Evaluating the Impact of Mineral Nutrient Concentration and Substrate Volume on the Development of Three Annual Coastal Plant Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental System
2.2. Plant Material and Cultivation
2.3. Nondestructive Physiological Measurements
2.4. Plant Harvest
2.5. Data Analysis
3. Results
3.1. Experiment with Ranunculus sceleratus
3.2. Experiment with Plantago coronopus
3.3. Experiment with Phleum arenarium
4. Discussion
4.1. The Model System
4.2. Physiological Aspects
4.3. Ecological Relevance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DM | Dry mass |
| EC | Electrical conductivity |
| Fv/Fm | Maximum quantum efficiency of photosystem II |
| GS | Garden soil |
| MNC | Mineral nutrient concentration |
| PC1 | Plantago coronopus accession 1 |
| PC2 | Plantago coronopus accession 2 |
| PC3 | Plantago coronopus accession 3 |
| PC4 | Plantago coronopus accession 4 |
| PIT | Performance Index Total |
| SV | Substrate volume |
References
- Watkinson, A.R.; Davy, A.J. Population biology of salt marsh and sand dune annuals. Vegetatio 1985, 62, 487–497. [Google Scholar] [CrossRef]
- Pemadasa, M.A.; Lovell, P.H. Effects of the timing of the life cycle on the vegetative growth of some dune annuals. J. Ecol. 1976, 64, 213–222. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Element nutrition of two contrasted dune annuals. J. Ecol. 1983, 71, 197–209. [Google Scholar] [CrossRef]
- Ernst, W.H.O. Some considerations of and perspectives in coastal ecology. Vegetatio 1985, 62, 533–545. [Google Scholar] [CrossRef]
- Lee, J.A.; Ignaciuk, R. The physiological ecology of strandline plants. Vegetatio 1985, 62, 319–326. [Google Scholar] [CrossRef]
- He, Q.; Silliman, B.R.; Cui, B. Incorporating thresholds into understanding salinity tolerance: A study using salt-tolerant plants in salt marshes. Ecol. Evol. 2017, 7, 6326–6333. [Google Scholar] [CrossRef]
- Arbelet-Bonnin, D.; Ben-Hamed-Louati, I.; Laurenti, P.; Abdelly, C.; Ben-Hamed, K.; Bouteau, F. Cakile maritima, a promising model for halophyte studies and a putative cash crop for saline agriculture. Adv. Agron. 2019, 155, 45–78. [Google Scholar]
