Abstract
Bacterial leaf blight (Xanthomonas oryzae pv. oryzae) is a significant rice disease. Aqueous crude extracts of Kalanchoe pinnata were shown to induce rice resistance against the disease. This study aims at testing the disease-reducing effects of K. pinnata leaf extracts using the liquid–liquid extraction method with three different solvents (dichloromethane, methanol, and water). This serves as a basis to select appropriate extracts for effective disease control. Three concentrations (1, 1.5, and 2%) of each extract were tested using seed soaking. The extracts did not show adverse effects on seed germination and seedling growth. Methanol extracts showed significantly different effects compared to those of the untreated control. The involvement of induced resistance in the disease reduction was shown through activities of the four defense-related and antioxidant enzymes, i.e., peroxidase (POX), catalase (CAT), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL). Using 1% methanol extract, activities of POX and CAT involved in hydrogen peroxide production in rice tissues increased 1–4 days after pathogen inoculation (DAI) and remained at high levels until 6 DAI. Activities of PPO and PAL involved in resistance signaling pathways significantly increased after pathogen inoculation. Activities of the four enzymes generally increased after pathogen inoculation and reached higher levels with extract applications.
1. Introduction
Bacterial leaf blight (BLB) caused by Xanthomonas oryzae pv. oryzae (Xoo) is one of the most notable diseases in rice fields, particularly in Vietnam [1,2]. Different means to control the disease have been studied, i.e., chemicals [3,4], antagonistic bacteria [5], genetic resistance [6,7,8], and induced resistance [9,10]. Induced resistance is defined as the process of active resistance dependent on the host plant’s physical and chemical barriers, activated by biotic or abiotic agents [11]. Physically, induced resistance may involve papilla formation, lignification, and callose accumulation which prevent the direct penetration of pathogens into plant tissues [12,13]. Induced resistance may also involve the production of phenolic compounds [14,15], phytoalexins [16,17], and defense-related enzymes like peroxidase (POX), polyphenol oxidase (PPO), and phenylalanine ammonia lyase (PAL) [18,19]. The induction of POX was associated with the production of toxic radicals, such as oxide (O2−) and hydrogen peroxide (H2O2) [20]. The increased production of both the superoxide radicals and H2O2 is a common feature of defense responses to plants challenged by pathogens and elicitors. There has been evidence indicating that H2O2 performs several important roles in plant pathogen interactions [21] where its metabolism is tightly correlated with the antioxidant enzyme catalase (CAT) which decomposes H2O2 at a rapid rate [22]. As a biotrophic pathogen, Xoo infection is inhibited by H2O2 [23]. PPO mediates phenolic oxidation in restricting plant disease development [24]. PAL and POX are the key enzymes involved in phenylpropanoid metabolism [25]. The increase in PAL and PPO activity could assist rice plants in inhibiting Xoo infection as they enhance the biosynthesis of secondary metabolites in rice tissues [9,10,25,26,27].
Numerous chemicals, bacteria and plant extracts can induce rice resistance against BLB. They include salicylic acid [28], vitamin B1 [29], rhizobacteria [30], Pseudomonas fluorescens [31], methanol extracts of Datura metel [9], aqueous and methanol extracts of Adhatoda vasica [26], aqueous extracts of Vitex negundo [27], and aqueous crude extracts of Kalanchoe pinnata [10,32]. Indeed, disease-reducing effects until 21 days after pathogen inoculation (DAI) were shown previously when rice seeds were soaked in aqueous K. pinnata crude extracts before sowing [10,32]. The protection involved induced resistance since activities of POX and CAT increased after extract applications, particularly with the presence of Xoo, while those of PPO and PAL increased at an early stage after pathogen inoculation [9,10,26,27].
As bioactive compounds have different characteristics and polarities, their contents and antioxidant power varied when extraction was conducted using different solvents [33]. It could be argued that different solvents and/or extraction methods could extract different resistance-inducing compounds against BLB. Previous studies showed the disease-reducing effects of aqueous crude extracts of K. pinnata against BLB [10,32]. This study aims at testing the disease-reducing effects against BLB, under greenhouse conditions, of K. pinnata leaf extracts using the liquid–liquid extraction method with three different solvents, i.e., dichloromethane, methanol, and water, thus investigating the involvement of induced resistance in disease reduction through the activities of the defense-related and antioxidant enzymes, i.e., POX, CAT, PPO, and PAL. These serve as a basis to select appropriate extracts to effectively control rice bacterial leaf blight and thus reduce the current use of chemical pesticides to protect human health and the ecosystem.
2. Materials and Methods
2.1. Materials
Whole healthy mature plants (one year old) grown in the greenhouse of Vinh Long University of Technology Education (Vinh Long City, Vietnam) were harvested at 7:00 a.m. Uniform mature leaves (approx. 10 cm in length) were collected from the plants under laboratory conditions to prepare extracts.
Rice cv. Jasmine 85 and Xoo isolates were provided by the Plant Pathology Group, Institute of Food and Biotechnology, Can Tho University (Can Tho City, Vietnam).
