Abstract
The current list of fungi from Colombia updated in the present review contains a total of 7619 species. The Ascomycota appears as the most diverse group, with 4818 species, followed by the Basidiomycota, with 2555 species. Despite this, we presume that the actual fungal diversity in Colombia could amount to between 105,600 and 300,000 species. Fungi represent an underestimated resource, indispensable for human well-being. Even though the current knowledge on potential applications of Colombian fungi is still limited, the number of studies on areas such as natural products discovery, biological control, and food and beverages, among other biotechnological applications, are increasing. With the current review, we aim to present a comprehensive update on the fungal diversity in Colombia and its potential applications. Colombia’s native fungal biodiversity holds much potential within the country’s current social-economical context, and the future must ensure efforts to preserve both the biodiversity and the untapped resources of the fungi in Colombia, which in alignment with the Sustainable Development Goals (SDGs) might result in new bioeconomy avenues for the country.
1. Introduction
Fungi are instrumental within diverse ecosystems across the globe. Their multifaceted and intriguing lifestyles give them unique characteristics, some of which offer tangible benefits to humanity [1,2,3]. Within this context, Colombia, a megadiverse country in the Neotropical region, occupies a privileged position, accounting for 10% of global biodiversity [4,5,6]. Colombia’s unique geographic location, which serves as a bridge between the northern and southern hemispheres, creates an ideal environment for fostering a rich and diverse fungal ecosystem [4,7].
In recent years, the state of mycology in Colombia has undergone a transformation, driven by advancements in molecular biology and DNA sequencing techniques. These developments have resulted in a better comprehension of the fungal diversity of the country. As a result, the Catalogue of Fungi of Colombia comprised 7044 species reported up to 2021 [4,7]. However, even though this number might appear substantial, compared to well-studied countries, including in temperate regions, such as Italy and Germany, it is evident that the fungal diversity in the country remains largely understudied [4]. For comparison, the number of plant, bird, and reptile species reported in Colombia is by far higher than in Italy or Germany (Figure 1), a difference attributed, in part, to the lower landscape diversity of these countries compared to Colombia and to the tropical climate, which through higher ecological dynamics and coevolutionary processes presumably generates and maintains higher diversity [8]. In contrast, the number of fungal species in the country is less than half the species reported for Germany and less than one-third the species known from Italy. This underscores the insufficient knowledge regarding fungal diversity in Colombia and emphasizes the importance of addressing this critical gap. The real diversity of fungi in the country is expected to be between 35,000 and 250,000 species [9]. This aligns with the patterns observed in countries situated in tropical regions, such as Brazil, with a similar biodiversity in well-known groups, such as birds and plants (Figure 1). Both countries have a diverse landscape, creating an optimal setting for a rich diversity of fungi.
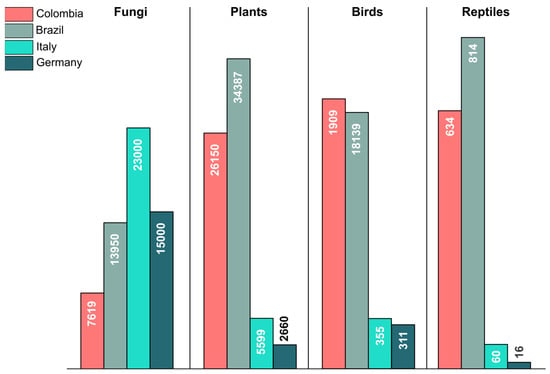
Figure 1.
Total number of reported species for fungi, plants, birds, and reptiles in Colombia, Brazil, Italy, and Germany according to the updated figures presented by Gaya et al. [9] and Butler [5,6].
2. Fungal Diversity in Colombia
Colombia can be divided into six biogeographical areas: the Andean and the Amazon region, the Caribbean coast, the Orinoco, the Pacific/Chocó, and insular areas. Most of the fungal studies have been carried out in the mountain areas of the Andean region, especially in the departments of Antioquia, Boyacá, Cundinamarca, and Valle del Cauca [9,10]. Nonetheless, recent studies in unexplored ecosystems, such as the Amazon region, demonstrate a high fungal diversity [11,12,13]. We highlight significant knowledge gaps regarding diversity and ecological research in the country, as the diversity patterns of many functional groups of fungi are not well understood.
The recent Catalogue of Fungi of Colombia included taxa found until 2021 [10]. In this work, 7044 species were reported, comprising 4535 Ascomycota, 2311 Basidiomycota, and 198 corresponding to early diverging fungi. This count excludes the reports of fossil fungi, Chromista, and Protozoa, all accounted for in the Catalogue. Here, we update the Catalogue, increasing the number to 7619 species, by almost 8%. Of these, 4818 belong to Ascomycota, 2555 to Basidiomycota, and 211 represent early diverging fungi (Figure 2). Moreover, 35 fossil fungi have been found in the country, yet their classification within extant phyla is unspecified, and these fossils are not further discussed. The updated database of species recorded in Colombia is provided as an accessible link (https://github.com/ECharria/ColFungi-updated.git, accessed on 1 October 2023).
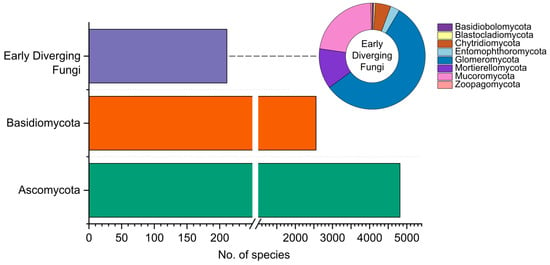
Figure 2.
Distribution of fungi recorded in Colombia, grouped by phyla.
2.1. Ascomycota
As mentioned above, the current number of ascomycete species reported for Colombia rises to 4818, representing the most diverse group of fungi in the country (Figure 3). The species are classified within three subphyla, i.e., Pezizomycotina, Saccharomycotina, and Taphrinomycotina. The largest group is Pezizomycotina, with 4701 species, while the subphyla Saccharomycotina and Taphromycotina are represented by only 111 species. Of the 173 yeast species reported in the country, 114 species belong to Ascomycota, while 56 are Basidiomycota. Most yeast reports are from natural environments and from products such as fermented fruits, fermented beverages, soils surrounding sugarcane crops, and soils contaminated with hydrocarbons [14,15,16,17].

Figure 3.
Fungi belonging to the phylum Ascomycota. (A) Cookeina sulcipes. (B) Rhytidhysteron columbiense. (C) Cordyceps tenuipes. (D) Cordyceps caloceroides. (E) Phillipsia dominguensis. (F) Leotia lubrica. (G) Xylaria multiplex. (H) Beauveria locustiphila. (I) Chlorociboria aeriginosa. (J) Camillea sulcata. Photos (B,G,I) taken by Robert Lücking.
Pezizomycotina contains 13 classes (Figure 4). The most diverse in Colombia is Lecanoromycetes, which includes almost exclusively lichenized fungi, with 2385 species, followed by Sordariomycetes with 856 and Dothideomycetes with 616.
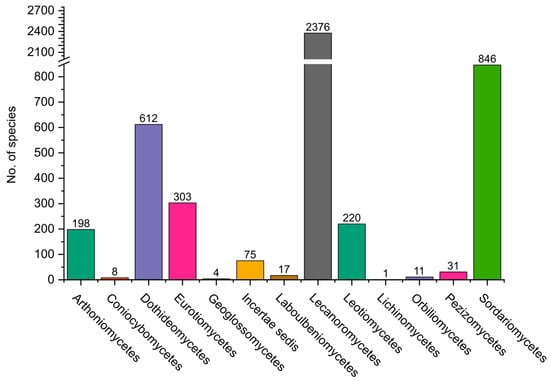
Figure 4.
Distribution of fungi belonging to the Pezizomycotina, grouped by classes.
Ascomycota includes 99% of the lichenized fungi, which constitute the most thoroughly investigated group of fungi in Colombia and make up 37% of the known Colombian fungi (Figure 5; see also Cossu et al. [10], Moncada et al. [18]). The importance of lichens in Colombia has been demonstrated in a variety of fields, such as their use as bioindicators of air pollution and environmental health [19,20] and as a potential source for the discovery of natural products [21]. Other recent works focused on their ecogeography [22] and their potential role of assessing climate change [23]. Understanding the drivers of and threats to lichen diversity is crucial for further assessment of the Colombian lichen biota, as they continue to be an important component of environmental impact assessments.

Figure 5.
Lichenized fungi. (A) Allographa lichexanthonica. (B) Astrothelium bireagens. (C) Coccocarpia pellita. (D) Yoshimuriella enfogoa. (E) Carbacanthographis submultiseptata. (F) Pyrenula asymmetrica. (G) Coenogonium velutinellum. (H) Glyphis lirellizans. (I) Pseudopyrenula daironii. (J) Lobariella foreroana. (K) Sticta henrici. Photos taken by Robert Lücking.
In the recent Catalogue, Moncada et al. [18] reported a total of 2670 lichenized species, but since then, 157 further taxa have been added, including 38 species new to science and 119 new records for the country [22,24,25,26,27,28,29,30,31]. Of these, 154 belong to the Ascomycota. Three further records correspond to lichenicolous fungi growing on lichens, all three Basidiomycota [32]. The total number of lichenized species known from Colombia thus raised to 2827, a further substantial increase (Figure 6), considering that only 15 years ago, the first checklist for the country included 1553 species [33] and the Catálogo de Plantas y Líquenes de Colombia, published seven years ago, listed 1674 [34]. Of the 2827 species now known, 2749 represent Ascomycota and 78 Basidiomycota.
