Abstract
Arbuscular mycorrhizal fungi (AMF) and vermicompost can be efficient in enhancing the accumulation of metabolites, whereas there are no reports about their effects on antioxidant bioactive molecules and Sun Protection Factor (SPF) in Anadenanthera colubrina, a species used in cosmetic formulations. We hypothesized that the combination of AMF inoculation and vermicompost supplementation would synergistically optimize the production of these compounds and improve the antioxidant capacity and SPF of the plant leaves. A completely randomized experiment was set up in a factorial design with three mycorrhizal inoculation treatments (control, Acaulospora longula, and Gigaspora albida) and two substrate proportions (soil alone and soil with 10% vermicompost). After 126 days, the leaves were harvested to evaluate the content of primary metabolites, phenolics, antioxidant capacity, and SPF. Vermicompost did not synergize with AMF to enhance biomolecule synthesis in A. colubrina; instead, it neutralized the mycorrhizal effects. However, plants grown in soil supplemented with vermicompost showed an increase in metabolite and SPF accumulation compared to those grown solely in soil. Seedlings colonized by G. albida and grown in soil also exhibited enhanced anabolism. Therefore, this is the first report in the literature regarding the mitigating effect of vermicompost application on the SPF of mycorrhizal plants. Future studies should consider analyzing these factors in field conditions to attest the need of these agricultural tools.
1. Introduction
Anadenanthera colubrina (Vell.) Brenan., known as cebil, is a legume found in the Brazilian Caatinga and Cerrado [1], which has antioxidant, anti-inflammatory [2], antimicrobial [3], immunomodulatory [4], and photoprotective properties [5], due to the health-promoting bioactive compounds present in the bark, leaves, and fruit, such as phenolic acids, flavonoids, and triterpenes [6]. In Brazil, this species is used in the formulation of cosmetics and antiseptics, such as those produced by Sanativo®.
This leguminous plant, used in several industrial sectors [7], has improved the accumulation of secondary metabolites when arbuscular mycorrhizal fungi (AMF) are applied during seedling production [5]. This benefit is attributed to Acaulospora and Gigaspora representatives due to the modulation of microbial respiration and soil pH, as these fungi reduce the soil acidity and enhance CO2 evolution [8]. In addition, the incorporation of superphosphate in A. colubrina cultivation also potentializes the synthesis of antioxidant compounds [9]. These findings highlight the potential of AMF and specific fertilizers in boosting the production of valuable compounds in A. colubrina, which can be beneficial for various industrial applications.
In the context of agricultural crops, soil fertilization is a common practice aimed at ensuring long-term soil health and nutrient availability for plants [10]. Thus, it is crucial to identify sustainable agricultural tools that can synergistically enhance the anabolism of crops like A. colubrina when used in conjunction with AMF. A practice that may be applied is the use of organic fertilizers, like vermicompost [11], a substrate that benefits the soil’s characteristics [12], promoting AMF activity [13,14].
It is expected that the vermicompost may act synergistically with the mycorrhizal symbiosis to increase the accumulation of plant metabolites [15], given its physical and chemical characteristics that favor soilborne microorganisms [16]. However, the response can vary from positive to neutral to even a reduction in metabolite biosynthesis in AMF-associated plants grown in organic substrates, depending on the dose and growth conditions [17,18]. Therefore, it is essential to define the role of vermicompost in the production of bioactive molecules in mycorrhizal A. colubrina, something that has not yet been established. Understanding this dynamic is vital for developing sustainable agricultural practices that can optimize the production of valuable bioactive compounds in crops.
Therefore, the hypothesis tested was that AMF inoculation and vermicompost supplementation act synergistically to boost the primary metabolites and phenolics production, the in vitro antioxidant capacity, and the Sun Protection Factor (SPF) in leaves of A. colubrina seedlings. The aim was to investigate if the combined application of mycorrhizal isolates and vermicompost can enhance the production of antioxidant compounds in A. colubrina leaves, aiming to identify the superior agricultural strategy for establishing a sustainable protocol to produce high-quality seedlings for the cosmetic and antiseptic industries.
2. Materials and Methods
The research was conducted over 126 days at the University of Pernambuco (UPE), Brazil, with the biochemical, phytochemical, and SPF assays carried out at the Laboratory of Analysis, Research, and Studies in Mycorrhizae (LAPEM/UPE) (8°2′47.143″ S; 34°53′15.086″ W).
2.1. Experimental Design
An experiment was established in a greenhouse, with completely randomized design, in a 3 × 2 factorial arrangement, with three mycorrhizal inoculation treatments (control without AMF, plants inoculated with Acaulospora longula Spain & N.C. Schenck, and plants inoculated with Gigaspora albida N.C. Schenck & G.S. Sm.) and two proportions of vermicompost supply (with and without 10% vermicompost), with four replicates each, totaling 24 experimental units.
2.2. Inoculum Multiplication
The initial isolates of A. longula and G. albida were sourced from the Mycology Department of the Federal University of Pernambuco, Brazil, and were propagated for 90 days in a greenhouse using disinfected soil plus 10% organic compost with Panicum miliaceum L. as the host. After this period, the aerial parts were discarded, while the underground parts were dried and roots were fragmented (±1 cm) and homogenized, resulting in the soil inoculum, which was collected and stored (4 °C) until the time of inoculation [19]. These inocula had an infectivity of around 60%, according to the methodology described by INVAM [20]. These isolates were chosen for their efficiency in improving the secondary metabolism of A. colubrina in previous studies [5,19].