2.2. Preparation of K. pinnata Extracts
K. pinnata leaf extracts were made by the liquid–liquid extraction method following the protocol of Kagale et al. [9]. The extraction was conducted using 300 g fresh leaves homogenized in 5 min in a blender containing 1500 mL solvent (1:5 w/v). Three different solvents were used to make the extracts including (1) dichloromethane [100%, polarity index (PI) 0.309, Sigma-Aldrich, Darmstadt, Germany], (2) methanol (100%, PI 0.762, Sigma-Aldrich, Germany) and (3) distilled water (PI 1.0). The mixtures were subjected to ultrasonication (Elmasonic S300, Elma Co., Singen, Germany) at 120 W power in 60 min, salted out by sodium sulphate (Na2SO4), and filtered through filter papers (Whatman Ø110 mm) to obtain filtrates. The filtrates were vacuum-evaporated at 50 °C using a rotary vacuum evaporator (IKA, Calgon Scientific Co., Kochi, India) operating at 5000 rpm. The resulting 100 mL products were dried at 50 °C to obtain three corresponding extracts. These were stored at −4 °C for further analyses in this study.
2.3. Effects of K. pinnata Leaf Extracts on Rice Seed Germination and Seedling Growth
The experiment was conducted in a completely randomized design with three replications. Three K. pinnata extracts [methanol (MeOH) extract, dichloromethane (CH2Cl2) extract, and distilled water (H2O) extract] were tested using three different concentrations [1, 1.5, and 2% (w/v)]. Rice seeds were soaked in the extracts for 24 h. Sterile distilled water was used as an untreated control. Effects of K. pinnata extracts on rice seed germination and seedling growth were tested using the protocol described by Singh and Rao [34] and modified by Khoa et al. [10]. A hundred seeds were evenly placed in ten rows (10 seeds/row) between two moist sheets of filter papers. The sheets were rolled and stored at 28 °C in plastic bags. After 7 days, shoot and root lengths of germinating seeds and germination rates (% of germinating seeds over total number of seeds) were recorded. Vigor indexes were calculated as described by Abdul-Baki and Anderson [35] and modified by Teixeira et al. [36].
Vigor index = (shoot length + root length) × germination rate
2.4. Effects of K. pinnata Leaf Extracts Using Seed Soaking on BLB Lesion Lengths under Greenhouse Conditions
The experiment was conducted in a completely randomized design with three replications. The MeOH extract, CH2Cl2 extract, and H2O extract were tested using three different concentrations [1, 1.5, and 2% (w/v)]. Rice seeds were soaked in K. pinnata extracts for 24 h. The seeds were then incubated at 28 °C for 48 h before sowing. Starner 20WP (oxolinic acid 20%) was used as a chemical control [10,32,37] and water as an untreated control.
As MeOH extracts provided the best protection among the extracts tested, higher concentrations (2, 4, 6, 8, and 10%) of MeOH extracts were further tested to determine whether the protection increased.
2.4.1. Inoculum Preparation
Xoo was grown at 28 °C on agar plates containing 25 mL of modified Wakimoto’s medium [1 L of the medium contains 20 g of sucrose, 5 g of peptone, 5 g of Ca(NO3)2·4H2O, 0.82 g of Na2HPO4 0.05 g of FeSO4·7H2O and 15 g of agar, pH 7.0] [38]. Inoculum was prepared by adding a loopful (2 mm diameter) of a 72 h old Xoo culture to 10 mL of sterile distilled water followed by homogenization using a vortex. The Xoo cell density used to inoculate rice plants was 109 colony forming unit/mL (CFU/mL) [10].
2.4.2. Pathogen Inoculation and Disease Assessment
Forty-five days after sowing, three mature fully expanded leaves counted from the top of each rice plant were inoculated using the leaf-clipping method [10,39]. Furthermore, 109-CFU/mL Xoo suspension was used for pathogen inoculation. Mean lesion lengths were recorded for three inoculated leaves/plant at 7, 14 and 21 DAI [10]. All experiments were conducted in a completely randomized design, and each treatment had three replications (five plants/replication).
2.5. Assays of Enzyme Activities
Four treatments were included in these assays, i.e., (1) seed soaking using 1% K. pinnata methanol extract and pathogen inoculation (MeOH + inoculated); (2) seed soaking using 1% K. pinnata methanol extract and no pathogen inoculation (MeOH + non-inoculated); (3) seed soaking using distilled water and pathogen inoculation (water + inoculated); (4) seed soaking using distilled water and no pathogen inoculation (water + non-inoculated).
2.5.1. Tissue Collection and Enzyme Extraction
Rice leaves were collected from 0 to 7 DAI (once a day) and quickly frozen in liquid nitrogen. For enzyme extraction, 10 g of rice leaves was ground in liquid nitrogen using a Retsch mixer mill (MM200, Retsch Co., Haan, Germany), and 0.1 g of the sample from each collection time point was subsequently homogenized in 1.5 mL of an extraction buffer solution corresponding to each enzyme. Indeed, sodium potassium phosphate buffer 0.1 M (pH 6.5) was used for the extraction of POX and PPO, sodium potassium phosphate buffer 0.1 M (pH 7.0) for CAT, and sodium borate buffer 0.1 M (pH 8.7) for PAL. The homogenates were then centrifuged at 10,000 rpm at 4 °C for 30 min. Enzyme samples were always kept on ice during the assays. Each assay was conducted with three replicates.
