Nuclear Expression of p-STAT3 Is Associated with Poor Prognosis in ER(−) Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Tissue Samples
2.2. Examination of Clinicopathological/Biological Characteristics
2.3. p-STAT3 Immunohistochemical Staining
2.4. Statistical Analysis
3. Results
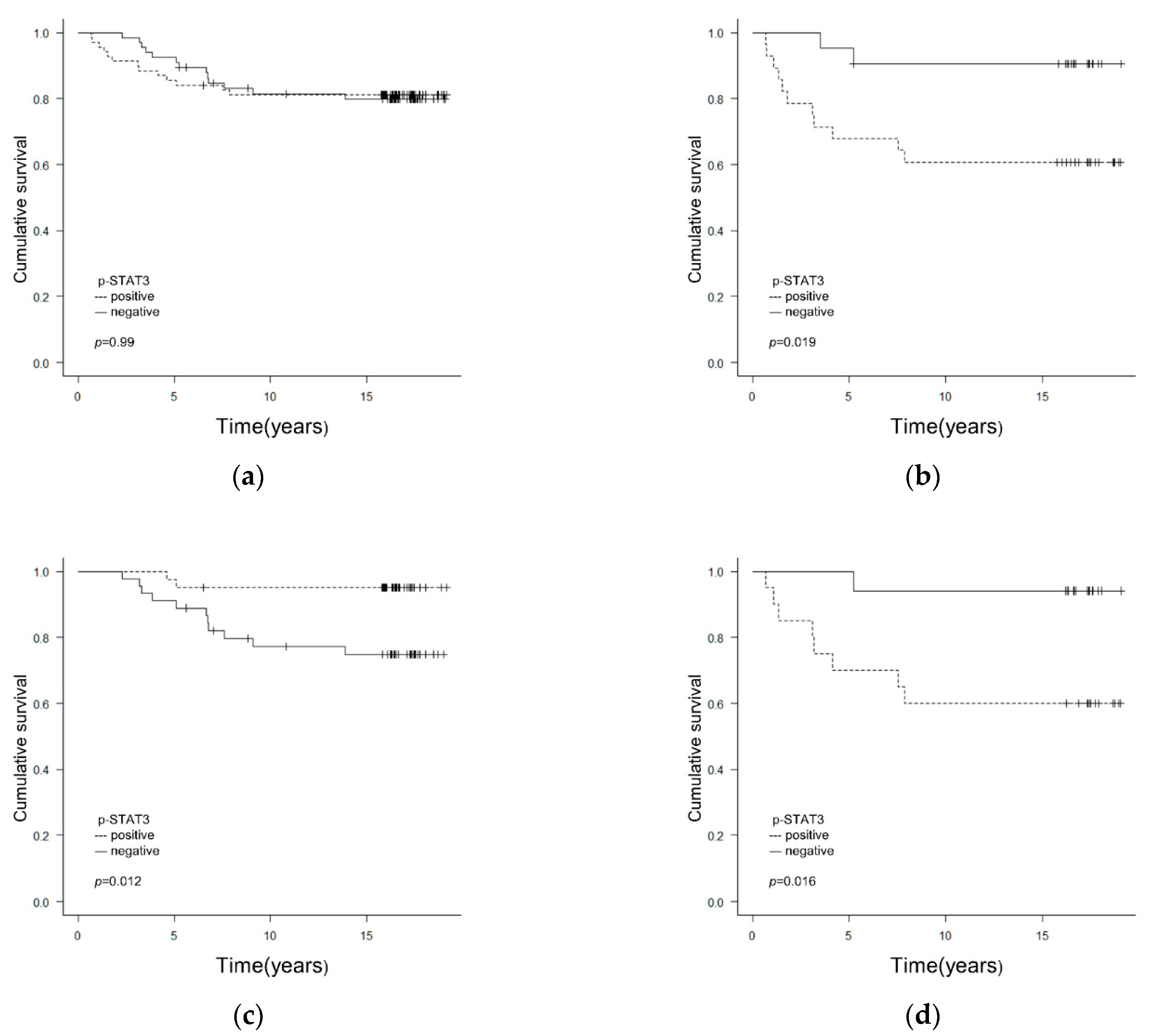
3.1. Correlation between Clinicopathological/Biological Factors including p-STAT3 and Prognosis
3.2. Correlation between p-STAT3 Expression and Pathological Factors
3.3. Correlations between p-STAT3 Expression and Clinicopathological/Biological Factors Affecting Relapse-Free Survival (RFS)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.; Albanese, C.; Darnell, J. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Shuai, K.; Liu, B. Regulation of JAK–STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Bowman, T.; Garcia, R.; Turkson, J.; Jove, R. STATs in oncogenesis. Oncogene 2000, 19, 2474–2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, R.; Mora, L.B.; Jove, R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin. Cancer Res. 2002, 8, 945–954. [Google Scholar] [PubMed]
- Yu, H.; Jove, R. The STATs of cancer—New molecular targets come of age. Nat. Rev. Cancer. 2004, 4, 97–105. [Google Scholar] [CrossRef]
- Haura, E.B.; Turkson, J.; Jove, R. Mechanisms of Disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol. 2005, 2, 315–324. [Google Scholar] [CrossRef]
- Niu, G.; Wright, K.L.; Huang, M.; Song, L.; Haura, E.; Turkson, J.; Zhang, S.; Wang, T.; Sinibaldi, D.; Coppola, D.; et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene 2002, 21, 2000–2008. [Google Scholar] [CrossRef] [Green Version]
- Arany, I.; Chen, S.-H.; Megyesi, J.K.; Adler-Storthz, K.; Chen, Z.; Rajaraman, S.; Ember, I.A.; Tyring, S.K.; Brysk, M.M. Differentiation-dependent expression of signal transducers and activators of transcription (STATs) might modify responses to growth factors in the cancers of the head and neck. Cancer Lett. 2003, 199, 83–89. [Google Scholar] [CrossRef]
- Masuda, M.; Suzui, M.; Yasumatu, R.; Nakashima, T.; Kuratomi, Y.; Azuma, K.; Tomita, K.; Komiyama, S.; Weinstein, I.B. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002, 62, 3351–3355. [Google Scholar]
- Watson, C.J.; Miller, W.R. Elevated levels of members of the STAT family of transcription factors in breast carcinoma nuclear extracts. Br. J. Cancer 1995, 71, 840–844. [Google Scholar] [CrossRef]
