Efferent Control in Musicians: A Review
Abstract
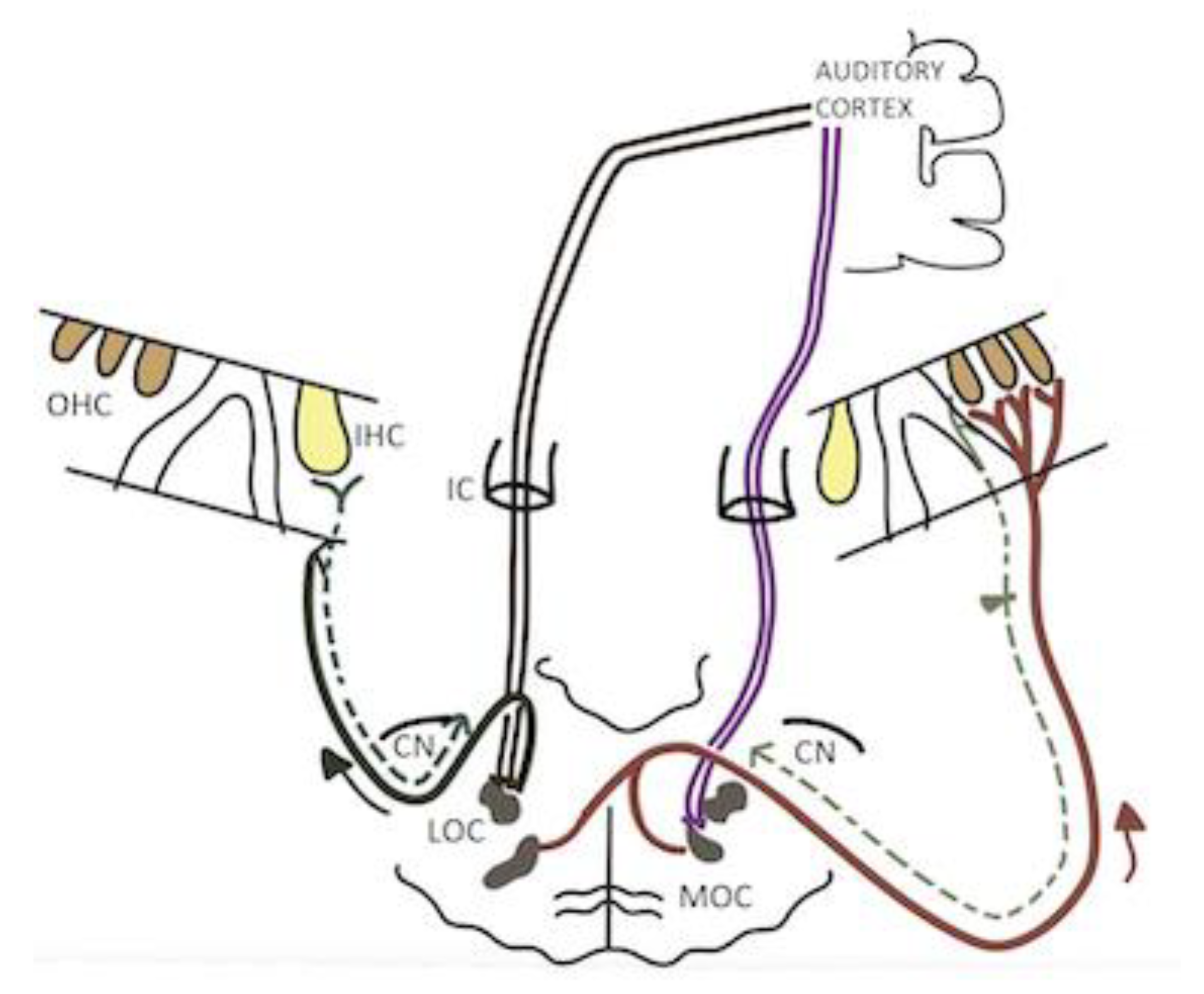
1. Introduction
2. Medial Olivocochlear Reflex Magnitude Measurement Techniques
3. Efferent Effect on Musicians
| Study | Criteria for Selection of Musicians Subjects | Age Range (Years) | Technique | Main Results |
|---|---|---|---|---|
| Micheyl et al., 1995 [44] | M: 12 normal hearing with musical training >12 years and 2 or more hours of daily practice (excluding percussionists) NM: 30 normal hearing | Not specified | TEOAEs suppression and loudness adaptation through the TDT | The M group showed great TEOAE suppression and less loudness adaptation than NM group |
| Micheyl et al., 1997 [45] | M: 16 subjects (8 females and 8 males) with at least 10 years of musical training. NM: 16 subjects (8 females and 8 males) normal hearing | M: 24.75 ± 2.86 NM: 24.06 ± 3.51 | TEOAEs suppression | The M group showed great TEOAE suppression |
| Perrot et al., 1999 [46] | M: who began their musical training between the ages of 3 and 11 and have played an instrument an average of 4 h a day for 20 years. NM: 32 normal hearing (18 women and 14 men) | M and NM: 26.66 ± 3.74 | TEOAEs suppression | The M group showed great TEOAE suppression in both right and left ear |
| Bidelman et al., 2017 [49] | M: 12 professional musicians (8 women and 4 men) with at least 9 years of experience. NM: 8 normal hearing subjects (3 women and 5 men) | M: 23.0 ± 4.1 NM: 23.3 ± 2.5 | Contralateral DPOAEs suppression and ipsilateral DPOAEs post-onset adaptation. | The M group showed great contralateral DPOAEs suppression and stronger ipsilateral DPOAE adaptation |
