Abstract
Background/Objectives: This study aimed to determine the prevalence of persistent symptoms and the radiological and laboratory evolution at 6 months and 5 years after discharge in patients hospitalized for SARS-CoV-2 pneumonia during the first wave of the pandemic in Spain and to estimate the healthcare impact of their follow-up. Methods: A retrospective longitudinal observational study was conducted at the “Hospital Central de la Defensa”. A total of 200 patients aged >18 years with a diagnosis of SARS-CoV-2 pneumonia were screened. Clinical, radiological, and laboratory data were collected from electronic medical records. Patients with symptoms or radiological abnormalities at discharge underwent in-person evaluations, while the remainder were assessed by telephone. Results: A total of 182 patients met the inclusion and exclusion criteria. Of these, 112 were assessed in the outpatient setting; 60.7% required in-person evaluations, with normal pulmonary auscultation in 93.6%, complete radiological resolution in 85%, and normalized laboratory parameters in almost all cases. At 6 months, 26.5% presented at least one residual symptom, whereas only three patients (4.5%) reported symptoms at 5 years. No risk factors associated with symptom persistence were identified. The estimated cumulative healthcare cost was EUR 21,627.50. Conclusions: Among patients hospitalized for SARS-CoV-2 pneumonia during the first wave of the pandemic, 26.7% and 4.46% presented at least one persistent symptom at 6 months and 5 years after discharge, respectively.
1. Introduction
The term “post-COVID-19 condition” (PCC), also commonly referred to as long COVID, refers to a range of signs and symptoms that appear or persist after the acute phase of SARS-CoV-2 infection, most commonly including fatigue, often exacerbated by physical or mental exertion, dyspnea, muscle and joint pain, chronic cough, and cognitive disturbances such as brain fog, impaired concentration, and memory problems [1,2,3,4]. According to the World Health Organization (WHO), PCC is characterized by symptoms that usually start within three months of the initial COVID-19 illness, last for at least two months, and cannot be explained by an alternative diagnosis [5]. It is a multisystem condition with a highly variable presentation. Currently, there is no unified consensus among international institutions regarding its definition, particularly concerning the terminology and the temporal criteria considered [6]. This lack of homogeneity, together with the nonspecific nature of many symptoms, hampers comparison across studies and the drawing of solid conclusions.
The World Health Organization (WHO) estimates that the prevalence of PCC in the general population ranges between 10% and 20%. However, these figures have been progressively revised, especially following the introduction of vaccination, with reported rates decreasing from 50 to 70% in hospitalized patients during the first pandemic waves to around 10% in the post-vaccination period [7]. It is estimated that 400 million people worldwide are affected [8].
The etiopathogenic mechanisms and precise risk factors of PCC have not been conclusively characterized, with considerable discrepancies in the literature that limit the development of optimal and specific therapeutic approaches [9]. To date, the severity of the acute COVID-19 episode remains one of the most consistently supported risk factors [10,11,12] along with other factors such as sex, age, and the presence of comorbidities [13,14,15]. This is closely related to both vaccination status and viral variant, which have also been identified as key determinants influencing disease severity and long-term outcomes [9,16]. Patients affected during the first waves, before the vaccination campaign and lack of immunity by natural infection, tended to experience more severe acute disease, which may partly explain the higher incidence of PCC observed in this population [17,18]. In particular, patients infected with the wild-type strain were managed according to non-standardized treatment protocols, potentially leading to unknown long-term consequences [19]. In addition, available evidence supports that the early SARS-CoV-2 variants are, per se, associated with a higher risk of developing post-COVID-19 condition compared with the Omicron variant, independently of vaccination status [20]. Nevertheless, other authors point out that, despite this lower individual risk, the very high number of Omicron infections has resulted in a substantial absolute burden of patients with persistent symptoms [21].
Further scientific evidence is needed to better characterize this condition and to guide healthcare resource allocation and follow-up strategies, considering its significant socioeconomic impact and the large number of individuals still affected worldwide.
In this context, the present study analyzes a cohort of patients hospitalized for COVID-19 pneumonia in a tertiary hospital, defined as a referral center providing specialized and advanced medical care, during the first pandemic wave in Spain, with follow-up assessments at 6 months and 5 years after discharge. The main objective was to determine the prevalence and nature of persistent symptoms at both time points. Secondary objectives included evaluating radiological and laboratory outcomes, identifying potential risk factors for PCC, and estimating the cumulative healthcare impact.
2. Materials and Methods
A longitudinal, retrospective, observational study was conducted. All patients hospitalized between February and March 2020 at the Central Defense Hospital “Gómez Ulla” were identified and reviewed through the electronic medical record system. A consecutive, non-probabilistic sampling method was applied.
