Abstract
Wheat production and grain quality are adversely affected by drought stress. The deployment of wheat genotypes with improved grain yield and grain quality assists in achieving food security and maintaining a balanced diet. Therefore, this study is aimed at evaluating the phenotypic traits and grain quality responses of wheat genotypes to drought-stressed conditions. Two field trials were conducted to evaluate ten wheat genotypes under drought-stressed (DS) and non-stressed (NS) conditions in 2022 and 2023. The grains of the genotypes were further evaluated for their quality. The recorded phenotypic traits include grain yield (GY), shoot biomass (SB), root biomass (RB), and harvest index (HI). The grain quality traits recorded were grain carbon content (C), nitrogen (N), and crude protein (CP). Significant (p < 0.05) genetic variation were observed for the recorded phenotypic and grain quality traits. The highest grain yield was recorded in LM48 (495.83 g m−2), and the least was observed in BW141 (131.48 g m−2) under DS conditions. The N ranged from 1.76% recorded in LM75 to 3.16% (BW141) under DS conditions. The wheat genotypes, LM48 and BW140, presented high harvest index percentages, which indicates that the genotypes were efficient in partitioning their biomass to GY production even under DS conditions. The overall mean values of C and CP were lower under DS than NS conditions. Furthermore, GY was positively associated with SB (r = 0.50 under DS; r = 0.49 under NS) and RB (r = 0.38 under DS conditions; r = 0.32 under NS conditions). Amongst all the evaluated quality traits, only CP was negatively correlated with GY (r = −0.02) under DS conditions. Based on high GY production performance under DS conditions, the wheat genotypes LM48 and BW140 are recommended for further evaluation across diverse environments and production under limited water conditions.
1. Introduction
Wheat has the second-highest production in grains after maize [] and the largest trade volume []. It provides approximately 20% of calories and protein consumed by more than 4.5 billion people in the form of bread, noodles and pasta [], whereas 20% of global wheat production is utilized for livestock feed. Despite this significance, the production of wheat is highly affected by climate change around the globe, with the increasing unavailability of water resources [] exacerbating the challenge. This has led to an increase in wheat demand from approximately 777.15 million to 791.40 million tons between 2022 and 2023 [].
The largest wheat-producing countries are Russia, India, and China, which account for about 41% of the world’s total wheat production []. On the other hand, the production of wheat in Africa is still very low. This is because out of 220 million ha of world agricultural land cultivated, only 4.55% of that land is utilized in Africa to produce approximately 25 million tons of grain per year. This causes African countries such as South Africa, Botswana and Zimbabwe to rely on importing wheat to meet the demands. For instance, the yield gap in South African dryland farming systems ranges between 1.58 and 3.13 t ha−1, which is only 38% of the yield potential []. The low grain yields in South Africa are attributed to the frequent occurrence of droughts associated with climatic variability []. Therefore, wheat characterization is vital to select genotypes with favorable yield and improved grain quality under DS conditions to achieve Sustainable Development Goals number 1 and 2, aiming to eradicate poverty and hunger, respectively.
Furthermore, different wheat researchers found a variation in phenotypic traits, such as grain yield (GY), shoot biomass (SB), and root biomass (RB), under different soil moisture environments. For instance, the studies conducted in South Africa highlighted that phenotypic traits such as GY, SB, and RB were lower under DS conditions as compared to NS conditions [,,,,]. That agrees with Li et al. [], who evaluated wheat genotypes under different soil moisture treatments and found that GY, SB, and RB were reduced under drought-stressed (DS) conditions. Another study conducted by Rossi et al. [] in Italy reported that the GY, SB, and RB were lower under DS conditions, but the root-to-shoot ratio was higher under DS as compared to NS conditions. These reported variations for phenotypic traits under DS and NS conditions are caused by differences in the evaluated germplasm and environmental factors []. Furthermore, some studies also suggested that DS conditions had an impact on grain quality. For instance, Wan et al. [] reported that limited soil moisture conditions under DS treatment lead to reduced GY and quality. This indicates that the lower water supply may result in the reduction of carbohydrate synthesis in plants, further leads to lower GY and grain protein content. On the other hand, Ozturk and Aydin [] revealed that long-term DS conditions increased grain protein concentration by 18%, whereas DS conditions from milking to physiological maturity increased grain protein concentration by 8.3% as compared to the NS conditions. On the contrary, Zörb et al. [] found that DS conditions from anthesis to grain filling have more severe impacts on grain protein quantity and quality. In addition, DS conditions cause changes in the balance between carbon assimilation and grain nitrogen accumulation and eventually result in higher nitrogen concentration in the grain []. That agrees with Liang et al. [], who found higher nitrogen content in winter wheat grain under limited water conditions. Moreover, nutrients such as carbon and nitrogen are reduced by limited water availability in wheat genotypes [,,]. This is due to the decrease in carbon fixation, which results in less carbohydrate production [] and decreased activity of nitrogen assimilation enzymes []. Consequently, the combined reduction in grain carbon and nitrogen content under DS conditions reduces grain protein content [].
The deployment of wheat genotypes with high GY potential and improved grain quality is a promising strategy to achieve food security and reduce malnutrition levels [,,]. However, there are still a few high-yielding cultivars that can grow under diverse environmental conditions. To exploit the genetic variation of wheat for improved GY potential and quality suitable for environmental conditions in South African conditions, the wheat breeding team at the African Center for Crop Improvement of the University of KwaZulu-Natal sourced a panel of 100 wheat genotypes from the International Maize and Wheat Improvement Centre (CIMMYT) heat and drought nursery []. These genotypes were evaluated for biomass production under limited water conditions by Mathew et al. []. Eight out of the hundred wheat genotypes from CIMMYT were selected based on biomass production, and two wheat genotypes locally adapted to South African conditions were evaluated in this study. The 10 wheat genotypes were further screened and characterized for improved yield performance and grain quality in order to enhance crop production and future breeding. Cultivating wheat genotypes with enhanced yield performance and superior grain nutritional quality will contribute to achieving food security and improving the nutritional status of populations. Therefore, the objective of the present study is to evaluate the phenotypic and grain quality responses of wheat genotypes under DS conditions.