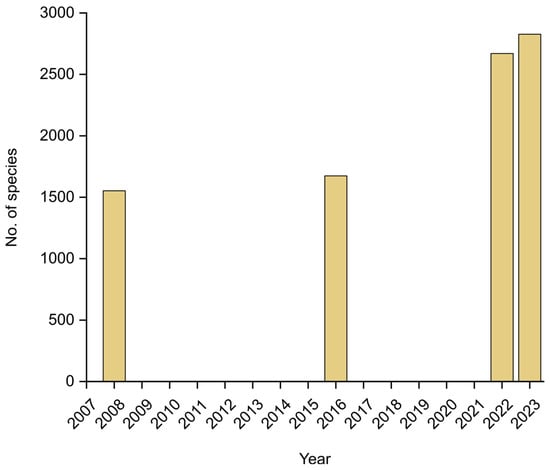
Figure 6.
Increase in the number of species of lichenized fungi known from Colombia since 2008.
Moncada et al. [23] showed that most records of lichenized fungi from Colombia originated from the Andean region, fewer from the Pacific, Orinoco, and Caribbean regions, and very few from the Amazon region. Notably, including data from environmental impact assessments considerably increased the number for the Caribbean region, making it the second best known after the Andean region. The 38 new species and 119 new country records added since then substantially change this picture, as most of these originate from the Amazon region, largely based on a study performed by Lücking et al. [31]. Consequently, the Amazon now represents the second-best-known region in terms of its lichen biota after the Andean region. Out of a total of 192 departmental records corresponding to 157 new species and new records added to the Catalogue, most correspond to the departments of Amazonas (59), Caquetá (45), Guainía (24), Guaviare (13), and Putumayo (11), all partly or completely within the Amazon region and making up nearly 80% of all records. This is largely the result of the continued, systematic collection efforts by the Instituto SINCHI, spearheaded by the late Dairon Cárdenas-López, who sadly passed away in early 2022 [31]. Likely, additional collection efforts in the Amazon region will reveal further new records and even species, and similar efforts in the understudied Pacific and Orinoco regions should increase the total of lichenized species known well beyond the estimate of 3600 species made by Lücking et al. [35]. Moncada et al. [23] already considered this estimate outdated, suggesting 5000 species as a more likely number, due to increasing evidence of hidden diversity in presumably known species with the application of molecular data [19]. The most recent example is the genus Coccocarpia, shown to contain many more species than currently believed in Colombia alone [36].
2.2. Basidiomycota
In Colombia, fungi belonging to the Basidiomycota (Figure 7) have been documented since 1882 [37]. A total of 2555 species has been compiled until today. Of these, 2477 belong to nonlichenized Basidiomycota and are distributed among three subphyla: Agaricomycotina (1861 species) including yeast (56 species), Pucciniomycotina (607 species), and Ustilaginomycotina (85 species) [10,13,14,38,39,40,41,42,43,44].

Figure 7.
Fungi belonging to the phylum Basidiomycota. (A) Cyathus striatus. (B) Cerioporus scutellatus. (C) Collybia plectophylla. (D) Hydnum repantum. (E) Ramaria stricta. (F) Geastrum pectinatum. (G) Laetiporus gilbertsonii. (H) Cantharellus coccolobae. (I) Sarcodon rufobrunneus. (J) Mutinus camimus. (K) Aquascypha hydrophora. (L) Campanella caesia. (M) Tremella mesenterica. Photos (B,G,L) taken by Robert Lücking.
In Basidiomycota, the most studied group is Agaricomycotina, especially from areas near large cities in the mountain region of the departments of Antioquia, Cundinamarca, Boyacá, Valle del Cauca, and Tolima, and some areas in the Amazon region (Figure 8). The dominant orders are Agaricales (832 species), Polyporales (279 species), Hymenochaetales (119 species), and Russulales (101 species) (Figure 9). Most of the studies are concentrated in oak forests dominated by ectomycorrhizal (EcM) fungi [45,46], whereas mixed mountain forests have not been studied in detail. Andean forests are under strong pressure due to the increase in the agricultural frontier, the expansion of cities, and habitat fragmentation, and therefore a high number species are threatened [47]. In the Amazon region, with more than 350 species reported, special surveys have been conducted with a particular focus on saprotrophs and EcM fungi associated with the host tree Pseudomonotes tropenbosii (Dipterocarpaceae) and the Fabaceae hosts Aldina sp. and Dicymbe uaiparuensis [11,12,13,46,48,49]. Despite the large number of species reported, this is still far from the expected fungal diversity, which is around 1000 species of macrofungi (including Ascomycota and Basidiomycota). Based on alpha diversity patterns and species accumulation curves analysis [50], a high diversity is expected in the Amazon lowlands and oak forests. It is important to note that this analysis lacks sufficient data from the Choco biogeographical area, an area we can also expect to be an important hotspot of fungal biodiversity, with a high endemism, similar to what has been observed for plants and animals [51,52,53].
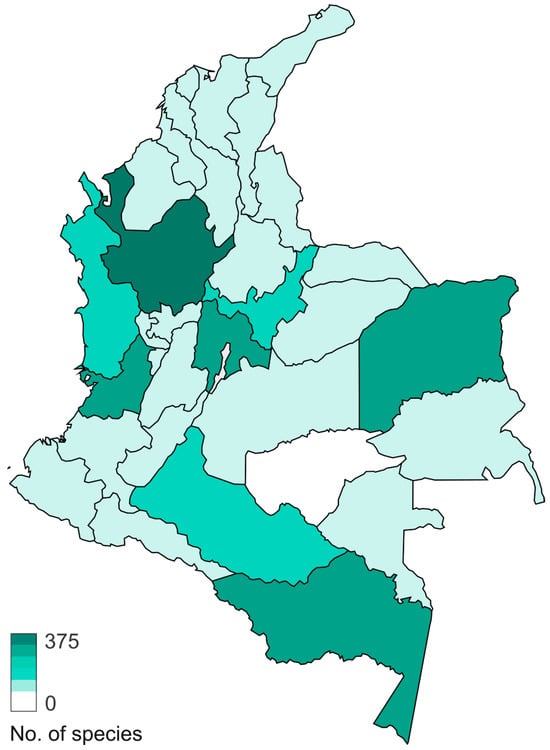
Figure 8.
Map showing the total number of species of Basidiomycota macrofungi, reported for each department in Colombia.
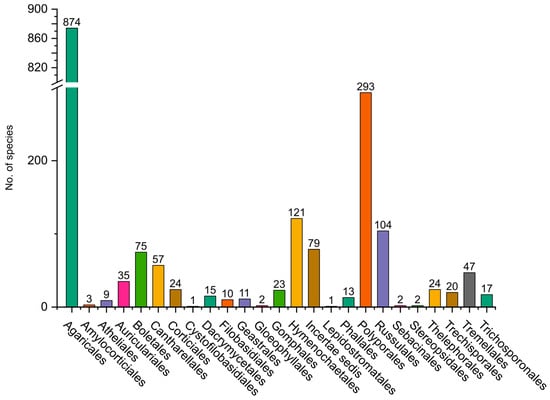
Figure 9.
Distribution of fungi belonging to the Agaromycotina, grouped by orders.
Rust fungi (Pucciniomycotina) and smut fungi (Ustilaginomycotina) are biotrophic and obligate pathogens of vascular plants. This group comprises species that cause economically relevant plant diseases, but also it is an important resource for the biological control of weeds. Although these groups have been widely studied, they are far from being completely documented, and departments such as Guaviare and Vichada, located in the Amazon and Orinoco region, have no reports of species yet [44].
The above highlights the substantial gap in knowledge about the diversity of Basidiomycota in Colombia, an issue that is not restricted to this group but is also evident in other phyla. Large areas of the country are still poorly explored, such as the Caribbean and the insular areas, the Andean slopes, and the highly biodiverse regions such as the Biogeographical Chocó, the Amazon, and the Orinoco [4,18,41,54,55]. Also, the country’s ecosystems are under pressure from extractive industries, livestock grazing, extensive oil palm cultivation, urbanization, and illegal activities such as mining and illicit drug cultivation [56]. Deforestation, primarily through conversion to pasture, has had a major impact on the Amazon, Caribbean, and, more recently, Andean regions. To date, 21 species of nonlichenized fungi with distribution in Colombia have been evaluated under the IUCN criteria. Of these, 17 found in mountain regions have been assessed as follows: five species under the category Vulnerable (VU); five under the category Near Threatened (NT); and four under the category Least Concern (LC). From the Amazon region, six species have been classified as follows: one Critically Endangered (CE), one NT, two LC, and two Data Deficient [47,57].
2.3. Early Diverging Fungi
The classification of early diverging fungi at different taxonomic levels is constantly changing, especially at the phylum level, for which the community is not arriving at a consensus yet. In the present study, we followed the classifications proposed by Tedersoo et al. [58] and Wijayawardene et al. [59].
In total, six different species have been reported from the phylum Entomophthoromycota. Taxa belonging to this phylum are animal pathogens, in general from insects. However, Conidiobolus coronatus is able to infect humans and other animals, causing rhinofacial zygomycosis [60,61].
