Abstract
Identifying optimal seed sources is critical for the propagation and restoration of Juglans neotropica Diels in the northern Ecuadorian Andes, where populations are declining due to habitat loss and overexploitation. This study evaluated the seed quality and germination performance of Juglans neotropica from three ecologically distinct provenances: a natural regeneration site (Cuyuja), a pure plantation (Natabuela), and an agroforestry system (Pimampiro). Five phenotypically superior trees were selected from each site, and germination was assessed under controlled nursery conditions over a 150-day period using a completely randomized design. Initial viability tests confirmed the physiological integrity of the seeds across all provenances. Germination onset ranged from day 55 to day 73, with significant differences in germination percentage, speed, and uniformity. The agroforestry provenance showed the highest germination rate (69%) and superior performance in all physiological indices, while natural regeneration had the lowest (15%). Post-trial viability assessments indicated that a substantial proportion of non-germinated seeds from Cuyuja remained dormant or deteriorated. These findings underscore the role of agroforestry systems in enhancing seed physiological quality and support their prioritization for large-scale propagation and ecological restoration initiatives involving Juglans neotropica.
1. Introduction
Juglans neotropica Diels, commonly referred to as Andean walnut, is a multipurpose forest tree species native to the South American Andes, with notable ecological and socioeconomic significance in Ecuador [1,2]. Its timber is highly prized for its durability, grain quality, and aesthetic appeal, serving both national and international markets [3]. Additionally, its edible nuts are valued for their nutritional properties and potential in specialty food markets [4], while the species holds cultural value through its use in artisanal crafts and religious items, reflecting the identity and traditions of highland communities [5].
From an ecological standpoint, J. neotropica contributes to biodiversity conservation and the provision of ecosystem services such as watershed protection, climate regulation, and habitat connectivity [6]. Its presence in fragmented Andean landscapes supports the persistence of endemic and migratory fauna. However, the species has experienced a marked population decline due to deforestation, overharvesting, and habitat degradation. In the past three decades, more than 50% of natural populations have been lost, prompting their classification as endangered (EN A2cd) by the IUCN [2]. In Ecuador, the loss of over 30% of Andean forest cover has further threatened its long-term viability [7].
In response, various conservation and restoration strategies have been implemented in regions such as Imbabura and Napo. These include the management of natural regeneration areas, protection of remnant stands, and establishment of agroforestry systems and monoculture plantations incorporating Juglans neotropica [8,9]. Despite these efforts, the success of restoration initiatives has been inconsistent, partly due to the use of non-local or genetically unsuitable seed sources [10].
In the context of forest genetic improvement and assisted restoration, the identification and use of genetically superior provenances—defined as populations originating from specific ecological or geographic zones—are critical to optimizing plantation performance and resilience [11,12]. Juglans neotropica displays considerable genetic and phenotypic variation across its distributional range, influenced by altitudinal, climatic, and ecological gradients [13]. This inherent variability provides an opportunity to select source populations with advantageous traits for specific environments, thereby increasing the likelihood of restoration success.
Ecuador’s wide range of elevations and climates contributes to significant local adaptation within J. neotropica, as documented in several population genetic studies [14,15]. In forest tree breeding programs, early-stage selection of optimal provenances is particularly effective, as it capitalizes on natural variation and offers the highest potential genetic gains relative to cost and effort [16,17].
To date, no studies have directly evaluated and compared germination behavior and physiological seed quality of Juglans neotropica across contrasting provenance types. This work addresses that gap by introducing a novel approach focused on seed performance under controlled conditions.
Specifically, this study aims to (i) evaluate differences in seed quality among three distinct provenances; (ii) analyze their germination performance under controlled conditions; and (iii) identify the provenance with the highest viability and adaptive potential.
These results are expected to inform seed sourcing strategies in ecological restoration and genetic conservation programs across Andean forest landscapes.
2. Materials and Methods
2.1. Study Sites and Environmental Conditions
The study was conducted in three contrasting sites in the northern Ecuadorian Andes, representing distinct ecophysiological conditions relevant for the assessment of seed source performance in Juglans neotropica (Figure 1).
Figure 1.
Geographic location of the three Juglans neotropica seed source sites (Cuyuja, Natabuela, Pimampiro) in the Northern Ecuadorian Andes.
- San Francisco de Natabuela (Plantation): Located in Antonio Ante canton, Imbabura Province (2255 m a.s.l.), this site features a pure plantation system with annual precipitation ranging from 750 to 1000 mm and an average temperature of 16 °C [18].
- Pimampiro (Agroforestry System): This site in Pimampiro canton (2165 m a.s.l.) integrates Juglans neotropica and Coffea arabica in an agroforestry matrix, with lower precipitation (500–750 mm) and comparable temperature (16 °C) [19].
- Cuyuja (Natural Regeneration): Located in Quijos canton, Napo Province (2310 m a.s.l.), this montane site has significantly higher rainfall (1750–2000 mm) and a cooler climate (10 °C), supporting natural forest regeneration [20].
Germination trials were conducted at the Yuyucocha Campus of Universidad Técnica del Norte (Ibarra, Imbabura), situated at 2256 m a.s.l. under relatively uniform climatic conditions (17 °C and 600 mm/year), as recorded by the INAMHI meteorological station M1240 [21].
2.2. Selection of Seed Trees
Seed tree selection was conducted through a purposive, non-probabilistic approach based on phenotypic superiority, following established procedures for identifying superior individuals [22]. Initial assessments covered 99 individuals in Natabuela, 145 in Pimampiro, and 40 in Cuyuja.
