Introduction
Over the past century humans have intro- duced a large number of chemical substances into the environment. Obviously some of these chemicals are useful but many are toxic and their harm to the environment and our health far outweighs their benefit. Man-made chemi- cals such as demulsifiers, dispersants, corro- sion inhibitors and pesticides contaminate land and water and may adversely affect terres- trial and aquatic biota. These chemicals are released into the environment through usage, spillage, transportation, disposal and uninten- tionally. Chemoreceptors of aquatic organisms have been known to be destroyed by toxic organics, thereby affecting their mating and reproduction process. There is growing scien- tific consensus that numerous industrial and agricultural chemicals have the ability to inter- fere with endocrine systems and hormonal activities of all animals. Many wildlife species are exposed to biologically active concentra-tions of endocrine-disrupting chemicals. For example, a greater number of the world’s amphibian species about 32% are threatened with extinction, 43% experiencing declines and another 22% with insufficient data[
1,
2] due to impaired reproduction and development causally linked to endocrine-disrupting chemi- cals. A well-documented evidence of masculin- ization (imposex) in periwinkle snails by trib- utyltin, a biocide used in antifouling paints[
3] and demasculinization and feminization of amphibians by atrazine[
4] have also be reported. All these could subsequently lead to reduction as well as extinction of some vital species.[5-9]
Industrial chemicals (demulsifiers, corro- sion inhibitors and dispersants) mainly of sur- factant-based commonly used by industrial petroleum operators may cause environmental problems including the toxicity of the surfac- tants to fish and invertebrates, foaming and euthrophication.[
10,
11] A foaming agent is a material that facilitates formation of foam such as a surfactant. A surfactant, when pres- ent in small amounts, reduces surface tension of a liquid (reduces the work needed to create the foam) or increases its colloidal stability by inhibiting coalescence of bubbles. Detergents that contain phosphate are highly caustic, and surfactant detergents are very toxic. They are widely used in daily activities and these deter- gents cause excess frothing and growth of floating aquatic weeds (eutrophication) on the water surface, affecting aeration and quality of fresh water and adversely affects the physio-logical and biochemical processes of fish. Similarly, pesticides present the only group of substances of chemical or biological origin that are purposely applied to the environment with aim to suppress plant and animal pests as well as to protect agricultural and industrial products. However, over 98% of sprayed pesti- cide reach a destination other than their target species, including non-target species, air, water, bottom sediments, and soil.[
12] Repeated application could lead to loss of biodiversity since they usually act by disrupting some com- ponent of the pest’s life processes to kill or inactivate it.[
13] Most of these chemicals espe- cially pesticides are not easily degradable, they persist in soil, leach to groundwater, surface water and contaminate the environment. Depending on their chemical properties, syn- thetic pesticides can enter an organism, bioac- cumulate in food chains and consequently influence human health.[
14] Thus, the overall intensive pesticide application results in sev- eral negative effects in the environment that cannot be ignored.[
15]
Presently in Nigeria, only a few chemicals have been ecologically tested for their safety in spite of their ecological and environment impact. Although the Federal Government of Nigeria is currently emphasizing the need for adequate environmental protection by strictly asking industrial operators to adequately char- acterise any chemical used in their operation before subsequent disposal into the environ- ment.[
16,
17] Retardation in growth and total anni-hilation of life could occur if these chemicals are discharged indiscriminately into the envi- ronment, hence the regulators in Nigeria; Department of Petroleum Resources (DPR) and Federal Ministry of Environment (FME) enforces laws guiding the disposal of these chemicals. However, apart from the direct effect, these chemicals can reduce the avail- ability of food for man’s consumption.[
9,
10,
18]
The objective of this study was to evaluate the acute effects of four chemicals (Rigwash, Oil eater, Nalco EC1304A/COT 505, Glycol™) and four pesticides (Propoxur, Deltamethrin, Atrazine, Furadan) using fish (
Tilapia guineensis) and earthworm (
Aporrectodea longa) with a view to determining their safe limits. The industrial chemicals were assessed due to their indiscriminate use in oil spill clean-up in Niger Delta waters. Similarly, the pesticides were tested because they are the major pesticides used in Nigeria to control a number of pests on food crops such as grains, tea, fruits and vegetable crops and on non-food crops such as tobacco and cotton.[
19] Fish and earthworm were used to represent the health of aquatic and terrestrial ecosystems.