Patients aged over 18 years who had a discharge diagnosis of SARS-CoV-2 pneumonia confirmed by a positive PCR test, or with compatible clinical and radiological findings, were included, in accordance with national recommendations at that time [22]. Patients whose medical records revealed alternative diagnoses or those with nosocomial SARS-CoV-2 pneumonia were excluded.
Clinical data from the hospitalization period and from a specific post-COVID-19 follow-up clinic created for these patients at the Central Defense Hospital were collected. The first assessment was conducted by telephone, during which the need for an in-person evaluation was determined using a structured checklist (Supplementary File S1). Patients who reported persistent symptoms during the telephone assessment or who presented radiological abnormalities on their last chest X-ray prior to discharge were reevaluated on an outpatient basis with clinical, laboratory, and radiological follow-up, according to a standardized protocol. Only symptoms that the attending physician attributed to PCC and recorded in the electronic medical record were considered. Patients could continue follow-up in this clinic or be referred to other specialists if necessary. To evaluate the symptoms attributed to long COVID during the long-term follow-up, including the 5-year assessment, the symptoms documented by specialists in the specific post-COVID-19 clinic at the Central Defense Hospital were considered. These diagnoses were included in the medical reports as part of the clinical assessment and were verified to meet the WHO criteria for PCC, which requires that symptoms appear within three months of the acute infection, persist for at least two months, and cannot be explained by alternative diagnoses, as mentioned in the Introduction.
The economic analysis was designed as an exploratory subanalysis aimed at estimating the cumulative healthcare impact of patient follow-up. To this end, the number of medical visits and complementary tests performed was recorded, and official tariffs published in the Boletín Oficial del Estado (Official State Gazette, BOE No. 105, 1 May 2025) were applied.
The study was approved by the Ethics Committee of the Central Defense Hospital “Gómez Ulla”, which granted a waiver of informed consent due to the retrospective nature of the study and the restrictive circumstances of the pandemic. Data were obtained from the electronic medical record system (HCIS), and when information was unavailable, the HORUS platform was consulted to access records from primary care or other healthcare centers.
Statistical analysis was performed using SPSS® software version 25 (IBM, Armonk, NY, USA). Both descriptive and analytical procedures were applied according to the nature of the variables. For quantitative variables, depending on whether the assumption of normality was met (assessed using the Kolmogorov–Smirnov test), data were expressed as mean and standard deviation (SD) for normally distributed variables or as median (Md) and interquartile range (IQR) otherwise. Categorical variables were summarized as absolute and relative frequencies (percentages).
To explore potential risk factors associated with the development of PCC, the chi-square test was used for categorical variables, and Student’s t-test or the Mann–Whitney U test was applied for quantitative variables, as appropriate. Chi-square tests were applied exclusively for exploratory purposes. No multivariate or adjusted analyses were performed due to sample size constraints. Odds ratios and 95% confidence intervals were not reported because the small cell sizes in the contingency tables would not yield reliable estimates.
Generative artificial intelligence (ChatGPT, GPT-5.2, OpenAI, San Francisco, CA, USA) was used solely to assist with minor grammatical and stylistic improvements. No AI tools were used for data analysis, interpretation, or content generation.
3. Results
During the study period, 200 patients were screened. After reviewing medical records, 182 patients with a diagnosis of SARS-CoV-2 pneumonia met the inclusion criteria, and none met the exclusion criteria. Among them, 68 patients (37.3%) died during hospitalization. Two additional patients (1.6%) died within the six months following hospital discharge (Figure 1).
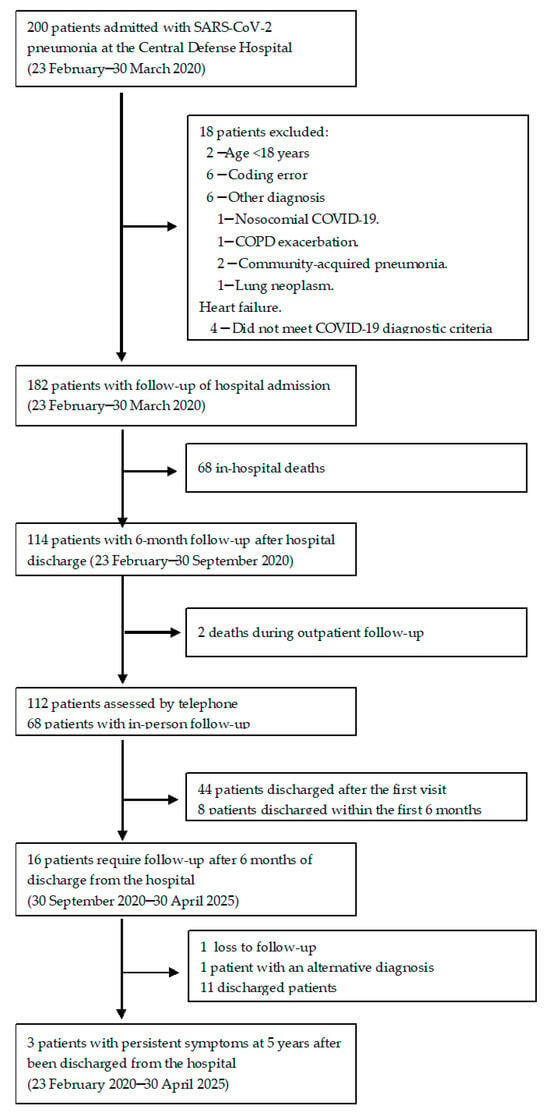
Figure 1.
