Abstract
Next-generation ektacytometry provided by the osmoscan module of the Laser Optical Rotational Red Cell Analyser (LoRRca) MaxSis is, so far, one of the best complementary diagnostic tools for congenital rare anaemias due to red blood cell defects. Osmotic gradient ektacytometry (OGE) is currently considered the gold standard for the diagnosis of red cell membrane disorders, especially hereditary spherocytosis (HS). Impairment of red cell deformability, leading to a decrease in red cell survival rate, is the common trait of hereditary haemolytic anaemias; in general, it is the consequence of an abnormal cell shape, increased rigidity or dehydration. Up to now, the next-generation ektacytometry has been mainly used for the differential diagnosis of red blood cell membranopathies, but experience with structural hemoglobinopathies and thalassemia is still scarce. However, recently, many new forms of therapy are being developed for the treatment of hemoglobinopathies, particularly sickle-cell disease and β-thalassemia; clinical interest in ektacytometry is increasing and should be further explored. Here, we have evaluated the OGE profiles provided by the osmoscan module of the LoRRca ektacytometer in 96 patients with different hemoglobinopathies, both structural and thalassemia, with the aim of analysing their usefulness for the early diagnosis of these disorders either individually or in co-inheritance with other hereditary RBC defects. In addition, this study aims to improve our knowledge of the contribution of red cell deformability, osmotic fragility and intracellular viscosity to the physiopathology of haemolysis, especially when these disorders are a cause of rare anaemia. From this study, we conclude that the osmoscan profile provides complementary information on red cell deformability and hydration homeostasis that may contribute to the better understanding of the physiopathology of decreased red cell survival and hemolysis which is present in some patients.
1. Introduction
Rare anaemias (RA) are, in more than 80% of cases, genetic disorders caused by mutations in the genes controlling erythropoiesis and/or red blood cell (RBC) structural components [1]. Abnormalities of RBC structural components constitute a cause of hereditary haemolytic anaemia (HHA) and are classified into three categories: hemoglobinopathies, membranopathies and enzymopathies. Hemoglobinopathies and enzymopathies are, in general, easily diagnosed by standardised laboratory tests such as electrophoresis, high-performance liquid chromatography (HPLC) and red cell enzyme activity measurement. However, though blood smear examination may be helpful in some cases, the diagnosis of membranopathies is frequently hampered by several interferences, mainly due to the overlapping of clinical variability, an intense reticulocytosis and/or the frequent blood transfusions in more severe cases, especially in new-born babies and children [2].
Structural hemoglobinopathies are due to qualitative changes caused by the substitution of one or more amino acids in globin chains. Because of the nature and location of the amino acid or of the substituted amino acids, different changes in the solubility, stability and function (affinity for oxygen) of the Hb molecule will be produced. In fact, the severity of the clinical expression depends on whether they are inherited as heterozygous, homozygous or double heterozygous [3]. Each hemoglobinopathy has its own complete blood count (CBC) picture, associated or not with a characteristic clinical phenotype. The hemoglobinopathies with a worldwide distribution are HbS, HbC, HbD, HbE and thalassemias, and they form a group of inherited rare anaemias in general with autosomal co-dominant character. Some of these hemoglobinopathies are characterised by a great heterogeneity from the clinical, pathophysiological and genetic point of view [3]. In thalassemias, a reduction of partial or total synthesis of one or more globin chains (α, β, γ, δβ, γδβ, δ, and εγδβ) is observed, and they are classified into two main groups: alpha thalassemias (α-Thal) and beta thalassemias (β-Thal) [4,5].
Hereditary red cell membrane defects, namely spherocytosis (HS), elliptocytosis (HE) and stomatocytosis (HSt) alter membrane cohesion, mechanical stability, and RBC hydration homeostasis, respectively. Consequently, RBC deformability is compromised, leading to their premature removal from circulation by the spleen, and haemolytic anaemia [6]. According to the British Committee for Standards Guidelines [7], a family history of HHA associated with a typical clinical phenotype and specific laboratory tests allow for an accurate diagnosis of RBC membranopathies in a high percentage of cases. However, the advent of next-generation sequencing (NGS) technologies has drastically changed the diagnostic workflow of HHA, and markedly decreased the frequency of undiagnosed cases. Moreover, the reduction in cost of these techniques has allowed the development and marketing of targeted NGS-based panels of known genes (t-NGS), and has facilitated genetic diagnosis in geographic regions with difficult access to highly specialized laboratories [8].