2. Materials and Methods
2.1. Plant Materials
Ten wheat genotypes, consisting of eight genotypes obtained from the International Maize and Wheat Improvement Centre (CIMMYT) heat and drought nursery [] and two genotypes locally adapted to dryland environments in South Africa (Table 1), were evaluated in this study. The eight genotypes were selected from a panel of a hundred diverse genotypes based on their high biomass production potential under water-limited conditions [].

Table 1.
List of wheat genotypes used under drought-stressed conditions.
2.2. Experimental Site Description
The experiment was conducted under field conditions at the University of KwaZulu-Natal’s Pietermaritzburg campus in South Africa. The climatic conditions in the area where the experiment was conducted are semi-arid with warm to hot summer months and cool winters. The long-term average temperature in the area of the experiment is 26.5 °C. The annual average precipitation is 738 mm, with approximately 20% falling during the winter months. The soil at the experimental area is clay-to-loamy clay.
2.3. Experimental Design and Establishment
The field experiments were laid out on a 2 × 5 alpha lattice design with two replicates per treatment in 2022 and 2023. The field was ploughed to a 30 cm depth, harrowed to achieve a fine tilth and ridges were prepared to enhance soil drainage and drip-line installation. The basal fertilizer was applied following the procedure by Mathew et al. [], using 300 kg ha−1 of compound fertilizer (NPK 10:20:10) applied during planting, and top-dressing fertilizer [urea (46% N)] at 100 kg ha−1 was applied at the tillering stage. The field experiment establishment was conducted following the procedure by Makhubu et al. []. Briefly, the field was covered with a custom-designed black plastic mulch to prevent rainfall infiltration. Small holes, each with a diameter of 5 cm, were created along the planting rows in the plastic mulch. Plants were spaced 10 cm apart within rows and 20 cm between rows. Three seeds per hole were sown at a depth of 3 cm and thinned to two seedlings per hole after two weeks. Each plot consisted of a single row of 7 planting holes, measured 60 cm in length and 30 cm in width (plot area = 1800 cm2). Two irrigation treatments were implemented side by side, both utilizing an automated drip irrigation system. Each planting row was equipped with a drip line beneath the mulch to ensure precision irrigation. To simulate terminal drought conditions, water stress was induced by reducing soil moisture to 35% field capacity from 50% heading to maturity. In the control treatment, soil moisture was maintained at 80% field capacity from planting to maturity. A HOBO UX120 water sensor was used to monitor every 30 min, and irrigation adjustments were made accordingly to maintain field capacity levels in both treatments []. Manual weeding and targeted application of insecticides were performed to control leaf miners and aphids.
2.4. Data Collection
2.4.1. Phenotypic Traits
The data for the phenotypic traits were recorded before harvesting: days to 50% heading (DTH) was recorded during the afternoon as the number of days from sowing until half of the plants in a plot had fully emerged spikes; days to 50% maturity (DTM) was measured daily during the afternoon as the number of days from planting until half of the plants in a plot have dried spikes. The data for the following traits were measured at maturity: plant height (PH) was measured from the base of the plant to the tip of the spike, and the units were normalized in centimeters; number of productive tillers (TN) per plant was determined by averaging the counted tillers from three sampled holes.
The data for the following phenotypic traits were recorded after harvesting: spike length (SL) was measured from the base of the spike to the tip of the spike, and the units were normalized in centimeters, and the number of spikelets per spike (SK) were counted and recorded as the mean of three randomly selected spikes per plot. Grain yield (GY) was measured as the dry weight of grain produced, and the units were normalized in g m−2. Shoot biomass (SB) was determined by drying the shoots (excluding spikes) in an oven drier at 70 °C for 48 h, after which the dry weight was recorded in g m−2. Root biomass (RB) was assessed after drying harvested roots at 70 °C for 48 h, and the units were expressed in g m−2. The root-to-shoot ratio (RS) was calculated as the ratio of the root to shoot biomass produced per plot. The harvest index (HI) was calculated following the equation by Mutanda et al. []:
where HI = harvest index.
2.4.2. Grain Carbon, Nitrogen and Protein Content Determination
The wheat grain samples were collected from all the plots planted in the 2022 growing season. The collected grain samples were oven-dried at 70 °C for 48 h and ground into fine powder using a pestle and mortar. The ground samples were analyzed for nitrogen (N) and carbon (C) content using a LECO TruMac CN analyzer, LECO Corporation, St. Joseph, MI, USA. The wheat samples were combusted at 1350 °C in the presence of oxygen, whereby the N and C are converted to carbon dioxide and NOx, respectively. Both gases were separated by chromatography, with argon acting as a carrier gas, and measured in a thermal conductivity cell. The crude protein content of samples was determined using the following equation:
where 6.25 is a common protein factor.
Crude protein = observed nitrogen content × 6.25
2.5. Data Analysis
The phenotypic traits data for two growing seasons were subjected to a combined analysis of variance using GenStat 24th edition by considering season, genotypes and water regimes as fixed factors. Fisher’s least significant difference at the 5% significance level was used to compare the mean values. Pearson’s correlation coefficients (r) were calculated separately for NS and DS conditions to examine the relationship and dependencies between phenotypic traits, grain carbon, nitrogen and crude protein using R statistical version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria, 2024. Principal component analysis was performed to visualize the relationship between phenotypic traits under DS and NS conditions using R statistical, version 4.4.1, R Foundation for Statistical Computing, Vienna, Austria, 2024.
3. Results
3.1. A Combined Analysis of Variances on Phenotypic Traits
Significant genotype variations were observed in all the recorded phenotypic traits (Table 2). The season was shown to have a significant effect on DTH, SL, SB, RB and RS (Table 2). The main effect of genotype × water regime × season interaction effect was non-significant on all phenotypic traits (Table 2).

Table 2.
Mean squares and significance tests for phenotypic traits of wheat genotypes evaluated across two seasons under drought-stressed and non-stressed conditions.
3.2. Analysis of Variance for the Grain Quality Traits
The findings of the present study show that the genotype × water regime interaction has a significant effect on C and N and a non-significant effect on CP (Table 3). Both genotype and water regime factors have significant effects on N and CP (Table 3).

Table 3.
Mean squares and significance tests for grain carbon, nitrogen, and crude protein of wheat lines evaluated during the 2022 growing season.