Flow diagram illustrating the inclusion, exclusion, and follow-up process of patients hospitalized with SARS-CoV-2 pneumonia during the first pandemic wave.
3.1. Clinical Assessment of Patients
A total of 112 patients were evaluated in the post-COVID-19 follow-up clinic by telephone. Among them, 60.7% (n = 68) required in-person follow-up at a mean of 119 days (SD = 101.3) after hospital discharge. Table 1 summarizes the clinical, radiological, and laboratory variables recorded during the first in-person assessment.

Table 1.
Description of clinical, radiological, and laboratory findings in patients evaluated in the post-COVID-19 clinic.
Overall, 26.5% (n = 30) of patients reported at least one residual symptom. Respiratory complaints were the most frequent, followed by myalgia and headache. On physical examination, pulmonary auscultation revealed preserved vesicular breath sounds in 93.6% of patients, while 6.4% presented with crackles. The mean baseline oxygen saturation was 97.5% (SD = 1.48).
Regarding radiological follow-up, 85% of patients showed complete resolution of lung lesions, whereas 14.5% exhibited improvement of infiltrates without deterioration. In the follow-up laboratory tests, mean inflammatory and prothrombotic markers were within normal ranges at six months; therefore, no further routine follow-up was deemed necessary.
Table 2 compares patients with persistent symptoms (n = 30) and asymptomatic patients (n = 82). No statistically significant differences were found between the two groups in anthropometric variables, pre-existing comorbidities, specific treatments received for SARS-CoV-2 pneumonia, length of hospital stay, proportion of patients requiring supplemental oxygen, or mean values of inflammatory and coagulation markers obtained during hospitalization.

Table 2.
Factors associated with the presence of symptoms at 180 days after hospital discharge.
Among the 68 patients with in-person follow-up, 64.7% (n = 44) were discharged after the first visit, and an additional 11.8% (n = 8) within the first six months. The remaining 23.5% (n = 16) required prolonged follow-up, with a median of 144 additional days (IQR = 1016) beyond the first six months and a mean of 2.16 (SD = 2.56) extra visits in the PCC clinic (range: 1–12).
Follow-up was completed once patients achieved resolution of symptoms and normalization of complementary tests. Only one patient was lost to follow-up, and one was discharged due to an alternative diagnosis of lung cancer and subsequently referred to oncology.
At five years, only three patients (4.46%) continued to experience symptoms, two of whom remained under active follow-up. Table 3 summarizes their baseline characteristics, the acute episode of SARS-CoV-2 pneumonia, findings from the initial post-COVID-19 assessment, and the long-term follow-up data.

Table 3.
Five-year follow-up characteristics of symptomatic patients.
One patient presented with persistent dyspnea and cough since the acute phase and developed arthralgia during the fourth year of follow-up. Another maintained constant anosmia and ageusia from onset, accompanied by fluctuating fatigue; this patient was eventually discharged during follow-up. The third patient developed post-COVID-19 myasthenia gravis and anterior ischemic optic neuropathy (AION) associated with post-COVID-19 choroiditis.
3.2. Healthcare Cost Assessment
During the follow-up period, a total of 177 evaluations were performed in the post-COVID-19 outpatient clinic, with a unit cost of EUR 84.98, resulting in a total expenditure of EUR 11,633.03.
Thirty-one referrals to other specialties were made, generating an accumulated cost of EUR 2634.38. Various complementary tests were requested according to patients’ symptoms and clinical evolution, with a total cost of EUR 7360.09. The complete distribution of referrals and diagnostic tests is presented in Table 4.

Table 4.
Distribution of healthcare costs associated with post-COVID-19 follow-up (n = 68).
The overall estimated healthcare expenditure for post-COVID-19 follow-up in this cohort amounted to EUR 21,627.50, corresponding to an average cost of approximately EUR 318 per patient who required in-person follow-up.
4. Discussion
SARS-CoV-2 infection has generated great interest regarding its medium- and long-term sequelae. The prevalence and characteristics of post-COVID-19 symptoms vary considerably across studies, particularly during the first pandemic waves and the pre-vaccination era, as in the case of our cohort. Follow-up in post-COVID-19 clinics has emerged as a key tool to identify, evaluate, and manage these sequelae.