During the last few years, the use of combined t-NGS with the osmotic gradient ektacytometry (OGE) provided by the new-generation laser-assisted optical rotational ektacytometer (LoRRca MaxSis Mechatronics Instruments®) has allowed further decreases in the number of undiagnosed cases, especially in patients with the previously mentioned diagnostic interfering factors [9,10]. The osmoscan module of the LoRRca ektacytometer provides several rheological parameters of clinical interest that reflect the maximal RBCs deformability (EImax), the osmotic fragility (Omin), the hydration state (Ohyper) and the area under curve (AUC), directly calculated from Omin and Ohyper at high osmolality rate. In a previous publication, we have studied a cohort of 42 unrelated non-transfusion-dependent (NTD) Spanish patients with HHA to better understand the influence of RBC deformability measurement on OGE parameters [11]. The present study intends to expand our experience with the LoRRca to the best known structural hemoglobinopathies and thalassemia, with the aim of better understanding red cells’ rheological behaviour under changing osmotic stress which may explain their loss of deformability.
2. Patients
In this study, we have included a total of 96 patients referred to our Rare Anemias Unit for diagnosis or diagnostic confirmation. According to clinical and hematological phenotype, they have been classified into Hemoglobin D (Hb D): 4 cases; Hemoglobin C (Hb C): 7 cases Hb C/HbO-Arab: 1 case; Hemoglobin E (Hb E): 5 cases; Hemoglobin S mono allelic (Hb S): 24 cases, Hemoglobin S Bi allelic (Hb SS): 7 cases/Hemoglobin SC (HbSC): 2 cases, Hb S/HbO-Arab): 1 case; beta-thalassemia (β-Thal): 41 cases; delta beta thalassemia (δβ-thal): 3 cases and alpha thalassemia (α-thal) 1 case. Whole blood from healthy control donors was anonymously obtained using the approved medical ethical protocol (Research Ethics Committee of the University Hospital Germans Trias i Pujol). Patients’ informed consent was gathered in agreement with the study protocol. Age at referral ranged from 18 to 67 years and the male/female ratio was 11:10. No patient required RBC transfusion and/or splenectomy, and their general hematological data are summarized in Table 1.

