Phytochemical Profile and Acute Toxicity in CD-1 Mice of the Hydroethanolic Extract and Butanolic Fraction of Piper marginatum Jacq.
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Preparation of Plant Material and Extract and Its Fractions
2.3. Experimental Design and Animal Work
2.3.1. Experimental Design
2.3.2. Experimental Animals
2.3.3. Experimental Groups and Treatments
2.3.4. Post-Treatment Clinical and Behavioral Observation
2.3.5. Variables Evaluated
2.3.6. Sampling and Analysis Unit
2.3.7. Ethical Aspects and Method of Slaughter
2.4. Preliminary Phytochemical Analysis
2.5. Chromatographic Profiles by HPLC/PDA and LC-QTOF/MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Post-Treatment Clinical and Behavioral Observation
3.2. Changes in the Body Mass of Mice
3.3. Histopathological Analysis
3.4. Preliminary Phytochemical Analysis
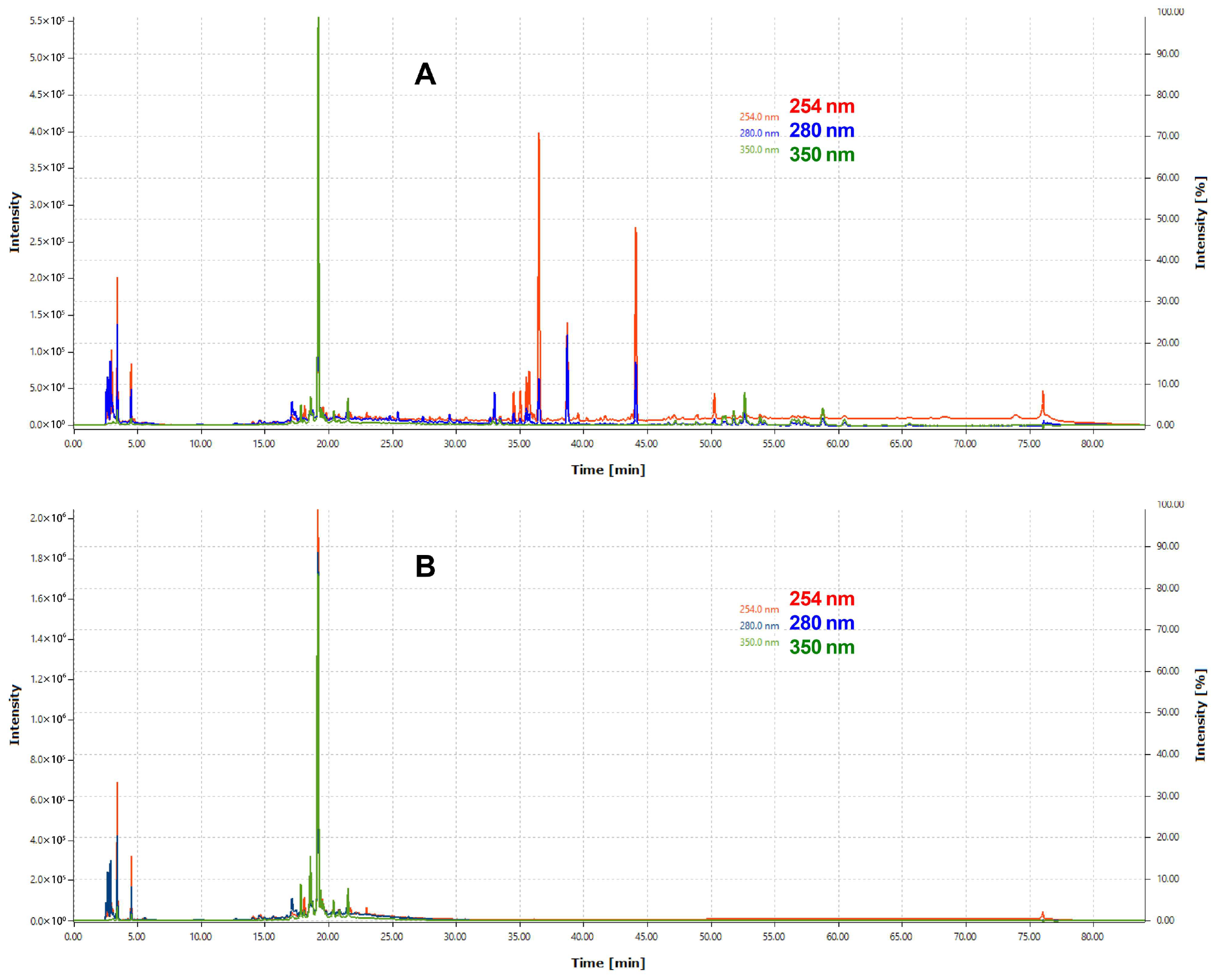
3.5. Chromatographic Profiling by HPLC/PDA and LC-QTOF/MS
3.6. Strengths and Weaknesses of This Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hartini, Y.S.; Utaminingsih; Julianus, J.; Patramurti, C.; Nugroho, L.H. Secondary Metabolite Profile in Mature and Old Leaves of Four Piper Species: Forest Betel (Piper aduncum L.), Red Betel (Piper crocatum Ruiz & Pav.), Javanese Chili Betel (Piper retrofractum Vahl.), and Green Betel (Piper betle L.). Plant Sci. Today 2024, 11, 546–552. [Google Scholar] [CrossRef]
- Parmar, V.; Jain, S.; Bisht, K.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.; Prasad, A.; Wengel, J.; Olsen, C.; et al. Phytochemistry of the genus Piper. Phytochemistry 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Salehi, B.; Zakaria, Z.; Gyawali, R.; Ibrahim, S.; Rajković, J.; Shinwari, Z.; Khan, T.; Sharifi-Rad, J.; Ozleyen, A.; Turkdonmez, E.; et al. Piper Species: A Comprehensive Review on Their Phytochemistry, Biological Activities and Applications. Molecules 2019, 24, 1364. [Google Scholar] [CrossRef] [PubMed]
- Scott, I.; Jensen, H.; Philogène, B.; Arnason, J. A review of Piper spp. (Piperaceae) phytochemistry, insecticidal activity and mode of action. Phytochem. Rev. 2008, 7, 65–75. [Google Scholar] [CrossRef]
- Tabopda, T.; Mitaine-Offer, A.; Miyamoto, T.; Tanaka, C.; Ngadjui, B.; Lacaille-Dubois, M. Secondary Metabolites from Polar Fractions of Piper umbellatum. Nat. Prod. Commun. 2012, 7, 595–596. [Google Scholar] [CrossRef]
- Da Silva, A.S.; Da Silva, J.M.; Ramos, C.S. Antimicrobial and antioxidant potential from Piper marginatum roots. PeerJ Org. Chem. 2023, 5, e8. [Google Scholar] [CrossRef]
- Jalalpure, S.; Patil, M.; Prakash, N.; Hemalata, K.; Manvi, F. Hepatoprotective Activity of the Fruits of Piper Longum Linn. Indian J. Pharm. Sci. 2003, 65, 363–366. [Google Scholar]
- Lopes, J.; Marx, C.; Ingrassia, R.; Picada, J.; Pereira, P.; Ferraz, A. Neurobehavioral and toxicological activities of two potentially CNS-acting medicinal plants of Piper genus. Exp. Toxicol. Pathol. 2012, 64, 9–14. [Google Scholar] [CrossRef]
- Bru, J.; Guzman, J. Folk medicine, phytochemistry and pharmacological application of Piper marginatum. Rev. Bras. De Farm. 2016, 26, 767–779. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, L.; Zhou, H.; Hou, G. Chemical composition and anti-inflammatory activity of n-butanol extract of Piper sarmentosum Roxb. In the intestinal porcine epithelial cells (IPEC-J2). J. Ethnopharmacol. 2021, 269, 113723. [Google Scholar] [CrossRef]
- Kulawe, D.; Abubakar, Z.; Hena, J. Toxicological and Histological Studies of the Ethyl Acetate, Aqueous and N-Butanol Fractions of the Leaf of Combretum Molle (R.Br. Ex. G. Don) to Wistar Rats. Int. J. Sci. Res. Publ. 2020, 10, 9794. [Google Scholar] [CrossRef]
- Ribeiro, N.; Camara, C.; Ramos, C. Toxicity of essential oils of Piper marginatum Jacq. against Tetranychus urticae Koch and Neoseiulus californicus (McGregor). Chil. J. Agric. Res. 2016, 76, 71–76. [Google Scholar] [CrossRef]
- Sequeda-Castañeda, L.G.; Celis-Zambrano, C.A.; Gutiérrez-Prieto, S.J. Piper marginatum Jacq. (Piperaceae): Phytochemical, therapeutic, botanical insecticidal and phytosanitary uses. Pharmacologyonline 2015, 3, 136–145. [Google Scholar]
- Gamboa, F.; Muñoz, C.-C.; Numpaque, G.; Sequeda-Castañeda, L.G.; Gutierrez, S.J.; Tellez, N. Antimicrobial Activity of Piper marginatum Jacq and Ilex guayusa Loes on Microorganisms Associated with Periodontal Disease. Int. J. Microbiol. 2018, 2018, 4147383. [Google Scholar] [CrossRef]
- Gutiérrez-Prieto, S.J.; Sequeda-Castañeda, L.G.; Penedo-Jaramillo, G.M.; Chacín-Nieto, A.V.; Contreras-Cáceres, D.R.; Moreno-Abello, G.C.; Galvis-Rincón, M.P.; Gamboa-Jaimes, F.O.; Luengas-Caicedo, P.E. In vitro mineral apposition analysis of two Colombian plant extracts on Amelogenesis imperfecta teeth. Clin. Exp. Dent. Res. 2022, 8, 336–349. [Google Scholar] [CrossRef]
- Sánchez, Y.; Correa, T.M.; Abreu, Y.; Martínez, B.; Duarte, Y.; Pino, O. Caracterización química y actividad antimicrobiana del aceite esencial de Piper marginatum Jacq. Rev. De Protección Veg. 2011, 26, 170–176. [Google Scholar]
- Santos, B.; De O Chaves, E.V.L.d.-C.M.C.; Gray, A. Phenylalkanoids from Piper marginatum. Phytochemistry 1998, 49, 1381–1384. [Google Scholar] [CrossRef]
- Sequeda-Castañeda, L.G. Phytochemical Study of Some Colombian Native Plants and Evaluation of Their Antimicrobial Activity and Remineralizing Effect on Dental Enamel. Ph.D. Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2021. [Google Scholar]
- Sequeda-Castañeda, L.G.; Suárez-Carvajal, L.F.; Téllez-Corral, M.A.; Gutiérrez-Prieto, S.J.; Méndez-Pinzón, H.A. Evaluation of Ilex guayusa and Piper marginatum Extract Cytotoxicity on Human Dental Pulp Mesenchymal Stem Cells. Dent. J. 2024, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahmad, N.; Amir, M.; AlJhisi, F.; Alamer, M.; Al-Shaban, H.; Alsadah, Z.; Alsultan, B.; Aldawood, N.; Chathoth, S.; et al. Quality Variation and standardization of black pepper (Piper nigrum): A comparative geographical evaluation based on instrumental and metabolomics analysis. Biomed. Chromatogr. 2019, 34, e4772. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 423: Acute Oral toxicity—Acute Toxic Class Method, OECD Guidelines for the Testing of Chemicals. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- Parasuraman, S. Toxicological screening. J. Pharmacol. Pharmacother. 2011, 2, 74–79. [Google Scholar] [CrossRef]
- Deng, Y.; Sriwiriyajan, S.; Tedasen, A.; Hiransai, P.; Graidist, P. Anti-cancer effects of Piper nigrum via inducing multiple molecular signaling in vivo and in vitro. J. Ethnopharmacol. 2016, 188, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Emon, N.U.; Alam, S.; Rudra, S.; Riya, S.R.; Paul, A.; Hossen, S.M.M.; Kulsum, U.; Ganguly, A. Antidepressant, anxiolytic, antipyretic, and thrombolytic profiling of methanol extract of the aerial part of Piper nigrum: In vivo, in vitro, and in silico approaches. Food Sci. Nutr. 2021, 9, 833–846. [Google Scholar] [CrossRef] [PubMed]
- Fatmawaty, F.; Anggreni, N.G.M.; Fadhil, N.; Prasasty, V.D. Potential In Vitro and In Vivo Antioxidant Activities from Piper crocatum and Persea Americana Leaf Extracts. Biomed. Pharmacol. J. 2019, 12, 661–667. [Google Scholar] [CrossRef]
- Mgbeahuruike, E.E.; Yrjönen, T.; Vuorela, H.; Holm, Y. Bioactive compounds from medicinal plants: Focus on Piper species. S. Afr. J. Bot. 2017, 112, 54–69. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Patahuddin, H.; Mohamad, A.S.; Israf, D.A.; Sulaiman, M.R. In vivo anti-nociceptive and anti-inflammatory activities of the aqueous extract of the leaves of Piper sarmentosum. J. Ethnopharmacol. 2010, 128, 42–48. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. J. Pharmacol. Pharmacother 2010, 1, 94–99. [Google Scholar] [CrossRef]
- OECD. Test No. 425. Acute Oral toxicity: Up-and-Down Procedure, OECD Guidelines for the Testing of Chemicals. In OECD Guidelines for the Testing of Chemicals; OECD Publishing: Paris, France, 2002. [Google Scholar] [CrossRef]
- D’Angelo, L.; Xavier, H.; Torres, L.; Lapa, A.; Souccar, C. Pharmacology of Piper marginatum Jacq. a folk medicinal plant used as an analgesic, antiinflammatory and hemostatic. Phytomedicine 1997, 4, 33–40. [Google Scholar] [CrossRef]
- Cáceres, A.; Kato, M.J. Importance of a multidisciplinary evaluation of Piper genus for development of new natural products in Latin America. Int. J. Phytocosmetics Nat. Ingred. 2014, 1, 4. [Google Scholar] [CrossRef]
- Sequeda-Castañeda, L.; Muñoz-Realpe, C.; Celis-Zambrano, C.; Gutiérrez-Prieto, S.; Luengas-Caicedo, P.; Gamboa, F. Preliminary Phytochemical Analysis of Berberis goudotii Triana & Planch. ex Wedd. (Berberidaceae) with Anticariogenic and Antiperiodontal Activities. Sci. Pharm. 2019, 87, 2. [Google Scholar] [CrossRef]
- Benavides, F.J.; Guénet, J.L. Manual de Genética de Roedores de Laboratorio: Principios Básicos y Aplicaciones; Universidad de Alcala de Henares. Servicio de Publicaciones: Alcalá de Henares, Spain, 2004. [Google Scholar]