- Hsieh, F.-C.; Cheng, G.; Lin, J. Evaluation of potential Stat3-regulated genes in human breast cancer. Biochem. Biophys. Res. Commun. 2005, 335, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Dolled-Filhart, M.; Camp, R.L.; Kowalski, D.P.; Smith, B.L.; Rimm, D.L. Tissue microarray analysis of signal transducers and activators of transcription 3 (Stat3) and phospho-Stat3 (Tyr705) in node-negative breast cancer shows nuclear localization is associated with a better prognosis. Clin. Cancer Res. 2003, 9, 594–600. [Google Scholar]
- Nakagawa, T.; Iida, S.; Osanai, T.; Uetake, H.; Aruga, T.; Toriya, Y.; Takagi, Y.; Kawachi, H.; Sugihara, K. Decreased expression of SOCS-3 mRNA in breast cancer with lymph node metastasis. Oncol. Rep. 2008, 19, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Neilson, L.M.; Peck, A.R.; Liu, C.; Tran, T.H.; Witkiewicz, A.; Hyslop, T.; Nevalainen, M.T.; Sauter, G.; Rui, H. Signal transducer and activator of transcription-3 and breast cancer prognosis. Am. J. Cancer Res. 2011, 1, 347–355. [Google Scholar] [PubMed]
- Chen, Y.; Wang, J.; Wang, X.; Liu, X.; Li, H.; Lv, Q.; Zhu, J.; Wei, B.; Tang, Y. STAT3, a Poor Survival Predicator, Is Associated with Lymph Node Metastasis from Breast Cancer. J. Breast Cancer 2013, 16, 40–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhir, R.; Ni, Z.; Lou, W.; DeMiguel, F.; Grandis, J.; Gao, A. Stat3 activation in prostatic carcinomas. Prostate 2002, 51, 241–246. [Google Scholar] [CrossRef]
- Torres-Roca, J.F.; DeSilvio, M.; Mora, L.B.; Khor, L.; Hammond, E.; Ahmad, N.; Jove, R.; Forman, J.; Lee, R.; Sandler, H.; et al. Activated STAT3 as a correlate of distant metastasis in prostate cancer: A secondary analysis of Radiation Therapy Oncology Group 86-10. Urology 2007, 69, 505–509. [Google Scholar] [CrossRef]
- Wei, D.; Le, X.; Zheng, L.; Wang, L.; Frey, J.; Gao, A.; Peng, Z.; Huang, S.; Xiong, H.; Abbruzzese, J.; et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene 2003, 22, 319–329. [Google Scholar] [CrossRef] [Green Version]
- Horiguchi, A.; Oya, M.; Shimada, T.; Uchida, A.; Marumo, K.; Murai, M. Activation of Signal Transducer and Activator of Transcription 3 in Renal Cell Carcinoma: A Study of Incidence and Its Association with Pathological Features and Clinical Outcome. J. Urol. 2002, 168, 762–765. [Google Scholar] [CrossRef]