| Kumar et al., 2016 [48] | M: 14 rock musicians, with >5 years of musical experience after the age of 10. With weekly musical practice equal to or greater than 15 h. NM: 14 Normal hearing subjects | M and NM: 18–25 | TEOAEs and DPOAE suppression | The M group shows greater suppression in the magnitude of TEOAEs and DPOAEs than the NM group. |
| Bulut et al., 2019 [47] | M: 26 musicians of the Balkan Symphony Orchestra with at least 5 years of experience. NM: 17 normal hearing. | M: 34.3 ± 1.4 NM: 37.7 ± 4.8 | TEOAEs suppression | The M group showed great TEOAE suppression |
| Braeshers et al., 2003 [50] | M: 29 professional musicians (17 women and 12 men) from the Louisiana Philharmonic Orchestra who had a weekly practice of 16–30 h. NM: 28 normal hearing | M: 24–61 NM: 25.4–62.8 | Binaural TEOAEs suppression | The M group subjects reached higher suppression values than in the NM group. |
| Tarnowska et al., 2020 [51] | M: 12 in musicians (10 women and 2 men) NM: 12 normal hearing | M: 24.4 ± 1.7 NM: 24.6 ± 3.4 | Measured PTCs at 2 and 4 kHz frequencies with contralateral pink noise of 60 dB SPL | PTCs were quantified using Q10; no significant difference was found between musicians and non-musicians. |
4. Discussion
4.1. The Strength of the Efferent Reflex
4.2. The Implications of the Strength of MOCR
4.3. Methodological Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feliciano, M.; Saldaña, E.M.E. Direct projections from the rat primary auditory neocortex to nucleus sagulum, paralemniscal regions, superior olivary complex and cochlear nuclei. Aud. Neurosci. 1995, 1, 287–308. Available online: https://www.researchgate.net/publication/280156410_Direct_projections_from_the_rat_primary_auditory_neocortex_to_nucleus_sagulum_paralemniscal_regions_superior_olivary_complex_and_cochlear_nuclei (accessed on 25 November 2020).
