Abstract
Root-synthesized cytokinins regulate the growth, development, and stress responses of aboveground tissues and follow the transport route via xylem tissue. Arabidopsis ATP-binding cassette (ABC) transporter G family member 14 (AtABCG14) is involved in the xylem loading of root-synthesized cytokinins. However, the phloem unloading of root-synthesized cytokinin and shoot distribution have remained elusive. The recent study by Zhao et al., (2021) proved that the AtABCG14 protein mediates the phloem unloading of cytokinins through the apoplastic pathway indicating the AtABCG14 is a master regulator of shoot cytokinin distribution.
Cytokinin regulates multiple biological processes in plants [1,2,3] Among the different cytokinin types, trans-zeatin (tZ-type) and isopentenyladenine (iP-type) are primarily synthesized in roots and shoots, respectively [4]. The shoot transport of the tZ-type is necessary for normal shoot development. ATP-binding cassette subfamily G (ABCG) are localized in the plasma membrane of vascular tissue and mobilize cytokinins at local and systemic levels [5,6,7,8]. ABCG proteins have been characterized as long-distance apoplastic cytokinin transporters in plants [4,6,8,9]. Mutants of ABCG in Arabidopsis (atabcg14) and rice (osabcg18) display a retarded shoot development phenotype, which is restored by exogenous tZ application [6]. Similarly, osabcg18 and Medicago truncatula, mtabcg56 mutants exhibit reduced grain yield and defective symbiosis, respectively. Therefore, ABCG has emerged as a cytokinin carrier that controls diverse functions such as shoot development, symbiosis, and grain yield [4,5]. Until recently, the role of ABCG transporters in xylem loading and the shoot transport of cytokinin has been investigated, but information on distribution to the leaves and other aerial parts remains elusive.
Recent work by Zhao et al. (2021) showed the role of AtABCG14 in the phloem unloading of root-synthesized cytokinin and its effect on shoot phenotypes. For a preliminary assessment of ABCG14 expression in Arabidopsis shoot and root, the authors performed promoter analyses (ABCG14pro:GUS/GFP) with GUS and GFP reporters. The expression pattern of ABCG14pro:GUS/GFP in young leaves overlapped with phloem companion cell (PCC)-specific and xylem-specific promoter expression, indicating AtABCG14 localization in PCC and xylem parenchyma cells. Further, AtABCG14 expression was observed in the minor veins of young leaves and only in the margins of old leaves. Enhanced AtABCG14 caused the accumulation of the tZ-type cytokinin in young leaves compared to mature leaves.
The contrasting leaf expression of AtABCG14 prompted authors to decipher the role of AtABCG14 in the shoot. A reciprocal micro-grafting experiment using the wild-type (WT) and mutant atabcg14, i.e., WT scion and atabcg14 rootstock (WT/atabcg14) or atabcg14 scion and WT rootstock (atabcg14/WT), revealed smaller shoots in heterografts than WT/WT. A reduction in the diameter of rosette leaves, the number of siliques, and shoot apical meristem activity was also observed in the order of atabcg14/atabcg14 > atabcg14/WT > WT/atabcg14, compared to WT/WT. These parameters were more restored in WT/atabcg14 versus atabcg14/WT, suggesting the importance of the aerial expression of AtABCG14 for shoot growth.
Moreover, cytokinin-responsive Arabidopsis Response Regulator 5 promoter-driven GUS/GFP expression in homo- and heterografts correlated shoot AtABCG14 expression and cytokinin distribution in the aerial tissues. The WT/atabcg14 displayed significantly high GFP and GUS expression in the leaves or inflorescences, whereas atabcg14/WT exhibited low expression. In addition, cytokinin-responsive gene expression was also high in WT/atabcg14 compared to the atabcg14/WT shoot. Thus, shoot cytokinin signaling recovered substantially in WT/atabcg14 and slightly in atabcg14/WT compared to WT/WT. Limited shoot cytokinin signaling in atabcg14/WT indicated that shoot AtABCG14 expression is indispensable for root-to-shoot cytokinin transport and signaling.
