Characterization and Biological Activities of Yeasts Isolated from Marine Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Isolation
2.2. DNA Extraction, PCR, and Identification
2.3. Culture Conditions
2.4. Microscopic Observation
2.5. Preparation of Fungal Extracts
2.6. Antioxidant Assays
2.6.1. ABTS Radical-Scavenging Assay
2.6.2. DPPH Radical-Scavenging Assay
2.7. Antimicrobial Activity
2.8. Tyrosinase Inhibition Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Identification and Phylogeny
3.2. Morphological Observation
3.3. Salt-Tolerant Ability
3.4. Antioxidant Activity
3.5. Antibacterial Activity
3.6. Tyrosinase Inhibition Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwon, Y.M.; Choi, H.S.; Lim, J.Y.; Jang, H.S.; Chung, D. Characterization of amylolytic activity by a marine-derived yeast Sporidiobolus pararoseus PH-Gra1. Mycobiology 2020, 48, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.M.; Liu, G.; Zhao, S.; Li, J.; Peng, Y. Marine yeasts as biocontrol agents and producers of bio-products. Appl. Microbiol. Biotechnol. 2010, 86, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Kutty, S.N.; Philip, R. Marine yeasts—A review. Yeast 2008, 25, 465–483. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, C.; Fell, J.W.; Boekhout, T. The Yeasts: A Taxonomic Study, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Zaky, A.S.; Tucker, G.A.; Daw, Z.Y.; Du, C. Marine yeast isolation and industrial application. FEMS Yeast Res. 2014, 14, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Hyde, K.D.; Jones, E.G.; Leaño, E.; Pointing, S.B.; Poonyth, A.D.; Vrijmoed, L.L. Role of fungi in marine ecosystems. Biodivers. Conserv. 1998, 7, 1147–1161. [Google Scholar] [CrossRef]
- Chi, Z.; Chi, Z.; Zhang, T.; Liu, G.; Li, J.; Wang, X. Production, characterization and gene cloning of the extracellular enzymes from the marine-derived yeasts and their potential applications. Biotechnol. Adv. 2009, 27, 236–255. [Google Scholar] [CrossRef]