3.3. Wheat Performance Under Drought-Stressed and Non-Stressed Conditions
The mean grain yield of the tested wheat genotypes was lower under DS conditions (304.83 g m−2) than under NS conditions (427.68 g m−2) (Table 4). The genotypes with the higher mean yield under DS conditions were LM48 (495.83 g m−2), BW140 (460.28 g m−2), LM47 (380.56 g m−2), LM75 (366.67 g m−2) and BW152 (331.94 g m−2), whilst the genotypes with higher mean grain under NS were LM48, LM70, LM47, BW140 and LM71, with mean yield values of 771.17 g m−2, 610.48 g m−2, 550.00 g m−2, 496.67 g m−2 and 466.67 g m−2, respectively. The mean grain yield for the evaluated genotypes under DS was lower during the 2022 growing season as compared to the 2023 growing season (Table S1). Furthermore, our findings showed that the average PH for the two seasons was lower under NS conditions than DS conditions. Drought-stressed conditions resulted in a reduction of RB (8.85%), SB (0.70%), and HI (24.50%). Despite the decrease in the overall genotype performance in terms of RB, genotypes BW152, BW162, LM26, LM48, and LM70 exhibited the highest RB production. The genotypes BW140 and LM75 exhibited the highest HI under DS conditions compared to other wheat genotypes. The overall mean DTM was lower by 0.73 days under NS conditions as compared to DS conditions. Similar observations were discovered in SPS and SL, which were reduced by 0.08 cm and 0.36 cm, respectively, under NS conditions (Table 4). The mean values are presented in Table 4, and most of the evaluated phenotypic traits, such as DTH, DTM, PH, TN, SL and SPS, showed lower differences between DS and NS conditions. However, GY, RB and HI were notably affected by DS conditions, reflecting their sensitivity to water stress during the reproductive stage.

Table 4.
Mean values of phenotypic traits of the ten wheat lines under drought-stressed and non-stressed conditions.
3.4. Grain Carbon, Nitrogen, and Protein Content Determination
In the present study, the mean nitrogen content was lower under DS conditions (2.74%) than under NS conditions (3.11%) (Table 5). The wheat genotypes with high grain carbon content under DS conditions included BW152 (41.95%), followed by BW141 (41.67%) and LM70 (41.63%), whilst under NS conditions, the genotypes with high grain carbon content were LM70 (41.91%), LM47 (41.74%) and BW152 (41.74%) (Table 5). In addition, the wheat grain crude protein was lower under DS conditions (17.48%) compared to NS conditions (19.45%) (Table 5).

Table 5.
Mean values for grain nitrogen, carbon, and crude protein content of ten wheat genotypes evaluated under drought-stressed and non-stressed conditions.
3.5. Correlation Analysis
3.5.1. Phenotypic Traits
Pearson’s correlation coefficient (r) among the recorded phenotypic traits of wheat genotypes was evaluated separately under DS (Figure 1) and NS (Figure 2) conditions. Under DS conditions, grain yield exhibited a strong positive correlation with SB (r = 0.50), HI (r = 0.79; p < 0.01) and SK (r = 0.47). In addition, SB exhibited a positive and significant correlation with RB (r = 0.73, p < 0.05) and SK (r = 0.75, p < 0.05) under DS conditions (Figure 1). Under NS conditions, GY showed a positive and significant correlation with SL (r = 0.68, p < 0.05), TN (r = 0.69, p < 0.05) and HI (r = 0.67, p < 0.05) (Figure 2). In both water regimes, GY exhibited a negative correlation with RS (r = −0.13 under DS conditions; r = −0.16 under NS conditions) (Figure 1 and Figure 2).
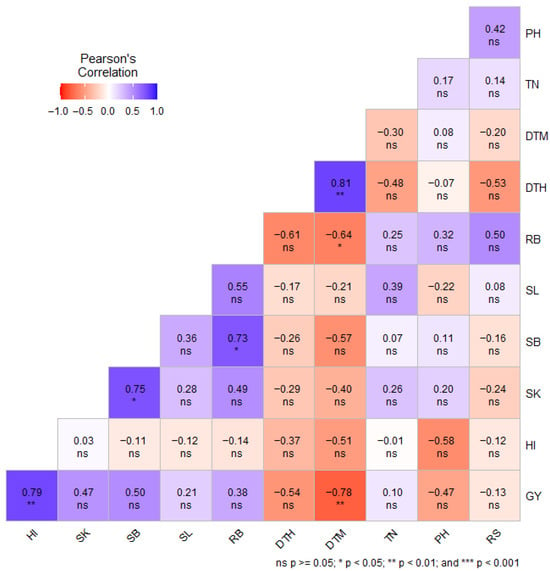
Figure 1.
Pearson’s correlation between phenotypic traits of wheat genotypes under drought-stressed conditions. DTH = days to 50% heading; DTM = days to 50% maturity; PH = plant height in centimeters; TN = number of productive tillers per plant; SL = spike length; SK = number of spikelets per spike; GY = grain yield in g m−2; SB = shoot biomass in g m−2; RB = root biomass in g m−2; RS = root-to-shoot ratio; HI = harvest index; ns = non-significant.
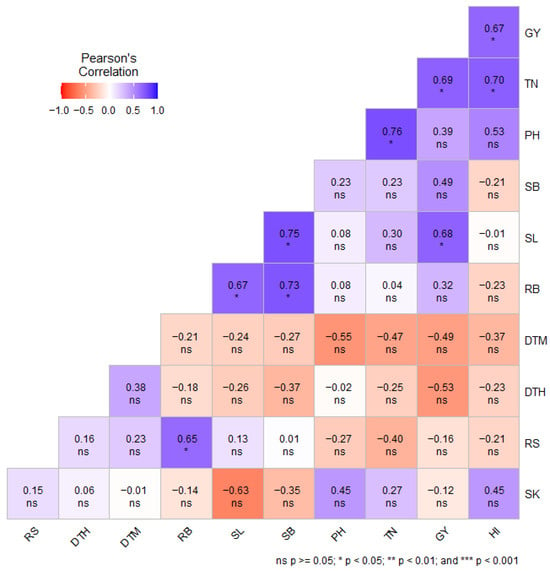
Figure 2.