Furthermore, the movement of root-synthesized cytokinins to the shoot was tracked by feeding the root with 14C-labeled tZ and 2H-labeled tZ. The transport of 14C-tZ or 2H5-tZ to the shoot was suppressed in atabcg14/atabcg14, fully rescued in atabcg14/WT, and partially rescued in WT/atabcg14. Root AtABCG14 restored shootward tZ transport in atabcg14/WT; however, cytokinin signaling and growth were hampered. Additionally, tZ expression spread from the petiole–midrib to the lamina in WT/WT. In contrast, in atabcg14/WT, its expression was primarily observed in the petiole–midrib, indicating impaired cytokinin distribution from the veins to the lamina of atabcg14/WT. Further, cytokinins’ presence was high in the apoplast of WT/WT and WT/atabcg14 and low in atabcg14/atabcg14 and atabcg14/WT. However, the phloem sap of atabcg14/WT retained much higher tZ-type compared to others. Thus, root-synthesized cytokinin reached the shoot, but aerial-tissue disruption of AtABCG14 disturbed phloem unloading and translocation to the lamina (Figure 1). Hence, AtABCG14 plays a pivotal role in shoot cytokinin distribution. The authors proved that under dysfunctional shoot AtABCG14 expression in atabcg14/WT, cytokinin is not distributed in the leaves and is in retrograde from shoot to root. This finding validates that AtABCG14 expression in PCCs is associated with root-synthesized cytokinin efflux phloem unloading to the apoplast.
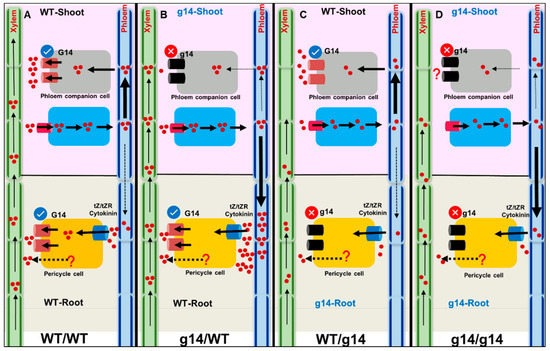
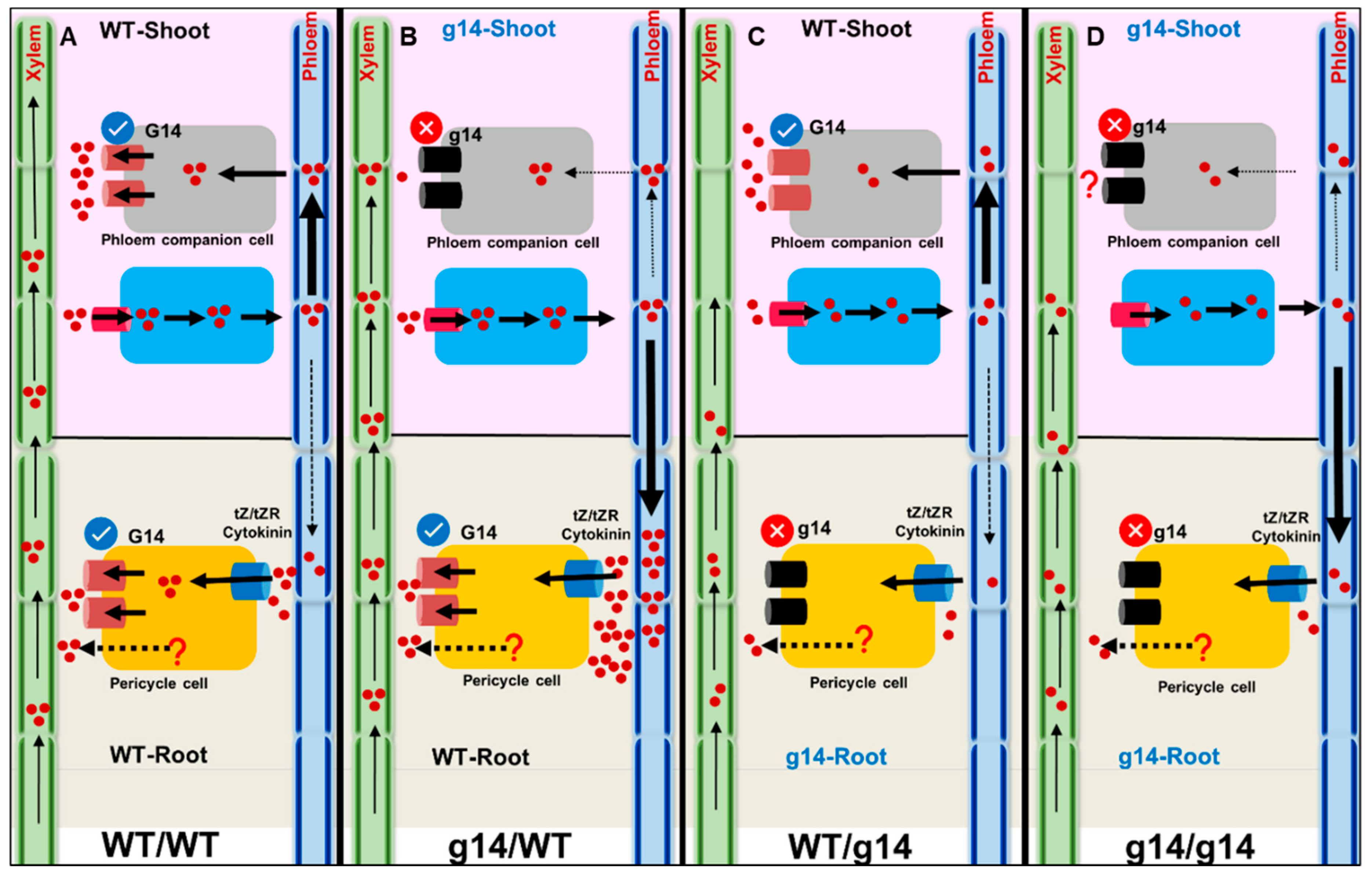
Figure 1.