- Wang, L.; Chi, Z.; Wang, X.; Ju, L.; Chi, Z.; Guo, N. Isolation and characterization of Candida membranifaciens subsp. flavinogenie W14-3, a novel riboflavin-producing marine yeast. Microbiol. Res. 2008, 163, 255–266. [Google Scholar] [CrossRef]
- Apte, M.; Sambre, D.; Gaikawad, S.; Joshi, S.; Bankar, A.; Kumar, A.R.; Zinjarde, S. Psychrotrophic yeast Yarrowia lipolytica NCYC 789 mediates the synthesis of antimicrobial silver nanoparticles via cell-associated melanin. AMB Express 2013, 3, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Saavedra, N.Y.; Ochoa, J.L. Copper–zinc superoxide dismutase from the marine yeast Debaryomyces hansenii. Yeast 1999, 15, 657–668. [Google Scholar] [CrossRef]
- Corinaldesi, C.; Barone, G.; Marcellini, F.; Dell’Anno, A.; Danovaro, R. Marine microbial-derived molecules and their potential use in cosmeceutical and cosmetic products. Mar. Drugs 2017, 15, 118. [Google Scholar] [CrossRef]
- Ding, J.; Wu, B.; Chen, L. Application of Marine Microbial Natural Products in Cosmetics. Front. Microbiol. 2022, 13, 892505. [Google Scholar] [CrossRef] [PubMed]
- Manohar, C.S.; Raghukumar, C. Fungal diversity from various marine habitats deduced through culture-independent studies. FEMS Microbiol. Lett. 2013, 341, 69–78. [Google Scholar] [CrossRef]
- Chung, D.; Baek, K.; Bae, S.S.; Jung, J. Identification and characterization of a marine-derived chitinolytic fungus, Acremonium sp. YS2-2. J. Microbiol. 2019, 57, 372–380. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Lai, H.Y.; Lim, Y.Y.; Tan, S.P. Antioxidative, tyrosinase inhibiting and antibacterial activities of leaf extracts from medicinal ferns. Biosci. Biotechnol. Biochem. 2009, 73, 1362–1366. [Google Scholar] [CrossRef]