Pearson’s correlation between phenotypic traits of wheat genotypes under non-stressed conditions. DTH = days to 50% heading; DTM = days to 50% maturity; PH = plant height in centimeters; TN = number of productive tillers per plant; SL = spike length; SK = number of spikelets per spike; GY = grain yield in g m−2; SB = shoot biomass in g m−2; RB = root biomass in g m−2; RS = root-to-shoot ratio; HI = harvest index; PB = total plant biomass in g m−2; ns = non-significant.
3.5.2. Phenotypic Traits, Grain Carbon, Nitrogen, and Crude Protein
Pearson’s correlation coefficient (r) among the selected phenotypic traits, grain carbon, nitrogen, and crude protein, was evaluated separately under DS (Figure 3) and NS conditions (Figure 4). Under DS conditions, RS showed a strong correlation with CP (r = 0.52) (Figure 3). Furthermore, C was positively correlated with N (r = 0.90, p < 0.001) and CP (r = 0.79, p < 0.01) under DS conditions. Amongst all the quality traits, only CP had a negative correlation with GY (r = −0.02) (Figure 3). Under DS conditions, GY exhibited positive correlation with C (r = 0.28), N (0.48), and CP (r = 0.48) (Figure 4).
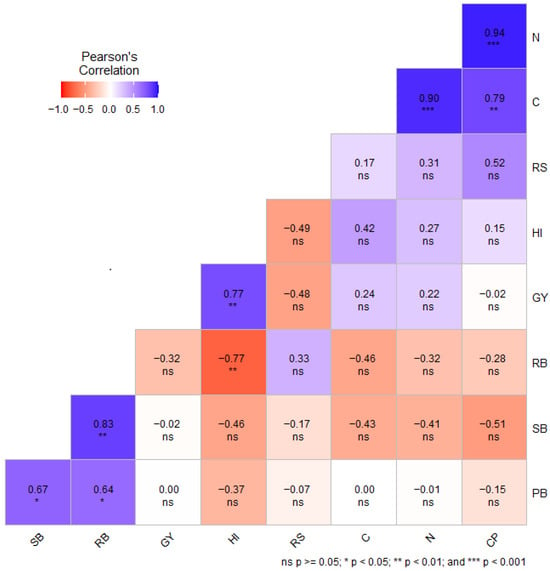
Figure 3.
Pearson’s correlation between selected phenotypic traits, grain carbon, nitrogen, and crude protein content of wheat genotypes under drought-stressed conditions. GY = grain yield in g m−2; SB = shoot biomass in g m−2; RB = root biomass in g m−2; RS = root-to-shoot ratio; HI = harvest index; PB = total plant biomass in g m−2; C = grain carbon content (%); N = grain nitrogen content; CP = grain crude protein content (%); ns = non-significant.
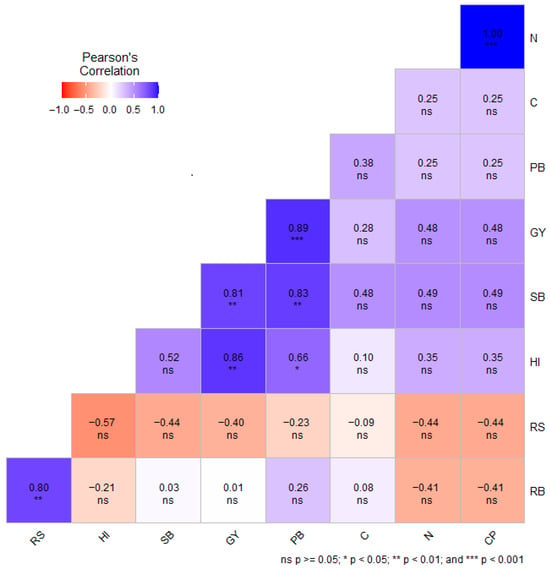
Figure 4.
Pearson’s correlation between selected phenotypic traits, grain carbon, nitrogen, and crude protein content of wheat genotypes under non-stressed conditions. GY = grain yield in g m−2; SB = shoot biomass in g m−2; RB = root biomass in g m−2; RS = root-to-shoot ratio; HI = harvest index; PB = total plant biomass in g m−2; C = grain carbon content (%); N = grain nitrogen content; CP = grain crude protein content (%); ns = non-significant.
3.6. Principal Component Analysis for the Phenotypic Traits Recorded over Two Seasons
The inter-relationship between phenotypic traits and wheat genotypes was explored using principal components analysis (PCA) under DS (Figure 5) and NS conditions (Figure 6), using R statistical software. The phenotypic trait data were first standardized (mean-centered and scaled to unit variance) to remove differences in measurement units and ensure equal contribution of each variable to the analysis. The PCA biplots were generated to visualize genotype distribution and the strength/direction of trait associations with principal components. The first two principal components (PC1 and PC2) were retained for interpretation as they jointly explained a substantial proportion of the total variation in the dataset and allowed for effective two-dimensional visualization of trait–genotype relationships. Under DS conditions (Figure 5), the first principal component (PC1) explained 38.28% of the total variation, while the second principal component (PC2) explained 22.11%, together accounting for 60.39% of the total variability. PC1 was positively associated with RB, PH, SK, SL, SB, TN and GY, suggesting these traits co-vary and contribute substantially to variation under DS. PC2 was positively correlated with PH, root-to-shoot ratio (RS), and harvest index (HI). The high-yielding genotype LM48 under DS conditions was positioned in the same vector direction as GY, indicating strong alignment between this genotype’s performance and yield-related traits under DS conditions. In contrast, low-yielding genotypes such as BW141 were located opposite to the GY vector, aligning more with traits that did not contribute positively to yield. Under NS conditions (Figure 6), PC1 and PC2 explained 36.22% and 26.74% of the variation, respectively, totaling 62.96%. The PC1 was positively associated with GY, TN, PH, RB, SL, SB and SK, while PC2 was positively associated with RS and DTM. Under both water regimes, grain yield (GY) was negatively correlated with RS, indicating that higher RS values tended to be associated with lower yields.
