What are the barriers to and facilitators of telehealth methods for sexual and reproductive healthcare services?
- What are the patient engagement strategies for telehealth?
2. Methods
We conducted our mapping review and report our methods and findings based on the methodology for evidence maps and the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidance for scoping reviews [15,16]. We registered our protocol on the Open Science Framework registry (https://doi.org/10.17605/OSF.IO/ZTX7R).
2.1. Selection Criteria
We developed our selection criteria based on the PICOTS framework; these criteria are shown in Table 1 [16]. Briefly, we included primary research studies for teleSRH in countries with HDI 0.8 or more published between 2017 and 2025. We used two broad conceptual definitions for SRH and telehealth, as described in Appendix A. In this review, we restricted our scope to telehealth involving only bi-directional communication between a provider and a patient. We included studies reporting at least one outcome related to SRH.

Table 1.
Populations, Interventions, Comparators, and Outcomes (PICOTS) and corresponding inclusion and exclusion criteria.
2.2. Peer-Reviewed Literature Search and Screening
We developed a search strategy with the help of a research librarian, following the Peer Review of Electronic Search Strategies (PRESS) guideline [17]. The search combined the two broad concepts as such: (sexual and reproductive healthcare services) AND (telehealth). For the concept of sexual/reproductive healthcare services, these specific concepts were used: (contraception OR family planning OR gynecology OR women’s health OR sexual health OR sexual wellness OR reproductive health OR sexually transmitted diseases OR fertility OR abortion OR unplanned pregnancy OR unwanted pregnancy OR preconception OR pre-pregnancy OR Papillomavirus vaccines). For the concept of telehealth, these specific concepts were used: (telemedicine OR telehealth OR telephone OR smartphone OR cellular device OR mobile device OR text messaging OR SMS OR virtual OR remote monitoring OR ehealth OR mhealth OR mobile health OR digital health). Telehealth was required to be a key concept by using title keywords or focused subject headings, and sexual/reproductive health was accepted as a general concept by using any subject heading or multi-placement keywords (title, abstract, etc.). Results were limited to journal articles in the English language, published between January 2017 and January 2025, to focus on recent evidence that may be applicable to current systems and as a continuation of a previous evidence map which included studies published through 2016 and the AHRQ systematic review that used a similar cut-off citing the same reason [4,5]. Searches were executed in three electronic databases: Medline (Ovid), Embase (Ovid), and CINAHL (Ebsco). The query used both keywords and subject headings for each concept, and a separate strategy was developed and optimized for each electronic database. The complete search strategies are available in Appendix B. All searches were conducted on 25 January 2025.
We uploaded citations to the PICO Portal™ (www.picoportal.net), an online tool for systematic reviews, and we removed duplicates using its software [18]. PICO Portal uses machine learning to prioritize citations most likely to meet eligibility criteria. Initially, two independent reviewers assessed titles and abstracts for relevance to the key questions based on predefined inclusion and exclusion criteria (Table 1). Any disagreements were resolved through team discussions. Once the algorithm achieved a 99% recall rate for eligible citations, we switched to one reviewer until a 100% recall rate and zero false negatives were achieved. Two independent reviewers then screened each article at the full-text level. Any disagreements were resolved by group discussion.
2.3. Data Management, Risk of Bias Assessment, and Synthesis
Data from included studies were extracted using Microsoft Excel [Microsoft Corporation. (2024)] sheets by one reviewer and checked for accuracy by a second reviewer (Supplemental Tables S1 and S2). We extracted the following data elements: study id, title, lead author contact details, geographical setting, study aim(s), study design, study period, participant characteristics (e.g., total N, population descriptions, age, race, ethnicity, etc.), service or intervention details, and summary of results. We focused only on outcomes directly related to sexual and reproductive health services, for example, rates for STI screening, STI treatment, STI incidence, STI prescription refill rates, rate of contraception uptake, relevant lab results (e.g., viral load), compliance and adherence to prescriptions, and process outcomes such as travel time, wait times, no-show rates, ability to receive confidential care in preferred settings, and rural/marginalized community outreach, etc.
In this review, we assessed the risk of bias (RoB) in the included RCTs using the Cochrane RoB-2 tool and in included cohort and quasi-experimental studies using the ROBINS-I tool [16,18]. For the ROBINS-I tool, full assessments were to be conducted only for studies which passed the pre-screener portion of the tool and were not assessed with critical RoB for the selection bias domain.
Data were analyzed descriptively in Microsoft Excel [Microsoft Corporation (2024), Redmond, WA, USA] and summarized narratively because the uniqueness of each telehealth intervention hindered quantitative pooling. Tableau [Salesforce (2025), Seattle, WA, USA] was used for data visualization. Narrative summaries presented in the Results Section are organized around the five key questions guiding the report.
3. Results
Searches resulted in 8306 references (Ovid MEDLINE: 2700 references; CINAHL: 2649 references; and Embase: 2957 references). The PRISMA flow diagram for screening and selection is presented in Figure 1. After deduplication, the initial sample totaled 5963 references for dual screening of title and abstract. In the full-text screening phase, 436 references were screened. A total of 142 references met study inclusion/exclusion criteria after the full-text screening [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160].
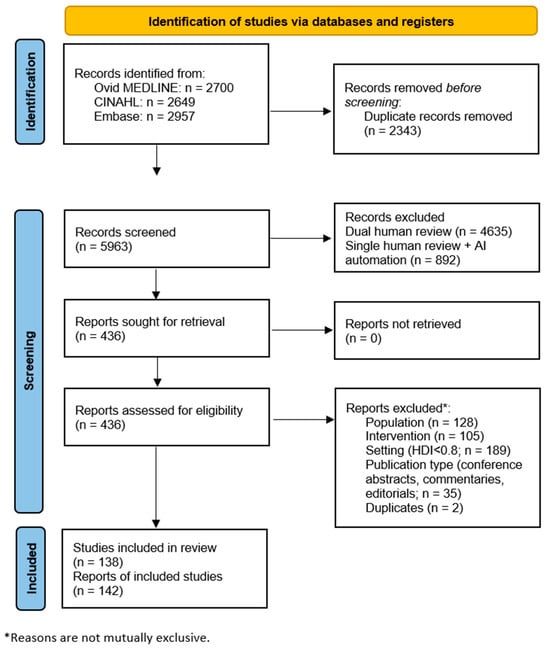
Figure 1.
PRISMA Flow Diagram for Study Selection. AI—artificial intelligence-assisted; HDI—human development index.
3.1. Results of the Literature Search
A total of 138 unique studies in 142 publications [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160] were included in the review [Supplemental Table S1]. The most common study designs were cohort studies (k = 48), cross-sectional studies (k = 34), mixed-methods studies (k = 23), and RCTs (k = 23). There were thirteen pre–post studies and one case–control study. Overall, as shown in Figure 2, contraceptive care and family planning was addressed in 64 studies; sexually transmitted infection prevention and treatment in 89 studies; and sexual wellness in 14 studies. Most of the RCTs (20 out of 23) focused on STI prevention or treatment [Figure 2]. Sexual wellness was the least evaluated service type, with only three RCTs and 9.9% of total studies (k = 14; Figure 2). Contraceptive care and family planning services were mostly evaluated in observational cohort (k = 25) and cross-sectional survey (k = 25) studies, with only one RCT evaluating this service type.
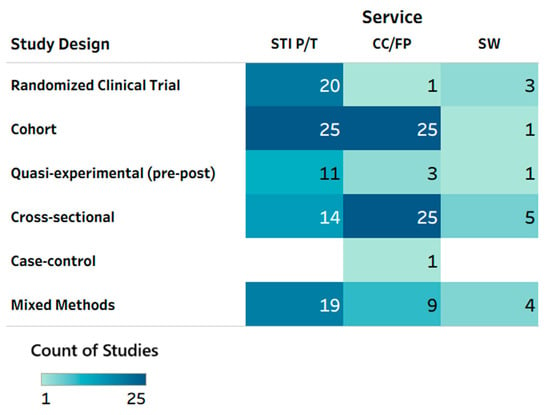
Figure 2.
Heat map: service type by study design. CC/FP—contraceptive care or family planning; STI P/T—sexually transmitted infection prevention and treatment; SW—sexual well-being. Note: Multiple service types could have been tested in a given study/publication, so total counts may not add up to the total number of reports included in this scoping review (n = 142 publications).
Most studies were conducted in the United States (106), with the majority of non-US contributions from the United Kingdom, Australia, and Canada. Among U.S.-based studies, all regional locations (the Northeast, South, Midwest, and West) had similar representation, with several studies covering multiple or unspecified regions. Patient rurality was described as urban in 31 studies, rural in 4 studies, and some combination of rural and urban and/or suburban in 16 studies; however, 83 studies did not report rurality status. Sexual and gender minority (LGBTQ+) representation was explicitly included in 41.5% studies [k = 59; Supplemental Table S1]. Among the 142 included publications, the majority reported the distribution of racial or ethnic groups in their study sample. At least 112 studies reported including racially/ethnically diverse populations, such as Black, White, Hispanic, Asian, Indigenous, Native American, Pacific Islander, “some other race”, or multiracial subgroups. These findings indicate that while studies varied widely in geography, design, and sample composition, many incorporated key dimensions of participant diversity relevant to equity in sexual and reproductive healthcare research.
Several different modes of telehealth were evaluated across service type, individually or as combinations, including synchronous modes of communication such as telephone and videoconferencing and asynchronous modes such as text messaging, mobile application, website, web portal, and electronic mail [Figure 3].
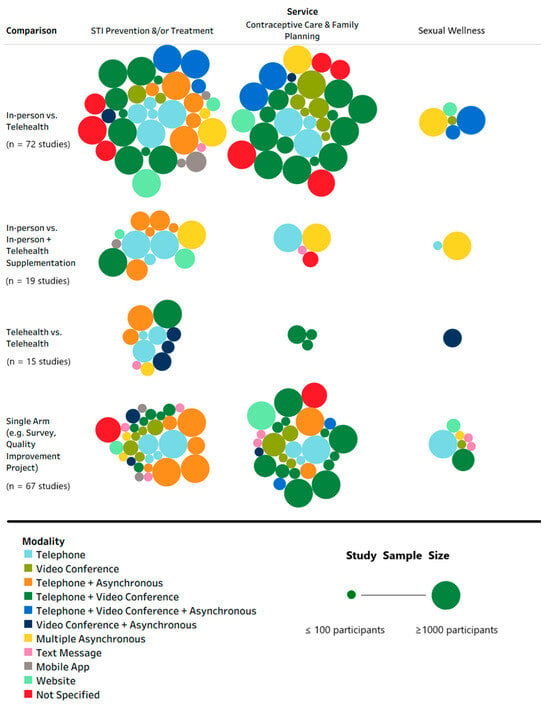
Figure 3.
Bubble plot: service type by comparisons by mode of telehealthcare delivery. STI—sexually transmitted infection; vs.—versus. Note: Asynchronous mode includes (two-way communication between provider and patient via) text message, mobile application, website, web-based portal, and e-mail. Each bubble represents a study and the size of the bubble corresponds to study sample size which, as shown in the legend, was winsorized at a minimum of 100 participants and a maximum of 1000 participants, for visual aid. Multiple service types could have been tested in a study, and multiple types of comparisons could have been made in the same study; therefore, row totals and column totals are not presented as their sum/total would not yield the total number of reports included in this scoping review (n = 142 publications).
3.2. Risk of Bias Assessment
Among RCTs, RoB ranged between moderate (some concerns) and high (Supplemental Table S3; Figure 4); common concerns, given the inherent difficulty of masking telehealth delivery, included lack of allocation concealment and challenges with blinding of participants and outcome assessors. Several trials relied on self-reported outcomes such as contraceptive adherence, satisfaction, or sexual health behaviors, which introduced potential reporting and social desirability biases. Attrition rates were often high, particularly in trials with longer follow-up periods or those targeting adolescent or marginalized populations, and few studies employed appropriate methods to address missing data. Additionally, fidelity of intervention delivery and variation in technological literacy were inconsistently assessed, limiting confidence in treatment effect estimates.
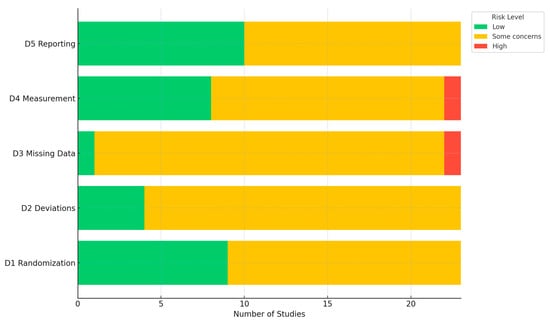
Figure 4.
Bar graph for domain-specific risk of bias assessments. Abbreviation: D—domain; D1—bias from the randomization process; D2—bias from deviations from intended interventions; D3—bias from missing outcome data; D4—bias in the measurement of the outcome; D5—bias in the selection of the reported result.