From each site, 10 trees were preselected based on qualitative traits (crown shape, phytosanitary condition, trunk form) and quantitative metrics (fruit yield, DBH, commercial height, total height). The five most outstanding trees per provenance were ultimately selected for seed collection.
2.3. Seed Quality Assessment
Seed quality was evaluated prior to germination. Parameters included physical purity, seed mass (seeds per kilogram), and moisture content, determined according to protocols of the International Seed Testing Association [23]. These assessments ensured the comparability and reliability of the germination data.
2.4. Germination Trials
The germination trial was conducted under nursery conditions between November 2022 and April 2023, following seed collection in October 2022. A completely randomized design was applied. From each of the 15 selected seed trees (five per provenance), 40 seeds were sown in four replicates of 10 seeds per tree, resulting in a total of 600 seeds.
Seeds were sown in 15 × 20 cm polyethylene bags filled with a standardized substrate composed of 60% black soil, 30% organic compost, and 10% pumice. Throughout the 150-day evaluation period, environmental conditions were rigorously controlled. Air temperature was maintained between 25 °C and 28 °C, while relative humidity was kept above 85% through the use of a transparent polyethylene enclosure and a timed misting system. Fine water sprays were applied for two minutes every three hours during the daytime period (08:00–17:00), creating a stable humid microenvironment that favored seed hydration and metabolic activation.
Seed viability was assessed both before and after the germination period. Prior to sowing, a random sample of 25 seeds per provenance was selected. Testa rupture was performed to expose the embryo, which was examined under a stereoscopic microscope. Seeds were classified as viable if they exhibited a well-defined embryonic axis, uniform coloration, and no signs of necrosis or malformation. At the end of the trial, the same procedure was applied to 25 non-germinated seeds from each provenance to determine residual viability.
Five germination parameters were used to evaluate the physiological response of seeds collected from different provenances. The germination percentage (PG) was calculated as shown in Equation (1) [23]. Additional indices related to germination rate and temporal dynamics were calculated following standard methodologies, as shown in Equations (2)–(5) [24]. A summary of these formulas is presented in Table 1.

Table 1.
Equations used to compute germination parameters in Juglans neotropica.
To illustrate the temporal dynamics of germination, cumulative germination curves were constructed for each provenance based on daily emergence records. The curves were plotted on standardized axes using the total number of seeds per provenance as the reference. Visualization was performed using the ggplot2 package in R version 4.1.0, applying a logistic smoothing function to improve interpretability while preserving the empirical structure of the data.
2.5. Statistical Analysis
Statistical analyses were conducted using complementary methods to rigorously assess the effects of provenance on seed quality and germination performance. A one-way analysis of variance (ANOVA) was applied to compare physical seed quality parameters—namely physical purity, seed mass, and moisture content—across provenances.
To evaluate germination dynamics, a multivariate analysis of variance (MANOVA) was performed using five dependent variables: final germination percentage, germination speed coefficient, germination index, germination speed index, and uniformity coefficient. Prior to MANOVA, assumptions of multivariate normality and homogeneity of covariance matrices were tested using Mardia’s test and Box’s M test, respectively [25,26].
In addition, linear mixed-effects models (LMMs) were implemented to account for the hierarchical structure of the experimental design. In these models, provenance was treated as a fixed effect, and individual seed tree identity was included as a random effect to capture intra-provenance variability. Model diagnostics confirmed that residuals met the assumptions of normality and homoscedasticity [27].
All analyses were conducted in R version 4.1.0, using the packages stats (for ANOVA), MVN (for multivariate normality tests), and lme4 and lmerTest (for LMMs) [28].
3. Results
3.1. Dasometric Characteristics of Selected Trees
Trees from the Cuyuja provenance exhibited the highest mean values for diameter at breast height (DBH), total height (TH), and commercial height (CH) among the evaluated sites (Figure 2). These superior growth patterns are likely driven by favorable climatic conditions in Cuyuja, notably higher annual precipitation (1750–2000 mm) and lower mean temperatures (10 °C), which enhance water availability and reduce thermal stress, thereby supporting the vigorous growth of Juglans neotropica. Moreover, the natural regeneration ecosystem at Cuyuja likely maintains greater genetic heterogeneity and competitive interactions that promote adaptive vigor in selected individuals.
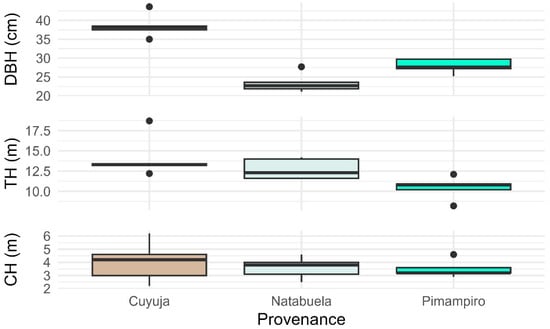
Figure 2.
Comparison of mean diameter at breast height (DBH), total height (TH), and commercial height (CH) among Juglans neotropica provenances.