- Jiang, R.; Jin, Z.; Liu, Z.; Sun, L.; Wang, L.; Li, K. Correlation of activated STAT3 expression with clinicopathologic features in lung adenocarcinoma and squamous cell carcinoma. Mol. Diagn. Ther. 2011, 15, 347–352. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, S. A meta-analysis of STAT3 and phospho-STAT3 expression and survival of patients with non-small-cell lung cancer. Eur. J. Surg. Oncol. 2014, 40, 311–317. [Google Scholar] [CrossRef]
- Lee, J.; Kang, W.K.; Park, J.O.; Park, S.H.; Park, Y.S.; Lim, H.Y.; Kim, J.; Kong, J.; Choi, M.G.; Sohn, T.S.; et al. Expression of activated signal transducer and activator of transcription 3 predicts poor clinical outcome in gastric adenocarcinoma. APMIS 2009, 117, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Kusaba, T.; Nakayama, T.; Yamazumi, K.; Yakata, Y.; Yoshizaki, A.; Nagayasu, T.; Sekine, I. Expression of p-STAT3 in human colorectal adenocarcinoma and adenoma; correlation with clinicopathological factors. J. Clin. Pathol. 2005, 58, 833–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusaba, T.; Nakayama, T.; Yamazumi, K.; Yakata, Y.; Yoshizaki, A.; Inoue, K.; Nagayasu, T.; Sekine, I. Activation of STAT3 is a marker of poor prognosis in human colorectal cancer. Oncol. Rep. 2006, 15, 1445–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheen-Chen, S.-M.; Huang, C.-C.; Tang, R.-P.; Chou, F.-F.; Eng, H.-L. Prognostic Value of Signal Transducers and Activators of Transcription 3 in Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2008, 17, 2286–2290. [Google Scholar] [CrossRef] [Green Version]
- Sparano, J.A.; Moulder, S.; Kazi, A.; Coppola, D.; Negassa, A.; Vahdat, L.; Li, T.; Pellegrino, C.; Fineberg, S.; Munster, P.; et al. Phase II Trial of Tipifarnib plus Neoadjuvant Doxorubicin-Cyclophosphamide in Patients with Clinical Stage IIB-IIIC Breast Cancer. Clin. Cancer Res. 2009, 15, 2942–2948. [Google Scholar] [CrossRef] [Green Version]
- Sonnenblick, A.; Uziely, B.; Nechushtan, H.; Kadouri, L.; Galun, E.; Axelrod, J.H.; Katz, D.; Daum, H.; Hamburger, T.; Maly, B.; et al. Tumor STAT3 tyrosine phosphorylation status, as a predictor of benefit from adjuvant chemotherapy for breast cancer. Breast Cancer Res. Treat. 2013, 138, 407–413. [Google Scholar] [CrossRef]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef] [Green Version]