- Mathiasen, J.R.; Moser, V.C. The Irwin Test and Functional Observational Battery (FOB) for Assessing the Effects of Compounds on Behavior, Physiology, and Safety Pharmacology in Rodents. Curr. Protoc. 2023, 3, e780. [Google Scholar] [CrossRef]
- Pariyani, R.; Ismail, I.S.; Azam, A.A.; Abas, F.; Shaari, K.; Sulaiman, M.R. Phytochemical Screening and Acute Oral Toxicity Study of Java Tea Leaf Extracts. Biomed Res. Int. 2015, 2015, 742420. [Google Scholar] [CrossRef]
- Scudamore, C.L. A Practical Guide to the Histology of the Mouse; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Bourhia, M.; Haj Said, A.A.; Chaanoun, A.; El Gueddari, F.; Naamane, A.; Benbacer, L.; Khlil, N. Phytochemical Screening and Toxicological Study of Aristolochia baetica Linn Roots: Histopathological and Biochemical Evidence. J. Toxicol. 2019, 2019, 8203832. [Google Scholar] [CrossRef]
- Winter, A.L.; Moses, M.A. Manual Merck de Veterinaria; Grupo Asis: Zaragoza, Spain, 2023. [Google Scholar]
- AVMA. Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Clarkson, J.M.; Martin, J.E.; McKeegan, D.E.F. A review of methods used to kill laboratory rodents: Issues and opportunities. Lab. Anim. 2022, 56, 419–436. [Google Scholar] [CrossRef]
- Shomer, N.H.; Allen-Worthington, K.H.; Hickman, D.L.; Jonnalagadda, M.; Newsome, J.T.; Slate, A.R.; Valentine, H.; Williams, A.M.; Wilkinson, M. Review of Rodent Euthanasia Methods. J. Am. Assoc. Lab. Anim. Sci. 2020, 59, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Burgot, G.; Burgot, J.L. General Analytical Chemistry: Separation and Spectral Methods; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- Di-Rienzo, J.A.; Casanoves, F.; Balzarini, M.G.; Gonzalez, L.; Tablada, M.; Robledo, C.W. InfoStat. Available online: http://www.infostat.com.ar/ (accessed on 27 July 2020).
- IBM-Corp. IBM SPSS Statistics 29.0, IBM SPSS Software; IBM Corporation: Armonk, NY, USA, 2022.
- Camacho, L.; Lewis, S.; Vanlandingham, M.; Juliar, B.; Olson, G.; Patton, R.; Da Costa, G.; Woodling, K.; Sepehr, E.; Bryant, M.; et al. Comparison of endpoints relevant to toxicity assessments in 3 generations of CD-1 mice fed irradiated natural and purified ingredient diets with varying soy protein and isoflavone contents. Food Chem. Toxicol. 2016, 94, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Clayton, Z.; McCurdy, C. Short-term thermoneutral housing alters glucose metabolism and markers of adipose tissue browning in response to a high-fat diet in lean mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R627–R637. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; Sablé, E.; Porsolt, R. Primary Observation (Irwin) Test in Rodents for Assessing Acute Toxicity of a Test Agent and its Effects on Behavior and Physiological Function. Curr. Protoc. Pharmacol. 2004, 27, 10. [Google Scholar] [CrossRef]
- Jogdand, S.V.; Jadhav, G.; Talekar, Y. Acute and sub-acute toxicity studies of hydro-alcoholic extract of dried fruits of Piper longum Linn in Wistar rats. Adv. Tradit. Med. 2023, 24, 179–190. [Google Scholar] [CrossRef]
- Murwanti, R.; Nurrochmad, A.; Gani, A.P.; Sasmito, E.; Edwina, A.E.; Chandra, M.K.; Suryawan, F.H.; Wardana, A.R.; Natalia Budiningsih, J.L. Acute and Subchronic Oral Toxicity Evaluation of Herbal Formulation: Piper crocatum Ruiz and Pav., Typhonium flagelliforme (Lodd.) Blume, and Phyllanthus niruri L. in Sprague–Dawley Rats. J. Toxicol. 2023, 2023, 7511397. [Google Scholar] [CrossRef]
- Daniel, N.; Ahmad, F.; Assim, Z.; Pin, C.H. Phytochemical, cytotoxicity and antioxidant studies on the stem bark of Piper arborescens. Malays. J. Fundam. Appl. Sci. 2017, 13, 840–845. [Google Scholar] [CrossRef]
- Sanchez-Aguirre, O.A.; Guevara-Valencia, M.; Juárez-Aguilar, E.; Flores, N.; Malagon-Aviles, O.; Sánchez-Medina, A.; Cano-Asseleih, L.M. Antioxidant, antimicrobial and antiproliferative activities of alcoholic extracts from Piper aequale Vahl leaves. Bol. Latinoam. Y Del Caribe De Plantas Med. Y Aromat. 2024, 23, 972–982. [Google Scholar] [CrossRef]
- Riani, L.; Macedo, A.; Chedier, L.; Pimenta, D. Chemical analysis of essential oil and hydrolates of leaves, inflorescences and stems of Piper chimonanthifolium Kunth. Rev. Virtual De Quimica 2017, 9, 1560–1569. [Google Scholar] [CrossRef]
- Onwidiwe, T.C.; Unekwe, P.C.; Chilaka, K.C.; Ilo, C.E.; Ughachukwu, P.O.; Aligwekwe, A.U. Evaluation of Gastroprotective Activities of Fraction Extracts of Piper guineense Leaf on Ethanol-Induced Ulcer in Wistar Rats. J. Pharm. Res. 2021, 21, 6–12. [Google Scholar] [CrossRef]
- Rosales, P.; Bordin, G.S.; Gower, A.; Moura, S. Indole alkaloids: 2012 until now, highlighting the new chemical structures and biological activities. Fitoterapia 2020, 143, 104558. [Google Scholar] [CrossRef] [PubMed]
- Thawabteh, A.; Juma, S.; Bader, M.; Karaman, D.; Scrano, L.; Bufo, S.; Karaman, R. The Biological Activity of Natural Alkaloids against Herbivores, Cancerous Cells and Pathogens. Toxins 2019, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Krenn, L. Plant Cardiotonic Glycosides. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: New York, NJ, USA, 2014; pp. 1–18. [Google Scholar]
- Elmusa, F.; Elmusa, M. Mini-Review on Coumarins: Sources, Biosynthesis, Bioactivity, Extraction and Toxicology. J. Turk. Chem. Soc. Sect. A Chem. 2024, 11, 933–944. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Adani, F.; Sciarria, P.; Squillace, P.; Scarafoni, A.; Iametti, S.; Scaglia, B. Antioxidant and Anti-Inflammatory Activities of the Crude Extracts of Raw and Fermented Tomato Pomace and Their Correlations with Aglycate-Polyphenols. Antioxidants 2020, 9, 179. [Google Scholar] [CrossRef]
- Leyva, E.; Loredo-Carrillo, S.E.; López, L.I.; Escobedo-Avellaneda, E.G.; Navarro-Tovar, G. Chemical and biological significance of naphthoquinones. Literature review. Afinidad 2017, 74, 39. [Google Scholar]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Salman, H.A.K.; Sulaiman, R. Extraction and quantification of saponins: A review. Food Res. Int. 2014, 59, 16–40. [Google Scholar] [CrossRef]
- Wutsqa, Y.U.; Suratman, S.; Sari, S.L.A. Detection of terpenoids and steroids in Lindsaea obtusa with thin layer chromatography. Asian J. Nat. Prod. Biochem. 2021, 19, 4. [Google Scholar] [CrossRef]
- Ozcelik, B.; Gurbuz, I.; Karaoglu, T.; Yesilada, E. Antiviral and antimicrobial activities of three sesquiterpene lactones from Centaurea solstitialis L. ssp. solstitialis. Microbiol. Res. 2009, 164, 545–552. [Google Scholar] [CrossRef]
- Banwart, W.; Porter, P.; Granato, T.; Hassett, J. HPLC separation and wavelength area ratios of more than 50 phenolic acids and flavonoids. J. Chem. Ecol. 1985, 11, 383–395. [Google Scholar] [CrossRef]
- Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. HPLC analysis of diverse grape and wine phenolics using direct injection and multidetection by DAD and fluorescence. J. Food Compos. Anal. 2007, 20, 618–626. [Google Scholar] [CrossRef]
- Motta, E.; Da Costa, J.; Bastos, J. A validated HPLC-UV method for the analysis of galloylquinic acid derivatives and flavonoids in Copaifera langsdorffii leaves. J. Chromatogr. B 2017, 1061–1062, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Guo, L.; Mandarakas, P.; Appleby, S. Determination of Dithiocarbamate and Its Breakdown Product Ethylenethiourea in Fruits and Vegetables. J. AOAC Int. 1995, 78, 1238–1243. [Google Scholar] [CrossRef]
- Hoagland, R.E.; Frear, D.S. Behavior and fate of ethylenethiourea in plants. J. Agric. Food Chem. 1976, 24, 129–133. [Google Scholar] [CrossRef]
- NIH. Report on Carcinogens; Ethylene Thiourea. CAS No. 96-45-7; U.S. Department of Health and Human Services: Durham, NC, USA, 2021. [CrossRef]
- PubChem. CID 2723650: Ethylenethiourea. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2723650 (accessed on 22 June 2025).
- Pungle, R.; Nile, S.H.; Makwana, N.; Singh, R.; Singh, R.P.; Kharat, A.S. Green Synthesis of Silver Nanoparticles Using the Tridax procumbens Plant Extract and Screening of Its Antimicrobial and Anticancer Activities. Oxidative Med. Cell. Longev. 2022, 2022, 9671594. [Google Scholar] [CrossRef]
- Vasanth Mp, V.; Kg Purushotham, P. Screening of Phytochemical Analysis and In vitro Bioactive of Polyherbal Formulation. Pharmacogn. J. 2020, 12, 1525–1533. [Google Scholar] [CrossRef]
- PubChem. CID 4201615: Lupanyl Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/4201615 (accessed on 22 June 2025).
- Kokubun, T.; Harborne, J.B. Phytoalexin induction in the sapwood of plants of the Maloideae (Rosaceae): Biphenyls or dibenzofurans. Phytochemistry 1995, 40, 1649–1654. [Google Scholar] [CrossRef]
- Miyakado, M.; Watanabe, K.; Nobuo, O.; Nonaka, F.; Morita, A. Isolation and Structural Determination of Eriobofuran, A New Dibenzofuran Phytoalexin from Leaves of Loquat, Eriobotrya japonica L. J. Pestic. Sci. 1985, 10, 101–106. [Google Scholar] [CrossRef][Green Version]
- PubChem. CID 178939: Eribofuran. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/178939 (accessed on 22 June 2025).
- Cumming, A. Flavonoid Composition. World Intellectual Property Organization WO2025/091065, 8 May 2025. Available online: https://patentscope.wipo.int/search/en/WO2025091065 (accessed on 22 June 2025).