3.2. Phenotypic Traits of Seed Trees
Phenotypic assessments revealed consistent expression of qualitative traits across the three provenances (Figure 3). In Pimampiro and Natabuela, 60% of evaluated trees were classified as superior, while in Cuyuja the proportion reached 50%. These classifications were based on the cumulative scoring of crown morphology, trunk straightness, and phytosanitary condition, applied to all measured individuals within each site. Despite exhibiting the highest mean values in dasometric parameters, trees from Cuyuja more frequently presented bifurcated crowns and trunk irregularities, resulting in lower phenotypic scores for structural traits. Overall, traits such as crown form and phytosanitary condition showed limited intra-site variation, suggesting a stable phenotypic expression that may favor their use in early selection for seed improvement programs.
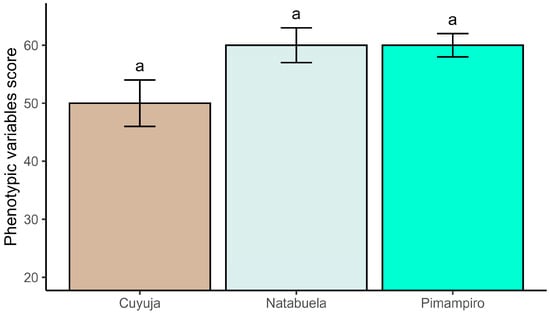
Figure 3.
Comparative phenotypic scores of selected Juglans neotropica trees based on crown shape, phytosanitary status, and trunk structure.
3.3. Seed Physical Quality
Seed purity showed statistically significant differences among provenances (ANOVA, p < 0.001), with Cuyuja presenting the highest physical purity (96.21% ± 2.59), exceeding values from Natabuela and Pimampiro (Figure 4). Despite this, purity was not strongly associated with germination success, suggesting that physiological dormancy may limit germination in natural stands.
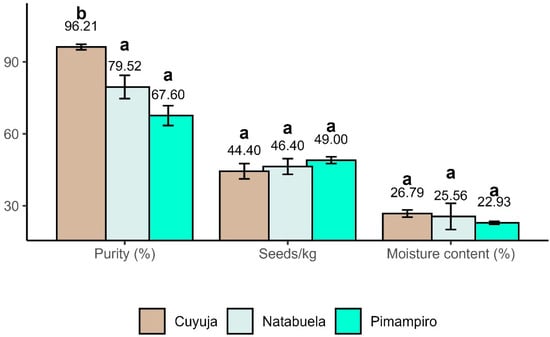
Figure 4.
Comparison of seed physical purity, moisture content, and density among provenances of Juglans neotropica. Different letters (a, b) indicate statistically significant differences among provenances (p < 0.05) according to Tukey’s HSD test.
3.4. Germination Performance
Initial viability assessments confirmed the physiological integrity of the seed lots used in the trial. None of the seeds analyzed exhibited signs of necrosis, structural malformation, or discoloration. Embryos were fully developed and morphologically intact across all provenances, indicating high initial viability and the absence of detectable deterioration prior to sowing.
Cumulative germination curves (Figure 5) reveal clear differences in germination timing and progression among provenances. Germination in Cuyuja began on day 55, followed by Pimampiro on day 57 and Natabuela on day 73. Although Cuyuja exhibited an earlier germination onset, its cumulative progression remained limited throughout the evaluation period. The agroforestry system (Pimampiro) demonstrated not only the earliest and most rapid germination but also the highest final accumulation.
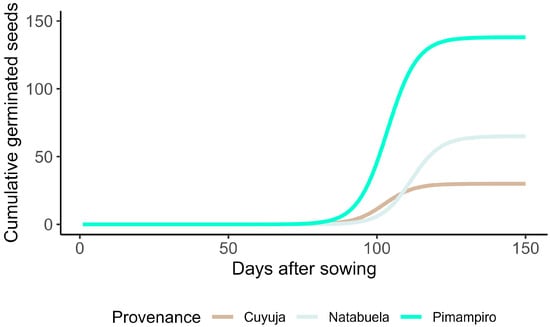
Figure 5.
Cumulative germination of Juglans neotropica over 150 days across three provenances in the northern Ecuadorian Andes.
At the end of the evaluation period, viability analysis of non-germinated seeds revealed patterns consistent with the cumulative germination profiles of each provenance. In Pimampiro, 30% of the non-germinated seeds showed signs of radicle emergence or testa rupture, 50% remained unchanged, and 20% exhibited tissue decomposition. In Natabuela, 40% showed evidence of germination activity, 40% showed no morphological changes, and 20% were deteriorated. In Cuyuja, only 20% of the non-germinated seeds exhibited signs of activation, while 50% remained unchanged and 30% showed signs of decomposition.
Multivariate analysis of variance (MANOVA) detected significant differences in germination variables among provenances (Pillai’s Trace = 1.07, F = 12.35, p < 0.001) (Table 2). These differences reflect the influence of environmental conditions and silvicultural systems on seed physiological quality.

Table 2.
(a) MANOVA summary of germination variables of Juglans neotropica across three provenances; (b) Multivariate comparison of provenance means and grouping based on Hotelling’s test (Bonferroni-adjusted, α = 0.05).
Canonical axis analysis (Figure 6) supported these findings, with germination percentage and germination index as the primary contributors to provenance differentiation. The agroforestry provenance showed stronger associations with faster and more uniform germination patterns.
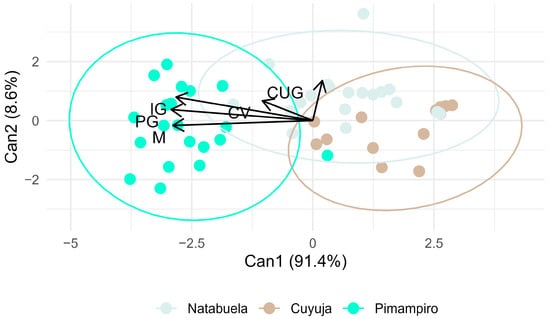
Figure 6.
