Follow-Up of the Immune Response and the Possible Presence of Brucella melitensis Strains in Peripheral Blood in Hoggets Vaccinated by Rev1 in Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm and Animals of the Study
2.2. Antibody Detection
2.3. Blood Culture
2.4. DNA Extraction
2.5. PCR Analyses
2.6. Whole Genome Sequencing
3. Results
3.1. Serological Analysis
3.2. PCR Testing of Blood Samples
3.3. Blood Cultures
3.4. Molecular Characterization of the Brucella spp. Isolates (PCR and WGS)
3.5. Association Between the Isolated B. melitensis Strains and the Immune Reaction
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wareth, G.; Abdeen, A.; Ali, H.; Bardenstein, S.; Blasco, J.-M.; Cardoso, R.; Sá, M.I.C.D.; Cvetnić, Ž.; Massis, F.; Diasty, M.E.; et al. Brucellosis in the Mediterranean Countries: History, Prevalence, Distribution, Current Situation and Attempts at Surveillance and Control. In OIE Book; World Organisation for Animal Health: Paris, France, 2019; Volume 12. [Google Scholar]
- Moreno, E.; Middlebrook, E.A.; Altamirano-Silva, P.; Dahouk, S.A.; Araj, G.F.; Arce-Gorvel, V.; Arenas-Gamboa, Á.; Ariza, J.; Barquero-Calvo, E.; Battelli, G.; et al. If You’re Not Confused, You’re Not Paying Attention: Ochrobactrum Is Not Brucella. J. Clin. Microbiol. 2023, 61, e00438-23. [Google Scholar] [CrossRef] [PubMed]
- Holzer, K.; Hoelzle, L.E.; Wareth, G. Genetic Comparison of Brucella spp. and Ochrobactrum spp. Erroneously Included into the Genus Brucella Confirms Separate Genera. Ger. J. Vet. Res. 2023, 3, 31–37. [Google Scholar] [CrossRef]
- Dadar, M.; Al-Khaza’leh, J.; Fakhri, Y.; Akar, K.; Ali, S.; Shahali, Y. Human Brucellosis and Associated Risk Factors in the Middle East Region: A Comprehensive Systematic Review, Meta-Analysis and Meta-Regression. Heliyon 2024, 10, e34324. [Google Scholar] [CrossRef] [PubMed]
- Alton, G.G. Control of Brucella Melitensis Infection in Sheep and Goats-a Review. Trop. Anim. Health Prod. 1987, 19, 65–74. [Google Scholar] [CrossRef]
- Akar, K.; Brangsch, H.; Jamil, T.; Öz, G.Y.; Baklan, E.A.; Eroğlu, B.; Atıl, E.; Gürbilek, S.E.; Keskin, O.; Tel, O.Y.; et al. Genomic Analysis of Brucella Isolates from Animals and Humans, Türkiye, 2010 to 2020. Euro. Surveill. 2024, 29, 2400105. [Google Scholar] [CrossRef]
- Holzhauer, M.; Wennink, G.J. Zoonotic Risks of Pathogens from Dairy Cattle and Their Milk-Borne Transmission. J. Dair. Res. 2023, 90, 325–331. [Google Scholar] [CrossRef]
- Laine, C.G.; Johnson, V.E.; Scott, H.M.; Arenas-Gamboa, A.M. Global Estimate of Human Brucellosis Incidence. Emerg. Infect. Dis. 2023, 29, 1789. [Google Scholar] [CrossRef]