- Ramirez, J.; Cartuche, L.; Morocho, V.; Aguilar, S.; Malagon, O. Antifungal activity of raw extract and flavanons isolated from Piper ecuadorense from Ecuador. Rev. Bras. De Farmacogn. 2013, 23, 370–373. [Google Scholar] [CrossRef]
- Rasul, A.; Millimouno, F.M.; Ali Eltayb, W.; Ali, M.; Li, J.; Li, X. Pinocembrin: A novel natural compound with versatile pharmacological and biological activities. Biomed Res. Int. 2013, 2013, 379850. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 68071: Pinocembrin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/68071 (accessed on 22 June 2025).
- Elshimy, R.; Khawagi, W.Y.; Naguib, I.A.; Bukhari, S.I.; El-Shiekh, R.A. 9-Methoxyellipticine: Antibacterial Bioactive Compound Isolated from Ochrosia elliptica Labill. Roots. Metabolites 2023, 13, 643. [Google Scholar] [CrossRef]
- Lin, Y.M.; Juichi, M.; Wu, R.Y.; Lee, K.H. Antitumor Agents LXIX. Alkaloids of Ochrosia acuminata. Planta Medica 1985, 51, 545–546. [Google Scholar] [CrossRef]
- Poisson, J.; Miet, C. 9-Methoxyellipticine, an alkaloid from “yellow wood,” Ochrosia borbonica, from Reunion Island. Ann. Pharm. Fr. 1967, 25, 523–524. [Google Scholar]
- PubChem. CID 72512: 9-Methoxyellipticine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/72512 (accessed on 22 June 2025).
- PubChem. CID 85447763: 4-Hydroxynornantenine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/85447763 (accessed on 22 June 2025).
- Saleem, H.; Zengin, G.; Locatelli, M.; Tartaglia, A.; Ferrone, V.; Htar, T.T.; Naidu, R.; Mahomoodally, M.F.; Ahemad, N. Filago germanica (L.) Huds. bioactive constituents: Secondary metabolites fingerprinting and in vitro biological assays. Ind. Crops Prod. 2020, 152, 112505. [Google Scholar] [CrossRef]
- Urzúa, A.; Cassels, B.K. 4-hydroxynornantenine, a 4-hydroxylated noraporphine. Tetrahedron Lett. 1978, 19, 2649–2652. [Google Scholar] [CrossRef]
- PubChem. CID 15102684: 5-Deoxystrigol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/15102684 (accessed on 22 June 2025).
- Ueno, K.; Nakashima, H.; Mizutani, M.; Takikawa, H.; Sugimoto, Y. Bioconversion of 5-deoxystrigol stereoisomers to monohydroxylated strigolactones by plants. J. Pestic. Sci. 2018, 43, 198–206. [Google Scholar] [CrossRef]
- Yoneyama, K.; Xie, X.; Kisugi, T.; Nomura, T.; Sekimoto, H.; Yokota, T.; Yoneyama, K. Characterization of strigolactones exuded by Asteraceae plants. Plant Growth Regul. 2011, 65, 495–504. [Google Scholar] [CrossRef]
- Hamburger, M. HPLC-based activity profiling for pharmacologically and toxicologically relevant natural products—Principles and recent examples. Pharm. Biol. 2019, 57, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, Y.; Nozaki, T.; Kamewada, Y.; Nakagawa, M.; Nakamura, Y.; Inaba, N.; Mimaki, Y. New quinolone alkaloids from Euodia Fruit, and their pancreatic lipase inhibitory and PPAR-gamma ligand-binding activities. Fitoterapia 2025, 180, 106322. [Google Scholar] [CrossRef] [PubMed]
- Nakasato, T.; Asada, S.; Marui, K. Dehydroevodiamine, Main Alkaloid from the Leaves of Evodia rutaecarpa HOOKER fil. et THOMSON. Yakugaku Zasshi 1962, 82, 619–626. [Google Scholar] [CrossRef] [PubMed][Green Version]
- PubChem. CID 9817839: Dehydroevodiamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9817839 (accessed on 22 June 2025).
- Kumar, V.; Sharma, N.; Mishra, V.K.; Mall, S.; Kumar, A.; Dev, K.; Patel, C.N. Computational Evaluation of Phytocompounds from Selective Medicinal Plants as Potential Antidiabetic Agents. Chem. Biodivers. 2025, 22, e202403368. [Google Scholar] [CrossRef]
- Mushagalusa Kasali, F.; Ahadi Irenge, C.; Murhula Hamuli, P.; Birindwa Mulashe, P.; Murhula Katabana, D.; Mangambu Mokoso, J.D.D.; Mpiana, P.T.; Ntokamunda Kadima, J. Ethnopharmacological Survey on Treatment of Hypertension by Traditional Healers in Bukavu City, DR Congo. Evid.-Based Complement. Altern. Med. 2021, 2021, 6684855. [Google Scholar] [CrossRef]
- PubChem. CID 5471459: Niazimicin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5471459 (accessed on 22 June 2025).
- Park, J.B. Identification and quantification of a major anti-oxidant and anti-inflammatory phenolic compound found in basil, lemon thyme, mint, oregano, rosemary, sage, and thyme. Int. J. Food Sci. Nutr. 2011, 62, 577–584. [Google Scholar] [CrossRef]
- Pedersen, H.A.; Steffensen, S.K.; Christophersen, C.; Mortensen, A.G.; Jørgensen, L.N.; Niveyro, S.; de Troiani, R.M.; Rodríguez-Enríquez, R.J.; Barba-de la Rosa, A.P.; Fomsgaard, I.S. Synthesis and Quantitation of Six Phenolic Amides in Amaranthus spp. J. Agric. Food Chem. 2010, 58, 6306–6311. [Google Scholar] [CrossRef]
- PubChem. CID 25245053: N-Trans-Sinapoyltyramine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/25245053 (accessed on 22 June 2025).
- Zheng, Y.-K.; Su, B.-J.; Wang, Y.-Q.; Wang, H.-S.; Liao, H.-B.; Liang, D. New Tyramine- and Aporphine-Type Alkamides with NO Release Inhibitory Activities from Piper puberulum. J. Nat. Prod. 2021, 84, 1316–1325. [Google Scholar] [CrossRef]
- Ibrahim, S.R.M.; Abdallah, H.M.; Mohamed, G.A.; Deshmukh, S.K. Exploring Potential of Aspergillus sclerotiorum: Secondary Metabolites and Biotechnological Relevance. Mycol. Prog. 2022, 22, 8. [Google Scholar] [CrossRef]
- Phainuphong, P.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Sakayaroj, J.; Srimaroeng, C.; Ontawong, A.; Duangjai, A.; Muangnil, P.; Muanprasat, C. Asperidines A–C, pyrrolidine and piperidine derivatives from the soil-derived fungus Aspergillus sclerotiorum PSU-RSPG178. Bioorganic Med. Chem. 2018, 26, 4502–4508. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 145985497: Asperidine C. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/145985497 (accessed on 22 June 2025).
- Berdyshev, E.V.; Gorshkova, I.A.; Garcia, J.G.N.; Natarajan, V.; Hubbard, W.C. Quantitative analysis of sphingoid base-1-phosphates as bisacetylated derivatives by liquid chromatography–tandem mass spectrometry. Anal. Biochem. 2005, 339, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Saba, J.D. Sphingosine-1-Phosphate. In Encyclopedia of Signaling Molecules; Choi, S., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 5128–5137. [Google Scholar]
- PubChem. CID 5283559: C17 Sphingosine-1-phosphate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5283559 (accessed on 22 June 2025).
- Wang, Q.; Meng, Q.; Xu, F.; Chen, Q.; Ma, C.; Huang, L.; Li, G.; Luo, M. Comparative Metabolomics Analysis Reveals Sterols and Sphingolipids Play a Role in Cotton Fiber Cell Initiation. Int. J. Mol. Sci. 2021, 22, 11438. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Díaz, I.; Ramírez-Cisneros, M.Á.; Alvarez, L.; Sánchez-Carranza, J.N.; Columba-Palomares, M.C.; Silva-Guzmán, J.A.; Cruz-Sosa, F.; Bernabé-Antonio, A. Metabolites Profile of Extracts and Fractions of Erythroxylum mexicanum Kunth by UHPLC-QTOF-MS/MS and its Antibacterial, Cytotoxic and Nitric Oxide Inhibitory Activities. Chem. Biodivers. 2024, 21, e202301474. [Google Scholar] [CrossRef]
- PubChem. CID 443717: N-Methyl-2,3,7,8-tetramethoxy-5,6-dihydrobenzophenathridine-6-ethanoic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/443717 (accessed on 22 June 2025).
- Krane, B.D.; Fagbule, M.O.; Shamma, M.; Gözler, B. The Benzophenanthridine Alkaloids. J. Nat. Prod. 1984, 47, 1–43. [Google Scholar] [CrossRef]
- Maslennikova, V.A.; Abubakirov, N.K. An investigation of the glycosides of jute. V. Coroloside and desglucocoroloside. Chem. Nat. Compd. 1975, 11, 553–554. [Google Scholar] [CrossRef]
- PubChem. CID 15559187: Desglucocoroloside. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/15559187 (accessed on 22 June 2025).
- Ain, Q.U.; Saleem, M.; Nazir, M.; Riaz, N.; Tousif, M.I.; Tauseef, S.; Hassan, L.; Zengin, G.; Sharifi-Rad, M.; Shah, S.A.A. Secondary metabolite profiling, antioxidant capacity, enzyme inhibitory potential and in silico studies of Launaea intybacea (Jacq.) Beauverd: A multifunctional approach to probe into the new nutraceuticals. J. Mol. Struct. 2023, 1294, 136480. [Google Scholar] [CrossRef]
- Hara, A.; Taketomp, T. Chemical Properties and Stereoisomerism of Heterogeneous Long Chain Bases in Lysosphingolipids by Positive Ion Fast Atom Bombardment Mass Spectrometry and Carbon-13 NMR Spectroscopy 1. J. Biochem. 1986, 100, 415–423. [Google Scholar] [CrossRef]
- Ines, C.; Parra-Lobato, M.C.; Paredes, M.A.; Labrador, J.; Gallardo, M.; Saucedo-Garcia, M.; Gavilanes-Ruiz, M.; Gomez-Jimenez, M.C. Sphingolipid Distribution, Content and Gene Expression During Olive-Fruit Development and Ripening. Front. Plant Sci. 2018, 9, 28. [Google Scholar] [CrossRef]
- Jala, R.C.R.; Vudhgiri, S.; Kumar, C.G. A comprehensive review on natural occurrence, synthesis and biological activities of glycolipids. Carbohydr. Res. 2022, 516, 108556. [Google Scholar] [CrossRef]
- PubChem. CID 440147: D-Glucosyldihydrosphingosine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/440147 (accessed on 22 June 2025).
- Aritomi, M.; Komori, T.; Kawasaki, T. Flavonol glycosides in leaves of Spinacia oleracea. Phytochemistry 1985, 25, 231–234. [Google Scholar] [CrossRef]
- Machado, P.G.; Londero, D.S.; Farias, C.A.A.; Pudenzi, M.A.; Barcia, M.T.; Ballus, C.A. Guabijú (Myrcianthes pungens): A comprehensive evaluation of anthocyanins and free, esterified, glycosylated, and insoluble phenolic compounds in its peel, pulp, and seeds. Food Chem. 2024, 432, 137296. [Google Scholar] [CrossRef]
- PubChem. CID 21722022: Spinatoside. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/21722022 (accessed on 22 June 2025).
- Jakupovic, J.; Paredes, L.; Bohlmann, F.; Watson, L. Prenyl flavanes from Marshallia species. Phytochemistry 1988, 27, 3273–3275. [Google Scholar] [CrossRef]
- Lv, H.-W.; Wang, Q.-L.; Luo, M.; Zhu, M.-D.; Liang, H.-M.; Li, W.-J.; Cai, H.; Zhou, Z.-B.; Wang, H.; Tong, S.-Q.; et al. Phytochemistry and pharmacology of natural prenylated flavonoids. Arch. Pharmacal Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Mohd Hazli, U.H.A.; Hwong, C.S.; Abdul-Aziz, A.; Mat-Junit, S.; Leong, K.H.; Kong, K.W. Effects of Alternanthera sessilis Red leaf extracts on hydrogen peroxide-induced oxidative stress in HepG2 cells and identification of phytochemicals using HPLC-QToF-MS/MS. S. Afr. J. Bot. 2022, 151, 440–450. [Google Scholar] [CrossRef]
- PubChem. CID 44257161: 3,4,7-Trihydroxy-5-methoxy-8-prenylflavan 7-O-beta-D-glucopyranoside. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/44257161 (accessed on 22 June 2025).
- Tan, H.M.; Leong, K.H.; Song, J.; Chew, L.Y.; Mohd-Sufian, N.S.F.; Kong, K.W. Antioxidant and LC-QToF-MS/MS analysis of polyphenols in polar and non-polar extracts from Strobilanthes crispus and Clinacanthus nutans. Int. Food Res. J. 2020, 27, 903–914. [Google Scholar]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS comprehensive classification system for lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 137323927: 1-(2-Methoxy-heneicosanyl)-sn-glycero-3-phosphoethanolamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/137323927 (accessed on 22 June 2025).