2.5.2. POX Assay
POX activity was recorded through the conversion of tetra-guaiacol and guaiacol at 470 nm in a UV/Vis spectrometer (Labomed 2602, PerkinElmer, Waltham, MA, USA) following the method described by Hammerschmidt et al. [40] and modified by Khoa et al. [10]. It was expressed as changes in absorbance at 470 nm per min per mg protein of fresh leaf tissue. The reaction mixture consisted of 1.6 mL H2O2 0.05 M, 0.15 mL guaiacol 0.15 M and 0.15 mL enzyme extract. A blank sample consisted of 1.6 mL H2O2 0.05 M, 0.15 mL guaiacol 0.15 M and 0.15 mL extraction buffer, the value of which was set 0 at 470 nm.
2.5.3. CAT Assay
CAT activity was expressed as changes in absorbance at 240 nm per min per mg protein of fresh leaf tissue of the concentration of H2O2 as described by Beers and Sizer [41] and modified by Khoa et al. [41]. The reaction mixture consisted of 1.75 mL H2O2 0.1 M and 0.15 mL enzyme extract. A blank sample consisted of 1.9 mL extraction buffer, the value of which was set 0 at 240 nm.
2.5.4. PPO Assay
PPO activity was recorded through the conversion of colorless catechol to brown benzoquinone at 490 nm per min per mg protein of fresh leaf tissue as described by Mayer et al. [42] and modified by Khoa et al. [10]. The reaction mixture consisted of 1.75 mL catechol 0.2 M and 0.15 mL enzyme extract. A blank sample consisted of 1.75 mL catechol 0.2 M and 0.15 mL extraction buffer, the value of which was set 0 at 240 nm.
2.5.5. PAL Assay
PAL activity was recorded through the deamination of L-phenylalanine to produce trans-cinnamic acid at 290 nm per min per mg protein of fresh leaf tissue as described by Dickerson et al. [43] and modified by Khoa et al. [10]. The reaction mixture consisted of 0.5 mL sodium borate buffer 0.1 M (pH 8.7), 1 mL L-phenylalanine 0.1 M, 0.15 mL distilled water and 0.2 mL enzyme extract. After allowing the reaction to occur in a test tube at 32 °C for 40 min, 0.2 mL HCl 5.0 N solution was added to stop the reaction. A blank sample consisted of 0.7 mL of extraction buffer, 1 mL L-phenylalanine 0.1 M and 0.15 mL distilled water, the value of which was set 0 at 290 nm.
2.6. Data Analyses
Data of rice seed germination and seedling growth, BLB lesion lengths, and enzyme activities were analyzed via an analysis of variance without transformation using PC-SAS® version 9.1 (SAS Institute Inc., Cary, NC, USA). All experiments were conducted in a completely randomized design with three replications. Three independent experiments were carried out.
3. Results
3.1. Effects of K. pinnata Leaf Extracts on Rice Seed Germination and Seedling Growth
The vigor indexes of rice seeds soaked in methanol extract (MeOH), dichloromethane extract (CH2Cl2), and distilled water extract (H2O) using three different concentrations (1, 1.5 and 2%) are shown in Table 1. The vigor indexes of rice seeds soaked in 2% extracts using MeOH, CH2Cl2 and H2O were significantly different compared to those of the untreated control. These extracts were therefore used to test their disease-reducing effects against BLB under greenhouse conditions.

Table 1.
Effect of Kalanchoe pinnata leaf extracts using three different solvents on seed vigor index of cv. Jasmine 85 at 7 days after soaking application.
3.2. Effects of K. pinnata Leaf Extracts Using Seed Soaking on BLB Lesion Lengths under Greenhouse Conditions
Seed soaking application using K. pinnata leaf extracts significantly reduced the lesion lengths of BLB under greenhouse conditions (Figure 1). At 14 DAI, there were no significant differences between BLB mean lesion lengths on rice leaves of the MeOH treatment using 1, 1.5, or 2% extracts (122.6 ± 5.4 mm, 124.4 ± 10.6 mm, 108.7 ± 9.8 mm, respectively) and those of the chemical control (123 ± 8.9 mm). At 21 DAI, mean lesion lengths of the MeOH treatment using 1, 1.5, and 2% extracts (308.2 ± 33 mm, 298.9 ± 18.2 mm, 268.2 ± 19.9 mm, respectively) were significantly different from those of the untreated control (350.0 ± 5.9 mm) and similar to those of the chemical control (280.5 ± 5.6 mm). As the 1.5% and 2% methanol extracts showed similar effects to those of the chemical control until 21 DAI, they were selected to investigate the involvement of induced resistance in the observed disease reduction.
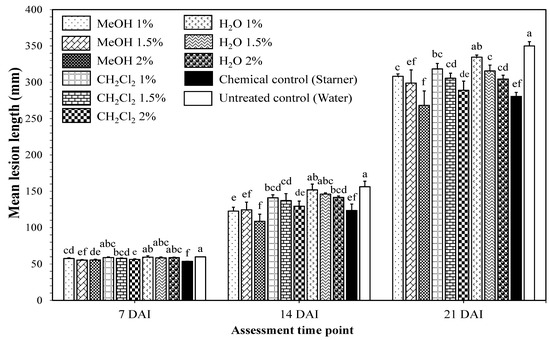
Figure 1.