- Elrashedy, A.; Nayel, M.; Salama, A.; Zaghawa, A.; Abdelsalam, N.R.; Hasan, M.E. Phylogenetic Analysis and Comparative Genomics of Brucella Abortus and Brucella Melitensis Strains in Egypt. J. Mol. Evol. 2024, 92, 338–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, L.; Wang, M.; Yuan, M.; Li, Z. Long Ignored but Making a Comeback: A Worldwide Epidemiological Evolution of Human Brucellosis. Emerg. Microbes. Infect. 2024, 13, 2290839. [Google Scholar] [CrossRef]
- Alton, G.G. Brucellla Melitensis. In Animal Brucellosis; CRC Press: Boston, MA, USA, 1990. [Google Scholar]
- Tittarelli, M.; Ventura, M.D.; Massis, F.D.; Scacchia, M.; Giovannini, A.; Nannini, D.; Caporale, V. The Persistence of Brucella Melitensis in Experimentally Infected Ewes through Three Reproductive Cycles. J. Vet. Med. B Infect. Dis. Vet. Public Health 2005, 52, 403–409. [Google Scholar] [CrossRef]
- Banai, M. Control of Small Ruminant Brucellosis by Use of Brucella Melitensis Rev.1 Vaccine: Laboratory Aspects and Field Observations. Vet. Microbiol. 2002, 90, 497–519. [Google Scholar] [CrossRef] [PubMed]
- Naseer, A.; Mo, S.; Olsen, S.C.; McCluskey, B. Brucella Melitensis Vaccines: A Systematic Review. Agriculture 2023, 13, 2137. [Google Scholar] [CrossRef]
- Alton, G.; Jones, L.; Angus, R.; Verger, J. Techniques for the Brucellosis Laboratory; INRA Publications: Versailles Cedex, France, 1988; pp. 192–195. [Google Scholar]
- Dahouk, S.A.; Nöckler, K.; Scholz, H.C.; Tomaso, H.; Bogumil, R.; Neubauer, H. Immunoproteomic Characterization of Brucella Abortus 1119-3 Preparations Used for the Serodiagnosis of Brucella Infections. J. Immunol. Methods. 2006, 309, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Durán-Ferrer, M.; Léon, L.; Nielsen, K.; Caporale, V.; Mendoza, J.; Osuna, A.; Perales, A.; Smith, P.; De-Frutos, C.; Gómez-Martín, B.; et al. Antibody Response and Antigen-Specific Gamma-Interferon Profiles of Vaccinated and Unvaccinated Pregnant Sheep Experimentally Infected with Brucella Melitensis. Vet. Microbiol. 2004, 100, 219–231. [Google Scholar] [CrossRef]
- Al-Garadia, M.A.; Khairani-B, S.; Zunita, Z.; Omar, A.R. Detection of Brucella Melitensis in Blood Samples Collected from Goats. J. Anim. Vet. Adv. 2011, 10, 1437–1444. [Google Scholar] [CrossRef]
- Fekete, A.; Bantle, J.A.; Halling, S.M.; Sanborn, M.R. Preliminary Development of a Diagnostic Test for Brucella Using Polymerase Chain Reaction. J. Appl. Bacteriol. 1990, 69, 216–227. [Google Scholar] [CrossRef]
- Keid, L.B.; Soares, R.M.; Vasconcellos, S.A.; Salgado, V.R.; Megid, J.; Richtzenhain, L.J. Comparison of a PCR Assay in Whole Blood and Serum Specimens for Canine Brucellosis Diagnosis. Vet. Rec. 2010, 167, 96–99. [Google Scholar] [CrossRef]