- Pemadasa, M.A.; Lovell, P.H. The mineral nutrition of some dune annuals. J. Ecol. 1974, 62, 647–657. [Google Scholar] [CrossRef]
- Madsen, J.D. Resource allocation at the individual plant level. Aquat. Bot. 1991, 41, 67–86. [Google Scholar] [CrossRef]
- Fitter, A.H. Influence of soil heterogeneity on the coexistence of grassland species. J. Ecol. 1982, 70, 139–148. [Google Scholar] [CrossRef]
- Fransen, B.; de Kroon, H.; Berendse, F. Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 1998, 11, 351–358. [Google Scholar] [CrossRef]
- Hinsinger, P.; Gobran, G.R.; Gregory, P.J.; Wenzel, W.W. Rhizosphere geometry and heterogeneity arising from root-mediated physical and chemical processes. New Phytol. 2005, 168, 293–303. [Google Scholar] [CrossRef]
- Wijesinghe, D.K.; John, E.A.; Hutchings, M.J. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. J. Ecol. 2005, 93, 99–112. [Google Scholar] [CrossRef]
- Ievinsh, G. Biological basis of biological diversity: Physiological adaptations of plants to heterogeneous habitats along a sea coast. Acta Univ. Latv. 2006, 710, 53–79. [Google Scholar]
- Karlsons, A.; Druva-Lusite, I.; Osvalde, A.; Necajeva, J.; Andersone-Ozola, U.; Samsone, I.; Ievinsh, G. Adaptation strategies of rare plant species to heterogeneous soil conditions on a coast of a lagoon lake as revealed by analysis of mycorrhizal symbiosis and mineral constituent dynamics. Environ. Exp. Biol. 2017, 15, 113–126. [Google Scholar]
- Druva-Lusite, I.; Karlsons, A.; Andersone-Ozola, U.; Ievina, B.; Necajeva, J.; Samsone, I.; Ievinsh, G. Physiological performance of a coastal marsh plant Hydrocotyle vulgaris in natural conditions in relation to mineral nutrition and mycorrhizal symbiosis. Proc. Latv. Acad. Sci. B 2020, 74, 252–262. [Google Scholar] [CrossRef]
- Karlsons, A.; Osvalde, A.; Ievinsh, G. Growth and mineral nutrition of two Triglochin species from saline wetlands: Adaptation strategies to conditions of heterogeneous mineral supply. Environ. Exp. Biol. 2011, 9, 83–90. [Google Scholar]
- Andersone-Ozola, U.; Gaile, L.; Ievinsh, G. Physiological responses of rare coastal salt marsh species Triglochin maritima L. to soil chemical heterogeneity. Acta Biol. Univ. Daugavp. 2017, 17, 149–155. [Google Scholar]