2.3. Inoculation and Cultivation of A. colubrina Seedlings
Anadenanthera colubrina seeds were disinfected with NaClO (25 mL/L) and germinated in chemically disinfected soil. Once the definitive leaves emerged, the seedlings were transplanted into pots with a soil capacity of approximately 2 kg. These pots contained either 10% vermicompost (9:1, v/v) or not, in which they received soil inoculum (containing 200 glomerospores of A. longula and G. albida), except for control plants. This proportion of vermicompost was chosen based on previous studies that evaluated the phytochemistry of mycorrhizal plants in vermicompost-based substrate with AMF [18,21]. The seedlings were kept in a greenhouse, watered daily to maintain 70% of the total pore volume (TPV) of the substrate filled with water [22] (TPV = 100 (1-DS/DP) (DS: soil density; DP: particle density), under environmental conditions of average temperature (25 °C) and relative humidity (77.5%).
The soil used for germination and cultivation was chemically characterized by the Brazilian Agricultural Research Corporation (EMBRAPA) (Table 1). Both the fungi and the plant species were officially registered in the National System for the Management of Genetic Heritage and Associated Traditional Knowledge (SisGen) under the number AA6688A.

Table 1.
Fertility of substrates used to cultivate Anadenanthera colubrina (Vell.) Brenan seedlings.
2.4. Extract Preparation
Following a 126-day period in a greenhouse, the leaves were collected, thermally stabilized at 45 °C for 72 h, and then sectioned. Aliquots of 500 mg of the plant material were then transferred to amber flasks, and 20 mL of ethanol (950 mL L−1) (Neon®, São Paulo, Brazil) were added. After 12 days of maceration (20 °C), the extracts were filtered twice and stored in a freezer at −18 °C, ready for use in biochemical, phytochemical, and antioxidant activity, and SPF assays. All data regarding metabolite production is expressed as content (mg plant−1) considering the values obtained from dry matter accumulation.
2.5. Biochemical Analyses
2.5.1. Total Soluble Carbohydrates
To quantify the total soluble carbohydrates, 50 µL of the plant extract was combined with 95 µL of distilled water, 50 µL of phenol (800 mg L−1) (Sigma-Aldrich®, São Paulo, Brazil), and 2 mL of H2SO4 (Isofar Ltd., Rio de Janeiro, Brazil). The mixture was then vortexed (Biomixer Ltd., São Paulo, Brazil) and allowed to stand for 10 min before spectrophotometer reading (Thermo Fisher Scientific, Waltham, MA, USA) (490 nm), using glucose (Vetec®, Rio de Janeiro, Brazil) as the standard curve [23] (Absorbance = 0.0006 × Concentration + 0.0605; R2 = 0.98630).
2.5.2. Total Proteins
The total proteins accumulated in A. colubrina leaves were analyzed by mixing 50 µL of extract and 2500 µL of Bradford reagent (composed of Coomassie Blue G-250 dye, distilled water, and phosphoric acid) (Sigma-Aldrich®, São Paulo, Brazil; Dinâmica®, São Paulo, Brazil). The samples were shaken in a vortex (Biomixer Ltd., São Paulo, Brazil) and, after 5 min, the samples were read in a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 595 nm. Bovine serum albumin (Sigma-Aldrich®, Saint Louis, MO, USA) was used as a standard curve [24] (Absorbance = 0.0007 × Concentration + 0.0036; R2 = 0.9972).
2.6. Phytochemical Analyses
2.6.1. Total Flavonones and Dihydroflavonols
To verify the potential of vermicompost and AMF in increasing the synthesis of antioxidant bioactive molecules, total flavonones and dihydroflavonols were measured using a method that involved reacting 100 µL of the extract with 200 µL of 2,4-dinitro-phenylhydrazine (10 g L−1) (Dinâmica®, São Paulo, Brazil). The mixture was transferred to microtubes (2 mL) and placed in a water bath (50 °C) for 50 min. After cooling to room temperature, an aliquot of 100 µL from this solution was transferred to amber flasks containing 1000 µL of KOH (100 mg L−1) (Fmaia®, São Paulo, Brazil), and 4900 µL of methanol (Dinâmica®, São Paulo, Brazil) were added. The samples were vortexed (Biomixer Ltd., São Paulo, Brazil) and then read in a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 486 nm, with methanol serving as the blank [25,26]. Naringenin was used as the standard curve (Sigma-Aldrich®, São Paulo, Brazil) (Absorbance = 0.1023 × Concentration + 0.0543; R2 = 0.9986).
2.6.2. Total Phenolic Compounds
Total phenolic compounds were quantified by adding 200 µL of plant extract and 1 mL of Folin-Ciocalteu reagent (100 mL L−1) (Merck®, Darmstadt, Germany), with the addition of 800 µL of sodium carbonate (75 g L−1) (Vetec Ltd., Duque de Caxias, Brazil) in amber flasks. The solution was vortex-stirred (Biomixer Ltd., São Paulo, Brazil) and left to stand for 30 min before being read spectrophotometrically (Thermo Fisher Scientific, Waltham, MA, USA) at 765 nm [27]. Gallic acid (Dinamica®, São Paulo, Brazil) was used to establish the standard curve (Absorbance = 0.0092 × Concentration − 0.0621; R2 = 0.9976).