In the present cohort, 26.7% of patients evaluated in the post-COVID-19 clinic reported at least one symptom six months after discharge, a proportion lower than that described by other authors in hospitalized patients from the same period [23]. This difference may be partly explained by the severity of the acute episode. Huang et al. published an observational study with a six-month follow-up in hospitalized COVID-19 patients [24]. In their cohort, the mean duration of hospital stay was 14 days; 68% required oxygen therapy via nasal cannula or Venturi mask, and 11% required high-flow nasal cannula, noninvasive ventilation, or invasive mechanical ventilation. In that study, 76% of patients reported at least one persistent symptom, with those who required intensive care showing a higher prevalence of long-term symptoms compared with patients who did not require respiratory support.
In our cohort, in-hospital mortality was 37%, similar to other Spanish series from the first wave, which reported rates around 28% [25]. However, our patients had shorter hospital stays and lower oxygen requirements, indicating milder clinical courses than those described in the study. These cases correspond to the earliest phase of the pandemic, when admission criteria were more lenient, just before the collapse of the healthcare system. Consequently, many of these patients might have been managed on an outpatient basis. The incidence of PCC among non-hospitalized and non-vaccinated patients during the first wave is between 10 and 37% [21,26]. Given that, globally, most COVID-19 cases were mild and managed on an outpatient basis, the absolute number of individuals with PCC is higher among those with initially mild disease compared with hospitalized patients [27,28]. For this reason, the NICE guidelines on PCC management emphasize the need to consider all patients, regardless of the severity of the acute illness [29].
Respiratory symptoms were the most frequent findings during post-COVID-19 follow-up in our cohort. The absence of anosmia and the low prevalence of “brain fog,” commonly reported by other authors, are notable [30,31]. In our study, symptoms were collected via telephone interviews with open-ended questions, followed by an in-person clinical evaluation. This method may have underestimated some symptoms, as patients might not spontaneously report them, and physicians did not use standardized questionnaires.
No specific factors were associated with the presence of persistent symptoms at six months. Several studies have identified age as a potential risk factor for developing PCC [32,33]. However, recent evidence suggests that the severity of the acute episode has a stronger influence on the development of PCC than age itself [34]. In a prospective multicenter analysis by V. Daitch et al., age was not an independent predictor of symptom persistence at five months, highlighting the influence of other variables such as in-hospital complications or pre-existing comorbidities [35]. Our findings are consistent with these results.
Regarding sex differences, our study observed a higher absolute number of symptomatic men, consistent with the distribution at admission. In contrast, Notarte et al. published a 2022 meta-analysis identifying female sex as a significant risk factor for PCC, with an odds ratio (OR) of 1.48 (95% CI: 1.17–1.86, p = 0.01) and moderate heterogeneity across studies [9]. This finding has been supported by multiple investigations, though methodological biases have also been noted [13,15]. Some authors suggest that women may be more likely to report symptoms or seek medical attention [36], a phenomenon documented in the literature on gender differences in healthcare access, which could bias results by increasing the apparent prevalence in women [37].
Regarding treatments used during the first wave, hydroxychloroquine and azithromycin were administered more frequently among symptomatic patients, though without statistically significant differences. The available literature on this aspect is limited. Among the pharmacological treatments evaluated, only the use of remdesivir has been associated with a significant reduction in persistent symptoms in some studies [19,38,39].
Complementary tests revealed complete or partial radiological resolution of lung lesions and normalization of laboratory parameters in most patients. Berry C. et al. evaluated 443 patients in nine months and compared them with controls from the general population [40]. They found no significant radiological abnormalities among patients with mild COVID-19 and reported similar NYHA functional class between groups. Currently, no reliable biomarkers have been identified for the detection of PCC, either during hospitalization or in follow-up [41]. Some authors have suggested that D-dimer could aid in post-discharge monitoring, although the evidence remains limited [42]. It is important to note that the absence of laboratory or imaging abnormalities does not exclude the presence of persistent clinical symptoms, as documented in numerous studies [43].
To date, no studies have reported five-year follow-up outcomes. Vallée et al. published a prospective cohort study with a 3.5-year follow-up of 85 patients, in which 25% continued to experience symptoms consistent with PCC [44]. Although symptom severity improved over time, patients reported a lower quality of life than controls.
In our cohort, only three patients continued to experience symptoms attributable to PCC five years after discharge, according to the assessment of the responsible physicians. Their profiles were highly heterogeneous in terms of age, all with a history of mild pneumonia and no relevant comorbidities. Two patients had persistent abnormal lung auscultation findings, and one had residual radiological abnormalities, with no laboratory alterations during the first post-COVID-19 assessment. These data highlight that the assessment of persistent symptoms, ranging from psychological to somatic manifestations, is complex in clinical practice and that patients should be managed in specialized post-COVID-19 clinics.