- PubChem. CID 100946793: 2-[2-[[(2R)-2,3-bis(docosa-9,11-diynylperoxy)propoxy]-hydroxyphosphoryl]oxyethyl-(carboxymethyl)amino]acetic acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/100946793 (accessed on 22 June 2025).
- Singh, A.; Markowitz, M.; Shoen, P.E.; Costellanos, C. Synthesis and characterization of self-organized microstructures with chemically active surfaces and evaluation of their technical utility. R. Soc. Chem. Spec. Publ. 1999, 235, 14–23. [Google Scholar]
- Gottlieb, O.R.; Magalhaes, M.T. Isolation of Piperonylic Acid from Ocotea pretiosa. Nature 1958, 182, 742–743. [Google Scholar] [CrossRef]
- Lee, D.; Lim, J.; Woo, K.C.; Kim, K.T. Piperonylic acid stimulates keratinocyte growth and survival by activating epidermal growth factor receptor (EGFR). Sci. Rep. 2018, 8, 162. [Google Scholar] [CrossRef]
- PubChem. CID 7196: Piperonylic Acid. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/7196 (accessed on 22 June 2025).
- Clarke, C.; Francis, F. Über einige Derivate dee Triaceton-amins. Berichte Der Dtsch. Chem. Ges. Zu Berl. 1912, 45, 2060–2065. [Google Scholar] [CrossRef]
- Mailey, E.A.; Day, A.R. Synthesis of Derivatives of Alkylated and Arylated Piperidones and Piperidinols. J. Org. Chem. 1957, 22, 1061–1065. [Google Scholar] [CrossRef]
- Yang, S.; Huang, J.; Tan, W.; Xia, X.; Gan, D.; Ren, Y.; Su, H.; Xiang, M. Xiaoyankangjun tablet alleviates dextran sulfate sodium-induced colitis in mice by regulating gut microbiota and JAK2/STAT3 pathway. Nat. Prod. Bioprospecting 2024, 14, 44. [Google Scholar] [CrossRef]
- PubChem. CID 75471: 2,2,6,6-Tetramethyl-4-piperidinol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/75471 (accessed on 22 June 2025).
- Euler, A.C.v. Über dem Lignin nahestehende Harne und Gerbsäuren der Fichtennadeln. Chem. Zentralblatt 1922, 93, 824–826. [Google Scholar]
- Ziting, L.; Xiaoqing, W.; Kemei, W.; Yachao, B.; Aimin, G.; Huang, L.; Bin, L.; Jun, Z. Systematical accumulating and regulating evaluations of leaf functional metabolites in geographically isolated edible medicinal plants of Piper sarmentosum. J. Plant Physiol. 2025, 310, 154512. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 5280536: 4-Hydroxy-3-methoxycinnamaldehyde. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280536 (accessed on 22 June 2025).
- Gao, E.; Zhou, Z.-Q.; Zou, J.; Yu, Y.; Feng, X.-L.; Chen, G.-D.; He, R.-R.; Yao, X.-S.; Gao, H. Bioactive Asarone-Derived Phenylpropanoids from the Rhizome of Acorus tatarinowii Schott. J. Nat. Prod. 2017, 80, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, L.L.; Shorygina, N.N.; Lopatin, B.V. Nitration of the lignin model compounds 3-(3,4-dimethoxyphenyl)-1-propanol and 3-(4-hydroxy-3-methoxyphenyl)-1-propanol. Bull. Acad. Sci. USSR Div. Chem. Sci. 1964, 13, 1163–1166. [Google Scholar] [CrossRef]
- PubChem. CID 77528: 3-(3,4-Dimethoxyphenyl)-1-propanol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/77528 (accessed on 22 June 2025).
- Hegnauer, R. Piperaceae. In Chemotaxonomie der Pflanzen: Eine Übersicht über die Verbreitung und die systematische Bedeutung der Pflanzenstoffe; Hegnauer, R., Ed.; Birkhäuser: Basel, Switzerland, 1990; pp. 226–240. [Google Scholar]
- Saiki, Y.; Saito, T.; Sasaki, H.; Fukushima, S. Gas-chromatography of natural volatile oils. I. Volatile oil of the Chinese drug chichin. Yakugaku Zasshi 1967, 87, 1524–1528. [Google Scholar] [CrossRef][Green Version]
- Hu, H.; Liu, G.; Li, Y. The isolation strategy and chemical analysis of oil cells from Asari Radix et Rhizoma. Plant Methods 2024, 20, 72. [Google Scholar] [CrossRef]
- PubChem. CID 596894: Kakuol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/596894 (accessed on 22 June 2025).
- Erdtman, H.; Robinson, R. Preparation of homopiperonal. J. Chem. Soc. 1933, 0, 1530–1531. [Google Scholar] [CrossRef]
- Yin, X.Y.; Luo, Y.; Jin, L.; Xu, F.H.; Luo, Y.M. Determination of Safrolglycol in by HPLC. Chin. J. Exp. Tradit. Med. Formulae 2012, 18, 113–115. [Google Scholar]
- PubChem. CID 197807: Safrolglycol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/97807 (accessed on 22 June 2025).
- Singh, R.K.; Rajput, R.; Singh, H.; Bharadwaj, P. Lancea tibetica Hook.f. & Thomson: Ethnobotany, Phytochemistry, and Pharmacology. In Immunity Boosting Medicinal Plants of the Western Himalayas; Sharma, A., Nayik, G.A., Eds.; Springer Nature: Singapore, 2023; pp. 309–316. [Google Scholar]
- Hodges, R.; Porte, A.L. The structure of loliolide: A terpene from lolium perenne. Tetrahedron 1964, 20, 1463–1467. [Google Scholar] [CrossRef]
- Huang, M.X.; Cai, Y.J.; Wang, J.; Wang, W.; Chen, L.; Han, P.D.; Lius, S.B. Chemical Constituents of Clinacanthus nutans. Chin. J. Exp. Tradit. Med. Formulae 2016, 22, 55–59. [Google Scholar]
- Lee, S.S.; Lee, S.H.; Kim, S.Y.; Lee, G.Y.; Han, S.Y.; Lee, B.H.; Yoo, Y.C. Endarachne binghamiae Ameliorates Hepatic Steatosis, Obesity, and Blood Glucose via Modulation of Metabolic Pathways and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 5103. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 100332: Loliolide. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/100332 (accessed on 22 June 2025).
- Dalko, M.; Gilbert, L. Use of Substituted Dimethoxyhydroxyphenylalkyl Derivatives as Preservative, Preserving Method, Compounds and Composition. U.S. Patent WO2012080153, 5 July 2016. [Google Scholar]
- Kratzl, K.; Schweers, W. The paper-chromatographic separation of monomeric ethanolysis products of lignin, III. Chem. Berichte 1956, 89, 186–192. [Google Scholar] [CrossRef]
- San-Emeterio, L.M.; Jiménez-Morillo, N.T.; Reina, L.; Vinciguerra, V.; Menéndez, P.; González-Pérez, J.A. Pyrolysis carbon compound-specific isotope analysis (Py-CSIA) of Eucalyptus spp. bark and the extracted lignin. J. Anal. Appl. Pyrolysis 2023, 170, 105896. [Google Scholar] [CrossRef]
- PubChem. CID 57382302: Syringylacetone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/57382302 (accessed on 22 June 2025).
- Klein, E.; Rojahn, W. Isolierung, struktur und synthese von dhelwangin. Tetrahedron Lett. 1969, 10, 2279–2280. [Google Scholar] [CrossRef]
- Zhen, Y.Q.; Tian, W.; Wang, Y.C.; Jiang, G.Z.; Niu, L.Y. Simultaneous Determination of Caffeic Acid, Acteoside, Isoacteoside and Pogostone in Pogostemonis Herba Formula Granules by HPLC with Changing Wavelength. Chin. J. Exp. Tradit. Med. Formulae 2017, 23, 79–83. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, P.; Zhang, X.; Fu, H.; Fu, C. Uncovering the pharmacological mechanisms of Patchouli essential oil for treating ulcerative colitis. J. Ethnopharmacol. 2025, 336, 118737. [Google Scholar] [CrossRef]
- PubChem. CID 54695756: Dhelwangin/Pogostone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/54695756 (accessed on 22 June 2025).
- Mazza, G. Gas chromatographic and mass spectrometric studies of the constituents of the rhizome of calamus: I. The volatile constituents of the essential oil. J. Chromatogr. A 1985, 328, 179–194. [Google Scholar] [CrossRef]
- Patra, A.; Mitra, A.K. Constituents of Acorus calamus: Structure of Acoramone. Carbon-13 NMR Spectra of Cis- and Trans-Asarone. J. Nat. Prod. 1981, 44, 668–669. [Google Scholar] [CrossRef]
- PubChem. CID 3083746: Acoramone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3083746 (accessed on 22 June 2025).
- Shao, J.; Yanxue, Z.; Yuanyuan, W.; Guohui, L.; Jinxia, W.; Wenbo, C.; Li, Y. Rapid classification and identification of chemical compositions of Pu-zhi-hui-ling decoction by UHPLC-Q-Orbitrap HRMS. Nat. Prod. Res. 2025, 39, 2453–2462. [Google Scholar] [CrossRef]
- Chen, Y. A kind of Method for the Intermediate 2,4,5-Trimethoxypropiophenone of Synthesizing α-Asarone. CN101891604A, 24 November 2010. Available online: https://eureka.patsnap.com/patent-CN101891604A (accessed on 22 June 2025).
- PubChem. CID 700861: Isoacoramone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/700861 (accessed on 22 June 2025).
- Fumarola, F. Process for extraction of the active biological principles from the ether extract of male fern. GB2075834A, 1926. [Google Scholar]
- Ingallina, C.; Capitani, D.; Mannina, L.; Carradori, S.; Locatelli, M.; Di Sotto, A.; Di Giacomo, S.; Toniolo, C.; Pasqua, G.; Valletta, A.; et al. Phytochemical and biological characterization of Italian “sedano bianco di Sperlonga” Protected Geographical Indication celery ecotype: A multimethodological approach. Food Chem. 2020, 309, 125649. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 122841: Aspidinol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/122841 (accessed on 22 June 2025).
- Chen, G.Y.; Wang, Y.F.; Yu, X.B.; Liu, X.Y.; Chen, J.Q.; Luo, J.; Tao, Q.W. Network Pharmacology-Based Strategy to Investigate the Mechanisms of Cibotium barometz in Treating Osteoarthritis. Evid. Based. Complement. Altern. Med. 2022, 2022, 1826299. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wu, Y.; Yin, H.; Du, L.; Chen, C. Senkyunolide I: A Review of Its Phytochemistry, Pharmacology, Pharmacokinetics, and Drug-Likeness. Molecules 2023, 28, 3636. [Google Scholar] [CrossRef]
- Tsuchida, T.; Kobayashi, M.; Kaneko, K.; Mitsuhashi, H. Studies on the Constituents of Umbelliferae Plants. XVI. Isolation and Structures of Three New Ligustilide Derivatives from Angelica acutiloba. Chem. Pharm. Bull. 1987, 35, 4460–4464. [Google Scholar] [CrossRef]
- PubChem. CID 11521428: Senkyunolide I. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11521428 (accessed on 22 June 2025).
- Shang, Y.; Zhu, Y.; Zhou, S.; Liu, Y.; Wei, S.; Zhou, H.; Jiang, Y.; Wang, Y.; Geng, T.; Wang, Q.; et al. A UPLC-MS/MS coupled with GC-MS method for quantification of twenty-one chemical ingredients from Suxiao Jiuxin pill in multiple tissue of rat and its application to tissue distribution study. J. Pharm. Biomed. Anal. 2025, 252, 116461. [Google Scholar] [CrossRef]
- Karimov, A.M.; Botirov, E.K. Structural Diversity and State of Knowledge of Flavonoids of the Scutellaria L. Genus. Russ. J. Bioorganic Chem. 2018, 43, 691–711. [Google Scholar] [CrossRef]
- Miyaichi, Y.; Imoto, Y.; Tomimori, T.; Namba, T. Studies on the Nepalese Crude Drugs. IX. On the Flavonoid Constituents of the Root of Scutellaria scandens BUCH.-HAM. ex D. DON. Chem. Pharm. Bull. 1988, 36, 2371–2376. [Google Scholar] [CrossRef]
- PubChem. CID 9816931: Dihydrobaicalein. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/9816931 (accessed on 22 June 2025).