- Nutaratat, P.; Boontham, W.; Khunnamwong, P. A novel yeast genus and two novel species isolated from pineapple leaves in Thailand: Savitreella phatthalungensis gen. nov., sp. nov. and Goffeauzyma siamensis sp. nov. J. Fungi 2022, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chi, Z.; Wang, G.Y.; Wang, Z.P.; Liu, G.L.; Lee, C.F.; Ma, Z.C.; Chi, Z.M. Taxonomy of Aureobasidium spp. and biosynthesis and regulation of their extracellular polymers. Crit. Rev. Microbiol. 2015, 41, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Perkins, V.; Vignola, S.; Lessard, M.H.; Plante, P.L.; Corbeil, J.; Dugat-Bony, E.; Frenette, M.; Labrie, S. Phenotypic and genetic characterization of the cheese ripening yeast Geotrichum candidum. Front. Microbiol. 2020, 11, 737. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Cheng, P.; Yu, G. Rhodotorula mucilaginosa—Alternative sources of natural carotenoids, lipids, and enzymes for industrial use. Heliyon 2022, 8, e11505. [Google Scholar] [CrossRef]
- Nicaud, J.M. Yarrowia lipolytica. Yeast 2012, 29, 409–418. [Google Scholar] [CrossRef]
- Butinar, L.; Santos, S.; Spencer-Martins, I.; Oren, A.; Gunde-Cimerman, N. Yeast diversity in hypersaline habitats. FEMS Microbiol. Lett. 2005, 244, 229–234. [Google Scholar] [CrossRef]
- Mamaev, D.; Zvyagilskaya, R. Yarrowia lipolytica: A multitalented yeast species of ecological significance. FEMS Yeast Res. 2021, 21, foab008. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.C.; Wu, Q.; Chen, G.Q. Halophiles, coming stars for industrial biotechnology. Biotechnol. Adv. 2015, 33, 1433–1442. [Google Scholar] [CrossRef]
- Camarasa, C.; Sanchez, I.; Brial, P.; Bigey, F.; Dequin, S. Phenotypic landscape of Saccharomyces cerevisiae during wine fermentation: Evidence for origin-dependent metabolic traits. PLoS ONE 2011, 6, e25147. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Cai, Y.Z.; Xing, J.; Corke, H.; Sun, M. A potential antioxidant resource: Endophytic fungi from medicinal plants. Econ. Bot. 2007, 61, 14–30. [Google Scholar] [CrossRef]
- Vitale, G.A.; Coppola, D.; Palma Esposito, F.; Buonocore, C.; Ausuri, J.; Tortorella, E.; de Pascale, D. Antioxidant molecules from marine fungi: Methodologies and perspectives. Antioxidants 2020, 9, 1183. [Google Scholar] [CrossRef] [PubMed]