Recent guidelines, such as those issued by the American Academy of Physical Medicine and Rehabilitation (AAPM&R) [45], recommend a holistic evaluation starting three months after the acute phase. They emphasize the importance of validating patients’ symptoms, performing targeted physical examinations, and maintaining a proactive diagnostic approach to identify alternative or concomitant pathologies.
These findings suggest a general trend toward spontaneous recovery while underscoring the clinical heterogeneity of the syndrome and the challenge of predicting its long-term course.
In this study, the mean time to spontaneous clinical resolution was 11 months after hospitalization, consistent with a meta-analysis including 1.2 million cases, which reported a mean of nine months among hospitalized patients (95% CI: 7–12). In that analysis, 15.1% of patients remained symptomatic after 12 months [46]. In our cohort, approximately half of the patients who were symptomatic at six months required follow-up beyond the initial visit, reinforcing the importance of monitoring patients with persistent early symptoms.
Although guidelines such as NICE [29] and AAPM&R [47] provide recommendations for PCC management, no standardized follow-up model currently exists, and the wide variability of symptoms complicates the design of uniform protocols. This situation carries significant healthcare and economic implications [48].
Beyond the individual clinical burden, PCC has been associated with a substantial socioeconomic impact at a population level, affecting healthcare systems, labor participation, and productivity [49]. Recent estimates suggest that millions of individuals worldwide experience reduced work capacity or prolonged work absence due to persistent post-COVID-19 symptoms, resulting in significant indirect costs related to loss of productivity and increased reliance on social support systems [8,49]. Economic analyses have estimated that the annual global economic toll of PCC may reach approximately 1% of the global gross domestic product, highlighting its relevance as a public health and economic challenge [8].
In the United Kingdom, a matched cohort study was conducted, identifying 52,988 individuals with a diagnosis of long COVID and 264,867 matched controls without long COVID. Healthcare utilization was assessed across primary care consultations, prescriptions, hospital admissions, emergency department visits, and outpatient appointments. During a 12-month follow-up, patients with long COVID showed a 49% higher overall use of healthcare services and approximately twice as many annual visits compared with controls (around 30 versus 16 visits per person per year), with statistically significant differences. Additionally, individuals with long COVID were more likely to incur healthcare expenditures and had 44% higher costs, with an average annual cost of approximately GBP 2500 per patient, compared with GBP 1500 in the comparator group [50].
David Cutler, in 2022, estimated the overall economic burden at USD 3.7 trillion in the United States. Nearly 60% of this cost was attributed to loss of quality of life, while the remaining proportion was related to decreased earnings and increased healthcare expenditure [49].
In our study, the total estimated healthcare expenditure exceeded EUR 21,000, with an average cost of EUR 318 per patient requiring in-person follow-up. Of all evaluated patients, 35.3% required prolonged follow-up, with an average of five visits per patient, 31 specialty referrals, and high-cost complementary tests such as CT, MRI, or transthoracic echocardiography. These costs were lower than those reported in other studies, mainly because many patients became asymptomatic within a short period of time, limiting direct comparability of results.
This healthcare burden highlights the need to establish follow-up protocols stratified by disease severity and clinical profile to optimize resource utilization and avoid unnecessary testing [51]. Furthermore, prolonged follow-up allowed the detection of alternative diagnoses, such as lung cancer or exacerbations of chronic diseases, underscoring the importance of maintaining diagnostic vigilance and avoiding misattribution of symptoms.
This study has several limitations that should be acknowledged. First, its retrospective design and single-center setting, and the number of symptomatic patients limit the generalizability of the findings and could have led to an underestimation of the actual burden of persistent symptoms and risk factors. Moreover, admission criteria during the first pandemic wave were not standardized and evolved over time, which may have biased the sample toward less severe cases.
Second, symptom collection was performed through telephone interviews using open-ended questions rather than validated questionnaires. This approach may have underestimated the prevalence of residual manifestations such as anosmia, cognitive impairment (“brain fog”), and fatigue.
Third, the absence of statistically significant associations between potential risk factors and the development of PCC should be interpreted with caution, as it may reflect limited statistical power rather than a true lack of correlation. The associations are purely descriptive, and causality cannot be inferred.
Finally, indirect costs such as work absenteeism, chronic medication use, or decreased productivity were not included in the economic analysis, and no systematic assessment of health-related quality of life was performed.
5. Conclusions
Among patients hospitalized for SARS-CoV-2 pneumonia during the first wave of the pandemic, 26.7% reported at least one persistent symptom at six months, while only 4.46% remained symptomatic at five years after discharge.
No clinical, laboratory, or radiological parameters were found to predict the long-term persistence of symptoms or the development of PCC.
The estimated direct healthcare cost of post-COVID-19 follow-up was EUR 381 per patient, underscoring the relevance of resource planning and the need for targeted, evidence-based follow-up strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/idr18010008/s1; Supplementary File S1: telephone consultation list.