- Délano, P.; Robles, I.; Robles, L. Sistema eferente auditivo Auditive efferent system. Rev. Otorrinolaringol. Cir. Cabeza Cuello 2005, 65, 55–62. [Google Scholar]
- Warr, W.B.; Guinan, J.J. Efferent innervation of the organ of corti: Two separate systems. Brain Res. 1979, 173, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Chambers, A.R.; Hancock, K.E.; Maison, S.F.; Liberman, M.C.; Polley, D.B. Sound-evoked olivocochlear activation in unanesthetized mice. JARO J. Assoc. Res. Otolaryngol. 2012, 13, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.; Gummer, M. Physiological and morphological characterization of efferent neurones in the guinea pig cochlea. Hear. Res. 1985, 20, 63–77. [Google Scholar] [CrossRef]
- Cooper, N.P.; Guinan, J.J. Efferent-mediated control of basilar membrane motion. J. Physiol. 2006, 576, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Guinan, J.J. Olivocochlear efferents: Their action, effects, measurement and uses, and the impact of the new conception of cochlear mechanical responses. Hear. Res. 2018, 362, 38–47. [Google Scholar] [CrossRef]
- Lopez-Poveda, E.A. Olivocochlear Efferents in Animals and Humans: From Anatomy to Clinical Relevance. Front. Neurol. 2018, 9, 197. [Google Scholar] [CrossRef]
- Kishon-Rabin, L.; Amir, O.; Vexler, Y.; Zaltz, Y. Pitch Discrimination: Are Professional Musicians Better than Non-Musicians? J. Basic Clin. Physiol. Pharm. 2001, 12, 125–144. [Google Scholar] [CrossRef]
- Micheyl, C.; Delhommeau, K.; Perrot, X.; Oxenham, A.J. Influence of musical and psychoacoustical training on pitch discrimination. Hear. Res. 2006, 219, 36–47. [Google Scholar] [CrossRef]
- Bidelman, G.M.; Gandour, J.T.; Krishnan, A. Musicians and tone-language speakers share enhanced brainstem encoding but not perceptual benefits for musical pitch. Brain Cogn. 2011, 77, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Slater, J.; Kraus, N. The role of rhythm in perceiving speech in noise: A comparison of percussionists, vocalists and non-musicians. Cogn. Process. 2016, 17, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Baer, L.H.; Park, M.T.M.; Bailey, J.A.; Chakravarty, M.M.; Li, K.Z.H.; Penhune, V.B. Regional cerebellar volumes are related to early musical training and finger tapping performance. Neuroimage 2015, 109, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Zarate, J.M.; Ritson, C.R.; Poeppel, D. Pitch-interval discrimination and musical expertise: Is the semitone a perceptual boundary? J. Acoust. Soc. Am. 2012, 132, 984–993. [Google Scholar] [CrossRef]
- Fujioka, T.; Trainor, L.J.; Ross, B.; Kakigi, R.; Pantev, C. Musical Training Enhances Automatic Encoding of Melodic Contour and Interval Structure. J. Cogn. Neurosci. 2004, 16, 1010–1021. [Google Scholar] [CrossRef]
- Hennessy, S.; Mack, W.J.; Habibi, A. Speech-in-noise perception in musicians and non-musicians: A multi-level meta-analysis. Hear. Res. 2022, 416, 108442. [Google Scholar] [CrossRef]
- Başkent, D.; Gaudrain, E. Musician advantage for speech-on-speech perception. J. Acoust. Soc. Am. 2016, 139, EL51–EL56. [Google Scholar] [CrossRef]
- Parbery-Clark, A.; Skoe, E.; Lam, C.; Kraus, N. Musician Enhancement for Speech-in-Noise. Ear. Hear. 2009, 30, 653–661. [Google Scholar] [CrossRef]
- Merten, N.; Fischer, M.E.; Dillard, L.K.; Klein, B.E.K.; Tweed, T.S.; Cruickshanks, K.J. Benefit of Musical Training for Speech Perception and Cognition Later in Life. J. Speech Lang. Hear. Res. 2021, 64, 2885–2896. [Google Scholar] [CrossRef]
- Rus-Oswald, O.G.; Benner, J.; Reinhardt, J.; Bürki, C.; Christiner, M.; Hofmann, E.; Schneider, P.; Stippich, C.; Kressig, R.W.; Blatow, M. Musicianship-Related Structural and Functional Cortical Features Are Preserved in Elderly Musicians. Front. Aging Neurosci. 2022, 14, 807971. [Google Scholar] [CrossRef]