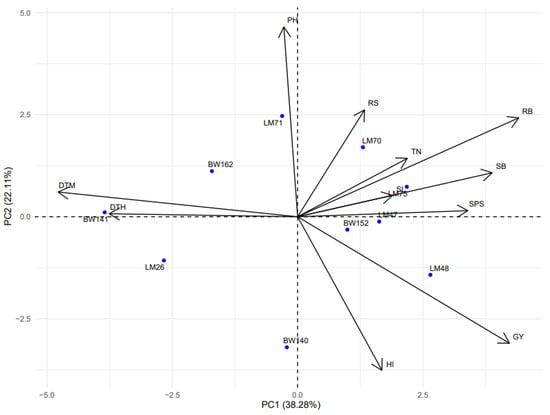
Figure 5.
Principal component analysis showing the relationship between the recorded phenotypic traits of wheat genotypes under drought-stressed conditions. DTH = days to 50% heading; DTM = days to 50% maturity; PH = plant height in centimeters; TN = number of productive tillers per plant; SL = spike length; SK = number of spikelets per spike; GY = grain yield in g m−2; SB = shoot biomass in g m−2; RB = root biomass in g m−2; RS = root-to-shoot ratio; HI = harvest index; PB = total plant biomass in g m−2.
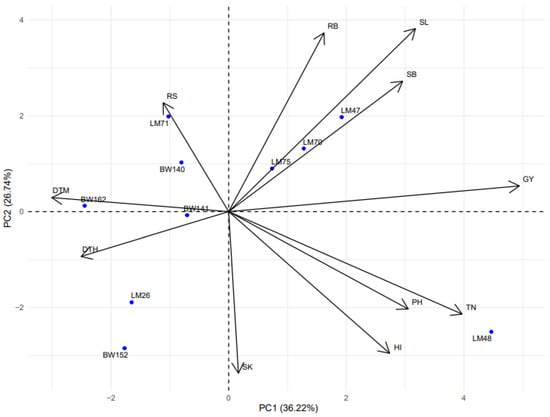
Figure 6.
Principal component analysis showing the relationship between the recorded phenotypic traits of wheat genotypes under non-stressed conditions. DTH = days to 50% heading; DTM = days to 50% maturity; PH = plant height in centimeters; TN = number of productive tillers per plant; SL = spike length; SK = number of spikelets per spike; GY = grain yield in g m−2; SB = shoot biomass in g m−2; RB = root biomass in g m−2; RS = root-to-shoot ratio; HI = harvest index.
4. Discussion
4.1. Performance of Wheat Genotypes
The wheat grain yield and yield components are adversely affected by DS conditions []. However, the impact of DS conditions on wheat cultivars varies as a result of genetic diversity []. Our findings showed significant variations among the recorded phenotypic traits of the evaluated wheat genotypes (Table 2), indicating that the presence of genetic variability is useful in the breeding of high-yielding wheat genotypes. The main effect interaction, water regime × genotype × season, was non-significant in all the recorded phenotypic traits (Table 2). This indicates that the environmental conditions across the two seasons did not strongly differentiate the wheat genotypes’ performance. Nonetheless, despite the non-significant three-way interaction, the genotype-specific responses across different water regimes revealed fundamental genotype × environment patterns, which are crucial for selection. For instance, wheat genotypes LM48 and BW140 maintained high GY under DS conditions, with LM48 surpassing the yields of several genotypes (including LM75, BW152, LM26, and BW162), even under NS conditions (Table 4). This stability suggests that these two genotypes possess physio-morphological traits, which enable efficient water-use and maintain high GY production under DS conditions. Therefore, it is recommended to include more seasons and diverse environments in future evaluations to gain a better understanding of the genotype × season interaction effects []. The mean GY for all the genotypes was reduced by 35.13% under DS conditions compared to NS conditions (Table 4). These findings align with Mwadzingeni et al. [] and Shamuyarira et al. [], who examined 100 wheat genotypes in South Africa and found that the lower mean GY was significantly reduced under DS conditions. That is possibly because DS conditions reduce the rate of photosynthesis and carbon uptake, leading to poor plant growth and development, and reduced yield production []. Furthermore, the findings of the present study highlighted that DS conditions reduced SB and RB by 0.70% and 8.85%, respectively. That agrees with Mathew et al. [] and Mutanda et al. [], who reported that RB and SB were reduced under DS conditions on the 100 wheat genotypes evaluated in their study.
Considering the mean values in Table 4, most of the evaluated traits (such as DTH, PH, TN, SL, and SPS) have shown lower differences between DS and NS conditions. This is due to the fact that the terminal drought imposed in the present study affected the grain filling and grain formation rather than earlier vegetative traits that are established before the onset of stress. These early vegetative traits tend to be less sensitive to late-stage DS conditions, which explains the limited variation observed among them. This may explain why some measured parameters showed minimal or no significant response to DS conditions. As previously reported in wheat and other cereals, the timing of DS conditions strongly determines which traits are most impacted [], with terminal stress often reducing grain filling duration and yield components [] rather than vegetative growth parameters established before stress onset. However, GY, RB, and HI were notably affected by DS conditions, highlighting their sensitivity to DS conditions during the reproductive stage. The GY reduction directly shows the impact of DS conditions on final productivity [], while reductions in RB indicate compromised water and nutrient uptake potential []. The changes in HI highlight the alterations in plant biomass partitioning towards GY production under DS conditions. These genetic variabilities observed for the phenotypic traits reveal their significance as targets for breeding of drought-resilient wheat genotypes.
When the performance of individual genotypes across different water regimes was considered, clear genotype-specific responses emerged, highlighting the importance of genotype × environment interactions when identifying superior genotypes. For instance, LM48 maintained high GY under DS conditions (495.83 g m−2), which was higher than the GY produced by wheat genotypes under NS conditions. Similarly, BW140 produced a DS yield of 460.28 g m−2, which is higher than several genotypes under NS conditions. These results indicate that certain genotypes possess inherent drought resilience that enables them to perform well even under water limited conditions. Conversely, some genotypes, such as BW152 and LM26, showed greater GY reduction under DS conditions, highlighting sensitivity to DS conditions. These differences in responses are critical for breeders because they allow the selection of genotypes with consistent performance and stability under different water regimes.