Effects of seed soaking using Kalanchoe pinnata leaf extracts on bacterial leaf blight lesion lengths on rice cv. Jasmine 85 inoculated 45 day after sowing under greenhouse conditions. Rice seeds were soaked in 1, 1.5, and 2% (w/v) K. pinnata leaf extracts using methanol (MeOH), dichloromethane (CH2Cl2), and distilled water (H2O). Starner 20WP (oxolinic acid 20%, 1 mg/mL) was used as a chemical control and distilled water as untreated control. At each assessment time point, bars with same letters are not significantly different at p ≤ 0.05 by Duncan’s multiple range test. Each value is the mean ± standard deviation of three replications. DAI: days after inoculation.
Higher concentrations of methanol K. pinnata leaf extracts (2, 4, 6, 8 and 10%) were tested to determine the concentration associated with the most efficient disease reduction. All methanol extracts showed significantly different disease-reducing effects until 21 DAI compared to t of the untreated control (Figure 2). Although the 8% extract provided best protection, the 1% extract was selected for further investigation as this was the lowest concentration where the effects were still observed until 21 DAI (Figure 1).
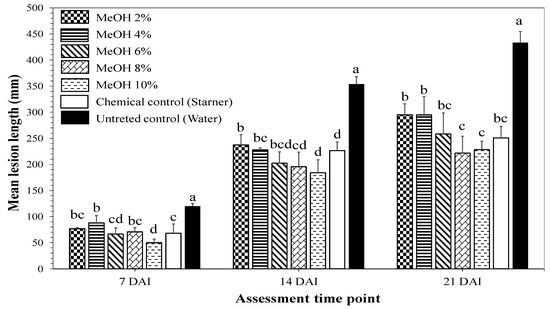
Figure 2.
Effects of seed soaking using methanol Kalanchoe pinnata leaf extracts on bacterial leaf blight lesion lengths on rice cv. Jasmine 85 inoculated 45 day after sowing under greenhouse conditions. Rice seeds were soaked in 2, 4, 6, 8, and 10% (w/v) methanol K. pinnata leaf extracts. Starner 20WP (oxolinic acid 20%, 1 mg/mL) was used as a chemical control and distilled water as untreated control. At each assessment time point, bars with same letters are not significantly different at p ≤ 0.05 by Duncan’s multiple range test. Each value is the mean ± standard deviation of three replications. DAI: days after inoculation.
3.3. Enzyme Activities
3.3.1. POX
POX activities generally increased after pathogen inoculation and reached higher levels with extract applications (Figure 3). The activities increased dramatically with pathogen inoculation 3–5 DAI, from 24.8 ± 0.8 to 58.1 ± 0.4 in the water + inoculated treatment. Extract applications induced POX activities 1–7 DAI, from 23.3 ± 3.0 to 30.5 ± 0.5 in the MeOH + non-inoculated treatment but reached high levels 1–6 DAI with the presence of both extract and pathogen, from 38.1 ± 0.4 to 59.2 ± 1.0 in the MeOH + inoculated treatment.
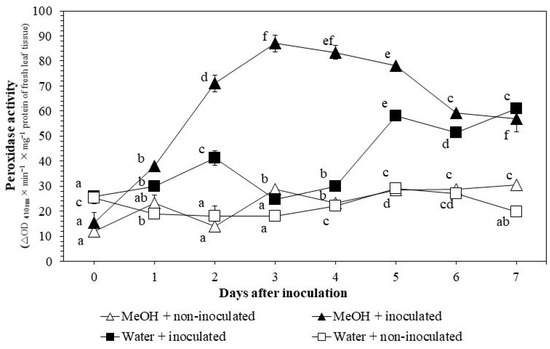
Figure 3.
Changes in peroxidase activities in rice leaf tissues under greenhouse conditions. MeOH + inoculated: rice seeds were soaked in 1% methanol K. pinnata leaf extract and leaves were inoculated with Xoo 45 days after sowing; MeOH + non-inoculated: seeds soaked in 1% extract and leaves not inoculated; Water + inoculated: seeds soaked in distilled water and leaves inoculated; Water + non-inoculated: seeds soaked in distilled water and leaves not inoculated. Within each treatment, values with same letters are not significantly different at p ≤ 0.05 by Duncan’s multiple range test. Each value is the mean ± standard deviation of three replications.
3.3.2. CAT
Similar to POX activities, CAT activities generally increased after pathogen inoculation and reached higher levels with extract applications (Figure 4). The activities increased dramatically with pathogen inoculation 1–4 DAI, from 17.0 ± 0.3 to 27.0 ± 1.3, in the MeOH + inoculated treatment and from 11.5 ± 0.6 to 20.3 ± 0.4 in the water + inoculated treatment. Extract applications induced POX activities 1–5 DAI, from 7.2 ± 0.8 to 13.0 ± 0.4, in the MeOH + non-inoculated treatment but reached high levels 3–7 DAI with the presence of both extract and pathogen, from 18.5 ± 0.6 to 24.3 ± 1.3, in the MeOH + inoculated treatment.