- Walker, S.R.; Nelson, E.A.; Zou, L.; Chaudhury, M.; Signoretti, S.; Richardson, A.; Frank, D. Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol. Cancer Res. 2009, 7, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Marotta, L.L.; Almendro, V.; Marusyk, A.; Shiptsin, M.; Schemme, J.; Walker, S.; Qimron, N.; Kim, J.; Choudhoury, S.; Maruyama, M.; et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24− stem cell-like breast cancer cells in human tumors. J. Clin. Investig. 2011, 121, 2723–2735. [Google Scholar] [CrossRef]
- Brierley, J.D.; Gospodararowicz, M.K.; Wittekind, C. Union for International. Cancer Control (UICC) TNM Classification of Malignant Tumours, 8th ed.; Wiley Blackwell: Oxford, UK, 2017; pp. 151–158. [Google Scholar]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.; Allred, D.; Cote, R.; Dowsett, M.; Fitzzgibons, R.; Hanna, W.; Langer, A.; et al. American Society of Clinical Oncology; College of American Pathologists: American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sirkisoon, S.R.; Carpenter, R.L.; Rimkus, T.; Anderson, A.; Harrison, A.; Lange, A.; Jin, G.; Watanabe, K.; Lo, H. Interaction between STAT3 and GLI1/tGLI1 oncogenic transcription factors promotes the aggressiveness of triple-negative breast cancers and HER2-enriched breast cancer. Oncogene 2018, 37, 2502–2514. [Google Scholar] [CrossRef]
- Sonnenblick, A.; Salgado, R.; Brohée, S.; Zahavi, T.; Peretz, T.; Eynden, G.V.D.; Rouas, G.; Salmon, A.; Francis, P.A.; Di Leo, A.; et al. p-STAT3 in luminal breast cancer: Integrated RNA-protein pooled analysis and results from the BIG 2-98 phase III trial. Int. J. Oncol. 2018, 52, 424–432. [Google Scholar] [CrossRef]
- Garcia, R.; Bowman, T.L.; Niu, G.; Yu, H.; Minton, S.; Muro-Cacho, C.; Cox, C.; Falcone, R.; Fairclough, R.; Parsons, S.; et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene 2001, 20, 2499–2513. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef]
- Song, Y.; Qian, L.; Song, S.; Chen, L.; Zhang, Y.; Yuan, G.; Zhang, H.; Xia, Q.; Hu, M.; Yu, M.; et al. Fra-1 and Stat3 synergistically regulate activation of human MMP-9 gene. Mol. Immunol. 2008, 45, 137–143. [Google Scholar] [CrossRef]
- Alvarez, J.V.; Febbo, P.G.; Ramaswamy, S.; Loda, M.; Richardson, A.L.; Frank, D.A. Identification of a Genetic Signature of Activated Signal Transducer and Activator of Transcription 3 in Human Tumors. Cancer Res. 2005, 65, 5054–5062. [Google Scholar] [CrossRef] [Green Version]
- Lo, H.W.; Hsu, S.C.; Xia, W.; Cao, X.; Shish, J.; Wei, Y.; Abbruzzese, J.; Hortobagyi, G. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007, 67, 9066–9076. [Google Scholar] [CrossRef] [Green Version]