All observational cohort and quasi-experimental studies demonstrated very high susceptibility to confounding and/or selection bias, and as a result, they did not merit complete assessments using the ROBINS-I tool. Many studies used convenience samples or recruited participants already engaged with telehealth services, potentially overestimating acceptability and effectiveness. Adjustment for key covariates such as age, socioeconomic status, prior healthcare access, and digital literacy was inconsistent, and most of the studies did not employ advanced methods (e.g., propensity score matching or inverse probability weighting) to mitigate these biases. Misclassification bias was also possible where telehealth exposure was not clearly defined or where hybrid in-person/virtual models were combined. Despite these limitations, most studies provided sufficient detail on intervention characteristics and outcomes to allow synthesis; however, the overall certainty of evidence remains limited due to methodological heterogeneity and risks of bias inherent in evaluating telehealth interventions in real-world sexual and reproductive health settings.
The results of the literature search [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160] are organized around the five key questions driving this study (i.e., effectiveness and acceptability of telehealth methods; patient experiences; provider experiences; barriers and facilitators; and effectiveness of patient engagement strategies), and study-level details are presented in Supplemental Tables S1 and S2. Within each subsection, evidence is reviewed, summarized, and synthesized as related to contraceptive care and family planning; sexually transmitted infection prevention and treatment; or sexual wellness (respectively) in alignment with the sexual and reproductive health conceptual definition guiding this report. Due to the overlap in articles discussing contraceptive care and family planning, these concepts were combined into a single construct for reporting results.
3.3. Key Question 1: Effectiveness, Harms, and Acceptability of Telehealth Interventions
Contraceptive care and family planning. Thirty-nine studies under KQ1 (KQ, key question) evaluated CC/FP delivered via telehealth. Overall, several studies examined whether teleSRH interventions improved the ability to provide access to care for people who may be missed in regular care settings. For example, one study focused on people with depression compared in-person visits and a 4-week ecological momentary intervention delivered via bi-directional text messaging to reduce sexual and reproductive health risks [71]. After the 4-week text intervention, participants reported lower condom-unprotected sex events and more consistency with condom use at three months [71]. Another small pilot study reported successful engagement of rural women with opioid use disorder [82]. Researchers in that study noted that of those who used the sexual and reproductive health services, over a quarter did so for contraceptive care and 47% for pregnancy counseling or testing. However, only a fraction of those referred to in-person care attended an in-person visit, limiting the effectiveness of the intervention [82].
Telehealth has varied effectiveness for contraceptive uptake and long-term use. For those attending telehealth visits for contraceptive needs, there are positive long-term trends. One study measured long-term follow up for Depo Provera in young people between the ages of 13 and 21 years [28]. They found that those receiving the text message intervention were 3.65 more likely to be using Depo Provera 20 months after the end of the intervention. Similarly, another study provided subcutaneous Depo-Provera self-injection counseling via telehealth and 21% of participants opted to use that form of birth control [56]. One study noted that almost 70% of those who had a contraceptive telemedicine visit were still using the method chosen six months later, and 44% would choose a telemedicine visit for their next contraceptive appointment [79]. Additionally, in that same study, 76% of people referred for an in-person visit were seen in the clinic within 30 days for contraceptive follow-up [79]. For men seeking vasectomy, uptake was also not different between men who underwent telehealth consults for the procedure versus those who completed the consultation in person, highlighting the effectiveness of contraceptive counseling via telehealth [37]. However, when studying contraceptive uptake postpartum, the telehealth trends were not as significant. Some studies found minimal changes in contraceptive uptake in postpartum patients [19,21]. One study noted that patients in telehealth and in-person visits selected contraception at similar rates, although those in the telehealth group were less likely to choose long-acting reversible contraceptive or permanent sterilization, which would require in-person visits [21].
Sexually transmitted infection prevention and treatment. Seventy-five studies under KQ1 evaluated STI-related services for telehealth. Telehealth was found to be an effective mode of care in the delivery of STI prevention and treatment to numerous specific populations. One RCT evaluated telephone-delivered motivational interviewing to reduce risky sexual behavior in racial–ethnic minority women living with HIV [55]. They found that telephone-delivered motivational interviewing focused on individualized care significantly reduced sexual risk behavior, such as reducing substance use in sexual contexts and condomless sex. One RCT [86] and one mixed-methods study [30] both reported that telehealth services offering free specimen self-collection kits and telephone or video consultation effectively increased STI testing uptake and treatment among populations at high risk for contracting HIV. In one RCT that provided home STI test kits with video consultation for transgender youth, the level of PrEP use was very low, and sexual risk behavior did not change significantly over the follow-up period [75]. They reported that the participants were worried about having to be seen on camera by a person they did not know and felt hesitant to talk about sensitive topics [75]. In addition to telephone and video consultations, a few studies also showed that SMS/text-based intervention with a two-way communications component or with a telephone call component was effective in enhancing PrEP adherence among young men who have sex with men [42,58]. Their findings also demonstrated that the intervention was associated with a reduction in missed doses measuring by pill count [42]. Mobile health applications were also reported as effective in lowering the proportion of condom-unprotected sex in adolescent and young adult women with depressive symptoms and high-risk sexual behavior [71].
Sexual wellness. Five studies in this review explicitly reported sexual wellness outcomes in the context of effectiveness of telehealth interventions. These studies targeted special populations with different population-specific outcomes, which does not allow comparison across studies.
In a secondary analysis of an RCT for testing the efficacy of HIV tele-counseling in 424 men who have sex with men (MSM) couples, one study reported the impact of the intervention on the couples’ formation and adherence to safer sexual agreements [76]. Couples in the intervention (tele-counseling) arm had significantly greater odds of reporting a safer sexual agreement (3 months OR 1.87, p-value = 0.005, and 6 months OR 1.84, p-value = 0.007), lower odds of reporting discordant sexual agreements at 6 months (OR 0.62, p-value = 0.048), and a significantly lower odds of reporting breaking their sexual agreement (3 months OR 0.51, p-value = 0.035, and 6 months OR 0.23, p-value < 0.001). Another secondary analysis study of an RCT reported effects of a mobile app and a website based online discussion forum for reducing HIV stigma among young Black MSM over 6 months [23]. Participants who used the discussion forum reported an overall significant decrease in perceived HIV stigma (p < 0.05). Only participants with at least a high school education had a significant reduction in internalized homophobia over time (p = 0.006). Participants whose discussions focused on experiencing sexuality-related stigma reported increases in internalized homophobia (p ≤ 0.01) and sexual prejudice (p ≤ 0.05) over time. One UK-based study evaluated a virtual youth center for sexual minority young men to discuss HIV, sexuality, relationships, and sex [22]. In this study, 11 participants were guided through discussions to introduce new knowledge and build emotional resilience. All participants either maintained or improved their level of HIV knowledge and felt more confident about their sexuality (11% increase). One RCT in Turkey evaluated the effect of telephone counseling on the sexual lives of individuals with a bowel stoma following ileostomy or colostomy surgery [81]. Participants in the intervention arm reported significantly greater sexual satisfaction, reduced concern about sexual performance, and lower chances of lack of sexual desire 12 weeks after surgery (p < 0.01). One RCT evaluated a multi-component mHealth intervention focused on black gay youth with other vulnerabilities (e.g., polydrug use, alcoholism, anxiety and depression, experience of racial discrimination or sexual minority stigma) and reported no significant improvement in sexual risk behavior, i.e., self-reported number of condomless anal sex acts with serodiscordant and unknown sero-status partners in the past 3 months [121].
3.4. Key Question 2: Patient Experiences, Preferences, and Choice
Contraceptive care and family planning. Twenty-four studies under KQ2 addressed CC/FP. Overall, patients had positive experiences with contraceptive/family planning telehealth visits. Those who are already comfortable with telemedicine and direct-to-consumer advertisements are more comfortable using telehealth services [54]. Similarly, those who were less likely to value long-term relationships with providers were more likely to prefer telehealth [32]. A cross-sectional study at a tertiary care center in the USA reported that 54% of telemedicine patients reported “high-quality” contraceptive counseling compared to 64% of office visit patients (p = 0.29) [70]. Telehealth patients also reported ease of communication, less scheduling difficulty, overall convenience, efficiency with shorter visit times, and privacy as reasons for preferring telehealth visits [31,60,62,70]. Multiple studies note that patients were satisfied with the care they received and would consider using telehealth in the future [20,51,78], although one study noted that post-pandemic women found telehealth less acceptable than in-person care [62]. Those who preferred in-person visits over telehealth cite privacy, communication concerns, and perceived limits related to patient-centered care as rationale for preferences [51,57,70]. During the pandemic, one small RCT (n = 87) evaluating tele-counseling for contraceptive care in pregnant women in their third trimester reported patient preference favoring telehealth over in-person care for family planning counseling and education [147]
Sexually transmitted infection prevention and treatment. Fifteen studies under KQ2 addressed STIs. The majority of patients both in the United States and international countries report having positive experiences with telehealth in STI services. A small mixed-methods evaluation study in the United States reported that partnered gay, bisexual, or other men who have sex with men who participated in telehealth delivered via video chat for STI testing reported a very high level of service acceptability and quality [80]. Another small mixed-methods study focused on patients’ parents’ experience with a mobile health application to enhance HPV vaccination decision-making [25]. Parents rated the app as helpful and useful and that the parents would recommend the app to others [25]. Patients who received pre-exposure prophylaxis (PrEP) care via telehealth reported that the service was very helpful, confidential, fast, convenient, and easy to use, and they would recommend the method to others [58,67]. Similar to the study in the UK, patients were very satisfied with similar PrEP care via telehealth and reported that telehealth increased access to STI services at a lower cost [46,66]. Moreover, patients who received telehealth for STI services also reported that telehealth provides greater anonymity than usual care [49]. For people living with HIV, one large survey reported that during the peak pandemic period, a vast majority of patients preferred telemedicine over in-person care [155]. The main benefits of telemedicine (compared to in person) reported were savings of time and money, convenience, and ability to complete appointments as scheduled [155]. Just over half of PLHIV said they would feel more comfortable discussing sensitive topics (e.g., substance use, relationship issues) in person than over telephone (60%, n = 164) or video (55%, n = 151). Despite limited experience with video telemedicine, half of all participants desired a mix of telephone and video visits as part of their future HIV care [155].
Sexual wellness. Nine studies under KQ2 addressed sexual wellness. Two studies in this review focused on sexual wellness care services for adolescent populations through confidential, bi-directional, text message-based services. One study which focused on adolescents’ service preferences reported that they prefer interactive texting services that connect them to a person, although automated messages might be useful to bring new topics to their attention [85]. The other adolescent-focused study evaluated utilization and preferences for a confidential sexual health text-based helpline to expand Planned Parenthood of Western Pennsylvania’s sexual health programming [65]. This service was highly rated by users and was viewed as acceptable and appropriate. Common topics for information seeking among users included topics such as relationships, sexual acts, contraception, body image, STIs, pregnancy, values, Planned Parenthood services, sexual identity, and PrEP. One study reported that in the US, erectile dysfunction and contraception were among the leading reasons for utilization of direct-to-consumer telemedicine services [52]. Notably, in comparison to primary care physician visits, direct-to-consumer telemedicine services were used more frequently in urban areas and less frequently by low-income households [52]. For men’s sexual health, a large survey study in Texas (USA) reported that older patients were less likely to prefer telemedicine (odds ratio [OR], 0.55; 95% confidence interval [CI], 0.36–0.80; p < 0.001), less likely to agree to a video visit because of privacy concerns (OR, 0.51; 95% CI, 0.35–0.75; p < 0.001), and less likely to recommend a telemedicine visit compared with their younger counterparts (OR, 0.37; 95% CI, 0.27–0.51; p < 0.001); these preferences were regardless of distance from the andrology clinic, which was up to 57.5 miles [146]. One small RCT focused on post-menopausal women evaluated a mindfulness-based intervention via videoconferencing [153]. This RCT reported greater patient satisfaction and significant reduction in sexual distress scores but no significant improvement in sexual function scores [153]. For people living with HIV, one large survey conducted during the peak pandemic period reported that a majority of the patients expressed comfort with discussing sensitive topics such as relationship issues with care providers via telehealth [155].
3.5. Key Question 3: Provider Experiences and Preferences
Contraceptive care and family planning. Six studies assessed providers’ experiences with contraceptive/family planning telehealth consultations. One cross-sectional survey study reported that the majority of family planning providers believed telehealth visits were effective and should continue post-pandemic [77]. Conversely, another survey noted that the majority of healthcare providers were less satisfied with telehealth visits but would be willing to continue them in the future [62]. Another survey reported that among the clinicians interviewed for their study, all believed that telehealth was a good option, particularly for time-critical services and for patients living with disabilities, particularly in rural areas [31]. However, another survey study noted that approximately one quarter of physicians in their study expressed concerns for confidentiality for their adolescent patients [74].