For Basidiobolomycota, there is only one taxon reported, Basidiobolus ranarum, which was isolated from air [62], even though this has been detected as a human pathogen worldwide [60]. Another phylum with only one species reported for Colombia is Zoopagomycotina. This species is Piptocephalis fimibriata, and its report appeared in GBIF [https://www.gbif.org/species/2559057 (accessed on 1 October 2023)]. However, we could not find the study in which it was reported to verify it and verify the substrate in which it was observed.
Chytridiomycota is a group of zoosporic fungi that includes mostly water-inhabiting organisms, although some are plant and animal pathogens [63,64,65]. To date, ten taxa have been reported from Colombia, all belonging to the class Chytridiomycetes. Chytridium schenkii and three different species of Synchytrium are plant pathogens [4]. Batrachochytrium dendrobatidis is an amphibian pathogen [66], and different species of Nephridiophaga are pathogens of cockroaches [67]. Other phyla containing zoosporic fungi are Blastocladiomycota and Neocallimastigomycota. From this latter phylum, no fungi have been ever reported in Colombia, while only Coelomomyces reticulatus is reported among Blastocladiomycota; it is an insect pathogen, particularly of mosquitoes [68]. Blastocladiella colombiensis, which belongs to Blastocladiomycota, has also been isolated and described from Colombia [69]. However, the name is invalid due to the lack of a Latin diagnosis or description of the genus name when it was introduced [70].
Mortierellomomycota comprises only one class, one order, one family, and six genera [59]. From this phylum, 26 taxa have been found in Colombia. All taxa belong to the genus Mortierella, except for Dissophora ornata, and were isolated from soil [71,72,73].
Hitherto, 47 species of Mucoromycota have been reported. These are included in two of the three classes currently accepted in the phylum, i.e., Mycoromycetes and Umbelopsidomycetes. The latter class is represented by only one genus, Umbelopsis, and seven species. The mucoromycete genus most commonly found in Colombia is Mucor, from which 14 different species have been reported. Members of this phylum are common saprobes or rarely facultative parasites [74]. In Colombia, most of them were isolated from soil [75] but also have been found responsible for human mucormycosis [61].
Finally, 119 different species belonging to Glomeromycota have been found in Colombia. The high number of reports compared to other early diverging fungi (Figure 2) is due to its agricultural importance. This group is one of the most studied ones since it comprises arbuscular mycorrhizal fungi [76]. Taxa are included in all three classes of the phylum, i.e., Archaeosporomycetes, Glomeromycetes, and Paraglomeromycetes, and 49 species belong to only three different genera, i.e., Acaulospora, Glomus, and Scutellospora, all of them belonging to Glomeromycetes. Even though the genus Rhizoglomus (Glomeraceae, Glomerales) has been recently synonymized to Rhizophagus [77], Rhizoglomus microaggregatum and Rhizoglomus vesiculiferum have not been transferred yet to that latter genus.
3. Applications of Colombian Fungal Biodiversity
The vast fungal diversity in Colombia holds untapped potential beyond mere taxonomic documentation. These organisms offer promising applications in fields such as medicine, agriculture, industry, and biomonitoring, yet this potential remains largely unexplored [7,78,79]. The concept of biodiversity valorization, which refers to the creation of value from biodiversity, is still an emerging idea in the country. However, in comparison to fungi, policies and research strategies for the development of plant-based value chains have increased in recent years, as in the case of value chains based on the processing of Lippia origanoides [80]. In this context, Colombian fungi emerge as pivotal components in the development of sustainable economic strategies and contributing to the Sustainable Development Goals (SDGs) [81,82]. New bioeconomy strategies highly rely on the dynamic evolution of biotechnological applications, and in this way, fungi have proven to be a biotechnologically valuable group of organisms [3]. Their exceptional versatility offers substantial potential for developing sustainable alternatives to traditional processes and products, while also fostering the creation of novel industrial processes and products. In the following sections, we summarize the reports on different applications of native fungi in Colombia, considering the country’s current socioeconomic landscape.
3.1. Natural Products from Colombian Fungi
Among the diverse beneficial properties of fungi, their ability to produce diverse secondary metabolites has rendered advancements in the development of medicines and pharmaceuticals [1,2,3]. Fungi represent a largely untapped source for the discovery of natural products with diverse applications, and efforts towards the study of native species from tropical countries such as Colombia remain particularly scarce. Over the past few years, encouraged by the lack of knowledge on the chemical diversity of Colombian fungi, our working groups have embarked on the search for biologically active secondary metabolites from native species [78,83,84]. It is expected that concurrent exploration of Colombian fungi as source of novel anti-infectives will lead to both the emergence of new opportunities to valorize fungi as well as to an enforcement of the importance of its preservation.
Traditionally, medicinal plants have been used by indigenous communities for treating tropical diseases. Despite the high number of documented medicinal plants in Colombia, there is an evident lack of knowledge regarding the identification of their bioactive principles [78]. Nevertheless, endophytic fungi derived from them have been shown to be useful in a broad range of applications, and they also represent a prolific source for the discovery of natural products [1,2,3]. During the investigation of secondary metabolite production by endophytes from Otoba gracilipes, a native medicinal plant from the Valle del Cauca river area, an isolate belonging to the ascomycete genus Diaporthe was found to exhibit promising antibacterial potential [78]. This particular fungus, later introduced as the new species D. caliensis, was prioritized for a systematic investigation using different metabolomics approaches in combination with classical chemical screening [83]. Insights into the complexity of the polyketide-lactones produced by this fungus revealed the production of the 10-membered lactone phomol (1), together with the new butenolides named caliensolides A (2) and B (3) (Figure 10). In addition, the biological activity found in the crude extract could be attributed to phomol, while caliensolides A and B did not show any biological activity against the tested microorganisms and cell lines. Diaporthe caliensis represents the tenth native species of this genus in Colombia, which reinforces the value of exploring Colombian biodiversity.
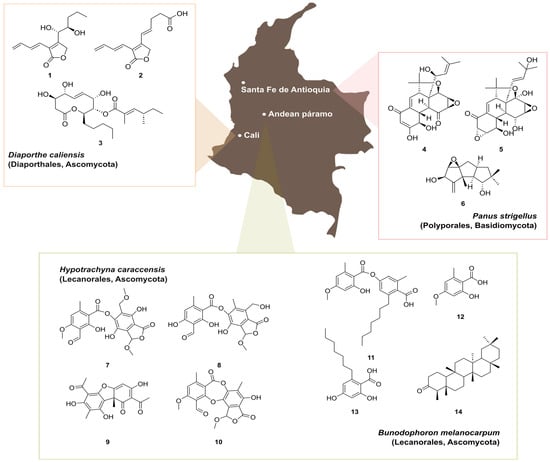
Figure 10.
Examples of secondary metabolites isolated from Colombian fungal species. Caliensolides A (1) and B (2) and phomol (3) produced by the endophyte Diaporthe caliensis (Diaporthales, Ascomycota) isolated from the medicinal plant Otoba gracilipes. Panapophenanthrin (4), Panepophenanthrin (5), and dihydrohypnophilin (6) produced by Panus strigellus (Polyporales, Basidiomycota). Hypotrachynin A (7) and B (8), (+)-(9b-R)-usnic acid (9), and methylstictic acid (10), isolated from Hypotrachyna caraccensis (Lecanorales, Ascomycota). Sphaerophorin (11), everninic acid (12), sphaerophorol carboxylic acid (13), and friedelin (14), isolated from Bunodophoron melanocarpum (Lecanorales, Ascomycota).
Similarly, the basidiomycete Panus strigellus, isolated from the municipality of Santa Fe de Antioquia, was found to produce oligocyclic diterpenoidal derivatives, panapophenanthrin (4) and panepophenanthrin (5), as well as dihydrohypnophilin (6) (Figure 10). Compounds 4 and 6 exhibited moderate antimicrobial activities when compared with compound 5, which did not show any biological activity in this study [84]. However, panepophenanthrin was the first reported inhibitor of the ubiquitin-activating enzyme (E1), fundamental in the activation process of the ubiquitin-proteasome pathway (UPP). This pathway is involved in the regulation of a variety of cellular functions, and the discovery of specific inhibitors for E1 are of great biological promise in the context of diseases like cancer, inflammation, and neurodegenerative disorders [85,86,87]. Its promising biological activity, together with the chemical complexity harbored by these epoxyquinoids, synthetized via a Diels–Alder-type dimerization and resulting in a highly substituted tetracyclic core have prompted several efforts towards the total synthesis of 5. Although several research groups investigated various synthetic approaches for panepophenanthrin, the search for an efficient synthetic route remains a challenge, primarily due to its structural complexity.
Colombian lichens also represent an untapped reservoir of unique secondary metabolites, which might arise from their intriguing ecological adaptations [88]. While reports on natural products from native lichens in Colombia are limited, it is expected that the interest in these organisms will increase as we expand our knowledge on their diversity and taxonomy. For instance, two β-orcinol depsides, namely, hypotrachynin A (7) and B (8), along with (+)-(9b-R)-usnic acid (9) and methylstictic acid (10), were isolated from Hypotrachyna caraccensis, a lichen growing in the páramo ecosystem (above 3500 m a.s.l.), unique to the north-west zone of South America (Figure 10). These compounds exhibited free radical scavenging activity, with 8 and 10 presenting optimal lipophilicity and permeability, making them potential candidates for topical application to prevent oxidative damage [89]. Similarly, sphaerophorin (11), everninic acid (12), sphaerophorol carboxylic acid (13), and friedelin (14) were isolated from Bunodophoron melanocarpum, a lichen from the Andean páramo [90]. Notably, compounds 11 and 13 are dual agents presenting antioxidant and photoprotective properties with also potential use as sunscreen ingredients. Finally, we mention that metabolomics approaches have been recently used to investigate the chemical diversity of species from the genera Usnea and Sticta, allowing for the identification of different chemotaxonomic markers for a fast identification of these lichens [20,91].