- Kamran, M.Z.; Patil, P.; Gude, R.P. Role of STAT3 in Cancer Metastasis and Translational Advances. BioMed Res. Int. 2013, 2013, 421821. [Google Scholar] [CrossRef] [PubMed]
- Tell, R.W.; Horvath, C.M. Bioinformatic analysis reveals a pattern of STAT3-associated gene expression specific to basal-like breast cancers in human tumors. Proc. Natl. Acad. Sci. USA 2014, 111, 12787–12792. [Google Scholar] [CrossRef] [Green Version]
- McDaniel, J.M.; Varley, K.; Gertz, J.; Savic, D.; Roberts, B.S.; Bailey, S.K.; Shevde, L.A.; Ramaker, R.C.; Lasseigne, B.; Kirby, M.K.; et al. Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget 2017, 8, 8226–8238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, J.-J.; Yan, L.; Zhang, J.; Zhang, W.-D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. 2019, 38, 1–16. [Google Scholar] [CrossRef] [PubMed]
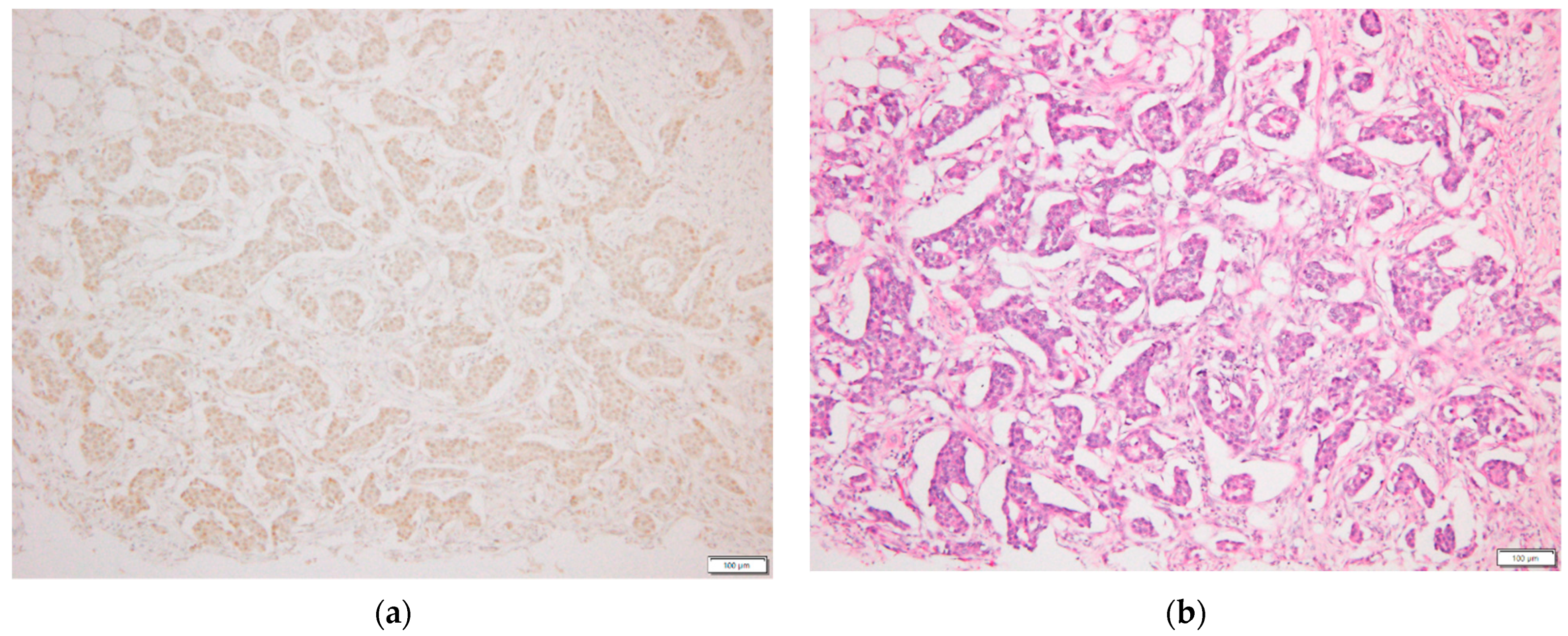
| Characteristics | No. | (%) |
|---|---|---|
| Age(years) | ||
| Median | 52 | |
| Range | 30–91 | |
| Tumor size | ||
| 1 | 63 | (46.7) |
| 2,3 | 72 | (53.3) |
| Lymph node metastasis | ||
| neg | 83 | (61.5) |
| pos | 52 | (38.5) |
| Nuclear grade | ||
| 1,2 | 109 | (80.7) |
| 3 | 26 | (19.3) |
| Blood vessel invasion | ||
| neg | 119 | (88.1) |
| pos | 16 | (11.9) |
| Lymphatic vessel invasion | ||
| neg | 104 | (77.0) |
| pos | 31 | (23.0) |
| Estrogen receptor | ||
| neg | 49 | (36.3) |
| pos | 86 | (63.7) |
| HER2 | ||
| neg | 114 | (84.4) |
| pos | 21 | (15.6) |
| Recurrence | ||
| neg | 109 | (80.7) |
| pos | 26 | (19.3) |
| Survival | ||
| alive | 114 | (84.4) |
| dead | 21 | (15.6) |
| Variables | All Cases (n = 135) | ER(−) Cases (n = 49) | ER(+) Cases (n = 86) | TNBC (n = 37) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-Recurrence | Recurrence | p-Value | Non-Recurrence | Recurrence | p-Value | Non-Recurrence | Recurrence | p-Value | Non-Recurrence | Recurrence | p-Value | |
| Tumor size | ||||||||||||
| 1 | 57 | 6 | 0.009 | 21 | 1 | 0.003 * | 36 | 5 | 0.56 * | 18 | 1 | 0.008 * |
| 2,3 | 52 | 20 | 15 | 12 | 37 | 8 | 10 | 8 | ||||
| Lymph node metastasis | ||||||||||||
| neg | 75 | 8 | <0.001 | 24 | 5 | 0.104 * | 51 | 3 | <0.001 * | 19 | 4 | 0.26 * |
| pos | 34 | 18 | 12 | 8 | 22 | 10 | 9 | 5 | ||||
| Nuclear grade | ||||||||||||
| 1,2 | 88 | 21 | 1 * | 23 | 9 | 1 * | 65 | 12 | 1 * | 17 | 6 | 1 * |
| 3 | 21 | 5 | 13 | 4 | 8 | 1 | 11 | 3 | ||||