- O’Leary, S.; Sheahan, M.; Sweeney, T. Brucella Abortus Detection by PCR Assay in Blood, Milk and Lymph Tissue of Serologically Positive Cows. Res. Vet. Sci. 2006, 81, 170–176. [Google Scholar] [CrossRef]
- Wareth, G.; El-Diasty, M.; Melzer, F.; Schmoock, G.; Moustafa, S.A.; El-Beskawy, M.; Khater, D.F.; Hamdy, M.E.R.; Zaki, H.M.; Ferreira, A.C.; et al. MLVA-16 Genotyping of Brucella Abortus and Brucella Melitensis Isolates from Different Animal Species in Egypt: Geographical Relatedness and the Mediterranean Lineage. Pathogens 2020, 9, 498. [Google Scholar] [CrossRef]
- Michaux, S.; Paillisson, J.; Carles-Nurit, M.J.; Bourg, G.; Allardet-Servent, A.; Ramuz, M. Presence of Two Independent Chromosomes in the Brucella Melitensis 16M Genome. J. Bacteriol. 1993, 175, 701–705. [Google Scholar] [CrossRef]
- Suárez-Esquivel, M.; Chaves-Olarte, E.; Moreno, E.; Guzmán-Verri, C. Brucella Genomics: Macro and Micro Evolution. Int. J. Mol. Sci. 2020, 21, 7749. [Google Scholar] [CrossRef] [PubMed]
- Katsiolis, A.; Papanikolaou, E.; Stournara, A.; Giakkoupi, P.; Papadogiannakis, E.; Zdragas, A.; Giadinis, N.D.; Petridou, E. Molecular Detection of Brucella Spp. in Ruminant Herds in Greece. Trop. Anim. Health Prod. 2022, 54, 173. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Brucellosis. In ECDC Annual Epidemiological Report for 2022; ECDC: Stockholm, Sweden, 2024. [Google Scholar]
- Kefaloudi, C.; Mellou, K.; Dougas, G.; Vorou, R.; Mitrou, K.; Kontopidou, F. Human Brucellosis in Greece, 2005-2020: A Persistent Public Health Problem. Vector-Borne Zoonotic Dis. 2022, 3, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Emmanouil, M.; Vourvidis, D.; Kyrma, A.; Makka, S.; Horefti, E.; Angelakis, E. Epidemiological Investigation of Animal Brucellosis in Domestic Ruminants in Greece from 2015 to 2022 and Genetic Characterization of Prevalent Strains. Pathogens 2024, 13, 720. [Google Scholar] [CrossRef]
- Minas, A.; Minas, M.; Stournara, A.; Tselepidis, S. The “Effects” of Rev-1 Vaccination of Sheep and Goats on Human Brucellosis in Greece. Prev. Vet. Med. 2004, 64, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dougas, G.; Katsiolis, A.; Linou, M.; Kostoulas, P.; Billinis, C. Modelling Human Brucellosis Based on Infection Rate and Vaccination Coverage of Sheep and Goats. Pathogens 2022, 11, 167. [Google Scholar] [CrossRef]
- Garin-Bastuji, B.; Benkirane, A. In FAO/WHO/OIE Round Table on the Use of Rev.1 Vaccine in Small Ruminants and Cattle; CNEVA: Alfort, France, 1995. [Google Scholar]
- Katsiolis, A.; Thanou, O.; Tzani, M.; Dile, C.; Korou, M.; Stournara, A.; Petridou, E.; Giadinis, N.D. Investigation of the human resources needs for the effective and efficient implementation of the sheep and goat brucellosis program in Greece. Vet. J. Repub. Srp. 2018, 18, 270–296. [Google Scholar] [CrossRef]