3.2. Biocontrol Strategies
In Colombia, where the agriculture sector is one of the major contributors within Colombia’s economy, the search for new biocontrol strategies against fungal pest and plant diseases is relevant. On the other hand, some fungi can be also effective agents of protecting crops, increasing agricultural productivity, and reducing the environmental impact of traditional biocontrol strategies.
For instance, it has been shown that the use of microbial consortia constitutes a promising alternative for managing pest diseases. One of these diseases is the fusarium wilt, which is caused by Fusarium oxysporum and is the main disease observed in cape gooseberry (Physalis peruviana) in Colombia. So far, no registered solutions or control agents are available for this disease, which has resulted in the migration of cropped areas within the country, even though the diseases persist in both old and new cropped areas. However, the use of different fungal-bacterial consortia under greenhouse conditions has exhibited high efficacy in the control of vascular wilt caused by this pathogen [92,93].
Similarly, anthracnose is a serious disease found in citrus and which results in negative phytosanitary effects and economic losses [94]. This disease is mainly caused by Colletotrichum gloeosporioides and C. acutatum, and despite the current availability of conventional fungicides to fight against it, their indiscriminate use might result in the generation of resistance mechanisms and negative environmental effects [95]. In this way, endophytic fungi from Tahiti limes have been explored for their antagonistic activity against C. acutatum, observing that the spore solutions of Xylaria adscendens and Trichoderma atroviride reduced the lesions caused by the phytopathogen in these in vivo tests [96].
A fungus belonging to the genus Purpureocillium (Ophiocordycipitaceae, Hypocreales) has demonstrated potent nematicidal activity against Meloidogyne spp. and Paratylenchus spp. in Colombian flower crops [97]. In Colombia, the flower industry, apart from generating substantial revenues each year, also significantly impacts the society and environment. This fungus was included on the 1000 fungal genomes project, and further studies await in order to comprehensively understand the potential of this native biocontrol agent [98].
Recently, the use of phytopathogenic fungi such as rust and smut for the biological control of invasive weed plant species has been seen. Such biological control could have valuable potential for both agriculture and noncultivated areas [99].
Some fungal products for agricultural applications have already been developed and commercialized in Colombia. Trichotec®, a product developed by Agrosavia [https://www.agrosavia.co/ (accessed on 1 October 2023)] based on Trichoderma koningiopsis, is available for the biocontrol of Fusarium oxysporum, Rhizoctonia solani, Sclerotinia sclerotium, S. minor, and Botrytis cinerea, in tomato, rice, lettuce, and red fruit crops. Fosfobiol®, produced by Biocultivos S.A. [https://www.biocultivos.com.co/ (accessed on 1 October 2023)] and based on Penicillium janthinellum, is a phosphorus solubilizer for cotton, rice, coffee, sugar cane, corn, pastures, and soybeans. Trifesol®, based on Trichoderma viride, is used as a biocontrol agent, plant growth promoter, and cellulose degrader. Companies such as Natural Control [https://naturalcontrol.com/ (accessed on 1 October 2023)] commercialize products based on native fungal strains, such as Anisagro (Metarhizium anisopliae), Vercani (Lecanicillium lecanii), Bassar (Beauveria bassiana), Fitotripen (Trichoderma harzianum, T. koningii and T. viride), Mycorrhizagro (Glomus, Acaulospora, Scutellospora and Entrophospora), and Safelomyces (Purpureocillium lilacinum and Cordyceps fumosorosea).
3.3. Food and Beverages from Fungi
Traditionally, yeasts have played an essential role in industrial processes such as food production, including bread and cheese, as well as in the production of fermented beverages. Notably, the global market value attributed to fermented food and beverages derived from fungi reaches USD 2075.04 billion [1]. The main yeast species involved in different biotechnological applications worldwide is the ascomycete fungus Saccharomyces cerevisiae. However, several native yeasts species have been demonstrated to be also involved in the production of traditional food and beverages in Colombia, such as “kumis”, “chicha”, and “champus”, among others [14,100,101,102].
While certain traditional fermentation processes have transitioned successfully into standardized industrial production, others, such as cocoa bean fermentation, remain based on spontaneous methods. The metabolic interactions among microbial communities within these processes contribute to the distinct set of qualities that define, for instance, fine-flavor cocoa. Among the groups of microorganisms involved in cocoa fermentations, yeasts and molds play an important role [103]. Their ability to hydrolyze pectin results in the release of organic acids, aldehydes, and esters that contribute significantly to the development of distinctive flavor profiles. Fernández-Niño et al. [103] used metagenomic approaches to dissect on-farm industrial fermentations of fine-flavor cocoa, identifying the shared presence of nine dominant microorganisms including four fungal species, Saccharomyces cerevisiae, Pestalotiopsis rhododendri, Malassezia restricta and M. globosa, between two farms located at completely different agroecological zones. Despite this finding, the specific role of these fungi in fine cocoa bean fermentation is unknown, and further research will be required in the future.
Similarly, several fungal species have been reported to have traditional uses in indigenous communities in Colombia, but still the beneficial properties of these organisms remain unexplored in a systematic manner [83,104,105]. This is the case of wild macrofungi, which are used by rural communities as food sources with beneficial properties. According to the Catalogue of Fungi of Colombia, there are at least 36 wild edible species (saprotrophic and EcM) used by these communities in Colombia, mostly in the departments of Boyacá, Amazonas, and Putumayo [7].
3.4. Other Biotechnological Applications
Biofertilizers are natural or microbial-based agents that can enhance soil fertility and plant nutrition. In Colombia, fungal biofertilizers offer a sustainable and ecological alternative to chemical fertilizers. Future research in this area will be needed to further expand the available products in comparison to conventional fertilizers. Circular processes within a sustainable economy will be necessary to minimize waste, maximize resource efficiency, and reduce environmental impact. As an example, at the greenhouse scale, it has been shown that Pleurotus ostreatus can degrade lignocellulosic biomass and low-density oxodegradable polyethylene, obtaining a processed substrate, which can be used to produce biochar as biofertilizer in the cultivation of Allium cepa [106].
Fungi can also be used in bioremediation to degrade or absorb contaminants. They represent a potential alternative for remediating polluted sites in Colombia, improving the quality of soil and water and restoring ecosystems affected by pollutants such as heavy metals, pesticides, and organic compounds [1,3]. Synthetic dyes, for instance, are produced in high quantities and often discharged into water bodies, presenting a threat for the environment. White-rot fungi such as Bjerkandera adusta, Leptosphaerulina sp., Irpex lacteus, Trametes pubescens and T. versicolor have been demonstrated to produce ligninolytic enzymes and to exert decolorizing activity [107,108,109]. Similarly, the ability of endophytes from Otoba gracilipes to produce laccases has been evaluated using a weighted screening system based on a semiquantitative classification [79]. This system allowed for the identification of the capacity of Phlebia floridensis to produce laccase at different pHs, as well the identification of the ability of Beltraniopsis sp., Neopestalotiopsis sp., and Phyllosticta sp. to decolorize. In addition, an endophytic strain of Fusarium oxysporum isolated from the same medicinal tree was found to produce exopolysaccharides with high antioxidant activity [110]. There has also been an increased interest in the search for fungal species able to absorb heavy metals from polluted ecosystems. The recently introduced new species Talaromyces santanderensis has been shown to tolerate cadmium concentrations over the permissible limits for contaminated soils, highlighting its potential use in bioremediation strategies to eliminate this metal from highly contaminated agricultural soils [111].
The biocatalytic potential of basidiomycete species has also been explored for the production of aromas and flavors [112,113]. For instance, Ganoderma webenarium, G. chocoense and G. stipitatum showed strong potential for creating aromas through de novo synthesis, particularly by decolorizing β,β-carotene. Additionally, Trametes elegans was shown to oxidize α-pinene, producing 2.9 mg of cis-Verbenol per gram of mycelium. This fungus was also found to generate flavor compounds in submerged cultures.
The production of carotenoid pigments has been investigated in naturally pigmented yeast such as Rhodotorula mucilaginosa, which after being subjected to various stress conditions was shown to have an increased production of these compounds [114,115].
Fungal collections are important sources of information and microorganisms for such developments in biotechnological processes and in the search for new compounds. Colombia has 18 fungal culture collections that house more than 8000 strains [116]. Countries like Colombia must focus their efforts on enriching these collections, as part of their programs to strengthen the protection and sustainable use of their natural resources.
4. Conclusions and Future Perspectives
In 2022, Colombia reported 7044 fungal species. Since then, 540 new species have been newly added to the Catalogue of Fungi of Colombia, highlighting the hidden biological treasures awaiting to be unearthed. Knowledge gaps persist, particularly in regions like Orinoco, Caribe, insular territories, Andean slopes, the Chocó Biogeographical Region, and the Amazon. The Amazon’s diverse ecosystems are expected to host numerous macro and microfungi, along with many endemisms. Although mycologists discover several new species each year, more effort and financial support are needed to expedite documentation and explore promising applications based on this fungal diversity.
Studying and preserving Colombia’s fungal diversity is of the utmost importance, given the imminent threats posed by habitat loss, deforestation, pollution, climate change, and invasive species. The potential loss of this biodiversity is not merely a threat to the ecosystems, but could also result in the loss of opportunities for valuable applications and products valuable for the economic growth of the country. Fungi, functioning as living repositories, not only safeguard reference material but also serve as witnesses to understanding the history of microbial biodiversity.