| Blood vessel invasion | ||||||||||||
| neg | 99 | 20 | 0.084 | 33 | 12 | 1 * | 66 | 6 | 0.016 * | 26 | 8 | 1 * |
| pos | 10 | 6 | 3 | 1 | 7 | 5 | 2 | 1 | ||||
| Lymphatic vessel invasion | ||||||||||||
| neg | 91 | 13 | 0.001 | 27 | 7 | 0.18 | 64 | 6 | 0.002 | 20 | 5 | 0.43 * |
| pos | 18 | 13 | 9 | 6 | 9 | 7 | 8 | 4 | ||||
| Estrogen receptor | ||||||||||||
| neg | 36 | 13 | 0.12 | |||||||||
| pos | 73 | 13 | ||||||||||
| HER2 | ||||||||||||
| neg | 95 | 19 | 0.13 | 28 | 9 | 0.71 * | 67 | 10 | 0.11 * | |||
| pos | 14 | 7 | 8 | 4 | 6 | 3 | ||||||
| p-STAT3 | ||||||||||||
| neg | 53 | 13 | 1 | 19 | 2 | 0.025 * | 34 | 11 | 0.015 * | 16 | 1 | 0.023 * |
| pos | 56 | 13 | 17 | 11 | 39 | 2 | 12 | 8 | ||||
| Variables | All Cases (n = 135) | ER(−) Cases (n = 49) | ER(+) Cases (n = 86) | TNBC (n = 37) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p-STAT3 | Negative | Positive | p-Value | Negative | Positive | p-Value | Negative | Positive | p-Value | Negative | Positive | p-Value |
| Tumor size | ||||||||||||
| 1 | 31 | 32 | 1 | 10 | 12 | 0.78 | 21 | 20 | 1 | 9 | 10 | 1 |
| 2,3 | 35 | 37 | 11 | 16 | 24 | 21 | 8 | 10 | ||||
| Lymph node metastasis | ||||||||||||
| neg | 42 | 41 | 0.72 | 13 | 16 | 0.78 | 29 | 25 | 0.83 | 11 | 12 | 1 |
| pos | 24 | 28 | 8 | 12 | 16 | 16 | 6 | 8 | ||||
| Nuclear grade | ||||||||||||
| 1,2 | 55 | 54 | 0.51 | 15 | 17 | 0.55 | 40 | 37 | 1 | 11 | 12 | 1 |
| 3 | 11 | 15 | 6 | 11 | 5 | 4 | 6 | 8 | ||||
| Blood vessel invasion | ||||||||||||
| neg | 57 | 62 | 0.6 | 18 | 27 | 0.3 * | 39 | 35 | 1 | 15 | 19 | 0.58 * |
| pos | 9 | 7 | 3 | 1 | 6 | 6 | 2 | 1 | ||||
| Lymphatic vessel invasion | ||||||||||||
| neg | 50 | 54 | 0.84 | 16 | 18 | 0.53 * | 34 | 36 | 0.17 | 13 | 12 | 0.32 * |
| pos | 16 | 15 | 5 | 10 | 11 | 5 | 4 | 8 | ||||
| Estrogen receptor | ||||||||||||
| neg | 21 | 28 | 0.37 | 21 | 28 | 0 | 0 | 17 | 20 | |||
| pos | 45 | 41 | 0 | 0 | 45 | 41 | 0 | 0 | ||||
| HER2 | ||||||||||||
| neg | 59 | 55 | 0.16 | 17 | 20 | 0.52* | 42 | 35 | 0.3* | 17 | 20 | |
| pos | 7 | 14 | 4 | 8 | 3 | 6 | 0 | 0 | ||||
| 10-year RFS rate (%) | 87.9 | 82.6 | 90.5 | 64.3 | 95.6 | 85.4 | 94.1 | 65 | ||||
| Covariate | Categories | All Cases (n = 135) | ER(−) Cases (n = 49) | ER(+) Cases (n = 86) | TNBC (n = 37) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | ||||||||||
| HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | HR (95%CI) | p-Value | ||