2.7. Antioxidant Activity Assay
To verify the antioxidant potential of the A. colubrina phytomass, the ability to reduce the phosphomolybdenum complex was assessed by adding 200 µL of the extract diluted (1:5) in ethanol (950 mL L−1) (Neon®, São Paulo, Brazil) to 2000 µL of the work solution, consisting of 588 µL of H2SO4 (Isofar Ltd., Rio de Janeiro, Brazil), 0.036 g of sodium phosphate (Dinâmica®, São Paulo, Brazil), and 0.046 g of ammonium molybdate (Dinâmica®, São Paulo, Brazil). The final solution was then placed in a water bath (95 °C) for 90 min and, after cooling to room temperature, the samples were read spectrophotometrically (695 nm). The results of this analysis were expressed as a percentage compared to that obtained for the positive control, tocopherol (0.0025 g/10 mL) (Sigma-Aldrich®, São Paulo, Brazil) [28,29].
2.8. Sun Protection Factor Assay
The SPF was evaluated using the extract diluted in ethanol (950 mL L−1) (Neon®, São Paulo, Brazil) (0.10 mg/mL), followed by vortex agitation for 10 s (Biomixer Ltd., São Paulo, Brazil). Then, 900 µL were transferred to a quartz cuvette (Thermo Fisher Scientific, Waltham, MA, USA) and scanned from 290 nm to 320 nm (at 5-nm intervals). Based on the absorbances obtained, the SPF was calculated using the equation SPF = CF × ABS × EE × I × AA, where CF = correction factor; ABS = absorbance (290–320 nm); EE = erythemogenic effect; I = solar intensity; and AA = absorbance obtained in the reading [30,31,32]. Benzophenone (2-hydroxy-4-methoxybenzophenone, 100 mg L−1) (Sigma-Aldrich®, Saint Louis, MO, USA), an active and synthetic ingredient in sunscreens, and quercetin (100 mg L−1) (Sigma-Aldrich®, Saint Louis, MO, USA), a flavonoid with recognized photoprotective activity, were used as positive controls.
2.9. Statistical Analysis
The data were evaluated using a two-way ANOVA, with the means compared using the Tukey test (p ≤ 0.05), using ASSISTAT version 7.7 beta (2016). Clustering algorithm analyses (K-means) and hierarchical dendrogram construction were carried out using Python language and Google Collaborative Platforms. The figures in the results were generated using GraphPad Prism 10.3.1—free demo.
3. Results and Discussion
Contrary to initial expectations, the results indicated that both AMF inoculation and organic fertilization with vermicompost independently enhanced compound production in A. colubrina. However, no synergism was observed when these agricultural technologies were applied together, as vermicompost mitigated the symbiotic efficiency reported in soil without fertilizer (Figure 1 and Figure 2).

Figure 1.
Content (mg plant-1) of total soluble carbohydrates (A), total proteins (B), total flavonones and dihydroflavonols (C), total phenolic compounds (D), antioxidant activity (phosphomolybdenum complex reduction) (E), and Sun Protection Factor (SPF) (F) in leaves of Anadenanthera colubrina (Vell.) Brenan seedlings inoculated or not with Acaulospora longula Spain & N.C. Schenck and Gigaspora albida N.C. Schenck & G.S. Sm. and cultivated in soil supplied or not with 10% vermicompost, after 126 days in a greenhouse. Positive controls SPF: benzophenone = 17.15; quercetin = 1.28. Means followed by the same letter do not differ by Tukey’s test (5%), lower case for mycorrhizal inoculation treatments, and upper case for substrate proportion. Reduction potential of the phosphomolybdenum complex in comparison to vitamin E (0.0025 g/10 mL). CV: coefficient of variation. Bars = standard deviation of the means.
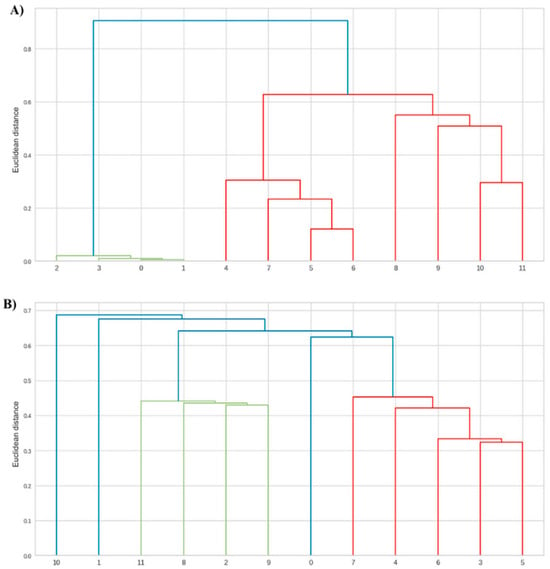
Figure 2.
Dendrogram showing the phytochemical variables of leaves from Anadenanthera colubrina (Vell.) Brenan seedlings inoculated or not with arbuscular mycorrhizal fungi (AMF), grown in soil (A) and soil with 10% vermicompost (B). 0–3 = control without AMF; 4–7 = plants inoculated with Acaulospora longula Spain & N.C. Schenck; 8–11 = plants inoculated with Gigaspora albida N.C. Schenck & G.S. Sm.
In this context, the presence of AMF, especially G. albida, was indispensable for increasing the content of primary and secondary metabolites and their antioxidant capacity in A. colubrina seedlings grown in soil, with an increase in inoculated plants being, on average, around 21,000% higher than in non-mycorrhizal A. colubrina grown without vermicompost supply (Figure 1). Therefore, seedlings associated with G. albida produced more total proteins, total soluble carbohydrates, total phenolic compounds, total flavonones, and dihydroflavonols, exhibiting outstanding antioxidant potential and SPF, with increases ranging from 2000 to 60,000%, compared to control seedlings (Figure 1). It is worth noting that A. colubrina seedlings colonized by A. longula also had their anabolism enhanced, although to a lesser extent (Figure 1).