In order to study the diversity of fungi in Colombia, it is relevant to cope with the international advances in this area. The identification of Colombian fungi is mainly based on morphological characters, and it is noteworthy that a high number of species are difficult or, in some cases, impossible to discriminate based on solely morphology. Therefore, molecular data must start to be derived from the reported fungi in the country to verify all the identifications appearing in the present Catalogue of Fungi of Colombia.
Exploring the potential of Colombian fungi as sources of natural products, particularly for the development of antimicrobial drugs, offers a promising avenue for economic growth in Colombia. Currently, several challenges are present, such as the limited infrastructure in academia. However, Colombia’s evolving policies in the field of bioeconomy emphasize the transition from basic scientific research to practical applications. To address these challenges, the integration of omics technologies such as metabolomics and genomics might present an innovative and viable solution for the prioritization of promising fungal producers. This transition from conventional methods to omics approaches not only aligns with the bioeconomic goals of Colombia but also positions it at the forefront of biotechnological innovation, linking economic development to the exploration of its fungal biodiversity.
In summary, it is crucial that the significance of fungi in various domains, including biodiversity studies, conservation efforts, and the burgeoning bioeconomy, is recognized by policy makers. Investments in dedicated funding for fungal research are crucial. Furthermore, we must actively promote initiatives that study and preserve fungi in the country, which not only unlocks vast scientific and economic potential but also protects their invaluable contributions to our planet.
Author Contributions
Conceptualization, E.C.-G., Y.M.-F. and A.M.V.-P.; database, B.M., Y.M.-F. and A.M.V.-P.; formal analysis, E.C.-G.; writing—original draft preparation, E.C.-G. and Y.M.-F.; writing—review and editing, E.C.-G., A.M.V.-P., B.M. and Y.M.-F.; project administration, Y.M.-F.; funding acquisition, E.C.-G., A.M.V.-P., B.M. and Y.M.-F. All authors have read and agreed to the published version of the manuscript.
Funding
E.C.-G. was supported by the HZI POF IV Cooperativity and Creativity Project Call. Y.M.-F. was supported by the Deutsche Forschungsgemeinschaft (DFG)—Project ID 490821847. A.M.V.-P. was supported by Fondo Primer Proyecto Universidad de Antioquia, Project: Exploración de la capacidad antimicrobiana de especies de macrohongos nativas de la Amazonía Colombiana, Project ID 2020-33675.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The complete updated database is accessible via https://github.com/ECharria/ColFungi-updated.git (accessed on 1 October 2023).
Acknowledgments
We are grateful to Robert Lücking who made the lichen photos available and assisted in compiling geographic distribution and other lichen data. Moreover, the authors wish to thank Robert Lücking and Marc Stadler for their comments on the draft. We also thank Ricardo Vargas-Carpintero for his assistance during the assessment of the implications of fungal-based applications for Colombian bioeconomy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Grace Niego, A.T.; Lambert, C.; Mortimer, P.; Thongklang, N.; Rapior, S.; Grosse, M.; Schrey, H.; Charria-Girón, E.; Hyde, K.D.; Stadler, M. The Contribution of Fungi to the Global Economy. Fungal Divers. 2023, 121, 95–137. [Google Scholar] [CrossRef]
- Mapook, A.; Hyde, K.D.; Hassan, K.; Kemkuignou, B.M.; Čmoková, A.; Surup, F.; Kuhnert, E.; Paomephan, P.; Cheng, T.; de Hoog, S.; et al. Ten Decadal Advances in Fungal Biology Leading towards Human Well-Being. Fungal Divers. 2022, 116, 547–614. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Xu, J.; Rapior, S.; Jeewon, R.; Lumyong, S.; Niego, A.G.T.; Abeywickrama, P.D.; Aluthmuhandiram, J.V.S.; Brahamanage, R.S.; Brooks, S.; et al. The Amazing Potential of Fungi: 50 Ways We Can Exploit Fungi Industrially. Fungal Divers. 2019, 97, 1–136. [Google Scholar] [CrossRef]
- Gaya, E.; Vasco-Palacios, A.M.; Vargas, N.; Lücking, R.; Carretero, J.; Sanjuan, T.; Moncada, B.; Allkin, B.; Bolaños-Rojas, A.C.; Castellanos-Castro, C.; et al. ColFungi: Colombian Resources for Fungi Made Accessible; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2021; p. 36. [Google Scholar] [CrossRef]
- Butler, R. The Top 10 Most Biodiverse Countries. 2016. Available online: https://news.mongabay.com/2016/05/top-10-biodiverse-countries (accessed on 1 October 2023).
- Butler, R. Countries with the Highest Biodiversity. 2019. Available online: https://rainforests.mongabay.com/03highest_biodiversity.htm (accessed on 1 October 2023).
- de Almeida, R.F.; Lücking, R.; Vasco-Palacios, A.M.; Gaya, E.; Diazgranados, M. (Eds.) Catalogue of Fungi of Colombia; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; p. 541. ISBN 978-1-84246-790-9. [Google Scholar]
- Brown, J.H. Why Are There so Many Species in the Tropics? J. Biogeogr. 2014, 41, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Gaya, E.; Motato-Vásquez, V.; Lücking, R. Diversity of Fungi of Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Cossu, T.; Lücking, R.; Vasco-Palacios, A.M.; Moncada, B.; Kirk, P.M.; de Almeida, R.; Gaya, E.; Coca, L.F.; De Souza, J.; Diaz Escandon, D. Annotated Checklist of Fungi of Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Vasco-Palacios, A.M.; Bahram, M.; Boekhout, T.; Tedersoo, L. Carbon Content and PH as Important Drivers of Fungal Community Structure in Three Amazon Forests. Plant Soil 2020, 450, 111–131. [Google Scholar] [CrossRef]
- Vasco-Palacios, A.M.; Hernandez, J.; Peñuela-Mora, M.C.; Franco-Molano, A.E.; Boekhout, T. Ectomycorrhizal Fungi Diversity in a White Sand Forest in Western Amazonia. Fungal Ecol. 2018, 31, 9–18. [Google Scholar] [CrossRef]
- Vasco-Palacios, A.M.; Boekhout, T. Pseudomonotes tropenbosii, an Endemic Dipterocarp Tree from a Neotropical Terra-Firme Forest in Colombian Amazonia that Hosts Ectomycorrhizal Fungi. In Mycorrhizal Fungi in South America. Fungal Biology; Lugo, M.A., Pagano, M.C., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Osorio-Cadavid, E.; Chaves-Lopez, C.; Tofalo, R.; Paparella, A.; Suzzi, G. Detection and Identification of Wild Yeasts in Champús, a Fermented Colombian Maize Beverage. Food Microbiol. 2008, 25, 771–777. [Google Scholar] [CrossRef]
- Salazar Alzate, B.C.; Cortés Rodríguez, M.; Montoya Campuzano, O. Identification of Some Kefir Microorganisms and Optimization of Their Production in Sugarcane Juice. Rev. Fac. Nac. Agron. Medellín 2016, 69, 7935–7943. [Google Scholar] [CrossRef]
- Ordoñez-Burbano, D.E.; Abella-Medina, C.A.; Echeverry-Tamayo, A.; Paz-Lasprilla, L.M.; Benitez-Campo, N. Biodegradación de hidrocarburos alifáticos saturados por microorganismos aislados de suelo contaminado con derivados del petroleo. Rev. Ciencias 2018, 22, 33–44. [Google Scholar] [CrossRef]
- Ramírez-Castrillón, M.; Barona-Colorado, A.; Bados-Lopez, M.C.; Bolaños-Burbano, D. Diversity of Environmental Yeasts of Colombia: A Systematic Review. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Moncada, B.; Coca, L.F.; Díaz-Escandón, D.; Jaramillo-Ciro, M.; Simijaca-Salcedo, D.; Soto-Medina, E.A.; Lücking, R. Diversity, Ecogeography, and Importance of Lichens of Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Correa-Ochoa, M.A.; Vélez-Monsalve, L.C.; Saldarriaga-Molina, J.C. Spatial Distribution of Lichen Communities and Air Pollution Mapping in a Tropical City: Medellín, Colombia. Rev. Biol. Trop. 2021, 69, 1107–1123. [Google Scholar] [CrossRef]
- Abril, M.A.Q.; Ospina, D.M.R.; Rave, M.I.D.; Valencia, J.L. Lichens as biosensors for the evaluation of urban and sub-urban air pollution in a tropical mountain valley, Rionegro, Antioquia. Rev. Bionatura 2021, 6, 1501–1509. [Google Scholar] [CrossRef]