Canonical axis analysis showing multivariate separation of provenances based on germination variables.
Linear mixed models further confirmed these trends (Figure 7), revealing that the agroforestry provenance exerted a significant positive effect on PG and IG, with minimal intra-provenance variability, indicating consistent seed performance within that system.
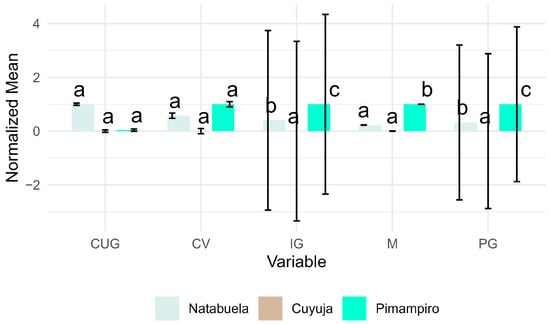
Figure 7.
Effects of provenance on germination metrics using linear mixed models, accounting for fixed (provenance) and random (tree) effects.
4. Discussion
The dasometric analysis revealed notable differences among provenances. Trees from the natural regeneration site in Cuyuja exhibited greater diameter at breast height (DBH), total height, and commercial height, likely due to higher precipitation and cooler temperatures that optimize water availability and reduce thermal stress. Additionally, natural regeneration environments promote greater genetic diversity and structural heterogeneity, which enhance competitive adaptation and resilience, as documented in studies on Quercus ilex and Zelkova serrata [29,30,31].
In contrast, individuals in the Natabuela plantation and Pimampiro agroforestry system exhibited lower growth metrics. In Natabuela, high tree density may lead to intraspecific competition for light and nutrients, favoring height growth over diameter expansion [32,33]. In Pimampiro, interspecific competition between Juglans neotropica and coffee (Coffea arabica) likely restricts resource acquisition [34]. However, agroforestry systems provide long-term benefits by enhancing soil structure, promoting biodiversity, and sustaining ecosystem services [35,36].
Qualitative traits—such as crown morphology, trunk straightness, and phytosanitary status—were consistent across provenances, indicating high heritability and limited phenotypic plasticity. These characteristics have proven to be reliable indicators for the selection of seed trees in Juglans species [37,38]. However, significant quantitative trait variation among provenances underscores the need to conserve diverse seed sources to maintain adaptive capacity and ensure long-term genetic improvement [39,40].
Seed purity differed significantly among provenances, with Cuyuja showing the highest values. This may reflect reduced anthropogenic disturbance and the self-cleaning effect of high humidity and organic matter decomposition, as previously observed under similar ecological conditions [41,42]. Nonetheless, the higher purity did not translate into superior germination, indicating that purity alone is insufficient to predict seed performance.
Seed mass and moisture content did not differ significantly among provenances; however, both parameters followed environmental gradients. The larger seed size observed in this study (46.6 seeds/kg) compared to prior reports from Loja [41] and Colombia [43] may reflect local genotypic adaptation to higher elevation and lower temperature conditions [44,45]. Moisture content was highest in Cuyuja, consistent with its humid montane environment, although elevated seed moisture can compromise storage longevity if not properly managed [46].
From a physiological perspective, classifying the storage behavior of Juglans neotropica seeds is essential for interpreting their germination dynamics. The measured moisture contents (22.93% to 26.79%) fall within the range typical of recalcitrant seeds, which are highly desiccation-sensitive and cannot withstand the drying required for long-term storage [43]. This contrasts sharply with orthodox seeds—such as those of Juglans regia—which naturally exhibit moisture contents as low as 3.11–3.98% at maturity [47], are tolerant to desiccation levels typical of orthodox seeds (i.e., below 10%) [43], and frequently require cold stratification to break physiological dormancy [48]. The absence of documented storage or stratification protocols for Juglans neotropica, combined with its high initial moisture content, supports the hypothesis that its seeds exhibit recalcitrant or possibly intermediate storage behavior.
These physiological traits emphasize the need to maintain optimal moisture conditions in the germination substrate. In our trials, the agroforestry provenance (Pimampiro) consistently outperformed the others across all germination metrics: germination percentage (PG), germination index (IG), germination speed index (M), and speed coefficient (CV). These results suggest that agroforestry systems create favorable microclimatic conditions—such as moderated temperature, partial shade, enhanced soil structure, and higher organic matter content—that promote seed metabolic activity, mitigate dormancy, and enhance germination performance [49,50,51].
Despite these favorable conditions, germination was not fully synchronous. Marked asynchrony was particularly evident in seeds from the natural regeneration provenance, and to a lesser extent in the agroforestry system. Although germination in Cuyuja began slightly earlier (day 55), its cumulative curve plateaued rapidly, reflecting a limited physiological response. In Pimampiro, which initiated germination on day 57, the progression was sustained and consistent, yielding the highest cumulative values. Natabuela exhibited a delayed onset (day 73) but maintained a moderate and progressive increase, stabilizing between the other two provenances.
This pattern aligns with previous findings in Juglans neotropica, where delayed germination is associated with both physical barriers—such as a hard endocarp—and physiological dormancy regulated by endogenous inhibitors [52]. In our study, the low uniformity coefficient (CUG), particularly in Cuyuja, supports the presence of dormancy-related mechanisms. Additionally, the high initial moisture content may have interfered with the partial dehydration required to trigger germination, contributing to the observed temporal dispersion.