- Kolman, S.; Maayan, M.C.; Gotesman, G.; Rozenszajn, L.A.; Wolach, B.; Lang, R. Comparison of the Bactec and Lysis Concentration Methods for Recovery of Brucella Species from Clinical Specimens. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 647–648. [Google Scholar] [CrossRef]
- Extramiana, A.B.; González, L.; Cortabarría, N.; García, M.; Juste, R.A. Evaluation of a PCR Technique for the Detection of Maedi-Visna Proviral DNA in Blood, Milk and Tissue Samples of Naturally Infected Sheep. Small Rumi. Res. 2002, 44, 109–118. [Google Scholar] [CrossRef]
- Babetsa, M.; Boukouvala, E.; Gelasakis, A.; Papadopoulos, A.; Zdragas, A.; Ekateriniadou, L. Detection of a Brucella Melitensis Rev1 Vaccine Strain and Rev1-Like Strains in Unvaccinated Small Ruminants and Aborted Fetuses. Open Access. J. Vet. Sci. Med. 2019, 1, 103. [Google Scholar]
- Baily, G.; Drasar, B.; Stoker, N. Detection of Brucella Melitensis and Brucella Abortus by DNA Amplification. J. Trop. Med. Hyg. 1992, 95, 271–275. [Google Scholar] [PubMed]
- Brangsch, H.; Sandalakis, V.; Babetsa, M.; Boukouvala, E.; Ntoula, A.; Makridaki, E.; Christidou, A.; Psaroulaki, A.; Akar, K.; Gürbilek, S.E.; et al. Genotype Diversity of Brucellosis Agents Isolated from Humans and Animals in Greece Based on Whole-Genome Sequencing. BMC Infect. Dis. 2023, 23, 529. [Google Scholar] [CrossRef]
- Stamatakis, A. RAxML Version 8: A Tool for Phylogenetic Analysis and Post-Analysis of Large Phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Argimón, S.; Abudahab, K.; Goater, R.J.E.; Fedosejev, A.; Bhai, J.; Glasner, C.; Feil, E.J.; Holden, M.T.G.; Yeats, C.A.; Grundmann, H.; et al. Microreact: Visualizing and Sharing Data for Genomic Epidemiology and Phylogeography. Microb. Genom. 2016, 2, e000093. [Google Scholar] [CrossRef] [PubMed]
- Fouskis, I.; Sandalakis, V.; Christidou, A.; Tsatsaris, A.; Tzanakis, N.; Tselentis, Y.; Psaroulaki, A. The Epidemiology of Brucellosis in Greece, 2007-2012: A “One Health” Approach. Trans. R. Soc. Trop. Med. Hyg. 2018, 112, 124–135. [Google Scholar] [CrossRef]
- Papaparaskevas, J.; Kandili, A.; Pantazatou, A.; Gartzonika, C.; Charalampakis, N.; Stathi, A.; Tarpatzi, K.; Refene, E.; Priftis, A.; Mageropoulou, A.; et al. Infections due to the Brucella melitensis REV-1 vaccine strain in Greece, 2001–2015. In Proceedings of the 26th ECCMID, Amsterdam, The Netherlands, 9–12 April 2016; Available online: https://www.escmid.org/escmid_publications/escmid_elibrary/material/?mid=46811 (accessed on 24 August 2021).