Materials and Methods
Test chemicals
The test chemicals namely, Rig wash, Oil eater, Nalco EC1304A/COT 505, Glycol™, Propoxur, Deltamethrin, Atrazine and Furadan were obtained from their respective manufac- turers. The selected chemicals are widely used in agriculture and oil industries around the world. Active ingredients were used instead of commercial formulations, aiming to document the acute effects of the active substances on the mortality rather than the effects of adju- vants added in the commercial products. Ethylene glycol, a versatile organic compound that, moderately viscous, hygroscopic liquid at room temperature is manufactured by Huntsman Chemicals. Rigwash a special aque- ous blend of anionic and non-ionic surfactant is produced by Global Drilling Fluids and Chemicals Limited in Gujarat, India. It is one of the most Popular Clean up Chemicals for the Oilfield Industry. It is an excellent emulsifier and may be used at any point in the drilling operation to improve emulsification and reduce torque and drag. It is highly effective in both fresh water and seawater. Oil Eater is ultra concentrated, biocatalytic multi-enzyme liquid concentrate. It is biodegradable and non-flammable and when combined with fresh or salt water and oxygen, it causes crude oil and other organic substances to rapidly decom- pose, eventually biodegrading them to carbon dioxide and water. It also safely dissolves grease and oils from asphalt and concrete. Oil Eater is perfect for pressure washing, parts cleaning, mopping and floor cleaning. It is pro- duced by Kafko International Ltd. Nalco EC1304A/COT 505 condensate corrosion inhibitor provides a full complement of volatile, filming and blended corrosion inhibitors to help arrest condensate corrosion. It addresses water conditions related to hard- ness, oxygen, silica, iron and more. It is manu- factured by Nalco Chemical Company, a corpo- ration of Delaware, USA.
Propoxur is a non-systemic carbamate insecticide widely used to kill all pests’ in house and public area, such as cockroaches, mosquitoes, flies, ants, fleas, louses, bedbugs, etc. It is especially effective on rice leafhoppers and plant hoppers. It blocks the production and action of cholinesterase, an essential nervous system enzyme, quickly paralyzes the nervous systems of those insects. It is produced by Shanghai Kima Chemical Company Ltd. Atrazine is a selective systemic herbicide used on pre- and post-emergence control of annual broad-leaved weeds and annual grasses in maize, sorghum, sugar cane, pineapples, chemical fallow, grassland, macadamia nuts, conifers, industrial weed control. It is manu- factured by Syngenta Corporation. Carbofuran is one of the most toxic carbamate pesticides. It is marketed under the trade names Furadan. Furadan is a systemic insecticide used to con- trol insects in a wide variety of field crops, including potatoes, corn and soybeans. It is manufactured by FMC Corporation. Deltamethrin is a pyrethroid insecticide that kills insects on contact and through digestion. It is used to control apple and pear suckers, plum fruit moth, caterpillars on brassicas, pea moth, aphids (apples, plums, hops), winter moth (apples and plums), codling and tortrix moths (apples). It is manufactured by Sumitomo Chemical Co. Ltd (Japan).