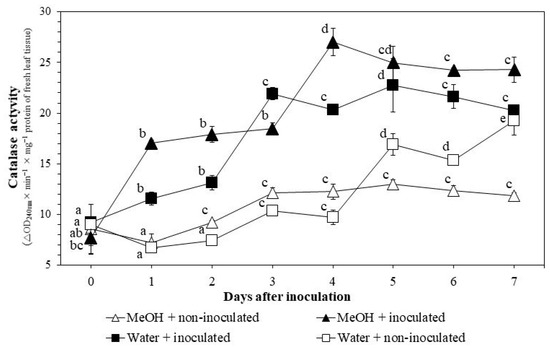
Figure 4.
Changes in catalase activities in rice leaf tissues under greenhouse conditions. MeOH + inoculated: rice seeds were soaked in 1% methanol K. pinnata leaf extract and leaves were inoculated with Xoo 45 days after sowing; MeOH + non-inoculated: seeds soaked in 1% extract and leaves not inoculated; Water + inoculated: seeds soaked in distilled water and leaves inoculated; Water + non-inoculated: seeds soaked in distilled water and leaves not inoculated. Within each treatment, values with same letters are not significantly different at p ≤ 0.05 by Duncan’s multiple range test. Each value is the mean ± standard deviation of three replications.
3.3.3. PPO
PPO activities increased with extract applications 2–5 DAI, from 4.4 ± 0.7 to 10.3 ± 0.6 in the MeOH + inoculated treatment and from 3.4 ± 0.8 to 4.3 ± 0.2 in the MeOH + non-inoculated treatment (Figure 5). The activities increased dramatically with pathogen inoculation 3–6 DAI, from 4.0 ± 0.7 to 8.0 ± 0.7, in the water + inoculated treatment. The activities reached high levels 2–5 DAI with the presence of both extract and pathogen, from 4.4 ± 0.7 to 10.3 ± 0.4 in the MeOH + inoculated treatment.
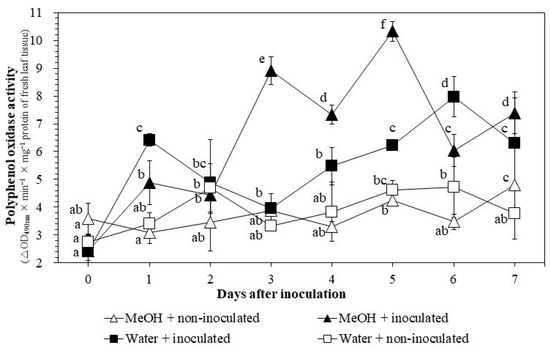
Figure 5.
Changes in polyphenol oxidase activities in rice leaf tissues under greenhouse conditions. MeOH + inoculated: rice seeds were soaked in 1% methanol K. pinnata leaf extract and leaves were inoculated with Xoo 45 days after sowing; MeOH + non-inoculated: seeds soaked in 1% extract and leaves not inoculated; Water + inoculated: seeds soaked in distilled water and leaves inoculated; Water + non-inoculated: seeds soaked in distilled water and leaves not inoculated. Within each treatment, values with same letters are not significantly different at p ≤ 0.05 by Duncan’s multiple range test. Each value is the mean ± standard deviation of three replications.
3.3.4. PAL
Activities of PAL decreased 0–7 DAI with pathogen inoculation, from 0.5 ± 0.01 to 0.3 ± 0.04, in the water + inoculated treatment, except a peak at 2 DAI (0.7 ± 0.01) (Figure 6). However, extract applications together with pathogen inoculation induced the activities of this enzyme dramatically 0–2 DAI, from 0.3 ± 0.03 to 0.8 ± 0.04, in MeOH + inoculated treatment.
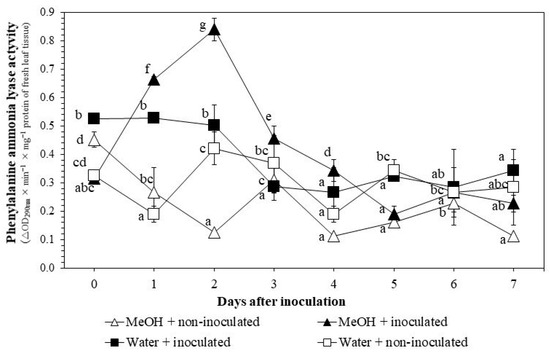
Figure 6.
Changes in phenylalanine ammonia lyase activities in rice leaf tissues under greenhouse conditions. MeOH + inoculated: rice seeds were soaked in 1% methanol K. pinnata leaf extract and leaves were inoculated with Xoo 45 days after sowing; MeOH + non-inoculated: seeds soaked in 1% extract and leaves not inoculated; Water + inoculated: seeds soaked in distilled water and leaves inoculated; Water + non-inoculated: seeds soaked in distilled water and leaves not inoculated. Within each treatment, values with same letters are not significantly different at p ≤ 0.05 by Duncan’s multiple range test. Each value is the mean ± standard deviation of three replications.
4. Discussion
Methanol K. pinnata leaf extracts significantly reduced lesion lengths of BLB under greenhouse conditions (Figure 1 and Figure 2). Numerous studies on the management of BLB using plant derivatives have been reported. Khoa et al. [10] presented that aqueous crude extracts of K. pinnata applied for seeds soaking reduced BLB by induced resistance. Nisha et al. [27] reported effective control of BLB using methanol extracts of Vitex negundo. The application of Datura metel methanol leaf extract was reported by Kagale et al. [9] to be able to effectively reduce the incidence of BLB under greenhouse conditions.