- Bartasiute, A.; Westerink, B.H.; Verpoorte, E.; Niederländer, H.A. Improving the in vivo predictability of an on-line HPLC stable free radical decoloration assay for antioxidant activity in methanol–buffer medium. Free Radic. Biol. Med. 2007, 42, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from marine organisms: Biological functions and industrial applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ambati, R.R.; Phang, S.M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Leyton, A.; Flores, L.; Mäki-Arvela, P.; Lienqueo, M.E.; Shene, C. Macrocystis pyrifera source of nutrients for the production of carotenoids by a marine yeast Rhodotorula mucilaginosa. J. Appl. Microbiol. 2019, 127, 1069–1079. [Google Scholar] [CrossRef]
- Cao, K.; Chen, J.; Lu, X.; Yao, Y.; Huang, R.; Li, L. Matrine-producing endophytic fungus Galactomyces candidum TRP-7: Screening, identification, and fermentation conditions optimization for Matrine production. Biotechnol. Lett. 2023, 45, 209–223. [Google Scholar] [CrossRef]
- Jiang, H.; Bao, J.; Xing, Y.; Li, X.; Chen, Q. Comparative genomic analyses provide insight into the pathogenicity of Metschnikowia bicuspidata LNES0119. Front. Microbiol. 2022, 13, 939141. [Google Scholar] [CrossRef]
- Jiang, H.; Bao, J.; Cao, G.; Xing, Y.; Feng, C.; Hu, Q.; Li, X.; Chen, Q. Experimental transmission of the yeast, Metschnikowia bicuspidata, in the Chinese mitten crab, Eriocheir sinensis. J. Fungi 2022, 8, 210. [Google Scholar] [CrossRef]
- Kubo, I.; Fujita, K.I.; Nihei, K.I.; Nihei, A. Antibacterial activity of akyl gallates against Bacillus subtilis. J. Agric. Food Chem. 2004, 52, 1072–1076. [Google Scholar] [CrossRef]
- Abdel-Lateff, A.; Elkhayat, E.S.; Fouad, M.A.; Okino, T. Aureobasidin, new antifouling metabolite from marine-derived fungus Aureobasidium sp. Nat. Prod. Commun. 2009, 4, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, K.M.; Leathers, T.D.; Price, N.P.; Manitchotpisit, P. Liamocin oil from Aureobasidium pullulans has antibacterial activity with specificity for species of Streptococcus. J. Antibiot. 2015, 68, 642–645. [Google Scholar] [CrossRef]