Table 1.
Hematological parameters of the patients with hemoglobinopathies included in this study.
3. Methods
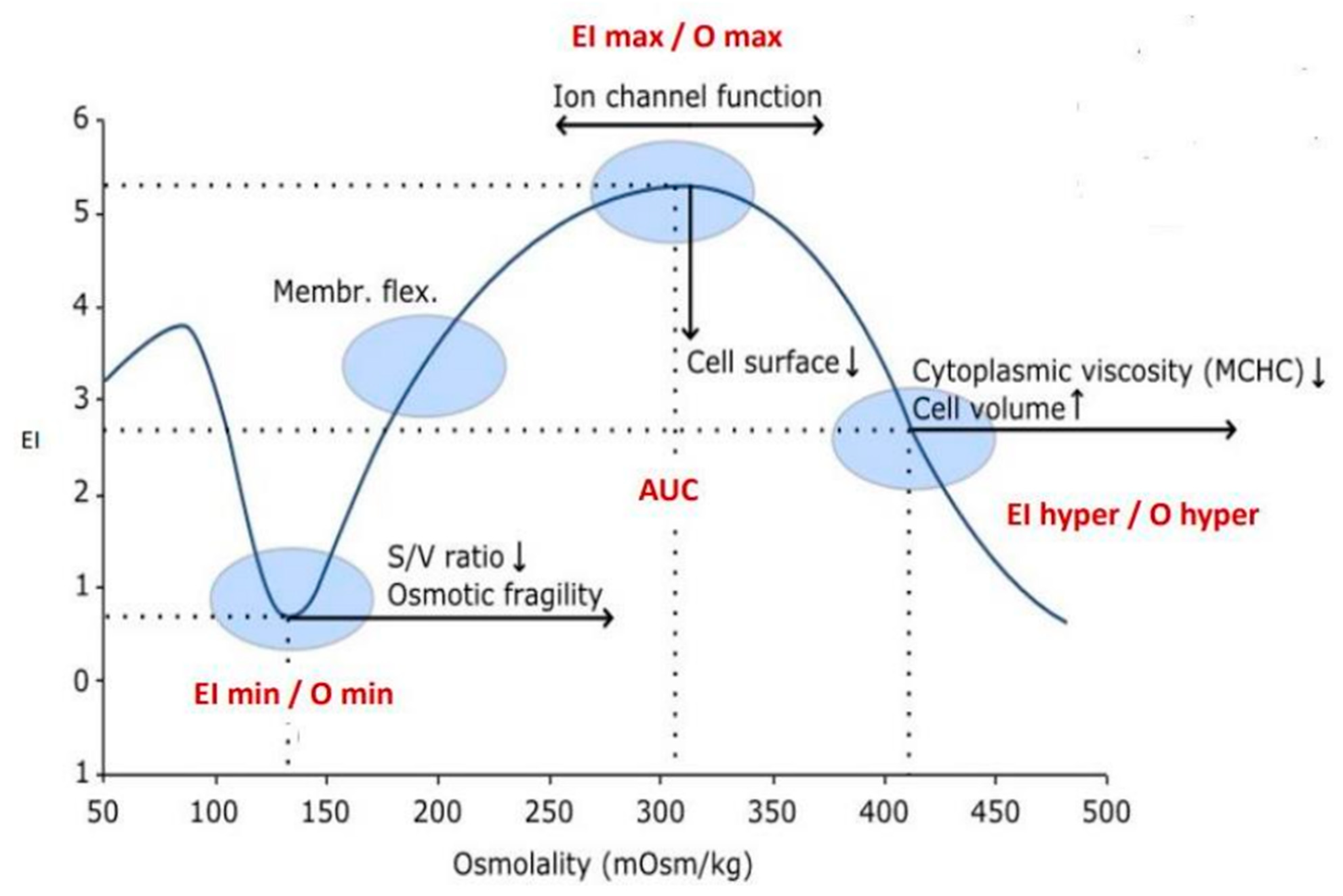
The diagnosis of patients was performed via a stepwise process including RBC morphology and high-performance liquid chromatography (HPLC), complemented by Hb electrophoresis and the measurement of key RBC enzyme activities [12]. For analytical purposes, 5 mL of whole blood was drawn in EDTA-K3 from the propositus and, when possible, from family members. When necessary, the genetic diagnosis of hemoglobinopathies was performed by t-NGS [11]. RBC deformability and other rheological parameters were studied by osmotic gradient ektacytometry (OGE) using the osmoscan module of the Laser-assisted Optical Rotational Deformability Cell Analyser (LoRRca; MaxSis. RR Mechatronics) as previously described [9]. OGE provides four distinct parameters of RBC rheological homeostasis: (1) Maximum elongation index (EImax) or red cell deformability measured by the EI at different osmotic gradients that reaches its maximum value near 300 mOsm/kg. This suggests that the normal red cell deforms optimally at the tonicity to which it is normally exposed. EImax depends mostly on cytoskeleton mechanics; (2) Osmolality value at minimum EI (Omin) that corresponds to the value of the hypotonic osmolality where 50 percent of the cells haemolyse, and provides information on the red cell surface-to-volume ratio (S/V) and osmotic fragility; (3) Osmolality value at half of EImax (Ohyper), that corresponds to the value of osmolality where the cells are at half of the EImax and provides information on intracellular viscosity and red cell hydration; and (4) The area under the curve (AUC), that is, the distance between the starting point in the hypo-osmolar region (Omin) and an ending point in the hyper-osmolar region (Ohyper) (Figure 1).
Figure 1.
Osmotic gradient ektacytometry (OGE) curve provides information on RBC deformabiliy (EI), osmotic fragility (Omin) and cell hydration (Ohyper). EI values and the membrane rigidity are depending on the RBCs shape and their position along the osmolality axis.