Investigations of mechanisms involved in observed disease reduction by plant derivatives have suggested that induced resistance in host plants results in the reduction of disease development [11]. The defense responses could involve pathogenesis-related proteins or metabolites [18]. Assays of activities of the defense-related and/or antioxidant enzymes, e.g., POX, CAT, PPO, and PAL have been used to show the involvement of induced resistance in plant protection against different diseases, among those BLB caused by Xoo [9,10,27]. In this study, seed soaking using methanol K. pinnata leaf extracts was shown to be able to induce rice resistance against BLB through the activities of these enzymes (Figure 3, Figure 4, Figure 5 and Figure 6). Indeed, activities of POX, CAT, PPO, and PAL coordinately increased in rice tissues after pathogen inoculation and reached higher levels with extract applications. Xoo multiplication commences 2–4 DAI; after sufficient growth, the pathogen invades xylem systems and continues to multiply until 14 DAI [6]. Increased activities of the defense-related and antioxidant enzymes 1–5 DAI could therefore reduce Xoo inoculum levels at the early infection stage, thus reducing BLB lesion lengths (Figure 1 and Figure 2).
Data obtained from the POX assays suggest the active role of this enzyme in induced resistance against BLB. Early and significant increase in the POX activity was observed with the presence of both extract application and pathogen inoculation (Figure 3). This is concordant with the results of previous studies. For instance, Kagale et al. [9] and Govindappa et al. [26] showed the continuous increase in POX activities from 24 to 96 h after inoculation (HAI) using seed soaking with methanol leaf extracts of Datura metel and Adhatoda vasica, respectively. Nisha et al. [27] reported the continuous increase in POX activities from 24 to 100 HAI using methanol extract of Vitex negundo. Khoa et al. [10] also observed an increase in POX activity 1–6 DAI using seed soaking with aqueous K. pinnata crude extracts.
In this study, CAT activities generally increased after pathogen inoculation and reached higher levels with extract applications (Figure 4). Xoo might have triggered the production of CAT to scavenge H2O2 which is toxic to the pathogen. CAT activities in the MeOH + non-inoculated treatment increased compared those in the water + non-inoculated plants 5 DAI. This means that extract applications also increase CAT activities. After 5 days, CAT activities in the MeOH + non-inoculated treatment were lower than those in the water + non-inoculated treatment. However, CAT activities in the MeOH + inoculated treatment were higher than those in the water + inoculated treatment 4–7 DAI. These results support those presented by Khoa et al. [10] where CAT activities increased 1–6 days using seed soaking with aqueous K. pinnata crude extracts. Pal et al. [44] also observed a continuous increase in CAT activities 0–96 HAI when rice seeds were soaked with extracts of Ocimum sanctum and Cymbopogan citrus.
With the presence of both extract application and Xoo inoculation, activities of PPO and PAL were higher compared to those of the water + non-inoculated treatment (Figure 5 and Figure 6). PPO was induced earlier (1–5 DAI) than PAL (1–4 DAI). These results support the findings of previous studies. For example, Kagale et al. [9] and Govindappa et al. [26] showed continuous increases of PAL activities 24–72 HAI. Khoa et al. [10] reported a similar increase 1–3 DAI when rice seeds were soaked with aqueous K. pinnata crude extracts. PAL is the key enzymes involved in phenylpropanoid metabolism [25]. The increase in PAL activity could therefore prevent Xoo infection. Phenolic compounds generated from the phenylpropanoid pathway are PPO substrates [45]. PPO is a copper-containing bifunctional enzyme which hydroxylases and oxidizes phenolic compounds to highly reactive ortho-quinones against biotrophic pathogens like Xoo [46,47].