- Giehl, R.F.H.; von Wirén, N. Root nutrient foraging. Plant Physiol. 2014, 166, 509–517. [Google Scholar] [CrossRef]
- Tumber-Dávila, S.J.; Schenk, H.J.; Du, E.; Jackson, R.B. Plant sizes and shapes above and belowground and their interactions with climate. New Phytol. 2022, 235, 1032–1056. [Google Scholar] [CrossRef]
- Hess, L.; de Kroon, H. Effects of rooting volume and nutrient availability as an alternative explanation for root self/non-self discrimination. J. Ecol. 2007, 95, 241–251. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, W.; Chen, C.; Chen, L. Plant root-shoot biomass allocation over diverse biomes: A global synthesis. Glob. Ecol. Conserv. 2019, 18, e00606. [Google Scholar] [CrossRef]
- Podar, D.; Maathuis, F.J.M. Primary nutrient sensors in plants. iScience 2022, 25, 104029. [Google Scholar] [CrossRef]
- Chen, J.W.; During, H.J.; Vermeulen, P.J.; de Kroon, H.; Poorter, H.; Anten, N.P.R. The analysis of plant root responses to nutrient concentration, soil volume and neighbour presence: Different statistical approaches reflect different underlying basic questions. Funct. Ecol. 2020, 34, 2210–2217. [Google Scholar] [CrossRef]
- Murphy, G.P.; File, A.I.; Dudley, S.A. Differentiating the effects of pot size and nutrient availability on plant biomass and allocation. Botany 2013, 91, 799–803. [Google Scholar] [CrossRef]
- O’Brien, E.E.; Brown, J.S. Games roots play: Effects of soil volume and nutrients. J. Ecol. 2008, 96, 438–446. [Google Scholar] [CrossRef]
- Bonser, S.P. High reproductive efficiency as an adaptive strategy in competitive environments. Funct. Ecol. 2013, 27, 876–885. [Google Scholar] [CrossRef]
- Chapin, F.S. The mineral nutrition of wild plants. Ann. Rev. Ecol. Syst. 1980, 11, 233–260. [Google Scholar] [CrossRef]
- Storm, C.; Süs, K. Are low-productive plant communities responsive to nutrient addition? Evidence from sand pioneer grassland. J. Veget. Sci. 2008, 19, 343–354. [Google Scholar] [CrossRef]
- Dodds, J.G. Plantago coronopus L. J. Ecol. 1953, 41, 467–478. [Google Scholar] [CrossRef]
- Noë, R.; Blom, C.W.P.M. Occurrence of three Plantago species in coastal dune grasslands in relation to pore-volume and organic matter content of the soil. J. Appl. Ecol. 1981, 19, 177–182. [Google Scholar] [CrossRef]