- Andrews, E.; Eierud, C.; Banks, D.; Harshbarger, T.; Michael, A.; Rammell, C. Effects of Lifelong Musicianship on White Matter Integrity and Cognitive Brain Reserve. Brain Sci. 2021, 11, 67. [Google Scholar] [CrossRef] [PubMed]
- Groussard, M.; Viader, F.; Landeau, B.; Desgranges, B.; Eustache, F.; Platel, H. The effects of musical practice on structural plasticity: The dynamics of grey matter changes. Brain Cogn. 2014, 90, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, L.; Hartmann, K.; Ripollés, P.; Rojo, N.; Sierpowska, J.; François, C.; Càmara, E.; van Vugt, F.T.; Mohammadi, B.; Samii, A.; et al. Structural neuroplasticity in expert pianists depends on the age of musical training onset. Neuroimage 2016, 126, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Karpati, F.J.; Giacosa, C.; Foster, N.E.V.; Penhune, V.B.; Hyde, K.L. Dance and music share gray matter structural correlates. Brain Res. 2017, 1657, 62–73. [Google Scholar] [CrossRef]
- Bermudez, P. Differences in Gray Matter between Musicians and Nonmusicians. Ann. N. Y. Acad. Sci. 2005, 1060, 395–399. [Google Scholar] [CrossRef]
- Liang, C.; Earl, B.; Thompson, I.; Whitaker, K.; Cahn, S.; Xiang, J.; Fu, Q.-J.; Zhang, F. Musicians Are Better than Non-musicians in Frequency Change Detection: Behavioral and Electrophysiological Evidence. Front. Neurosci. 2016, 10, 464. [Google Scholar] [CrossRef]
- Tierney, A.T.; Krizman, J.; Kraus, N. Music training alters the course of adolescent auditory development. Proc. Natl. Acad. Sci. USA 2015, 112, 10062–10067. [Google Scholar] [CrossRef]
- Wong, P.C.M.; Skoe, E.; Russo, N.M.; Dees, T.; Kraus, N. Musical experience shapes human brainstem encoding of linguistic pitch patterns. Nat. Neurosci. 2007, 10, 420–422. [Google Scholar] [CrossRef]
- White-Schwoch, T.; Carr, K.W.; Anderson, S.; Strait, D.L.; Kraus, N. Older Adults Benefit from Music Training Early in Life: Biological Evidence for Long-Term Training-Driven Plasticity. J. Neurosci. 2013, 33, 17667–17674. [Google Scholar] [CrossRef]
- Kraus, N.; Slater, J.; Thompson, E.C.; Hornickel, J.; Strait, D.L.; Nicol, T.; White-Schwoch, T. Music Enrichment Programs Improve the Neural Encoding of Speech in At-Risk Children. J. Neurosci. 2014, 34, 11913–11918. [Google Scholar] [CrossRef]
- Brown, C.J.; Jeon, E.-K.; Driscoll, V.; Mussoi, B.; Deshpande, S.B.; Gfeller, K.; Abbas, P. Effects of Long-Term Musical Training on Cortical Auditory Evoked Potentials. Ear Hear. 2017, 38, e74–e84. [Google Scholar] [CrossRef] [PubMed]
- Arkhipova, A.; Hok, P.; Valošek, J.; Trnečková, M.; Všetičková, G.; Coufalová, G.; Synek, J.; Zouhar, V.; Hluštík, P. Changes in Brain Responses to Music and Non-music Sounds Following Creativity Training within the “Different Hearing” Program. Front. Neurosci. 2021, 15, 703620. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Hjortkjær, J.; Santurette, S.; Zatorre, R.J.; Siebner, H.R.; Dau, T. Subcortical and cortical correlates of pitch discrimination: Evidence for two levels of neuroplasticity in musicians. Neuroimage 2017, 163, 398–412. [Google Scholar] [CrossRef] [PubMed]
- Herholz, S.C.; Zatorre, R.J. Musical Training as a Framework for Brain Plasticity: Behavior, Function, and Structure. Neuron 2012, 76, 486–502. [Google Scholar] [CrossRef]
- Oxenham, A.J.; Bacon, S.P. Cochlear Compression: Perceptual Measures and Implications for Normal and Impaired Hearing. Ear Hear. 2003, 24, 352–366. [Google Scholar] [CrossRef]
- Miranda, F.A.; Aguilar-Vidal, E. Magnitude of the contralateral efferent olivocochlear effect as a function of the frequency. J. Otol. 2022, 17, 67–71. [Google Scholar] [CrossRef]