- Corpa, J.M.; Pérez, V.; García Marín, J.F. Differences in the Immune Responses in Lambs and Kids Vaccinated against Paratuberculosis, According to the Age of Vaccination. Vet. Microbiol. 2000, 77, 475–485. [Google Scholar] [CrossRef]
- Grilló, M.J.; Barberán, M.; Blasco, J.M. Transmission of Brucella Melitensis from Sheep to Lambs. Vet. Rec. 1997, 140, 602–605. [Google Scholar] [CrossRef]
- Hensel, M.E.; Garcia-Gonzalez, D.G.; Chaki, S.P.; Hartwig, A.; Gordy, P.W.; Bowen, R.; Ficht, T.A.; Arenas-Gamboa, A.M. Vaccine Candidate Brucella Melitensis 16M ΔvjbR Is Safe in a Pregnant Sheep Model and Confers Protection. mSphere 2020, 5, e00120-20. [Google Scholar] [CrossRef]
- Nguyen, T.C. The Immune Response in Sheep: Analysis of Age, Sex and Genetic Effects on the Quantitative Antibody Response to Chicken Red Blood Cells. Vet. Immunol. Immunopathol. 1984, 5, 237–245. [Google Scholar] [CrossRef]
- Arellano-Reynoso, B.; Suárez-Güemes, F.; Estrada, F.M.; Michel-GómezFlores, F.; Hernández-Castro, R.; Acosta, R.B.; Díaz-Aparicio, E. Isolation of a Field Strain of Brucella Abortus from RB51-Vaccinated- and Brucellosis-Seronegative Bovine Yearlings That Calved Normally. Trop. Anim. Health Prod. 2013, 45, 695–697. [Google Scholar] [CrossRef]
- El-Diasty, M.; Wareth, G.; Melzer, F.; Mustafa, S.; Sprague, L.D.; Neubauer, H. Isolation of Brucella Abortus and Brucella Melitensis from Seronegative Cows Is a Serious Impediment in Brucellosis Control. Vet. Sci. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- El Idrissi, A.H.; Benkirane, A.; El Maadoudi, M.; Bouslikhane, M.; Berrada, J.; Zerouali, A. Comparison of the Efficacy of Brucella Abortus Strain RB51 and Brucella Melitensis Rev. 1 Live Vaccines against Experimental Infection with Brucella Melitensis in Pregnant Ewes. Rev. Sci. Tech. 2001, 20, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Barrio, M.B.; Grilló, M.J.; Muñoz, P.M.; Jacques, I.; González, D.; Miguel, M.J.D.; Marín, C.M.; Barberán, M.; Letesson, J.J.; Gorvel, J.P.; et al. Rough Mutants Defective in Core and O-Polysaccharide Synthesis and Export Induce Antibodies Reacting in an Indirect ELISA with Smooth Lipopolysaccharide and Are Less Effective than Rev 1 Vaccine against Brucella Melitensis Infection of Sheep. Vaccine 2009, 27, 1741–1749. [Google Scholar] [CrossRef]
- Minas, A.; Stournara, A.; Christodoulopoulos, G.; Katsoulos, P.D. Validation of a Competitive ELISA for Diagnosis of Brucella Melitensis Infection in Sheep and Goats. Vet. J. 2008, 177, 411–417. [Google Scholar] [CrossRef]
- Mathur, S.; Banai, M.; Cohen, D. Natural Brucella Melitensis Infection and Rev. 1 Vaccination Induce Specific Brucella O-Polysaccharide Antibodies Involved in Complement Mediated Brucella Cell Killing. Vaccines 2022, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Kojouri, G.A.; Gholami, M. Post Vaccination Follow-up of Brucella Melitensis in Blood Stream of Sheep by PCR Assay. Comp. Clin. Path. 2009, 18, 439–442. [Google Scholar] [CrossRef]