- Mefteh, F.B.; Daoud, A.; Chenari Bouket, A.; Alenezi, F.N.; Luptakova, L.; Rateb, M.E.; Kadri, A.; Gharsallah, N.; Belbahri, L. Fungal root microbiome from healthy and brittle leaf diseased date palm trees (Phoenix dactylifera L.) reveals a hidden untapped arsenal of antibacterial and broad spectrum antifungal secondary metabolites. Front. Microbiol. 2017, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Dieuleveux, V.; Van Der Pyl, D.; Chataud, J.; Gueguen, M. Purification and characterization of anti-Listeria compounds produced by Geotrichum candidum. Appl. Environ. Microbiol. 1998, 64, 800–803. [Google Scholar] [CrossRef]
- Vidya, P.; Kutty, S.N.; Sebastian, C.D. Extraction, characterization and antimicrobial properties of pigments from yeast, Rhodotorula mucilaginosa isolated from the mangrove sediments of North Kerala, India. Asian J. Biol. Life Sci. 2021, 10, 559. [Google Scholar] [CrossRef]
- Yoo, A.Y.; Alnaeeli, M.; Park, J.K. Production control and characterization of antibacterial carotenoids from the yeast Rhodotorula mucilaginosa AY-01. Process Biochem. 2016, 51, 463–473. [Google Scholar] [CrossRef]
- Gramisci, B.R.; Lutz, M.C.; Lopes, C.A.; Sangorrín, M.P. Enhancing the efficacy of yeast biocontrol agents against postharvest pathogens through nutrient profiling and the use of other additives. Biol. Control 2018, 121, 151–158. [Google Scholar] [CrossRef]
- Sørensen, A.B.; Harholt, J.; Arneborg, N. Application of Yarrowia lipolytica in fermented beverages. Front. Food Sci. Technol. 2023, 3, 1190063. [Google Scholar] [CrossRef]
- Vega, F.E.; Posada, F.; Aime, M.C.; Pava-Ripoll, M.; Infante, F.; Rehner, S.A. Entomopathogenic fungal endophytes. Biol. Control 2008, 46, 72–82. [Google Scholar] [CrossRef]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 2011, 6, 175–191. [Google Scholar] [CrossRef]