Sexually transmitted infection prevention and treatment. Six studies discussed the providers’ experience with telehealth services for STI prevention or treatment; however, the feedback provided was generally positive. Due to the COVID-19 pandemic, a reduction in walk-in hours and in-person visits was reported [74]. Health providers rated telehealth platforms as excellent or good in delivering STI services in response to the rapid change from the pandemic [46]. They expressed that telehealth allowed them to assess patients safely and confidentially during the COVID-19 outbreak [46]. Some physicians noted that telehealth improved access for people with disabilities and those living in remote locations to services [31]. A small survey study in seven community-based organizations in two Southern states in the US reported that more than half of health providers working in community-based HIV/AIDS service organizations in the US were very interested in using mHealth apps to communicate and share general health tips with their patients in the future [84].
Sexual wellness. In this review, no studies related to sexual wellness evaluated provider perspectives or experience of telehealth services.
3.6. Key Question 4: Barriers and Facilitators for TeleSRH
Contraceptive care and family planning. Forty-four studies under KQ4 addressed CC/FP. During the pandemic, access to contraceptive/family planning telehealth visits drastically increased [20,33,74]. Patients without insurance were more likely to use telehealth services [57]. Barriers to telehealth for patients included not having a device [88], technology issues during the visit or concerns for miscommunication [51,70], or language discordance between the patient and provider [31]. In terms of miscommunication, an inability to read the providers’ body language or facial expressions emerged as a concern [31], while issues communicating with providers in general was cited as the reason to choose an in-person visit over telehealth [70]. Only one study found telehealth visit completion rates by race [87].
Multiple studies noted that telehealth visits facilitated care for underserved patient groups. For parenting teenagers and their families, telehealth filled a gap in reproductive and sexual health services [72]. Telehealth also connected rural-living women with opioid use disorders to care [82]. Participants in multiple studies noted that telehealth was particularly helpful when they lived at a distance from the clinic and had difficulty accessing care [32,51,82]. Additionally, other research teams found that patients chose telehealth visits to avoid a physical exam, to reduce wait times, and for privacy concerns [51,60].
Clinic-based barriers to providing telehealth stemmed from a lack of existing policies and procedures, reduced staff availability, and gaps in technology infrastructure [24,32]. There was an overall increase in the number of telehealth visits during the pandemic in states with insurance reimbursement, although it did not increase the overall number of contraceptive encounters [38]. Providers found that three quarters of contraceptive telehealth visits were covered by insurance, although this was slightly less than coverage for non-contraception visits (p < 0.001) [60]; however, interestingly, Medicare reimbursement was seen as a facilitator [24].
Sexually transmitted infection prevention and treatment. Forty-three studies under KQ2 addressed STIs. Although telehealth has been described as a silver lining in the midst of the COVID-19 outbreak, there were some significant challenges that limited the use of the telehealth method in STI services. Patients noted that technical difficulties and digital inequalities were barriers to delivering STI services via telephone [66]. Some patients, such as elderly people or people with economic disadvantages, may not have access to the internet or a phone/smartphone. Patients who were assessed for STI prescription via SMS text message were less likely to have an agreement with safe prescribing information than those assessed via telephone, and they were not likely to use the service in the future [61]. Patients were willing to use the telehealth platforms that they got used to, such as telephone, mobile applications, video calls, or SMS, for future service [31,84].
Patients’ and healthcare providers’ experiences and preferences with related technology were one of the facilitators of telehealth. According to clinicians and patients’ interviews, they felt that language barriers were a challenge faced by utilizing telephone consultation, particularly among new patients, leading to communication issues [31,66].
The current review noted some facilitators of telehealth methods for STI prevention and management services. Privacy and confidentiality were described as facilitators of using telehealth for STI services. A survey study in seven community-based organizations in two Southern states in the US reported that healthcare staff who were confident that safeguards are in place to keep electronically shared information from being seen by other people were very interested in using telehealth in future STI services [84].
Sexual wellness. Only a single study in this review evaluated any barriers for telehealth services for sexual wellness. This study reported that during the initial phase of the COVID-19 pandemic, compared to in-person care, adoption of telehealth services was lower among African Americans and multi-racial patients in Arkansas, Kansas, Missouri, and Oklahoma [48]. This study found no differences between adoption of telehealth or in-person care among Hispanic patients and reported an increase in adoption of telehealth services among White patients, as compared to in-person care during the initial phase of the COVID-19 pandemic. This study did not empirically evaluate any reasons to explain why race was a barrier to adoption of telehealth services during the initial phase of the pandemic [48].
3.7. Key Question 5: Patient Engagement Strategies
Contraceptive care and family planning. Eighteen studies under KQ5 addressed CC/FP. Engaging patients in contraceptive/family planning telehealth visits may depend on the patient’s comfort with technology. Those with higher levels of comfort with technology or prior experience with telehealth were more likely to choose a contraceptive/family planning telehealth visit [32,54]. Even with that comfort level, it is important to offer other accessible options to limit additional burden. One study effectively used telehealth visits in conjunction with mail order pharmacies and curbside contraceptive services, limiting the amount of time patients would spend on accessing care [33]. Another study provided regular text message reminders with case manager follow-up, resulting in a significant increase in contraceptive use at 20 months post-intervention [28]. Text message or smartphone alerts were used in other studies and showed patients were willing to engage with technology and were simultaneously satisfied with the intervention [69,71].
Sexually transmitted infection prevention and treatment. Twenty-four studies under KQ5 addressed sexual wellness. Multiple strategies were used to engage patients in telehealth for STI prevention and management services. Based on some studies found in this review, a possible strategy to enhance engagement of telePrEP includes weekly text/SMS messages with the option to set up reminders and a platform that supports two-way communication, such as telephone or video calls [30,42,58]. Video or telephone consultations were commonly utilized for STI testing with the services of free home test-kit delivery [30,36,67,75]. However, some patients prefer receiving text/SMS only for a negative result or phone calls to consult about the treatment if they receive a positive result [49]. To enhance STI/HIV-preventive attitudes, knowledge, and skills and reduce sexual risk behavior, the intervention commonly employs tailored telephone/video counseling that focuses on individualized care [29,59]. Additionally, standardized phone calls and mobile applications that provide information about vaccination with reminders and chat functions could be considered to be a good method for enhancing patient engagement in the HPV vaccination program [25,26].
Sexual wellness. Two studies in this review evaluated patient engagement strategies for telehealth [44,64]. One study evaluated videoconferencing as a strategy to deliver mindfulness-based intervention for sexual wellness among breast and gynecologic cancer survivors, the efficacy of which had been established before in in-person settings [44]. This study reported that adapting such an intervention for delivery via videoconferencing was feasible, perceived as appropriate, and acceptable among patients during the COVID-19 pandemic. In an adult population in Italy, one study evaluated patient engagement with a telephone helpline service between 2010 and 2017, which included sexual wellness counseling [64]. This study reported a decrease in requests over time for information about specific sexual dysfunctions, and general sexual health. Despite the advent of social media, there was an increase in the number of service users over the years, particularly among men who called most frequently for erectile dysfunction, suggesting that a telephone-based helpline may still be an important resource for patent engagement and delivery of sexual wellness care [64].
4. Discussion
There is relatively robust evidence that prescription of contraception via telehealth is feasible and acceptable to patients and providers. While comparable effectiveness (telehealth vs. in-person care) was not well established in the literature, patients generally found telehealth comparable in terms of acceptability and in many cases preferred telehealth over in-person contraception care. Studies on sexual wellness were scant and merit more research. The few studies on sexual wellness included in this review mostly reported positive results; however, this could potentially be due to publication bias. For contraceptive care/family planning services, we found only one small RCT (n = 87, Turkey) which evaluated effectiveness of telehealth for family planning counseling and education for third trimester pregnant women [147]. This RCT reported patient preference favoring telehealth over in-person care for counseling, education, and family planning consultation services [147]. We found no other RCTs evaluating telehealth for contraceptive care/family planning, but we found three quasi-experimental studies [51,56,82] and several observational studies which indicated that providing contraceptive care via telehealth is a feasible option in regard to clinical and patient-reported outcomes. By reducing barriers to care (e.g., transportation to clinics, childcare, etc.), telehealth provides methods of contraception to populations who may otherwise not receive consistent or any contraceptive care [31,72,82]. Arias et al. noted that long-acting reversible contraceptive methods may be selected less often by telehealth patients compared to patients seen in person [21]. However, telehealth patients receive contraceptive counseling on the full range of options and can select a long-acting reversible contraceptive, though it requires an in-person follow-up visit for placement.
The finding with perhaps the strongest evidence suggests that telehealth may be an important tool in the prevention of HIV. Two RCT articles reported on telehealth services to increase PrEP uptake and provide prescriptions among groups at high risk for contracting HIV [58,75]. These studies showed that telehealth may be an effective method of providing PrEP and/or promoting regular use. Similar results were found in four quasi-experimental studies [34,42,50,67] and two observational studies [46,66]. Some RCTs studies suggest that telehealth interventions may be an acceptable method of educating young people and their families about HPV vaccination [26,41,73,83]. The quasi-experimental study by Becker [25] further supports this finding. There were no observational studies that support these findings. However, there is not sufficient evidence to suggest that telehealth interventions are as effective or more effective in promoting HPV vaccine uptake compared to in-person interventions.
Although we did not conduct formal grading of evidence, our review team internally discussed the overall strength of the body of evidence following the GRADE/CERQual principles [161]. Overall confidence in the evidence on teleSRH is low to moderate, limited by study design, inconsistency, and indirectness. Most studies were observational and single-site, with heterogeneous telehealth models and outcome measures, leading to reduced confidence due to potential bias and limited comparability. Confidence in findings related to equity and quality of care was very low, reflecting incomplete and inconsistent reporting. Despite these limitations, the direction of the effect in the body of evidence was generally favorable, i.e., telehealth appeared to expand access and maintain safety and effectiveness across diverse SRH domains. However, uncertainty about the magnitude and durability of these effects remains. Future research should prioritize well-designed, adequately powered studies using standardized outcomes and equity-focused measures to strengthen certainty and guide sustainable, patient-centered implementation of teleSRH.
4.1. Applicability of Findings
Title X-funded clinics serve populations that differ markedly from the general U.S. SRH-seeking population. Title X clients are disproportionately low-income, racially and ethnically diverse, uninsured or underinsured, and more likely to experience structural barriers to obtaining in-person SRH care. Many rely on public transportation, shift-based employment, or caregiving responsibilities that constrain their ability to attend in-clinic visits. Telehealth may therefore offer unique value in this context by reducing logistical burdens and expanding access to contraception, STI services, and pregnancy-related care. The set of studies included in this review examined teleSRH interventions in study populations whose demographic characteristics (Supplemental Table S1) approximately resemble Title X patient profiles, including high representation of Black and Hispanic individuals, LGBTQ+ populations, and patients receiving care in safety-net or urban clinics.
It is also important to note that the countries represented in our review differ in their broader levels of telehealth adoption and digital health system maturity. For example, the U.S. is considered a high-capacity telehealth environment, yet adoption varies markedly across states, health systems, and reimbursement structures. Other very-high-HDI countries included in this review (e.g., Canada, Australia, Western and Northern European countries) have more uniform national telehealth frameworks, often supported by centralized governance and universal health coverage. The teleSRH interventions in our included studies therefore reflect localized programmatic implementation rather than national readiness. These differences underscore that while the synthesized evidence is relevant to U.S. Title X clinics, transferability may depend on local digital infrastructure, reimbursement pathways, and patient access to technology.
Several factors may influence the generalizability of our findings. First, sexual and reproductive health services are often provided alongside primary care, which means our review might not have encompassed all telehealth services related to sexual and reproductive health. Second, our review focused exclusively on high-income countries, with most of the studies originating from the United States. However, due to differences in healthcare and insurance systems, findings from other high-income countries may have limited and conditional applicability in US-based title X clinic settings, especially in regard to patient and provider experiences and barriers and facilitators to telehealth implementation. Relatedly, our choice to limit inclusion to very-high-HDI countries may have excluded informative teleSRH models from high-HDI settings with emerging digital health infrastructure. Expanding the geographic scope to include high-HDI countries (HDI 0.7–0.79) in future reviews could provide a broader perspective on telehealth implementation across diverse resource levels while still retaining relevance to U.S. Title X settings. Third, most of the studies included in the review were conducted during the COVID-19 pandemic, a period marked by a rapid expansion of telehealth services and the adoption of various communication methods between patients and providers. This resulted in a steep learning curve for healthcare systems, and initial data may not fully reflect the long-term effectiveness of telehealth services after the pandemic. Additionally, during the pandemic, even if some services were available via telehealth, many others were still disrupted; our findings are limited to the services covered by telehealth. Fourth, fewer studies on sexual wellness were identified for inclusion in the review, highlighting the need for caution when considering telehealth as a means to improve sexual wellness due to the limited amount of data available. Lastly, while this review included all accessible studies, it is important to acknowledge that there may be additional unpublished research related to telehealth in this domain that was not available at the time of our review.