- Gao, S.; Li, T.; Li, Z.R.; Liao, B.; Zhao, M.; Zhou, C.; Luo, D.; Jia, R.B. Gradient ethanol extracts of Coreopsis tinctoria buds: Chemical and in vitro fermentation characteristics. Food Chem. 2025, 464, 141894. [Google Scholar] [CrossRef] [PubMed]
- Levene, P.A.; Jacobs, W.A. On sphingosine. Chem. Zentralblatt 1912, 83, 527. [Google Scholar] [CrossRef]
- Mamode Cassim, A.; Grison, M.; Ito, Y.; Simon-Plas, F.; Mongrand, S.; Boutté, Y. Sphingolipids in plants: A guidebook on their function in membrane architecture, cellular processes, and environmental or developmental responses. FEBS Lett. 2020, 594, 3719–3738. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 656816: C16 Sphinganine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/656816 (accessed on 22 June 2025).
- Zhang, D. Characterization of Sphingolipid Biosynthesis and Modification in Plants. Ph.D. Thesis, University of Nebraska, Lincoln, NE, USA, 2021. [Google Scholar]
- Baba, K.; Xiao, Y.-Q.; Taniguchi, M.; Ohishi, H.; Kozawa, M. Isocoumarins from Coriandrum sativum. Phytochemistry 1991, 30, 4143–4146. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, S.; Huang, X.; Luo, J.; Wang, J.; Kong, L. Supercritical fluid extraction of Coriandrum sativum and subsequent separation of isocoumarins by high-speed counter-current chromatography. Food Chem. 2009, 117, 504–508. [Google Scholar] [CrossRef]
- Ouelbani, R.; Bensari, S.; Mouas, T.N.; Khelifi, D. Ethnobotanical investigations on plants used in folk medicine in the regions of Constantine and Mila (North-East of Algeria). J. Ethnopharmacol. 2016, 194, 196–218. [Google Scholar] [CrossRef]
- PubChem. CID 131752232: Coriandrone B. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/131752232 (accessed on 22 June 2025).
- PubChem. CID 6430518: N-(14-Methylhexadecanoyl)pyrrolidine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6430518 (accessed on 22 June 2025).
- Li, Y.; Wang, X.; Sa, Y.; Li, L.; Wang, W.; Yang, L.; Ding, S.; Wilson, G.; Yang, Y.; Zhang, Y.; et al. A comparative UHPLC-QTOF-MS/MS-based metabolomics approach reveals the metabolite profiling of wolfberry sourced from different geographical origins. Food Chem. X 2024, 21, 101221. [Google Scholar] [CrossRef]
- Tofern, B.; Mann, P.; Kaloga, M.; Jenett-Siems, K.; Witte, L.; Eich, E. Aliphatic pyrrolidine amides from two tropical convolvulaceous species. Phytochemistry 1999, 52, 1437–1441. [Google Scholar] [CrossRef]
- Nakatani, N.; Inatani, R.; Fuwa, H. Structures and Syntheses of Two Phenolic Amides from Piper nigrum L. Agric. Biol. Chem. 2014, 44, 2831–2836. [Google Scholar] [CrossRef]
- Shukla, R.; Singh, S.; Singh, A.; Misra, K. Two pronged approach for prevention and therapy of COVID-19 (Sars-CoV-2) by a multi-targeted herbal drug, a component of ayurvedic decoction. Eur. J. Integr. Med. 2021, 43, 101268. [Google Scholar] [CrossRef]
- PubChem. CID 5280537: N-trans-feruloiltramina. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280537 (accessed on 22 June 2025).
- Khan, Z.; Hong, S.-M.; Lee, J.-W.; Moon, E.-Y.; Huh, J.; Chang, K.-A.; Kim, S.Y. Potential of N-trans feruloyl tyramine from Lycium barbarum fruit extract on neurogenesis and neurotrophins; targeting TrkA/ERK/CREB signaling pathway. J. Funct. Foods 2021, 80, 104432. [Google Scholar] [CrossRef]
- Carter, H.E.; Celmer, W.D.; Lands, W.E.M.; Mueller, K.L.; Tomizawa, H.H. Biochemistry of the sphingolipides: VIII. Occurrence of a long chain base in plant phosphatides. J. Biol. Chem. 1954, 206, 613–623. [Google Scholar] [CrossRef]
- Glenz, R.; Kaiping, A.; Gopfert, D.; Weber, H.; Lambour, B.; Sylvester, M.; Froschel, C.; Mueller, M.J.; Osman, M.; Waller, F. The major plant sphingolipid long chain base phytosphingosine inhibits growth of bacterial and fungal plant pathogens. Sci. Rep. 2022, 12, 1081. [Google Scholar] [CrossRef]
- PubChem. CID 122121: Phytosphingosine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/122121 (accessed on 22 June 2025).
- Zhu, H.; Duan, Y.; Qin, K.; Jin, J.; Liu, X.; Cai, B. A UPLC-Q-TOF-MS-Based Metabolomics Approach to Screen out Active Components in Prepared Rhubarb for Its Activity on Noxious Heat Blood Stasis Syndrome. Front. Pharmacol. 2022, 13, 907831. [Google Scholar] [CrossRef]
- Asano, N.; Tomioka, E.; Kizu, H.; Matsui, K. Sugars with nitrogen in the ring isolated from the leaves of Morus bombycis. Carbohydr. Res. 1994, 253, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Ogawa, K.; Higuchi, O.; Kimura, T.; Miyazawa, T.; Hori, M. Determination of iminosugars in mulberry leaves and silkworms using hydrophilic interaction chromatography-tandem mass spectrometry. Anal. Biochem. 2010, 404, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Parida, I.S.; Soo, T.; Nakagawa, K. A comprehensive review on the production, pharmacokinetics and health benefits of mulberry leaf iminosugars: Main focus on 1-deoxynojirimycin, d-fagomine, and 2-O-ɑ-d-galactopyranosyl-DNJ. Crit. Rev. Food Sci. Nutr. 2023, 63, 3468–3496. [Google Scholar] [CrossRef] [PubMed]
- PubChem. CID 5317441: Galactosyl-DNJ. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5317441 (accessed on 22 June 2025).
- Serino, E.; Pollastro, F.; Luciano, P.; Touboul, D.; Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Analytically Unsupervised Metabolomic Profile of the Premium Malgasy Pepper Voatsipérifery (Piper borbonense): Identification of Marker Components. J. Agric. Food Chem. 2025, 73, 9854–9866. [Google Scholar] [CrossRef]
- Siddiqui, B.S.; Gulzar, T.; Begum, S.; Afshan, F.; Sattar, F.A. Two New Insecticidal Amide Dimers from Fruits of Piper nigrum Linn. Helv. Chim. Acta 2004, 87, 660–666. [Google Scholar] [CrossRef]
- PubChem. CID 12575258: Pipercitine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/12575258 (accessed on 22 June 2025).
- Marquez-Flores, Y.K.; Ayala-Velasco, J.; Correa-Basurto, J.; Estrada-Perez, A.; Melendez-Camargo, M.E. Peperomia campylotropa A.W. Hill: Ethnobotanical, Phytochemical, and Metabolomic Profile Related to Its Gastroprotective Activity. Molecules 2025, 30, 772. [Google Scholar] [CrossRef]
- Adzhar Kamarulzaman, F.; Shaari, K.; Siong Hock Ho, A.; Haji Lajis, N.; Hwang Teo, S.; Boon Lee, H. Derivatives of Pheophorbide-a and Pheophorbide-b from Photocytotoxic Piper penangense Extract. Chem. Biodivers. 2011, 8, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Willstätter, R.; Utzinger, M. Untersuchungen über Chlorophyll. über die ersten Umwandlungen des Chlorophylls. Justus Liebigs Ann. Der Chem. 1911, 382, 129–194. [Google Scholar] [CrossRef]