4. Results
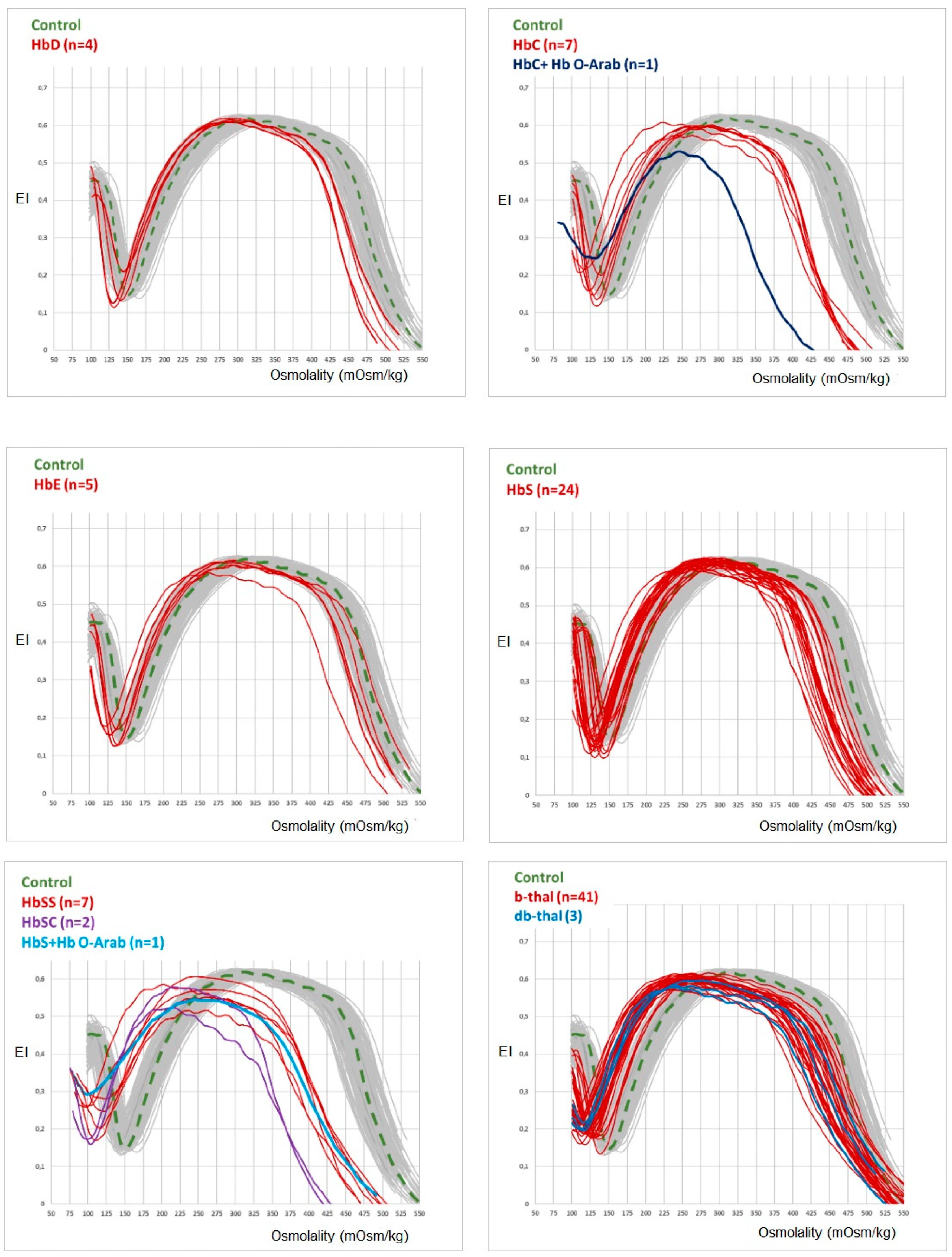
The results of osmoscan parameters in the different hemoglobinopathies are summarised in Table 2. When compared to the controls, significant (p < 0.05) differences are observed for EImax, Omin, Ohyper and AUX in Hb C Hb SS, and β-thal, indicating that in these hemoglobinopathies, decreased red cell deformability is associated with a decreased osmotic fragility (OF) and an increased red cell dehydration. In accordance with the values of osmoscan parameters, all the curves of hemoglobinopathies shift to the left to a different degree, depending on the clinical severity of the hemoglobinopathy (Figure 2).

Table 2.
Osmoscan parameters of the hemoglobinopathies included in this study.
Figure 2.
OGE curve profile of the different hemoglobinopathies studied here.