This mechanism may explain the paradox observed in Cuyuja, where the highest seed purity coincided with the lowest germination rates. In montane ecosystems, seed dormancy often functions as a bet-hedging strategy, delaying germination until environmental conditions are predictably favorable [53]. These findings highlight the importance of incorporating pre-germination treatments when using seeds from natural stands in restoration efforts.
Canonical axis analysis indicated that final germination percentage (PG) and germination index (IG) were the most discriminating variables among provenances, particularly favoring the Pimampiro agroforestry system. These indices are robust indicators of seed vigor and viability [24]. Meanwhile, the germination uniformity coefficient (CUG) was most strongly associated with Cuyuja, reflecting synchronized yet constrained germination, which aligns with dormancy-regulated behavior in montane species.
Linear mixed models further confirmed the significant effect of provenance on germination dynamics, with Pimampiro exhibiting superior and more consistent performance. This statistical robustness highlights its reliability for large-scale nursery propagation and forest restoration under variable environmental conditions.
To date, most research on Juglans neotropica has emphasized population structure, distribution patterns, or conservation status [13,14,41], with minimal attention to the physiological seed traits that underpin propagation success. This study is the first to integrate phenotypic selection of seed trees with multivariate germination analysis across ecologically contrasted provenances. The use of advanced statistical methods (MANOVA and LMMs) to assess germination behavior provides a methodological innovation, offering a more predictive approach for selecting seed sources with adaptive potential.
The superior performance of seeds from the Pimampiro agroforestry system supports its prioritization as a source for propagation and ecological restoration. Agroecological management appears to enhance seed physiological quality, possibly through improved soil conditions, microclimate buffering, and maternal effects—factors especially relevant under climate uncertainty [11,15].
Future research should explore the genetic basis of these differences using genomic tools such as SNP genotyping or GWAS, to identify markers associated with germination traits [13,14]. Parallel assessments of phenotypic plasticity across elevation and management gradients could elucidate the stability and adaptability of selected provenances in the face of environmental change [54,55].
5. Conclusions
The results of this study demonstrate that provenance exerts a significant influence on the seed quality and germination performance of Juglans neotropica in the northern Ecuadorian Andes. While the natural regeneration site in Cuyuja exhibited the most vigorous tree growth and highest seed purity, these advantages did not translate into germination success, likely due to dormancy-related constraints.
In contrast, seeds collected from the Pimampiro agroforestry system exhibited the highest values for germination percentage, index, and speed, along with reduced intra-provenance variability. These outcomes highlight the effectiveness of agroforestry management in enhancing seed physiological performance through favorable microclimatic and edaphic conditions.
The uniform expression of qualitative phenotypic traits across all provenances supports their reliability as selection criteria for seed tree identification. However, the observed variation in quantitative traits and germination behavior underscores the importance of maintaining genetic diversity through the conservation and integration of multiple seed sources.
Given its superior and consistent germination performance, the Pimampiro agroforestry system is identified as a priority seed source for propagation and restoration initiatives involving Juglans neotropica. Future research should incorporate genomic tools and phenotypic plasticity assessments to strengthen seed sourcing strategies and guide climate-resilient reforestation efforts in Andean landscapes.
Author Contributions
Conceptualization, J.-L.R.-L., M.A. and H.V.; methodology, J.-L.R.-L., M.A. and H.V.; software, J.-L.R.-L.; validation, J.-L.R.-L.; formal analysis, J.-L.R.-L.; investigation, M.A., H.V., K.E. and C.A.; resources, M.A., H.V., K.E. and C.A.; data curation, J.-L.R.-L., H.V. and K.E.; writing—original draft preparation, J.-L.R.-L.; writing—review and editing, J.-L.R.-L., M.A., H.V. and C.A.; visualization, J.-L.R.-L.; supervision, J.-L.R.-L., M.A. and H.V.; project administration, H.V. and C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DBH | Diameter at Breast Height |
| TH | Total Height |
| CH | Commercial Height |
| PG | Germination Percentage |
| IG | Germination Index |
| M | Germination Speed Index |
| CV | Germination Speed Coefficient |
| CUG | Germination Uniformity Coefficient |
| LMM | Linear Mixed Model |
| MANOVA | Multivariate Analysis of Variance |
| ISTA | International Seed Testing Association |
| INAMHI | Instituto Nacional de Meteorología e Hidrología (Ecuador) |
| PDOT | Plan de Desarrollo y Ordenamiento Territorial |
References
- Palacios-Herrera, B.; Pereira-Lorenzo, S.; Pucha-Cofrep, D. Natural and Artificial Occurrence, Structure, and Abundance of Juglans neotropica Diels in Southern Ecuador. Agronomy 2023, 13, 2531. [Google Scholar] [CrossRef]
- Toro Vanegas, E.; Roldán Rojas, I.C. State of the Art, Propagation and Conservation of Juglans neotropica Diels in Andean Zones. Madera Bosques 2018, 24, e11560. [Google Scholar] [CrossRef]
- Valverde-Rodríguez, J.X. Composición química de la madera de Juglans neotropica Diels y su relación con las propiedades químicas del suelo en la parroquia Valladolid, provincia de Zamora Chinchipe, Ecuador. Rev. Investig. Agrar. 2020, 2, 68–82. [Google Scholar] [CrossRef]
- Vilcacundo, E.; Alvarez, M.; Silva, M.; Carpio, C.; Morales, D.; Carrillo, W. Walnut protein concentrate (Juglans neotropica Diels), gastrointestinal digests and their antioxidant capacity. Asian J. Pharm. Clin. Res. 2018, 11, 395–398. [Google Scholar] [CrossRef]
- Bourque, N. Ritual and remembrance in the Ecuadorian Andes. Mt. Res. Dev. 2012, 32, 489–490. [Google Scholar] [CrossRef]
- Medina, J.; Quizhpe, W.; Déleg, J.; Gonzalez, K.; Aguirre, Z.; Aguirre, N.; Montaño, L.; Benítez, Á. Are Juglans neotropica plantations useful as a refuge of bryophytes diversity in tropical areas? Life 2021, 11, 434. [Google Scholar] [CrossRef] [PubMed]
- Ministerio del Ambiente [MAE]. Estadísticas del Patrimonio Natural del Ecuador Continental, 2nd ed.; Ministerio del Ambiente: Quito, Ecuador, 2018; Available online: https://proamazonia.org/wp-content/uploads/2019/10/ECUADOR_Folleto_Patrimonio_Natural_compressed.pdf (accessed on 4 November 2024).