- PubChem. CID 253193: Pheophorbide A. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/253193 (accessed on 22 June 2025).
- Chen, K.; Li, Y.; Zhou, C.; Wang, Y.; Zalán, Z.; Cai, T. Inhibitory effects of chlorophyll pigments on the bioaccessibility of β-carotene: Influence of chlorophyll structure and oil matrix. Food Chem. 2024, 451, 139457. [Google Scholar] [CrossRef] [PubMed]
- Djoumbou Feunang, Y.; Eisner, R.; Knox, C.; Chepelev, L.; Hastings, J.; Owen, G.; Fahy, E.; Steinbeck, C.; Subramanian, S.; Bolton, E.; et al. ClassyFire: Automated chemical classification with a comprehensive, computable taxonomy. J. Cheminformatics 2016, 8, 61. [Google Scholar] [CrossRef]
- Kind, T.; Fiehn, O. Advances in structure elucidation of small molecules using mass spectrometry. Bioanal. Rev. 2010, 2, 23–60. [Google Scholar] [CrossRef]
- Scalbert, A.; Andres-Lacueva, C.; Arita, M.; Kroon, P.; Manach, C.; Urpi-Sarda, M.; Wishart, D. Databases on food phytochemicals and their health-promoting effects. J. Agric. Food Chem. 2011, 59, 4331–4348. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies—Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis. Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Dunn, W.B.; Wilson, I.D.; Nicholls, A.W.; Broadhurst, D. The importance of experimental design and QC samples in large-scale and MS-driven untargeted metabolomic studies of humans. Bioanalysis 2012, 4, 2249–2264. [Google Scholar] [CrossRef]
- Dettmer, K.; Aronov, P.A.; Hammock, B.D. Mass spectrometry-based metabolomics. Mass Spectrom. Rev. 2007, 26, 51–78. [Google Scholar] [CrossRef]
- Bino, R.J.; Hall, R.D.; Fiehn, O.; Kopka, J.; Saito, K.; Draper, J.; Nikolau, B.J.; Mendes, P.; Roessner-Tunali, U.; Beale, M.H.; et al. Potential of metabolomics as a functional genomics tool. Trends Plant Sci. 2004, 9, 418–425. [Google Scholar] [CrossRef]
- Yin, X.; Struik, P.C.; Kropff, M.J. Role of crop physiology in predicting gene-to-phenotype relationships. Trends Plant Sci. 2004, 9, 426–432. [Google Scholar] [CrossRef]
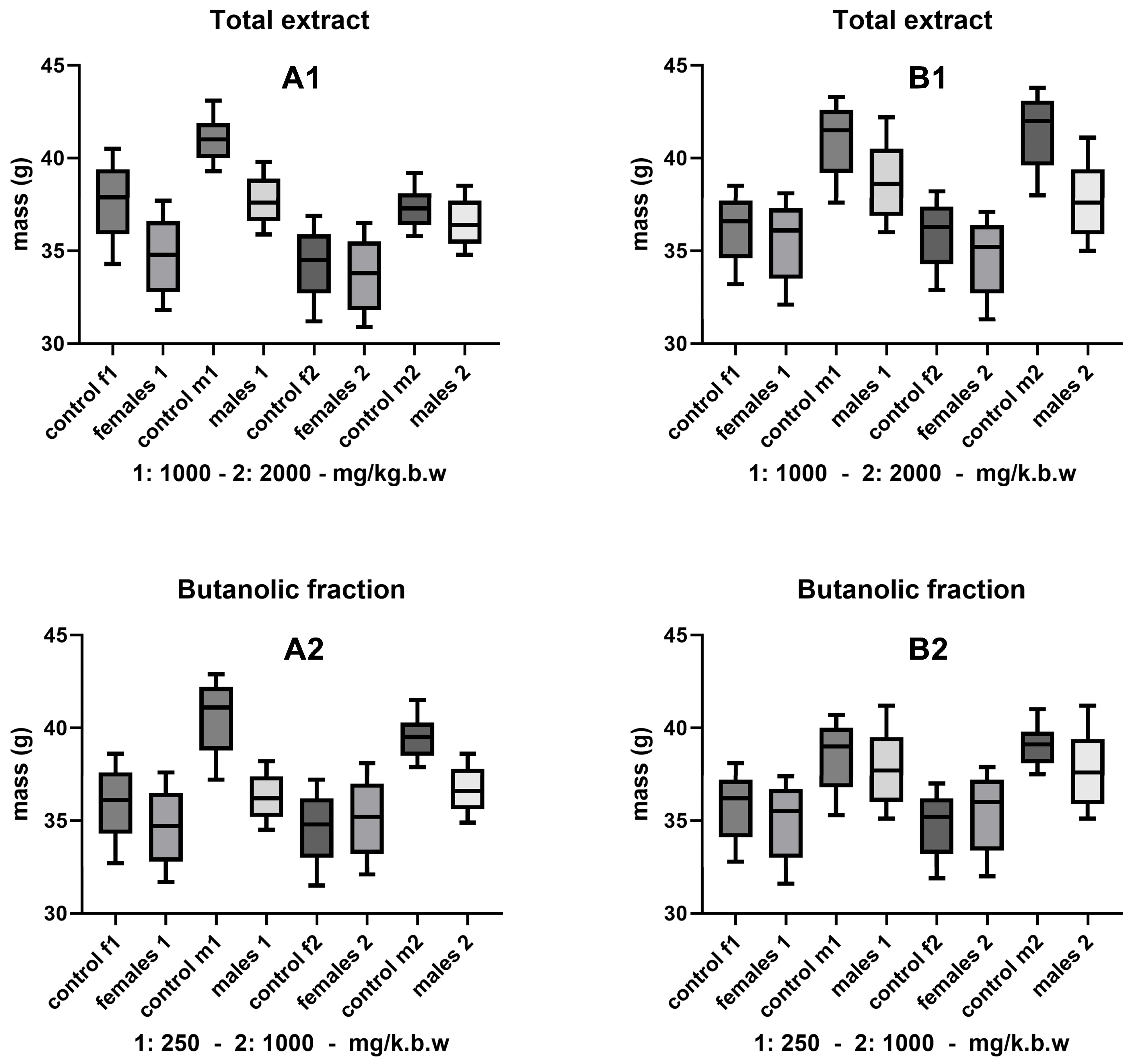
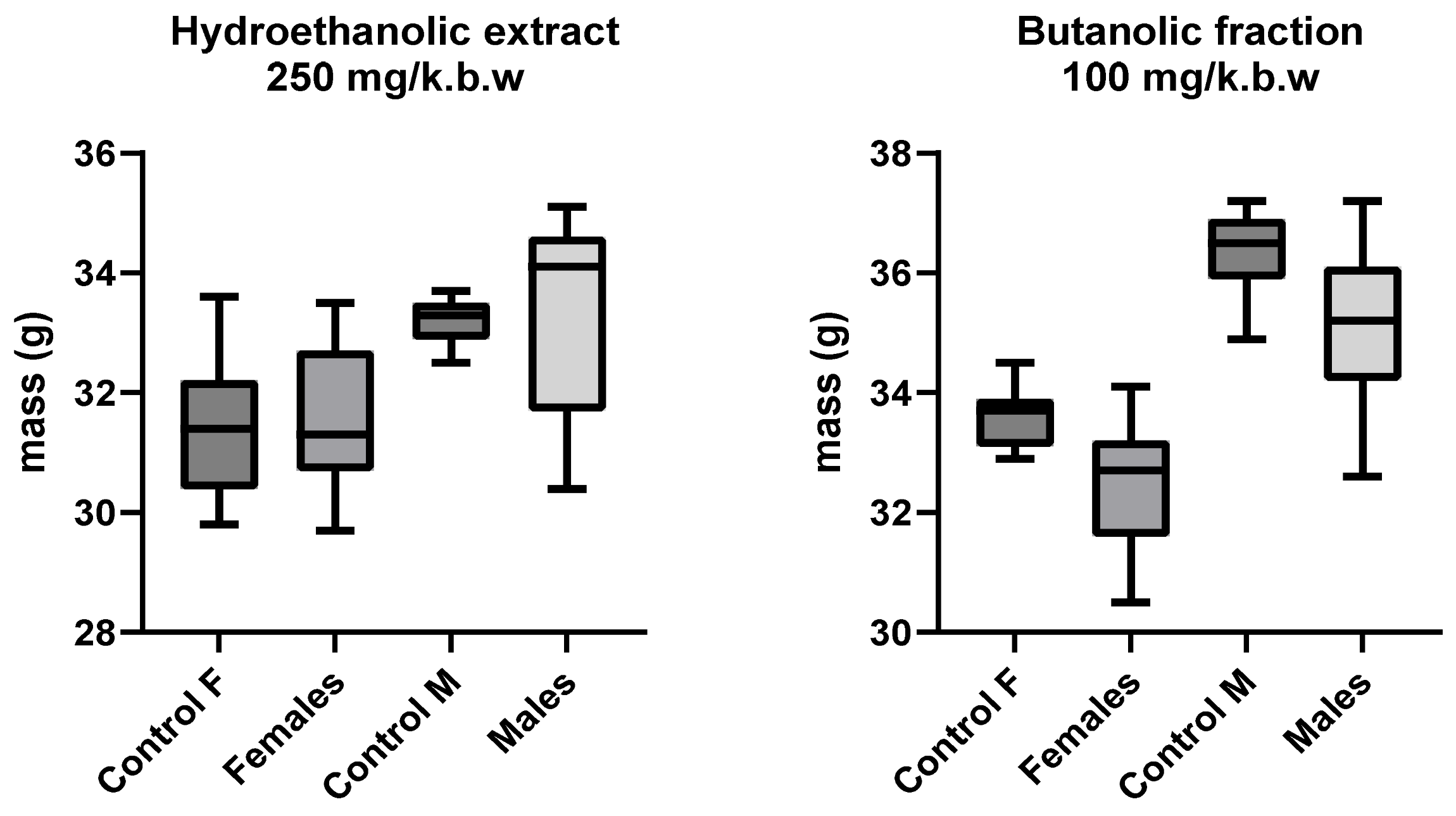
| Dose | Route of Administration | Substance | mg/kg bw | Number of Animals * | Age (Weeks) |
|---|---|---|---|---|---|
| Unique | Oral e intraperitoneal | Total extract | 2000 | 8 | 8–9 |
| 1000 | 8 | ||||
| Butanolic fraction | 1000 | 8 | |||
| 250 | 8 | ||||
| Repeated | Oral | Total extract | 250 | 8 | |
| Butanolic fraction | 100 | 8 | |||
| Control | Oral and intraperitoneal | Vehicle | - | 8 |
| Metabolite Class (Test) (*, **) | Total Extract | Butanolic Fraction | Control | |
|---|---|---|---|---|
| A (−) | B (+) | |||
| Alkaloids (ammonium reineckate) * | + | + | − | + |
| Alkaloids (Dragendorff) * | + | + | − | + |
| Alkaloids (Mayer) * | + | + | − | + |
| Alkaloids (Valser) * | + | + | − | + |
| Alkaloids (Wagner) * | + | + | ||
| Cardiotonic glycosides (Kedde) * | − | − | − | + |
| Cardiotonic glycosides (Vanillin/H3PO4/Kedde) ** | − | − | − | + |
| Coumarins (fluorescence) * | − | − | − | + |
| Coumarins (Vainillin/H3PO4/FeCl3/HCl) ** | + | + | − | + |
| Flavonoids (NP-PEG) ** | + | + | − | + |
| Flavonoids (Shinoda) * | + | + | − | + |
| Leucoanthocyanin (Rosenhein) * | − | − | − | + |
| Naphtho- and/or anthraquinones (Borntränger-Krauss) * | + | − | − | + |
| Naphtho- and/or anthraquinones (KOH/EtOH) ** | + | − | − | + |
| Phenols (ferric chloride) * | + | + | ||
| Proanthocyanidins (BuOH/HCl) * | + | + | − | + |
| Saponins (Anisaldheido/H2SO4) ** | − | − | − | + |
| Saponins (foam) * | − | − | − | + |
| Saponins (Hemolysis) * | − | − | − | + |
| Steroids and/or triterpenes (Liebermann-Burchard) * | + | − | − | + |
| Steroids and/or triterpenes (Vanillin) ** | + | − | ||
| Tannins (gelatin, salt and ferric chloride) * | + | + | − | + |
| Tannins (potassium ferrocyanide and ferric chloride) ** | + | + | − | + |
| Terpenic lactones (ferric hydroxamate) * | + | − | − | + |
| Terpenic lactones (Vainillin/H3PO4/FeCl3/HCl) ** | + | − | − | + |
| Extract/Fraction | Parameters | 254 nm | 280 nm | 350 nm |
|---|---|---|---|---|
| Hydroethanolic extract | Number of peaks | 191 | 180 | 108 |
| Total Area * | 8.30 × 109 | 8.10 × 109 | 2.40 × 109 | |
| Butanolic fraction | Number of peaks | 77 | 77 | 47 |
| Total area * | 5.10 × 109 | 4.80 × 109 | 1.40 × 109 |
| tR min | m/z | Adduct | Formula | Tentative Identification | Metabolite Class | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Theoric | Error ppm | Common Name | IUPAC Name | ID Level | |||||
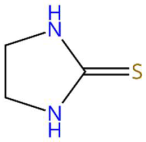
| 20.12 | 125.0149 | 102.0252 | 5 | M+Na | C3H6N2S | Ethylenethiourea | Imidazolidine-2-thione | 3 | Sulphur and heterocyclic compound (imidazole) | [68,69,70,71] |
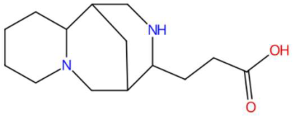
| 15.19 | 235.1810 | 234.1732 | 1 | M+H | C14H22N2O | Lupanyl acid | 3-(7,11-diazatricyclo[7.3.1.0~2,7~]tridecan-10-yl)propanoic acid | 3 | Triterpenoid derivative (lupanoid) | [72,73,74] |
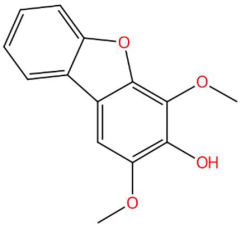
| 20.12 | 243.0683 | 244.0736 | 8 | M−H | C14H12O4 | Eriobofuran | 2,4-dimethoxydibenzofuran-3-ol | 3 | Dibenzofuran derivative (furanopheno) | [75,76,77] |
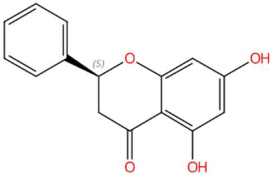
| 18.16 | 255.0682 | 256.0736 | 7 | M−H | C15H12O4 | Pinocembrin | (2S)-5,7-dihydroxy-2-phenyl-2,3-dihydrochromen-4-one | 3 | Flavonoid (flavanonol) | [78,79,80,81] |
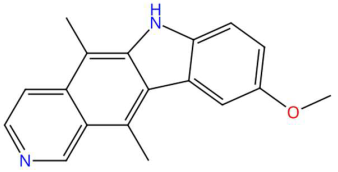
| 23.63 | 277.1339 | 276.1263 | 1 | M+H | C18H16N2O | 9-Methoxyellipticine | 9-methoxy-5,11-dimethyl-6H-pyrido[4,3-b]carbazole | 3 | Alkaloid (pyrido carbazole) | [82,83,84,85] |
| 18.06 | 324.1265 | 341.1263 | 9 | M+H−H2O | C19H19NO5 | 4-Hydroxynornantenine | 18,19-dimethoxy-5,7-dioxa-13-azapentacyclo[10.7.1.0~2,10~.0~4,8~.0~16,20~]icosa-1(20),2,4(8),9,16,18-hexaen-15-ol | 3 | Alkaloid indólico | [86,87,88] |
| 18.16 | 329.1425 | 330.1467 | 9 | M−H | C19H22O5 | 5-Deoxystrigol | (3E,3aR,8bS)-8,8-dimethyl-3-[[(2R)-4-methyl-5-oxo-2H-furan-2-yl]oxymethylidene]-3a,4,5,6,7,8b-hexahydroindeno[1,2-b]furan-2-one | 3 | Strigolactone and indenofuran | [89,90,91] |
| 22.46 | 336.0901 | 301.1215 | 3 | M+Cl | C19H15N3O | Dehydroevodiamine | 21-methyl-3,13,21-triazapentacyclo[11.8.0.0~2,10~.0~4,9~.0~15,20~]henicosa-1,3,5,7,9,15,17,19-octaen-14-one | 3 | Alkaloid (quinazoline) | [92,93,94,95] |