- Kumar, V.; Jain, L.; Kaushal, P.; Soni, R. Fungal endophytes and their applications as growth promoters and biological control agents. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology, 1st ed.; Sharma, V.K., Shah, M.P., Parmar, S., Kumar, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 315–337. [Google Scholar] [CrossRef]
- Fernandes, M.S.; Kerkar, S. Microorganisms as a source of tyrosinase inhibitors: A review. Ann. Microbiol. 2017, 67, 343–358. [Google Scholar] [CrossRef]
- Chang, T.S. An updated review of tyrosinase inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef]
- Balboa, E.M.; Conde, E.; Soto, M.L.; Pérez-Armada, L.; Domínguez, H. Cosmetics from Marine Sources. In Springer Handbook of Marine Biotechnology, 1st ed.; Springer Handbooks; Kim, S.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1015–1042. [Google Scholar] [CrossRef]
- Goetghebeur, M.; Kermasha, S. Inhibition of polyphenol oxidase by copper-metallothionein from Aspergillus niger. Phytochemistry 1996, 42, 935–940. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Yamada, K.; Minoura, K.; Miyamoto, K.; Usami, Y.; Kobayashi, T.; Hamada-Sato, N.; Imada, C.; Tsujibo, H. Purification and determination of the chemical structure of the tyrosinase inhibitor produced by Trichoderma viride strain H1-7 from a marine environment. Biol. Pharm. Bull. 2008, 31, 1618–1620. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kim, M.K.; Lee, U.; Kim, S.K.; Kang, J.S.; Choi, H.D.; Son, B.W. Myrothenones A and B, cyclopentenone derivatives with tyrosinase inhibitory activity from the marine-derived fungus Myrothecium sp. Chem. Pharm. Bull. 2005, 53, 453–455. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Park, S.J.; Lee, J.E.; Lee, Y.J.; Song, C.H.; Choi, S.H.; Ku, S.K.; Kang, S.J. Anti-skin-aging benefits of exopolymers from Aureobasidium pullulans SM2001. J. Cosmet. Sci. 2014, 65, 285–298. [Google Scholar] [PubMed]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Yuan, Y.; Jin, W.; Nazir, Y.; Fercher, C.; Blaskovich, M.A.; Cooper, M.A.; Ross, T.B.; Ziora, Z.M. Tyrosinase inhibitors as potential antibacterial agents. Eur. J. Med. Chem. 2020, 187, 111892. [Google Scholar] [CrossRef]