Author Contributions
Conceptualization, A.R.C. and M.E.M.; methodology, A.R.C. and M.E.M.; software, F.J.M.d.N.; formal analysis, A.R.C.; writing—original draft preparation, A.R.C. and M.E.M.; writing—review and editing, C.G.O. and M.N.T.; visualization, A.R.C.; supervision, M.E.M., M.N.T. and F.J.M.d.N. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by Foundation SEIMC-GESIDA.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of “Hospital Central de la Defensa Gómez Ulla” 51/20. tfr. ref. COVID-19 Research Area (approved on 29 December 2025).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the restrictive circumstances of the pandemic.
Data Availability Statement
The data presented in this study are available on reasonable request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
Generative artificial intelligence tools (ChatGPT, GPT-5.2, OpenAI, San Francisco, CA, USA)were used exclusively for minor language editing. The authors are fully responsible for the content of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PCC | Post-COVID-19 condition |
| AAPM&R | American Academy of Physical Medicine and Rehabilitation |
| AION | Anterior ischemic optic neuropathy |
| AST | Aspartate aminotransferase |
| COVID-19 | Coronavirus disease 2019 |
| CRP | C-reactive protein |
| CT | Computed tomography |
| HCIS | Hospital Clinical Information System |
| IQR | Interquartile range |
| LDH | Lactate dehydrogenase |
| MD | Median |
| MRI | Magnetic resonance imaging |
| PCR | Polymerase chain reaction |
| PCT | Procalcitonin |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SD | Standard deviation |
| SPSS | Statistical Package for the Social Sciences |
| WHO | World Health Organization |
References
- Hope, A.A.; Evering, T.H. Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Infect. Dis. Clin. N. Am. 2022, 36, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, M.; Elliott, J.; Chadeau-Hyam, M.; Riley, S.; Darzi, A.; Cooke, G.; Ward, H.; Elliott, P. Persistent COVID-19 symptoms in a community study of 606,434 people in England. Nat. Commun. 2022, 13, 1957. [Google Scholar] [CrossRef] [PubMed]
- Chudzik, M.; Babicki, M.; Kapusta, J.; Kałuzińska-Kołat, Ż.; Kołat, D.; Jankowski, P.; Mastalerz-Migas, A. Long-COVID Clinical Features and Risk Factors: A Retrospective Analysis of Patients from the STOP-COVID Registry of the PoLoCOV Study. Viruses 2022, 14, 1755. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid—Mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Post COVID-19 Condition (Long COVID). Fact Sheet. WHO, Geneva. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/post-covid-19-condition-(long-covid) (accessed on 7 November 2025).
- Sociedad Española de Médicos Generales y de Familia (SEMG). Guía Clínica Para la Atención al Paciente Long COVID/COVID Persistente; SEMG: Madrid, Spain, 2021; ISBN 978-84-18576-44-0. [Google Scholar]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Al-Aly, Z.; Davis, H.; McCorkell, L.; Soares, L.; Wulf-Hanson, S.; Iwasaki, A.; Topol, E.J. Long COVID science, research and policy. Nat. Med. 2024, 30, 2148–2164. [Google Scholar] [CrossRef]
- Notarte, K.I.; de Oliveira, M.H.S.; Peligro, P.J.; Velasco, J.V.; Macaranas, I.; Ver, A.T.; Pangilinan, F.C.; Pastrana, A.; Goldrich, N.; Kavteladze, D.; et al. Age, Sex and Previous Comorbidities as Risk Factors Not Associated with SARS-CoV-2 Infection for Long COVID-19: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 7314. [Google Scholar] [CrossRef]
- Blomberg, B.; Mohn, K.G.-I.; Brokstad, K.A.; Zhou, F.; Linchausen, D.W.; Hansen, B.-A.; Lartey, S.; Onyango, T.B.; Kuwelker, K.; Sævik, M.; et al. Long COVID in a prospective cohort of home-isolated patients. Nat. Med. 2021, 27, 1607–1613. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Tolba, M.; Omirah, M.A.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef]