The symbiotic benefit highlighted in seedlings forming mycorrhiza with G. albida (Figure 1) can be explained by the presence of tubular vacuoles in the mycelium, which are capable of efficiently transporting P, a feature well-documented in Gigaspora species [33]. This condition may have promoted the improved production of carbohydrates and proteins (Figure 2), which are essential for the anabolism of antioxidant phenolics, a process dependent on several ATP molecules [34]. Similar results were observed by Silva et al. [35], in Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz var. ferrea, considering that the enhanced synthesis of proteins and flavonoids occurred when this leguminous tree was associated with G. albida.
On the other hand, the presence of vermicompost mitigated the mycorrhization benefits reported in plants grown in soil (Figure 1). In general, when the seedlings were kept in an organic-based substrate, there was an optimized accumulation of primary and secondary metabolites (Figure 1). Thus, the production of both soluble carbohydrates and proteins in plants grown in soil with vermicompost increased at least 320 times compared to plants cultivated in unfertilized control (Figure 1). Similarly, fertilization also increased the biosynthesis of phenolics, flavonones, and dihydroflavonols by more than 13,000% compared to seedlings grown in soil without vermicompost (Figure 1). This contrasts with the findings of Yusof et al. [36], who observed a decrease in the antioxidant activity of Clinacanthus nutans (Burm.f) Lindau with vermicompost, and Souffront et al. [37], who found that vermicompost did not influence phenolic compound production.
The increased production of antioxidant molecules found in the leaves of seedlings grown in soil fertilized with vermicompost (Figure 1) may be related to the elevated nutrient availability, considering the chemical properties of the fertilized soil (Table 1), as better plant nutrition can influence mycorrhizal dependency [38]. In previous studies, under conditions of inorganic fertilization, the greater availability of P can make the use of AMF to promote certain biomolecules such as flavonoids, soluble carbohydrates, and total proteins [19] to increase the production of antioxidant compounds unessential [9]. This behavior has also been observed with organic sources in seedling cultivation, though not for plant SPF. Therefore, future studies should focus on understanding the level of mycorrhizal dependence of this legume, which appears to be facultative [39,40].
Even though the characteristics of vermicompost may favor the mycorrhizal activity itself, such as spore production [41], colonization [38], and deposition of glomalin-related soil proteins [42], due to the presence of humic acid and mineralizing soil microorganisms, no synergism was observed in this research. Despite the occurrence of Acaulospora and Gigaspora isolates with similar or higher organic matter content [43,44], AMF species can be negatively influenced by the soil organic matter content [45], and this may be one of the reasons that mycorrhizal A. colubrina seedlings did not surpass metabolite accumulation and SPF when cultivated in soil supplemented with vermicompost.
Comparable to bioactive compound accumulation, the antioxidant capacity of the extracts was increased by approximately 3000% in plants grown in soil plus vermicompost compared to those kept just in soil, irrespective of mycorrhization status (Figure 1). This pattern was consistent with the findings of Falcão et al. [9], in which seedlings grown in soil supplemented with phosphorus (50 mg dm−3) showed an antioxidant potential twice as high as plants inoculated with AMF and grown in soil with lower P levels.
Even with a less expressive antioxidant activity value compared to the plants grown in fertilized soil, it is worth noting that the mycorrhizal seedlings also had an antioxidant capacity on average 2000% greater than the control plants without any fertilizer supply (Figure 1). This demonstrates the potential of mycorrhizal legumes to integrate cosmetic formulations with antioxidant action, considering that the values obtained are higher than those of other Fabaceae, such as Hymenaea martiana Hayne [32] and Centrosema coriaceum Benth [46]. This highlights the benefits of sustainable cultivation strategies, such as the use of AMF or vermicompost, in producing antioxidant-rich phytomass.
Similarly, mycorrhization led to an increase in SPF content by over 17,000% compared with non-mycorrhizal seedlings grown in soil (Figure 1). As verified by Falcão et al. [5], the benefits of mycorrhization on photoprotective activity are associated with the biosynthesis of antioxidant metabolites (Figure 3).
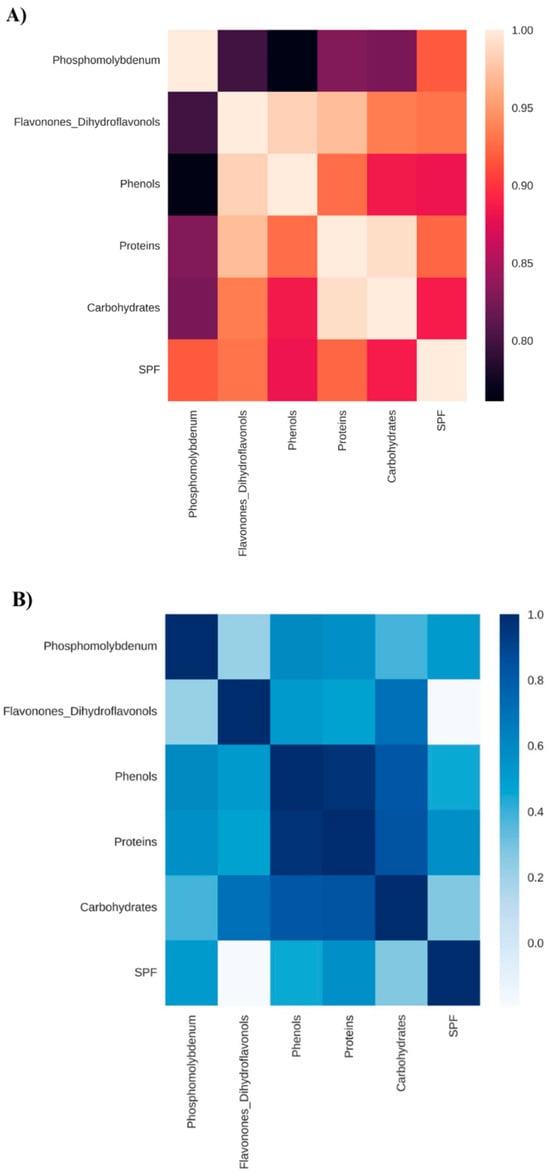
Figure 3.