5. Discussion
The LoRRca ektacytometer osmoscan module allows the measurement of the continuous variation of the erythrocyte elongation index (EI) in an osmotic solution that ranges from 50 to 550 mOsm/kg. RBC deformability depends on MCV and ion/water content, both regulated by ion pumps, ion channels, symporters and antiporters [13]. The RBC shape, S/V ratio and the mean cellular haemoglobin concentration (MCHC) are also important factors that contribute to maintaining the hydration/dehydration equilibrium, the osmotic fragility and the cell rigidity [13,14]. As can be seen in Figure 2, the osmoscan curves obtained from the patients studied here have in common a left shift of both curve tails, suggesting the existence of a different degree of red cell dehydration, depending on the type of hemoglobinopathy. The most severe decrease of EImax and left shift of the osmoscan curve is observed in patients with Hb SS, HbSC and HbS/HbO-Arab, all associated with sickle-cell anaemia (SCA) and/or vase-occlusive crises. The substitution of one of the amino acids of the globin chains (glutamine for valine in position 6 of the β chain) implies that sickle cells are formed in hypoxic conditions [15]; although the osmoscan module does not consider the oxygenation of the sample (this is considered by the Oxyscan module), the possible sample deoxygenation during the analytical process may explain a partial Hb S polymerisation and, in turn, the increase of red cell rigidity and dehydration [16]. The AUC, which is an important marker of decreased deformability in RBC membranopathies, is also decreased in all hemoglobinopathies studied here.
Hb S, Hb C and β-thal show a similar osmoscan profile with an intermediate left shift of the curve and a decrease in EImax (deformability) suggesting a lower red cell dehydration compared with Hb SS and HbSC. Finally, Hb D, Hb E and Hb S show an osmoscan curve with a slight decrease in EImax and Ohyper and an almost normal profile in accordance with their low or absent clinical expression.
Beta-thalassemias (β-thal and δβ-thal) show a characteristic left shift of osmoscan curve that facilitates its differentiation from iron deficiency anaemia [17,18]. Probably, the decrease of the synthesis of one of the globin chains may lead to the imbalance of α and β chains’ equilibrium, with overproduction of the normal chain that may increase the red cell rigidity and dehydration [19,20]. Concerning alpha-thalassemia (α-thal) we have studied only one patient with Hb H disease (α-/α-) that exhibited an osmoscan profile suggesting slight overhydration). Probably, the excess of beta globin chains may modify the red cell membrane permeability, leading to an increase in red cell water. The study of more patients with α-thalassemia is necessary to confirm the existence of a different osmoscan profile when compared to patients with β or δβ thalassemia.
From this study we can conclude that, in contrast with RBC membrane defects [11,21], the osmoscan profile, when used as an isolated test, does not allow the differential diagnosis of hemoglobinopathies; however, it provides complementary information on RBC rheological properties such as cell deformability (EImax) and hydration (Ohyper). This contributes to better understanding of the physiopathology of decreased RBC survival and haemolysis present in some patients. Moreover, despite the low frequency of structural hemoglobinopathies, our results may contribute to the diagnosis of patients with rare anaemias of unknown etiology.
Author Contributions
Investigation and writing-original draft preparation (E.K.). Investigation and formal analysis (M.M.; Á.A.). Project administration (I.H.), Investigation and writing-original draft preparation (J.-L.V.-C.). All authors have read and agreed to the published version of the manuscript.
Funding
This research has been partially supported by the European Commission EQUALITY PLUS Grant (2019-1-TR01-KA202-076789).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of the Institute for Leukaemia Research Josep Carreras (IJC).
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study (Project identification code: PI-21-308).