- Stournara, A.; Minas, A.; Bourtzi-Chatzopoulou, E.; Stack, J.; Koptopoulos, G.; Petridou, E.; Sarris, K. Assessment of Serological Response of Young and Adult Sheep to Conjunctival Vaccination with Rev-1 Vaccine by Fluorescence Polarization Assay [FPA] and Other Serological Tests for B. Melitensis. Vet. Microbiol. 2007, 119, 53–64. [Google Scholar] [CrossRef]
- Garin-Bastuji, B.; Blasco, J.M.; Grayon, M.; Verger, J.M. Brucella Melitensis Infection in Sheep: Present and Future. Vet. Res. 1998, 29, 255–274. [Google Scholar]
- Crespo-León, F. Brucellosis Ovina Y Caprina; World Organisation for Animal Health (OIE): Paris, France, 1994; pp. 1–450. [Google Scholar]
- Díaz Aparicio, D. Epidemiology of Brucellosis in Domestic Animals Caused by Brucella Melitensis, Brucella Suis and Brucella Abortus. Rev. Sci. Tech. 2013, 32, 53–60. [Google Scholar] [CrossRef]
- Sutherland, S.S.; Searson, J. The Immune Response to Brucella Abortus: The Humoral Immune Response. In Animal Brucellosis; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- Tittarelli, M.; Giovannini, A.; Conte, A.; Ventura, M.D.; Nannini, D.; Caporale, V. The Use of Homologous Antigen in the Serological Diagnosis of Brucellosis Caused by Brucella Melitensis. J. Vet. Med. Ser. B Infect. Dis. Vet. Public Health 2005, 52, 75–81. [Google Scholar] [CrossRef]
- More, S.; Bøtner, A.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Schmidt, C.G.; Michel, V.; et al. Assessment of Listing and Categorisation of Animal Diseases within the Framework of the Animal Health Law [Regulation (EU) No 2016/429]: Infection with Brucella Abortus, B. Melitensis and B. Suis. EFSA J. 2017, 15, e04889. [Google Scholar] [CrossRef]
- Wareth, G.; Melzer, F.; El-Diasty, M.; Schmoock, G.; Elbauomy, E.; Abdel-Hamid, N.; Sayour, A.; Neubauer, H. Isolation of Brucella Abortus from a Dog and a Cat Confirms Their Biological Role in Re-Emergence and Dissemination of Bovine Brucellosis on Dairy Farms. Transbound. Emerg. Dis. 2017, 64, 27–30. [Google Scholar] [CrossRef]
- El-Sayed, A.; Awad, W. Brucellosis: Evolution and Expected Comeback. Int. J. Vet. Sci. Med. 2018, 6, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Hikal, A.F.; Wareth, G.; Khan, A. Brucellosis: Why is it eradicated from domestic livestock in the United States but not in the Nile River Basin countries? Ger. J. Microbiol. 2023, 3, 19–25. [Google Scholar] [CrossRef]