Test organisms
Fish (Tilapia guineensis)
Tilapia guineensis (fish) from fresh water environment were collected from a cultured farm in Ekrheranwhen in the Niger Delta eco- logical zone of Nigeria. Ekrheranwhen is locat- ed in Ughelli North Local Government Area of Delta State, Nigeria (latitude 05° 32’ 43.6” N and longitude 005° 55’ 04.6” E). The test organisms were acclimated to laboratory con- ditions for seven days in large holding tanks before the experiment. The acute toxicity was determined in terms of 96-hr LC
50 and lethal concentrations according to the OECD Guideline # 203 protocol for Testing of Chemicals 210, Fish, Early Life Stage Toxicity Test.[
20]
Earthworms (Aporrectodea longa)
The Oligochaete
Aporrectodea longa is cur- rently used invertebrate species for ecotoxico-logical assessment of substances in soil, which is the OECD and International Standardization Organization (ISO) recommended earthworms test species.[
21,
22,
23] Adult earthworms (weighing between 400–600 mg) with well-developed clitella were collected by gentle digging and hand sorting from sub surface litters from the Plant and Animal Biotechnology Garden at the Faculty of Life Sciences University of Benin, Nigeria and cultured in the laboratory in artifi- cial soil according to OECD guidelines.[
21,
22] The acute toxicity of earthworm exposed to the test chemicals was determined using the OECD # 207 protocol.21 The soil samples were prepared by mixing clean dry soil with 5 g of cellulose and 80 ml (test solution) homogenized in a glass container. Soil water content was meas- ured every week and moisture was adjusted to 35% of the maximum water-holding capacity by adding distilled water whenever necessary. Thereafter, ten voided earthworms (400–600 mg) were cleaned and transferred from their holding containers with a sterilized platinum wire to the soils spiked with concentrations of the test chemicals in three replicates. The con- trol experiment contained ten (10) organisms, cellulose, water and clean soil.[
24]
Experimental design
The semi-static renewal bioassay procedure started with a range finding test. This was used to determine the range of concentrations for the definitive test. The test concentrations for the definitive test were prepared by appro- priate dilution of the stock solutions. Five dif- ferent concentrations of the test industrial chemicals were prepared by dissolving in 5 ml of deionised water in geometric series of 31.25, 62.5, 125, 250, and 500 mg/l while four different concentrations of 2.5, 5, 10 and 15mg/l for pesticides were also prepared. A total of five (5) litres of the test medium and control (dilution water) was used to test 10 test organisms in three replicates.[
20] After approximately 24 hours, the test solutions were renewed and their physico-chemical con- stituents namely pH, temperature, total dis- solved solids (TDS), salinity and conductivity were measured at test initiation and termina- tion. The organisms were not fed during the 96 h experimental period. The weight and length of the fish were 0.471±0.03 g and 1.83±0.12 cm respectively (
Table 1).
Two replicates were made for each treat- ment.
Aporrectodea longa were segregated from the culture beds, washed with deionised water and kept in a trough on moist filter paper for three hours to devoid their gut contents. These worms were rinsed again with deionised water, blotted on a filter paper and placed one in each Petri plate. Each Petri plate was covered with a perforated plastic film. Test Petri plates were kept at laboratory tempera- ture of 20±2ºC for 48 hours and worm’s mortal-ity was observed by giving a gentle mechanical stimulus to their prostomial part. Fingerlings were exposed to different concentrations according to the Organization for Economic Co-operation and Development (OECD) stan- dard method, to determine the LC
50 96 h of fin- gerlings. Number of dead fish was recorded at time 24, 48, 72, and 96 h.[
25]
Statistical analysis
The vulnerability of the test species to the various test chemicals was determined using a computerized probit method of analysis according to Finney[
26] for the LC
50 at day 4 and 14 respectively. In addition, the analysis of variance (ANOVA) in Statistical Package for Social Science (SPSS) statistical software in Version 15.0 was also used to test the variables at P<0.05 level of significance.
Results
Mortality and fish behaviour
The toxicological endpoint used in this study was mortality. Mortality in the test tanks for each concentration was recorded at 24, 48, 72 and 96 h when the fish failed to show the evidence of opercula activity and do not respond to gentle prodding. The dead organ-isms were removed immediately. There was no death or physiological change in the control tanks throughout the 96 h test period. The influence of concentration, exposure duration and environmental conditions were observed in this study. Death rate increased with increase in concentration and exposure dura- tion. Varying behavioural patterns observed in the fish include: erratic swimming, loss of reflexes, loss of equilibrium, paleness of skin and gasping for air.
The 96 h mean % mortality recorded in the industrial test chemicals for four varying con- centrations ranging between 1.25 and 200 mg/l are indicated as follows: Rigwash (57, 80, 100, 100%), Oil eater (13, 30, 100, 100%), Nalco EC1304A/COT 505 (53, 77, 100, 100%) and Glycol™ (40, 100, 100, 100%). In the pesticide test, values recorded for Propoxur was 0, 7, 27, 60% Atrazine (27.1, 36.7, 52.6, 100%), Furadan (47.53, 49.29, 50.32, 78.70%) while Deltamethrin was 30, 80, 100, 100%. However, from the results obtained, the mean % mortal- ity at 96 h exposure in the test chemicals and control groups was significantly different at P<0.05.