| 21.83 | 340.1218 | 357.1246 | 1 | M+H−H2O | C16H23NO6S | Niazimicin | O-ethyl N-[[4-(3,4,5-trihydroxy-6-methyloxan-2-yl)oxyphenyl]methyl]carbamothioate | 3 | Sulphur compound | [96,97,98] |
| 17.73 | 342.1371 | 343.1420 | 7 | M−H | C19H21NO5 | Sinapoyltyramine | (E)-3-(4-hydroxy-3,5-dimethoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]prop-2-enamide | 3 | Phenylpropanoid derivative | [99,100,101,102] |
| 21.68 | 366.2182 | 331.2511 | 7 | M+Cl | C21H33NO2 | Asperidine C | (2R,4S,5R,6S)-2-nonyl-6-phenyl-7-oxa-1-azabicyclo[3.2.1]octan-4-ol | 3 | Alkaloid (azabicyclic) | [103,104,105] |
| 19.01 | 400.2030 | 365.2331 | 1 | M+Cl | C17H36NO5P | C17 Sphingosine-1-phosphate | [(E,2S,3R)-2-amino-3-hydroxyheptadec-4-enyl] dihydrogen phosphate | 3 | Sphingolipid | [106,107,108,109] |
| 23.89 | 422.1639 | 423.1682 | 7 | M−H | C24H25NO6 | N-methyl tetramethoxy chrysoaranoic acid * | 2-[(6S)-2,3,7,8-tetramethoxy-5-methyl-6H-benzo[c]phenanthridin-6-yl]acetic acid | 3 | Alkaloid (benzo[c]phenanthridine) | [110,111,112] |
| 34.69 | 485.2924 | 504.3087 | 4 | M−H−H2O | C29H44O7 | Desglucocoroloside | 3-[3-(4,5-dihydroxy-6-methyloxan-2-yl)oxy-14-hydroxy-10,13-dimethyl-1,2,3,4,5,6,7,8,9,11,12,15,16,17-tetradecahydrocyclopenta[a]phenanthren-17-yl]-2H-furan-5-one | 3 | Cardenolide glycoside | [113,114,115] |
| 34.67 | 502.3178 | 463.3509 | 7 | M+K | C24H49NO7 | D-glucosyldihydrosphingosine | (2R,3R,4S,5S,6R)-2-(2-amino-3-hydroxyoctadecoxy)-6-(hydroxymethyl)oxane-3,4,5-triol | 3 | Glycosphingolipid | [116,117,118,119] |
| 28.52 | 540.1365 | 522.1010 | 3 | M+NH4 | C23H22O14 | Spinatoside | (2S,3S,4S,5R,6S)-6-[4-(5,7-dihydroxy-3,6-dimethoxy-4-oxochromen-2-yl)-2-hydroxyphenoxy]-3,4,5-trihydroxyoxane-2-carboxylic acid | 3 | Flavonoid glycoside (O-glycosylated type with glucuronic acid) | [120,121,122] |
| 22.85 | 557.1967 | 534.2101 | 5 | M+Na | C27H34O11 | Prenylflavan-7-O-glucosideo ** | (2S,4S,5S)-2-[[(2R,3S,4S)-3,4-dihydroxy-2-(4-hydroxyphenyl)-5-methoxy-8-(3-methylbut-2-enyl)-3,4-dihydro-2H-chromen-7-yl]oxy]-6-(hydroxymethyl)oxane-3,4,5-triol | 3 | Flavandiol prenylated glucoside | [123,124,125,126,127] |
| 34.70 | 584.3969 | 539.3951 | 7 | M+HCOOH−H | C27H58NO7P | Methoxyalkyalkylated phosphatidylethanolamine (PE-OCH3 C21:0) *** | 2-aminoethyl [(2R)-2-hydroxy-3-(2-methoxyhenicosoxy)propyl] hydrogen phosphate | 3 | Ether-type phosphatidylethanolamine | [128,129] |
| 34.69 | 986.6010 | 963.6201 | 9 | M+Na | C53H90NO12P | Carboxymethylated phosphatidylperoxydinoxyenoylglycerol **** | 2-[2-[[(2R)-2,3-bis(docosa-9,11-diynylperoxy)propoxy]-hydroxyphosphoryl]oxyethyl-(carboxymethyl)amino]acetic acid | 3 | Phospholipid | [130,131] |
 |  |  | ||||||||
| Ethylenethiourea (tR 20.12 min; C3H6N2S) 102.0252 Da | Lupanyl acid (tR 15.19 min; C14H22N2O) 234.1732 Da | Eriobofuran (tR 20.12 min; C14H12O4) 244.0736 Da | ||||||||
 |  | 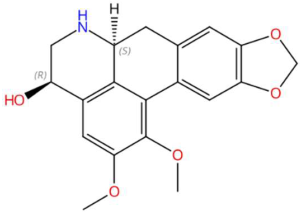 | ||||||||
| (-)-Pinocembrin (tR 18.16 min; C15H12O4) 256.0736 Da | 9-Methoxyellipticine (tR 23.63 min; C18H16N2O) 276.1263 Da | 4-Hydroxynornantenine (tR 18.06 min; C19H19NO5) 341.1263 Da | ||||||||
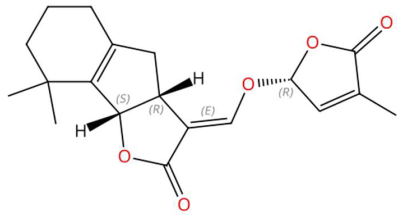 | 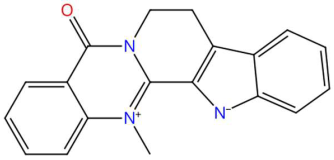 | 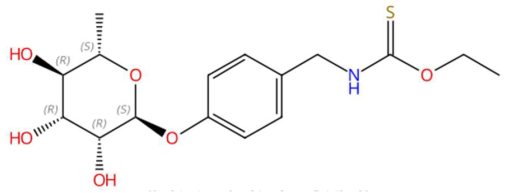 | ||||||||
| 5-Deoxystrigol (tR 18.16 min; C19H22O5) 330.1467 Da | Dehydroevodiamine (tR 22.46 min; C19H15N3O) 301.1215 Da | Niazimicin (tR 21.83 min; C16H23NO6S) 357.1246 Da | ||||||||
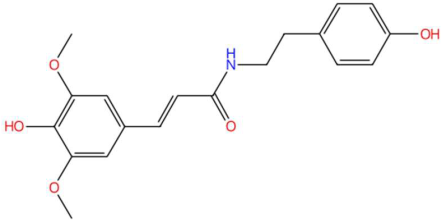 | 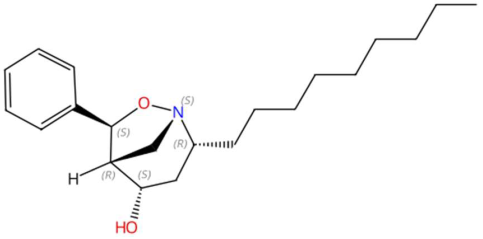 | 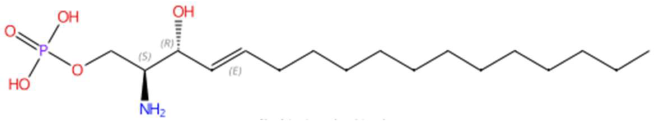 | ||||||||
| Sinapoyltyramine (tR 17.73 min; C19H21NO5) 343.1420 Da | Asperidine C (tR C 21.68 min; C21H33NO2) 331.2511 Da | C17 Sphingosine-1-phosphate (tR 19.01 min; C17H36NO5P) 365.2331 Da | ||||||||
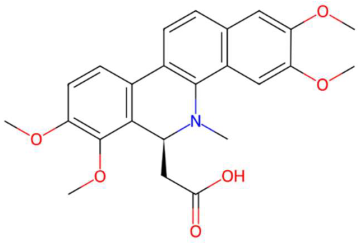 | 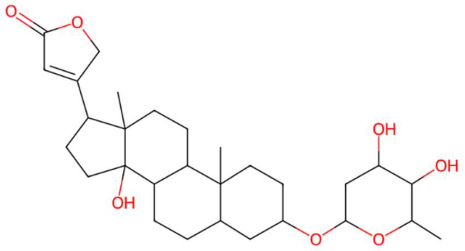 | 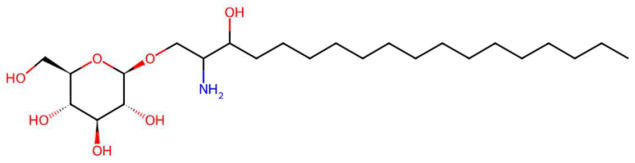 | ||||||||
| N-methyl tetramethoxy chrysoaranoic acid (tR 23.89 min; C24H25NO6)—423.1682 Da | Desglucocoroloside (tR 34.69 min; C29H44O7) 504.3087 Da | D-glucosyldihydrosphingosine (tR 34.67 min; C24H49NO7) 463.3509 Da | ||||||||
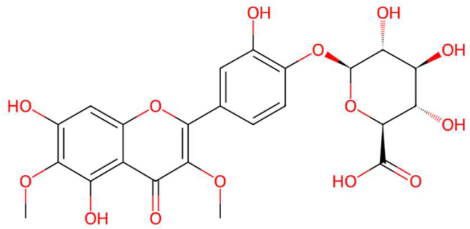 | 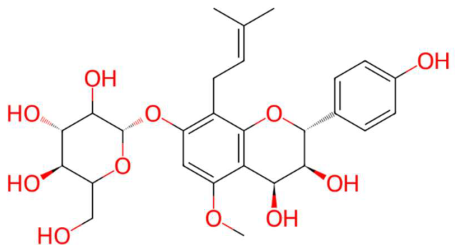 | 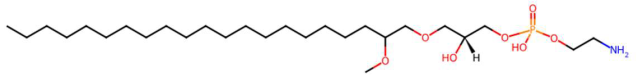 | ||||||||
| Spinatoside (tR 28.52 min; C23H22O14) 522.1010 Da | Prenylflavan-7-O-glucosideo (tR 22.85 min; C27H34O11) 534.2101 Da | Methoxyalkyalkylated phosphatidylethanolamine (tR 34.70 min; C27H58NO7P)—539.3951 Da | ||||||||
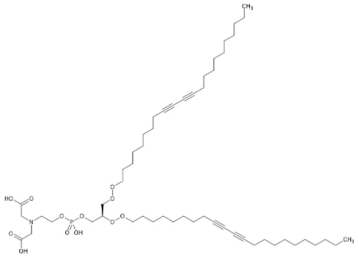 | ||||||||||
| Carboxymethylated phosphatidylperoxydinoxyenoylglycerol (tR 34.69 min; C53H90NO)—963.6201 Da | ||||||||||
| tR min | m/z | Adduct | Formula | Tentative Identification | Metabolite Class | References | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp | Theoric | Error ppm | Common Name | IUPAC Name | ID Level | |||||
| 30.32 | 149.0226 | 166.0266 | 5 | M+H−H2O | C8H6O4 | Piperonylic acid | 1,3-benzodioxole-5-carboxylic acid | 3 | Benzodioxol acid derivative | [132,133,134] |
| 14.86 | 158.1529 | 157.1467 | 7 | M+H | C9H19NO | Lastar A | 2,2,6,6-tetramethyl-4-piperidinol | 3 | Alkaloid (piperidine derivative) | [135,136,137,138] |
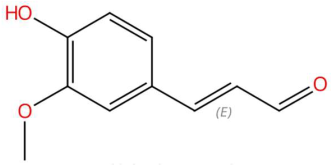
| 16.48 | 179.0699 | 178.0630 | 2 | M+H | C10H10O3 | Coniferyl aldehyde | (E)-3-(4-hydroxy-3-methoxyphenyl)prop-2-enal | 3 | Phenylpropanoid (cinnamaldehyde derivative) | [139,140,141] |
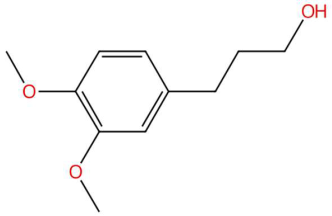
| 18.38 | 179.1060 | 196.1099 | 4 | M+H−H2O | C11H16O3 | 3-veratril-1-propanol | 3-(3,4-dimethoxyphenyl)propan-1-ol | 3 | Phenylpropanoid (phenylpropane alcohol) | [142,143,144] |
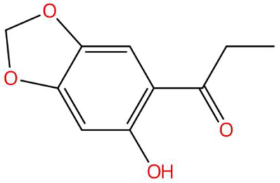
| 18.21 | 195.0660 | 194.0579 | 4 | M+H | C10H10O4 | Kakuol | 1-(6-hydroxy-1,3-benzodioxol-5-yl)propan-1-one | 3 | Benzodioxol derivative | [145,146,147,148] |
| 19.56 | 197.0802 | 196.0736 | 3 | M+H | C10H12O4 | Safrolglycol | 3-(1,3-benzodioxol-5-yl)propane-1,2-diol | 3 | Benzodioxol derivative | [149,150,151,152] |
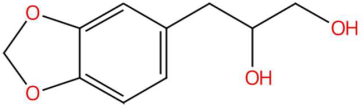
| 18.38 | 197.1170 | 196.1099 | 1 | M+H | C11H16O3 | Loliolide | (6S,7aR)-6-hydroxy-4,4,7a-trimethyl-6,7-dihydro-5H-1-benzofuran-2-one | 3 | Benzofuranonic lactone | [153,154,155,156] |
| 23.45 | 225.1115 | 224.1049 | 3 | M+H | C12H16O4 | Syringylacetone | 4-(4-Hydroxy-3,5-dimethoxyphenyl)butan-2-one | 3 | Phenylpropanoids (cinnamic acid derivative) | [157,158,159,160] |
| 24.18 | 225.1115 | 224.1049 | 3 | M+H | C12H16O4 | Pogostone/Dhelwangin | 4-Hydroxy-6-methyl-3-(4-methylpentanoyl)-2H-pyran-2-one | 3 | Pyrone/polyketoid lactone | [161,162,163,164] |