Schematic representation of the role of AtABCG14 (G14) in cytokinin transport and corresponding leaf distribution and shoot phenotype. AtABCG14, along with an unknown protein, loads tZR/tZ into the root xylem. (A) In WT homografts, phloem transports most cytokinins to the minor veins, and AtABCG14 in PCCs mediates cytokinin release to the apoplast. A small amount of phloem cytokinin retrogrades to the root. The functional AtABCG14 in root and shoot distribute cytokinins from veins to the lamina, resulting in GUS expression and better shoot phenotype. (B) In the heterograft atabcg14/WT, functional AtABCG14 in roots transports most of the cytokinin to shoots; however, phloem unloading is suppressed in AtABCG14-deficient shoots, and the majority of root-synthesized cytokinin is retrograded to the roots. The suppressed phloem unloading results in a defective shoot phenotype and no GUS signals in the leaf. (C) In the heterograft WT/atabcg14, an unknown protein loads cytokinin into the xylem. Functional shoot AtABCG14 translocates cytokinin into the apoplast, improving the shoot phenotype and GUS expression compared to atabcg14/WT. (D) In homograft atabcg14/atabcg14, a disrupted root-and-shoot AtABCG14 results in completely suppressed shoot phenotype and absence of GUS staining. There could be a different unknown mechanism for the transport of cytokinin and further unloading at phloem. tZ—trans-zeatin; tZR—tZ-riboside; G14—AtABCG14; PCC—phloem companion cells; PC—pericycle cells, ?-unknown mechanism. The grey and blue rectangle box denotes the phloem companion cell. The figure is modified from Zhao et al., 2021.