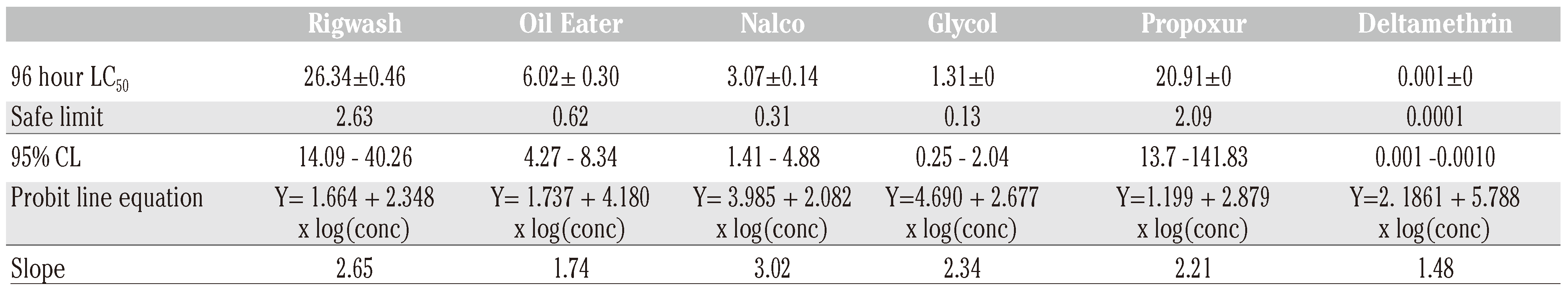
The acute toxicity of the chemicals was also evaluated using estimated 96 h LC50 values in varying concentrations. The estimated 96 h LC50 values for Rigwash, Oil eater, Nalco EC1304A/COT 505, Glycol, Propoxur and Deltamethrin were 26.34±0.46, 6.02±0.30, 3.07±0.14, 1.31±0.01, 20.91±0 and 0.001±0 mg/l respectively. The estimated safe limits for these chemicals are 10% of the 96 h LC50. The fish were sensitive to the test chemicals how- ever; the control organisms were active and responded to stimuli throughout the experi- mental period.
Changes in behaviour were observed already after one hour of exposure to the industrial chemicals and pesticides in the groups of fish in the highest concentrations (250 and 500 mg/l for industrial chemicals and 10 and 15 mg/l for pesticides). The behavioural changes were also observed in the group exposed to 62.5 mg/l for industrial chemicals and 5 mg/l for pesticides after 24 hours, but these changes were not so intensive. Abnormal behaviour included reduced reflexes, erratic swimming, loss of equilibrium, and accelerat- ed respiration. In the highest concentration, the fish were lying on their side and were mov- ing only in this position. No changes in behav- iour were observed in the control group and experimental group exposed to 2.5 mg/l of atrazine as well as in all groups during the recovery period.
Toxicological effects to earthworms
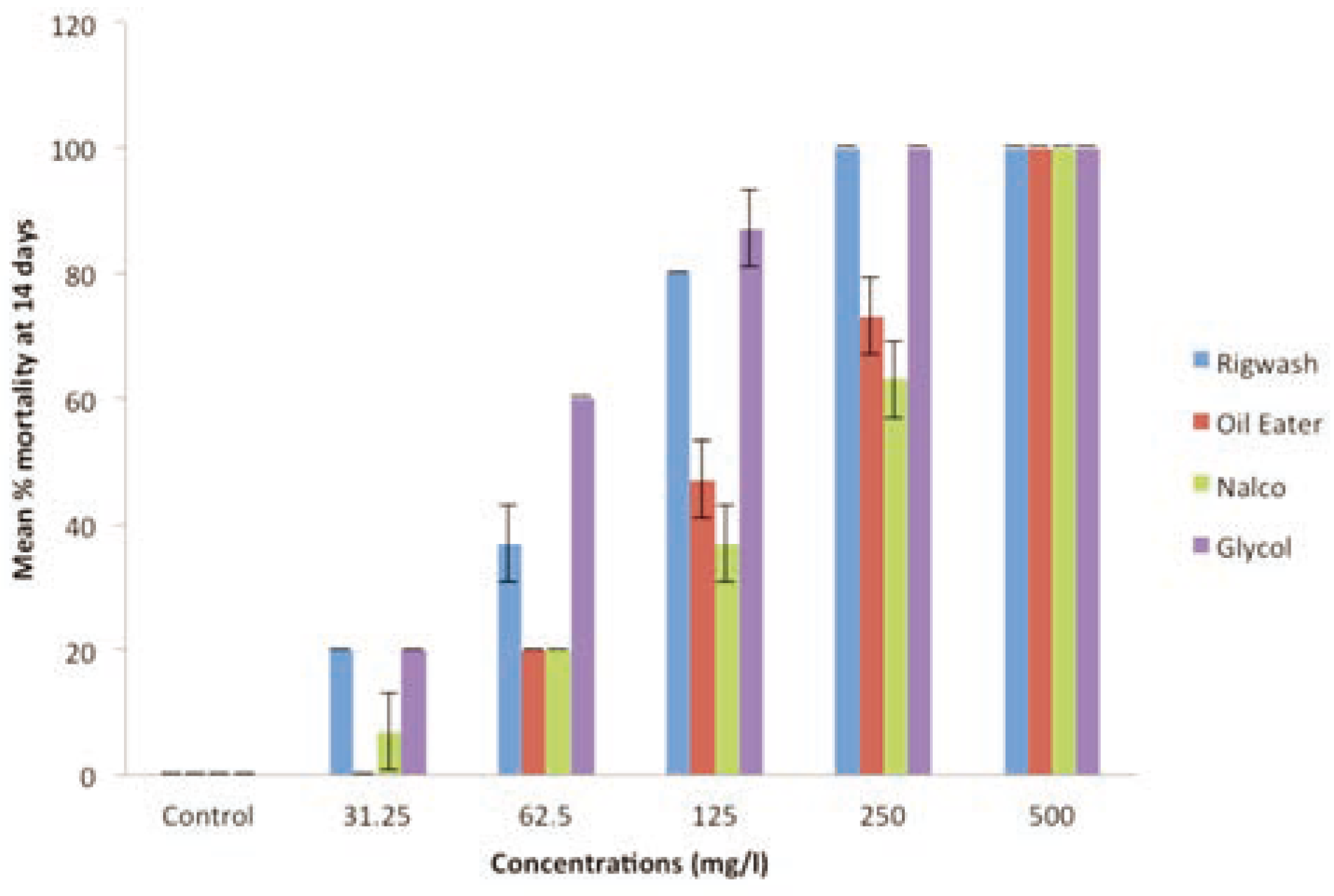
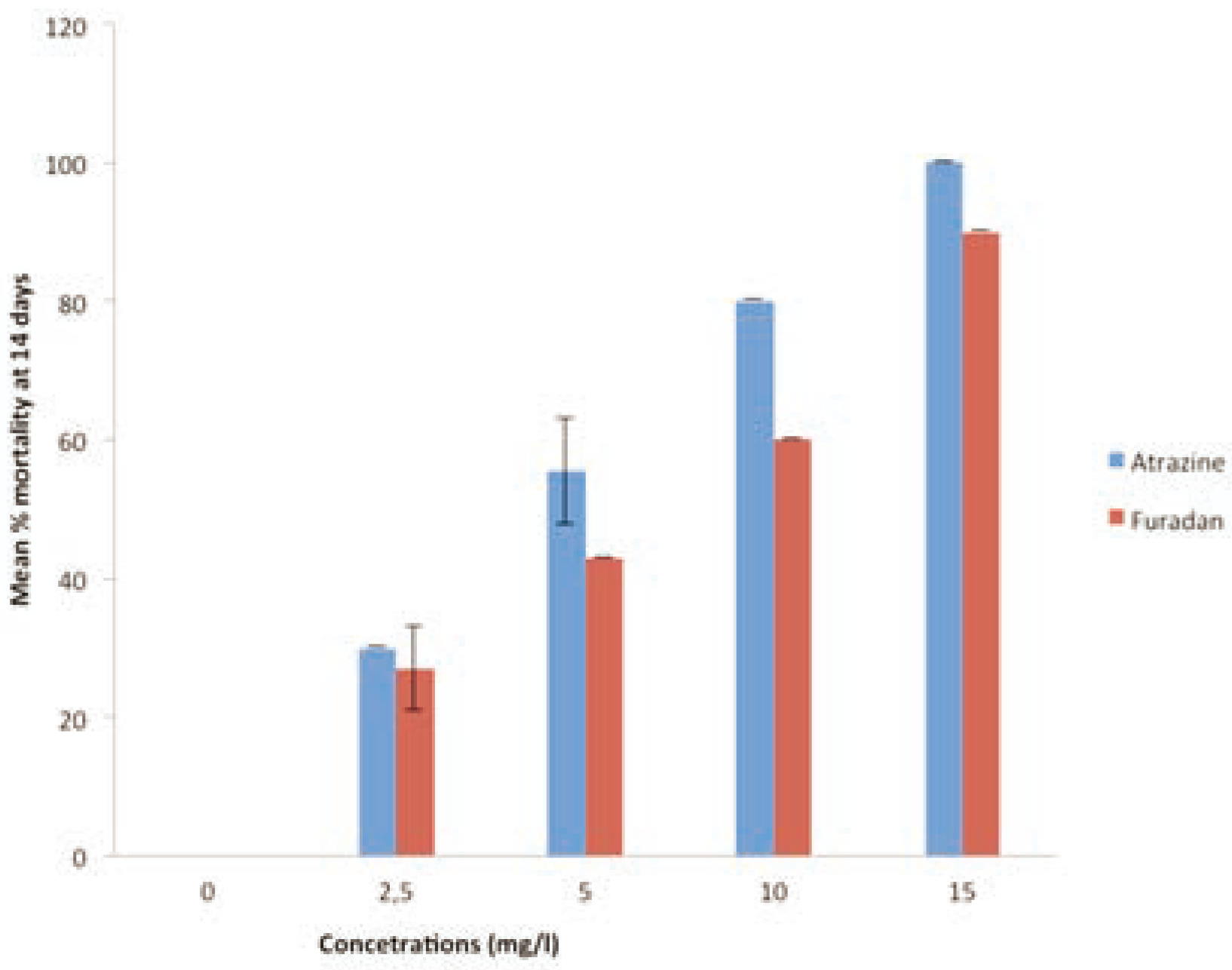
The test organisms exposed to the various test concentrations of the industrial chemicals (62.5 - 500 mg/kg) and pesticides (2.5 - 15 mg/kg) at test termination (day 14) are shown in the
Figure 1 and
Figure 2 respectively. The observed results exhibited a clear concentra- tion-dependent relationship, which indicated that worms exposed to the test chemicals had mean % mortality that increased with increased concentration and exposure time for all industrial chemicals and pesticides tested. The mean mortality for the control experiment was significantly different from the results obtained for the test chemicals at levels of P<0.05. The acute toxicity profile of the chem- icals was also evaluated using estimated 14- day LC
50 values in varying concentrations. The estimated 14-day LC
50 values for Rig wash, Oil eater, Nalco EC1304A/COT 505, Glycol, Atrazine, Furadan were 80.05±3.5, 151.55±10.7, 172.63±14.2, 63.72±2.43, 4.97±0