| Tumor size | 1 vs. 2,3 | 3.31 (1.33–8.25) | 0.01 | 1.88 (0.70–5.08) | 0.21 | 12.43 (1.61–95.80) | 0.016 | 13.03 (1.69–100.50) | 0.014 | 1.52 (0.50–4.63) | 0.47 | 10.32 (1.29–82.67) | 0.028 | 11.50 (1.43–92.51) | 0.022 | ||
| Lymph node metastasis | neg vs. pos | 4.10 (1.78–9.44) | <0.001 | 2.31 (0.87–6.09) | 0.092 | 2.53 (0.83–7.75) | 0.1 | 6.53 (1.80–23.73) | 0.0044 | 3.96 (0.87–17.84) | 0.073 | 2.14 (0.57–7.97) | 0.26 | ||||
| Nuclear grade | 1,2 vs. 3 | 1.03 (0.63–1.67) | 0.92 | 0.93 (0.52–1.68) | 0.81 | 0.84 (0.30–2.34) | 0.74 | 0.94 (0.47–1.87) | 0.85 | ||||||||
| Blood vessel invasion | neg vs. pos | 2.60 (1.04–6.49) | 0.041 | 0.96 (0.12–7.39) | 0.97 | 5.01 (1.63–15.37) | 0.0048 | 1.76 (0.50–6.17) | 0.38 | 1.50 (0.19–12.04) | 0.7 | ||||||
| Lymphatic vessel invasion | neg vs. pos | 4.13 (1.91–8.94) | <0.001 | 2.23 (0.92–5.40) | 0.075 | 2.12 (0.71–6.31) | 0.18 | 6.97 (2.33–20.80) | <0.001 | 2.76 (0.81–9.36) | 0.1 | 1.80 (0.48–6.71) | 0.38 | ||||
| Estrogen receptor | neg vs. pos | 0.50 (0.23–1.08) | 0.079 | ||||||||||||||
| HER2 | neg vs. pos | 2.34 (0.98–5.57) | 0.055 | 1.57 (0.48–5.11) | 0.45 | 2.85 (0.78–10.37) | 0.11 | ||||||||||
| p-STAT3 | neg vs. pos | 1.00 (0.46–2.15) | 0.99 | 5.05 (1.12–22.83) | 0.035 | 5.37 (1.19–24.29) | 0.029 | 0.18 (0.04–0.81) | 0.027 | 0.18 (0.03–0.82) | 0.026 | 8.41 (1.05–67.32) | 0.045 | 9.48 (1.18–76.21) | 0.034 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, T.; Oda, G.; Kawachi, H.; Ishikawa, T.; Okamoto, K.; Uetake, H. Nuclear Expression of p-STAT3 Is Associated with Poor Prognosis in ER(−) Breast Cancer. Clin. Pract. 2022, 12, 157-167. https://doi.org/10.3390/clinpract12020020
Nakagawa T, Oda G, Kawachi H, Ishikawa T, Okamoto K, Uetake H. Nuclear Expression of p-STAT3 Is Associated with Poor Prognosis in ER(−) Breast Cancer. Clinics and Practice. 2022; 12(2):157-167. https://doi.org/10.3390/clinpract12020020
Chicago/Turabian StyleNakagawa, Tsuyoshi, Goshi Oda, Hiroshi Kawachi, Toshiaki Ishikawa, Kentaro Okamoto, and Hiroyuki Uetake. 2022. "Nuclear Expression of p-STAT3 Is Associated with Poor Prognosis in ER(−) Breast Cancer" Clinics and Practice 12, no. 2: 157-167. https://doi.org/10.3390/clinpract12020020
APA StyleNakagawa, T., Oda, G., Kawachi, H., Ishikawa, T., Okamoto, K., & Uetake, H. (2022). Nuclear Expression of p-STAT3 Is Associated with Poor Prognosis in ER(−) Breast Cancer. Clinics and Practice, 12(2), 157-167. https://doi.org/10.3390/clinpract12020020