Even though the RB of the evaluated wheat genotypes was reduced under DS conditions, LM48 managed to produce high GY compared to other genotypes under DS conditions. This suggests that LM48 may possess other physio-biochemical traits that enhance drought resilience, including osmotic adjustment and enhanced photosynthetic capacity, which were not evaluated in this study. Interestingly, BW140 recorded a comparable GY despite having lower RB, indicating that RB alone may not fully account for GY under DS conditions, and yield stability depends on aboveground traits and source–sink dynamics. These genotype-specific patterns highlight the value of evaluating the yield behavior beyond mean values, as they help identify genotypes with superior performance under DS conditions. These insights are critical for breeders aiming to combine biomass-related traits and genetic adaptability into new varieties suited for DS conditions. This agrees with suggestions by Shamuyarira et al. [], Mathew et al. [], and Mutanda et al. []. The authors highlighted that the wheat genotypes with high RB production have the potential to produce high yields. Therefore, there is a need for wheat root phenotyping to improve wheat productivity under DS conditions.
4.2. Grain Carbon, Nitrogen, and Protein Content Determination
Drought stress has an impact on both wheat grain yield production and grain quality. Our findings indicated that the mean grain carbon content of the tested wheat genotypes was lower under DS than NS conditions (Table 5). This is because DS conditions reduce the photosynthesis rate, leading to carbon assimilation in the plant []. The %CV for grain nitrogen content of the tested wheat genotypes was lower under DS conditions than NS conditions (Table 5). This indicates that these wheat genotypes had more consistent or uniform nitrogen content across different genotypes when subjected to DS conditions. Even though several studies reported an increase in grain nitrogen content under DS conditions [,], our findings highlighted that under DS conditions, the grain nitrogen content was reduced by 11.90% as compared to NS conditions. That is possibly because DS conditions disrupt assimilate partitioning of assimilates between shoot and root, limit root carbohydrate use, and reduce leaf photosynthetic nitrogen utilization, leading to a functional imbalance between shoot and root [], which may eventually reduce grain nitrogen content. Furthermore, the changes caused by DS conditions on grain carbon and nitrogen coordination play a significant role in reducing grain crude protein percentage under DS conditions as compared to NS conditions, as found in the present study (Table 5). The results agree with Yang et al. [], who reported a decrease in grain protein content under DS conditions as compared to NS conditions.
4.3. Association Between Phenotypic Traits
The findings of our study showed a positive association between grain yield and shoot biomass production under DS (Figure 1 and Figure 5) and NS conditions (Figure 2 and Figure 6). That indicates the importance of aboveground biomass production in improving wheat GY potential. These findings agree with Mathew et al. [] and Shamuyarira et al. [], who reported a positive correlation between GY and aboveground biomass of 100 wheat genotypes evaluated under different water regimes in South Africa. That is possibly because high aboveground biomass production offers a large surface area for photosynthesis, which supports carbon uptake and carbon assimilation []. In addition, the grain yield has a positive relationship with root biomass under DS and NS conditions (Figure 5 and Figure 6). This suggests the importance of root development in improving wheat grain yield under DS and NS conditions. Mathew et al. [] and Mutanda et al. [] have reported a similar trend of correlation on the 100 wheat genotypes evaluated under limited water conditions and NS conditions. In both water regimes, grain yield was negatively associated with RS. These findings align with Qi et al. [] and Asadullah et al. [], who reported a negative correlation between grain yield and root-to-shoot ratio. Therefore, the increasing root-to-shoot ratio in plants has a negative impact on grain yield production, as reflected in wheat genotypes such as LM71 and LM70, which are in the opposite direction and the same direction as GY under DS conditions.
4.4. Link Between Selected Phenotypic Traits and Grain Quality Traits
The results showed that GY had a positive correlation with N and C under DS and NS conditions. This suggests that the wheat genotypes, which are more efficient in nitrogen and carbon uptake, transport and utilization, tend to produce high yields. These results agree with Li et al. [], who revealed that grain yield was positively correlated with carbon accumulation (r = 0.96) and nitrogen accumulation (r = 0.95). In addition, crude protein content was positively associated with grain yield under NS conditions and negatively correlated with grain yield under NS conditions. This suggests that under DS conditions, plants prioritize yield production over protein synthesis. Furthermore, all the grain quality traits were positively correlated under both water regimes (Figure 3 and Figure 4). This is because these quality traits are intrinsically linked through plant metabolic processes. Nitrogen is a crucial element for the synthesis of amino acids, which are the building blocks of proteins; thus, higher nitrogen content typically leads to increased protein content. Carbon, being a fundamental part of all organic compounds in plants, is involved in the synthesis of both proteins and other metabolic products. Under both water regimes, efficient nutrient uptake and assimilation processes ensure that nitrogen and carbon are effectively used to produce proteins, maintaining a positive correlation between these quality traits.
5. Conclusions
This study showed a substantial and wide variation in phenotypic traits of the evaluated wheat genotypes, indicating that these are vital genetic resources for the development of drought-tolerant varieties. The results showed that both GY and quality traits were lower under DS conditions than NS conditions. However, an important exception was observed in certain genotypes: BW140 and LM48 achieved high GY under DS conditions, surpassing the yields of wheat genotypes, and BW152, BW162, LM26, and LM75 under NS conditions. This suggests that some genotypes possess better yield stability and superior drought resilience mechanisms to maintain productivity despite water scarcity. The correlation analysis revealed that GY was positively associated with SB, RB, C, and N under DS and NS conditions. Based on high GY production under DS conditions, the wheat genotypes LM48 and BW140 are recommended for further evaluation across diverse environments and production under limited water conditions.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijpb16040119/s1, Table S1: The agronomic traits of the wheat genotypes evaluated under drought stressed and non-stressed conditions during the 2022 growing season; Table S2: The agronomic traits of the wheat genotypes evaluated under drought stressed and non-stressed conditions during the 2023 growing season.
Author Contributions
M.M.: Conceptualization; Draft preparation; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Writing—original draft; Writing—review and editing. S.F.: Conceptualization; Data curation; Funding acquisition; Supervision; Investigation; Methodology; Resources; Validation; Writing—review and critical editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was based on generous financial support from the Water Research Commission of the Republic of South Africa (WRC Project No. C2020/2021-00646).
Data Availability Statement
The datasets generated and analyzed during the present study are available from the corresponding author upon reasonable request.