- Waite, S.; Hutchings, M.J. Plastic energy allocation patterns in Plantago coronopus. Oikos 1982, 38, 333–342. [Google Scholar]
- Rushton, B.S. Morphological variation in some populations of Plantago coronopus. Ir. Nat. J. 1977, 19, 61–66. [Google Scholar]
- Rushton, B.S. Variation in reproductive allocation of Plantago coronopus L. related to habitat type and geographical location. Proc. R. Ir. Acad. Sect. B Biol. Geol. Chem. Sci. 1990, 90B, 175–192. [Google Scholar]
- Smekens, M.J.; van Tienderen, P.H. Genetic variation and plasticity of Plantago coronopus under saline conditions. Acta Oecol. 2001, 22, 187–200. [Google Scholar] [CrossRef]
- Tyler, T.; Herbertsson, L.; Olofsson, J.; Olsson, P.A. Ecological indicator and traits values for Swedish vascular plants. Ecol. Indic. 2021, 120, 106923. [Google Scholar] [CrossRef]
- Ievinsh, G.; Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Karlsons, A.; Osvalde, A. Salinity and heavy metal tolerance, and phytoextraction potential of Ranunculus sceleratus plants from a sandy coastal beach. Life 2022, 12, 1959. [Google Scholar] [CrossRef]
- Borhidi, A. Social behaviour types, the naturalness and relative ecological indicator values of the higher plants in the Hungarian flora. Acta Bot. Hung. 1995, 39, 97–181. [Google Scholar]
- Neiceniece, L.; Jēkabsone, A.; Andersone-Ozola, U.; Banaszczyk, L.; Osvalde, A.; Karlsons, A.; Ievinsh, G. Effect of salinity and nitrogen on heavy metal tolerance and accumulation potential in Rumex maritimus. Stresses 2025, 5, 29. [Google Scholar] [CrossRef]
- Pierce, S.; Negreiros, D.; Cerabolini, B.E.L.; Kattge, J.; Diaz, S.; Kleyer, M.; Shipley, B.; Wright, S.J.; Soudzilovskaia, N.A.; Onipchenko, V.G.; et al. A global method for calculating plant CSR ecological strategies applied across biomes world-wide. Funct. Ecol. 2017, 31, 444–457. [Google Scholar] [CrossRef]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Yang, T.; Samarakoon, U.; Altland, J.; Ling, P. Influence of electrical conductivity on plant growth, nutritional quality, and phytochemical properties of kale (Brassica napus) and collard (Brassica oleracea) grown using hydroponics. Agronomy 2024, 14, 2704. [Google Scholar] [CrossRef]