- Cuenca, P.; Robalino, J.; Arriagada, R.; Echeverría, C. Are government incentives effective for avoided deforestation in the tropical Andean forest? PLoS ONE 2018, 13, e0203545. [Google Scholar] [CrossRef]
- Jones, K.W.; Holland, M.B.; Naughton-Treves, L.; Morales, M.; Suarez, L.; Keenan, K. Forest conservation incentives and deforestation in the Ecuadorian Amazon. Environ. Conserv. 2017, 44, 56–65. [Google Scholar] [CrossRef]
- Mohebalian, P.M.; Aguilar, F.X. Additionality and design of forest conservation programs: Insights from Ecuador’s Socio Bosque Program. For. Policy Econ. 2016, 71, 103–114. [Google Scholar] [CrossRef]
- Fremout, T.; Thomas, E.; Bocanegra-González, K.T.; Aguirre-Morales, C.A.; Morillo-Paz, A.T.; Atkinson, R.; Kettle, C.; González-M, R.; Alcázar-Caicedo, C.; González, M.A.; et al. Dynamic seed zones to guide climate-smart seed sourcing for tropical dry forest restoration in Colombia. For. Ecol. Manage. 2021, 490, 119127. [Google Scholar] [CrossRef]
- Tsegaye, M.; Lemage, B.; Hido, A. Seedling performance of different provenances of selected indigenous tree species in Debub Ari District, Southern Ethiopia. Glob. J. Earth Environ. Sci. 2021, 6, 38–43. [Google Scholar] [CrossRef]
- Stevens, K.A.; Woeste, K.; Chakraborty, S.; Crepeau, M.W.; Leslie, C.A.; Martínez-García, P.J.; Puiu, D.; Romero-Severson, J.; Coggeshall, M.; Dandekar, A.M.; et al. Genomic variation among and within six Juglans species. G3 Genes Genomes Genet. 2018, 8, 2153–2165. [Google Scholar] [CrossRef] [PubMed]
- Collevatti, R.G.; Novaes, E.; Silva-Junior, O.B.; Vieira, L.D.; Lima-Ribeiro, M.S.; Grattapaglia, D. A genome-wide scan shows evidence for local adaptation in a widespread keystone Neotropical forest tree. Heredity 2019, 123, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Cortés, A.J.; Restrepo-Montoya, M.; Bedoya-Canas, L.E. Modern strategies to assess and breed forest tree adaptation to changing climate. Front. Plant Sci. 2020, 11, 583323. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, S.; Chen, S.; Xia, D.; Yang, C.; Zhao, X. Genetic variation and superior provenances selection for wood properties of Larix olgensis at four trials. J. For. Res. 2022, 33, 1867–1879. [Google Scholar] [CrossRef]
- Rodríguez-Vásquez, M.E.; Rodríguez-Ortiz, G.; Enríquez-Del Valle, J.R.; Campos-Ángeles, G.V.; Velasco-Velasco, V.A.; Hernández-Hernández, A. Ensayos de progenies y huertos semilleros de especies forestales en México. Rev. Mex. Agroecosistemas 2021, 8, 79–88. [Google Scholar]
- GADP San Francisco de Natabuela. Plan de Desarrollo y Ordenamiento Territorial (PDOT) 2019–2023; Gobierno Autónomo Descentralizado Parroquial de San Francisco de Natabuela: Imbabura, Ecuador, 2019; Available online: https://www.gadnatabuela.gob.ec/documents/PDOT-OFICIAL-NATABUELA-2019-2023-.pdf (accessed on 4 November 2024).
- GADM Pimampiro. Actualización del Plan de Desarrollo y Ordenamiento Territorial del Cantón San Pedro de Pimampiro 2014–2027; Gobierno Autónomo Descentralizado Municipal de Pimampiro: Imbabura, Ecuador, 2015; Available online: https://www.imbabura.gob.ec/index.php/componente-territorial/instrumentos-de-planificacion/pdot-cantonal/file/506-pdot-pimampiro (accessed on 21 October 2024).