4.2. Implications for Practice and Policy
This review has several important implications for clinical practice and policy development. The COVID-19 pandemic significantly disrupted clinical practice, making the use of technology for basic care essential [12]. Our findings show that patients are generally more open to telehealth for sexual and reproductive health services than healthcare providers. Given that telehealth is now integrated into many healthcare systems, providers must become more proficient in using telehealth tools. Health training programs should incorporate telehealth-specific training to prepare providers effectively. Moreover, clinics and hospitals must continue enhancing measures to protect patient confidentiality. Several studies raised concerns about confidentiality during sexual and reproductive health telehealth sessions, which could be compromised by either patients or providers [4,5,6]. Health systems should offer more guidance on maintaining confidentiality during these visits, such as ensuring private environments for both patients and providers. Documentation of how confidentiality was ensured should be a standard practice. Although telehealth is often seen as a solution for reaching rural or underserved populations, current research predominantly reflects experiences of urban populations. To improve telehealth accessibility in rural areas and for vulnerable groups, additional financial support is needed. Policy changes at the national and state levels will be essential to support these adjustments [12]. The 2010 Affordable Care Act (ACA) expanded access to sexual and reproductive health services, and the COVID-19 pandemic further accelerated telehealth adoption. Future research and funding should focus on evaluating telehealth outcomes, particularly among vulnerable populations, and assess its cost-effectiveness for health systems.
Of note, we recognize that teleSRH care exists within broader sociocultural and political contexts, including gender, sexuality, stigma, and structural inequities, that are beyond the scope of this implementation-focused review but remain critical areas for future research. Although AI is not within the scope of this review, it merits discussion. The use of AI in healthcare is accelerating at a time when AI use is not regulated [162]. There is an increasing use of virtual assistants, chatbots, and AI translation in healthcare services [162]. A lot of how those services work and how they are used is unclear to the patients [162,163]. The increasing adoption of AI technologies increases the risk of dehumanizing healthcare, eroding the already strained patient–provider relationship [163]. These are tools that should help the provider get closer to the patient, not tools replace the provider or create distance between the provider and the community. This would not benefit vulnerable communities and would increase the existing inequities [163]. As the AI landscape continues to evolve, integration of AI into telehealth warrants more research in regard to safety, efficacy, effectiveness, and provider + patient preferences.
5. Conclusions
This scoping review synthesizes a rapidly growing body of evidence on the use of telehealth in the delivery of sexual and reproductive health (SRH) services, revealing both promise and persistent challenges. Overall, telehealth demonstrates effectiveness and acceptability across key SRH service categories including contraceptive care/family planning, STI prevention and treatment, and sexual wellness, particularly in expanding access for underserved populations, including rural residents, adolescents, and individuals with stigmatized health needs. Patients largely report positive experiences, citing convenience, confidentiality, and efficiency, though preferences for in-person care remain among those valuing relational continuity or requiring procedures. Provider perspectives were generally supportive but reflect concerns about confidentiality and communication limitations, especially for adolescents and patients with language barriers. Barriers to telehealth include digital inequities, technology literacy, and infrastructural gaps, whereas facilitators include insurance coverage, flexible service modalities (e.g., text messaging, video, mail-order delivery), and tailored engagement strategies. Evidence on patient engagement strategies, such as SMS reminders, app-based counseling, and hybrid care models, suggests potential to enhance uptake and adherence to SRH care, though further study is needed to evaluate impact across diverse populations. Notably, sexual wellness remains understudied, particularly from the provider perspective. Overall, our findings underscore the need for policy and practice innovations that address structural and technological barriers, promote equity in digital access, and support the development of evidence-based, patient-centered telehealth models for SRH care. Future research should explore long-term outcomes, cost-effectiveness, and integration of telehealth into hybrid care models to ensure comprehensive, equitable delivery of sexual and reproductive healthcare in the post-pandemic landscape.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/clinpract16010014/s1. Table S1: Descriptive characteristics of included studies; Table S2: Summary of Findings; Table S3: Risk of bias assessments
Author Contributions
R.R.P.: design, data acquisition, analysis, interpretation, drafting manuscript, final approval; N.U.S.: design, data acquisition, analysis, interpretation, drafting manuscript, final approval; C.S.: data acquisition, analysis, interpretation, critical editing of manuscript, final approval, P.P.: design, data acquisition, analysis, interpretation, final approval; P.M.: data acquisition, analysis, interpretation, final approval; A.A.P.: design, data acquisition, analysis, interpretation, drafting manuscript, final approval; L.A.C.-P.: design, data acquisition, analysis, interpretation, drafting manuscript, final approval; M.E.B.: design, analysis, interpretation, critically editing manuscript, final approval. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Key Definitions
Two key constructs were identified as focus points for this review: (1) telehealth and (2) sexual and reproductive health services.
Telehealth. In this manuscript, the term “telehealth” is used to define services that may include the use of information and telecommunications technology in healthcare delivery for a specific patient involving a provider across distance or time, such as remote real-time clinical visits and remote monitoring. For this review, we will refer to “telehealth” when considering interventions that use technology to facilitate interactions at a distance between specific patients and providers.
Interactions could occur over time (asynchronous) and over distance. We will consider telephone conversations, e-mail, and short message service (SMS) texts to be telehealth if they allow interaction between patient and provider and could replace or supplement an in-person interaction. These interventions will not be included if they occur only in one direction or if they are not personalized (e.g., phone, email or text message notifications; generic messages sent to a group of patients).
Sexual and reproductive health services. In this manuscript, sexual and reproductive health (SRH) services considered for this review include contraceptive care, family planning, sexually transmitted infection (STI) prevention and treatment services, and sexual wellness. For this review, contraceptive care includes screening, counseling, provision of contraception, and follow-up care. Family planning services include pre-conception/pre-pregnancy counseling (including birth spacing), pregnancy testing and options counseling, and basic infertility counseling. STI prevention and treatment services include STI counseling, testing, treatment, and prophylaxis, including Human Papilloma Virus (HPV) vaccination counseling and education. Sexual wellness services include education, counseling and treatment related to sexual self-esteem, sexual satisfaction, sexual awareness, and body esteem. We will consider sexual and reproductive health services that can be delivered via telehealth by a broad range of healthcare workers (e.g., physicians, nurses, pharmacists, counselors).
Appendix B
Search Strategy
Ovid MEDLINE(R) and Epub Ahead of Print, In-Process, In-Data-Review & Other Non-Indexed Citations and Daily <25 January 2025>
- exp Reproductive Control Agents/
- exp Contraception Behavior/or exp Contraception/
- (contracept* or birth control).mp.
- exp Family Planning Services/
- family planning.mp.
- exp Gynecology/or exp Women’s Health/
- (gyn?ecolog* or (women* adj2 health)).mp.
- exp reproductive health/or exp sexual health/
- ((reproduct* adj health*) or (sexual* adj health*)).mp.
- exp Sexually Transmitted Diseases/
- (sexually transmitted disease* or sexually transmitted infection* or STD or STDs or STI or STIs).mp.
- exp Fertility/
- fertility.mp.
- exp Abortion, Induced/
- abortion*.mp.
- exp pregnancy, unplanned/or exp pregnancy, unwanted/
- ((unplanned or “not planned” or unwanted or “not want*”) adj3 pregnan*).mp.
- ((sexual or reproductive) adj2 (wellness or wellbeing or well-being)).mp.
- (preconception or pre-conception or prepregnancy or pre-pregnancy).mp.
- exp Papillomavirus Vaccines/or (papillomavirus vaccin* or HPV vaccin* or gardasil or papillomavirus immunis* or papillomavirus immuniz* or HPV immunis* or HPV immuniz*).mp.
- or/1–20
- (telemedicine or telemedical or telehealth or telephone or phone or phones or smartphone* or cell* device* or mobile device* or text messag* or SMS or texting or virtual* or remote* monitor* or ehealth or e-health or mhealth or m-health or mobile health or digital health).ti.
- exp *Telemedicine/or exp *Mobile Applications/or exp *Cell Phone/
- 22 or 23
- 21 and 24
- limit 25 to (English language and yr = “2017-Current”)
Embase
- exp contraceptive agent/or exp contraceptive behavior/or exp contraception/or birth control/
- (contracept* or birth control).mp.
- exp family planning/
- family planning.mp.
- exp gynecology/or exp women’s health/
- (gyn?ecolog* or (women* adj2 health)).mp.
- exp reproductive health/or exp sexual health/
- ((reproduct* adj health*) or (sexual* adj health*)).mp.
- exp sexually transmitted disease/
- (sexually transmitted disease* or sexually transmitted infection* or STD or STDs or STI or STIs).mp.
- exp fertility/
- fertility.mp.
- exp induced abortion/or exp abortive agent/
- abortion*.mp.
- exp unplanned pregnancy/or exp unwanted pregnancy/
- ((unplanned or “not planned” or unwanted or “not want*”) adj3 pregnan*).mp.
- ((sexual or reproductive) adj2 (wellness or wellbeing or well-being)).mp.
- (preconception or pre-conception or prepregnancy or pre-pregnancy).mp.
- exp Human papilloma virus vaccine/or (papillomavirus vaccin* or HPV vaccin* or gardasil or papillomavirus immunis* or papillomavirus immuniz* or HPV immunis* or HPV immuniz*).mp.
- or/1–19
- (telemedicine or telemedical or telehealth or telephone or phone or phones or smartphone* or cell* device* or mobile device* or text messag* or SMS or texting or virtual* or remote* monitor* or ehealth or e-health or mhealth or m-health or mobile health or digital health).ti.
- exp *telehealth/or exp *mobile application/or exp *mobile phone/
- 21 or 22
- 20 and 23
- limit 24 to (english language and yr = “2017-Current”)
- limit 25 to conference abstract status
- 25 not 26
CINAHL
- S1.
- (MH “Reproductive Control Agents+”)
- S2.
- (MH “Contraception+”)
- S3.
- (contracept* OR birth control)
- S4.
- (MH “Family Planning+”)
- S5.
- family planning
- S6.
- (MH “Gynecology”) OR (MH “Gynecologic Nursing”) OR (MH “Gynecologic Care”) OR (MH “OB-GYN Nurse Practitioners”)
- S7.
- (gyn#ecolog* OR (women* N2 health))
- S8.
- (MH “Reproductive Health”) OR (MH “Sexual Health”)
- S9.
- ((reproduct* N1 health*) OR (sexual* N1 health*))
- S10.
- (MH “Sexually Transmitted Diseases+”)
- S11.
- (sexually transmitted disease* OR sexually transmitted infection* OR STD OR STDs OR STI OR STIs)
- S12.
- (MH “Fertility+”)
- S13.
- fertility
- S14.
- (MH “Abortion, Induced+”)
- S15.
- abortion*
- S16.
- (MH “Pregnancy, Unplanned”) OR (MH “Pregnancy, Unwanted”)
- S17.
- ((unplanned OR “not planned” OR unwanted OR “not want*”) N3 pregnan*)
- S18.
- ((sexual OR reproductive) N2 (wellness OR wellbeing OR well-being))
- S19.
- (preconception OR pre-conception OR prepregnancy OR pre-pregnancy)
- S20.
- (MH “Papillomavirus Vaccine”)
- S21.
- (papillomavirus vaccin* OR HPV vaccin* OR gardasil or papillomavirus immunis* OR papillomavirus immuniz* OR HPV immunis* OR HPV immuniz*)
- S22.
- S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21
- S23.
- (MM “Telehealth+”)
- S24.
- (MM “Mobile Applications”)
- S25.
- (MM “Cellular Phone+”)
- S26.
- TI (telemedicine OR telemedical OR telehealth OR telephone* OR phone OR phones OR smartphone* OR cell* device* OR mobile device* OR text messag* OR SMS OR texting OR virtual* OR remote* monitor* OR ehealth OR e-health OR mhealth OR m-health OR mobile health OR digital health)
- S27.
- S23 OR S24 OR S25 OR 26
- S28.