- Scoggins, H.L.; van Iersel, M.W. In situ probes for measurement of electrical conductivity of soilless substrates: Effects of temperature and substrate moisture content. HortScience 2006, 41, 210–214. [Google Scholar] [CrossRef]
- Rhoades, J.D. Salinity, electrical conductivity and total dissolved solids. In Methods of Soil Analysis, Part 3, Chemical Methods; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 417–435. [Google Scholar]
- Rubio, G.; Zhu, J.; Lynch, J.P. A critical test of the two prevailing theories of plant response to nutrient availability. Amer. J. Botany 2003, 90, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Weiner, J.; Campbell, L.G.; Pino, J.; Echarte, L. The allometry of reproduction within plant populations. J. Ecol. 2009, 97, 1220–1233. [Google Scholar] [CrossRef]
- Kolář, J.; Seňková, J. Reduction of mineral nutrient availability accelerates flowering of Arabidopsis thaliana. J. Plant Physiol. 2008, 165, 1601–1609. [Google Scholar] [CrossRef]
- Pigliucci, M.; Schlichting, C.D. Reaction norms of Arabidopsis (Brassicaceae). III. Response to nutrients in 26 populations from a worldwide collection. Am. J. Bot. 1995, 82, 1117–1125. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Tsay, Y.-F. Influence of differing nitrate and nitrogen availability on flowering control in Arabidopsis. J. Exp. Bot. 2017, 68, 2603–2609. [Google Scholar] [CrossRef]
- Bresson, J.; Bieker, S.; Riester, L.; Doll, J.; Zentgraf, U. A guideline for leaf senescence analyses: From quantification to physiological and molecular investigations. J. Exp. Bot. 2018, 69, 769–786. [Google Scholar] [CrossRef]
- Li, Z.; Liu, H.; Zhang, Y. A review on chlorophyll meter application on nitrogen fertilizer recommendation. Plant Nutr. Fert. Sci. 2006, 1, 125–132. [Google Scholar]
- Wang, S.; Guan, K.; Wang, Z.; Ainsworth, E.A.; Zheng, T.; Townsend, P.A.; Li, K.; Moller, C.; Wu, G.; Jiang, C. Unique contributions of chlorophyll and nitrogen to predict crop photosynthetic capacity from leaf spectroscopy. J. Exp. Bot. 2018, 72, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Vuletić, M.V.; Španić, V. Characterization of photosynthetic performance during natural leaf senescence in winter wheat: Multivariate analysis as a tool for phenotypic characterization. Photosynthetica 2020, 58, 301–313. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef]