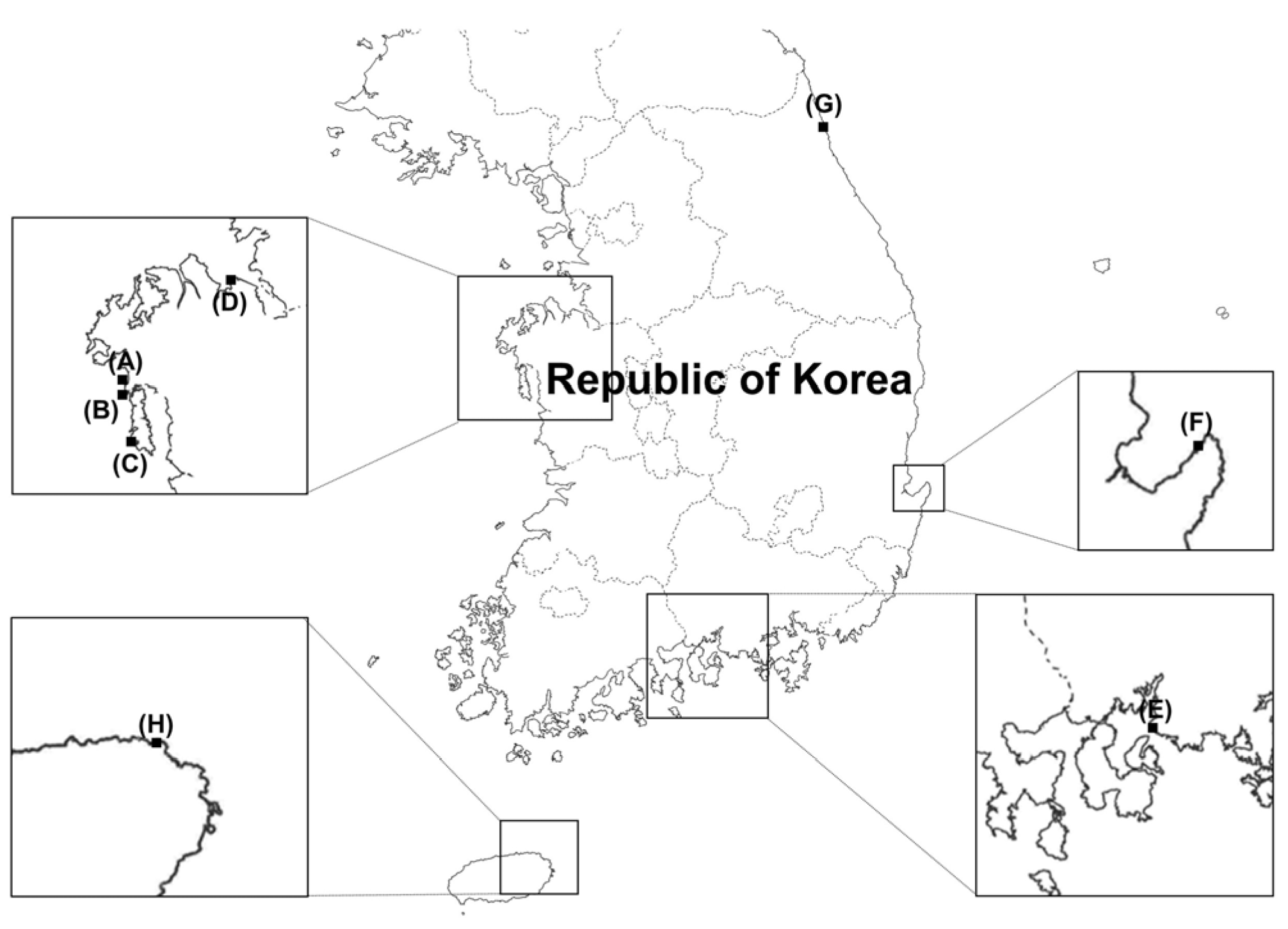

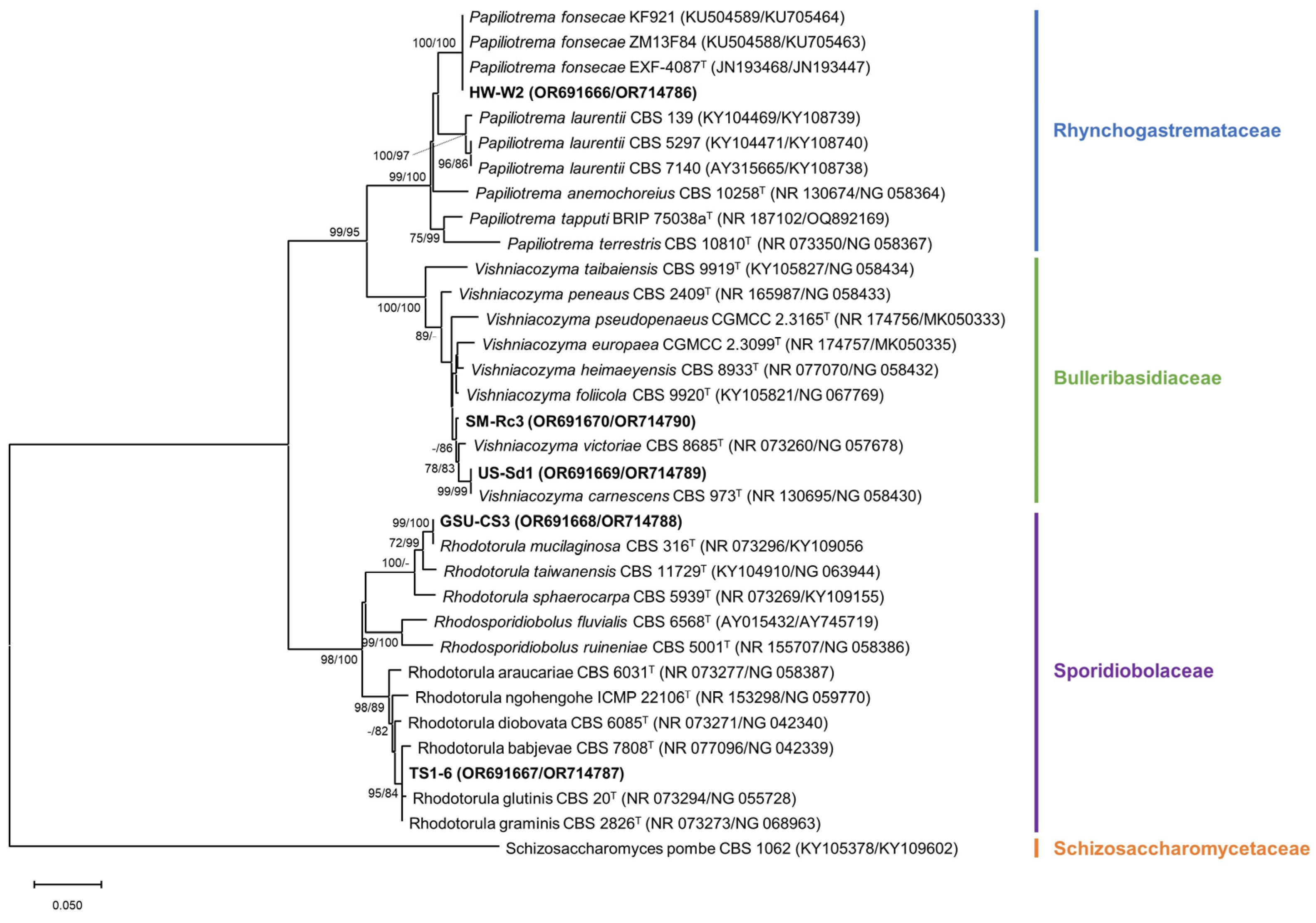

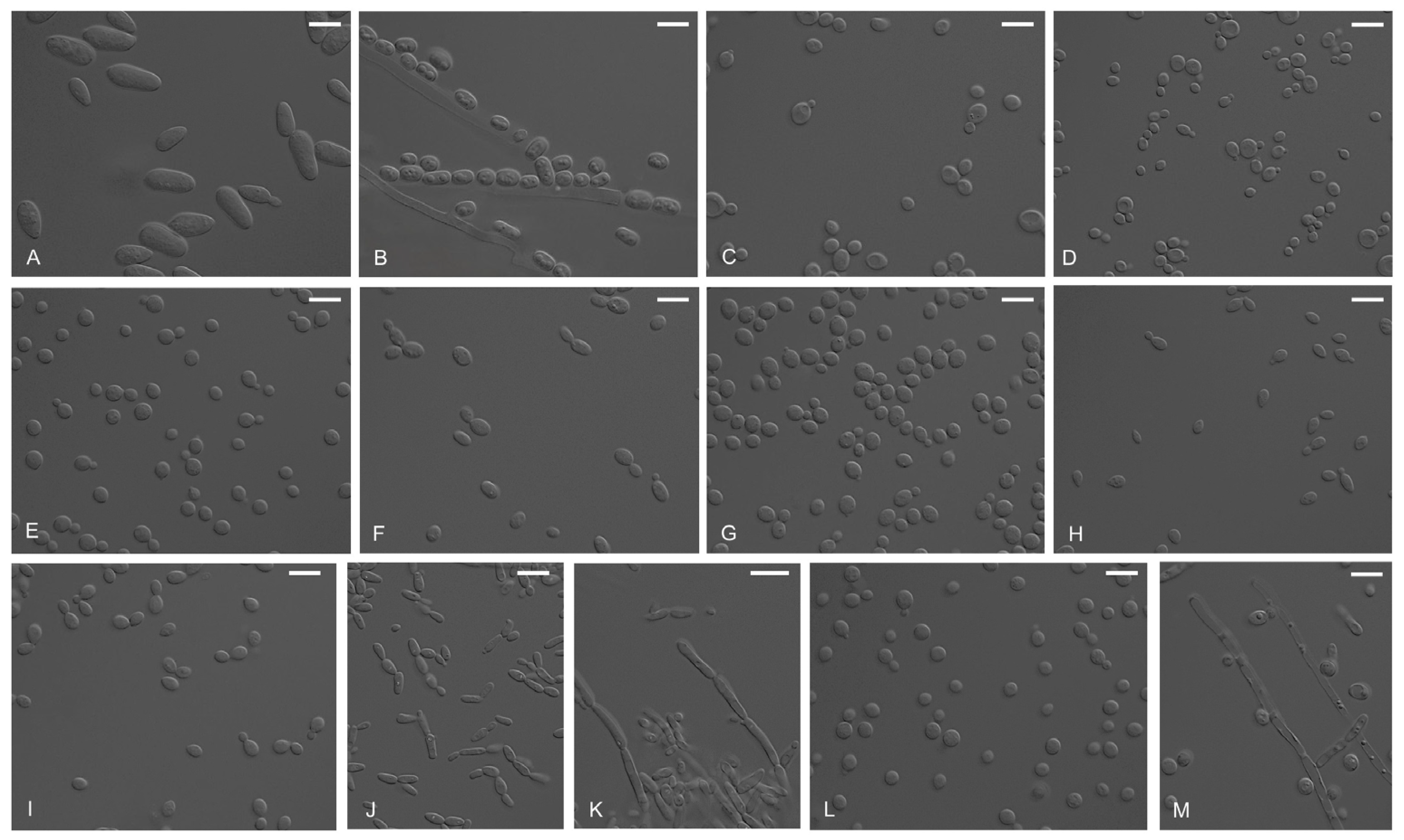
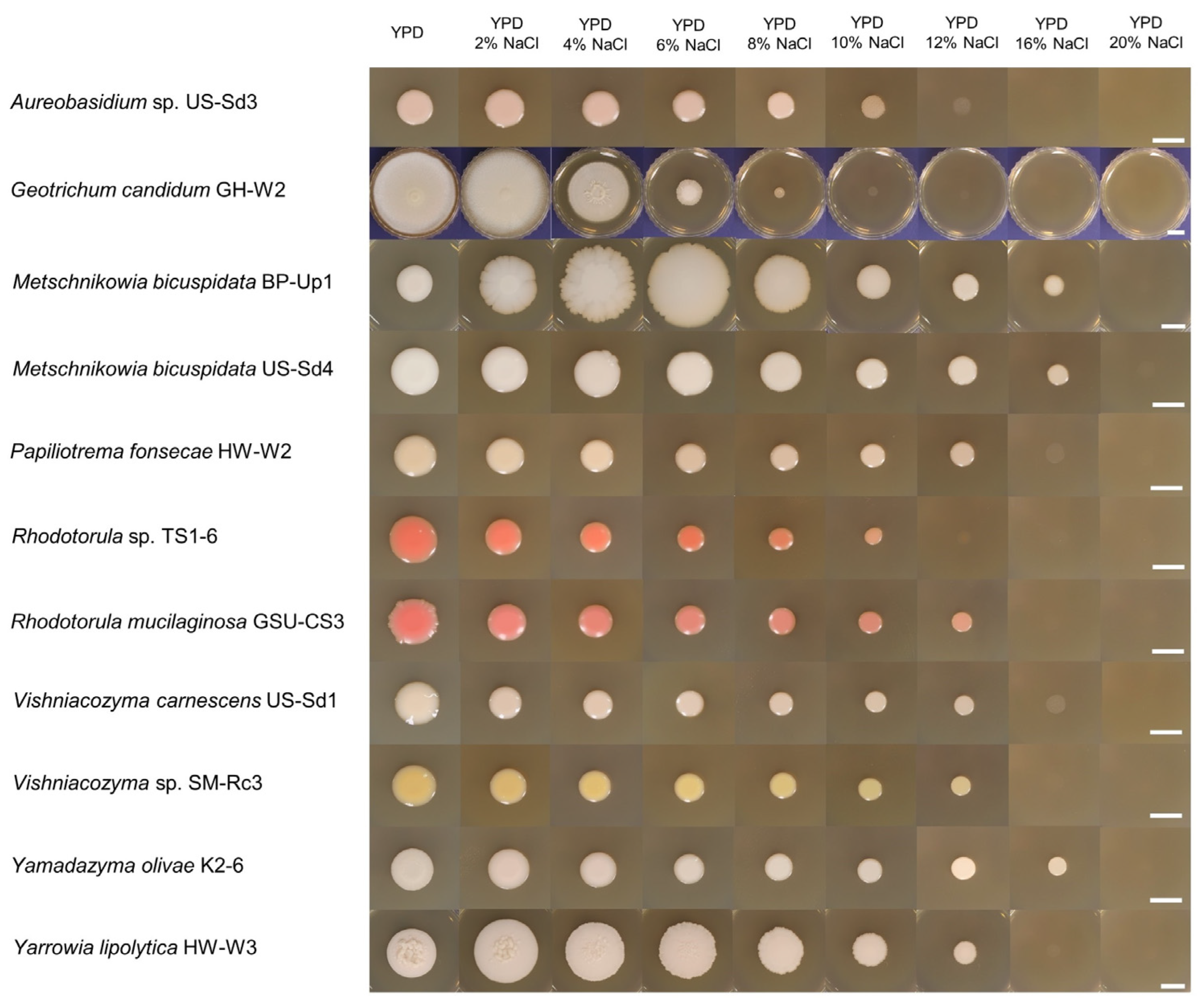
| Identity | ID | Sampling Site * | Isolation Source | Culture ID in MMBB of MABIK |
|---|---|---|---|---|
| Aureobasidium sp. | US-Sd3 | Nam-myeon, Taean-gun, Chungcheongnam-do A | Sediment | MABIK FU00001254 |
| Geotrichum candidum | GH-W2 | Daebang-dong, Sacheon-si, Gyeongsangnam-do E | Seawater | MABIK FU00001121 |
| Metschnikowia bicuspidata | BP-Up1 | Toseong-myeon, Goseong-gun, Gangwon-do G | Ulva australis | MABIK FU00001255 |
| US-Sd4 | Nam-myeon, Taean-gun, Chungcheongnam-do A | Sediment | MABIK FU00001256 | |
| Papiliotrema fonsecae | HW-W2 | Gujwa-eup, Jeju-si, Jeju-do H | Seawater | MABIK FU00001257 |
| Rhodotorula sp. | TS1-6 | Anmyeon-eup, Taean-gun, Chungcheongnam-do C | Seawater | MABIK FU00001258 |
| Rhodotorula mucilaginosa | GSU-CS3 | Nam-myeon, Taean-gun, Chungcheongnam-do B | Venerupis philippinarum | MABIK FU00001259 |
| Vishniacozyma carnescens | US-Sd1 | Nam-myeon, Taean-gun, Chungcheongnam-do A | Sediment | MABIK FU00001260 |
| Vishniacozyma sp. | SM-Rc3 | Seokmun-myeon, Dangjeon-si, Chungcheongnam-do D | Rumex crispus | MABIK FU00001179 |
| Yamadazyma olivae | K2-6 | East sea of South Korea F | Guts of Pagrus major | MABIK FU00001261 |
| Yarrowia lipolytica | HW-W3 | Gujwa-eup, Jeju-si, Jeju-do H | Seawater | MABIK FU00001262 |
| Fungal Name | ID | Radical-Scavenging Activity (%) | Antibacterial Activity (MIC 3, µg/mL) | Tyrosinase Inhibition (%) | |
|---|---|---|---|---|---|
| ABTS 1 | DPPH 2 | B. subtilis | |||
| Aureobasidium sp. | US-Sd3 | 9.78 ± 0.40 a,A | 20.90 ± 1.65 ab,B | 100 a | 27.65 ± 0.26 |