- GADP Cuyuja. Plan de Desarrollo y Ordenamiento Territorial 2030; Gobierno Autónomo Descentralizado Parroquial de Cuyuja: Napo, Ecuador, 2019; Available online: https://sil.napo.gob.ec/wp-content/uploads/2022/09/PDOT-Cuyuja.pdf (accessed on 4 November 2024).
- Instituto Nacional de Meteorología e Hidrología [INAMHI]. Visor de Estaciones Meteorológicas e Hidrológicas—Estación Meteorológica Ibarra-1 (M1240). Available online: http://www.inamhi.gob.ec/visor/estaciones (accessed on 28 May 2024).
- Ordóñez, L.; Aguirre, N.; Hofstede, R. Sitios de Recolección de Semillas Forestales Andinas del Ecuador; Ediciones Abya-Yala: Quito, Ecuador, 2001; ISBN 9978-04-745-X. [Google Scholar]
- International Seed Testing Association (ISTA). Reglas Internacionales Para El Análisis de Las Semillas 2016 (International Rules for Seed Testing, 2016 ed.); ISTA: Bassersdorf, Switzerland, 2016; ISSN 2310-3655. [Google Scholar]
- González-Zertuche, L.; Orozco-Segovia, A. Métodos de análisis de datos en la germinación de semillas, un ejemplo: Manfreda brachystachya. Bot. Sci. 1996, 58, 15–30. [Google Scholar] [CrossRef]
- Ahmad, R. A robustness evaluation of homogeneity test of covariance matrices. In Lecture Notes on Data Engineering and Communications Technologies, Proceedings of the Fifteenth International Conference on Management Science and Engineering Management (ICMSEM 2021), Toledo, Spain, 2–3 August 2021; Xu, J., García Márquez, F.P., Ali Hassan, M.H., Duca, G., Hajiyev, A., Altiparmak, F., Eds.; Springer: Cham, Switzerland, 2021; Volume 78, pp. 313–324. [Google Scholar] [CrossRef]
- Kim, N. Omnibus tests for multivariate normality based on Mardia’s skewness and kurtosis using normalizing transformation. Commun. Stat. Appl. Methods 2020, 27, 501–510. [Google Scholar] [CrossRef]
- Tanaka, E.; Hui, F.K.C. Symbolic formulae for linear mixed models. In Statistics and Data Science: Research School on Statistics and Data Science, RSSDS 2019, Melbourne, Australia, 24–26 July 2019, Proceedings; Nguyen, H., Ed.; Communications in Computer and Information Science; Springer: Singapore, 2019; Volume 1150, pp. 3–21. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.1.0; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 4 November 2024).
- Gouveia-Barrocas, E.; Gonçalves, A.C. A new methodology to evaluate natural regeneration: A case study of Quercus ilex in the Montado in Portugal. Front. For. Glob. Chang. 2023, 6, 1123248. [Google Scholar] [CrossRef]
- Iwaizumi, M.G.; Takahashi, M.; Yano, K. Temporal variation in regeneration events affecting population structure in different size- and life-stages contributes to overall genetic diversity of natural Zelkova serrata population. J. For. Res. 2021, 26, 32–42. [Google Scholar] [CrossRef]
- Verbylaitė, R.; Aravanopoulos, F.A.; Baliuckas, V.; Juškauskaitė, A. Genetic monitoring of Alnus glutinosa natural populations using two generation cohorts. Forests 2023, 14, 330. [Google Scholar] [CrossRef]
- Del Río, M.; Condés, S.; Pretzsch, H. Analyzing size-symmetric vs. size-asymmetric and intra- vs. inter-specific competition in beech (Fagus sylvatica L.) mixed stands. For. Ecol. Manag. 2014, 325, 90–98. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, X.; Wu, R.; Han, H.; Kang, F.; Zhu, J.; Tian, P. Effect of intraspecific competition on biomass partitioning of Larix principis-rupprechtii. J. Plant Interact. 2018, 13, 1–8. [Google Scholar] [CrossRef]
- Defrenet, E.; Roupsard, O.; Van Den Meersche, K.; Charbonnier, F.; Pérez-Molina, J.P.; Khac, E.; Prieto, I.; Stokes, A.; Roumet, C.; Rapidel, B.; et al. Root biomass, turnover and net primary productivity of a coffee agroforestry system in Costa Rica: Effects of soil depth, shade trees, distance to row and coffee age. Ann. Bot. 2016, 118, 833–851. [Google Scholar] [CrossRef]
- Santos, P.Z.F.; Crouzeilles, R.; Sansevero, J.B.B. Can agroforestry systems enhance biodiversity and ecosystem service provision in agricultural landscapes? A meta-analysis for the Brazilian Atlantic Forest. For. Ecol. Manag. 2019, 433, 140–145. [Google Scholar] [CrossRef]
- Udawatta, R.P.; Rankoth, L.M.; Jose, S. Agroforestry for Biodiversity Conservation; Springer: Cham, Switzerland, 2021; ISBN 978-3-030-80060-4. [Google Scholar]
- Raurau Quisiyupanqui, M.N. Caracterización de Fuentes Semilleras Para uso Sostenible y Conservación de Recursos Forestales de los Bosques Andinos de Loja, Ecuador. Master’s Thesis, Centro Agronómico Tropical de Investigación y Enseñanza (CATIE), Turrialba, Costa Rica, 2012. [Google Scholar]