- Ose, A.; Andersone-Ozola, U.; Ievinsh, G. Substrate-dependent effect of vermicompost on yield and physiological indices of container-grown Dracocephalum moldavica plants. Agriculture 2021, 11, 1231. [Google Scholar] [CrossRef]
- Ievinsh, G. Water content of plant tissues: So simple that almost forgotten? Plants 2023, 12, 1238. [Google Scholar] [CrossRef]
- Friedman, J. The evolution of annual and perennial plant life histories: Ecological correlated and genetic mechanisms. Annu. Rev. Ecol. Evol. Syst. 2020, 51, 461–481. [Google Scholar] [CrossRef]
- Gormally, C.L.; Donovan, L.A. No evidence of local adaptation in Uniola paniculata L. (Poaceae), a coastal dune grass. Southeast. Nat. 2011, 10, 751–760. [Google Scholar] [CrossRef]
- Castillo, J.M.; Grewell, B.J.; Pickart, A.; Bortolus, A.; Peña, C.; Figueroa, E.; Sytsma, M. Phenotypic plasticity of invasive Spartina densiflora (Poaceae) along a broad latitudinal gradient on the Pacific coast of North America. Am. J. Bot. 2014, 101, 448–458. [Google Scholar] [CrossRef]
- Gratani, L. Plant phenotypic plasticity in response to environmental factors. Adv. Bot. 2014, 2014, 208747. [Google Scholar] [CrossRef]
- Kohn, D.; Murrell, G.; Parker, J.; Whitehorn, M. What Henslow taught Darwin. How a herbarium helped to lay the foundations of evolutionary thinking. Nature 2005, 436, 643–645. [Google Scholar] [CrossRef] [PubMed]
- Pagel, J.; Treurnicht, M.; Bond, W.J.; Kraaij, T.; Nottebrock, H.; Schutte-Vlok, A.; Tonnabel, J.; Esler, K.J.; Schurr, F.M. Mismatches between demographic niches and geographic distributions are strongest in poorly dispersed and highly persistent plant species. Proc. Natl. Acad. Sci. USA 2020, 117, 3663–3669. [Google Scholar] [CrossRef]
- Sporbert, M.; Keil, P.; Seidler, G.; Bruelheide, H.; Jandt, U.; Aćić, S.; Biurrun, I.; Campos, J.A.; Čarni, A.; Chytrý, M.; et al. Testing macroecological abundance patterns: The relationship between local abundance and range size, range position and climatic suitability among European vascular plants. J. Biogeogr. 2020, 47, 2210–2222. [Google Scholar] [CrossRef]