and 0.29±0 mg/kg respectively (
Table 2). Discussion
Following exposure to the experimental organisms, the test chemicals resulted in mor- tality of the fish fingerlings when compared with the control, indicating an effect induced by the chemicals. Fish mortality increased with increased concentrations and exposure time. This study used laboratory test, which could be extrapolated to possible field concentration since the chemicals usually find their way into waters or land
ia indiscriminate disposal and unintentional release into the environment of the Nigeria Niger Delta. In Nigeria, atrazine for example is a common herbicide used in farmlands and to date there seems to be dearth of information regarding its effect on earth- worms. The mortality values reported for
Tilapia guineensis were influenced by toxicity modifying factors such as exposure duration, concentrations, type of chemicals and environ- mental conditions. The estimated 96 h LC
50 val- ues obtained in this study compared with GESAMP rating showed that the test chemicals were toxic.[
27]
Industrial chemicals (demulsifiers, disper- sants, corrosion inhibitors) and their metabo- lites contain active ingredients which could be absorbed through respiratory membranes of aquatic organisms that can prevents the exchange of gases and ultimately leads to death.[
11] Most industrial chemicals and pesti- cides are persistent and bioaccumulative. This implies that they remain in the food chain, often absorbed in body fat where they can wreak havoc for a long time. However, the faster a given chemical or pesticide breaks down in the environment, the less threat it poses to aquatic life.[
28]
Some pesticides that are applied to water to kill plants are toxic to fish and other water ani- mals at concentrations similar to those used to kill the plants.[
29] As have been noted earlier, repeated exposure to sub-lethal doses of some pesticides can cause physiological and behav- ioural changes in fish that reduce populations, by causing abandonment of nests and broods, decreased immunity to disease and increased failure to avoid predators.[
29] Pesticides acted by blocking the organism’s nervous system, caus- ing malfunction, tremors, and death. Most of which are relatively insoluble, persist in soils and aquatic sediments, bioconcentrate in the tissues of invertebrates and vertebrates from their food, move up trophic chains, and affect top predators.[