- Zaazouee, M.S.; Nada, E.A.; Al-Kafarna, M.; Shaheen, A.; Ramu, S.K.; Hafez, A.H.; Matar, S.G.; Assar, A.; Elshennawy, M.; Abu El-Enien, H.; et al. A multinational cross-sectional study on the prevalence and predictors of long COVID across 33 countries. Sci. Rep. 2025, 15, 28299. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat. Med. 2022, 28, 1706–1714. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Xu, M.; Li, J.; Liu, Y.; Zhang, J.; Xu, Y.; Dong, W. Clinical sequelae of COVID-19 survivors in Wuhan, China: A single-centre longitudinal study. Clin. Microbiol. Infect. 2021, 27, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Cegolon, L.; Mauro, M.; Sansone, D.; Tassinari, A.; Gobba, F.M.; Modenese, A.; Casolari, L.; Liviero, F.; Pavanello, S.; Scapellato, M.L.; et al. A Multi-Center Study Investigating Long COVID-19 in Healthcare Workers from North-Eastern Italy: Prevalence, Risk Factors and the Impact of Pre-Existing Humoral Immunity—ORCHESTRA Project. Vaccines 2023, 11, 1769. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Boglione, L.; Meli, G.; Poletti, F.; Rostagno, R.; Moglia, R.; Cantone, M.; Esposito, M.; Scianguetta, C.; Domenicale, B.; Di Pasquale, F.; et al. Risk factors and incidence of long-COVID syndrome in hospitalized patients: Does remdesivir have a protective effect? QJM Int. J. Med. 2021, 114, 865–871. [Google Scholar] [CrossRef]
- Diexer, S.; Klee, B.; Gottschick, C.; Xu, C.; Broda, A.; Purschke, O.; Binder, M.; Frese, T.; Girndt, M.; Hoell, J.I.; et al. Association between virus variants, vaccination, previous infections, and post-COVID-19 risk. Int. J. Infect. Diseases. 2023, 136, 14–21. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J. Long COVID: A clinical update. Lancet 2024, 404, 707–724. [Google Scholar] [CrossRef]
- Spanish Ministry of Health. Strategy for Early Detection, Surveillance and Control of COVID-19 (Updated 22 December 2021). Ministry of Health, Madrid, Spain. 2021. Available online: https://www.sanidad.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos.htm (accessed on 8 November 2025).
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Acute Sequelae of COVID-19 (PASC) or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2021, 226, 1593–1607. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-year outcomes in hospital survivors with COVID-19: A longitudinal cohort study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, J.; Ryan, P.; Jarrín, I.; Carratalà, J.; Pachón, J.; Yllescas, M.; Arriba, J.R.; Muñoz, E.A.; Gil Divasson, P.; Muñiz, P.G.; et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin. Microbiol. Infect. 2020, 26, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA 2021, 325, 2015–2016. [Google Scholar] [CrossRef] [PubMed]
- Peluso, M.J.; Deeks, S.G. Mechanisms of long COVID and the path toward therapeutics. Cell 2024, 187, 5500–5529. [Google Scholar] [CrossRef]
- Long COVID. Household Pulse Survey. National Center for Health Statistics. Available online: https://www.cdc.gov/nchs/covid19/pulse/long-covid.htm (accessed on 6 March 2023).
- National Institute for Health and Care Excellence (NICE). COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19 (NG188). London: NICE. 2020. Available online: https://www.nice.org.uk/guidance/ng188 (accessed on 28 January 2023).
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Long COVID syndrome-associated brain fog. J. Med. Virol. 2022, 94, 979–984. [Google Scholar] [CrossRef]
- Graham, E.L.; Clark, J.R.; Orban, Z.S.; Lim, P.H.; Szymanski, A.L.; Taylor, C.; DiBiase, R.M.; Jia, D.T.; Balabanov, R.; Ho, S.U.; et al. Persistent neurologic symptoms and cognitive dysfunction in non-hospitalized COVID-19 “long haulers”. Ann. Clin. Transl. Neurol. 2021, 8, 1073–1085. [Google Scholar] [CrossRef]
- Finamore, P.; Arena, E.; Lupoi, D.; Savito, L.; Di Nunzio, F.; Furbatto, M.; Dragonieri, S.; Incalzi, R.A.; Scarlata, S. Long COVID Syndrome: A Narrative Review on Burden of Age and Vaccination. J. Clin. Med. 2024, 13, 4756. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Zheng, H.; Liu, L. Risk Factors for Long COVID in Older Adults. Biomedicines 2023, 11, 3002. [Google Scholar] [CrossRef]
- Evans, R.A.; McAuley, H.J.C.; Harrison, E.M.; Shikotra, A.; Singapuri, A.; Sereno, M.; Elneima, O.; Docherty, A.B.; Lone, N.I.; Leavy, O.C.; et al. Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): A UK multicentre, prospective cohort study. Lancet Respir. Med. 2021, 9, 1275–1287. [Google Scholar] [CrossRef]