- Wilson, J.B.; Lee, W.G. C-S-R triangle theory: Community-level predictions, tests, evaluation of criticisms, and relation to other theories. Oikos 2000, 91, 77–96. [Google Scholar] [CrossRef]
- Koyro, H.-W. Effect of salinity on growth, photosynthesis, water relations and solute composition of the potential cash crop halophyte Plantago coronopus (L.). Environ. Exp. Bot. 2006, 56, 136–146. [Google Scholar] [CrossRef]
- Ltaeif, H.B.; Sakhraoui, A.; González-Orenga, S.; Faz, A.L.; Boscaiu, M.; Vicente, O.; Rouz, S. Responses to salinity in four Plantago species from Tunisia. Plants 2021, 10, 1392. [Google Scholar] [CrossRef]



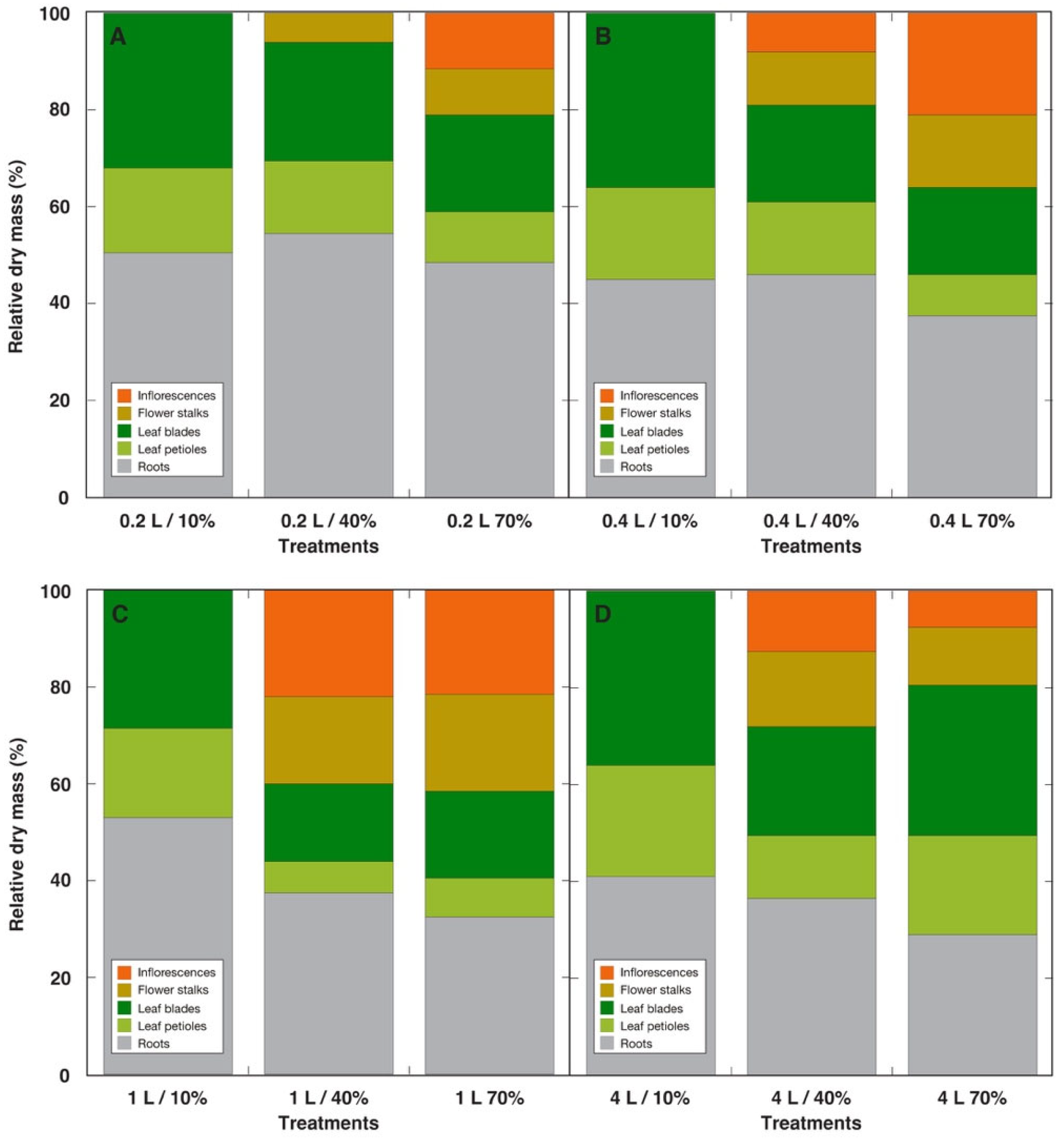

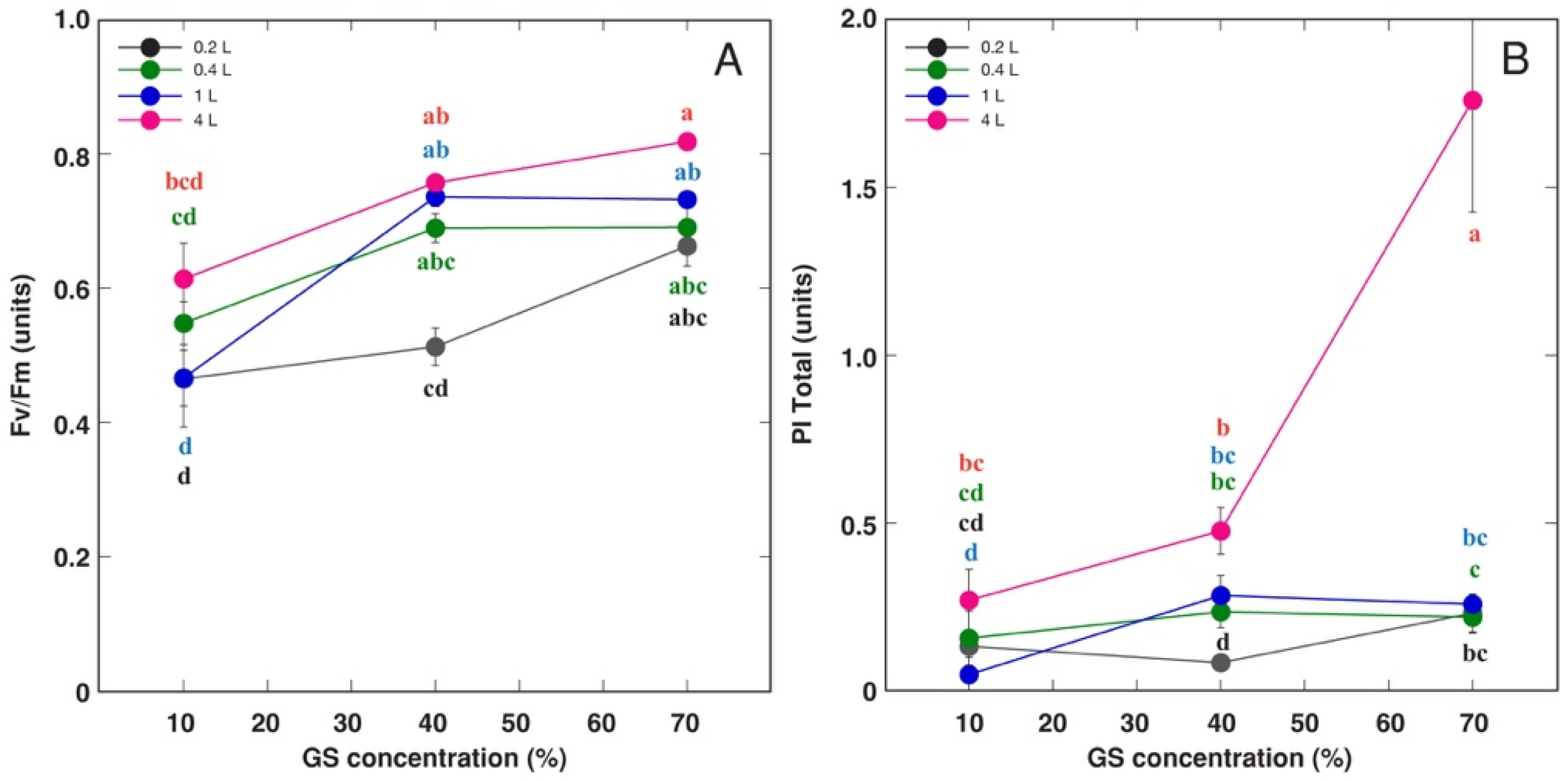


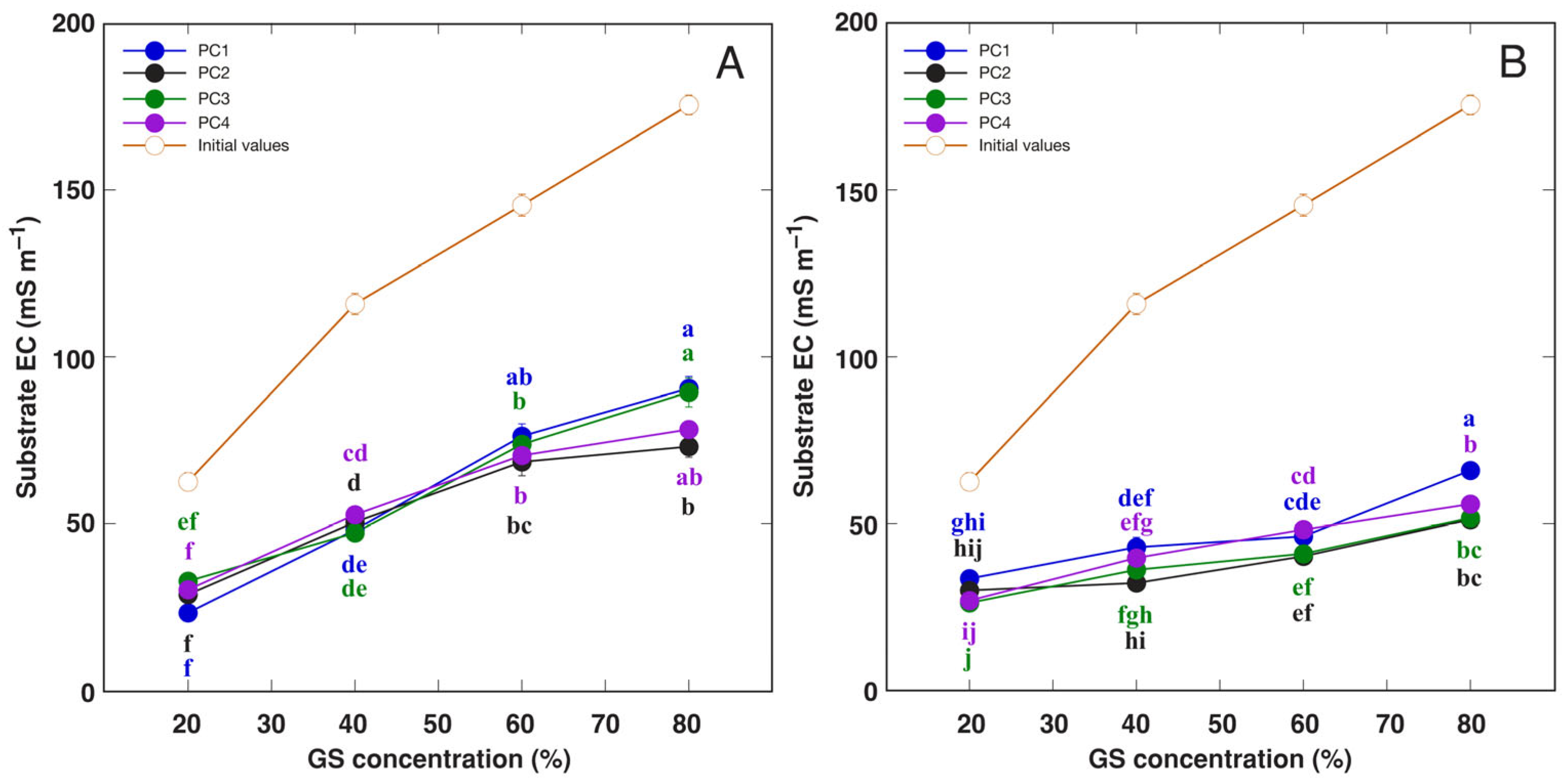
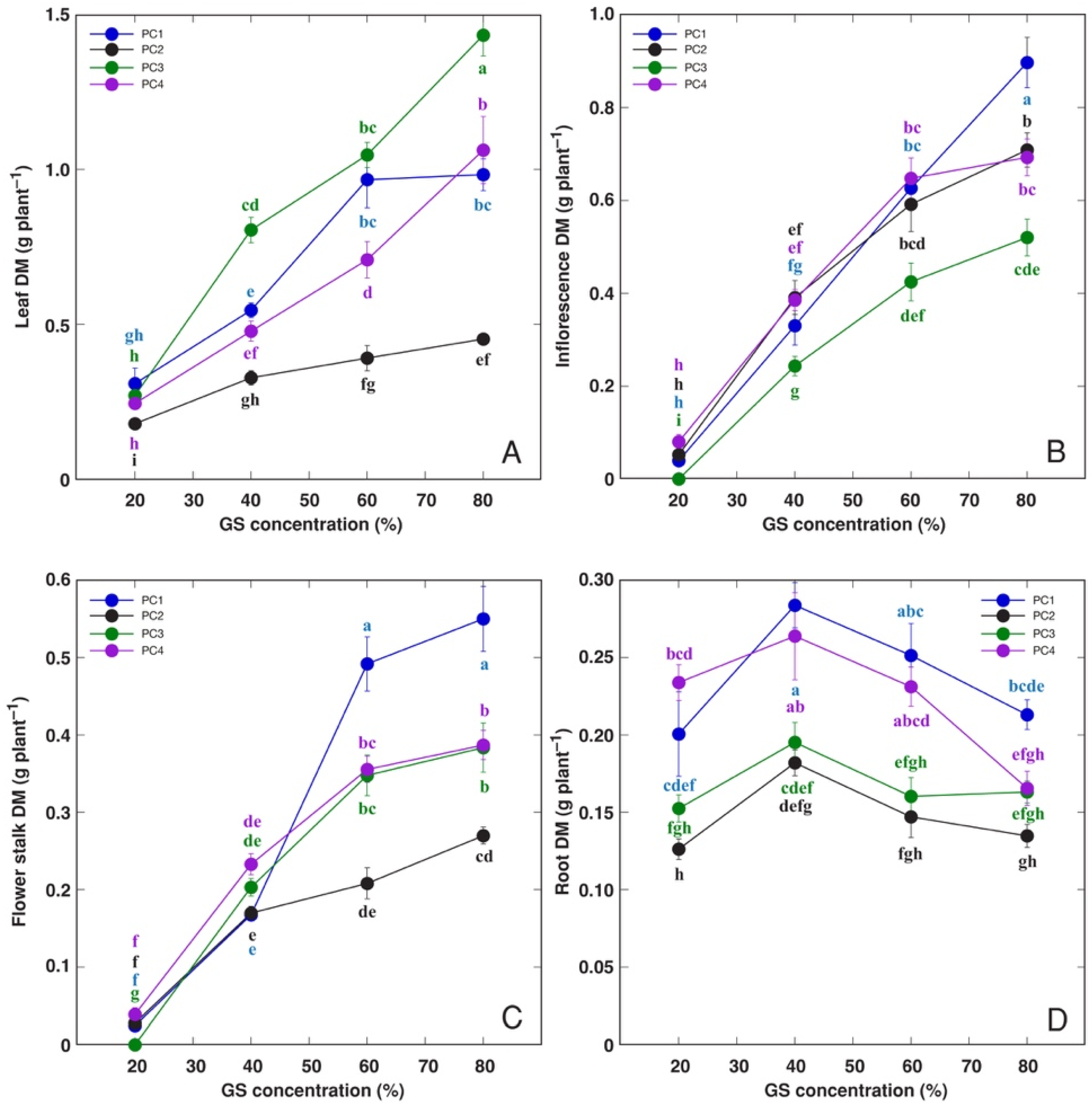



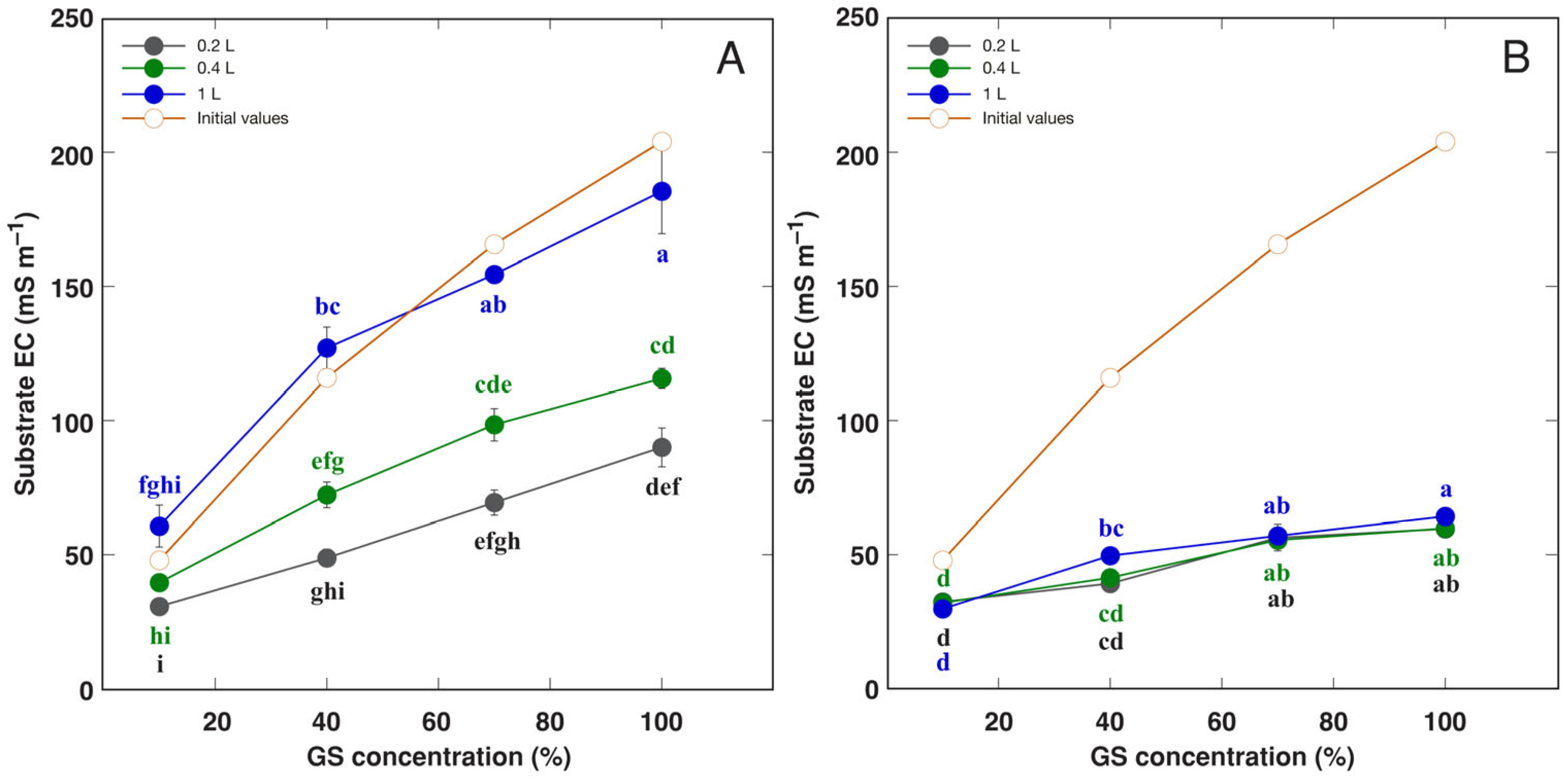

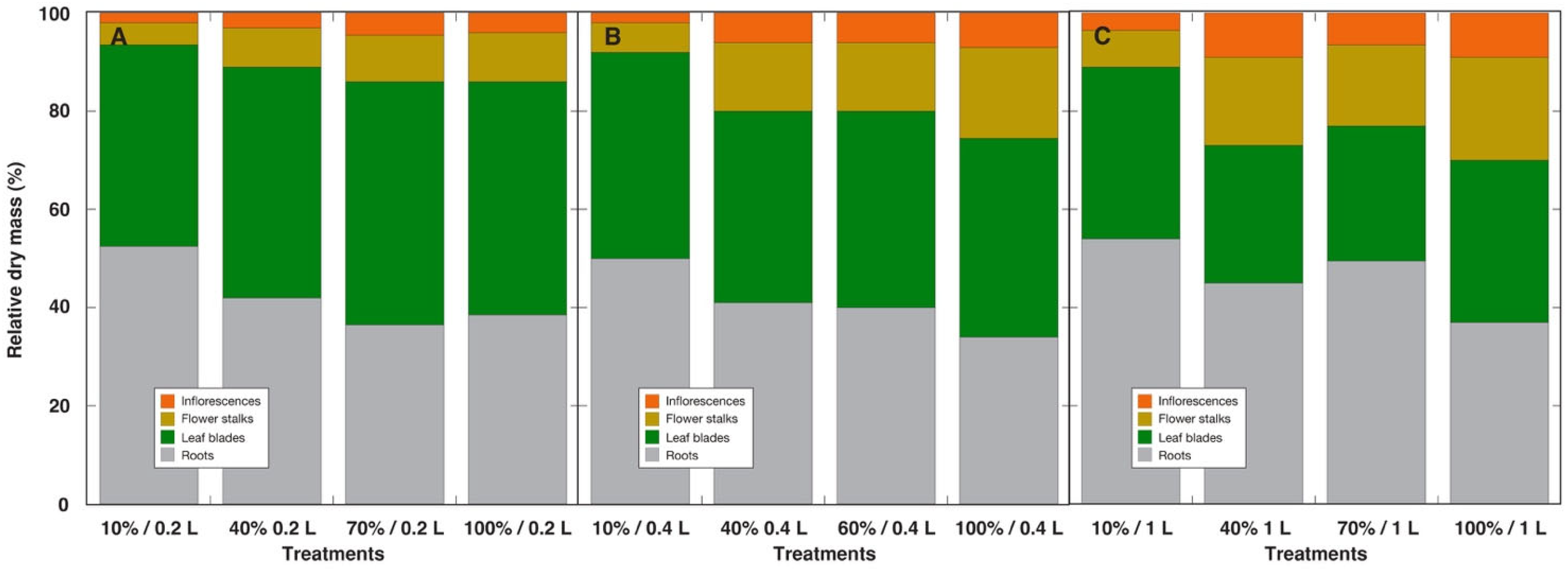
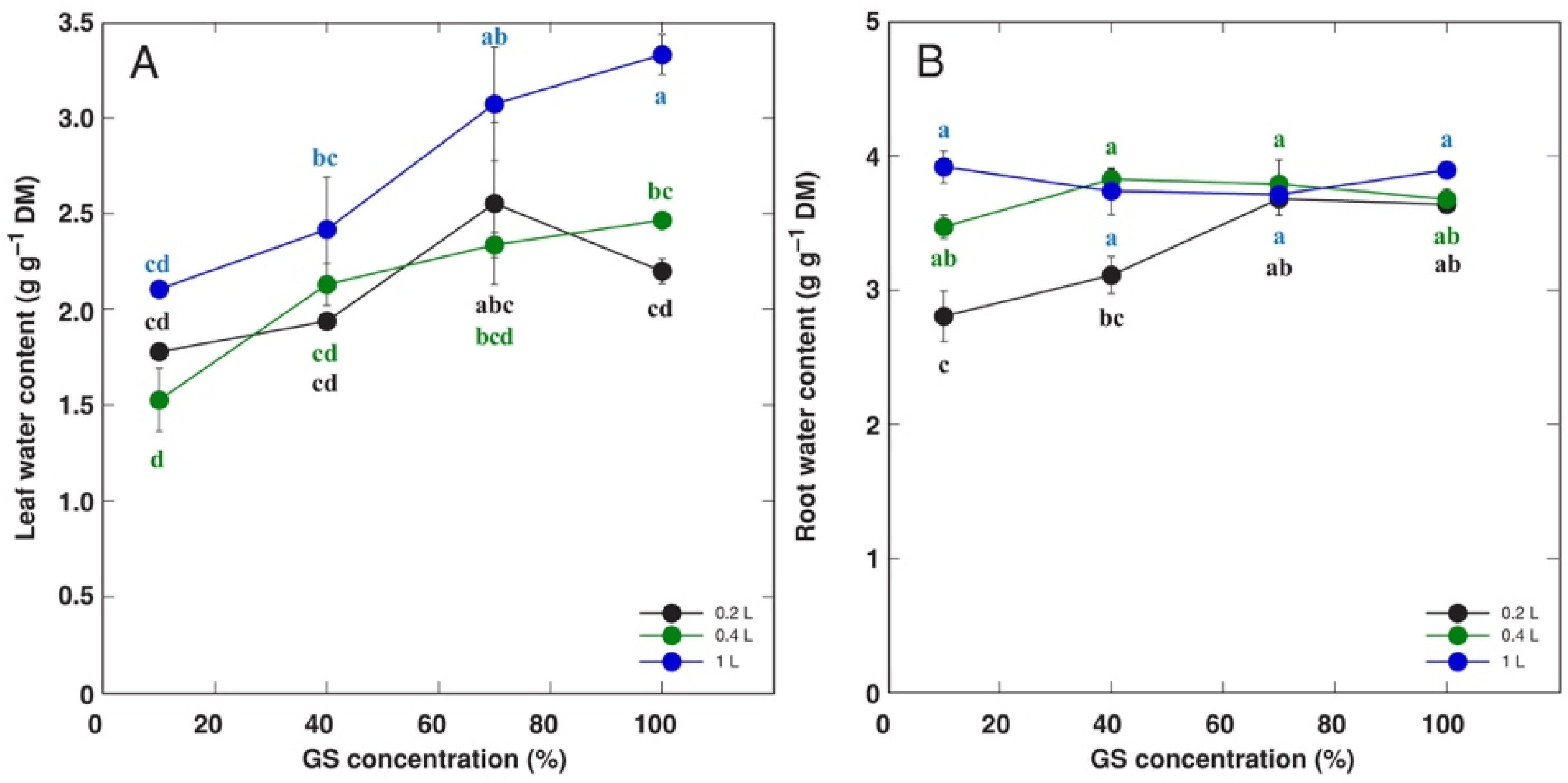

| Species | Competitor (%) | Stress Tolerator (%) | Ruderal (%) | Strategy | GS Substitution Rate (%) | Container Volume (L) |
|---|---|---|---|---|---|---|
| Phleum arenarium | 5.49 | 75.15 | 19.37 | S/SR | 10, 40, 70, 100 | 0.2, 0.4, 1 |
| Plantago coronopus | 32.35 | 0 | 67.65 | R/CR | 20, 40, 60, 80 | 0.2 |
| Ranunculus sceleratus | 46.32 | 0 | 53.68 | CR | 10, 40, 70 | 0.2, 0.4, 1, 4 |
| Response | Phleum arenarium | Plantago coronopus | Ranunculus sceleratus |
|---|---|---|---|
| Relative root biomass (% DM) | 34–54 | 7–38 | 29–55 |
| Relative biomass of generative structures (% DM) | 2–30 | 0–50 | 0–40 |
| Vegetative growth | Growth inhibited by low MNC, more at higher SV | – | Growth inhibited by low MNC and by low SV |
| Changes in root biomass | Increases by increasing MNC, lower at high SV | Weakly affected by MNC | Increases both by MNC and SV |
| Allocation to roots | Decreases with increasing MNC | Decreases with increasing MNC | Decreases with increasing MNC at high SV, decreases with SV at high MNC |