5. Conclusions
Methanol K. pinnata leaf extracts did not show adverse effects on seed germination and seedling growth. It was efficient to use the 1% extract to induce rice resistance against BLB. Although methanol was an adequate solvent to prepare K. pinnata extracts, other extraction methods and/or fractionation of the extracts could be further investigated. These will serve as a basis to select appropriate extracts or bioactive compound(s) in the extracts to effectively control rice bacterial leaf blight and thus reduce the current use of chemical pesticides to protect human health and the ecosystem.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by T.V.X., T.K.T., N.Đ.Đ. and N.Đ.K. The first draft of the manuscript was written by T.V.X. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mew, T.W. Focus on Bacterial Blight of Rice. Plant Disease 1993, 77, 5–12. [Google Scholar] [CrossRef]
- Khoa, N.Đ. Rice bacterial leaf blight. In Plant Diseases in Vietnam; Mân, V.T., Tuyến, B.C., Tuất, N.V., Kim, P.V., Eds.; Publishing House of the Vietnam National University of Agriculture: Ha Noi, Vietnam, 2018; pp. 107–113. [Google Scholar]
- Khan, J.A.; Siddiq, R.; Arshad, H.M.I.; Anwar, H.S.; Saleem, K.; Jamil, F.F. Chemical control of bacterial leaf blight of rice caused by Xanthomonas oryzae pv. oryzae. Pak. J. Phytopathol. 2012, 24, 97–100. [Google Scholar]
- Syed-Ab-Rahman, S.F.; Sijam, K.; Omar, D. Chemical composition of Piper sarmentosum extracts and antibacterial activity against the plant pathogenic bacteria Pseudomonas fuscovaginae and Xanthomonas oryzae pv. oryzae. J. Plant Dis. Prot. 2014, 121, 237–242. [Google Scholar] [CrossRef]
- Khoa, N.Đ.; Giàu, N.Đ.N.; Tuấn, T.Q. Effects of Serratia nematodiphila CT-78 on rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2016, 103, 1–10. [Google Scholar] [CrossRef]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, R.; Sengupta, D.; Das, S.N.; Pandey, M.K.; Bohra, A.; Sharma, N.K.; Sinha, P.; Sok, H.; Ghazi, I.A.; et al. Deployment of genetic and genomic tools toward gaining a better understanding of rice Xanthomonas oryzae pv. oryzae interactions for development of durable bacterial blight resistant rice. Front. Plant Sci. 2020, 11, 1152. [Google Scholar]
- Zhang, F.; Zhuo, D.L.; Zhang, F.; Huang, L.Y.; Wang, W.S.; Xu, J.L.; Vera Cruz, C.; Li, Z.K.; Zhou, Y.L. Xa39, a novel dominant gene conferring broad-spectrum resistance to Xanthomonas oryzae pv. oryzae in rice. Plant Pathol. 2015, 64, 568–575. [Google Scholar] [CrossRef]
- Kagale, S.; Marimuthu, T.; Thayumanavan, B.; Nandakumar, R.; Samiyappan, R. Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol. Mol. Plant Pathol. 2004, 65, 91–100. [Google Scholar] [CrossRef]
- Khoa, N.Đ.; Xạ, T.V.; Hào, L.T. Disease-reducing effects of aqueous leaf extract of Kalanchoe pinnata on rice bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae involve induced resistance. Physiol. Mol. Plant Pathol. 2017, 100, 57–66. [Google Scholar] [CrossRef]
- Kloepper, J.W.; Tuzun, S.; Kuć, J.A. Proposed definitions related to induced disease resistance. Biocontrol Sci. Technol. 1992, 2, 349–351. [Google Scholar] [CrossRef]
- Kang, Z.; Huang, L.; Buchenauer, H. Ultrastructural changes and localization of lignin and callose in compatible and incompatible interactions between wheat and Puccinia striiformis. J. Plant Dis. Prot. 2002, 109, 25–37. [Google Scholar]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic fungi as biocontrol agents: Elucidating mechanisms in disease suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Khan, J.A.; Afroz, S.; Arshad, H.M.I.; Sarwar, N.; Anwar, H.S.; Saleem, K.; Jamil, F.F. Biochemical basis of resistance in rice against Bacterial leaf blight disease caused by Xanthomonas oryzae pv. oryzae. Adv. Life Sci. 2014, 1, 181–190. [Google Scholar]
- Fan, S.; Tian, F.; Li, J.; Hutchins, W.; Chen, H.; Yang, F.; Yuan, X.; Cui, Z.; Yang, C.; He, C. Identification of phenolic compounds that suppress the virulence of Xanthomonas oryzae on rice via the type III secretion system. Mol. Plant Pathol. 2017, 18, 555–568. [Google Scholar] [CrossRef]
- Cho, M.H.; Lee, S.W. Phenolic Phytoalexins in Rice: Biological Functions and Biosynthesis. Int. J. Mol. Sci. 2015, 16, 29120–29133. [Google Scholar] [CrossRef]
- Singh, R.; Chandrawat, K.S. Role of phytoalexins in plant disease resistance. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 125–129. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M.J. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Hiraga, S.; Sasaki, K.; Ito, H.; Ohashi, Y.; Matsui, H. A large family of class III plant peroxidases. Plant Cell Physiol. 2001, 42, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Shetty, N.P.; Jørgensen, H.J.L.; Jensen, J.D.; Collinge, D.B.; Shetty, H.S. Roles of reactive oxygen species in interactions between plants and pathogens. Eur. J. Plant Pathol. 2008, 121, 267–280. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Yu, C.; Wang, N.; Wu, M.; Tian, F.; Chen, H.; Yang, F.; Yuan, X.; Yang, C.H.; He, C. OxyR-regulated catalase CatB promotes the virulence in rice via detoxifying hydrogen peroxide in Xanthomonas oryzae pv. oryzae. BMC Microbiol. 2016, 16, 269. [Google Scholar] [CrossRef]