Correlation matrix considering the reduction of the phosphomolybdenum complex, total flavonones and dihydroflavonols, total phenols, total proteins, total soluble carbohydrates, and in vitro Sun Protection Factor (SPF) of Anadenanthera colubrina (Vell.) Brenan, inoculated or not with arbuscular mycorrhizal fungi and grown in soil (A) or in soil with 10% vermicompost (B).
On the other hand, in an organic-based substrate, the foliar SPF of A. colubrina seedlings was not enhanced by AMF (Figure 2). This result showed a reduction in the beneficial effect of the fungus on the SPF of A. colubrina seedlings in response to higher levels of available P (Figure 4). This aligns with the findings of Falcão et al. [9], in which inorganic soil fertilization could achieve SPF promotion without the need of AMF. On the other hand, negative effects of vermicompost in plant SPF have been described [21].
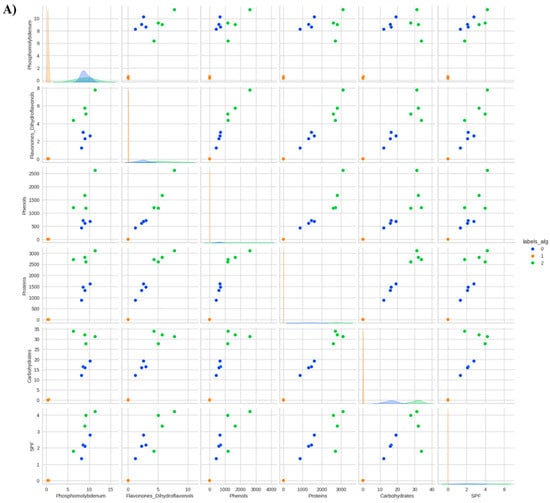
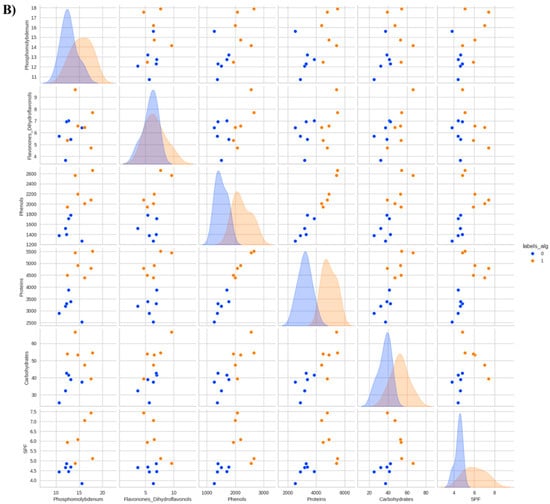
Figure 4.
Data distribution by cluster considering the reduction of the phosphomolybdenum complex, total flavonones and dihydroflavonols, total phenols, total proteins, total soluble carbohydrates, and in vitro Sun Protection Factor (SPF) of Anadenanthera colubrina (Vell.) Brenan, inoculated or not with arbuscular mycorrhizal fungi (K-means = 3), grown in soil (A) or soil with 10% vermicompost (B) (K-means = 2).
In short, to offer a cultivation protocol for this legume tree, the vermicompost application appears more attractive due to its positive effects on both the plant and soil in the short term [47,48], coupled with its well-established commercialization. In this case, the approximate cost for this approach is 0.20 cents per cultivation pot. Comparatively, the producer can opt for mycorrhizal technology as an equally sustainable but less efficient and more cost-effective option, with the production of G. albida inoculum costing around 0.08 cents per plant [49]. However, it is important to test whether this biotechnology can offer superior long-term benefits over vermicompost.
Therefore, the hypothesis of the study was not supported, as no synergistic effect was observed between vermicompost and AMF in increasing the primary and secondary anabolism of A. colubrina seedlings. Moreover, both agrotechnologies can be used separately for the sustainable cultivation of A. colubrina seedlings, providing phytomass enriched with higher levels of antioxidant metabolites and SPF. This is particularly relevant for the formulation of cosmetics with anti-aging, photoprotective, and anti-inflammatory activities [50], especially since this species is already used in several cosmetic products in Brazil [51].
Further studies with alternative fertilizers are essential to verify the role of organic fertilization on the metabolism of A. colubrina seedlings. Also, it is essential to test if the same pattern of this research is achieved in field conditions. Furthermore, it is also crucial to explore the potential of other AMF that were effective in improving growth in Anadenanthera [52], both as individual strains and in a consortium, to enhance the production of antioxidant and photoprotective bioactive compounds in this Fabaceae phytomass.