- Ortiz Muñoz, E.; Acosta Hernández, C.C.; Linares Márquez, P.; Morales Romero, Z.; Rebolledo Camacho, V. Selección de árboles semilleros de Juglans pyriformis Liebm. en poblaciones naturales de Coatepec y Coacoatzintla, Veracruz. Rev. Mex. Cienc. For. 2016, 7, 43–58. [Google Scholar] [CrossRef]
- Olsson, S.; Dauphin, B.; Jorge, V.; Grivet, D.; Farsakoglou, A.M.; Climent, J.; Alizoti, P.; Faivre-Rampant, P.; Pinosio, S.; Milesi, P.; et al. Diversity and enrichment of breeding material for resilience in European forests. For. Ecol. Manag. 2023, 530, 120748. [Google Scholar] [CrossRef]
- Ruņgis, D.; Luguza, S.; Baders, E.; Šķipars, V.; Jansons, A. Comparison of genetic diversity in naturally regenerated Norway spruce stands and seed orchard progeny trials. Forests 2019, 10, 926. [Google Scholar] [CrossRef]
- Herrera Herrera, C.M. Evaluación de Fuentes Semilleras de Especies Forestales Nativas Como Apoyo a Programas y Políticas de Reforestación de la Provincia de Loja. Master’s Thesis, Universidad Nacional de Loja, Loja, Ecuador, 2016. [Google Scholar]
- Marinho, O.A.; Martinelli, L.A.; Duarte-Neto, P.J.; Mazzi, E.A.; King, J.Y. Photodegradation influences litter decomposition rate in a humid tropical ecosystem, Brazil. Sci. Total Environ. 2020, 715, 136601. [Google Scholar] [CrossRef]
- Ceballos-Freire, Á.J.; López-Ríos, J.A. Conservación de la calidad de semillas forestales nativas en almacenamiento. Cenicafé 2007, 58, 265–292. [Google Scholar]
- Wu, H.; Meng, H.; Wang, S.; Wei, X.; Jiang, M. Geographic patterns and environmental drivers of seed traits of a relict tree species. For. Ecol. Manag. 2018, 422, 59–68. [Google Scholar] [CrossRef]
- Cuyckens, G.E.E.; Hensen, I.; López, V.L.; Cellini, J.M.; Renison, D. Germination of high Andean treeline species of contrasting environments and along elevational gradients in northwest Argentina. Neotrop. Biodivers. 2021, 7, 111–120. [Google Scholar] [CrossRef]
- Garcias-Morales, C.; Orozco-Segovia, A.; Soriano, D.; Zuloaga-Aguilar, S. Effects of in situ burial and sub-optimal storage on seed longevity and reserve resources in sub-tropical mountain cloud forest tree species of Mexico. Trop. Conserv. Sci. 2021, 14, 1940082921989196. [Google Scholar] [CrossRef]
- Simsek, M.; Gulsoy, E.; Beyhan, O.; Osmanoglu, A.; Turgut, Y. Determination of some botanical, phenological, physical and chemical characteristics of walnut (Juglans regia L.) genotypes grown in Turkey. Appl. Ecol. Environ. Res. 2017, 15, 1279–1291. [Google Scholar] [CrossRef]
- Keshavarzian, M.; Gerivani, Z.; Sadeghipour, H.R.; Aghdasi, M.; Azimmohseni, M. Suppression of mitochondrial dehydrogenases accompanying post-glyoxylate cycle activation of gluconeogenesis and reduced lipid peroxidation events during dormancy breakage of walnut kernels by moist chilling. Sci. Hortic. 2013, 161, 314–323. [Google Scholar] [CrossRef]
- Vijay, D.; Gupta, S.K.; Mishra, S.M. Seed yield and quality enhancement of pollarded subabul (Leucaena leucocephala) by nutrient supplementation. Agrofor. Syst. 2017, 91, 613–621. [Google Scholar] [CrossRef]
- Stöcker, C.M.; Bamberg, A.L.; Stumpf, L.; Monteiro, A.B.; Cardoso, J.H.; de Lima, A.C.R. Short-term soil physical quality improvements promoted by an agroforestry system. Agrofor. Syst. 2020, 94, 2053–2064. [Google Scholar] [CrossRef]
- de Carvalho, A.F.; Fernandes-Filho, E.I.; Daher, M.; Gomes, L.C.; Cardoso, I.M.; Fernandes, R.B.A.; Schaefer, C.E.G.R. Microclimate and soil and water loss in shaded and unshaded agroforestry coffee systems. Agrofor. Syst. 2021, 95, 119–134. [Google Scholar] [CrossRef]
- Cué-García, J.L.; Ramírez-López, J.-L.; Chagna Ávila, E.J. Tratamientos pregerminativos y diferentes sustratos en la germinación de semillas de Juglans neotropica Diels, Ecuador. Ciênc. Florest. 2024, 34, e83757. [Google Scholar] [CrossRef]
- Klupczyńska, E.A.; Pawłowski, T.A. Regulation of seed dormancy and germination mechanisms in a changing environment. Int. J. Mol. Sci. 2021, 22, 1357. [Google Scholar] [CrossRef]
- Zeng, X.; Durka, W.; Welk, E.; Fischer, M. Heritability of early growth traits and their plasticity in 14 woody species of Chinese subtropical forest. J. Plant Ecol. 2017, 10, 222–231. [Google Scholar] [CrossRef]
- Engelhardt, K.A.M.; Lloyd, M.W.; Neel, M.C. Effects of genetic diversity on conservation and restoration potential at individual, population, and regional scales. Biol. Conserv. 2014, 179, 6–16. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).