- S22 AND S27
References
- Mawson, R.L.; Hodges, V.; Salway, S.; Mitchell, C. Understanding access to sexual and reproductive health in general practice using an adapted Candidacy Framework: A systematic review and qualitative evidence synthesis. Br. J. Gen. Pract. 2025. Epub ahead of printing. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hart, J.; Crear-Perry, J.; Stern, L. US Sexual and Reproductive Health Policy: Which Frameworks Are Needed Now, and Next Steps Forward. Am. J. Public Health 2022, 112, S518–S522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Adler, A.; Biggs, M.A.; Kaller, S.; Schroeder, R.; Ralph, L. Changes in the Frequency and Type of Barriers to Reproductive Health Care Between 2017 and 2021. JAMA Netw. Open 2023, 6, e237461. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cantor, A.; Nelson, H.D.; Pappas, M.; Atchison, C.; Hatch, B.; Huguet, N.; Flynn, B.; McDonagh, M. Effectiveness of Telehealth for Women’s Preventive Services [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2022; (Comparative Effectiveness Review, No. 256). Available online: https://www.ncbi.nlm.nih.gov/books/NBK581511/ (accessed on 1 January 2024). [CrossRef]
- Goldstein, K.M.; Zullig, L.L.; Dedert, E.A.; Alishahi Tabriz, A.; Brearly, T.W.; Raitz, G.; Sata, S.S.; Whited, J.D.; Bosworth, H.B.; Gordon, A.M.; et al. Telehealth Interventions Designed for Women: An Evidence Map. J. Gen. Intern Med. 2018, 33, 2191–2200. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Muheriwa-Matemba, S.R.; Alcena-Stiner, D.C.; Glazier, A.; LeBlanc, N.M. Telehealth use for sexual and reproductive health promotion and care during the early phase of COVID-19 pandemic: A descriptive-interpretive qualitative study of healthcare providers’ perspectives and experiences in Western-Central New York State. PLoS Glob. Public Health 2024, 4, e0003259. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gajarawala, S.N.; Pelkowski, J.N. Telehealth Benefits and Barriers. J. Nurse Pract. 2021, 17, 218–221. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Polinski, J.M.; Barker, T.; Gagliano, N.; Sussman, A.; Brennan, T.A.; Shrank, W.H. Patients’ Satisfaction with and Preference for Telehealth Visits. J. Gen. Intern. Med. 2016, 31, 269–275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mair, F.; Whitten, P. Systematic review of studies of patient satisfaction with telemedicine. BMJ 2000, 320, 1517–1520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kruse, C.S.; Krowski, N.; Rodriguez, B.; Tran, L.; Vela, J.; Brooks, M. Telehealth and patient satisfaction: A systematic review and narrative analysis. BMJ Open 2017, 7, e016242. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wosik, J.; Fudim, M.; Cameron, B.; Gellad, Z.F.; Cho, A.; Phinney, D.; Curtis, S.; Roman, M.; Poon, E.G.; Ferranti, J.; et al. Telehealth transformation: COVID-19 and the rise of virtual care. J. Am. Med. Inf. Assoc. 2020, 27, 957–962. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shaver, J. The State of Telehealth Before and After the COVID-19 Pandemic. Prim. Care 2022, 49, 517–530. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ezeamii, V.C.; Okobi, O.E.; Wambai-Sani, H.; Perera, G.S.; Zaynieva, S.; Okonkwo, C.C.; Ohaiba, M.M.; William-Enemali, P.C.; Obodo, O.R.; Obiefuna, N.G. Revolutionizing Healthcare: How Telemedicine Is Improving Patient Outcomes and Expanding Access to Care. Cureus 2024, 16, e63881. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abernethy, A.; Adams, L.; Barrett, M.; Bechtel, C.; Brennan, P.; Butte, A.; Faulkner, J.; Fontaine, E.; Friedhoff, S.; Halamka, J.; et al. The Promise of Digital Health: Then, Now, and the Future. NAM Perspect. 2022, 2022, 10–31478. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khalil, H.; Welch, V.; Grainger, M.; Campbell, F. Methodology for mapping reviews, evidence maps, and gap maps. Res. Synth. Methods 2025, 16, 786–796. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- McGowan, J.; Sampson, M.; Salzwedel, D.M.; Cogo, E.; Foerster, V.; Lefebvre, C. PRESS Peer Review of Electronic Search Strategies: 2015 Guideline Statement. J. Clin. Epidemiol. 2016, 75, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.R.; Troester, A.; Southwell, B.; Ester, E.; Sultan, S.; Claussen, A.M.; Greeno, E.; Arsoniadis, E.; Church, T.R.; Wilt, T.J.; et al. Treatment of Stages I–III Squamous Cell Anal Cancer: A Systematic Review [Internet]; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2024; Report No.: AHRQ 24-EHC026; Report No.: PCORI 2024-SR-03. [Google Scholar] [PubMed]
- Adams, A.M.; Wu, H.; Zhang, F.R.; Wajsberg, J.R.; Bruney, T.L. Postpartum Care in the Time of COVID-19: The Use of Telemedicine for Postpartum Care. Telemed. J. e-Health 2023, 29, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Coskun, R.; Jimenez, P.; Omurtag, K. Satisfaction with new patient telehealth visits for reproductive endocrinology patients in the era of COVID-19. J. Assist. Reprod. Genet. 2022, 39, 1571–1576. [Google Scholar] [CrossRef]
- Arias, M.P.; Wang, E.; Leitner, K.; Sannah, T.; Keegan, M.; Delferro, J.; Iluore, C.; Arimoro, F.; Streaty, T.; Hamm, R.F. The impact on postpartum care by telehealth: A retrospective cohort study. Am. J. Obs. Gynecol. MFM 2022, 4, 100611. [Google Scholar] [CrossRef]
- Baker, D.P.; Ussher, G.R.; Rimes, K.A. Development of a text-based chatroom HIV prevention and confidence-building intervention for same-sex attracted young males in South England. J. HIVAIDS Soc. Serv. 2021, 20, 262–270. [Google Scholar] [CrossRef]
- Bauermeister, J.A.; Muessig, K.E.; LeGrand, S.; Flores, D.D.; Choi, S.K.; Dong, W.; Sallabank, G.; Hightow-Weidman, L.B. HIV and Sexuality Stigma Reduction Through Engagement in Online Forums: Results from the HealthMPowerment Intervention. AIDS Behav. 2019, 23, 742–752. [Google Scholar] [CrossRef]
- Beatty, K.E.; Smith, M.G.; Khoury, A.J.; Ventura, L.M.; Ariyo, T.; de Jong, J.; Surles, K.; Rahman, A.; Slawson, D. Telehealth for Contraceptive Care During the Initial Months of the COVID-19 Pandemic at Local Health Departments in 2 US States: A Mixed-Methods Approach. J. Public Health Manag. Pract. 2022, 28, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.R.; Shegog, R.; Savas, L.S.; Frost, E.L.; Coan, S.P.; Healy, C.M.; Spinner, S.W.; Vernon, S.W. Parents’ Experience with a Mobile Health Intervention to Influence Human Papillomavirus Vaccination Decision Making: Mixed Methods Study. JMIR Pediatr. Parent. 2022, 5, e30340. [Google Scholar] [CrossRef] [PubMed]
- Bernard-Genest, M.P.; Ruel-Laliberte, J.; Lapointe-Milot, K. Effect of educative reminder telephone calls on human papillomavirus immunization rate: A randomized controlled trial. Womens Health Lond. Engl. 2021, 17, 17455065211003820. [Google Scholar] [CrossRef] [PubMed]
- Bissessor, M.; Bradshaw, C.S.; Fairley, C.K.; Chen, M.Y.; Chow, E.P. Provision of HIV test results by telephone is both safe and efficient for men who have sex with men. Int. J. STD AIDS 2017, 28, 39–44. [Google Scholar] [CrossRef]
- Buchanan, C.R.M.; Tomaszewski, K.; Chung, S.E.; Upadhya, K.K.; Ramsey, A.; Trent, M.E. Why Didn’t You Text Me? Poststudy Trends from the DepoText Trial. Clin. Pediatr. 2018, 57, 82–88. [Google Scholar] [CrossRef]
- Carey, M.P.; Dunne, E.M.; Norris, A.; Dunsiger, S.; Rich, C.; Rosen, R.K.; Chan, P.; Salmoirago-Blotcher, E. Telephone-Delivered Mindfulness Training to Promote Medication Adherence and Reduce Sexual Risk Behavior Among Persons Living with HIV: An Exploratory Clinical Trial. AIDS Behav. 2020, 24, 1912–1928. [Google Scholar] [CrossRef]
- Chasco, E.E.; Hoth, A.B.; Cho, H.; Shafer, C.; Siegler, A.J.; Ohl, M.E. Mixed-Methods Evaluation of the Incorporation of Home Specimen Self-Collection Kits for Laboratory Testing in a Telehealth Program for HIV Pre-exposure Prophylaxis. AIDS Behav. 2021, 25, 2463–2482. [Google Scholar] [CrossRef]
- Cheng, Y.; Boerma, C.; Peck, L.; Botfield, J.R.; Estoesta, J.; McGeechan, K. Telehealth sexual and reproductive health care during the COVID-19 pandemic. Med. J. Aust. 2021, 215, 371–372. [Google Scholar] [CrossRef]
- Clure, C.; Sheeder, J.; Teal, S.; Cohen, R. Telemedicine to improve reproductive health care for rural Coloradans: Perceptions of interest and access. J. Rural Health 2023, 39, 172–178. [Google Scholar] [CrossRef]
- Comfort, A.B.; Rao, L.; Goodman, S.; Raine-Bennett, T.; Barney, A.; Mengesha, B.; Harper, C.C. Assessing differences in contraceptive provision through telemedicine among reproductive health providers during the COVID-19 pandemic in the United States. Reprod. Health 2022, 19, 99. [Google Scholar] [CrossRef] [PubMed]
- Comstock, E.; James, C.; Hegarty, R.; Flores, Y.; Amoroso, A.; Talwani, R.; Wilson, E. Pre-Exposure Prophylaxis (PrEP) Safety and Tolerability in Individuals ≥ 45 Years Old. J. AIDS HIV Treat. 2021, 3, 27–30. [Google Scholar] [CrossRef]
- Day, S.; Kinsella, R.; Jones, S.; Tittle, V.; Suchak, T.; Forbes, K. Safeguarding outcomes of 16 and 17-year-old service users of Sexual Health London (SHL.uk), a pan-London online sexual health service. Int. J. STD AIDS 2020, 31, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Day, S.; Smith, J.; Perera, S.; Jones, S.; Kinsella, R. Beyond the binary: Sexual health outcomes of transgender and non-binary service users of an online sexual health service. Int. J. STD AIDS 2021, 32, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Doolittle, J.; Jackson, E.M.; Gill, B.; Vij, S.C. The Omission of Genitourinary Physical Exam in Telehealth Pre-Vasectomy Consults Does Not Reduce Rates of Office Procedure Completion. Urology 2022, 167, 19–23. [Google Scholar] [CrossRef]
- Ellison, J.; Cole, M.B.; Thompson, T.A. Association of Telehealth Reimbursement Parity with Contraceptive Visits During the COVID-19 Pandemic. JAMA Netw. Open 2022, 5, e226732. [Google Scholar] [CrossRef]
- Engen, J.; Black, A.; Holdsworth, G.; Howroyd, C.; Courtenay, M.; Baraitser, P. Photodiagnosis of genital herpes and warts within a specialist online sexual health service: An observational (mixed methods) study of user experience and clinical outcomes. BMJ Open 2021, 11, e042160. [Google Scholar] [CrossRef]
- Estcourt, C.S.; Gibbs, J.; Sutcliffe, L.J.; Gkatzidou, V.; Tickle, L.; Hone, K.; Aicken, C.; Lowndes, C.M.; Harding-Esch, E.M.; Eaton, S.; et al. The eSexual Health Clinic system for management, prevention, and control of sexually transmitted infections: Exploratory studies in people testing for Chlamydia trachomatis. Lancet Public Health 2017, 2, e182–e190. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Savas, L.S.; Atkinson, J.S.; Ricks, K.B.; Ibekwe, L.N.; Jackson, I.; Castle, P.E.; Jobe, D.; Vernon, S.W. Evaluation of a 2-1-1 Telephone Navigation Program to Increase Cancer Control Behaviors: Results from a Randomized Controlled Trial. Am. J. Health Promot. 2022, 36, 1083–1093. [Google Scholar] [CrossRef]
- Fuchs, J.D.; Stojanovski, K.; Vittinghoff, E.; Fuchs, J.D.; Stojanovski, K.; Vittinghoff, E.; McMahan, V.M.; Hosek, S.G.; Amico, K.R.; Kouyate, A.; et al. A Mobile Health Strategy to Support Adherence to Antiretroviral Preexposure Prophylaxis. AIDS Patient Care STDs 2018, 32, 104–111. [Google Scholar] [CrossRef]
- Glasner, S.; Patrick, K.; Ybarra, M.; Reback, C.J.; Ang, A.; Kalichman, S.; Bachrach, K.; Garneau, H.C.; Venegas, A.; Rawson, R.A. Promising outcomes from a cognitive behavioral therapy text-messaging intervention targeting drug use, antiretroviral therapy adherence, and HIV risk behaviors among adults living with HIV and substance use disorders. Drug Alcohol. Depend. 2022, 231, 109229. [Google Scholar] [CrossRef]