30] The amount of pesticide that migrates from the intended application area is influenced by the particular chemical’s proper- ties such as its propensity for binding to soil, water solubility, and resistance to being bro- ken down over time.[
31]
The estimated LC
50 obtained were quiet comparable with previous and related studies. Ezemonye
et al. [
32] reported a range of 13.79 –20.41 mg/l for Neatex (industrial detergent) and 12.43 – 14.73 mg/l for Norust CR 486 (cor- rosion inhibitor) exposed to 14-day old
Tilapia guineensis at 96 h. Similarly, Nwoko
et al. [
33] reported an acute toxicity value of a corrosion inhibitor exposed to African catfish (
Clarias Gariepinus) at 96 h as 0.228±0.007 mg/l. Other studies which showed similar comparison include the research works by several other Authors.[
9,
10,
11,
18,
34,
35,
36,
37,
38,
39]
Soil organisms enhance soil aggregation and porosity, thus increasing infiltration and reducing runoff. Earthworms are an important component of the soil system, and can enhance plant growth by improving soil fertili- ty and nutrient recycling.[
40] They are important for conditioning soil since they aerate and ingest soil and other organic matter as they tunnel. They break up thatch, a layer of dead matter that can accumulate on the soil surface and excrete material, called castings, which enrich the soil and provide many benefits to gardeners and farmers. They represent the greatest part of biomass of terrestrial inverte- brates (>80%) and play an important role in soil ecosystem. They are used as bioindicator of soil contamination providing an early warn- ing of decline in soil quality and serve as model organisms in toxicity testing. However, pesti- cides and fungicides used to control insects and fungus may pose a great threat to earth- worms and harm them. This could reduce earthworm populations significantly, causes rigidity and immobility.[
41]
Earthworms are characterized by high abili- ty to cumulate a lot of pollutants from soil in their tissues, thus they are used for studying of toxicity potential of chemicals. The differential acute toxicity levels of the chemicals as shown in the LC
50 values for the soil toxicity may be attributed to the toxic constituents of the chemicals.[