- Daitch, V.; Yelin, D.; Awwad, M.; Guaraldi, G.; Milić, J.; Mussini, C.; Falcone, M.; Tiseo, G.; Carrozzi, L.; Pistelli, F.; et al. Characteristics of long-COVID among older adults: A cross-sectional study. Int. J. Infect. Dis. 2022, 125, 287–293. [Google Scholar] [CrossRef]
- Redondo-Sendino, Á.; Guallar-Castillón, P.; Banegas, J.R.; Rodríguez-Artalejo, F. Gender differences in the utilization of health-care services among the older adult population of Spain. BMC Public Health 2006, 6, 155. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.S.R.; Jawahir, S.; Manual, A.; Mutalib, N.E.A.; Noh, S.N.M.; Ab Rahim, I.; Ab Hamid, J.; Nordin, A.A. Gender differences in health-seeking behaviour: Insights from the National Health and Morbidity Survey 2019. BMC Health Serv. Res. 2025, 25, 900. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Franco-Moreno, A.; Ruiz-Ruigómez, M.; Arrieta-Ortubay, E.; Ryan-Murua, P.; Lumbreras-Bermejo, C.; Del-Valle-Loarte, P.; Pellicer-Valero, O.J.; Giordano, R.; Arendt-Nielsen, L.; et al. Is Antiviral Treatment with Remdesivir at the Acute Phase of SARS-CoV-2 Infection Effective for Decreasing the Risk of Long-Lasting Post-COVID Symptoms? Viruses 2024, 16, 947. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.; Kong, A.M.; Paredes, R.; Paone, J.; Shah, R.; Taylor, R.; Mozaffari, E.; Gupta, R.; Gottlieb, R.L.; Mateu, L.; et al. Risk of Long COVID in hospitalized individuals treated with remdesivir for acute COVID-19. Sci. Rep. 2025, 15, 27441. [Google Scholar] [CrossRef]
- Berry, C.; Bayes, H.K. Post-COVID-19 illness trajectory in community patients: Mostly reassuring results. Eur. Heart J. 2022, 43, 1138–1140. [Google Scholar] [CrossRef]
- Yong, S.J. Long COVID or post-COVID-19 syndrome: Putative pathophysiology, risk factors, and treatments. Infect. Dis. 2021, 53, 737–754. [Google Scholar] [CrossRef]
- Pasini, E.; Corsetti, G.; Romano, C.; Scarabelli, T.M.; Chen-Scarabelli, C.; Saravolatz, L.; Dioguardi, F.S. Serum Metabolic Profile in Patients with Long-Covid (PASC) Syndrome: Clinical Implications. Front. Med. 2021, 8, 714426. [Google Scholar] [CrossRef]
- Huerne, K.; Filion, K.B.; Grad, R.; Ernst, P.; Gershon, A.S.; Eisenberg, M.J. Epidemiological and clinical perspectives of long COVID syndrome. Am. J. Med. Open 2023, 9, 100033. [Google Scholar] [CrossRef]
- Vallée, G.; Xi, D.; Avramovic, G.; O’Kelly, B.; Lambert, J.S. Evaluating the longitudinal physical and psychological health effects of persistent long Covid 3.5 years after infection. PLoS ONE 2025, 20, e0326790. [Google Scholar] [CrossRef]
- Cheng, A.L.; Herman, E.; Abramoff, B.; Anderson, J.R.; Azola, A.; Baratta, J.M.; Bartels, M.N.; Bhavaraju-Sanka, R.; Blitshteyn, S.; Fine, J.S.; et al. Multidisciplinary collaborative guidance on the assessment and treatment of patients with Long COVID: A compendium statement. PM&R 2025, 17, 684–708. [Google Scholar] [CrossRef]
- Global Burden of Disease Long COVID Collaborators; Hanson, S.W.; Abbafati, C.; Aerts, J.G.; Al-Aly, Z.; Ashbaugh, C.; Ballouz, T.; Blyuss, O.; Bobkova, P.; Bonsel, G.; et al. Estimated Global Proportions of Individuals with Persistent Fatigue, Cognitive, and Respiratory Symptom Clusters Following Symptomatic COVID-19 in 2020 and 2021. JAMA 2022, 328, 1604–1615. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Menges, D.; Ballouz, T.; Anagnostopoulos, A.; Aschmann, H.E.; Domenghino, A.; Fehr, J.S.; Puhan, M.A. Burden of post-COVID-19 syndrome and implications for healthcare service planning: A population-based cohort study. PLoS ONE 2021, 16, e0254523. [Google Scholar] [CrossRef]
- Katz, G.M.; Bach, K.; Bobos, P.; Cheung, A.; Décary, S.; Goulding, S.; Herridge, M.S.; McNaughton, C.D.; Palmer, K.S.; Razak, F.A.; et al. Understanding How Post–COVID-19 Condition Affects Adults and Health Care Systems. JAMA Health Forum 2023, 4, e231933. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Henderson, A.D.; Carlile, O.; Dillingham, I.; Butler-Cole, B.F.C.; Marks, M.; Briggs, A.; Jit, M.; Tomlinson, L.A.; Bates, C.; et al. Healthcare utilisation in people with long COVID: An OpenSAFELY cohort study. BMC Med. 2024, 22, 255. [Google Scholar] [CrossRef]
- Koumpias, A.M.; Schwartzman, D.; Fleming, O. Long-haul COVID: Healthcare utilization and medical expenditures 6 months post-diagnosis. BMC Health Serv. Res. 2022, 22, 1010. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.