- Albornoz, L.; Torres-Benítez, A.; Moreno-Palacios, M.; Simirgiotis, M.J.; Montoya-Serrano, S.A.; Sepulveda, B.; Stashenko, E.; García-Beltrán, O.; Areche, C. Phylogenetic Studies and Metabolite Analysis of Sticta Species from Colombia and Chile by Ultra-High Performance Liquid Chromatography-High Resolution-Q-Orbitrap-Mass Spectrometry. Metabolites 2022, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Soto-Medina, E.A.; Díaz, D.; Montaño, J. Biogeography and richness of lichens in Colombia [Biogeografía y riqueza de los líquenes de Colombia]. Rev. Acad. Colomb. Cienc. Exactas Fís. Nat. 2021, 45, 122–135. [Google Scholar] [CrossRef]
- Lasso, E.; Matheus-Arbeláez, P.; Gallery, R.E.; Garzón-López, C.; Cruz, M.; Leon-Garcia, I.V.; Aragón, L.; Ayarza-Páez, A.; Curiel Yuste, J. Homeostatic Response to Three Years of Experimental Warming Suggests High Intrinsic Natural Resistance in the Páramos to Warming in the Short Term. Front. Ecol. Evol. 2021, 9, 615006. [Google Scholar] [CrossRef]
- Moncada, B.; Sipman, H.; Lücking, R. Testing DNA Barcoding in Usnea (Parmeliaceae) in Colombia Using the Internal Transcribed Spacer (ITS). Plant Fungal Syst. 2020, 65, 358–385. [Google Scholar] [CrossRef]
- Moncada, B.; Rincón-Murillo, D.; Lücking, R. Three New Lobarioid Lichens (Lichenized Ascomycota: Peltigeraceae) from Colombia in Memory of Enrique Forero. Rev. Acad. Colomb. Cienc. Exactas Fís. Nat. 2023, 47, 619–640. [Google Scholar] [CrossRef]
- Wilk, K. Calogaya miniata comb. nov., Huneckia crocina comb. nov., and New Neotropical Records of Wetmoreana Brouardii. Mycotaxon 2021, 136, 387–400. [Google Scholar] [CrossRef]
- Simijaca, D.; Lücking, R.; Moncada, B. Two New Species of Astrothelium (Trypetheliaceae) with Amyloid Ascospores Inhabiting the Canopy of Quercus humboldtii Trees in Colombia. Phytotaxa 2021, 508, 229–234. [Google Scholar] [CrossRef]
- Soto-Medina, E.A.; Aptroot, A.; Lücking, R. New Species of Lichen for Colombia Tropical Dry Forest. Cryptogam. Mycol. 2023, 44, 104–107. [Google Scholar] [CrossRef]
- Soto-Medina, E.A. New Records and a Key for Species of Synarthonia (Lichenized Scomycota: Arthoniaceae) in Colombia. Rev. Acad. Colomb. Cienc. Exactas Fís. Nat. 2022, 46, 165–168. [Google Scholar] [CrossRef]
- Ossowska, E.A.; Moncada, B.; Kukwa, M.; Flakus, A.; Rodriguez-Flakus, P.; Olszewska, S.; Lücking, R. New Species of Sticta (Lichenised Ascomycota, Lobarioid Peltigeraceae) from Bolivia Suggest a High Level of Endemism in the Central Andes. MycoKeys 2022, 92, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Lücking, R.; Álvaro-Alba, W.R.; Moncada, B.; Marín-Canchala, N.L.; Tunjano, S.S.; Cárdenas-López, D. Lichens from the Colombian Amazon: 666 Taxa Including 28 New Species and 157 New Country Records Document an Extraordinary Diversity. Bryologist 2023, 126, 242–303. [Google Scholar] [CrossRef]
- Diederich, P.; Millanes, A.M.; Wedin, M.; Lawrey, J. Flora of Lichenicolous Fungi 1; National Museum of Natural History: Luxembourg, 2022. [Google Scholar]
- Sipman, H.J.M.; Hekking, W.; Aguirre, C.J. Checklist of Lichenised and Lichenicolous Fungi from Colombia; Universidad Nacional de Colombia: Bogotá, Colombia, 2008; 242p. [Google Scholar]
- Sipman, H.J.M.; Aguirre, C.; Líquenes, J. Catálogo de Plantas y Líquenes de Colombia; Bernal, R., Gradstein, S.R., Celis, M., Eds.; Universidad Nacional de Colombia, Facultad de Ciencias, Instituto de Ciencias Naturales: Bogotá, Colombia, 2016; Volume 1, pp. 159–281. [Google Scholar]
- Lücking, R.; Rivas Plata, E.; Chaves, J.L.; Umaña, L.; Sipman, H.J.M. How many tropical lichens are there... really? Bibl. Lichenol. 2009, 100, 399–418. [Google Scholar]
- Coca, L.F.; Lumbsch, T.H.; Mercado-Díaz, J.A.; Widhelm, T.J.; Goffinet, B.; Kirika, P.; Lücking, R. Diversity, Phylogeny, and Historical Biogeography of the Genus Coccocarpia (Lichenized Ascomycota: Peltigerales) in the Tropics; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Vasco-Palacios, A.M.; Moncada, B. Two Centuries of Mycological History in Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Traversa, G.; Sosa, A.J.; Chantre, G.R.; Bianchinotti, M.V. Biological Studies of Puccinia Lantanae, a Potential Biocontrol Agent of “Lippia” (Phyla Nodiflora Var. Minor). Agron. Colomb. 2022, 40, 383–394. [Google Scholar] [CrossRef]
- Álvarez-Morales, L.C.; Morales-Osorio, J.G.; Salazar-Yepes, M. First report of Puccinia lagenophorae Cooke on Senecio spp. in Colombia. Can. J. Plant Pathol. 2022, 44, 689–694. [Google Scholar] [CrossRef]
- Cruz-O’Byrne, R.; Gamez-Guzman, A.; Piraneque-Gambasica, N.; Aguirre-Forero, S. Genomic sequencing in Colombian coffee fermentation reveals new records of yeast species. Food Biosci. 2023, 52, 102415. [Google Scholar] [CrossRef]
- Gómez-Montoya, N.; Ríos-Sarmiento, C.; Zora-Vergara, B.; Benjumea-Aristizabal, C.; Santa-Santa, D.J.; Zuluaga-Moreno, M.; Franco-Molano, A.E. Diversidad de macrohongos (Basidiomycota) de Colombia: Listado de especies. Actual. Biol. 2022, 44, 116. [Google Scholar] [CrossRef]
- Pinzón-Osorio, C.A.; Schuster, M.H.B.; Carrero-Torres, C.C. Nuevos registros del género Geastrum (Agaricomycetes, Basidiomycota) para Colombia. Hoehnea 2022, 49, e242021. [Google Scholar] [CrossRef]
- Ramírez-Castrillón, M.; Barona-Colorado, A.; Bados-Lopez, M.C.; Bolaños-Burbano, D. Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Salazar-Yepes, M.; Piepenbring, M. Diversity of Rust and Smut Fungi of Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Peña-Venegas, C.P.; Vasco-Palacios, A.M. Endo- and Ectomycorrhizas in Tropical Ecosystems of Colombia. In Mycorrhizal Fungi in South America. Fungal Biology; Pagano, M., Lugo, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Vasco-Palacios, A.M.; Lücking, R.; Moncada, B.; Palacio, M.; Motato-Vásquez, V. A Critical Assessment of Biogeographic Distribution Patterns of Colombian Fungi. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Simijaca, D.; Mueller, G.; Vasco-Palacios, A.M. Fungal Conservation in Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- López-Quintero, C.A.; Straatsma, G.; Franco-Molano, A.E.; Boekhout, T. Macrofungal Diversity in Colombian Amazon Forests Varies with Regions and Regimes of Disturbance. Biodivers. Conserv. 2012, 21, 2221–2243. [Google Scholar] [CrossRef]
- Vasco-Palacios, A.M.; Franco-Molano, A.E.; López-Quintero, C.A.; Boekhout, T. Macromycetes (Ascomycota, Basidiomycota) de la región del medio Caquetá, departamentos de Caquetá y Amazonas (Colombia). Biota Colomb. 2005, 6, 127–140. [Google Scholar]
- Corrales, A.; Benjumea, C.; Gomez-Montoya, N. Diversity, Functional Groups, and Community Structure of Fungi of Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Vargas, C.J.I.; Salazar, E.C.A. Biodiversidad y Mariposas en una Región del Alto Chocó, San José del Palmar, Colombia. Bol. Científico Cent. Mus. Mus. Hist. Nat. 2014, 18, 259–284. Available online: https://revistasojs.ucaldas.edu.co/index.php/boletincientifico/article/view/4480 (accessed on 1 October 2023).
- Bernal, R.; Gradstein, S.R.; Celis, M. (Eds.) Catálogo de Plantas y Líquenes de Colombia; Instituto de Ciencias Naturales, Universidad Nacional de Colombia: Bogotá, Colombia, 2019; Available online: https://catalogoplantasdecolombia.unal.edu.co (accessed on 1 October 2023).
- Pérez-Escobar, O.A.; Lucas, E.; Jaramillo, C.; Monro, A.; Morris, S.K.; Bogarín, D.; Greer, D.; Dodsworth, S.; Aguilar-Cano, J.; Sanchez Meseguer, A.; et al. The Origin and Diversification of the Hyperdiverse Flora in the Chocó Biogeographic Region. Front. Plant. Sci. 2019, 10, 1328. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan, T.; Brothers, K. Diversity of Non-Lichenised Macro-Ascomycota of Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Vasco-Palacios, A.M.; Franco-Molano, A.E. Diversity of Colombian macrofungi (Ascomycota-Basidiomycota). Mycotaxon 2013, 121, 100–158. [Google Scholar]
- Clerici, N.; Armenteras, D.; Kareiva, P.; Botero, R.; Ramírez-Delgado, J.P.; Forero-Medina, G.; Ochoa, J.; Pedraza, C.; Schneider, L.; Lora, C.; et al. Deforestation in Colombian Protected Areas Increased during Post-Conflict Periods. Sci. Rep. 2020, 10, 4971. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species, Version 2022-2. 2022. Available online: https://www.iucnredlist.org (accessed on 1 October 2023).