42] This is because organisms are known to react differently to varying stressors depending on their toxicity profile. There was high indication that the chemicals induced death in the test organisms due to the fact that there was significant difference between the test concentrations and the control groups. Soil contaminated with organic pollutants (
e.g. dispersant, corrosion inhibitors, pesticides, detergents
etc) can be detrimental to earth- worm populations. In concentrations of 50-100 ppm surfactants would not only denature the cells proteins, but also totally inactivate the enzyme and actually alter the cell wall perme- ability. The hazardous effects of chemicals and pesticides on aquatic and terrestrial organ- isms have been reported by other Authors.[
43,
44,
45] Ezemonye
et al. [
43] reported a 14-day LC
50 value of 511.32 and 207.61 mg/kg for earthworms exposed to an industrial detergent and corro- sion inhibitor respectively.
Many of the substances used in the formula-tion of these chemicals are persistent soil con- taminants, whose impact may endure for decades and adversely affect soil conservation. Similarly, the use of pesticides decreases the general biodiversity in the soil. Not using the chemicals results in higher soil quality, thus the factors in the soil, such as its texture, its ability to retain water, and the amount of organic matter contained in it, also affect the amount of pesticide that would be degraded. With the additional effect that more organic matter in the soil allows for higher water retention. A smaller content of organic matter in the soil increases the amount of pesticide that will leave the area of application, because organic matter binds to and helps break down pesticides.[
46]
Pesticides enter the soil
ia spray drift dur-ing foliage treatment, wash-off from treated foliage, release from granulates or from treat- ed seeds in soil. Some pesticides such as soil fumigants and nematocides are applied direct- ly into soil to control pests and plant diseases presented in soil. As earlier indicated, the transport, persistence or degradation of pesti- cides in soil depends on their chemical proper- ties as well as physical, chemical and biologi- cal properties of the soil. These factors affect sorption/desorption, volatilisation, degrada- tion, uptake by plants, run-off, and leaching of pesticides.[
47]