Acknowledgments
The University of South Africa (UNISA) and the African Centre of Crop Improvement (ACCI) of the University of KwaZulu Natal (UKZN) are thanked for the overall research support. The research staff of the University of KwaZulu Natal’s Ukulinga Research Farm, Fikile N Makhubu, Mkoyi Hosana, and Sandra Maluleke, are thanked for their technical support.
Conflicts of Interest
There are no personal interests that could have appeared to influence the work reported in this study.
References
- Yang, J.; Yang, R.; Liang, X.; Marshall, J.M.; Neibling, W. Impact of Drought Stress on Spring Wheat Grain Yield and Quality. Agrosyst. Geosci. Environ. 2023, 6, e20351. [Google Scholar] [CrossRef]
- Huang, X.; Wang, C.; Hou, J.; Du, C.; Liu, S.; Kang, J.; Lu, H.; Xie, Y.; Guo, T.; Ma, D. Coordination of Carbon and Nitrogen Accumulation and Translocation of Winter Wheat Plant to Improve Grain Yield and Processing Quality. Sci. Rep. 2020, 10, 10340. [Google Scholar] [CrossRef]
- Gooding, M.J.; Shewry, P.R. Wheat: Environment, Food and Health; John Wiley & Sons: Hoboken, NJ, USA, 2022; ISBN 9781119652557. [Google Scholar]
- Fathian, M.; Bazrafshan, O.; Jamshidi, S.; Jafari, L. Impacts of Climate Change on Water Footprint Components of Rainfed and Irrigated Wheat in a Semi-Arid Environment. Environ. Monit. Assess. 2023, 195, 324. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization. 2023. Available online: https://sdgs.un.org/un-system-sdg-implementation/food-and-agriculture-organization-fao-54096 (accessed on 12 June 2024).
- Dadrasi, A.; Chaichi, M.; Nehbandani, A.; Soltani, E.; Nemati, A.; Salmani, F.; Heydari, M.; Yousefi, A.R. Global Insight into Understanding Wheat Yield and Production through Agro-Ecological Zoning. Sci. Rep. 2023, 13, 15898. [Google Scholar] [CrossRef]
- Soba, D.; Shu, T.; Runion, G.B.; Prior, S.A.; Fritschi, F.B.; Aranjuelo, I.; Sanz-Saez, A. Effects of Elevated [CO2] on Photosynthesis and Seed Yield Parameters in Two Soybean Genotypes with Contrasting Water Use Efficiency. Environ. Exp. Bot. 2020, 178, 104154. [Google Scholar] [CrossRef]
- Tadesse, W.; Bishaw, Z.; Assefa, S. Wheat Production and Breeding in Sub-Saharan Africa. Int. J. Clim. Change Strateg. Manag. 2019, 11, 696–715. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Tesfay, S.; Tsilo, T.J. Screening of Bread Wheat Genotypes for Drought Tolerance Using Phenotypic and Proline Analyses. Front. Plant Sci. 2016, 7, 1276. [Google Scholar] [CrossRef]
- Mathew, I.; Shimelis, H.; Mutema, M.; Clulow, A.; Zengeni, R.; Mbava, N.; Chaplot, V. Selection of Wheat Genotypes for Biomass Allocation to Improve Drought Tolerance and Carbon Sequestration into Soils. J. Agron. Crop Sci. 2019, 205, 385–400. [Google Scholar] [CrossRef]
- Shamuyarira, K.W.; Shimelis, H.; Figlan, S.; Chaplot, V. Combining Ability Analysis of Yield and Biomass Allocation Related Traits in Newly Developed Wheat Populations. Sci. Rep. 2023, 13, 11832. [Google Scholar] [CrossRef]
- Shamuyarira, K.W.; Shimelis, H.; Tapera, T.; Tsilo, T.J. Genetic Advancement of Newly Developed Wheat Populations Under Drought-Stressed and Non-Stressed Conditions. J. Crop Sci. Biotechnol. 2019, 22, 169–176. [Google Scholar] [CrossRef]
- Makebe, A.; Shimelis, H.; Mashilo, J. Selection of M5 Mutant Lines of Wheat (Triticum aestivum L.) for Agronomic Traits and Biomass Allocation under Drought Stress and Non-Stressed Conditions. Front. Plant Sci. 2024, 15, 1314014. [Google Scholar] [CrossRef]
- Li, P.; Ma, B.; Palta, J.A.; Wei, X.; Guo, S.; Ding, T.; Ma, Y. Distinctive Root System Adaptation of Ploidy Wheats to Water Stress: A Cue to Yield Enhancement. J. Agron. Crop Sci. 2023, 209, 566–577. [Google Scholar] [CrossRef]
- Rossi, R.; Bochicchio, R.; Labella, R.; Amato, M.; De Vita, P. Phenotyping Seedling Root Biometry of Two Contrasting Bread Wheat Cultivars under Nutrient Deficiency and Drought Stress. Agronomy 2024, 14, 775. [Google Scholar] [CrossRef]
- Wan, C.; Dang, P.; Gao, L.; Wang, J.; Tao, J.; Qin, X.; Feng, B.; Gao, J. How Does the Environment Affect Wheat Yield and Protein Content Response to Drought? A Meta-Analysis. Front. Plant Sci. 2022, 13, 896985. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, A.; Aydin, F. Effect of Water Stress at Various Growth Stages on Some Quality Characteristics of Winter Wheat. J. Agron. Crop Sci. 2004, 190, 93–99. [Google Scholar] [CrossRef]
- Zörb, C.; Becker, E.; Merkt, N.; Kafka, S.; Schmidt, S.; Schmidhalter, U. Shift of Grain Protein Composition in Bread Wheat under Summer Drought Events. J. Plant Nutr. Soil Sci. 2016, 180, 49–55. [Google Scholar] [CrossRef]
- Foulkes, M.J.; Hawkesford, M.J.; Barraclough, P.B.; Holdsworth, M.J.; Kerr, S.; Kightley, S.; Shewry, P.R. Identifying Traits to Improve the Nitrogen Economy of Wheat: Recent Advances and Future Prospects. Field Crops Res. 2009, 114, 329–342. [Google Scholar] [CrossRef]
- Liang, X.; Liu, Y.; Chen, J.; Adams, C. Late-season Photosynthetic Rate and Senescence Were Associated with Grain Yield in Winter Wheat of Diverse Origins. J. Agron. Crop Sci. 2017, 204, 1–12. [Google Scholar] [CrossRef]