- Wareth, G.; Melzer, F.; Tomaso, H.; Roesler, U.; Neubauer, H. Detection of Brucella abortus DNA in aborted goats and sheep in Egypt by real-time PCR. BMC Res. Notes 2015, 8, 212. [Google Scholar] [CrossRef] [PubMed]
- Dagan, T.; Blekhman, R.; Graur, D. The “Domino Theory” of Gene Death: Gradual and Mass Gene Extinction Events in Three Lineages of Obligate Symbiotic Bacterial Pathogens. Mol. Biol. Evol. 2006, 23, 310–316. [Google Scholar] [CrossRef]
- Radhakrishnan, G.; Yu, Q.; Harms, J.; Splitter, G. Brucella TIR Domain-containing Protein Mimics Properties of the Toll-like Receptor Adaptor Protein TIRAP. J. Biol. Chem. 2009, 10, 9892–9898. [Google Scholar] [CrossRef]
- Audic, S.; Lescot, M.; Claverie, J.; Scholz, H. Brucella microti: The genome sequence of an emerging pathogen. BMC Genom. 2009, 10, 352. [Google Scholar] [CrossRef]
- Dahouk, S.A.; Hofer, E.; Tomaso, H.; Vergnaude, G.; Flèchee, P.L.; Cloeckaertim, A.; Koylass, M.S.; Whatmore, A.M.; Nöckler, K.; Scholz, H.C. Intraspecies Biodiversity of the Genetically Homologous Species Brucella microti. Appl. Environ. Microbiol. 2012, 78, 1534–1543. [Google Scholar] [CrossRef]

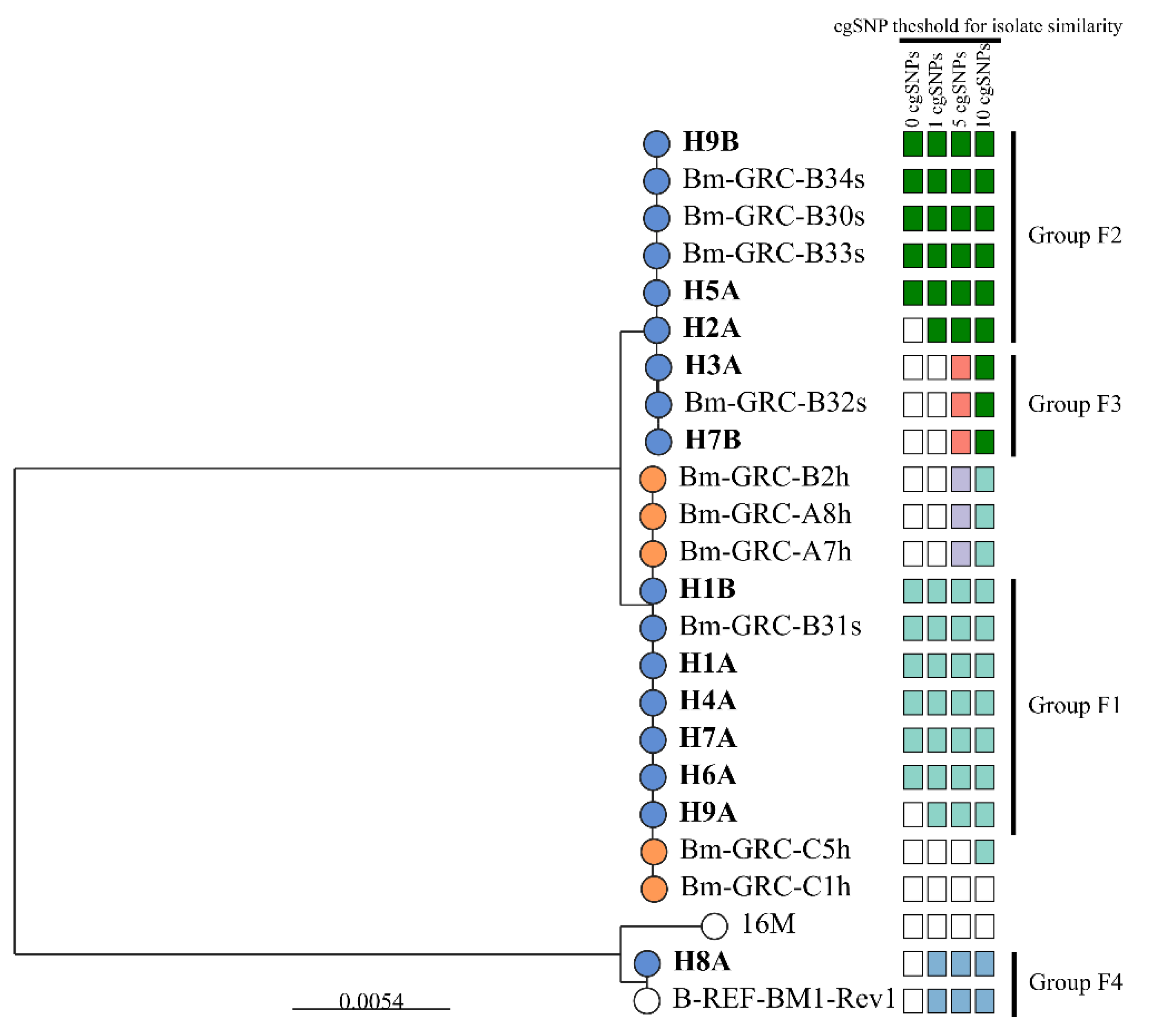

| Days Post-Vaccination with the Rev1 Vaccine Strain | |||||
|---|---|---|---|---|---|
| Animal ID | 0 | 30 | 60 | 90 | 120 |
| H1 | 4.1 ± 0.2 | 139.6 ± 5.2 | 156.3 ± 6.3 | 154.5 ± 4.8 | 117.9 ± 3.9 |
| H2 | 4.1 ± 0.4 | 125.9 ± 4.3 | 142.0 ± 4.4 | 91.0 ± 3.1 | 96.4 ± 4.4 |
| H3 | 4.1 ± 0.6 | 56.7 ± 1.8 | 137.5 ± 6.1 | 108.8 ± 3.9 | 98.6 ± 3.7 |
| H4 | 3.7 ± 0.3 | 96.8 ± 3.6 | 82.6 ± 2.8 | 43.7 ± 1.4 | 21.9 ± 1.5 |
| H5 | 4.0 ± 0.5 | 102.8 ± 3.4 | 129.7 ± 4.1 | 89.2 ± 4.1 | 62.0 ± 2.1 |
| H6 | 4.0 ± 0.7 | 156.4 ± 6.2 | 177.6 ± 7.3 | 165.9 ± 4.4 | 162.1 ± 4.4 |
| H7 | 4.2 ± 0.9 | 135.4 ± 3 | 163.7 ± 5.5 | 167.5 ± 7.6 | 200.4 ± 7.3 |
| H8 | 4.5 ± 0.5 | 121.9 ± 4.5 | 79.5 ± 4.4 | 30.2 ± 2.1 | 23.0 ± 4.3 |
| H9 | 4.0 ± 0.1 | 156.4 ± 3.3 | 82.6 ± 3.9 | 19.2 ± 3 | 16.7 ± 2.2 |
| H10 | 4.9 ± 0.5 | 167.4 ± 3 | 171.1 ± 5.4 | 148.7 ± 4.3 | 121 ± 4 |
| Hogget No. | 0 d | 30 d | 60 d | 90 d | 120 d | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | M | E | C | Is. ID | M | E | C | M | E | C | Is. ID | M | E | C | Is. ID | M | E | |
| H1 | − | − | − | + | H1A | + | + | − | + | + | + | H1B | + | + | − | − | + | |
| H2 | − | + | − | + | H2A | + | + | − | + | + | − | − | + | − | − | + | ||
| H3 | − | + | − | + | H3A | + | + | − | − | + | − | − | + | − | − | + | ||
| H4 | − | − | − | + | H4A | + | + | − | − | + | − | − | + | − | − | − | ||
| H5 | − | − | − | − | + | + | − | − | + | + | H5A | + | + | − | − | + | ||
| H6 | − | − | − | − | + | + | − | − | + | + | H6A | + | + | − | − | + | ||
| H7 | − | + | − | + | H7A | + | + | − | − | + | + | H7B | + | + | − | − | + | |