4. Conclusions
Three main conclusions/considerations were obtained in this research. The first one relies on the application of AMF without organic fertilization. In this case, inoculation with G. albida is recommended to improve the biosynthesis of phenolic compounds and plant SPF that outstands the accumulation of non-inoculated plants and A. longula inoculated seedlings cultivated in soil. Secondly, the soil supplementation with vermicompost can often surpass the metabolite content and SPF seen in mycorrhizal plants, providing an accessible alternative for supplying A. colubrina phytomass to the cosmetic industries. Thirdly, no synergistic effect is observed when AMF and vermicompost are combined in A. colubrina cultivation, since the vermicompost mitigates AMF benefits and makes the use of these biostimulants unnecessary in this scenario. Thus, both mycorrhizal technology and vermicompost increase the production of antioxidant compounds and SPF in A. colubrina leaves, indicating their potential as valuable options for the production chain of cosmetics and antiseptics based on A. colubrina phytomass. For future studies, it is important to evaluate the effect of other AMF combined with various organic matrices in A. colubrina cultivation, while also investigating their role under field conditions.
Author Contributions
J.G.L.d.C.: Writing—review & editing, original draft, Methodology, Investigation, Formal analysis. E.L.F.: Writing—review & editing, original draft, Methodology, Investigation, Formal analysis. C.J.A.B.F.: Writing—review & editing, original draft, data analysis. Q.-S.W.: Writing—review & editing. F.S.B.d.S.: Writing—review & editing, original draft, Supervision, Resources, Methodology, Investigation, Funding acquisition, Formal analysis, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) (finance code 001) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process number: 140405/2024-0).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the productivity fellowship granted to Fábio Sérgio Barbosa da Silva and Carmelo José Albanez Bastos Filho, the doctoral scholarship to Eduarda Lins Falcão (process number: 140405/2024-0), and the undergraduate scholarship (PIBIC/CNPq/UPE) to João Gabriel Lira de Carvalho. The authors also thank Ângelo Souto de Santana for their help in conducting the experiment.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Anadenanthera in Flora e Funga do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB18071 (accessed on 7 December 2024).
- Cardoso Junior, O.; Lima, N.M.; Silva, M.G.A.; Aguiar, V.B.; Carli, G.P.; Scherrer, E.C.; Castro, S.B.R.; Alves, C.C.S.; Oliveira, M.A.L.; Carli, A.P. In vitro and in vivo evaluation of anti-inflammatory activity and free radical scavenging potential of leaves extract from Anadenanthera colubrina. Nat. Prod. Res. 2021, 35, 4819–4823. [Google Scholar] [CrossRef] [PubMed]
- Maia, C.M.D.A.; Pasetto, S.; Nonaka, C.F.W.; Costa, E.M.M.D.B.; Murata, R.M. Yeast-host interactions: Anadenanthera colubrina modulates virulence factors of C. albicans and inflammatory response in vitro. Front. Pharmacol. 2021, 12, 629778. [Google Scholar] [CrossRef]
- Ramos, K.A.; Soares, I.G.M.; Oliveira, L.M.A.; Braga, M.A.; Soares, P.P.C.; Guarneire, G.J.; Scherrer, E.C.; Silva, F.S.; Lima, N.M.; La Porta, F.A.; et al. Immunomodulatory effects of Anadenanthera colubrina bark extract in experimental autoimmune encephalomyelitis. Curr. Issues Mol. Biol. 2024, 46, 8726–8740. [Google Scholar] [CrossRef]
- Falcão, E.L.; Bastos Filho, C.J.A.; Silva, F.S.B. Arbuscular mycorrhizal fungi enhance the Sun Protection Factor (SPF) biosynthesis in Anadenanthera colubrina (Vell.) Brenan leaves. Rhizosphere 2022, 24, 100595. [Google Scholar] [CrossRef]
- Delices, M.; Muller, J.D.A.I.; Arunachalam, K.; de Oliveira Martins, D.T. Anadenanthera colubrina (Vell) Brenan: Ethnobotanical, phytochemical, pharmacological and toxicological aspects. J. Ethnopharmacol. 2023, 300, 115745. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.M.B.; Barros, A.J.M.; Silva, J.N.S.; Ribeiro, G.N. Prospecção tecnológica do angico-branco (Anadenanthera colubrina (Vell) Brenan). In Engenharia, Agronomia e Geociência 2014–2021, 1st ed.; Santos, D., Furtado, A.D., Eds.; Eptec: Campina Grande, Brazil, 2021; Volume 1, pp. 67–78. [Google Scholar]
- Falcão, E.L.; Muniz, B.C.; Bastos Filho, C.J.A.; Kapoor, R.; Silva, F.S.B. Soil microbial respiration and pH modulated by arbuscular mycorrhizal fungi influence the biosynthesis of health-promoting compounds in Anadenanthera colubrina (Vell.) Brenan. Rhizosphere 2023, 26, 100685. [Google Scholar] [CrossRef]
- Falcão, E.L.; Barreto, C.B.; Hijri, M.; Bastos Filho, C.J.A.; Silva, F.S.B. No synergy between P and AMF Inoculation to improve sun protection factor production in Anadenanthera colubrina (Vell.) Brenan leaves. Rhizosphere 2024, 30, 100916. [Google Scholar] [CrossRef]