- He, M.; Dijkstra, F.A. Drought Effect on Plant Nitrogen and Phosphorus: A Meta-analysis. New Phytol. 2014, 204, 924–931. [Google Scholar] [CrossRef]
- Peters, W.; van der Velde, I.R.; van Schaik, E.; Miller, J.B.; Ciais, P.; Duarte, H.F.; van der Laan-Luijkx, I.T.; van der Molen, M.K.; Scholze, M.; Schaefer, K.; et al. Increased Water-Use Efficiency and Reduced CO2 Uptake by Plants during Droughts at a Continental Scale. Nat. Geosci. 2018, 11, 744–748. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Xie, F. Effect of Drought Stress at Reproductive Stages on Growth and Nitrogen Metabolism in Soybean. Agronomy 2020, 10, 302. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, N.; Lu, H.; Zhu, L. Molecular Mechanism of Organic Pollutant-Induced Reduction of Carbon Fixation and Biomass Yield in Oryza sativa L. Environ. Sci. Technol. 2022, 56, 4162–4172. [Google Scholar] [CrossRef]
- Aluko, O.O.; Liu, Z.; Sun, X. The Interplay of Carbon and Nitrogen Distribution: Prospects for Improved Crop Yields. Mod. Agric. 2023, 1, 57–75. [Google Scholar] [CrossRef]
- Husenov, B.; Makhkamov, M.; Garkava-Gustavsson, L.; Muminjanov, H.; Johansson, E. Breeding for Wheat Quality to Assure Food Security of a Staple Crop: The Case Study of Tajikistan. Agric. Food Secur. 2015, 4, 9. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F. Effects of Elevated CO2 and Heat on Wheat Grain Quality. Plants 2021, 10, 1027. [Google Scholar] [CrossRef]
- Makhubu, F.N.; Mutanda, M.; Madala, N.E.; Figlan, S. Metabolite Profiling in Ten Bread Wheat (Triticum aestivum L.) Genotypes in Response to Drought Stress. Plant Stress 2024, 14, 100680. [Google Scholar] [CrossRef]
- Mutanda, M.; Shimelis, H.; Chaplot, V.; Shamuyarira, K.W.; Figlan, S. Agronomic Performance and Water Use Efficiency of Newly Developed Wheat Populations under Drought-Stressed and Non-Stressed Conditions. Discov. Appl. Sci. 2025, 7, 176. [Google Scholar] [CrossRef]
- Mutanda, M.; Shimelis, H.; Chaplot, V.; Figlan, S. Managing Drought Stress in Wheat (Triticum aestivum L.) Production: Strategies and Impacts. S. Afr. J. Plant Soil 2025, 1–12. [Google Scholar] [CrossRef]
- Mutanda, M.; Shimelis, H.; Chaplot, V.; Madala, N.E.; Figlan, S. Association between Agronomic Traits and Metabolite Profiles on Yield Response and Water Use Efficiency in Newly Developed Wheat Populations under Drought Stressed Conditions. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2025, 75, 2454389. [Google Scholar] [CrossRef]
- Robertson, M.J.; Fukai, S.; Peoples, M.B. The Effect of Timing and Severity of Water Deficit on Growth, Development, Yield Accumulation and Nitrogen Fixation of Mungbean. Field Crops Res. 2004, 86, 67–80. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, J.; Zhang, R.; Li, F.; Liang, L.; Liu, S.; Xu, B.; Chen, Y. Assessing the Impact of Early and Terminal Droughts on Root Growth, Grain Yield and Yield Stability in Old and Modern Wheat Cultivars on the Loess Plateau. Agric. Water Manag. 2024, 301, 108940. [Google Scholar] [CrossRef]
- Xu, Z.; Lai, X.; Ren, Y.; Yang, H.; Wang, H.; Wang, C.; Xia, J.; Wang, Z.; Yang, Z.; Geng, H.; et al. Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat. Agronomy 2023, 13, 2968. [Google Scholar] [CrossRef]
- Shamuyarira, K.W.; Shimelis, H.A.; Mathew, I.; Tsilo, T.J. Correlation and Path Coefficient Analyses of Yield and Yield Components in Drought-Tolerant Bread Wheat Populations. S. Afr. J. Plant Soil 2019, 36, 367–374. [Google Scholar] [CrossRef]
- Gous, P.W.; Warren, F.; Mo, O.W.; Gilbert, R.G.; Fox, G.P. The Effects of Variable Nitrogen Application on Barley Starch Structure under Drought Stress. J. Inst. Brew. 2015, 121, 502–509. [Google Scholar] [CrossRef]
- He, J.; Hu, W.; Li, Y.; Zhu, H.; Zou, J.; Wang, Y.; Meng, Y.; Chen, B.; Zhao, W.; Wang, S.; et al. Prolonged Drought Affects the Interaction of Carbon and Nitrogen Metabolism in Root and Shoot of Cotton. Environ. Exp. Bot. 2022, 197, 104839. [Google Scholar] [CrossRef]
- Shamuyarira, K.W.; Shimelis, H.; Figlan, S.; Chaplot, V. Path Coefficient and Principal Component Analyses for Biomass Allocation, Drought Tolerance and Carbon Sequestration Potential in Wheat. Plants 2022, 11, 1407. [Google Scholar] [CrossRef]
- Reynolds, M.P.; Pellegrineschi, A.; Skovmand, B. Sink-Limitation to Yield and Biomass: A Summary of Some Investigations in Spring Wheat. Ann. Appl. Biol. 2005, 146, 39–49. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant Root-Shoot Biomass Allocation over Diverse Biomes: A Global Synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Asadullah Kalhoro, S.A.; Farhad, W.; Iqbal, A.; Sultan Waheed, A.; Rashid, M.; Shah, S.R.U. Exploring the Variability of Root System Architecture under Drought Stress in Heat-Tolerant Spring-Wheat Lines. Plant Soil 2024, 502, 103–119. [Google Scholar] [CrossRef]
- Li, Q.; Ren, Y.; Fu, H.; Li, Z.; Kong, F.; Yuan, J. Cultivar Differences in Carbon and Nitrogen Accumulation, Balance, and Grain Yield in Maize. Front. Plant Sci. 2022, 13, 992041. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).