- Li, L.; Steffens, J.C. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta 2002, 215, 239–247. [Google Scholar] [CrossRef]
- Solekha, R.; Susanto, F.A.; Joko, T.; Nuringtyas, T.R.; Purwestri, Y.A. Phenylalanine ammonia lyase (PAL) contributes to the resistance of black rice against Xanthomonas oryzae pv. oryzae. J. Plant Pathol. 2020, 102, 359–365. [Google Scholar] [CrossRef]
- Govindappa, M.; Umesha, S.; Lokesh, S. Adhatoda vasica leaf extract induces resistance in rice against bacterial leaf blight disease (Xanthomonas oryzae pv. oryzae). Int. J. Plant Physiol. Biochem. 2011, 3, 6–14. [Google Scholar]
- Nisha, S.; Revathi, K.; Chandrasekaran, R.; Kirubakaran, S.A.; Sathish-Narayanan, S.; Stout, M.J.; Senthil-Nathan, S. Effect of plant compounds on induced activities of defense-related enzymes and pathogenesis related protein in bacterial blight disease susceptible rice plant. Physiol. Mol. Plant Pathol. 2012, 80, 1–9. [Google Scholar] [CrossRef]
- Mohan Babu, R.; Sajeena, A.; Vijaya, S.A.; Sreedhar, A.; Vidhyasekaran, P.; Seetharaman, K.; Reddy, M.S. Induction of systemic resistance to Xanthomonas oryzae pv. oryzae by salicylic acid in Oryza sativa (L.). J. Plant Dis. Prot. 2003, 110, 419–431. [Google Scholar]
- Ahn, I.P.; Kim, S.; Lee, Y.H. Vitamin B1 functions as an activator of plant disease resistance. Plant Physiol. 2005, 138, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Chithrashree, U.; Udayashankar, A.C.; Nayaka, S.C.; Reddy, M.S.; Srinivas, C. Plant growth-promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 2011, 59, 114–122. [Google Scholar] [CrossRef]
- Lingaiah, S.; Umesha, S. Pseudomonas fluorescens inhibits the Xanthomonas oryzae pv. oryzae the bacterial leaf blight pathogen in rice. Can. J. Plant Prot. 2013, 1, 147–153. [Google Scholar]
- Hương, N.T.T.; Hào, L.T.; Khoa, N.Đ. Effects of foliar spraying of Kalanchoe pinnata leaf extract on rice bacterial leaf blight involve phenylalanine ammonia-lyase and polyphenol oxidase activities in induced resistance. Can. Tho Univ. J. Sci. 2018, 54, 13. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Rajauria, G.; Abu-Ghannam, N.; Gupta, S. Effect of different solvents on polyphenolic content, antioxidant capacity and antibacterial activity of irish york cabbage. J. Food Biochem. 2012, 36, 344–358. [Google Scholar] [CrossRef]
- Singh, R.A.; Rao, M.H.S. A simple technique for detection of Xanthomonas oryzae in rice seeds. Seed Sci. Technol. 1997, 5, 123–127. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor determination in soybean seed by multiple criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Teixeira, S.B.; Pires, S.N.; Asvila, G.E.; Silva, B.E.P.; Schmitz, V.N.; Deuner, C.; Silva Armesto, R.; Silva Moura, D.; Deuner, S. Application of vigor indexes to evaluate the cold tolerance in rice seeds germination conditioned in plant extract. Sci. Rep. 2021, 11, 11038. [Google Scholar] [CrossRef] [PubMed]
- Kleitman, F.; Shtienberg, D.; Blachinsky, D.; Oppenheim, D.; Zilberstaine, M.; Dror, O.; Manulis, S. Erwinia amylovora populations resistant to oxolinic acid in Israel: Prevalence, persistence and fitness. Plant Pathol. 2005, 54, 108–115. [Google Scholar] [CrossRef]
- Karganilla, A. A comparative study of culture media for Xanthomonas oryzae. Philipp. Agric. 1973, 57, 141–152. [Google Scholar]
- Kauffman, H.E. An improved technique for evaluating resistance to rice varieties of Xanthomonas oryzae. Plant Dis. Rep. 1973, 57, 537–541. [Google Scholar]
- Hammerschmidt, R.; Nuckles, E.M.; Kuć, J. Association of enhanced peroxidase activity with induced systemic resistance of cucumber to Colletotrichum lagenarium. Physiol. Plant Pathol. 1982, 20, 73–82. [Google Scholar] [CrossRef]
- Beers, R.D.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, D.P.; Pascholati, S.F.; Hagerman, A.E.; Butler, L.G.; Nicholson, R.L. Phenylalanine ammonia-lyase and hydroxycinnamate: CoA ligase in maize mesocotyls inoculated with Helminthosporium maydis or Helminthosporium carbonum. Physiol. Plant Pathol. 1984, 25, 111–123. [Google Scholar] [CrossRef]
- Pal, T.K.; Bhattacharya, S.; Chakraborty, K.; Highway, O.B.; Bengal, W.; Road, B.C. Induction of systemic resistance in rice by leaf extract of Cymbopogan citrus and Ocimum sanctum against sheath blight disease. Science 2011, 3, 392–400. [Google Scholar]
- Araji, S.; Grammer, T.A.; Gertzen, R.; Anderson, S.D.; Mikulic-Petkovsek, M.; Veberic, R.; Phu, M.L.; Solar, A.; Leslie, C.A.; Dandekar, A.M. Novel roles for the polyphenol oxidase enzyme in secondary metabolism and the regulation of cell death in walnut. Plant Physiol. 2014, 164, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Yoruk, R.; Marshall, M.R. Physicochemical properties and function of plant polyphenol oxidase: A review. J. Food Biochem. 2003, 27, 361–422. [Google Scholar] [CrossRef]
- Constabel, C.P.; Barbehenn, R. Defensive roles of polyphenol oxidase in plants. In Induced Plant Resistance to Herbivory; Schaller, A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 253–270. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).