- Tedersoo, L.; Sánchez-Ramírez, S.; Kõljalg, U.; Bahram, M.; Doring, M.; May, T.; Ryberg, M.; Abarenkov, K. High-level classification of the Fungi and a tool for evolutionary ecological analyses. Fungal Divers. 2018, 90, 135–159. [Google Scholar] [CrossRef]
- Wijayawardene, N.N.; Pawłowska, J.; Letcher, P.M.; Kirk, P.M.; Humber, R.A.; Schüßler, A.; Wrzosek, M.; Muszewska, A.; Okrasińska, A.; Istel, Ł.; et al. Notes for Genera: Basal Clades of Fungi (Including Aphelidiomycota, Basidiobolomycota, Blastocladiomycota, Calcarisporiellomycota, Caulochytriomycota, Chytridiomycota, Entomophthoromycota, Glomeromycota, Kickxellomycota, Monoblepharomycota, Mortierellomyc. Fungal Divers. 2018, 92, 43–129. [Google Scholar] [CrossRef]
- Barta, M.; Cagáň, L. Aphid-Pathogenic Entomophthorales (Their Taxonomy, Biology and Ecology). Biologia 2006, 61, S543–S616. [Google Scholar] [CrossRef]
- Morales-López, S.; Ceballos-Garzón, A.; Parra-Giraldo, C.M. Zygomycete Fungi Infection in Colombia: Literature Review. Curr. Fungal Infect. Rep. 2018, 12, 149–154. [Google Scholar] [CrossRef]
- Méndez Puentes, C.A.; Camacho Suarez, J.G.; Echeverry Hernandez, S. Identificación de Bacterias y Hongos en El Aire de Neiva, Colombia. Rev. Salud Pública 2016, 17, 728–737. [Google Scholar] [CrossRef]
- Grossart, H.-P.; Van den Wyngaert, S.; Kagami, M.; Wurzbacher, C.; Cunliffe, M.; Rojas-Jimenez, K. Fungi in Aquatic Ecosystems. Nat. Rev. Microbiol. 2019, 17, 339–354. [Google Scholar] [CrossRef]
- van de Vossenberg, B.T.L.H.; Warris, S.; Nguyen, H.D.T.; van Gent-Pelzer, M.P.E.; Joly, D.L.; van de Geest, H.C.; Bonants, P.J.M.; Smith, D.S.; Lévesque, C.A.; van der Lee, T.A.J. Comparative Genomics of Chytrid Fungi Reveal Insights into the Obligate Biotrophic and Pathogenic Lifestyle of Synchytrium endobioticum. Sci. Rep. 2019, 9, 8672. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Garner, T.W.J. Chytrid Fungi and Global Amphibian Declines. Nat. Rev. Microbiol. 2020, 18, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Flechas, S.V.; Paz, A.; Crawford, A.J.; Sarmiento, C.; Acevedo, A.A.; Arboleda, A.; Bolívar-García, W.; Echeverry-Sandoval, C.L.; Franco, R.; Mojica, C.; et al. Current and Predicted Distribution of the Pathogenic Fungus Batrachochytrium dendrobatidis in Colombia, a Hotspot of Amphibian Biodiversity. Biotropica 2017, 49, 685–694. [Google Scholar] [CrossRef]
- Radek, R.; Wellmanns, D.; Wolf, A. Two New Species of Nephridiophaga (Zygomycota) in the Malpighian Tubules of Cockroaches. Parasitol. Res. 2011, 109, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Gleason, F.H.; Marano, A.V.; Johnson, P.; Martin, W.W. Blastocladian Parasites of Invertebrates. Fungal Biol. Rev. 2010, 24, 56–67. [Google Scholar] [CrossRef]
- Karling, J.S. Zoosporic soil fungi of La Guajiera, Colombia. Nova Hedwig. 1984, 40, 329–340. [Google Scholar]
- Matthews, V.D. A new genus of the Blastocladiaceae. J. Elisha Mitchell Sci. Soc. 1937, 53, 191–195. [Google Scholar]
- Landinez-Torres, A.; Panelli, S.; Picco, A.M.; Comandatore, F.; Tosi, S.; Capelli, E. A Meta-Barcoding Analysis of Soil Mycobiota of the Upper Andean Colombian Agro-Environment. Sci. Rep. 2019, 9, 10085. [Google Scholar] [CrossRef]
- Veerkamp, J.; Gams, W. Los Hongos de Colombia-VIII some new species of soil fungi from Colombia. Caldasia 1983, 13, 709–717. [Google Scholar]
- Gualdrón-Arenas, C.; Suárez-Navarro, A.L.; Valencia-Zapata, H. Hongos del suelo aislados de zonas de vegetación natural del páramo de Chisacá, Colombia. Caldasia 1997, 19, 235–245. [Google Scholar]
- Kirk, P.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Ainsworth & Bisby’s Dictionary of the Fungi, 10th ed.; CAB International: Wallingford, UK, 2008. [Google Scholar]
- Landínez-Torres, A.Y.; Becerra Abril, J.L.; Tosi, S.; Nicola, L. Soil Microfungi of the Colombian Natural Regions. Int. J. Environ. Res. Public Health 2020, 17, 8311. [Google Scholar] [CrossRef] [PubMed]
- Schüßler, A.; Schwarzott, D.; Walker, C. A new fungal phylum, the Glomeromycota: Phylogeny and evolution. Mycol. Res. 2001, 105, 1413–1421. [Google Scholar] [CrossRef]
- Walker, C.; Schüßler, A.; Vincent, B.; Cranenbrouck, S.; Declerck, S. Anchoring the Species Rhizophagus intraradices (Formerly Glomus Intraradices). Fungal Syst. Evol. 2021, 8, 179–201. [Google Scholar] [CrossRef] [PubMed]
- Charria-Girón, E.; Espinosa, M.C.; Zapata-Montoya, A.; Méndez, M.J.; Caicedo, J.P.; Dávalos, A.F.; Ferro, B.E.; Vasco-Palacios, A.M.; Caicedo, N.H. Evaluation of the Antibacterial Activity of Crude Extracts Obtained from Cultivation of Native Endophytic Fungi Belonging to a Tropical Montane Rainforest in Colombia. Front. Microbiol. 2021, 12, 716523. [Google Scholar] [CrossRef] [PubMed]
- Hoyos, L.V.; Chaves, A.; Grandezz, D.; Medina, A.; Correa, J.; Ramirez-castrillon, M.; Valencia, D.; Caicedo-ortega, N.H. Systematic Screening Strategy for Fungal Laccase Activity of Endophytes from Otoba gracilipes with Bioremediation Potential. Fungal Biol. 2023, 127, 1298–1311. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Carpintero, R.; Romero-Perdomo, F.; Martínez, J.F.; Lewandowski, I. A Review of the Knowledge Base for the Development of Natural Ingredients Value Chains for a Sustainable Biobased Economy in Colombia. Discov. Sustain. 2023, 4, 33. [Google Scholar] [CrossRef]
- Huddart, J.E.A.; Crawford, A.J.; Luna-Tapia, A.L.; Restrepo, S.; Di Palma, F. EBP-Colombia and the Bioeconomy: Genomics in the Service of Biodiversity Conservation and Sustainable Development. Proc. Natl. Acad. Sci. USA 2022, 119, e2115641119. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, A. The Road Ahead: Narratives and Imaginaries of the Value of Biodiversity in Shaping Bioeconomy Policy in Colombia. Tapuya Lat. Am. Sci. Technol. Soc. 2022, 5, 2059137. [Google Scholar] [CrossRef]
- Charria-Girón, E.; Marin-Felixa, Y.; Beutling, U.; Franke, R.; Brönstrup, M.; Vasco-Palacios, A.M.; Caicedo, N.H.; Surup, F. Metabolomics insights into the polyketide-lactones produced by Diaporthe caliensis sp. nov., an endophyte of the medicinal plant Otoba gracilipes. Microbiol. Spectr. 2023, e0274323. [Google Scholar] [CrossRef]
- Llanos-López, N.A.; Ebada, S.S.; Vasco-Palacios, A.M.; Sánchez-Giraldo, L.M.; López, L.; Rojas, L.F.; Mándi, A.; Kurtán, T.; Marin-Felix, Y. Panapophenanthrin, a Rare Oligocyclic Diterpene from Panus strigellus. Metabolites 2023, 13, 848. [Google Scholar] [CrossRef]
- Sekizawa, R.; Ikeno, S.; Nakamura, H.; Naganawa, H.; Matsui, S.; Iinuma, H.; Takeuchi, T. Panepophenanthrin, from a Mushroom Strain, a Novel Inhibitor of the Ubiquitin-Activating Enzyme. J. Nat. Prod. 2002, 65, 1491–1493. [Google Scholar] [CrossRef] [PubMed]
- Moses, J.E.; Commeiras, L.; Baldwin, J.E.; Adlington, R.M. Total Synthesis of Panepophenanthrin. Org. Lett. 2003, 5, 2987–2988. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Johnson, R.P.; Porco, J.A. Total Synthesis of the Ubiquitin-Activating Enzyme Inhibitor (+)-Panepophenanthrin. Angew. Chem. Int. Ed. 2003, 42, 3913–3917. [Google Scholar] [CrossRef] [PubMed]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary Metabolism in the Lichen Symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef] [PubMed]