- Gorman, J.R.; Drizin, J.H.; Smith, E.; Corey, S.; Temple, M.; Rendle, K.A. Feasibility of Mindful After Cancer: Pilot Study of a Virtual Mindfulness-Based Intervention for Sexual Health in Cancer Survivorship. J. Sex. Med. 2022, 19, 1131–1146. [Google Scholar] [CrossRef]
- Groos, A.; Peardon-Freeman, S.; McFarlane, K.; Braithwaite, S.; Gajjar, D.; Murch, P. Free online chlamydia and gonorrhoea urine test request in Queensland: Sexually transmissible infections testing can be hard for young people even if the process is easy. Sex. Health 2020, 17, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Henderson, L.; Gibbs, J.; Quinn, J.; Ramasami, S.; Estcourt, C. Maintaining access to HIV pre- exposure prophylaxis in a pandemic: A service evaluation of telephone-based pre-exposure prophylaxis provision. Int. J. STD AIDS 2022, 33, 718–721. [Google Scholar] [CrossRef]
- Heumann, C.L.; Katz, D.A.; Dombrowski, J.C.; Bennett, A.B.; Manhart, L.E.; Golden, M.R. Comparison of In-Person Versus Telephone Interviews for Early Syphilis and Human Immunodeficiency Virus Partner Services in King County, Washington (2010–2014). Sex. Transm. Dis. 2017, 44, 249–254. [Google Scholar] [CrossRef]
- Hill, B.J.; Lock, L.; Anderson, B. Racial and ethnic differences in family planning telehealth use during the onset of the COVID-19 response in Arkansas, Kansas, Missouri, and Oklahoma. Contraception 2021, 104, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Horwood, J.; Brangan, E.; Manley, P.; Horner, P.; Muir, P.; North, P.; Macleod, J. Management of chlamydia and gonorrhoea infections diagnosed in primary care using a centralised nurse-led telephone-based service: Mixed methods evaluation. BMC Fam. Pract. 2020, 21, 265. [Google Scholar] [CrossRef]
- Hoth, A.B.; Shafer, C.; Dillon, D.B.; Mayer, R.; Walton, G.; Ohl, M.E. Iowa TelePrEP: A Public- Health-Partnered Telehealth Model for Human Immunodeficiency Virus Preexposure Prophylaxis Delivery in a Rural State. Sex. Transm. Dis. 2019, 46, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, A.C.M.; Crowley, S.M.; Landry, K.M.; Landry, M.S. Telehealth contraceptive care in 2018: A quality improvement study of barriers to access and patient satisfaction. Contraception 2022, 112, 81–85. [Google Scholar] [CrossRef]
- Jain, T.; Mehrotra, A. Comparison of Direct-to-Consumer Telemedicine Visits with Primary Care Visits. JAMA Netw. Open 2020, 3, e2028392. [Google Scholar] [CrossRef]
- Jasper, W.; Macdonald, M.; Luxmanan, D.; Foley, E.; Patel, R. Telephone-triage services do not lead to an increased wait time for assessment of gonorrhoea in symptomatic patients. Int. J. STD AIDS 2021, 32, 852–855. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.K. Telemedicine and direct to consumer advertising attitudes and the future of telehealth: Women report telemedicine as a comfortable option for accessing birth control. Health Mark. Q. 2023, 40, 309–325. [Google Scholar] [CrossRef]
- Kalichman, S.C.; Cherry, C.; Kalichman, M.O.; Eaton, L.A.; Kohler, J.J.; Montero, C.; Schinazi, R.F. Mobile Health Intervention to Reduce HIV Transmission: A Randomized Trial of Behaviorally Enhanced HIV Treatment as Prevention (B-TasP). J. Acquir. Immune Defic. Syndr. 2018, 78, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Katz, M.; Newmark, R.L.; Aronstam, A.; O’Grady, N.; Strome, S.; Rafie, S.; Karlin, J. An implementation project to expand access to self-administered depot medroxyprogesterone acetate (DMPA). Contraception 2020, 102, 392–395. [Google Scholar] [CrossRef]
- Lindberg, L.D.; Mueller, J.; Haas, M.; Jones, R.K. Telehealth for Contraceptive Care During the COVID-19 Pandemic: Results of a 2021 National Survey. Am. J. Public Health 2022, 112, S545–S554. [Google Scholar] [CrossRef]
- Liu, A.Y.; Vittinghoff, E.; von Felten, P.; Rivet Amico, K.; Anderson, P.L.; Lester, R.; Andrew, E.; Estes, I.; Serrano, P.; Brothers, J.; et al. Randomized Controlled Trial of a Mobile Health Intervention to Promote Retention and Adherence to Preexposure Prophylaxis Among Young People at Risk for Human Immunodeficiency Virus: The EPIC Study. Clin. Infect. Dis. 2019, 68, 2010–2017. [Google Scholar] [CrossRef]
- Llewellyn, C.D.; Abraham, C.; Pollard, A.; Jones, C.I.; Bremner, S.; Miners, A.; Smith, H. A randomised controlled trial of a telephone administered brief HIV risk reduction intervention amongst men who have sex with men prescribed post-exposure prophylaxis for HIV after sexual exposure in the UK: Project PEPSE. PLoS ONE 2019, 14, e0216855. [Google Scholar] [CrossRef]
- Martinez, K.A.; Rastogi, R.; Lipold, L.; Rothberg, M.B. Response to requests for contraception in one direct-to-consumer telemedicine service. Contraception 2020, 101, 350–352. [Google Scholar] [CrossRef]
- McCulloch, H.; Syred, J.; Holdsworth, G.; Howroyd, C.; Ardines, E.; Baraitser, P. Communication strategies used to obtain clinical histories before remotely prescribing antibiotics for postal treatment of uncomplicated genital chlamydia: Service evaluation. J. Med. Internet Res. 2020, 22, e15970. [Google Scholar] [CrossRef]
- McLaughlin, E.J.; Ellett, L.C.; Readman, E.; Mooney, S. Telehealth for gynaecology outpatients during the COVID-19 pandemic: Patient and clinician experiences. Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Nadarzynski, T.; Bayley, J.; Llewellyn, C.; Kidsley, S.; Graham, C.A. Acceptability of artificial intelligence (AI)-enabled chatbots, video consultations and live webchats as online platforms for sexual health advice. BMJ Sex. Reprod. Health 2020, 46, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Nimbi, F.M.; Rossi, R.; Simonelli, C. Telephone sexual counseling and current technologies: Are helplines still effective in the social media era? Sexologies 2018, 27, 162–170. [Google Scholar] [CrossRef]
- O’Malley, T.L.; Horowitz, K.R.; Burke, J.G. Information seeking from a sexual health textline: Utilisation and perceptions of helpfulness among young people. Sex. Educ. 2022, 22, 217–227. [Google Scholar] [CrossRef]
- Quirke, S.; Quinn, L.; Hegarty, D.; Loy, A.; Lyons, F.; Mulcahy, F.; Devitt, E. Virtual HIV pre-exposure prophylaxis outpatient service in the era of COVID-19. Int. J. STD AIDS 2021, 32, 100–103. [Google Scholar] [CrossRef]
- Refugio, O.N.; Kimble, M.M.; Silva, C.L.; Lykens, J.E.; Bannister, C.; Klausner, J.D. Brief Report: PrEPTECH: A Telehealth-Based Initiation Program for HIV Pre-exposure Prophylaxis in Young Men of Color Who Have Sex with Men. A Pilot Study of Feasibility. J. Acquir. Immune Defic. Syndr. 2019, 80, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.B.; Garrett, S.M.; Hutchings, D.; Lund, K.; Kennedy, J.; Pullon, S.R.H. Clinician education, advice and SMS/text reminders improve test of reinfection rates following diagnosis of Chlamydia trachomatis or Neisseria gonorrhoeae: Before and after study in primary care. BMJ Sex. Reprod. Health 2020, 46, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Shanks, S.; Morelli, A.; Ardines, E.; Holdsworth, G.; Baraitser, P. Two-Way Text Messaging to Support Self-Care and Delivery of an Online Sexual Health Service: Mixed Methods Evaluation. JMIR mHealth uHealth 2020, 8, e17191. [Google Scholar] [CrossRef]
- Shin, R.J.; Yao, M.; Akesson, C.; Blazel, M.; Mei, L.; Brant, A.R. An exploratory study comparing the quality of contraceptive counseling provided via telemedicine versus in-person visits. Contraception 2022, 112, 86–92. [Google Scholar] [CrossRef]
- Shrier, L.A.; Burke, P.J.; Parker, S.; Edwards, R.; Jonestrask, C.; Pluhar, E.; Harris, S.K. Development and pilot testing of a counseling-plus- mHealth intervention to reduce risk for pregnancy and sexually transmitted infection in young women with depression. mHealth 2020, 6, 17. [Google Scholar] [CrossRef]
- Smiley, Y.; Sadeghi, N.; Jolda, C.; Chokshi, B. Parenting in a Pandemic: Needs of Teen Parents During COVID-19. Clin. Pediatr. 2021, 60, 559–563. [Google Scholar] [CrossRef]
- Staras, S.A.S.; Richardson, E.; Merlo, L.J.; Bian, J.; Thompson, L.A.; Krieger, J.L.; Gurka, M.J.; Sanders, A.H.; Shenkman, E.A. A feasibility trial of parent HPV vaccine reminders and phone-based motivational interviewing. BMC Public Health 2021, 21, 109. [Google Scholar] [CrossRef]
- Steiner, R.J.; Zapata, L.B.; Curtis, K.M.; Whiteman, M.K.; Brittain, A.W.; Tromble, E.; Keys, K.R.; Fasula, A.M. COVID-19 and Sexual and Reproductive Health Care: Findings from Primary Care Providers Who Serve Adolescents. J. Adolesc. Health 2021, 69, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.; Todd, K.; Kahle, E.; Sullivan, S.P.; Miller-Perusse, M.; Sharma, A.; Horvath, K.J. Project Moxie: Results of a Feasibility Study of a Telehealth Intervention to Increase HIV Testing Among Binary and Nonbinary Transgender Youth. AIDS Behav. 2020, 24, 1517–1530. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, R.; Sullivan, S.P.; Mitchell, J.W.; Johnson, B.A.; Sullvian, P.S. Efficacy of a Telehealth Delivered Couples’ HIV Counseling and Testing (CHTC) Intervention to Improve Formation and Adherence to Safer Sexual Agreements Among Male Couples in the US: Results from a Randomized Control Trial. AIDS Behav. 2022, 26, 2813–2824. [Google Scholar] [CrossRef]
- Stifani, B.M.; Avila, K.; Levi, E.E. Telemedicine for contraceptive counseling: An exploratory survey of US family planning providers following rapid adoption of services during the COVID-19 pandemic. Contraception 2021, 103, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Stifani, B.M.; Smith, A.; Avila, K.; Boos, E.W.; Ng, J.; Levi, E.E.; Benfield, N.C. Telemedicine for contraceptive counseling: Patient experiences during the early phase of the COVID-19 pandemic in New York City. Contraception 2021, 104, 254–261. [Google Scholar] [CrossRef]
- Stifani, B.M.; Smith, A.; Avila, K.; Levi, E.E.; Benfield, N.C. Telemedicine for Contraceptive Counseling During the COVID-19 Pandemic: Referral Patterns and Attendance at Follow-Up Visits. Telemed. J. e-Health 2022, 28, 1517–1524. [Google Scholar] [CrossRef]
- Sullivan, S.P.; Sullivan, P.S.; Stephenson, R. Acceptability and Feasibility of a Telehealth Intervention for STI Testing Among Male Couples. AIDS Behav. 2021, 25, 4029–4043. [Google Scholar] [CrossRef] [PubMed]
- Taylan, S.; Akıl, Y. The Effect of Postoperative Telephone Counseling on the Sexual Life of Patients with a Bowel Stoma: A Randomized Controlled Trial. Ostomy Wound Manag. 2019, 65, 14–29. [Google Scholar] [CrossRef]
- Thompson, T.A.; Ahrens, K.A.; Coplon, L. Virtually possible: Using telehealth to bring reproductive health care to women with opioid use disorder in rural Maine. mHealth 2020, 6, 41. [Google Scholar] [CrossRef]
- Wang, Z.; Lau, J.T.F.; Ip, T.K.M.; Yu, Y.; Fong, F.; Fang, Y.; Mo, P.K. Two Web-Based and Theory-Based Interventions with and without Brief Motivational Interviewing in the Promotion of Human Papillomavirus Vaccination Among Chinese Men Who Have Sex with Men: Randomized Controlled Trial. J. Med. Internet Res. 2021, 23, e21465. [Google Scholar] [CrossRef] [PubMed]
- Wigfall, L.T. eHealth Communication with Clients at Community-Based HIV/AIDS Service Organizations in the Southern United States: Cross-Sectional Survey. JMIR Form. Res. 2020, 4, e17154. [Google Scholar] [CrossRef] [PubMed]