- Khan, M.T.; Aleinikovienė, J.; Butkevičienė, L.M. Innovative organic fertilizers and cover crops: Perspectives for sustainable agriculture in the era of climate change and organic agriculture. Agronomy 2024, 14, 2871. [Google Scholar] [CrossRef]
- Cruz, G.S.J.; Hermes, P.H.; Miriam, S.V.; López, A.M. Benefits of vermicompost in agriculture and factors affecting its nutrient content. J. Soil. Sci. Plant Nutr. 2024, 24, 4898–4917. [Google Scholar] [CrossRef]
- Rehman, S.; Castro, F.; Aprile, A.; Benedetti, M.; Fanizzi, F.P. Vermicompost: Enhancing plant growth and combating abiotic and biotic stress. Agronomy 2023, 13, 1134. [Google Scholar] [CrossRef]
- Liu, R.C.; Meng, L.L.; Zou, Y.N.; He, X.H.; Wu, Q.S. Introduction of earthworms into mycorrhizosphere of white clover facilitates N storage in glomalin-related soil protein and contribution to soil total N. Appl. Soil. Ecol. 2022, 179, 104597. [Google Scholar] [CrossRef]
- Koskey, G.; Avio, L.; Turrini, A.; Sbrana, C.; Bàrberi, P. Biostimulatory effect of vermicompost extract enhances soil mycorrhizal activity and selectively improves crop productivity. Plant Soil. 2023, 484, 183–199. [Google Scholar] [CrossRef]
- Sarathambal, C.; Srinivasan, V.; Jeevalatha, A.; Sivaranjani, R.; Alagupalamuthirsolai, M.; Peeran, M.F.; Sankar, S.M.; George, P.; Dilkush, F. Unravelling the synergistic effects of arbuscular mycorrhizal fungi and vermicompost on improving plant growth, nutrient absorption, and secondary metabolite production in ginger (Zingiber officinale Rosc.). Front. Sustain. Food Syst. 2024, 8, 1412610. [Google Scholar] [CrossRef]
- Yatoo, A.M.; Ali, M.N.; Baba, Z.A.; Hassan, B. Sustainable management of diseases and pests in crops by vermicompost and vermicompost tea. A review. Agron. Sustain. Dev. 2021, 41, 7. [Google Scholar] [CrossRef]
- Boutasknit, A.; Ait-El-Mokhtar, M.; Fassih, B.; Ben-Laouane, R.; Wahbi, S.; Meddich, A. Effect of arbuscular mycorrhizal fungi and rock phosphate on growth, physiology, and biochemistry of carob under water stress and after rehydration in vermicompost-amended soil. Metabolites 2024, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.S.; Campos, M.A.; Silva, F.S. Arbuscular Mycorrhizal fungi and vermicompost to maximize the production of foliar biomolecules in Passiflora Alata Curtis seedlings. J. Sci. Food Agric. 2015, 95, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Pedone-Bonfim, M.V.; Lins, M.A.; Coelho, I.R.; Santana, A.S.; Silva, F.S.; Maia, L.C. Mycorrhizal technology and phosphorus in the production of primary and secondary metabolites in cebil (Anadenanthera colubrina (Vell.) Brenan) seedlings. J. Sci. Food Agric. 2013, 93, 1479–1484. [Google Scholar] [CrossRef]
- INVAM. Available online: https://invam.ku.edu/methods/infectivity-assays/mean-infection-percentage-mip (accessed on 14 August 2024).
- Muniz, B.C.; Kapoor, R.; Almeida, J.R.G.S.; Bastos Filho, C.J.A.; Silva, F.S.B. Arbuscular mycorrhizae increase but vermicompost decrease the sun protection factor (SPF) in leaves of Hymenaea martiana Hayne seedlings. Rhizosphere 2023, 27, 100781. [Google Scholar] [CrossRef]
- Vomocil, J.A. Porosity. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, New Jersey, USA, 1965; pp. 299–314. ISBN 978-0-89118-203-0. [Google Scholar]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nagy, M.; Grancai, D. Colorimetric determination of flavanones in propolis. Pharmazie 1996, 51, 100–101. [Google Scholar]
- Popova, A.; Christov, M.; Raicheva, S.; Sokolova, E. Adsorption and inhibitive properties of benzimidazole derivatives in acid mild steel corrosion. Corros. Sci. 2004, 46, 1333–1350. [Google Scholar] [CrossRef]
- Orujei, Y.; Shabani, L.; Sharifi-Tehrani, M. Induction of glycyrrhizin and total phenolic compound production in licorice by using arbuscular mycorrhizal fungi. Russ. J. Plant Physiol. 2013, 60, 855–860. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Sarmah, N.; Handique, A.K. Antioxidant activities of the unripen and ripen Citrus aurantifolia of Assam. IJIRSET 2013, 2, 4811–4816. [Google Scholar]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef]
- Mansur, J.d.S.; Breder, M.N.R.; Mansur, M.C.d.; Azulay, R.D. Determinação do fator de proteção solar por espectrofotometria. An. Bras. Dermatol. 1986, 61, 121–124. [Google Scholar]
- Oliveira, F.G.S.; Veras, B.O.; Silva, A.P.S.; Araújo, A.D.; Barbosa, D.C.S.; Silva, T.C.M.; Ribeiro, E.R.F.R.; Maia, M.M.L.; Júnior, U.P.S.; Lima, V.L.M.; et al. Photoprotective activity and HPLC-MS-ESI-IT profile of flavonoids from the barks of Hymenaea martiana Hayne (Fabaceae): Development of topical formulations containing the hydroalcoholic extract. Biotechnol. Biotechnol. Equip. 2021, 35, 504–516. [Google Scholar] [CrossRef]