The authors used a mutant complementation assay to define the role of the AtABCG14 gene. AtABCG14 expression using a PCC-specific promoter complemented the atabcg14 mutant and rescued the apoplastic cytokinin levels and defective phenotypes; however, xylem-specific promoter expression was not successful in mutant complementation. These results, in summary, prove that AtABCG14 expression in the shoot is required for the distribution of cytokinin in all aboveground plant parts.
The short- and/or long-distance transport of cytokinin governs plant growth, development, and biotic–abiotic stress responses. The elucidation of cytokinin transport and the signaling nexus has substantially provided deep insights into cytokinin’s physiological roles [9]. The authors utilized grafting experiments between the mutant and WT and isotope-labeled tZ feeding, followed by the profiling of phloem and apoplastic extract and mutant complementation studies, to determine that AtABCG14 coordinates the root loading and shoot distribution of root-synthesized cytokinins (Figure 1). Cytokinin transport from root to shoot and translocation determines the developmental fate of different plant tissues. Thus, the spatio-temporal expression of cytokinin signaling in the root and shoot determines the balance between cell division, elongation, and differentiation. This reprograms molecular machinery and optimizes transcriptional dynamics to favor proper organogenesis, as in shoot-and-root nodule development and enhanced grain yield [2,4,5,8]. Thus, the ABCG protein subfamily can be targeted as a potential genetic engineering candidate for regulating the spatio-temporal distribution and signaling of cytokinin to enhance stress tolerance, with better plant phenotypes and enhanced yields.
Author Contributions
G.K. and D.M. summarized and wrote the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No Data is provided.
Conflicts of Interest
There is no conflict of interest.
References
- Kang, J.; Lee, Y.; Sakakibara, H.; Martinoia, E. Cytokinin transporters: GO and STOP in signaling. Trends Plant Sci. 2017, 22, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Yadav, M.; Singh, B.; Pandey, V.; Nawaz, K.; Bhatia, S. Evolutionary and functional analysis of Two-Component System in chickpea reveals CaRR13, a TypeB RR, as positive regulator of symbiosis. Plant Biotechnol. J. 2021, 19, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Pandey, V.; Singh, B.; Bhatia, S. Evolutionary and expression dynamics of LRR-RLKs and functional establishment of KLAVIER homolog in shoot mediated regulation of AON in chickpea symbiosis. Genomics 2021, 113, 4313–4326. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Pandey, V.; Singh, B.; Bhatia, S. Dynamics of miRNA mediated regulation of legume symbiosis. Plant Cell Environ. 2021, 44, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Jarzyniak, K.; Banasiak, J.; Jamruszka, T.; Pawela, A.; Di Donato, M.; Novák, O.; Geisler, M.; Jasiński, M. Early stages of legume–rhizobia symbiosis are controlled by ABCG-mediated transport of active cytokinins. Nat. Plants 2021, 7, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Ko, D.; Kang, J.; Kiba, T.; Park, J.; Kojima, M.; Do, J.; Kim, K.Y.; Kwon, M.; Endler, A.; Song, W.-Y.; et al. Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Proc. Natl. Acad. Sci. USA 2014, 111, 7150–7155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ding, B.; Zhu, E.; Deng, X.; Zhang, M.; Zhang, P.; Wang, L.; Dai, Y.; Xiao, S.; Zhang, C.; et al. Phloem unloading via the apoplastic pathway is essential for shoot distribution of root-synthesized cytokinins. Plant Physiol. 2021, 186, 2111–2123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yu, N.; Ju, M.; Fan, B.; Zhang, Y.; Zhu, E.; Zhang, M.; Zhang, K. ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J. Exp. Bot. 2019, 70, 6277–6291. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-J.; Zhao, Y.; Zhang, K. Cytokinin transporters: Multisite players in cytokinin homeostasis and signal distribution. Front. Plant Sci. 2019, 10, 693. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).