- Willoughby, J.F.; Muldrow, A. SMS for sexual health: A comparison of service types and recommendations for sexual health text message service providers. Health Educ. J. 2017, 76, 231–243. [Google Scholar] [CrossRef]
- Wilson, E.; Free, C.; Morris, T.P.; Syred, J.; Ahamed, I.; Menon-Johansson, A.S.; Palmer, M.J.; Barnard, S.; Rezel, E.; Baraitser, P. Internet-accessed sexually transmitted infection (e-STI) testing and results service: A randomised, single-blind, controlled trial. PLoS Med. 2017, 14, e1002479. [Google Scholar] [CrossRef]
- Wood, S.M.; White, K.; Peebles, R.; Pickel, J.; Alausa, M.; Mehringer, J.; Dowshen, N. Outcomes of a Rapid Adolescent Telehealth Scale-Up During the COVID-19 Pandemic. J. Adolesc. Health 2020, 67, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Yarger, J.; Hopkins, K.; Elmes, S.; Rossetto, I.; De La Melena, S.; McCulloch, C.E.; White, K.; Harper, C.C. Perceived Access to Contraception via Telemedicine Among Young Adults: Inequities by Food and Housing Insecurity. J. Gen. Intern. Med. 2023, 38, 302–308. [Google Scholar] [CrossRef]
- Allison, W.E.; Choi, A.N.; Kawasaki, K.; Desai, A.; Melhado, T.V. Accessing Care During the COVID-19 Pandemic Using Telemedicine: Perspectives from People with HIV. Health Promot. Pract. 2023, 24, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Andrade, E.A.; Betancourt, G.; Morales, G.; Zapata, O.; Marrero, L.; Rivera, S.; Nieves, E.; Miranda, C.; Diaz, C.; Beil, R.; et al. A Community-Based Pre-Exposure Prophylaxis Telehealth Program Focused on Latinx Sexual Minority Men. AIDS Patient Care STDs 2023, 37, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Avalos, L.A.; Oberman, N.; Gomez, L.; Quesenberry, C.P.; Sinclair, F.; Kurtovich, E.; Gunderson, E.P.; Hedderson, M.M.; Stark, J. Group Multimodal Prenatal Care and Postpartum Outcomes. JAMA Netw. Open 2024, 7, e2412280. [Google Scholar] [CrossRef] [PubMed]
- Barth, S.K.; Saulters, K.J.; Balba, G.P.; Monroe, A.K.; Horberg, M.A.; Kumar, P.N.; Greenberg, A.E.; Castel, A.D.; DC Cohort Executive Committee. Mixed Methods Analysis of Telehealth Experience, Satisfaction, and Quality of Care During the COVID Pandemic Among Persons with HIV in Washington, DC. AIDS Behav. 2024, 28, 912–923. [Google Scholar] [CrossRef] [PubMed]
- Beatty, K.; Smith, M.G.; Khoury, A.J.; Ventura, L.M.; Ariyo, O.; de Jong, J.; Surles, K.; Slawson, D. Contraceptive care service provision via telehealth early in the COVID-19 pandemic at rural and urban federally qualified health centers in 2 southeastern states. J. Rural Health 2023, 39, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Beck, E.; Mandalia, S.; Yfantopoulos, P.; Jones, C.; Bremner, S.; Fatz, D.; Vera, J.; Whetham, J. The efficiency of the EmERGE pathway of care for people living with HIV in England. AIDS Care 2023, 35, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Bielick, C.; Canan, C.; Ingersoll, K.; Waldman, A.L.; Schwendinger, J.; Dillingham, R. Three-Year Follow-up of PositiveLinks: Higher Use of mHealth Platform Associated with Sustained HIV Suppression. AIDS Behav. 2024, 28, 2708–2718. [Google Scholar] [CrossRef] [PubMed]
- Bryson, A.E.; Milliren, C.E.; Borzutzky, C.; Golub, S.A.; Pitts, S.A.B.; Di Vasta, A.D. Telemedicine for Adolescent and Young Adult Long-Acting Reversible Contraception Follow-Up Care amidst a Global Pandemic. J. Pediatr. Adolesc. Gynecol. 2023, 36, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Bryson, A.E.; Milliren, C.E.; Borzutzky, C.; Golub, S.A.; Pitts, S.A.B.; Di Vasta, A.D. Adolescent and young adult long-acting reversible contraception post-insertion visit attendance before and after COVID-19. Int. J. Adolesc. Med. Health 2024, 36, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bryson, A.E.; Milliren, C.E.; Golub, S.A.; Maslyanskaya, S.; Escovedo, M.; Borzutzky, C.; Pitts, S.A.; Di Vasta, A.D. Telemedicine for Adolescent and Young Adult Long-Acting Reversible Contraception Post-insertion Visits: Outcomes over 1 Year. J. Pediatr. Adolesc. Gynecol. 2024, 37, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Bullo, M.; Kierszenowicz, T.; Acosta, M.C.; Rolon, M.J.; Cecchini, D.; Rodriguez, C.; Scapellato, P.; Bottaro, E.; Losso, M.H. Telemedicine in HIV health care during the COVID-19 pandemic: An implementation research study in Buenos Aires, Argentina. HIV Med. 2024, 25, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Chernick, L.S.; Bugaighis, M.; Hochster, D.; Daylor, V.; Gorroochurn, P.; Schnall, R.; Stockwell, M.S.; Bell, D. A Randomized Controlled Trial of a Digital Intervention to Improve the Sexual Health of Adolescent and Young Adult Male Emergency Department Patients. J. Adolesc. Health 2025, 76, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.; Lynn, T.; van der Werff, L.; Kennedy, J. Does telemedicine hold the key for reproductive health care? A quantitative examination of women’s intentions toward use and accurate information disclosure. Health Serv Res. 2025, 60, e14403. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cox, A.L.; Tsang, D.; Spacek, L.A.; Daskalakis, C.; Coppock, D. The Impact of Telemedicine on Human Immunodeficiency Virus (HIV)-Related Clinical Outcomes During the COVID-19 Pandemic. AIDS Behav. 2024, 28, 2438–2443. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.A.; Knoll, H.; Ireland, C.; Frayne, D. A Quality Improvement Innovation for Reproductive Health Planning in the Time of COVID. Matern. Child Health J. 2023, 27, 1450–1453. [Google Scholar] [CrossRef] [PubMed]
- Dunn, R.C.; Stegall, C.J.; Creel, C.; Fuchs, C.J.; Menzies, B.E.; Summers, N.A. Evaluating the delivery of care by telemedicine for incarcerated people living with HIV: A cohort study. BMC Infect. Dis. 2024, 24, 717. [Google Scholar] [CrossRef] [PubMed]
- El-Nahal, W.G.; Chander, G.; Jones, J.L.; Fojo, A.T.; Keruly, J.C.; Manabe, Y.C.; Moore, R.D.; Gebo, K.A.; Lesko, C.R. Telemedicine Use Among People with HIV in 2021: The Hybrid-Care Environment. J. Acquir. Immune Defic. Syndr. 2023, 92, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Ereme, K.; Akullo, K.; Class, Q.A.; Hinz, E. Patient Perceived Quality of Virtual Group Contraception Counseling. Open Access J. Contracept. 2024, 15, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Erenrich, R.K.; Braun, R.A.; Torres-Mendoza, D.M.; Stevenson, O.L.; Doan, T.-H.P.; Klausner, J.D. Effectiveness of PrEPTECH: Findings from a 180-Day Randomized Controlled Trial of a Pre-Exposure Prophylaxis Telehealth Intervention. J. Acquir. Immune Defic. Syndr. 2024, 95, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Fitch, K.; Bohn, J.A.; Emerson, J.B.; Boniface, E.R.; Bruegl, A. Acceptability of human papillomavirus self-collection and the role of telehealth: A prospective, randomized study stratified by menopausal status. Int. J. Gynecol. Cancer 2024, 34, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Flynn, A.N.; Koelper, N.C.; Sonalkar, S. Telephone-Based Intervention to Improve Family Planning Care in Pregnancies of Unknown Location: Retrospective Pre-Post Study. J. Med. Internet Res. 2023, 25, e42559. [Google Scholar] [CrossRef] [PubMed]
- Fonner, V.; Agostini, T.; Desai, R.; Hartzell, P.; Martin, L.; Meissner, E.G. Implementation of free-draft text messaging to enhance care retention and satisfaction for persons living with HIV infection. AIDS Care 2024, 36, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.; Paul, R.; Dorsey, M.; Madden, T. Comparison of interpersonal quality of contraceptive counseling delivered via telehealth versus in person. Contraception 2023, 128, 110129. [Google Scholar] [CrossRef] [PubMed]
- Freeman, E.; Paul, R.; Dorsey, M.; Nigaglioni Rivera, A.; Reeves, J.A.; Madden, T. Demographic differences between patients selecting video or telephone for contraceptive counseling via telehealth. Contraception 2025, 141, 110699. [Google Scholar] [CrossRef] [PubMed]
- Gass, S.-J.; Yelverton, V.; Ostermann, J.; Weissman, S.; Albrecht, H. Which Features of Telehealth in HIV Care Are Most Important? A Mixed-Methods Study with HIV Care Providers and People Living with HIV in South Carolina. Sex. Transm. Dis. 2024, 51, e17–e25. [Google Scholar] [CrossRef] [PubMed]
- Gerke, D.R.; Glotfelty, J.; Slovacek, S.; Freshman, M.; Schlueter, J.; Plax, K. More than just Reminders: Using text Messaging to Improve HIV care Outcomes Among Youth and Young Adults Living with HIV. AIDS Behav. 2023, 27, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, K.; Fugit, R.; Nicholson, L.; Wright, J. A Retrospective Comparison of HIV Pre-exposure Prophylaxis (PrEP) Outcomes Between a Pharmacist-led Telehealth Clinic and In-person Clinic in a Veteran Population. AIDS Behav. 2023, 27, 3678–3686. [Google Scholar] [CrossRef] [PubMed]
- Ham, L.; Montoya, J.L.; Serrano, V.; Yeager, S.; Paltin, D.; Pasipanodya, E.C.; Marquine, M.J.; Hoenigl, M.; Ramers, C.B.; Kua, J.; et al. High Psychosocial Burden Relates to Poorer Antiretroviral Treatment Adherence Among Black/African American People with HIV. AIDS Patient Care STDs 2023, 37, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, H.; Bernstein, A.P.; Zhu, E.; Saba, B.; Rapoport, E.; Najari, B.B. No Detectable Association Between Virtual Setting for Vasectomy Consultation and Vasectomy Completion Rate. Urol. Pract. 2024, 11, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.D.; Sergi, F.; Zhang, K.; Spinelli, M.A.; Black, D.; Sola, C.; Blaz, V.; Nguyen, J.Q.; Oskarsson, J.; Gandhi, M.; et al. Pragmatic randomized trial of a pre-visit intervention to improve the quality of telemedicine visits for vulnerable patients living with HIV. J. Telemed. Telecare 2023, 29, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Higgins, D.M.; Riba, A.; Alderton, L.; Wendel, K.A.; Scanlon, J.; Weise, J.; Gibson, N.; Obafemi, O. Evaluation of the Impact and Outcomes of a Rapid Transition to Telehealth PrEP Delivery at a Sexual Health Clinic During the COVID-19 Pandemic. Sex. Transm. Dis. 2023, 50, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Homkham, N.; Manojai, N.; Patpeerapong, P.; Apiputthipan, R.; Srikummoon, P.; Kummaraka, U.; Chiawkhun, P.; Rankantha, A.; Traisathit, P. A comparative study of transgender women accessing HIV testing via face-to-face and telemedicine services in Chiang Mai, Thailand during the COVID-19 pandemic and their risk of being HIV-positive. BMC Public Health 2023, 23, 2161. [Google Scholar] [CrossRef] [PubMed]
- Houang, S.T.; Kafka, J.M.; Choi, S.K.; Meanley, S.P.; Muessig, K.E.; Bauermeister, J.A.; Hightow-Weidman, L.B. Co-occurring Epidemic Conditions Among Southern U.S. Black Men Who Have Sex with Men in an Online eHealth Intervention. AIDS Behav. 2023, 27, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Kalichman, S.C.; Kalichman, M.O.; Eaton, L.A. Phone-Delivered Intervention to Improve HIV Care for Young People Living with HIV: Trial to Inform Implementation and Utility. J. Acquir. Immune Defic. Syndr. 2023, 94, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.E.; Anderson-Bialis, J.; Citro, L.; Gracia, C.R. Patient satisfaction with telemedicine and in-person visits in reproductive endocrinology and infertility clinics. Reprod. Biomed. Online 2023, 47, 103286. [Google Scholar] [CrossRef] [PubMed]