- Leal, A.; Rojas, J.L.; Valencia-Islas, N.A.; Castellanos, L. New β-Orcinol Depsides from Hypotrachyna caraccensis, a Lichen from the Páramo Ecosystem and Their Free Radical Scavenging Activity. Nat. Prod. Res. 2018, 32, 1375–1382. [Google Scholar] [CrossRef] [PubMed]
- Valencia-Islas, N.A.; Arguello, J.J.; Rojas, J.L. Antioxidant and Photoprotective Metabolites of Bunodophoron melanocarpum, A Lichen from the Andean Páramo. Pharm. Sci. 2020, 27, 281–290. [Google Scholar] [CrossRef]
- Salgado, F.; Albornoz, L.; Cortéz, C.; Stashenko, E.; Urrea-Vallejo, K.; Nagles, E.; Galicia-Virviescas, C.; Cornejo, A.; Ardiles, A.; Simirgiotis, M.; et al. Secondary Metabolite Profiling of Species of the Genus Usnea by UHPLC-ESI-OT-MS-MS. Molecules 2017, 23, 54. [Google Scholar] [CrossRef]
- Izquierdo-García, L.F.; González-Almario, A.; Cotes, A.M.; Moreno-Velandia, C.A. Trichoderma virens Gl006 and Bacillus velezensis Bs006: A Compatible Interaction Controlling Fusarium Wilt of Cape Gooseberry. Sci. Rep. 2020, 10, 6857. [Google Scholar] [CrossRef]
- García, D.; González-Almario, A.; Cotes, A.M. Controlling Fusarium Wilt of Cape Gooseberry by Microbial Consortia. Lett. Appl. Microbiol. 2023, 76, 72. [Google Scholar] [CrossRef]
- Wang, W.; de Silva, D.D.; Moslemi, A.; Edwards, J.; Ades, P.K.; Crous, P.W.; Taylor, P.W.J. Colletotrichum Species Causing Anthracnose of Citrus in Australia. J. Fungi 2021, 7, 47. [Google Scholar] [CrossRef]
- Forcelini, B.B.; Peres, N.A. Widespread Resistance to QoI Fungicides of Colletotrichum acutatum from Strawberry Nurseries and Production Fields. Plant Health Prog. 2018, 19, 338–341. [Google Scholar] [CrossRef]
- Muñoz-Guerrero, J.; Guerra-Sierra, B.E.; Alvarez, J.C. Fungal Endophytes of Tahiti Lime (Citrus Citrus × Latifolia) and Their Potential for Control of Colletotrichum acutatum J. H. Simmonds Causing Anthracnose. Front. Bioeng. Biotechnol. 2021, 9, 650351. [Google Scholar] [CrossRef] [PubMed]
- Cardona, N.L.; Franco-Sierra, N.D.; Correa Alvarez, J. Complete Mitogenome of the Biocontroller Fungus Purpureocillium sp. (Ascomycota, Ophiocordycipitaceae, Hypocreales). Mitochondrial DNA Resour. 2018, 3, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- Robey, M.T.; Caesar, L.K.; Drott, M.T.; Keller, N.P.; Kelleher, N.L. An Interpreted Atlas of Biosynthetic Gene Clusters from 1000 Fungal Genomes. Proc. Natl. Acad. Sci. USA 2021, 118, e2020230118. [Google Scholar] [CrossRef]
- Mira Taborda, Y.D.; Castañeda Sánchez, D.A.; Morales Osorio, J.G.; Patiño Hoyos, L.F. Ecological, phytosanitary, and agronomic aspects of target weeds for biological control studies in Antioquia, Colombia. Acta Agronóm. 2022, 71, 195–206. [Google Scholar] [CrossRef]
- López-Arboleda, W.A.; Ramírez-Castrillón, M. Diversidad de Levaduras Asociadas a Chichas Tradicionales de Colombia. Rev. Colomb. Biotecnol. 2010, 12, 176–186. [Google Scholar]
- Chaves-López, C.; Serio, A.; Grande-Tovar, C.D.; Cuervo-Mulet, R.; Delgado-Ospina, J.; Paparella, A. Traditional Fermented Foods and Beverages from a Microbiological and Nutritional Perspective: The Colombian Heritage. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1031–1048. [Google Scholar] [CrossRef]
- Sandoval-Lozano, C.J.; Caballero-Torres, D.; López-Giraldo, L.J. Screening Wild Yeast Isolated from Cocoa Bean Fermentation Using Volatile Compounds Profile. Molecules 2022, 27, 902. [Google Scholar] [CrossRef]
- Fernández-Niño, M.; Rodríguez-Cubillos, M.J.; Herrera-Rocha, F.; Anzola, J.M.; Cepeda-Hernández, M.L.; Aguirre Mejía, J.L.; Chica, M.J.; Olarte, H.H.; Rodríguez-López, C.; Calderón, D.; et al. Dissecting Industrial Fermentations of Fine Flavour Cocoa through Metagenomic Analysis. Sci. Rep. 2021, 11, 8638. [Google Scholar] [CrossRef]
- Vargas, N.; Gómez-Montoya, N.; Peña-Cañón, R.; Torres-Morales, G. Useful Fungi of Colombia. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
- Franco–Molano, A.E.; Vasco-Palacios, A.M.; López-Quintero, C.A.; Boekhout, T. Macrohongos de la Región del Medio Caquetá, Colombia; Guía de Campo Multimpresos: Medellín, Colombia, 2005. [Google Scholar]
- Moreno-Bayona, D.A.; Gómez-Méndez, L.D.; Blanco-Vargas, A.; Castillo-Toro, A.; Herrera-Carlosama, L.; Poutou-Piñales, R.A.; Salcedo-Reyes, J.C.; Díaz-Ariza, L.A.; Castillo-Carvajal, L.C.; Rojas-Higuera, N.S.; et al. Simultaneous Bioconversion of Lignocellulosic Residues and Oxodegradable Polyethylene by Pleurotus ostreatus for Biochar Production, Enriched with Phosphate Solubilizing Bacteria for Agricultural Use. PLoS ONE 2019, 14, e0217100. [Google Scholar] [CrossRef]
- Copete-Pertuz, L.S.; Alandete-Novoa, F.; Plácido, J.; Correa-Londoño, G.A.; Mora-Martínez, A.L. Enhancement of Ligninolytic Enzymes Production and Decolourising Activity in Leptosphaerulina sp. by Co–Cultivation with Trichoderma viride and Aspergillus terreus. Sci. Total Environ. 2019, 646, 1536–1545. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Otálvaro, F.; Merino-Restrepo, A.; Hormaza-Anaguano, A. Evaluation of a Trametes pubescens Laccase Concentrated Extract on Allura Red AC Decolorization without the Addition of Synthetic Mediators. J. Environ. Manag. 2021, 285, 112117. [Google Scholar] [CrossRef] [PubMed]
- Merino-Restrepo, A.; Mejía-Otálvaro, F.; Velásquez-Quintero, C.; Hormaza-Anaguano, A. Evaluation of Several White-Rot Fungi for the Decolorization of a Binary Mixture of Anionic Dyes and Characterization of the Residual Biomass as Potential Organic Soil Amendment. J. Environ. Manag. 2020, 254, 109805. [Google Scholar] [CrossRef] [PubMed]
- Caicedo, N.H.; Davalos, A.F.; Puente, P.A.; Rodríguez, A.Y.; Caicedo, P.A. Antioxidant Activity of Exo-Metabolites Produced by Fusarium oxysporum: An Endophytic Fungus Isolated from Leaves of Otoba gracilipes. Microbiologyopen 2019, 8, e903. [Google Scholar] [CrossRef] [PubMed]
- Guerra Sierra, B.E.; Arteaga-Figueroa, L.A.; Sierra-Pelaéz, S.; Alvarez, J.C. Talaromyces santanderensis: A New Cadmium-Tolerant Fungus from Cacao Soils in Colombia. J. Fungi 2022, 8, 1042. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.J.; Caicedo, N.H.; Salamanca, C. Trametes elegans: A Fungal Endophytic Isolate from Otoba gracilipes as Biocatalyst for Natural Flavors Production. N. Biotechnol. 2018, 44, S75. [Google Scholar] [CrossRef]
- Jaramillo, D.A.; Méndez, M.J.; Vargas, G.; Stashenko, E.E.; Vasco-Palacios, A.-M.; Ceballos, A.; Caicedo, N.H. Biocatalytic Potential of Native Basidiomycetes from Colombia for Flavour/Aroma Production. Molecules 2020, 25, 4344. [Google Scholar] [CrossRef]
- Garcia-Cortes, A.; Garcia-Vásquez, J.A.; Aranguren, Y.; Ramirez-Castrillon, M. Pigment Production Improvement in Rhodotorula mucilaginosa AJB01 Using Design of Experiments. Microorganisms 2021, 9, 387. [Google Scholar] [CrossRef]
- Vargas-Sinisterra, A.F.; Ramírez-Castrillón, M. Yeast Carotenoids: Production and Activity as Antimicrobial Biomolecule. Arch. Microbiol. 2021, 203, 873–888. [Google Scholar] [CrossRef]
- Ortiz-Moreno, M.L.; Moncada, B.; Vasco-Palacios, A.M.; de Almeida, R.F.; Gaya, E. Fungi in Colombian and international biological collections. In Catalogue of Fungi of Colombia; De Almeida, R.F., Lücking, R., Vasco-Palacios, A.M., Gaya, E., Diazgranados, M., Eds.; Kew Publishing Royal Botanic Gardens: Richmond, UK, 2022; ISBN 978-1-84246-790-9. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).