| Allocation to generative structures | Increases with increasing both MNC and SV | Increases by increasing MNC up to saturation | Decreases with increasing MNC at high SV, decreases with SV |
| Flowering and flowering time | Low MNC at low SV delays flowering | Low MNC inhibits flowering in a genotype-specific manner | Low MNC inhibits flowering irrespective of SV, high MNC delays flowering |
| Chlorophyll concentration during the first measurement | Increases with increasing MNC, in part by SV | – | Increases with increasing MNC, no changes with increasing SV |
| Chlorophyll concentration during the second measurement | Increases with increasing both MNC and SV | – | Indicates differences in leaf senescence |
| Leaf water content | Increases with increasing MNC | Decreases with increasing MNC in a genotype-specific manner | Low water content indicates leaf senescence |
| Root water content | Increases with increasing SV only at low MNC | No significant changes | Decreases with increasing MNC only at low SV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jēkabsone, A.; Andersone-Ozola, U.; Banaszczyk, L.; Ievinsh, G. Evaluating the Impact of Mineral Nutrient Concentration and Substrate Volume on the Development of Three Annual Coastal Plant Species. Int. J. Plant Biol. 2025, 16, 118. https://doi.org/10.3390/ijpb16040118
Jēkabsone A, Andersone-Ozola U, Banaszczyk L, Ievinsh G. Evaluating the Impact of Mineral Nutrient Concentration and Substrate Volume on the Development of Three Annual Coastal Plant Species. International Journal of Plant Biology. 2025; 16(4):118. https://doi.org/10.3390/ijpb16040118
Chicago/Turabian StyleJēkabsone, Astra, Una Andersone-Ozola, Lidia Banaszczyk, and Gederts Ievinsh. 2025. "Evaluating the Impact of Mineral Nutrient Concentration and Substrate Volume on the Development of Three Annual Coastal Plant Species" International Journal of Plant Biology 16, no. 4: 118. https://doi.org/10.3390/ijpb16040118
APA StyleJēkabsone, A., Andersone-Ozola, U., Banaszczyk, L., & Ievinsh, G. (2025). Evaluating the Impact of Mineral Nutrient Concentration and Substrate Volume on the Development of Three Annual Coastal Plant Species. International Journal of Plant Biology, 16(4), 118. https://doi.org/10.3390/ijpb16040118