- Kishkovich, T.P.; James, K.E.; Orona, K.C.; Bernstein, S.N.; Cohen, J.L.; Clapp, M.A. Telehealth use and receipt of recommended services within one-year postpartum. J. Telemed. Telecare 2025, 31, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Klotz, S.A.; Chan, C.B.; Bianchi, S.; Egurrola, C.; York, L.D. The Genie Is Out of the Bottle: Telemedicine Is More Effective Than Brick-and-Mortar Clinics in the Care of HIV-Infected Outpatients. Am. J. Med. 2023, 136, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.; Aharonovich, E.; Zingman, B.S.; Stohl, M.; Walsh, C.; Elliott, J.C.; Fink, D.S.; Durant, S.; Menchaca, R.; Sharma, A.; et al. HealthCall: Smartphone Enhancement of Brief Interventions to Improve HIV Medication Adherence Among Patients in HIV Care. AIDS Behav. 2024, 28, 1912–1922. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.R.; Arias, M.P.; Leitner, K.; Wang, E.; Clement, E.G.; Hamm, R.F. Assessing the impact of telehealth implementation on postpartum outcomes for Black birthing people. Am. J. Obstet. Gynecol. MFM 2023, 5, 100831. [Google Scholar] [CrossRef] [PubMed]
- Lagon, E.P.; Mauney, L.; Onwuzurike, C.; Shahawy, S.; Schaefer, K.; Starosta, A.; Ye, S.; Bartz, D.; Schantz-Dunn, J. An assessment of postpartum contraception rates with evolving care during the COVID-19 pandemic. Sex. Reprod. Healthc. 2023, 36, 100844. [Google Scholar] [CrossRef] [PubMed]
- Lersten, I.; Fought, A.; Yannetsos, C.; Sheeder, J.; Roeca, C. Patient perspectives of telehealth for fertility care: A national survey. J. Assist. Reprod. Genet. 2023, 40, 1369–1376. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.C.; Gold, M.A.; Vacca, S.H.; Garbers, S. Mixed-methods Exploration of Telehealth-supported Long-acting Reversible Contraceptive Services in School-based Health Centers: How Much Added Value? J. Pediatr. Healthc. 2023, 37, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Andres, J.; Fairley, C.K.; Krulic, T.; Ong, J.J.; Owen, L.; McNulty, A.; Bissessor, M.; Thng, C.; Bell, C.; Ratnayake, M.; et al. Telehealth for HIV care and management among people living with HIV in Australia: Results from an online survey. Sex. Health 2024, 21, SH24067. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Riveros, H.; Díaz, Y.; Montoro-Fernandez, M.; Moreno-Fornés, S.; González, V.; Muntada, E.; Romano-deGea, P.; Muñoz, R.; Hoyos, J.; Casabona, J.; et al. An Online HIV Self-Sampling Strategy for Gay, Bisexual and Other Men Who Have Sex with Men and Trans Women in Spain. J. Community Health 2024, 49, 535–548. [Google Scholar] [CrossRef] [PubMed]
- Masters, M.C.; Rivera, J.; Calamari, M.; Wright, K.; Janulis, P.; Rusie, L.; Bannon, J.; Milne, P.; Galvin, S.R.; Molina, E.G.; et al. Telemedicine and HIV Care Quality Measures During the COVID-19 Pandemic. J. Acquir. Immune Defic. Syndr. 2023, 94, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Mastorino, L.; Delmonte, S.; Ribero, S.; Quaglino, P.; Testi, R.; Dal Conte, I. The Limitation of Accessing Hospital Services Due to COVID-19 Pandemic: A Pilot Study of the Telephone Triage to Re-Organize the Access to a Center for Sexual Health in Northwest Italy. Sex. Transm. Dis. 2023, 50, 603–606. [Google Scholar] [CrossRef] [PubMed]
- McKellar, M.S.; Des Marais, A.C.; Chen, H.; Choi, Y.; Lilly, R.; Ayers, D.; Bennett, J.; Kestner, L.; Perry, B.; Poley, S.; et al. Providing medication for opioid use disorder and HIV pre-exposure prophylaxis at syringe services programs via telemedicine: A pilot study. Harm Reduct. J. 2024, 21, 69. [Google Scholar] [CrossRef] [PubMed]
- Merz-Herrala, A.A.; Kerns, J.L.; Logan, R.; Gutierrez, S.; Marshall, C.; Diamond-Smith, N. Contraceptive care in the United States during the COVID-19 pandemic: A social media survey of contraceptive access, telehealth use and telehealth quality. Contraception 2023, 123, 110000. [Google Scholar] [CrossRef] [PubMed]
- Meurice, M.E.; Mody, S.K.; Nodora, J.; Marengo, A.; Averbach, S. Social determinants of choosing telemedicine for contraceptive care: A retrospective cohort study. Contraception 2024, 134, 110414. [Google Scholar] [CrossRef] [PubMed]
- Monaco, A.E.; Westhoff, C.L. Evaluating virtual visits for contraceptive counseling: An exploratory study using the Person-Centered Contraceptive Counseling (PCCC) scale, a patient-reported outcome measure. Contraception 2024, 137, 110443. [Google Scholar] [CrossRef] [PubMed]
- Phianphitthayakul, O.-A.; Li, J.; Rongkapich, R.; Karroon, P.; Vatrasresth, J.; Jaisamrarn, U.; Santibenchakul, S. Client experiences with telehealth using LINE for consultation and assessment of adverse effects of contraceptive implants during the COVID-19 pandemic in Thailand. Digit. Health 2023, 9, 20552076231203877. [Google Scholar] [CrossRef] [PubMed]
- Raphael, M.; Abacan, A.; Cotter, S.; Smith, P.B.; Chacko, M.R. Telehealth for Reproductive Health Services for Economically Disadvantaged Youth. J. Adolesc. Health 2024, 75, 958–964. [Google Scholar] [CrossRef] [PubMed]
- Ratcliffe, J.; Paer, J.; Quigee, D.; Carnevale, C.; Richards, P.; Lasota, E.; Dandan, N.; Scherer, M.; Gordon, P.; Cohall, A.; et al. Examining Patient Preferences for Express, Telemedicine, and Standard Visits in a Sexual Health Clinic in New York City. Sex. Transm. Dis. 2024, 51, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Reback, C.J.; Cain, D.; Rusow, J.A.; Benkeser, D.; Schader, L.; Gwiazdowski, B.A.; Skeen, S.J.; Hannah, M.; Belzer, M.; Castillo, M.; et al. Technology-Based Interventions, with a Stepped Care Approach, for Reducing Sexual Risk Behaviors and Increasing PrEP Initiation Among Transgender and Gender Expansive Youth and Young Adults. AIDS Behav. 2024, 28, 3956–3969. [Google Scholar] [CrossRef] [PubMed]
- Reyes, E.; Silvis, J.; Gandhi, M.; Shi, Y.; Greene, M. Telehealth access and experiences of older adults with HIV during the COVID-19 pandemic: Lessons for the future. J. Am. Geriatr. Soc. 2024, 72, 2816–2824. [Google Scholar] [CrossRef] [PubMed]
- Ridgway, J.P.; Massey, R.; Mason, J.A.; Devlin, S.; Friedman, E.E. Measuring Retention in HIV Care in the First Year of the COVID-19 Pandemic: The Impact of Telehealth. AIDS Behav. 2023, 27, 1403–1408. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.; Tasker, S.; Nichols, K.; Tweed, M.; Darking, M.; Whetham, J.; Richardson, D. Returning home sampling kits for STI and HIV testing in people using a digital health HIV-PrEP pathway (PrEP-EmERGE). Sex. Transm. Infect. 2023, 99, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Saffati, G.; Naeem, T.; Kassab, J.; Orozco Rendon, D.; Green, C.; Liphsultz, L.I.; Khera, M. Will you go the distance? A satisfaction survey of telemedicine in sexual medicine. Sex. Med. 2023, 11, qfad060. [Google Scholar] [CrossRef] [PubMed]
- Aksut, R.S.; Inam, O. Evaluation of telemedicine for contraceptive counseling given to pregnant women during the COVID-19 pandemic: A randomized controlled trial. Health Care Women Int. 2024, 46, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.; Long, D.; Kay, E.; Levitan, E.B.; Batey, D.S.; Reed-Pickens, H.; Rana, A.; Carodine, A.; Nevin, C.; Eady, S.; et al. Role of Visit Modality in the HIV-Related No-Shows During the COVID-19 Pandemic: A Multisite Retrospective Cohort Study. AIDS Behav. 2023, 27, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Sonubi, T.; Sheik-Mohamud, D.; Ratna, N.; Bell, J.; Talebi, A.; Mercer, C.H.; Sinka, K.; Migchelsen, S.J.; Folkard, K.; Mohammed, H. STI testing, diagnoses and online chlamydia self-sampling among young people during the first year of the COVID-19 pandemic in England. Int. J. STD AIDS 2023, 34, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Sothornwit, J.; Kaewrudee, S.; Somboonporn, W.; Seanbon, O.; Ngamjarus, C. Implementing the individualized postpartum care with telemedicine during the COVID-19 pandemic at tertiary hospital in Thailand. Heliyon 2023, 9, e16242. [Google Scholar] [CrossRef] [PubMed]
- Stigliani, S.; Massarotti, C.; Maccarini, E.; Sozzi, F.; Rebora, S.; Scaruffi, P.; Anserini, P. Telehealth for infertile patients during SARS-CoV-2 pandemic: Far, and yet close. Minerva Obstet. Gynecol. 2023, 75, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Tewari, S.; Coyne, K.D.; Weinerman, R.S.; Findley, J.; Kim, S.T.; Flyckt, R.L.R. Racial disparities in telehealth use during the coronavirus disease 2019 pandemic. Fertil. Steril. 2023, 120, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Thomas, H.N.; Brotto, L.A.; de Abril Cameron, F.; Yabes, J.; Thurston, R.C. A virtual, group-based mindfulness intervention for midlife and older women with low libido lowers sexual distress in a randomized controlled pilot study. J. Sex. Med. 2023, 20, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Vatrasresth, J.; Prapaisilp, P.; Sukrong, M.; Sinthuchai, N.; Karroon, P.; Maitreechit, D.; Ittipuripat, S.; Kuptarak, A.; Sathitloetsakun, S.; Santibenchakul, S.; et al. Acceptability of telemedicine for follow up after contraceptive implant initiation at an obstetrics and gynecologic training center. BMC Health Serv. Res. 2023, 23, 817. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Moucheraud, C.; Butler, D.; Takayama, C.; Shoptaw, S.; Currier, J.S.; Gladstein, J.; Hoffman, R. Telemedicine for HIV care: A cross-sectional survey of people living with HIV receiving care at two federally qualified health centers in Los Angeles during a mature phase of the COVID-19 pandemic. BMC Infect. Dis. 2024, 24, 1481. [Google Scholar] [CrossRef] [PubMed]
- White, J.; Rahman, F.; Petrella, F.; Rivero, M.-J.; Deebel, N.; Arbeleaz, M.C.S.; Ledesma, B.; Kohn, T.; Ramasamy, R. Telehealth Sterilization Consultation Does Not Impact Likelihood of Vasectomy: A Retrospective Institutional Analysis. Urology 2023, 176, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Wollum, A.; Zuniga, C.; Grindlay, K.; Grossman, D. Who Accesses Birth Control Online? An Analysis of Requests for Contraception Submitted to an Online Prescribing Platform in the United States. Women’s Health Issues 2023, 33, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Wray, T.B.; Chan, P.A.; Klausner, J.D.; Ward, L.M.; Ocean, E.M.S.; Carr, D.J.; Guigayoma, J.P.; Nadkarni, S. The effects of regular home delivery of HIV self-testing and follow-up counselling on HIV testing and prevention outcomes in men who have sex with men who test infrequently in the United States: A pragmatic, virtual randomized controlled trial. J. Int. AIDS Soc. 2024, 27, e26318. [Google Scholar] [CrossRef] [PubMed]
- Yarger, J.; Hopkins, K.; Elmes, S.; Rossetto, I.; Van Liefde, D.; De La Melena, S.; Harper, C.C. Use of telemedicine to obtain contraception among young adults: Inequities by health insurance. Contraception 2024, 134, 110419. [Google Scholar] [CrossRef] [PubMed]
- Yelverton, V.; Ostermann, J.; Natafgi, N.; Olatosi, B.; Weissman, S.; Albrecht, H. Disparities in Telehealth Use in HIV Care During the COVID-19 Pandemic: Study Findings from South Carolina. Telemed. J. e-Health 2024, 30, 1594–1599. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.; Booth, A.; Glenton, C.; Munthe-Kaas, H.; Rashidian, A.; Wainwright, M.; Bohren, M.A.; Tunçalp, Ö.; Colvin, C.J.; Garside, R.; et al. Applying GRADE-CERQual to qualitative evidence synthesis findings: Introduction to the series. Implement. Sci. IS 2018, 13, 2. [Google Scholar] [CrossRef]
- Bajwa, J.; Munir, U.; Nori, A.; Williams, B. Artificial intelligence in healthcare: Transforming the practice of medicine. Future Healthc. J. 2021, 8, e188–e194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mennella, C.; Maniscalco, U.; De Pietro, G.; Esposito, M. Ethical and regulatory challenges of AI technologies in healthcare: A narrative review. Heliyon 2024, 10, e26297. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.