- Aguilar, E.; Eustaquio-Martin, A.; Lopez-Poveda, E.A. Contralateral Efferent Reflex Effects on Threshold and Suprathreshold Psychoacoustical Tuning Curves at Low and High Frequencies. J. Assoc. Res. Otolaryngol. 2013, 14, 341–357. [Google Scholar] [CrossRef]
- Aguilar, E.; Johannesen, P.T.; Lopez-Poveda, E.A. Contralateral efferent suppression of human hearing sensitivity. Front. Syst. Neurosci. 2015, 8, 251. [Google Scholar] [CrossRef]
- Yasin, I.; Drga, V.; Plack, C.J. Effect of Human Auditory Efferent Feedback on Cochlear Gain and Compression. J. Neurosci. 2014, 34, 15319–15326. [Google Scholar] [CrossRef]
- Fletcher, M.D.; Krumbholz, K.; de Boer, J. Effect of Contralateral Medial Olivocochlear Feedback on Perceptual Estimates of Cochlear Gain and Compression. J. Assoc. Res. Otolaryngol. 2016, 17, 559–575. [Google Scholar] [CrossRef]
- Wegel, R.L.; Lane, C.E. The auditory masking of one pure tone by another and its probable relation to the dynamics of the inner ear. Phys. Rev. 1924, 23, 266–285. [Google Scholar] [CrossRef]
- Jennings, S.G.; Strickland, E.A. Evaluating the effects of olivocochlear feedback on psychophysical measures of frequency selectivity. J. Acoust. Soc. Am. 2012, 132, 2483–2496. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Poveda, E.A.; Aguilar, E.; Johannesen, P.T.; Eustaquio-Martín, A. Contralateral Efferent Regulation of Human Cochlear Tuning: Behavioural Observations and Computer Model Simulations. In Basic Aspects of Hearing; Springer: Berlin/Heidelberg, Germany, 2013; pp. 47–54. [Google Scholar] [CrossRef]
- Micheyl, C.; Carbonnel, O.; Collet, L. Medial olivocochlear system and loudness adaptation—Differences between musicians and non-musicians. Brain Cogn. 1995, 29, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Micheyl, C.; Khalfa, S.; Perrot, X.; Collet, L. Difference in cochlear efferent activity between musicians and non-musicians. Neuroreport 1997, 8, 1047–1050. [Google Scholar] [CrossRef]
- Perrot, X.; Micheyl, C.; Khalfa, S.; Collet, L. Stronger bilateral efferent influences on cochlear biomechanical activity in musicians than in non-musicians. Neurosci. Lett. 1999, 262, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Bulut, E.; Öztürk, G.; Taş, M.; Türkmen, M.; Gülmez, Z.; Öztürk, L. Medial olivocochlear suppression in musicians versus non-musicians. Physiol. Int. 2019, 106, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Grover, V.; Publius, A.S.; Sanju, H.K.; Sinha, S. Assessment of rock musician’s efferent system functioning using contralateral suppression of otoacoustic emissions. World J. Otorhinolaryngol. Head Neck. Surg. 2016, 2, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Bidelman, G.M.; Schneider, A.D.; Heitzmann, V.R.; Bhagat, S.P. Musicianship enhances ipsilateral and contralateral efferent gain control to the cochlea. Hear Res. 2017, 344, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Brashears, S.M.; Morlet, T.G.; Berlin, C.I.; Hood, L.J. Olivocochlear Efferent Suppression in Classical Musicians. J. Am. Acad. Audiol. 2003, 14, 314–324. [Google Scholar] [CrossRef]
- Tarnowska, E.; Wicher, A.; Moore, B.C.J. No Influence of Musicianship on the Effect of Contralateral Stimulation on Frequency Selectivity. Trends Hear. 2020, 24, 233121652093977. [Google Scholar] [CrossRef] [PubMed]