| 17.90 | 225.1116 | 224.1049 | 2 | M+H | C12H16O4 | Acoramone | 1-(2,4,5-Trimethoxyphenyl)propan-2-one | 3 | Phenylpropanoid (keto-phenylpropane) | [165,166,167,168] |
| 18.75 | 225.1117 | 224.1049 | 2 | M+H | C12H16O4 | Isoacoramone | 1-(2,4,5-Trimethoxyphenyl)propan-1-one | 3 | Phenylpropanoid (keto-phenylpropane) | [165,168,169,170] |
| 21.31 | 225.1117 | 224.1049 | 2 | M+H | C12H16O4 | Aspidinol | 1-(2,6-dihydroxy-4-methoxy-3-methylphenyl)butan-1-one | 3 | Phenylpropanoid (keto-phenylpropane) | [171,172,173,174] |
| 19.97 | 225.1118 | 224.1049 | 1 | M+H | C12H16O4 | Senkyunolide I | (3Z,6S,7S)-3-butylidene-6,7-dihydroxy-4,5,6,7-tetrahydro-2-benzofuran-1-one | 3 | Lactone | [175,176,177,178] |
| 15.27 | 235.1810 | 234.1732 | 2 | M+H | C14H22N2O | Lupanyl acid | 3-(7,11-diazatricyclo[7.3.1.0~2,7~]tridecan-10-yl)propanoic acid | 3 | Triterpenoid derivative (lupanoid) | [72,73,74] |
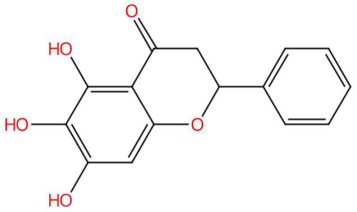
| 18.45 | 255.0679 | 272.0685 | 9 | M+H−H2O | C15H12O5 | Dihydrobaicalein | 5,6,7-trihydroxy-2-phenyl-2,3-dihydrochromen-4-one | 3 | Flavonoid (flavanone) | [179,180,181,182] |
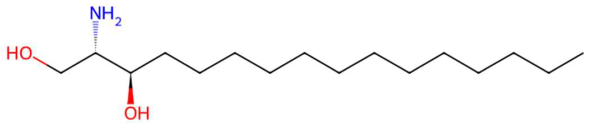
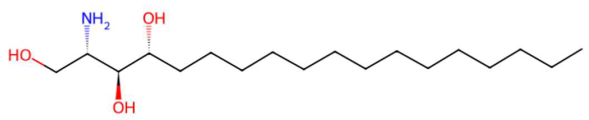
| 20.51 | 274.2737 | 273.2668 | 1 | M+H | C16H35NO2 | C16 Sphinganine | (2S,3R)-2-aminohexadecane-1,3-diol | 3 | Sphingolipid (phytosphingolipid/ceramide base) | [183,184,185,186] |
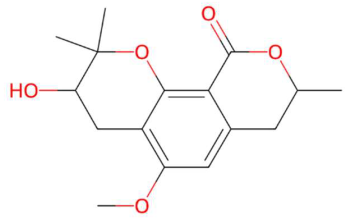
| 21.31 | 293.1357 | 292.1311 | 9 | M+H | C16H20O5 | Coriandrone B | 3-hydroxy-5-methoxy-2,2,8-trimethyl-3,4,7,8-tetrahydropyrano[4,3-h]chromen-10-one | 3 | Benzopyran derivatives | [187,188,189,190] |
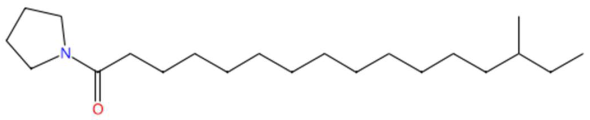
| 24.59 | 304.2992 | 323.3188 | 4 | M−H−H2O | C21H41NO | N-isopalmitoylpyrrolid * | 14-methyl-1-pyrrolidin-1-ylhexadecan-1-one | 3 | Fatty acid-derived aliphatic amide | [191,192,193] |
| 18.58 | 314.1384 | 313.1314 | 1 | M+H | C18H19NO4 | Feruloyl tyramine | (E)-3-(4-hydroxy-3-methoxyphenyl)-N-[2-(4-hydroxyphenyl)ethyl]prop-2-enamide | 3 | Phenylpropanoid amide (hydroxycinnamamide acid derivative) | [194,195,196,197] |
| 20.65 | 318.2996 | 317.293 | 2 | M+H | C18H39NO3 | Phytosphingosine | (2S,3S,4R)-2-aminooctadecane-1,3,4-triol | 3 | Sphingolipid (phytosphingolipid/hydroxy-sphingosine) | [198,199,200,201] |
| 18.46 | 326.1417 | 325.1373 | 9 | M+H | C12H23NO9 | Galactosyl-DNJ | (2S,3R,4S,5R,6R)-2-[(3S,4S,5R,6R)-4,5-dihydroxy-6-(hydroxymethyl)piperidin-3-yl]oxy-6-(hydroxymethyl)oxane-3,4,5-triol | 3 | Glucopyranosylated iminosaccharide | [202,203,204,205] |
| 26.07 | 332.3305 | 349.3345 | 2 | M+H−H2O | C23H43NO | Pipercitine | (E)-1-piperidin-1-yloctadec-2-en-1-one | 3 | Aliphatic azacycloalkane | [206,207,208,209] |
| 27.93 | 593.2764 | 592.2686 | 1 | M+H | C35H36N4O5 | Pheophorbide A | 3-[(3R,21S,22S)-16-ethenyl-11-ethyl-4-hydroxy-3-methoxycarbonyl-12,17,21,26-tetramethyl-7,23,24,25-tetrazahexacyclo[18.2.1.15,8.110,13.115,18.02,6]hexacosa-1,4,6,8(26),9,11,13(25),14,16,18(24),19-undecaen-22-yl]propanoic acid | 3 | Tetrapyrrole | [210,211,212,213] |
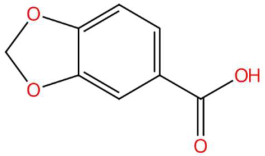 | 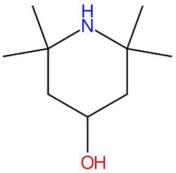 |  | ||||||||
| Piperonylic acid (tR 30.32 min; C8H6O4) 166.0266 Da | Lastar A (tR 14.86 min; C9H19NO) 157.1467 Da | Coniferyl aldehyde (tR 16.48 min; C10H10O3) 178.0630 Da | ||||||||
 |  |  | ||||||||
| 3-veratril-1-propanol (tR 18.38 min; C11H16O3) 196.1099 Da | Kakuol (tR 18.21 min; C10H10O4) 194.0579 Da | Safrolglycol (tR 19.56 min; C10H12O4) 196.0736 Da | ||||||||
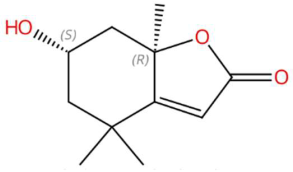 | 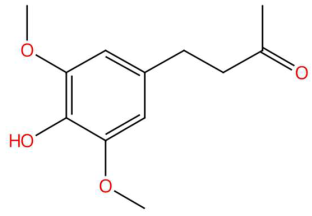 | 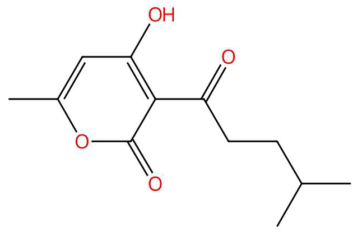 | ||||||||
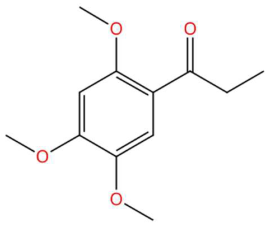
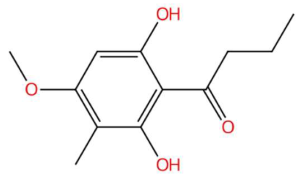
| Loliolide (tR 18.38 min; C11H16O3) 196.1099 Da | Syringylacetone (tR 23.45 min; C12H16O4) 224.1049 Da | Dhelwangin/Pogostone (tR 24.18 min; C12H16O4) 224.1049 Da | ||||||||
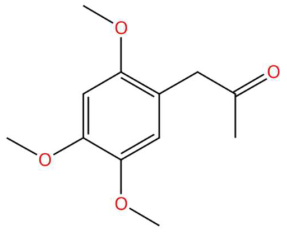 |  |  | ||||||||
| Acoramone (tR 17.90 min; C12H16O4) 224.1049 Da | Isoacoramone (tR 18.75 min; C12H16O4) 224.1049 | Aspidinol (tR 21.31 min; C12H16O4) 224.1049 Da | ||||||||
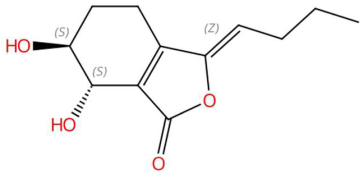 | 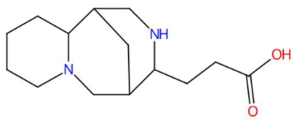 |  | ||||||||
| Senkyunolide I (tR 19.97 min; C12H16O4) 224.1049 Da | Lupanyl acid (tR 15.27 min; C14H22N2O) 234.1732 Da | Dihydrobaicalein (tR 18.45 min; C15H12O5) 272.0685 Da | ||||||||
 |  |  | ||||||||
| C16 Sphinganine (tR 20.51 min; C16H35NO2) 273.2668 Da | Coriandrone B (tR 21.31 min; C16H20O5) 292.1311 Da | N-isopalmitoylpyrrolid (tR 24.59 min; C21H41NO) 323.3188 Da | ||||||||
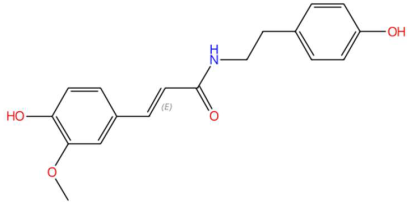 |  | 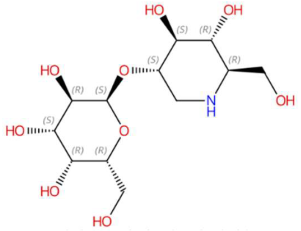 | ||||||||
| Feruloyl tyramine (tR 18.58 min; C18H19NO4) 313.1314 Da | Phytosphingosine (tR 20.65 min; C18H39NO3) 317.293 Da | Galactosyl-DNJ (tR 18.46 min; C12H23NO9) 325.1373 Da | ||||||||
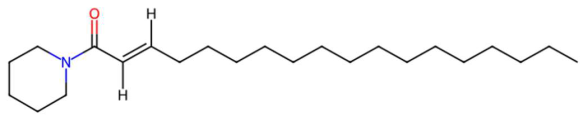 | 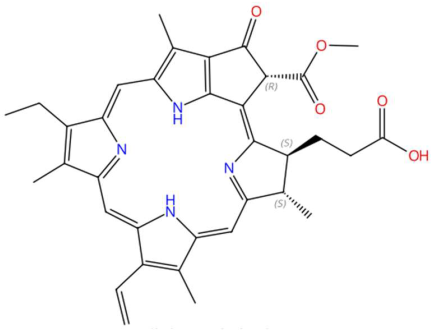 | |||||||||
| Pipercitine (tR 26.07 min; C23H43NO) 349.3345 Da | Pheophorbide A (tR 27.93 min; C35H36N4O5) 592.2686 Da | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sequeda-Castañeda, L.G.; Ospina-Giraldo, L.F.; Gutiérrez-Prieto, S.J.; Luengas-Caicedo, P.E. Phytochemical Profile and Acute Toxicity in CD-1 Mice of the Hydroethanolic Extract and Butanolic Fraction of Piper marginatum Jacq. J. Xenobiot. 2025, 15, 156. https://doi.org/10.3390/jox15050156
Sequeda-Castañeda LG, Ospina-Giraldo LF, Gutiérrez-Prieto SJ, Luengas-Caicedo PE. Phytochemical Profile and Acute Toxicity in CD-1 Mice of the Hydroethanolic Extract and Butanolic Fraction of Piper marginatum Jacq. Journal of Xenobiotics. 2025; 15(5):156. https://doi.org/10.3390/jox15050156
Chicago/Turabian StyleSequeda-Castañeda, Luis Gonzalo, Luis Fernando Ospina-Giraldo, Sandra Janeth Gutiérrez-Prieto, and Pilar Ester Luengas-Caicedo. 2025. "Phytochemical Profile and Acute Toxicity in CD-1 Mice of the Hydroethanolic Extract and Butanolic Fraction of Piper marginatum Jacq." Journal of Xenobiotics 15, no. 5: 156. https://doi.org/10.3390/jox15050156
APA StyleSequeda-Castañeda, L. G., Ospina-Giraldo, L. F., Gutiérrez-Prieto, S. J., & Luengas-Caicedo, P. E. (2025). Phytochemical Profile and Acute Toxicity in CD-1 Mice of the Hydroethanolic Extract and Butanolic Fraction of Piper marginatum Jacq. Journal of Xenobiotics, 15(5), 156. https://doi.org/10.3390/jox15050156