Data Availability Statement
This manuscript conform to MDPI’s policies as described in the Committee on Publication Ethics (COPE) principles of publication ethics laid out in its core practices documents.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vives-Corrons, J. The Rare Anaemias. In Rare Diseases [Internet]; Wu, Z.H., Ed.; IntechOpen: London, UK, 2019; Available online: https://www.intechopen.com/chapters/69673 (accessed on 13 February 2022). [CrossRef]
- Mohandas, N.J. Red cell membrane disorders. Int. J. Lab. Hematol. 2017, 39 (Suppl. 1), 47. [Google Scholar]
- Kohne, E. Hemoglobinopathies: Clinical manifestations, diagnosis, and treatment. Dtsch. Ärzteblatt Int. 2011, 108, 532–540. [Google Scholar]
- Brancaleoni, V.; Di Pierro, E.; Motta ICappellini, M.D. Laboratory diagnosis of thalassemia. Int. J. Lab. Hematol. 2016, 38, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Polin, R.A.; Steven, H.; Abman, D.; David Rowitch, F. Fetal and Neonatal Physiology, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2017; Volume 2. [Google Scholar]
- Andolfo, I.; Russo, R.; Gambale, A.; Iolascon, A. New insights on hereditary erythrocyte membrane defects. Haematologica 2016, 101, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Bolton-Maggs, P.H.; Langer, J.C.; Iolascon, A. General Haematology Task Force of the British Committee for Standards in H. Guidelines for the diagnosis and management of hereditary spherocytosis—2011 Update. Br. J. Haematol. 2011, 2012, 37–49. [Google Scholar]
- Bianchi, P.; Vercellati, C.; Fermo, E. How will next generation sequencing (NGS) improve the diagnosis of congenital hemolytic anemia? Ann. Transl. Med. 2020, 8, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Llaudet-Planas, E.; Vives-Corrons, J.L.; Rizzuto, V.; Gómez-Ramírez, P.; Sevilla Navarro, J.; Coll Sibina, M.T.; García-Bernal, M.; Ruiz Llobet, D.; Badell, I.; Velasco-Puyó, P.; et al. Osmotic gradient ektacytometry: A valuable screening test for hereditary spherocytosis and other red blood cell membrane disorders. Int. J. Lab. Hematol. 2018, 40, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zaninoni, A.; Fermo, E.; Vercellati, C.; Consonni, D.; Marcello, A.P.; Zanella, A.; Cortelezzi, A.; Barcellini, W.; Bianchi, P. Use of laser assisted optical rotational cell analyzer (LoRRca MaxSis) in the diagnosis of RBC membrane disorders, enzyme defects, and congenital dyserythropoietic anemias: A monocentric study on 202 patients. Front. Physiol. 2018, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Vives-Corrons, J.L.; Krishnevskaya, E.; Rodriguez, I.H.; Ancochea, A. Characterization of hereditary red blood cell membranopathies using combined targeted next-generation sequencing and osmotic gradient ektacytometry. Int. J. Hematol. 2021, 113, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Vives Corrons, L.; Bascompte, A. Technical Manual for Hematology Diagnosis, 4th ed.; Elsevier-Masson: Amsterdam, The Netherlands, 2014. (In Spanish) [Google Scholar]
- Huisjes, R.; Bogdanova, A.; van Solinge, W.W.; Schiffelers, R.M.; Kaestner, L.; van Wijk, R. Squeezing for Life—Properties of Red Blood Cell Deformability. Front. Physiol. 2018, 9, 656. [Google Scholar] [CrossRef] [PubMed]
- Berga, L.; Feliu, E.; Vives Corrons, J.L. Deformabilidad Eritrocitaria y Anemias Hemolíticas. Rev. Obras Publicas 1989, 3285, 825–838. [Google Scholar]
- Ilesanmi, O.O. Pathological basis of symptoms and crises in sickle cell disorder: Implications for counseling and psychotherapy. Hematol. Rep. 2010, 2, e2. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef] [PubMed]
- Krishnevskaya, E.; Payán-Pernía, S.; Hernández-Rodríguez, I.; Remacha Sevilla, Á.F.; Ancochea Serra, Á.; Morales-Indiano, C.; Serra Ferrer, M.; Vives-Corrons, J.L. Distinguishing iron deficiency from beta-thalassemia trait by new generation ektacytometry. Int. J. Lab. Hematol. 2021, 43, e58–e60. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Mora, L.; Cabello-Fusarés, M.; Ferré-Torres, J.; Riera-Llobet, C.; Krishnevskaya, E.; Trejo-Soto, C.; Payán-Pernía, S.; Hernández-Rodríguez, I.; Morales-Indiano, C.; Alarcón, T.; et al. Blood Rheological Characterization of β-Thalassemia Trait and Iron Deficiency Anemia Using Front Microrheometry. Front. Physiol. 2021, 12, 761411. [Google Scholar] [CrossRef] [PubMed]
- Bunn, H.F. Pathogenesis and treatment of sickle cell disease. N. Engl. J. Med. 1997, 337, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Barabino, G.A.; Platt, M.O.; Kaul, D.K. Sickle cell biomechanics. Annu. Rev. Biomed. Eng. 2010, 12, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, E.; Gulbis, B.; Oirschot, B.V.; van Wijk, R. Next-generation osmotic gradient ektacytometry for the diagnosis of hereditary spherocytosis: Interlaboratory method validation and experience. Clin. Chem. Lab. Med. 2017, 55, 394–402. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).