- Stuart, A.; Daughtrey, E.R. On the Relationship between Musicianship and Contralateral Suppression of Transient-Evoked Otoacoustic Emissions. J. Am. Acad. Audiol. 2016, 27, 333–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-B.; Liu, L.-M.; Yu, N.; Zhu, Y.; Mei, L.; Chen, J.; Liang, C. Efferent neurons control hearing sensitivity and protect hearing from noise through the regulation of gap junctions between cochlear supporting cells. J. Neurophysiol. 2022, 127, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Boero, L.E.; Castagna, V.C.; di Guilmi, M.N.; Goutman, J.D.; Elgoyhen, A.B.; Gómez-Casati, M.E. Enhancement of the Medial Olivocochlear System Prevents Hidden Hearing Loss. J. Neurosci. 2018, 38, 7440–7451. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, S.; Tsuzaki, M.; Sonoda, J.; Tanaka, S.; Furukawa, S. A role of medial olivocochlear reflex as a protection mechanism from noise-induced hearing loss revealed in short-practicing violinists. PLoS ONE 2016, 11, e0146751. [Google Scholar] [CrossRef] [PubMed]
- Behar, A.; Chasin, M.; Mosher, S.; Abdoli-Eramaki, M.; Russo, F.A. Noise exposure and hearing loss in classical orchestra musicians: A five-year follow-up. Noise Health 2018, 20, 42–46. [Google Scholar] [CrossRef]
- Aedo, C.; Aguilar, E. Cochlear synaptopathy: New findings in animal and human research. Rev. Neurosci. 2020, 31, 605–615. [Google Scholar] [CrossRef]
- Couth, S.; Prendergast, G.; Guest, H.; Munro, K.J.; Moore, D.R.; Plack, C.J.; Ginsborg, J.; Dawes, P. Investigating the effects of noise exposure on self-report, behavioral and electrophysiological indices of hearing damage in musicians with normal audiometric thresholds. Hear. Res. 2020, 395, 108021. [Google Scholar] [CrossRef]
- Hyde, K.L.; Lerch, J.; Norton, A.; Forgeard, M.; Winner, E.; Evans, A.C.; Schlaug, G. Musical Training Shapes Structural Brain Development. J. Neurosci. 2009, 29, 3019–3025. [Google Scholar] [CrossRef]
- Perrot, X.; Collet, L. Function and plasticity of the medial olivocochlear system in musicians: A review. Hear. Res. 2014, 308, 27–40. [Google Scholar] [CrossRef]
- Lisowska, G.; Smurzynski, J.; Morawski, K.; Namyslowski, G.; Probst, R. Influence of Contralateral Stimulation by Two-tone Complexes, Narrow-band and Broad-band Noise Signals on the 2f 1-f 2 Distortion Product Otoacoustic Emission Levels in Humans. Acta Otolaryngol. 2002, 122, 613–619. [Google Scholar] [CrossRef]
- Maison, S.; Micheyl, C.; Andéol, G.; Gallégo, S.; Collet, L. Activation of medial olivocochlear efferent system in humans: Influence of stimulus bandwidth. Hear. Res. 2000, 140, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Velenovsky, D.S.; Glattke, T.J. The effect of noise bandwidth on the contralateral suppression of transient evoked otoacoustic emissions. Hear. Res. 2002, 164, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Lilaonitkul, W.; Guinan, J.J. Frequency tuning of medial-olivocochlear-efferent acoustic reflexes in humans as functions of probe frequency. J. Neurophysiol. 2012, 107, 1598–1611. [Google Scholar] [CrossRef]
- Lilaonitkul, W.; Guinan, J.J. Human Medial Olivocochlear Reflex: Effects as Functions of Contralateral, Ipsilateral, and Bilateral Elicitor Bandwidths. J. Assoc. Res. Otolaryngol. 2009, 10, 459–470. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acuña, F.; Jeria, R.; Pavez, E.; Aguilar-Vidal, E. Efferent Control in Musicians: A Review. Audiol. Res. 2023, 13, 76-85. https://doi.org/10.3390/audiolres13010007
Acuña F, Jeria R, Pavez E, Aguilar-Vidal E. Efferent Control in Musicians: A Review. Audiology Research. 2023; 13(1):76-85. https://doi.org/10.3390/audiolres13010007
Chicago/Turabian StyleAcuña, Francisca, Rodrigo Jeria, Elisabeth Pavez, and Enzo Aguilar-Vidal. 2023. "Efferent Control in Musicians: A Review" Audiology Research 13, no. 1: 76-85. https://doi.org/10.3390/audiolres13010007
APA StyleAcuña, F., Jeria, R., Pavez, E., & Aguilar-Vidal, E. (2023). Efferent Control in Musicians: A Review. Audiology Research, 13(1), 76-85. https://doi.org/10.3390/audiolres13010007