- Ashford, A. Tubular vacuoles in arbuscular mycorrhizas. New Phytol. 2002, 154, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Lemos, V.C.; Reimer, J.J.; Wormit, A. Color for life: Biosynthesis and distribution of phenolic compounds in pepper (Capsicum annuum). Agriculture 2019, 9, 81. [Google Scholar] [CrossRef]
- Silva, F.A.; Silva, F.S.B.; Maia, L.C. Biotechnical application of arbuscular mycorrhizal fungi used in the production of foliar biomolecules in ironwood seedlings [Libidibia Ferrea (Mart. Ex Tul.) L.P. Queiroz var. ferrea]. J. Med. Plant Res. 2014, 8, 814–819. [Google Scholar] [CrossRef]
- Yusof, Z.; Ramasamy, S.; Mahmood, N.Z.; Yaacob, J.S. Vermicompost supplementation improves the stability of bioactive anthocyanin and phenolic compounds in Clinacanthus nutans Lindau. Molecules 2018, 23, 1345. [Google Scholar] [CrossRef] [PubMed]
- Souffront, D.K.S.; Salazar-Amoretti, D.; Jayachandran, K. Influence of vermicompost tea on secondary metabolite production in tomato crop. Sci. Hortic. 2022, 301, 111135. [Google Scholar] [CrossRef]
- Salari, H.; Amooaghaie, R.; Mozafari, H. Synergistic effects of vermicompost and mycorrhizal inoculation on arsenic tolerance and phytostabilization in safflower (Carthamus tinctorius L.). Environ. Sci. Pollut. Res. 2024, 31, 21947–21961. [Google Scholar] [CrossRef] [PubMed]
- Habte, M.; Manjunath, A. Categories of vesicular-arbuscular mycorrhizal dependency of host species. Mycorrhiza 1991, 1, 3–12. [Google Scholar] [CrossRef]
- Carvalho, L.G.V.; Santos, S.C.; Lourente, E.R.P.; Trovato, V.W.; Santos, C.C.; Rui, R.F. Jatobazeiro seedlings associated with arbuscular mycorrhizal fungi. Rev. Bras. Frutic. 2022, 44, e-006. [Google Scholar] [CrossRef]
- Coelho, I.R.; Pedone-Bonfim, M.V.; Silva, F.S.B.; Maia, L.C. Optimization of the production of mycorrhizal inoculum on substrate with organic fertilizer. Braz. J. Microbiol. 2014, 45, 1173–1178. [Google Scholar] [CrossRef]
- Agnihotri, R.; Pandey, A.; Bharti, A.; Chourasiya, D.; Maheshwari, H.S.; Ramesh, A.; Billore, S.D.; Sharma, M.P. Soybean processing mill waste plus vermicompost enhances arbuscular mycorrhizal fungus inoculum production. Curr. Microbiol. 2021, 78, 2595–2607. [Google Scholar] [CrossRef] [PubMed]
- Nopphakat, K.; Runsaeng, P.; Klinnawee, L. Acaulospora as the dominant arbuscular mycorrhizal fungi in organic lowland rice paddies improves Phosphorus availability in soils. Sustainability 2022, 14, 31. [Google Scholar] [CrossRef]
- Pontes, J.S.; Oehl, F.; Pereira, C.D.; Machado, C.T.T.; Coyne, D.; Silva, D.K.A.; Maia, L.C. Heterogeneity in arbuscular mycorrhizal fungi and plant communities of the brazilian Cerrado, transitional areas toward the Caatinga, and the Atlantic Forest. Microb. Ecol. 2024, 87, 29. [Google Scholar] [CrossRef]
- Jiang, S.; An, X.; Shao, Y.; Kang, Y.; Chen, T.; Mei, X.; Dong, C.; Xu, Y.; Shen, Q. Responses of arbuscular mycorrhizal fungi occurrence to organic fertilizer: A meta-analysis of field studies. Plant Soil. 2021, 469, 89–105. [Google Scholar] [CrossRef]
- Lemos, A.S.O.; Campos, L.M.; Souza, T.F.; Granato, J.T.; Oliveira, E.E.; Aragão, D.M.O.; Apolônio, A.C.M.; Ferreira, A.P.; Fabri, R.L. Combining UFLC-QTOF-MS analysis with biological evaluation of Centrosema coriaceum (Fabaceae) leaves. An. Acad. Bras. Ciênc. 2022, 94, e20200491. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Bakker, M.R.; Milin, S.; Graham, D. Enhancement in soil fertility, early plant growth and nutrition and mycorrhizal colonization by vermicompost application varies with native and exotic tree species. J. Soils Sediments 2022, 22, 1662–1676. [Google Scholar] [CrossRef]
- Song, W.; Shu, A.; Liu, J.; Shi, W.; Li, M.; Zhang, W.; Li, Z.; Liu, G.; Yuan, F.; Zhang, S.; et al. Effects of long-term fertilization with different substitution ratios of organic fertilizer on paddy soil. Pedosphere 2022, 32, 637–648. [Google Scholar] [CrossRef]
- Silva, F.S.B.; Silva, F.A. A low cost alternative, using mycorrhiza and organic fertilizer, to optimize the production of foliar bioactive compounds in pomegranates. J. Appl. Microbiol. 2020, 128, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Bharadvaja, N.; Gautam, S.; Singh, H. Natural polyphenols: A promising bioactive compounds for skin care and cosmetics. Mol. Biol. Rep. 2023, 50, 1817–1828. [Google Scholar] [CrossRef] [PubMed]
- Sanativo. Available online: https://www.sanativo.com.br/nossos-produtos/ (accessed on 9 December 2024).
- Gomes, M.P.; Marques, R.Z.; Nascentes, C.C.; Scotti, M.R. Synergistic effects between arbuscular mycorrhizal fungi and rhizobium isolated from As-contaminated soils on the As-phytoremediation capacity of the tropical woody legume Anadenanthera peregrina. Int. J. Phytoremediat. 2020, 22, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).