| Geotrichum candidum | GH-W2 | 67.89 ± 1.93 d,A | 22.34 ± 2.72 ab,B | >100 b | N.D. 4 |
| Metschnikowia bicuspidata | BP-Up1 | 85.88 ± 0.42 e,A | 27.28 ± 1.61 bc,B | N.D. | N.D. |
| US-Sd4 | 65.26 ± 3.74 d,A | 27.47 ± 0.74 bc,B | N.D. | N.D. | |
| Papiliotrema fonsecae | HW-W2 | 37.44 ± 0.84 b,A | 36.04 ± 0.83 c,A | N.D. | N.D. |
| Rhodotorula sp. | TS1-6 | 17.23 ± 0.89 a,A | 15.14 ± 1.62 a,A | N.D. | N.D. |
| Rhodotorula mucilaginosa | GSU-CS3 | 63.67 ± 1.86 d,A | 25.23 ± 2.98 b,B | >100 b | N.D. |
| Vishniacozyma carnescens | US-Sd1 | 45.15 ± 3.61 bc,A | 17.30 ± 1.43 a,B | >100 b | N.D. |
| Vishniacozyma sp. | SM-Rc3 | 50.94 ± 0.63 c,A | 28.65 ± 1.43 bc,B | >100 c | N.D. |
| Yamadazyma olivae | K2-6 | 79.19 ± 1.68 e,A | 26.31 ± 0.62 b,B | >100 d | N.D. |
| Yarrowia lipolytica | HW-W3 | 40.10 ± 1.11 bc,A | 26.85 ± 2.18 b,B | >100 b | N.D. |
| Ascorbic acid † | 13.70 ± 0.06 * | 6.80 ± 0.27 * | |||
| Kojic acid † | 49.32 ± 0.35 * | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, W.-J.; Chung, D.; Bae, S.S.; Kwon, Y.M.; Cho, E.-S.; Choi, G. Characterization and Biological Activities of Yeasts Isolated from Marine Environments. Microbiol. Res. 2023, 14, 1984-1999. https://doi.org/10.3390/microbiolres14040134
Yu W-J, Chung D, Bae SS, Kwon YM, Cho E-S, Choi G. Characterization and Biological Activities of Yeasts Isolated from Marine Environments. Microbiology Research. 2023; 14(4):1984-1999. https://doi.org/10.3390/microbiolres14040134
Chicago/Turabian StyleYu, Woon-Jong, Dawoon Chung, Seung Seob Bae, Yong Min Kwon, Eun-Seo Cho, and Grace Choi. 2023. "Characterization and Biological Activities of Yeasts Isolated from Marine Environments" Microbiology Research 14, no. 4: 1984-1999. https://doi.org/10.3390/microbiolres14040134
APA StyleYu, W.-J., Chung, D., Bae, S. S., Kwon, Y. M., Cho, E.-S., & Choi, G. (2023). Characterization and Biological Activities of Yeasts Isolated from Marine Environments. Microbiology Research, 14(4), 1984-1999. https://doi.org/10.3390/microbiolres14040134