| H8 | − | − | − | − | − | + | − | − | + | − | + | + | + | H8A | + | − | ||
| H9 | − | − | − | + | H9A | + | + | − | − | + | + | H9B | + | − | − | − | − | |
| H10 | − | − | − | − | + | + | − | − | + | − | + | + | − | − | + | |||
| H1A | H2A | H3A | H4A | H7A | H9A | H1B | H5A | H6A | H7B | H9B | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H1A | 0 | 248 | 254 | 0 | 0 | 1 | 0 | 247 | 0 | 254 | 247 |
| H4A | 0 | 248 | 254 | 0 | 0 | 1 | 0 | 247 | 0 | 254 | 247 |
| H7A | 0 | 248 | 254 | 0 | 0 | 1 | 0 | 247 | 0 | 254 | 247 |
| H1B | 0 | 248 | 254 | 0 | 0 | 1 | 0 | 247 | 0 | 254 | 247 |
| H6A | 0 | 248 | 254 | 0 | 0 | 1 | 0 | 247 | 0 | 254 | 247 |
| H9A | 1 | 249 | 255 | 1 | 1 | 0 | 1 | 248 | 1 | 255 | 248 |
| H5A | 247 | 1 | 7 | 247 | 247 | 248 | 247 | 0 | 247 | 7 | 0 |
| H9B | 247 | 1 | 7 | 247 | 247 | 248 | 247 | 0 | 247 | 7 | 0 |
| H2A | 248 | 0 | 8 | 248 | 248 | 249 | 248 | 1 | 248 | 8 | 1 |
| H3A | 254 | 8 | 0 | 254 | 254 | 255 | 254 | 7 | 254 | 2 | 7 |
| H7B | 254 | 8 | 2 | 254 | 254 | 255 | 254 | 7 | 254 | 0 | 7 |
| H8A | 2389 | 2403 | 2409 | 2389 | 2389 | 2390 | 2389 | 2402 | 2389 | 2409 | 2402 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babetsa, M.; Brangsch, H.; Wareth, G.; Bouzalas, I.; Gelasakis, A.I.; Zdragas, A.; Ekateriniadou, L.V.; Boukouvala, E.; Papadopoulos, A.I. Follow-Up of the Immune Response and the Possible Presence of Brucella melitensis Strains in Peripheral Blood in Hoggets Vaccinated by Rev1 in Greece. Microbiol. Res. 2025, 16, 124. https://doi.org/10.3390/microbiolres16060124
Babetsa M, Brangsch H, Wareth G, Bouzalas I, Gelasakis AI, Zdragas A, Ekateriniadou LV, Boukouvala E, Papadopoulos AI. Follow-Up of the Immune Response and the Possible Presence of Brucella melitensis Strains in Peripheral Blood in Hoggets Vaccinated by Rev1 in Greece. Microbiology Research. 2025; 16(6):124. https://doi.org/10.3390/microbiolres16060124
Chicago/Turabian StyleBabetsa, Maria, Hanka Brangsch, Gamal Wareth, Ilias Bouzalas, Athanasios I. Gelasakis, Antonios Zdragas, Loukia V. Ekateriniadou, Evridiki Boukouvala, and Athanasios I. Papadopoulos. 2025. "Follow-Up of the Immune Response and the Possible Presence of Brucella melitensis Strains in Peripheral Blood in Hoggets Vaccinated by Rev1 in Greece" Microbiology Research 16, no. 6: 124. https://doi.org/10.3390/microbiolres16060124
APA StyleBabetsa, M., Brangsch, H., Wareth, G., Bouzalas, I., Gelasakis, A. I., Zdragas, A., Ekateriniadou, L. V., Boukouvala, E., & Papadopoulos, A. I. (2025). Follow-Up of the Immune Response and the Possible Presence of Brucella melitensis Strains in Peripheral Blood in Hoggets Vaccinated by Rev1 in Greece. Microbiology Research, 16(6), 124. https://doi.org/10.3390/microbiolres16060124








